Characterization, Source and Risk of Pharmaceutically Active Compounds (PhACs) in the Snow Deposition Near Jiaozhou Bay, North China
Abstract
Abstract
Capsule Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Study Area and Sampling
2.3. Analytical Method
2.3.1. Physicochemical Parameters
2.3.2. Pharmaceutically Active Compounds (PhACs)
2.4. Quality Assurance and Quality Control
2.5. Risk Characterization
PNEC = (LC50 or EC50)/AF
2.6. Statistical Analyses
3. Results and Discussion
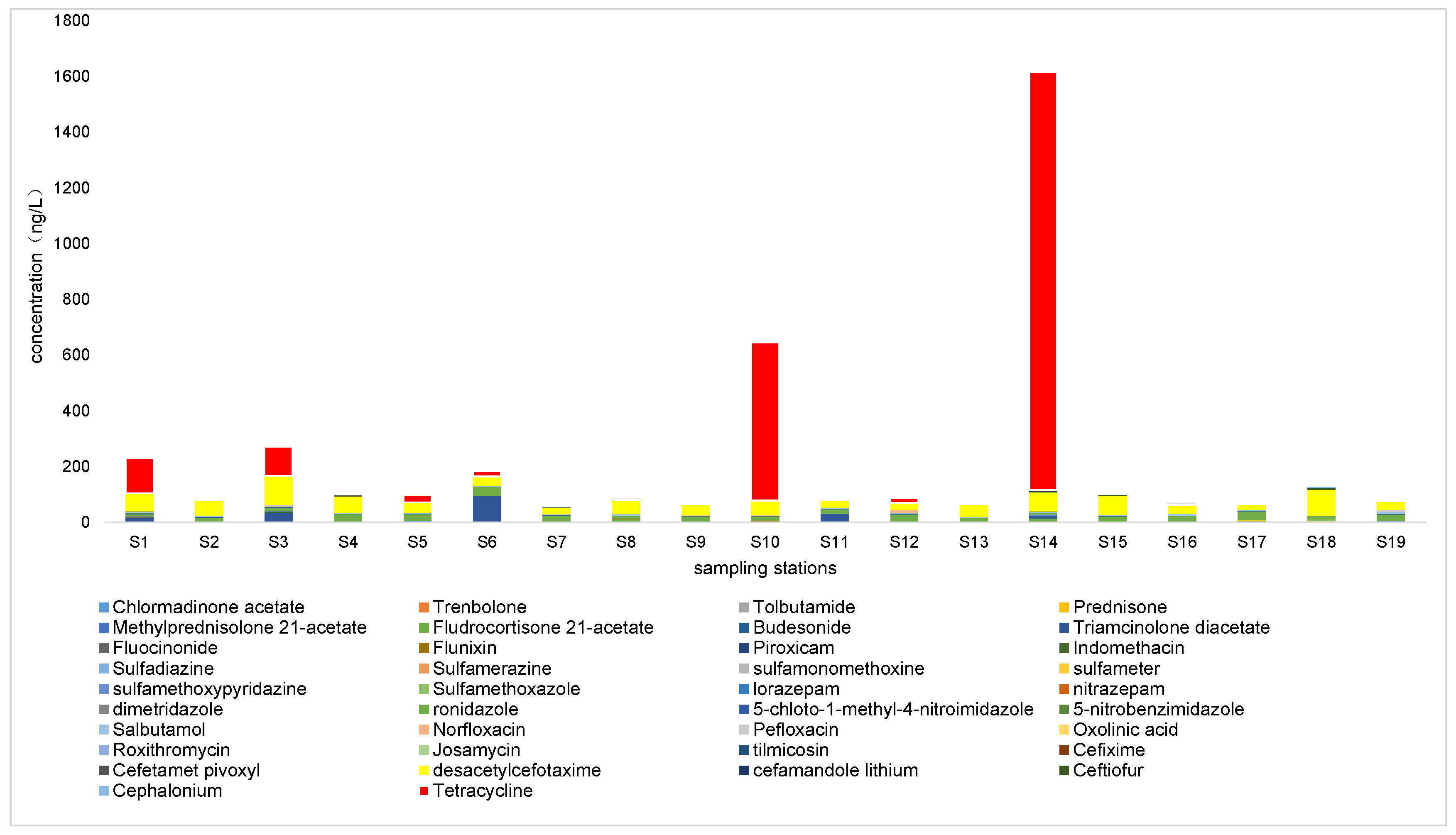
3.1. Composition Characteristics of Pharmaceuticals in Snow
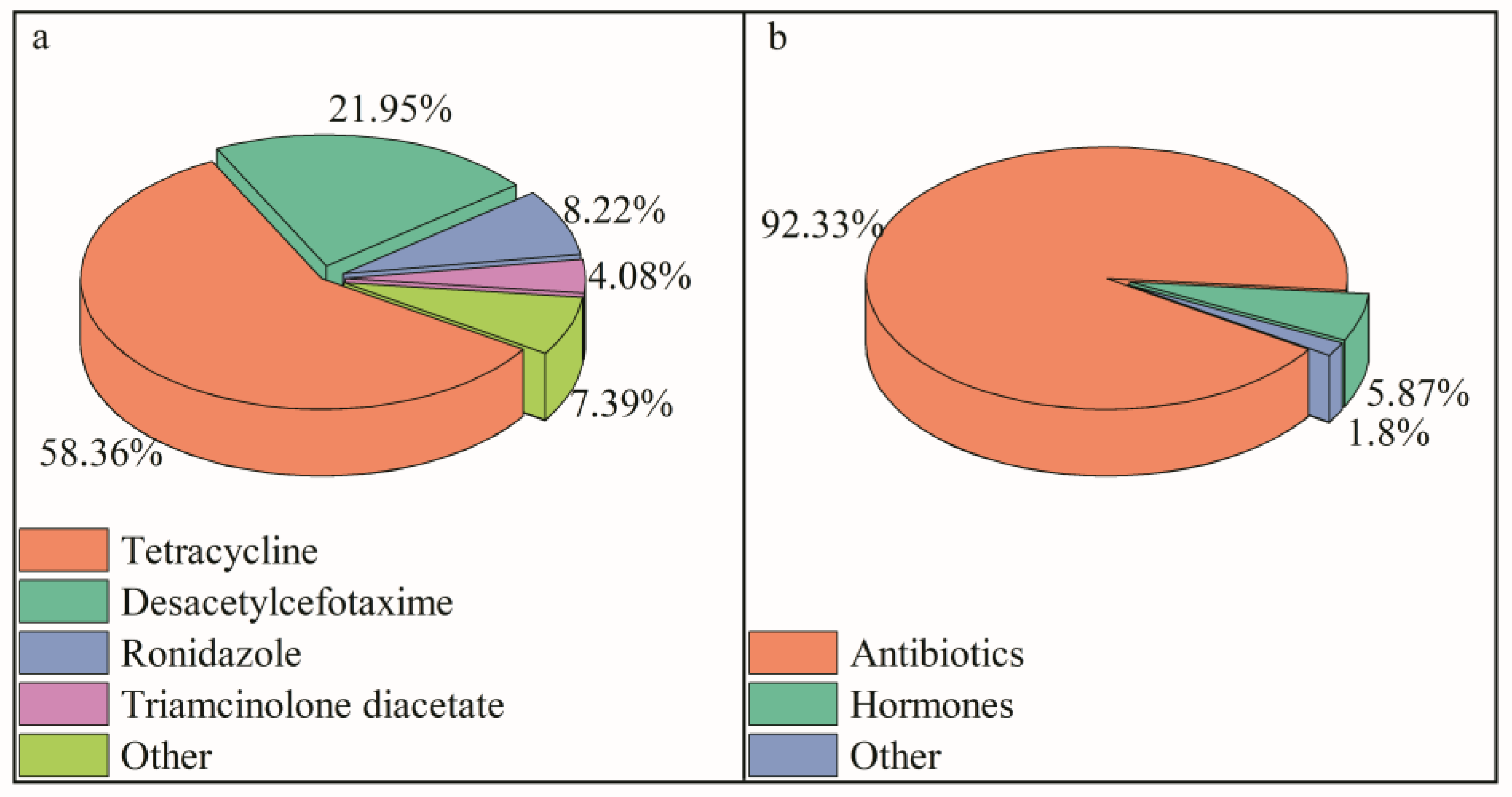
3.1.1. Antibiotics
3.1.2. Nonsteroidal Anti-Inflammatory Drugs (NSAIDs)
3.1.3. Hormones
3.1.4. Other Pharmaceuticals
3.2. Source Analysis
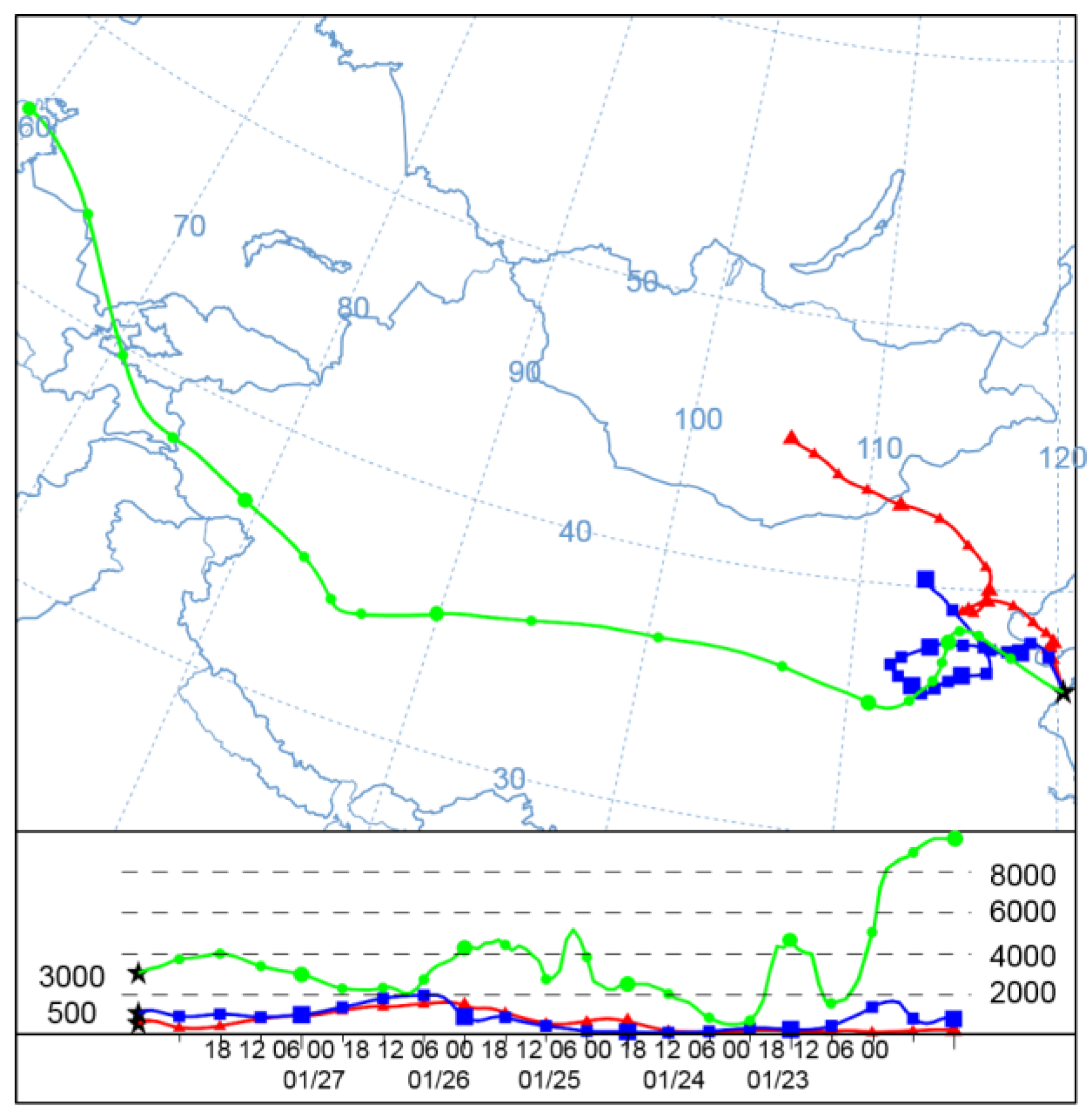
3.2.1. Path Source
3.2.2. Composition Source
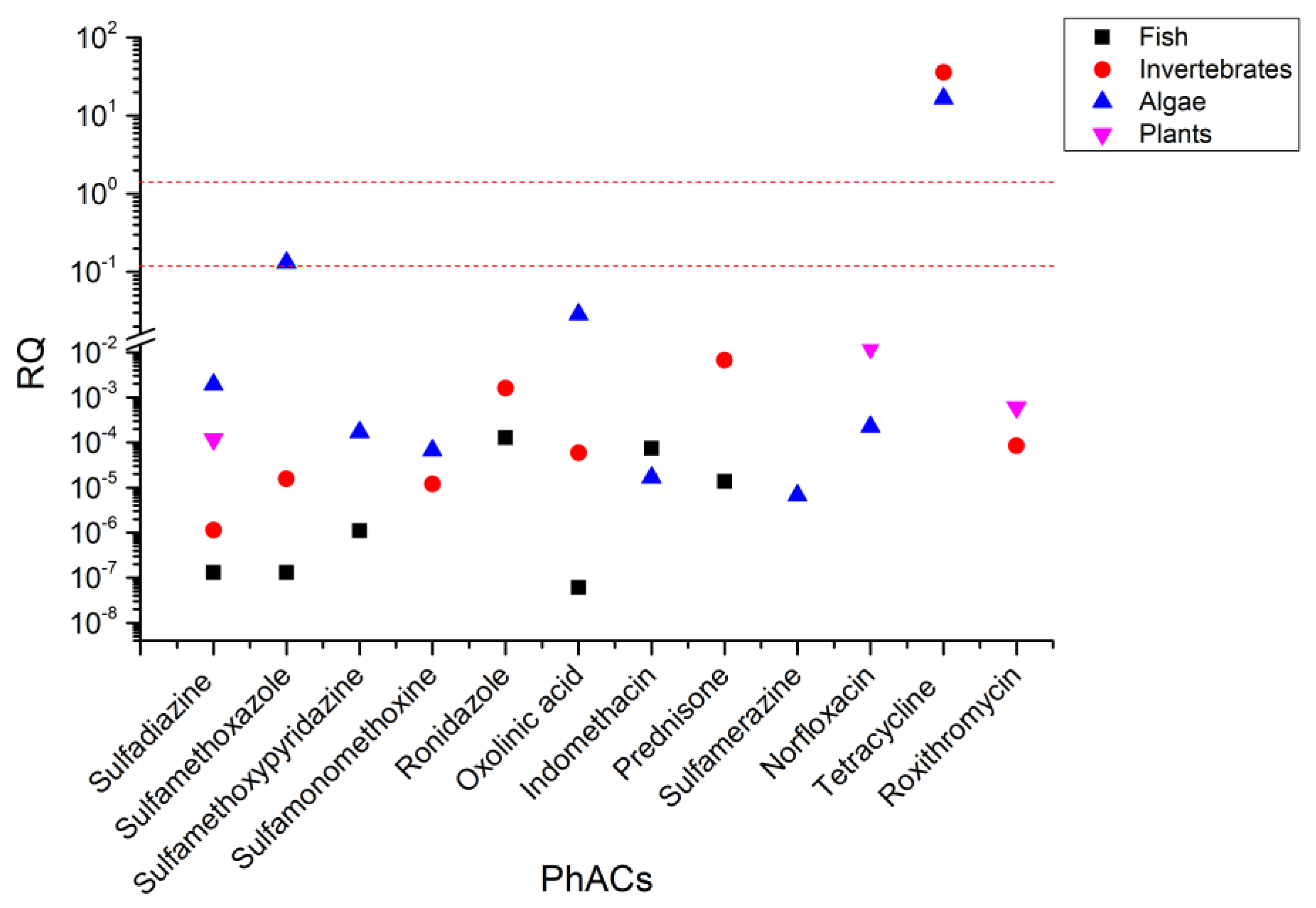
3.3. Environmental Risk Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Daughton, C.G.; Ternes, T.A. Pharmaceuticals and personal care products in the environment: Agents of subtle change. Environ. Health Perspect. 1999, 107, 907–938. [Google Scholar] [CrossRef] [PubMed]
- Beaumont, C.; Young, G.C.; Cavalier, T.; Young, M.A. Human absorption, distribution, metabolism and excretion properties of drug molecules: A plethora of approaches. Br. J. Clin. Pharmacol. 2015, 78, 1185–1200. [Google Scholar] [CrossRef] [PubMed]
- Silva, B.F.D.; Jelic, A.; López-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barceló, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.W.; Wang, B.; Huang, J.; Gang, Y. Pharmaceuticals and personal care products in the aquatic environment in China: A review. J. Hazard Mater. 2013, 262C, 189–211. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, D.E.; Lee, J.H.; Ha, U.H.; Kim, S. The effects of antibiotic on the biofilm formation and antibiotic resistance gene transfer. Desalin. Water Treat. 2015, 54, 3582–3588. [Google Scholar] [CrossRef]
- Song, J.M.; Qu, B.X.; Li, X.G.; Yuan, H.M.; Li, N.; Duan, L.Q. Carbon sinks/sources in the Yellow and East China Seas-Air-sea interface exchange, dissolution in seawater, and burial in sediments. Sci. China Earth Sci. 2018, 61, 1583–1593. [Google Scholar] [CrossRef]
- Sbardella, L.; Comas, J.; Fenu, A.; Rodriguez-Roda, I.; Weemaes, M. Advanced biological activated carbon filter for removing pharmaceutically active compounds from treated wastewater. Sci. Total Environ. 2018, 636, 519–529. [Google Scholar] [CrossRef]
- He, K.; Hain, E.; Timm, A.; Tarnowski, M.; Blaney, L. Occurrence of antibiotics, estrogenic hormones, and UV-filters in water, sediment, and oyster tissue from the Chesapeake Bay. Sci. Total Environ. 2019, 650, 3101–3109. [Google Scholar] [CrossRef] [PubMed]
