Featured Application
This specific application of the research aims to monitor residual chlorpyrifos in crops and agricultural soils.
Abstract
This study was conducted to investigate the residual loss of chlorpyrifos and the amount transferred to lettuce from soil. Field trials on lettuce were conducted in two different greenhouses located in Yongin (field 1) and Gwangju (field 2) in Korea. Soil and lettuce samples were collected on different days after the treatment of chlorpyrifos at two different levels. The initial residue of chlorpyrifos (0.86 and 2.31 mg/kg) in soils decreased to 0.06 and 0.18 mg/kg, respectively, at 36 days after treatment (DAT) in field 1, and the initial residue with values of 11.10 and 22.59 mg/kg decreased to 1.20 and 3.04 mg/kg, respectively, at 43 DAT in field 2. In field 1, the half-lives of chlorpyrifos were approximately 8.4 and 9.0 days for soils treated with 0.86 and 2.31 mg/kg, respectively, while in field 2, the half-lives of chlorpyrifos were approximately 18.7 and 13.9 days for soils treated with 11.10 and 22.59 mg/kg, respectively. Residue levels of chlorpyrifos on lettuce were 0.66–5.98% and 2.71–13.26% compared to the initial concentration in the soil. Therefore, the management guideline of chlorpyrifos for lettuce-cultivating soils could be suggested to be 0.75 mg/kg with regards to the Positive List System level of chlorpyrifos on lettuce of 0.01 mg/kg.
1. Introduction
Pesticides are generally utilized to manage agricultural pests, such as fungi, insects, and weeds, in order to increase crop productivity. Chlorpyrifos is a broad-spectrum chlorine-containing organophosphorus pesticide that is used for controlling agricultural and household insect pests [1,2].
This compound possesses strong inhibition on acetylcholinesterase activity and is also potentially toxic to non-target animals or humans [3]. With the results of some studies appraising the safety of chlorpyrifos for mammals, it was found that chlorpyrifos might be related to various health disorders [4,5]. Chlorpyrifos is registered in Korea for formulating a single or mixed product with some pyrethroids to control mites, aphids, and lepidopteran insects on apples, grapes, pears, peaches, cucumbers, cabbage, and mandarins [6].
After pesticides are applied, they can retain in the crop; thus, residuals have caused anxiety amongst consumers. In particular, foliar-sprayed pesticides can remain on the leaves of the target plant or drop onto the nearby soil, while the crop is growing [7]. The levels of pesticides in cultivation soils are periodically monitored by the government agency. Three pesticides such as endosulfan, procymidone, and chlorpyrifos, were frequently detected in Korean upland soil. In addition to this result, chlorpyrifos has also been shown to be one of the most detected pesticides in Korean orchard soil and in China [8,9]. Results from a four-year analysis of pesticide residues in Good Agricultural Practices (GAP) certified agricultural products reported that chlorpyrifos was one of the pesticides that showed over a 5% detection rate from samples analyzed. Besides, chlorpyrifos is one of the violating compounds that were not registered on the detected crops [10]. These results could have been due to the use of an unregistered pesticide on the crops or translocation from soil contaminated with the pesticide because some of the detected pesticides were systemic chemicals [11].
In addition to the concern of residual unregistered pesticides in crops, the Positive List System (PLS) has been introduced. In order to ensure the safety of the consumption of foodstuffs presented in pesticide residues, the maximum residue limits (MRL), which is a non-risk level, has been established for each pesticide and crop by scientific evaluation using toxicity data and food intake. However, since MRL values for the registered pesticides are varied for each crop, it is hard to secure safety when the pesticide, that is not registered for use, is sprayed or left in a crop. Therefore, the PLS has been used on unregistered pesticides for crops on a non-detected basis and the standard of non-detection in PLS is established as 0.01 ppm (mg/kg) or less.
In this regard, various reports have well-documented the levels of retained pesticides in crops/plants translocated from contaminated soils [12,13,14,15,16,17,18], especially for persistent organochlorine insecticides. The long-term persistence of chlorpyrifos results from its high stability in acidic and neutral pH conditions, while hydrolytic degradation is more rapid at alkaline pH conditions [19]. It is persistent in conditions with organic matter, leaf characteristics, decreased temperature, and ultraviolet light [20,21].
