Abstract
Geopolymer-based grouting materials often have a higher early strength, better durability, and lower environmental impact than those of traditional cement-based grouts. However, existing geopolymer grouts face common challenges such as rapid setting and low compatibility with treated substrates. This study develops a new grouting material using industrial byproducts to overcome these limitations while optimizing performance for reinforcing silty mudstone slopes. The base materials used were ground granulated blast furnace slag (GGBFS) and zeolite powder, with calcium lignosulphonate (CL) serving as the retarding agent and NaOH as the alkali activator. The investigation focused on the effects of the mix ratio and water–binder ratio on the setting time, flowability, bleeding rate, concretion rate, and compressive strength of the new grouting material. Scanning electron microscope (SEM) and X-ray diffraction (XRD) analyses were employed to examine the action mechanism of the material components in the slurry. The one-factor standard deviation method and Grey Relational Analysis (GRA) were used to assess the influence of each material component on the slurry performance indices and the correlation between each performance index and its optimal mix ratio. Subsequently, the optimal mix ratio of the new grouting material was ascertained. The results indicate that the setting time is positively correlated with the zeolite powder and CL dosages and the water–binder ratio, while it is inversely related to the NaOH dosage. The flowability is significantly enhanced with increasing zeolite powder and NaOH dosages, but decreases at a higher CL dosage and water–binder ratio. This insight is crucial for optimizing the workability of the grouting material under various conditions. The optimal ratio of the grout is zeolite powder:GGBFS:CL:NaOH = 30:70:5:7, with a water–binder ratio of 0.6. Compared to existing commercial grouting materials, the compressive strength of this new grout is comparable to that of silty mudstone. This significantly reduces the problem of stress concentration at the grout–rock interface due to strength differences, thus effectively reducing the risk of secondary cracking at the interface. These findings provide a new material solution for grouting and repairing fractured silty mudstone slopes.
1. Introduction
The continuous development of transportation infrastructure in China has led to a substantial increase in the number of silty mudstone slopes along roads and railways in the hot and humid southern regions. However, frequent rainfall and evapotranspiration can significantly contribute to the deterioration of silty mudstone slopes, potentially leading to slope failures [1]. This poses a significant threat to the safety of road operations and the well-being of individuals and property. Grouting techniques are commonly used to repair fractured silty mudstone slopes, and their effectiveness depends largely on the type of grouting material used. Presently, silicate cement-based grouting material remains the predominant material employed for slope grouting due to its inherent advantages, including stability and high strength. However, its production incurs substantial costs and results in substantial carbon dioxide emissions during the manufacturing process, a factor that is incongruent with China’s commitment to achieving its strategic ‘dual-carbon’ target. Therefore, there is an urgent need to develop green, low-carbon, cost-effective grouting materials that are efficient for restoration and environmentally friendly.
Geopolymer is a gelling material with a three-dimensional mesh structure, consisting of AlO4 and SiO4 tetrahedral structural units. It is made from minerals or industrial wastes rich in silica and aluminum through the chemical reaction process of depolymerization, reorganization, coalescence, and setting in an alkaline environment [2,3]. In recent years, a significant number of studies have been conducted on the use of geopolymer grouting materials as a replacement for silicate cement-based grouting materials. Yingli Khan et al. [4] investigated the feasibility of using waste glass powder (GP) as a partial precursor in alkali-activated mortars made from fly ash (FA) and ground granulated blast furnace slag (GGBFS). The findings demonstrate that the FA-GGBFS mortar exhibits a dense and compact microstructure at a 20% GP dosing. Mukhtar et al. [5] used mechanochemical activation to prepare geopolymer grouting material from GGBFS and fly ash, and found that the flowability and mechanical properties of the slurry significantly improved with an increase in the slag dosage. In the aforementioned studies on the preparation of geopolymer grouting materials, the majority of the main cementitious materials selected include fly ash, GGBFS, and other highly reactive solid waste materials. However, fly ash is associated with several disadvantages, including a protracted setting time and inadequate durability. Additionally, it contains high levels of radioactive substances and heavy metals, which can easily pollute soil and groundwater if not properly managed, posing challenges to addressing the root of environmental issues [6,7,8]. This limits its use in engineering applications [9]. Although GGBFS has high activity, rich resource reserves, and a low cost [10,11], its dry shrinkage is significant, and it is difficult to meet engineering requirements when using it alone [12]. Zeolite powder is selected as a composite material with GGBFS powder for the preparation of geopolymer grout slurry. Zeolite powder is a material produced by grinding natural zeolites. It is of interest because of its local availability and low cost [13]. The unique spatial mesh structure of this material provides a large internal surface area and reactivity [14], which can effectively improve the performance and quality of the grouting material [15], and, at the same time, overcome the difficulties of low raw material activity and poor alkali activation in building materials from solid wastes.