- Omar, T.F.T.; Aris, A.Z.; Yusoff, F.M.; Mustafa, S.H. Risk assessment of pharmaceutically active compounds (PhACs) in the Klang River estuary, Malaysia. Environ. Geochem. Health 2018. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, U.; Schlosser, D. Biochemical and physicochemical processes contributing to the removal of endocrine-disrupting chemicals and pharmaceuticals by the aquatic ascomycete Phoma sp UHH 5-1-03. Appl. Microbiol. Biotechnol. 2016, 100, 2381–2399. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Michael, C.; Fatta-kassinos, D.; Fotopoulos, V. Can the pharmaceutically active compounds released in agroecosystems be considered as emerging plant stressors. Environ. Int. 2018, 114, 360–364. [Google Scholar] [CrossRef]
- Reoyo-Prats, B.; Aubert, D.; Sellier, A.; Roiq, B.; Palacios, C. Dynamics and sources of pharmaceutically active compounds in a coastal Mediterranean river during heavy rains. Environ. Sci. Pollut. Res. Int. 2017, 25, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murray, K.J.; Parker, W.J.; Bragg, L.M.; Servos, M.R. Fate of selected pharmaceutically active compounds in the integrated fixed film activated sludge process. Water Sci. Technol. 2017, 75, 2680–2691. [Google Scholar] [CrossRef]
- Singh, R.K.; Philip, L.; Ramanujam, S. Rapid degradation, mineralization and detoxification of pharmaceutically active compounds in aqueous solution during pulsed corona discharge treatment. Water Res. 2017, 121, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.J.; Chen, L.L.; Li, H.P.; Luo, Z.F.; Lu, J.; Yang, Z.G. Pharmaceutically active compounds in the Xiangjiang River, China: Distribution pattern, source apportionment, and risk assessment. Sci. Total Environ. 2018, 636, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.X.; Lu, G.H.; Liu, JC.; Yan, Z.H.; Ma, B.N.; Zhang, Z.H.; Chen, W. Occurrence, bioaccumulation, and trophic magnification of pharmaceutically active compounds in Taihu Lake, China. Chemosphere 2015, 138, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.C.; Lu, G.H.; Zhang, Z.H.; Bao, Y.J.; Liu, F.L.; Wu, D.H.; Wang, Y.H. Biological effects and bioaccumulation of pharmaceutically active compounds in crucian carp caged near the outfall of a sewage treatment plant. Environ. Sci. Process. Impacts 2015, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.F.; Mclain, J.E.T. Soil Persistence and Fate of Carbamazepine, Lincomycin, Caffeine, and Ibuprofen from Wastewater Reuse. J. Environ. Qual. 2012, 41, 1473. [Google Scholar] [CrossRef]