In addition to this persistence of chlorpyrifos, other parameters can affect its uptake, such as soil type. Despite the observed effect of soil type on crop uptake, there have been no apparent means of estimating the absorbed level of contaminants in various plants in different types of soils [16,17,22,23]. The uptake of O-methylcarbamoyloximes and substituted ureas with numerous lipophilicities from water solutions was studied by using barley roots [24]. They concluded that root factors (RCFs, Croot/Cwater) and translocation were not proportional to the octanol-water partition coefficient (Kow) values of those compounds. Similarly, results of empirical correlations between pollutants and plants and leaves have also been reported [25,26,27,28].
A recent report exhibited that the amount of chlorpyrifos absorbed by cucumber roots ranged from 1.0 to 1.3% of the spiked concentration in soil, and that it was not transferred to the fruits [11]. Some experiments have analyzed the levels of pesticides absorbed from soil contaminated with boscalid, chlorfenapyr, fluquinconazole, and tetraconazole. These studies showed that the levels of these pesticides in Korean cabbage or onion were less than 2.5% in comparison to the spiked concentration in the soil [29,30].
Even if the translocated pesticides in plants depends on the degree of the compounds in the soil, other parameters affecting the absorption of pesticides are the physicochemical properties of the pesticide, the type of plant, and the soil type that sustains the plant [31,32,33].
On the other hand, the Korean government agency has required the performing of an efficacy test and a phytotoxicity test on the crops in order to assess the effect of pesticides. However, the results on residual levels of pesticides on succeeding crops have not yet been requested. Therefore, if residual pesticides are transferred into the crop after use, it is likely to remain above 0.01 ppm. There have been no studies on the absorption or translocation of chlorpyrifos from soil contaminated with chlorpyrifos to lettuce, even though Hwang et al. [11] reported similar studies using the same insecticide on cucumbers. In our study, we attempted to compare the uptake rates of chlorpyrifos from chlorpyrifos-contaminated soils to lettuce, which possessed a short-growth cycle as well as significant potential to uptake soil pesticide residues due to the proximity between edible leaf parts and roots, which could be significantly influenced by soil contamination. In this paper, we reported the decline in residue in the soil after treatment with chlorpyrifos at different application rates, as well as the level of chlorpyrifos in lettuce grown in the treated soil to ensure the safety of lettuce products.
2. Materials and Methods
Chemicals. Chlorpyrifos (99.7%) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). A granule formulation (2%) for chlorpyrifos was supplied by Enbio Co. (Gunpo, Korea). A HPLC- grade of solvents such as acetone, dichloromethane (DCM), and n-hexane were purchased from Burdick and Jackson® (Ulsan, Korea). A florisil solid phase extraction (1 g) cartridge and filter paper (Whatman No. 2) were acquired from Phenomenex (Torrance, CA, USA) and Whatman International Ltd. (Maidstone, England), respectively. A rotary vacuum evaporator made by Heidolph Instruments GmbH & Co. (Schwabach, Germany) and a nitrogen evaporator designated as Hurricane-Lite made by Chongmin Tech (Seoul, Korea) were equipped for removing organic solvents. All other chemicals are of highest purity and reagent grade. Chlorpyrifos used in this study was formulated as 2% GR.
Field experiments. Greenhouses were selected for the field experiments and they were located in two different cities, which were Yongin City (field 1) and Gwangju City (field 2) in Gyeonggi Province, Korea. Soil treatment of chlorpyrifos in field 1 was conducted in triplicate plots of 5 m2 for a total of 15 m2 for each trial. The chlorpyrifos formulation was scattered over the soil at an application rate of 1.0 mg/kg and 2.0 mg/kg. Field 2 was treated with 10.0 mg/kg and 20.0 mg/kg to 10 m2 on each test plot. The lettuce seeds were sowed immediately after treatment with the pesticide. The seeding density was 20 × 20 cm. Two environmental parameters (temperature and relative humidity) during the cultivation period were recorded daily using an electric data logger (Lascar, China). The chlorpyrifos-spiked soil was collected at five different days after treatment (DAT) as 0, 7, 14, 22, 28, and 36 DAT for field 1, and 0, 7, 14, 25, 35, 39, and 43 DAT for field 2 to assess the dissipation pattern. Soil samples were left for shadow air-dryness and sieved using a 10-mesh sieve. After sieving, dried soil samples were kept in a refrigerator under −20 °C until chlorpyrifos analysis.