As demonstrated in previous studies, geopolymer grouting materials are most commonly deployed in road construction, filling hollow areas, and reinforcement tunnels. Rahman et al. [16] conducted a study on fly ash (FA) and ground granulated blast furnace slag (GGBFS)-based self-compacting geopolymer concrete (SCGC), and assessed its efficacy in marine applications. The findings indicated that the mixture can be effectively used in marine environments, with a design strength of 40 MPa attained under harsh marine conditions. Ni et al. [17] developed a new type of synchronous grouting material for tunnel grouting and determined the optimal mix ratio of geopolymer grouting material through a series of performance tests and Response Surface Methodology (RSM). This material was subsequently applied to metro projects. In summary, geopolymer grouting materials have demonstrated excellent performance and application potential. However, existing research on grouting materials does not fully meet the reinforcement needs for silty mudstone slopes. The strength disparity of existing geopolymer- and cement-based grouting materials and silty mudstone is too significant. This renders the process of grouting repair susceptible to secondary cracking, which has the potential to compromise the efficacy of the repair and the long-term stability of the slope [18]. As a result, there is a research gap in the development and application of geopolymer grouting materials for silty mudstone slope reinforcement.
To address this gap, this study develops a new environmentally friendly grouting material using GGBFS and zeolite powder as the base materials, CL as the retarder, and NaOH solution as the alkali activator. The tests focused on the setting time, flowability, bleeding rate, concretion rate, and compressive strength and used scanning electron microscopy (SEM) and X-ray diffraction (XRD). The one-factor standard deviation and Grey Relational Analysis (GRA) were used to quantitatively analyze the influence of different material components and performance indicators on the mix ratio, and determine the optimal mix ratio of alkali-activated GGBFS–zeolite powder grouting materials. These findings could provide new materials for the grouting reinforcement of fractured rocky slopes in the southern region of China.
2. Material Properties
2.1. Base Materials
GGBFS and zeolite powder were selected as geopolymer base materials due to their environmental friendliness, optimized properties, and wide range of applications [19,20]. Compared with the elevated energy consumption of metakaolin, the restricted availability of silica fume, and the concomitant environmental hazards linked with fly ash, GGBFS possesses the benefit of water-hardening and performance-enhancing properties as a byproduct of iron and steel. The zeolite powder stands out for its porosity, adsorption, and energy-saving features that do not require calcination. Therefore, industrial solid waste (S95 GGBFS powder and 200 mesh zeolite powder) was used as the base material for the preparation of the grouting material, and the materials are shown in Figure 1.
Figure 1.
Basic materials: (a) GGBFS powder; (b) zeolite powder.
S95 GGBFS powder, with a specific surface area of 412 m2/kg, a density of 2.9 g/cm3, a specific gravity of 2.45, and a particle size range of 5–20 μm, was purchased from Hengyuan New Material Company, located in Zhengzhou City, Henan Province, China. The 200-mesh natural zeolite powder was purchased from Henan Gongyi Hengxin Co., Ltd., China (located in Gongyi City, Henan Province, China), with a density range of 1.75 g/cm3, a specific surface area of 560 m2/kg, a specific gravity of 2.1, and a particle size range of 74–100 μm. Figure 2 shows the XRD patterns of the primary base materials, while Table 1 provides a comprehensive overview of their chemical compositions. As shown in Figure 2 and Table 1, the primary chemical composition of GGBFS powder consists of SO2 and CaO, accounting for 72.35%, followed by Al2O3, at 15.04%. The predominant minerals include calcite (CaCO3), anhydrite (CaSO4), and calcium alumina feldspar (Ca2Al2SiO7). The primary chemical composition of zeolite powder consists of SO2, accounting for 73.35% of the total, followed by Al2O3, at 12.45%. The predominant mineral compositions are gismondine (CaAl2Si2O8·4H2O) and wairakite (CaAl2Si6O16·6H2O).