- Ma, R.X.; Wang, B.; Lu, S.Y.; Zhang, Y.Z.; Yin, L.; Huang, J.; Deng, S.B.; Wang, Y.J.; Yu, G. Characterization of pharmaceutically active compounds in Dongting Lake, China: Occurrence, chiral profiling and environmental risk. Sci. Total Environ. 2016, 557–558, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Casal, P.; Castro-Jiménez, J.; Pizarro, M.; Katsoyiannis, A.; Dachs, J. Seasonal soil/snow-air exchange of semivolatile organic pollutants at a coastal arctic site (Tromso, 69 degrees N). Sci. Total Environ. 2018, 636, 1109–1116. [Google Scholar] [CrossRef] [PubMed]
- Song, J.M.; Duan, L.Q.; Yuan, H.M. Chemical Environmental Evolution in the Jiaozhou Bay; Science Press: Beijing, China, 2016. [Google Scholar]
- Liang, X.M.; Song, J.M.; Duan, L.Q.; Yuan, H.M.; Li, X.G.; Li, N.; Qu, B.; Wang, Q. Metals in size-fractionated core sediments of Jiaozhou Bay, China: Records of recent anthropogenic activities and risk assessments. Mar. Pollut. Bull. 2018, 127, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.C.; Song, J.M.; Li, N. Compositions, sources and ecological risk of 6 antibacterial agents in surface water of the Jiaozhou Bay, China. Haiyang Xuebao 2018, 40, 71–83. [Google Scholar]
- Wei, Y.; Liu, S.S.; Wang, Z.Q.; Wang, Z.C.; Wang, S.Z. The distribution variation of polycyclic aromatic hydrocarbons between fresh snow and seasonal snowpack in campus in Changchun City, Northeast China. Water Air Soil Pollut. 2017, 228, 233. [Google Scholar] [CrossRef]
- Sun, X.H.; Sun, S.; Li, C.L.; Wang, M.X. Seasonal change in body length of important small copepods and relationship with environmental factors in Jiaozhou Bay, China. Chin. J. Oceanol. Limnol. 2012, 30, 404–409. [Google Scholar] [CrossRef]
- Xing, J.W.; Song, J.M.; Yuan, H.M.; Li, X.G.; Li, N.; Duan, L.Q.; Kang, X.M.; Wang, Q.D. Fluxes, seasonal patterns and sources of various nutrient species (nitrogen, phosphorus and silicon) in atmospheric wet deposition and their ecological effects on Jiaozhou Bay, North China. Sci. Total Environ. 2017, 576, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography–tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Technical Guidance Document in Support of Commission Directive 93/67/EEC on Risk Assessment for New Notified Substances and Commission Regulation (EC) no. 1488/94 on Risk Assessment for Existing Substance, Part II. Office for Official Publications of the European Communities; UNIPUB [distributor]: Luxembourg, 2003; pp. 100–103. [Google Scholar]