Additionally, a random collection was employed for selecting crop samples from each chlorpyrifos-treated plot at 22, 25, 28, 32, and 36 DAT for field 1, and at 35, 37, 39, 41, and 43 DAT for field 2. Immediately after picking, the crop samples were washed with tap water two times and the remaining water was removed with a paper tissue to remove surface contaminants. The samples were then placed into polyethylene bags and transported with ice to the laboratory where the roots were removed and aerial samples were homogenized. Then, each sample was placed into a polyethylene bottle and frozen at −20 °C until use for analysis.
Instrumental analysis and calibration curve. A stock solution for chlorpyrifos (99.7%, 10.03 mg) was prepared in 100 mL of acetone to a concentration of 100 mg/L. A working standard solution at a concentration of 20 mg/L was made by dilution using acetone and was then serially diluted to obtain various standard solutions of 0.1, 0.5, 1.0, 5.0, and 10.0 mg/L. An aliquot of 1.0 μL was used to analyze chlorpyrifos using a gas chromatography-electron capture detector (GC-ECD). A standard calibration curve was prepared based on the peak area vs. concentration. As shown in Table 1, the residual analysis conditions were modified according to the experimental conditions previously reported [11].

Table 1.
Instrumental parameters for the analysis of chlorpyrifos.
Recovery of chlorpyrifos and sample analysis. For each sample (soil: 20 g; lettuce: 10 g), a standard solution of chlorpyrifos was fortified at levels of 0.2 mg/kg and 1.0 mg/kg. Then, the sample was extracted with acetone (100 mL) by shaking at 200 rpm with a mechanical mixer for 1 h, followed by filtering through Whatman filter paper No. 2 under a vacuum. The container and the filtrate were washed again with acetone (30 mL), and the extracts were transferred to a 500 mL separatory funnel. Then, a saturated NaCl solution (10 mL), water (90 mL), and DCM (70 mL) were put into the separatory funnel and the funnel was shaken. After shaking, the lower DCM layer was drained into a 500 mL flask through anhydrous sodium sulfate. This partitioning was repeated two times using 70 mL of DCM. After this procedure, the extracts were collected, pooled, and concentrated to dryness using a rotary vacuum evaporator at 40 °C. The residue was re-dissolved in 2 mL of a mixture of acetone and hexane (20/80, v/v). The concentrated extract was loaded into a florisil SPE cartridge, which was prewashed with 5 mL of 20% acetone in hexane. Then, chlorpyrifos was eluted with 8 mL of 20% acetone in hexane. The eluted fraction was concentrated using a nitrogen evaporator, and the residue was re-dissolved in 4 mL of acetone (2 mL of acetone was used for lettuce) for analysis.
Sensitivity of the analytical method. The method limit of quantitation (MLOQ) is a practical limit of quantitation (LOQ) for the total analytical method using samples. It is calculated using the LOQ, injection volume, final solution volume, and sample weight in the analytical procedure [11].
MLOQ (mg/kg) = (LOQ × final solution volume)/(injection volume × sample weight)
MLOQ (mg/kg) = (0.1 ng × 4 mL)/(1.0 μL × 20 g) = 0.02 mg/kg (soil)
Dissipation and half-life in soil. The dissipation of chlorpyrifos in the soil was determined using a first-order kinetics regression analysis with the following model [34]:
where Ct is the concentration of the pesticide at any time t, C0 is the initial concentration, and k is the rate constant (d−1).
The half-life (DT50), which is the time at which the concentration of the initial deposit reaches 50%, was determined by the following equation:
Calculation of the crop bioconcentration ratio (CBR). The crop-to-soil bioconcentration ratio is used to relate the concentration of the chemical measured in the crop to the concentration in the soil supporting the crop. The CBR was calculated by dividing the residue concentration of the pesticide in the crop by the concentration in the soil at 0 DAT according to the following equation [35]:
3. Results and Discussion
Analytical method validation. The recovery, reproducibility, MLOQ, and linearity of the calibration curve were prepared to evaluate the analytical performance.