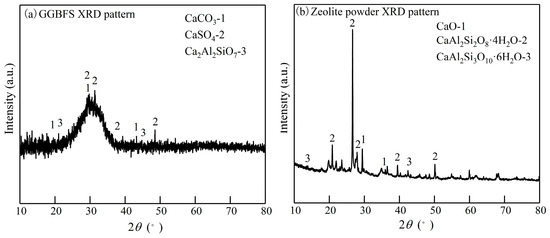
Figure 2.
XRD of base materials: (a) GGBFS powder; (b) zeolite powder.

Table 1.
Chemical composition of base materials.
2.2. Additives
White NaOH granules were used to prepare the solution as an alkali activator, aimed at stimulating the activity of GGBFS and zeolite powder and promoting the coagulation and hardening of the slurry. NaOH broke the Si-O and Al-O bonds by providing OH- ions, breaking them and producing ions with activity (e.g., silicate ions and aluminate ions) which, under certain conditions, polymerised to form C-(A)-S-H or N-A-S-H gels. This would promote the coagulation and hardening of the slurry [21].
The setting time of the GGBFS–zeolite powder grouting material was generally rapid, failing to meet the criteria in the ‘Common Portland cement’ (GB 175-2023) standard [22]. To address this, CL powder was used as a retarder, extending the hydration reaction time and increasing the setting time of the slurry. Calcium lignosulphonate is a multi-component polymer anionic surfactant with the molecular formula C20H24CaO10S2. It is a dark brown powder with an aromatic odor and is easily soluble in water. It is also stable, with strong dispersibility, adhesion, and chelating properties.
3. Test Schemes
A reasonable setting time ensures sufficient working time during construction while allowing the slurry to set and cure quickly after grouting. Good flowability enables the slurry to uniformly diffuse and effectively fill the complex cracks in the rock slope. A low bleeding rate maintains the slurry’s volume stability and homogeneity during solidification, preventing internal pores and strength loss from water dissipation. When the compressive strength of the slurry closely matches that of the silty mudstone, stress concentration is minimized, reducing the risk of secondary cracking. Therefore, grouting materials with appropriate compressive strength, good flowability, low bleeding rate, and reasonable setting time are crucial for effective slope reinforcement and long-term stability [23,24].
To determine the optimal mix ratio, preliminary tests on setting time and compressive strength were conducted on specimens with various mix ratios. The results of these preliminary tests indicated the following ranges for further optimization: 0% to 50% zeolite powder, 50% to 100% GGBFS, 1% to 5% CL, 1% to 9% NaOH, and 0.4 to 0.8 of the water–binder ratios. In the formal experiment, the influence of the zeolite powder-to-GGBFS ratio on grout performance was initially evaluated at 2% CL, 7% NaOH, and a 0.6 water–binder ratio. Subsequently, the dosages of CL and NaOH, as well as the water–binder ratio, were varied, respectively, while maintaining the optimal zeolite powder-to-GGBFS ratio. A total of 18 mix ratio schemes were designed for the formal experiment, as shown in Table 2.

Table 2.
Alkali-activated GGBFS–zeolite powder grouting material ratio.
Although there is no well-developed and unified standard system for testing alkali-activated materials, as there is for conventional cementitious materials, the workability testing principles for alkali-activated materials are similar to those for conventional materials. Therefore, the setting time tests, flow tests, and bleeding rate tests of alkali-activated GGBFS–zeolite powder grouting materials were conducted in strict accordance with the following test methods: the Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of Portland Cement (GB/T 1346-2011) [25]; the Test Method for Fluidity of Cement Mortar (GB/T 2419-2005) [26]; and the Testing Methods of Cement and Concrete for Highway Engineering (JTG 3420-2020) [27]. To reduce the potential impact of test errors, a total of three parallel specimens were designed for each group, and the mean values were subsequently calculated after the removal of outliers. The compressive strength of the slurry stone body was evaluated in accordance with the Test Method of Cement Mortar Strength (GB/T 17671-2021) [28]. Demolded specimens were then subjected to a curing process for 7, 14, and 28 days under standard conditions (temperature of 20 ± 2 °C and relative humidity >95%). Subsequently, the mechanical properties were determined using a WDW-100C microcomputer-controlled electronic universal testing machine. This tester was produced by Shanghai Hualong Testing Instrument Co. China (Shanghai, China). The specimen size was 40 mm × 40 mm × 40 mm, using a three-test mold casting block. Following the compressive strength test, the specimen fragments were subjected to grinding, and the powder was subsequently analyzed using XRD. Fragments with dimensions less than 12 mm were selected for the SEM test, which was performed using a Zeiss EVO10 instrument to observe the surface micro-morphology of the stone bodies. The tester was produced by Carl Zeiss and is located in Jena, Germany.