- Yao, L.L.; Wang, Y.X.; Tong, L.; Deng, Y.M.; Li, Y.G.; Gan, Y.Q.; Guo, W.; Dong, C.J.; Duan, Y.H.; Zhao, K. Occurrence and risk assessment of antibiotics in surface water and groundwater from different depths of aquifers: A case study at Jianghan Plain, central China. Ecotoxicol. Environ. Saf. 2017, 135, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Jia, A.; Hu, J.Y.; Wu, X.Q.; Peng, H.; Wu, S.M.; Dong, Z.M. Occurrence and source apportionment of sulfonamides and their metabolites in Liaodong Bay and the adjacent Liao River Basin, North China. Environ. Toxicol. Chem. 2011, 30, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Ying, G.G.; Pan, C.G.; Liu, Y.S.; Zhao, J.L. Comprehensive Evaluation of Antibiotics Emission and Fate in the River Basins of China: Source Analysis, Multimedia Modeling, and Linkage to Bacterial Resistance. Environ. Sci. Technol. 2015, 49, 6772–6782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.J.; Zhang, G.; Zheng, Q.; Tang, J.H.; Chen, Y.J.; Xu, W.H.; Zou, Y.D.; Chen, X.X. Occurrence and risks of antibiotics in the Laizhou Bay, China: Impacts of river discharge. Ecotoxicol. Environ. Saf. 2012, 80, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.W.; Tao, Y.; Hu, J.Y.; Tan, Y.M. Determination of cephalosporin antibiotics in water samples by optimised solid phase extraction and high performance liquid chromatography with ultraviolet detector. Int. J. Environ. Anal. Chem. 2011, 91, 1267–1281. [Google Scholar]
- Niu, Z.G.; Zhang, K.; Zhang, Y. Occurrence and distribution of antibiotic resistance genes in the coastal area of the Bohai Bay, China. Mar. Pollut. Bull. 2016, 107, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Jing, L.J.; Teng, Y.G.; Wang, J.S. Characterization of antibiotics in a large-scale river system of China: Occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018, 618, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Minh, T.B.; Leung, H.W.; Loi, I.H.; Chan, W.H.; So, M.K.; Mao, J.Q.; Choi, D.; Lam, J.C.; Zheng, G.; Martin, M.; Lee, J.H.; et al. Antibiotics in the Hong Kong metropolitan area: Ubiquitous distribution and fate in Victoria Harbour. Mar. Pollut. Bull. 2009, 58, 1052–1062. [Google Scholar] [CrossRef] [PubMed]
- Hernández, F.; Montory, M.; Gómez-Fuentes, C. Occurrence of antibiotics and bacterial resistance in wastewater and sea water from the Antarctic. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Li, S.P.; Guo, C.N.; Meng, L.; Huang, X.H. Determination of cefalonium residue in milk by high performance liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2014, 32, 519–523. [Google Scholar] [CrossRef]