A calibration curve of chlorpyrifos exhibited good linearity ranging from 0.1 mg/L to 10 mg/L with the regression equation of y = 428022.4x + 35931.7. The value of the correlation coefficient (r2) was 0.9998. According to the analysis of standard solutions, 0.1 ng was determined as the LOQ, which was satisfactory for the residual analysis of chlorpyrifos. The MLOQ value of chlorpyrifos was calculated as 0.02 mg/kg for both soil and lettuce. These values met the criteria of the Korea Food and Drug Administration as it was below 0.05 mg/kg, or half of the maximum residue limit [36].
For obtaining the recovery rate, the control samples were spiked with chlorpyrifos standard solution at two different concentrations and were analyzed according to an established analytical method. Average recovery rates from the two types of soils ranged from 102.3 to 109.1%, while recoveries from lettuce ranged from 90.2% to 92.3% with low coefficients of variation of less than 3.6% and 4.6%, respectively (Table 2). In the gas chromatography chromatograms of soil and lettuce samples, there were no interfering peaks in the untreated sample and the retention time of chlorpyrifos was 4.8 min. Thus, the residual analytical method employed in this study was adequate to quantify residual chlorpyrifos in samples.

Table 2.
Recovery of chlorpyrifos from the tested soil and lettuce.
Dissipation of chlorpyrifos in the treated soil. Chlorpyrifos residue in field 1 soil treated with 1.0 mg/kg was 0.86 ± 0.09 mg/kg on the day of application, and decreased to 0.06 ± 0.01 mg/kg at 36 DAT. Meanwhile, the initial concentration of chlorpyrifos with an application of 2.0 mg/kg was 2.31 ± 0.09 mg/kg, which decreased to 0.18 ± 0.05 mg/kg after the same period (Figure 1). The regression equations of dissipation were C = 0.8437e−0.0825t (r2 = 0.9596) and C = 2.3877e−0.0766t (r2 = 0.9789) with DT50 values of 8.4 d and 9.0 d, respectively.
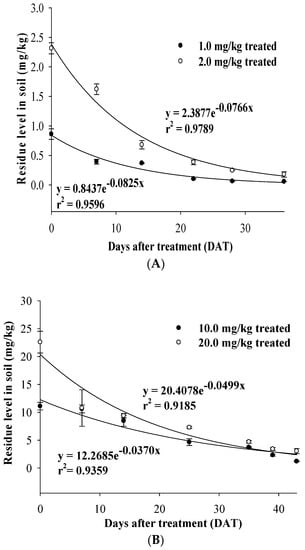
Figure 1.
Dissipation of chlorpyrifos in the treated soils. (A) Soil samples from field 1; (B) Soil samples from field 2.
In field 2 treated with applications of 10.0 mg/kg and 20.0 mg/kg of pesticide, the residue levels were reduced to 1.20 ± 0.05 mg/kg at 43 DAT from 11.10 ± 0.68 mg/kg with a regression equation of C = 12.2685e−0.0370t (r2 = 0.9359), and 22.59 ± 1.95 mg/kg at 0 DAT was degraded to 3.04 ± 0.47 mg/kg at 43 DAT with an equation of C = 20.4078e−0.0499t (r2 = 0.9185), respectively. The DT50 values were 18.7 d and 13.9 d with the application of 10.0 mg/kg and 20.0 mg/kg, respectively (Figure 1).
The degradation of chlorpyrifos in a field where cucumbers were grown showed a DT50 of 25.7–73 d. The DT50 values of chlorpyrifos in soils were reported to be 0.4–2.1 d in a cabbage field and 175–1576 d in five different soils treated with 1000 mg/kg of pesticide. The dissipation of pesticides is affected by climate conditions, treatment amount, soil conditions, and cultivation method [36]. The high log Kow and low water solubility values of chlorpyrifos can result in long retention in soil with a long DT50.