4. Results and Discussion
4.1. Setting Time
As demonstrated in Figure 3, the setting time of the slurry was influenced by various factors. The present study investigated the effect of different dosages of zeolite powder on the setting time of the slurry. The findings indicated a direct correlation between the increased dosage of zeolite powder and the rise in setting time. The initial setting time increased from 20 min to 36 min, an 80% increase, while the final setting time rose from 62 min to 109 min, indicating an increase of 75.81%. The phenomenon was due to the porous nature of zeolite powder. This characteristic facilitated the absorption of water molecules, which were then introduced into the internal pores. Consequently, the hydration reaction rate of the slurry was inhibited, resulting in a prolonged setting time.
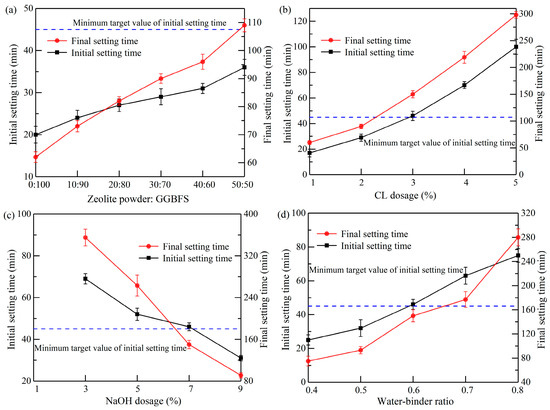
Figure 3.
Effects of different factors on setting time: (a) zeolite powder: GGBFS; (b) CL dosage; (c) NaOH dosage; (d) water–binder ratio.
Figure 3b shows that when the CL dosage was set at 5%, the initial and final setting times of the slurry were maximized to 100 and 298 min, respectively, with an increased rate of 488.24% and 396.67%. This is because the CL molecule contains a large number of hydrophilic groups (e.g., sulphonic acid groups and hydroxyl groups), which promote the interaction between the CL molecules and the surface of solid particles [29,30]. When mixed with slurry containing solid particles, such as zeolite powder and GGBFS, the CL molecules were adsorbed onto the surface of the solid particles by chemisorption. During this process, a complexation reaction occurred between the CL molecules and Ca2+, forming a gelling film. This film hindered the direct contact of zeolite powder and GGBFS with water molecules. Consequently, the hydration reaction was slowed down.
It was established that as the quantity of NaOH increased, the setting time correspondingly decreased. The initial and final setting times decreased by 55.07% and 74.37%, respectively. Notably, the slurry remained unsolidified at a NaOH dosage of 1%. This outcome demonstrates that the alkaline environment was not sufficient to stimulate the production of a gelatinous substance in the slurry when the NaOH dosage was set at 1%, as per the mix ratio established in Group 11. An increased water–binder ratio resulted in an increased setting time for the slurry. Furthermore, when the water–binder ratio was between 0.7 and 0.8, the rate of increase in the slurry setting time was accelerated, with a rise of 19.05% and 58.19%, respectively. The hydration reaction of the slurry was delayed as the free water molecules in the slurry increased, leading to an increased setting time.
The final results showed that, among the 18 groups of ratios, the initial and final setting times of the slurries in Groups 1–7, 11, and 14–16 did not meet the requirements of the Chinese technical specification standard GB 175-2023 (initial setting time was not less than 45 min and final setting time was not more than 600 min).
4.2. Flowability
The flowability was identified as the primary metric for evaluating the performance of the grouting material, with the hydration reaction rate serving as a key factor influencing flowability. Figure 4 demonstrates the impact of various factors on slurry flowability. It was established that, as the dosage of zeolite powder increased, the flowability of the slurry gradually decreased, reaching a minimum value of 28.43 cm. The increase in the specific surface area of the compound grouting material, resulting from the substantial replacement of GGBFS by zeolite powder, led to a decrease in the average water content per unit surface area. This, in turn, led to excessively dry or viscous characteristics in the prepared slurry, thereby reducing its flowability. Moreover, an increase in slurry flowability was observed with a 17.86% rise in the CL dosage. In the field of construction materials, CL is used as a water-reducing agent. Under constant water–binder ratio conditions, the dosage of CL is increased, resulting in elevated levels of free water molecules within the slurry.