- Szultka-Mlynska, M.; Pomastowski, P.; Buszewski, B. Application of solid phase microextraction followed by liquid chromatography-mass spectrometry in the determination of antibiotic drugs and their metabolites in human whole blood and tissue samples. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1086, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Goi, H.; Watanabe, T.; Hara, T.; Ishii, T.; Kazuno, Y.; Inouye, S. Antibacterial activity of cefminox in human urine. Jpn. J. Antibiot. 1988, 41, 25–36. [Google Scholar] [PubMed]
- Grellet, A.; Makhlouf, S.; Desquilbet, L.; Hovhannessian, F.; Boogaerts, C.; Dore, V.; Espana, B.; Prouillac, C.; Kirilov, P.; Polack, B.; et al. Efficacy of guar gum-based ronidazole capsules as a treatment for Tritrichomonas foetus infection in cats. J. Feline Med. Surg. 2015, 19, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.; Miao, T.T.; Hua, D.W.; Jin, X.Y.; Tao, X.B.; Huang, C.B.; Wang, S.F. Synthesis and in vitro cytotoxic evaluation of new 1H-benzo[d]imidazole derivatives of dehydroabietic acid. Bioorganic and Med. Chem. Lett. 2017, 27, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Mezzelani, M.; Gorbi, S.; Da, R.Z.; Fattorini, D.; d’Errico, G.; Milan, M.; Bargelloni, L.; Regoli, F. Ecotoxicological potential of non-steroidal anti-inflammatory drugs (NSAIDs) in marine organisms: Bioavailability, biomarkers and natural occurrence in Mytilus galloprovincialis. Mar. Environ. Res. 2016, 121, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Vidal-Dorsch, D.E.; Bay, S.M.; Maruya, K.; Snyder, S.A.; Trenholm, R.A.; Vanderford, B.J. Contaminants of emerging concern in municipal wastewater effluents and marine receiving water. Environ. Toxicol. Chem. 2012, 31, 2674–2682. [Google Scholar] [CrossRef]
- Conkle, J.L.; White, J.R.; Metcalfe, C.D. Reduction of pharmaceutically active compounds by a lagoon wetland wastewater treatment system in Southeast Louisiana. Chemosphere 2008, 73, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Collado Alsina, N. Multi-scale investigation of occurrence, fate, removal and biodegradation of pharmaceutical contamination in wastewater treatment and river systems. Int. Acad. Bus. Econ. 2013, 8, L131–L133. [Google Scholar]
- Walraven, N.; Laane, R.W.P.M. Assessing the discharge of pharmaceuticals along the Dutch coast of the North Sea. Rev. Environ. Contam. Toxicol. 2009, 199, 1–18. [Google Scholar] [PubMed]
- Lianou, A.; Frontistis, Z.; Chatzisymeon, E.; Antonopoulou, M.; Konstantinou, I. Sonochemical oxidation of piroxicam drug: Effect of key operating parameters and degradation pathways. J. Chem. Technol. Biotechnol. 2017, 93, 1–33. [Google Scholar] [CrossRef]