Concentration of chlorpyrifos in lettuce. Chlorpyrifos residue in lettuce grown in the field treated at the 1.0 mg/kg application rate ranged from 0.04 ± 0.00 mg/kg at 22 DAT to 0.02 ± 0.00 mg/kg at 32 DAT, while chlorpyrifos in the lettuce grown in the field with an application rate of 2.0 mg/kg ranged from 0.02 ± 0.01 mg/kg at 22 DAT to 0.05 ± 0.00 mg/kg at 32 DAT. In field 2 treated with application rates of 10.0 mg/kg and 20.0 mg/kg, residue levels were 1.47 ± 0.20 mg/kg to 0.79 ± 0.03 mg/kg and 1.03 ± 0.10 mg/kg to 0.66 ± 0.07 mg/kg, respectively (Table 3). The residue decreased in proportion to the time after treatment. The lettuce cultivated in field 2 was larger than that grown in field 1 at harvest, and showed higher residual chlorpyrifos levels (Figure 2). The residue was proportional to the amount of chemical applied. The average residue in the plot treated with an application of 10.0 mg/kg was the highest at 1.47 mg/kg, followed by 1.11 mg/kg in the plot treated with 20.0 mg/kg, and 0.12 mg/kg in the plot treated with 2.0 mg/kg.

Table 3.
Residue levels of chlorpyrifos in lettuce grown in treated soil.
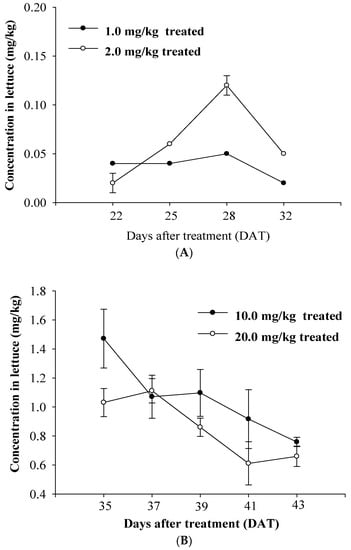
Figure 2.
Residual amount of chlorpyrifos in lettuce grown in the treated soils. (A) Lettuce samples from field 1; (B) Lettuce samples from field 2.
Crop bioconcentration ratio of chlorpyrifos. The CBR was obtained after calculation by comparing the concentration in the crop to the initial concentration in the soil. The initial concentrations of chlorpyrifos in soil were 0.86, 2.31, 11.10, and 22.59 mg/kg. The calculated CBRs are shown in Table 3. The highest average residue was 1.47 mg/kg at 35 DAT in field 2. This meant that 13.26% of the chlorpyrifos concentration compared to the initial soil concentration was observed in the lettuce. Although the MRL of chlorpyrifos for lettuce is not set, in order for concentrations in lettuce to remain under the non-detection criteria of 0.01 mg/kg required by the Positive List System, it could be suggested that the MRL be 0.75 mg/kg using the highest CBR (0.13) in soils used for the cultivation of lettuce.
The residual pesticides in soil could be absorbed by lettuce depending on the physicochemical properties of the target pesticide, especially its water solubility. Lettuce took 2.2% and 2.4% of boscalid residue, when it was grown in the soil spiked with boscalid. The uptake rates of chlorfenapyr by cabbages were 1.3% to 1.5% of the soil concentration of chlorfenapyr. In this study, lettuce absorbed 0.6–3.4% of the initial concentration in the soil. The uptake of boscalid by the lettuce was higher than that of chlorfenapyr. The water solubility of boscalid, chlorfenapyr, and chlorpyrifos were 4.6, 0.14, and 1.4 mg/L, respectively, and the log P values of these chemicals were 2.96, 4.83, and 4.7, respectively. Boscalid is more polar and water-soluble; thus, it is absorbed and translocated to the upper part of lettuce through the evaporation stream to a greater extent [29,37]. Apart from water solubility, the systemic properties of a pesticide are critical parameters for understanding the uptake and translocation of the chemical to a plant. Chlorpyrifos is a systemic insecticide absorbed from the roots that move to the upper part of the plant. Chlorfenapyr is a limited systemic, and boscalid is a foliar fungicide; however, the systemicity of boscalid is not well known [2].