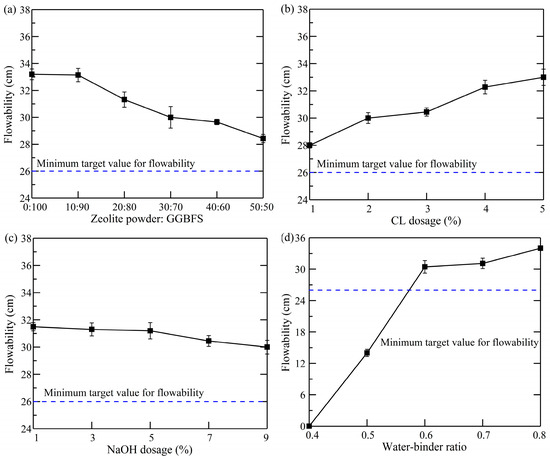
Figure 4.
Effects of different factors on flowability: (a) zeolite powder–GGBFS; (b) CL dosage; (c) NaOH dosage; (d) water–binder ratio.
As the NaOH dosage increased, the slurry flowability decreased more gradually, from 31.5 cm to 30 cm. Accordingly, the impact of the NaOH dosage on flowability was found to be negligible, and the resulting flowability values met the established minimum standard criteria. When the water–binder ratio was 0.6, the flowability of the slurry showed a substantial enhancement, exhibiting a 117.5% increase in comparison to the water–binder ratio of 0.5. The test pattern was similar to that in the results reported by Gasperi et al. [31]. The greater number of water molecules in the slurry led to their attraction to each other, forming a smooth interface. As a result, this enhanced the flowability of the slurry. According to the Chinese technical specification GB/T 50448-2015 [32], the flowability of the slurry should be not less than 260 mm. Therefore, Groups 15 and 16 did not meet this standard.
4.3. Bleeding and Concretion Rate
The bleeding rate directly influenced the stability and filling effect of the slurry, while the concretion rate directly affected the strength and durability of the grouting material [33]. Figure 5 provides a visual representation of the effect of different factors on the slurry’s bleeding and concretion rates. As the dosage of zeolite powder increased, the bleeding rate of the slurry gradually increased, while the concretion rate steadily decreased. When the ratio of zeolite powder to GGBFS was 50:50, the bleeding rate of the slurry peaked, with a reduction of 100%. The slurry bleeding rate increased uniformly with elevated CL dosage. Due to the retardation and water-reducing properties of CL, the slurry hydration reaction was retarded, leading to an increase in free water molecules and the bleeding rate. The slurry bleeding rate gradually decreased as the NaOH dosage increased. The increased alkali concentration in the solution promoted a variety of chemical reactions in the slurry [34]. Concurrently, a significant amount of heat was released, thereby facilitating the progression of the reaction. Consequently, the bleeding rate was minimal, while the concretion rate was maximal at a NaOH dosing of 9%.
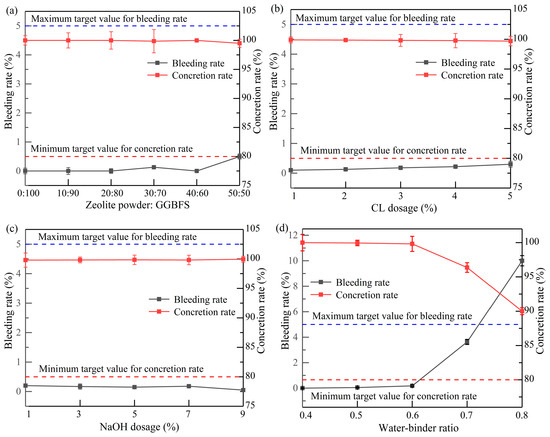
Figure 5.
Effects of different factors on bleeding and concretion rate: (a) zeolite powder–GGBFS; (b) CL dosage; (c) NaOH dosage; (d) water–binder ratio.