- Fizazi, K.; Tran, N.; Fein, L.E.; Matsubara, N.; Bjartell, A.; Galli, L.; Lee, J.; Mazhar, D.; North, S.A.; Olmos, D.; et al. Abiraterone acetate (AA) plus prednisone (P) 5 mg QD in metastatic castration-naïve prostate cancer (mCNPC): Detailed safety analyses from the LATITUDE phase 3 trial. J. Clin. Oncol. 2018, 36 (Suppl. 6), 182. [Google Scholar] [CrossRef]
- Macikova, P.; Groh, K.J.; Ammann, A.A.; Schirmer, K.; Suter, M.J.F. Endocrine Disrupting Compounds Affecting Corticosteroid Signaling Pathways in Czech and Swiss Waters: Potential Impact on Fish. Environ. Sci. Technol. 2014, 48, 12902–12911. [Google Scholar] [CrossRef] [PubMed]
- De-Almeida, C.A.A.; Oliveira, M.; Mallmann, C.A.; Martins, A. Determination of the psychoactive drugs carbamazepine and diazepam in hospital effluent and identification of their metabolites. Environ. Sci. Pollut. Res. 2015, 22, 17192–17201. [Google Scholar] [CrossRef]
- Beyn, F.; Matthias, V.; Aulinger, A.; Dähnke, K. Do N-isotopes in atmospheric nitrate deposition reflect air pollution levels. Atmos. Environ. 2015, 10, 281–288. [Google Scholar] [CrossRef]
- Qiao, X.; Xiao, W.Y.; Jaffe, D.; Kota, S.H.; Ying, Q.; Tang, Y. Atmospheric wet deposition of sulfur and nitrogen in Jiuzhaigou National Nature Reserve, Sichuan Province, China. Sci. Total Environ. 2015, 511, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yan, X.Y.; Xiong, Z.Q.; Xie, Y.X.; Xing, G.X.; Shi, S.L.; Zhu, Z.L. Spatial and temporal variation of inorganic nitrogen wet deposition to the Yangtze River Delta Region, China. Water Air Soil Pollut. 2009, 203, 277–289. [Google Scholar] [CrossRef]
- Gu, B.J.; Ge, Y.; Ren, Y.; Xu, B.; Luo, W.D.; Jiang, H.; Gu, B.H.; Chang, J. Atmospheric reactive nitrogen in China: Sources, recent trends, and damage costs. Environ. Sci. Technol. 2012, 46, 9420–9427. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.W.; Hong, H.S.; Huang, Q.J.; Wu, J.Z. Atmospheric nitrogen deposition and its long-term dynamics in a southeast China coastal area. J. Environ. Manag. 2011, 92, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Zhou, J.; Peng, Y.; He, Y.Q.; Yang, H.; Mao, J.D.; Zhang, M.L.; Wang, Y.H.; Wang, S.W. Atmospheric wet deposition of nitrogen and sulfur in the agroecosystemin developing and developed areas of southeastern China. Atmos. Environ. 2014, 89, 102–108. [Google Scholar] [CrossRef]