Hwang et al. [11] demonstrated that plant growth, concentration dependency, and duration are important parameters in understanding the transportation pattern of chlorpyrifos in cucumbers. They found that approximately 0.03% to 0.04% of the initial concentration of chlorpyrifos in the soil was transported to the cucumbers in a green house study. With regard to this report, our results were significantly different from the results suggested by Hwang et al. [11] due to the difference in plant species. Lettuce has wider leaves and grows quickly. The residue of chlorpyrifos in lettuce was determined as approximately 0.66–5.98% and 2.71–13.26% compared to the initial concentration in field 1 soil and field 2 soil, respectively. Stevens and Baker [38] demonstrated that different uptake levels of prochloraz between plant species were dependent on the addition of surfactant, while 2,4-dichlorophenoxyacetic acid (2.4-D) was variable. Furthermore, they suggested that the uptake of chemicals might be correlated to the combined interactions of the active ingredient, surfactant, and the leaf surfaces as well as cuticular morphology, as they conducted foliar-sprayed treatment. According to the results of the previous study by [39], differences between Hwang et al. [11] and our study on the residue level of chlorpyrifos might have been caused by the different morphology of roots because of the use of pesticide pre-treated soil samples or the use of different surfactants in the formulation. As the residue levels of chlorpyrifos varied between cucumbers and lettuce, Ge et al. [40] exhibited similar results, as the translocation factor of chlorpyrifos was very different when they determined the root uptake rate using pak choi (Brassica chinensis) and lettuce at high concentration treatments of chlorpyrifos.
Brigg et al. [24] concluded that RCFs and translocation were not proportional to the Kow values of the compounds from the results of the uptakes of O-methylcarbamoyloximes and substituted ureas with different lipophilicities from a water solution by barley roots. The accumulation of non-ionic compounds by barley roots was described by the partitioning of the chemical to the root lipophilic part and uptake into the aqueous part in the roots. The lipophilic compound had a considerable partition value, but the uptake of chemicals to the polar phase was demonstrated for polar compounds. Translocation to the shoot was a passive process and was shown to have a maximum value with intermediate polarity, having log Kow values of 1.5–2.0 [24,27,28].
The contaminant in the soil could be absorbed by soil particles and by the plant root after eluting into soil water. This means that the partitioning of the pesticide between soil particles and water as well as between soil water and the plant is important for the translocation of the pesticide to the plant. The estimation of pesticide concentrations in crops based on the water extractable residue can prevent the production of crops contaminated with pesticide residues from soils.
In conclusion, chlorpyrifos residue was transferred into lettuce, and ranged from 0.66% to 13.26% of the initial concentrations in soils. Therefore, when lettuce is grown in soil treated repeatedly with chlorpyrifos, transportation of residual chlorpyrifos in the soil into the lettuce should be carefully considered because lettuce is a leafy vegetable. In addition to this, a management guideline in soil for chlorpyrifos should be established concerning its transportation regardless of foliar spray treatment. Further studies should be conducted into the edible parts of vegetables to predict plant uptake and to provide information regarding the safety of crops grown in soil contaminated with various pesticides, and differing soil type. Additionally, contaminant concentration in the soil, type of crop, cultivation period, and distribution of the absorbed pesticide should also be considered.
Author Contributions
S.-E.L. and J.-K.M. conceived and designed all of the experiments; K.-W.H. performed the experiments; S.-C.Y. and J.-K.M. analyzed the results and interpreted the data; and S.-E.L. and J.-K.M. wrote the paper.
Funding