As shown in Figure 5d, an increase in the water–binder ratio led to a gradual increase in the slurry’s bleeding rate. As the water–binder ratio increased from 0.7, a significant increase in the bleeding rate was observed, reaching its maximum at a water–binder ratio of 0.8. Due to the increased number of free water molecules in the slurry that did not participate in the chemical reaction, the setting time increased, particles precipitated faster, and the bleeding rate increased. The final results showed that only the bleeding rate of Group 18 exceeded the upper limit value specified in the Chinese technical specification DL/T 5148-2021 [35].
4.4. Compressive Strength of Stone Bodies
The compressive strength is a pivotal metric for assessing the material properties of alkali-activated GGBFS–zeolite powder slurry. As shown in Figure 6, the compressive strength of the slurry is influenced by various factors. When the ratio of zeolite powder to GGBFS was 50:50, the 28 d compressive strength of the stone bodies reached a minimum value of 12.49 MPa. The incorporation of zeolite powder significantly reduced the total pore volume of the slurry. Furthermore, the internal structure and composition of the pores were optimized, and the stone bodies were densified. However, due to the zeolite powder’s high water absorption and storage capacity [36], a consequent high level of water absorption occurred during the chemical reaction, which hindered the hydration reaction. It was observed that when CL was dosed at 5%, the 28 d compressive strength of the slurry stone bodies reached a maximum of 17.19 MPa, 26.30% higher than the lowest value. CL was hypothesized to exert an air-entraining effect [37], which was theorized to promote the hydration reaction, increase the product, decrease porosity, and improve the compressive strength of the slurry stone bodies.
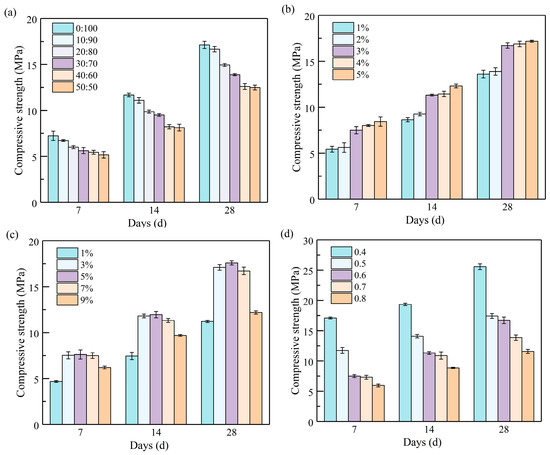
Figure 6.
Effects of different factors on the compressive strength of grouting slurry stone: (a) zeolite powder–GGBFS; (b) CL dosage; (c) NaOH dosage; (d) water–binder ratio.
As the NaOH dosage, increased, the compressive strength of the slurry stone bodies first increased, then decreased. A maximum value of the 28 d compressive strength of 17.59 MPa was attained at a dosage of 5%, and the maximum increase in the 28 d compressive strength was 52.49% at a dosage of 3%. Thus, the correct amount of alkali activator promoted the binding of active molecules in the chemical reaction [38]. As the NaOH dosage exceeded 5%, the elevated OH− concentration resulted in a rapid reaction rate, leading to the generation of numerous hydration products in a short period. These products accumulated rapidly on the surface of the active particles, impeding the ensuing reaction and consequently diminishing the strength of the stone bodies. This observation is consistent with the results reported by Mahfoud et al. [39] and Huang et al. [40].
The compressive strength of the stone bodies decreased with the water–binder ratio. The 28 d compressive strength decreased most significantly at a water–binder ratio of 0.5, reaching 31.80%. As the water–binder ratio increased, the free water molecules in the slurry also increased. In addition, the excess free water molecules diluted the alkaline material in the system and reduced the OH− concentration. This reduction slowed the rate of the hydration reaction, limiting the formation of hydration products and their ability to adequately fill the pores, further reducing the compressive strength of the stone bodies [41,42].
4.5. XRD and SEM Analysis
As shown in Figure 7, the XRD analysis was conducted on samples with varying zeolite powder-to-slag powder ratios (0:100, 30:70, and 50:50), CL retarder dosages (2% and 5%), and NaOH alkali activator dosages (3% and 9%). The main products of the alkali-activated GGBFS–zeolite powder grouting materials were CaSO4·2H2O, C-S-H, SiO2, and CaCO3. As C-(A)-S-H is an amorphous phase [43,44], its diffraction peaks were not evident in the XRD analysis.