- Gu, Y.G.; Gao, Y.P. Bioaccessibilities and health implications of heavy metals in exposed-lawn soils from 28 urban parks in the megacity Guangzhou inferred from an in vitro physiologically-based extraction test. Ecotoxicol. Environ. Saf. 2018, 148, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.G.; Ke, C.L.; Liu, Q. Characterization, sources, and ecological hazards of polycyclic aromatic hydrocarbons in the intertidal sediments of Zhelin Bay, the biggest mariculture area on the eastern Guangdong coast of China. Mar. Pollut. Bull. 2018, 130, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.G.; Ning, J.J.; Ke, C.L.; Huang, H.H. Bioaccessibility and human health implications of heavy metals in different trophic level marine organisms: A case study of the South China Sea. Ecotoxicol. Environ. Saf. 2018, 163, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.L.; Gu, Y.G.; Liu, Q. Polycyclic aromatic hydrocarbons (PAHs) in exposed-Lawn soils from 28 urban parks in the megacity Guangzhou: Occurrence, sources, and human health implications. Arch. Environ. Contam. Toxicol. 2017, 72, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Yayan, J.; Ghebremedhin, B.; Rasche, K. No Resistance to Penicillin, Cefuroxime, Cefotaxime, or Vancomycin in Pneumococcal Pneumonia. Int. J. Med Sci. 2015, 12, 980–986. [Google Scholar] [CrossRef] [PubMed]
- Kaymakoglu, S.; Eraksoy, H.; Okten, A.; Demir, K.; Calangu, S.; Cakaloglu, Y.; Boztas, G.; Besisik, F. Spontaneous ascitic infection in different cirrhotic groups: Prevalence, risk factors and the efficacy of cefotaxime therapy. Eur. J. Gastroenterol. Hepatol. 1997, 9, 71–76. [Google Scholar] [CrossRef]
- Hou, J.; Wan, W.; Mao, D.; Wang, C.; Mu, Q.H.; Qin, S.Y.; Luo, Y. Occurrence and distribution of sulfonamides, tetracyclines, quinolones, macrolides, and nitrofurans in livestock manure and amended soils of Northern China. Environ. Sci. Pollut. Res. 2014, 22, 4545–4554. [Google Scholar] [CrossRef]
- Kissell, L.W.; Brinson, P.D.; Gehring, R.; Tell, L.A.; Wetzlich, S.E.; Baynes, R.E.; Riviere, J.E.; Smith, G.W. Pharmacokinetics and tissue elimination of flunixin in veal calves. Am. J. Vet. Res. 2016, 77, 634–640. [Google Scholar] [CrossRef] [PubMed]
- David, T.C.; Khaled, T.C.; Juan, P.V.; Samuel, C.B.; Josep, E.R. Determination of oxolinic acid, danofloxacin, ciprofloxacin, and enrofloxacin in porcine and bovine meat by micellar liquid chromatography with fluorescence detection. Food Chem. 2017, 221, 1277–1284. [Google Scholar]
- Martande, S.S.; Pradeep, A.R.; Kumari, M.; Priyanka, N.; Singh, S.P.; Naik, S.B.; Patel, S.P.; Bagchi, P. Clinical and microbiological efficacy of systemic roxithromycin as an adjunct to non-surgical periodontal therapy in treatment of chronic periodontitis. A randomized, double-blinded, placebo-controlled clinical trial. Am. J. Dent. 2015, 28, 137–142. [Google Scholar] [PubMed]