This work was financially supported by Cooperative Project PJ010876012017 of the Rural Development Administration of Korea.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sasikala, C.; Jiwal, S.; Rout, P.; Ramya, M. Biodegradation of chlorpyrifos by bacterial consortium isolated from agriculture soil. World J. Microbiol. Biotechnol. 2012, 28, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Tomlin, C.D.S. The Pesticide Manual: A World Compendium, 15th ed.; British Crop Protection Council: Hampshire, UK, 2015. [Google Scholar]
- Oliver, G.R.; Bolles, H.G.; Shurdut, B.A. Chlorpyrifos: Probabilistic assessment of exposure and risk. Neurotoxicology 2000, 21, 203–208. [Google Scholar] [PubMed]
- Johnson, D.E.; Seidler, F.J.; Slotkin, T.A. Early biochemical detection of delayed neurotoxicity resulting from developmental exposure to chlorpyrifos. Brain Res. Bull. 1998, 45, 143–147. [Google Scholar] [CrossRef]
- Randhawa, M.A.; Anjum, F.M.; Ahmed, A.; Randhawa, M.S. Field incurred chlorpyrifos and 3,5,6-trichloro-2-pyridinol residues in fresh and processed vegetables. Food Chem. 2007, 103, 1016–1023. [Google Scholar] [CrossRef]
- Korea Crop Protection Association (KCPA). Using Guideline of Crop Protection Agents; Samjung Inc.: Seoul, Korea, 2016. [Google Scholar]
- Paterson, S.; Mackay, D. A model of organic chemical uptake by plants from soil and the atmosphere. Environ. Sci. Technol. 1994, 28, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Park, B.J.; Lee, B.M.; Kim, C.S.; Park, K.H.; Park, S.W.; Kwon, H.; Kim, J.H.; Choi, G.H.; Lim, S.J. Long-term monitoring of pesticide residues in arable soils in Korea. Korean J. Pestic. Sci. 2013, 17, 283–292. [Google Scholar] [CrossRef]
- Yuan, Y.; Chen, C.; Zheng, C.; Wang, X.; Yang, G.; Wang, Q.; Zhang, Z. Residue of chlorpyrifos and cypermethrin in vegetables and probabilistic exposure assessment for consumers in Zhejiang Province, China. Food Control 2014, 36, 63–68. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, D.S.; Kim, S.G. Analysis of recent four years situation for pesticide residues in the GAP certified agricultural products analyzed by national agricultural cooperative federation. Korean J. Pestic. Sci. 2013, 17, 271–282. [Google Scholar] [CrossRef]
- Hwang, J.I.; Jeon, S.O.; Lee, S.H.; Lee, S.E.; Hur, J.H.; Kim, K.R.; Kim, J.E. Distribution patterns of organophosphorous insecticide chlorpyrifos absorbed from soil into cucumber. Korean J. Pestic. Sci. 2014, 18, 148–155. [Google Scholar] [CrossRef]
- Li, H.; Sheng, G.; Chiou, C.T.; Xu, O. Relationship of organic contaminant equilibrium sorption and kinetic uptake in plants. Environ. Sci. Technol. 2005, 39, 4864–4870. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, E.P. Plant absorption of insecticides, absorption of some chlorinated hydrocarbon insecticide form soil into various crops. J. Agric. Food Chem. 1959, 7, 430–434. [Google Scholar] [CrossRef]
- Lichtenstein, E.P. Insecticide uptake from soils, insecticidal residues in various crops grown in soils treated with abnormal rates of aldrin and heptachlor. J. Agric. Food Chem. 1960, 8, 448–451. [Google Scholar] [CrossRef]
- Lichtenstein, E.P.; Myrdal, G.R.; Schulz, K.R. Insecticide uptake from soils, absorption of insecticidal residues from contaminated soils into five carrot varieties. J. Agric. Food Chem. 1965, 13, 126–131. [Google Scholar] [CrossRef]
- Lim, D.H.; Lim, D.S.; Keum, Y.S. Translocation of polychlorinated biphenyls in carrot-soil systems. Korean J. Pestic. Sci. 2016, 20, 203–210. [Google Scholar] [CrossRef]
- Chiou, C.T.; Sheng, G.; Manes, M. A partition-limited model for the plant uptake of organic contaminants from soil and water. Environ. Sci. Technol. 2001, 35, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Montemurro, N.; Grieco, F.; Lacertosa, G.; Visconti, A. Chlorpyrifos decline curve and residue levels from different commercial formulations applied to oranges. J. Agric. Food Chem. 2002, 50, 5975–5980. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, S.; Kookana, R.S.; Naidu, R. Degradation of bifenthrin, chlorpyifos and imidacloprid in soil and bedding materials at termiticidal application rates. Pestic. Sci. 1999, 55, 1222–1228. [Google Scholar]
- Muir, D.C.G.; Teixeira, C.A.; Alaee, M.; Hermanson, M. Persistent organohalogens and current use pesticides in remote lake waters, sediments, and ice caps. In Persistent Organic Pollutants (POPs) in the European Atmosphere: An Updated Overview; EUR 22876 EN; Institute for Environment and Sustainability, European Commission, Directorate-General, Joint Research Centre, European Commissio: Luxembourg, 2007; pp. 88–95. [Google Scholar]
- Lu, M.X.; Jiang, W.W.; Wang, J.L.; Jian, Q.; Shen, Y.; Liu, X.J.; Yu, X.Y. Persistence and dissipation of chlorpyrifos in brassica chinensis, lettuce, celery, asparagus lettuce, eggplant and pepper in a greenhouse. PLoS ONE 2014, 9, e100556. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.; McFarlane, C.; Matthies, M. Model for uptake of xenobiotics into plant: Validation with bromacil experiment. Environ. Toxicol. Chem. 1994, 13, 413–422. [Google Scholar] [CrossRef]
- Alkorta, I.; Garbisu, C. Phytoremediation of organic contaminants in soils. Bioresour. Technol. 2001, 79, 273–276. [Google Scholar] [CrossRef]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A. Relationship between lipophilicity and root uptake and translocation of nonionized chemicals by barley. Pestic. Sci. 1982, 13, 495–504. [Google Scholar] [CrossRef]
- Trapp, S. Plant Contamination: Modeling and Simulation of Organic Chemical Processes; Lewis Publishers: Boca Raton, FL, USA, 1995. [Google Scholar]
- Briggs, G.G.; Bromilow, R.H.; Evans, A.A.; Williams, M. Relationship between lipophilicity and the distribution of nonionized chemicals in barley shoots following uptake by the roots. Pestic. Sci. 1983, 14, 492–500. [Google Scholar] [CrossRef]
- Hsu, F.C.; Marxmiller, R.L.; Yang, A.Y.S. Study of root uptake and xylem translocation of cinmethylin and related compounds in detopped soybeans roots using a pressure chamber technique. Plant Physiol. 1990, 93, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Burken, J.G.; Schnoor, J.L. Predictive relationships for uptake of organic contaminants by hybrid poplar trees. Environ. Sci. Technol. 1998, 32, 3379–3385. [Google Scholar] [CrossRef]
- Jeon, S.O.; Hwang, J.I.; Lee, S.H.; Kim, J.E. Uptake of boscalid and chlorfenapyr residues in soil into Korean cabbage. Korean J. Pestic. Sci. 2014, 18, 314–320. [Google Scholar] [CrossRef]
- Lee, E.H.; Hwang, J.I.; Kim, J.E. Patterns of uptake and removal by processing types of triazole fungicides in onion. Korean J. Pestic. Sci. 2014, 19, 248–254. [Google Scholar] [CrossRef]
- Motoki, Y.; Iwafune, T.; Seike, N.; Otani, T.; Akiyama, Y. Relationship between plant uptake of pesticides and water-extractable residue in Japanese soils. J. Pestic. Sci. 2015, 40, 175–183. [Google Scholar] [CrossRef]
- Collins, C.; Fryer, M.; Grosso, A. Plant uptake of nonionic organic chemicals. Environ. Sci. Technol. 2006, 40, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Dowdy, D.L.; McKone, T.E. Predicting the bioconcentration of organic chemicals from soil or air into plants using quantitative structure-activity relationships. Environ. Toxicol. Chem. 1997, 16, 2448–2456. [Google Scholar] [CrossRef]
- Lee, Y.D. Practical Guide for Food Code Pesticide Residue Analysis (Extended Ed.); KFDA: Osong, Korea, 2013.
- Hwang, K.W.; Bang, W.S.; Jo, H.W.; Moon, J.K. Dissipation and removal of the etofenprox residue during processing in spring onion. J. Agric. Food Chem. 2015, 63, 6675–6680. [Google Scholar] [CrossRef] [PubMed]
- McKone, T.E.; Maddalena, R.L. Plant uptake of organic pollutants from soil: Bioconcentration estimates based on models and experiment. Environ. Toxicol. Chem. 2007, 26, 2494–2504. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.S. Behavior of pesticides in soil. Korean J. Pestic. Sci. 2010, 14, 303–317. [Google Scholar]
- Stevens, P.J.G.; Baker, E.A. Factors affecting the foliar absorption and redistribution of pesticides. 1. Properties of leaf surfaces and their interactions with spray droplets. Pest Manag. Sci. 1987, 19, 243–252. [Google Scholar] [CrossRef]
- Juraske, R.; Vivas, C.S.M.; Velesquez, A.E.; Santos, G.G.; Moreno, M.B.B.; Gomez, J.D.; Binder, C.R.; Hellweg, S.; Dallos, J.A.D. Pesticide uptake in potatoes: Model and field experiments. Environ. Sci. Technol. 2011, 45, 651–667. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lu, M.; Wang, D.; Zhang, Z.; Liu, X.; Yu, X. Dissipation and distribution of chlorpyrifos in selected vegetables through foliage and root uptake. Chemosphere 2016, 144, 201–206. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).