- Liu, X.H.; Liu, Y.; Lu, S.Y.; Guo, X.C.; Lu, H.B.; Qin, P.; Bi, B.; Wan, Z.F.; Xi, B.D.; Zhang, T.T.; et al. Occurrence of typical antibiotics and source analysis based on PCA-MLR model in the East Dongting Lake, China. Ecotoxicol. Environ. Saf. 2018, 163, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Deka, J.; Sarma, K.P.; Hoque, R.R. Source contributions of Polycyclic Aromatic Hydrocarbons in soils around oilfield in the Brahmaputra Valley. Ecotoxicol. Environ. Saf. 2016, 133, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.J.; Jiao, N.Z.; Wu, C.W.; Liang, B.; Zhang, S.Y. Evolution of nutrient structure and phytoplankton composition in the Jiaozhou Bay ecosystem. J. Environ. Sci. 2005, 17, 95–102. [Google Scholar]


| PhACs | Component | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Sulfamonomethoxine | 0.853 | −0.239 | −0.114 |
| Sulfameter | 0.891 | −0.089 | 0.259 |
| Nitrazepam | 0.735 | 0.207 | 0.275 |
| Ronidazole | −0.533 | −0.162 | 0.33 |
| Salbutamol | −0.575 | −0.492 | 0.291 |
| Desacetylcefotaxime | 0.755 | 0.245 | −0.223 |
| Ceftiofur | 0.858 | −0.02 | 0.248 |
| Flunixin | 0.02 | −0.675 | 0.198 |
| Sulfamethoxazole | 0.012 | 0.836 | 0.33 |
| Tetracycline | 0.101 | 0.832 | 0.227 |
| 5-chloto-1-methyl-4-nitroimidazole | −0.053 | 0.029 | −0.76 |
| 5-nitrobenzimidazole | −0.12 | −0.136 | −0.726 |
| Oxolinic acid | −0.376 | −0.197 | 0.632 |
| Roxithromycin | 0.066 | 0.232 | 0.478 |
| Eigenvalues | 4.34 | 2.52 | 2.09 |
| % Variance | 30.98 | 17.97 | 14.94 |
| Cumulative % | 30.98 | 48.95 | 63.89 |
| Extraction Method: Principal component analysis. | |||
| Rotation Method: Varimax with Kaiser normalization. | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, Q.; Song, J.; Li, X.; Yuan, H.; Yang, G. Characterization, Source and Risk of Pharmaceutically Active Compounds (PhACs) in the Snow Deposition Near Jiaozhou Bay, North China. Appl. Sci. 2019, 9, 1078. https://doi.org/10.3390/app9061078
Peng Q, Song J, Li X, Yuan H, Yang G. Characterization, Source and Risk of Pharmaceutically Active Compounds (PhACs) in the Snow Deposition Near Jiaozhou Bay, North China. Applied Sciences. 2019; 9(6):1078. https://doi.org/10.3390/app9061078
Chicago/Turabian StylePeng, Quancai, Jinming Song, Xuegang Li, Huamao Yuan, and Guang Yang. 2019. "Characterization, Source and Risk of Pharmaceutically Active Compounds (PhACs) in the Snow Deposition Near Jiaozhou Bay, North China" Applied Sciences 9, no. 6: 1078. https://doi.org/10.3390/app9061078
APA StylePeng, Q., Song, J., Li, X., Yuan, H., & Yang, G. (2019). Characterization, Source and Risk of Pharmaceutically Active Compounds (PhACs) in the Snow Deposition Near Jiaozhou Bay, North China. Applied Sciences, 9(6), 1078. https://doi.org/10.3390/app9061078