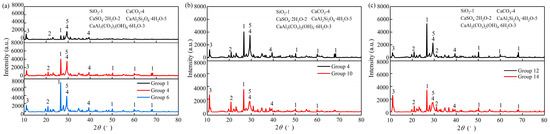
Figure 7.
Effects of different factors on XRD: (a) zeolite powder–GGBFS; (b) CL dosage; (c) NaOH dosage.
As the dosage of zeolite powder increased from 0% to 30%, both the CaCO3 and SiO2 diffraction peaks increased, with a rise of 79.51% and 344.29%, respectively. When the dosage of zeolite powder reached 50%, the SiO2 diffraction peaks continued to enhance, reaching a level of 76.92%, while the CaCO3 diffraction peaks remained largely unchanged, increasing by only 9.46%. The findings indicated that in an alkaline environment, where all other factors remained constant, increasing the dosage of zeolite powder resulted in an increased content of unconsumed SiO2. This, in turn, led to a decreased pH value of the slurry, thereby hindering the effective hydration of the gelling material. In this case, the high-energy-state aluminum–oxygen bond (-Al-O-) and silicon–oxygen bond (-Si-O-) exhibited difficulty in dissolution [45], resulting in a decline in the generated C-S-H and C-(A)-S-H cementitious hydrides. This, in turn, contributed to the gradual decrease in the compressive strength of the slurry stone bodies with increased dosage of zeolite powder.
It was demonstrated that as the dosage of CL increased, the intensities of the SiO2 and CaCO3 diffraction peaks decreased significantly, with a degree of reduction of 12.74% and 52.70%., indicating that SiO2 and CaCO3 were gradually converted into other hydration products. CL, an anionic surfactant, enhances the water dispersibility of adsorbent materials [46]. This improvement was attributed to the ability of CL to wet the surface of the particles, thereby facilitating the hydration reaction. Furthermore, the diffraction peak intensities of the monocalcium aluminate hydration products exhibited a concomitant enhancement with increasing CL doping, showing an increase of 114.90%. This suggests that the monocalcium aluminate hydration products play a substantial role in enhancing the densification of stone bodies [47]. This was the same as the conclusion reached in Section 4.4. Keeping all other conditions constant, the strength of the stone bodies doped with 5% CL exceeded that of those doped with 2%.
The SEM images of the slurry stone bodies in Figure 8 combined with Figure 6b clearly show that the compressive strength of the stone bodies was higher at a 5% CL dosage compared to 3%. The specimen with 5% CL doping had a more compact structure, fewer holes, denser gels, and stronger bonding of the gels to the skeleton. In addition to the Calcium Sulfoaluminate hydrate (C-(A)-S-H) gelation phase and Cement Silicate Hydrogel (C-S-H) gelation phase inside the stone bodies [48], the mineral backbone in the stone bodies consisted of a number of unreacted minerals such as quartz and calcite.

Figure 8.
SEM image of alkali-activated GGBFS–zeolite powder grouting slurry stone: (a) 2% CL; (b) 5% CL.
In the presence of the NaOH alkali activator, the SiO2 and Al2O3 crystal structures dissolved to form aluminum–silicon complexes, which were rearranged to form a stable three-dimensional mesh structure. On the other hand, gypsum (CaSO4), the main mineral in GGBFS, reacted with NaOH and ambient CO2 to form CaCO3. As depicted in Figure 7c, when the NaOH dosage increased, the XRD peak area of CaCO3 rose by 20.51%, accompanied by an increase in its crystal content. Moreover, the intensity of the diffraction peaks of the monocalcium aluminate hydration products also witnessed a significant growth of 226.19%. When the alkali content was too high, OH− would be consumed during the hydration of the geopolymer and there would be an excess of Na+ ions. This makes it difficult to maintain charge equilibrium, preventing the polymerization reaction from progressing and leading to the formation of a less robust stone body. This phenomenon explained why the overall intensity remained unaltered, despite the decreased intensity of the SiO2 diffraction peaks by 35.38%.
5. Evaluation of Material Factors and Performance Indicators by One-Way Standard Deviation and GRA
The objective of this section is to ascertain the impact of each material factor on the slurry performance indicators and the correlation between each performance indicator of the slurry and its optimal slurry ratio. The ultimate objective was to determine the optimal mix ratio of the grout. The key to determining the optimal mix ratio of alkali-activated GGBFS–zeolite powder grouting material was quantifying the effect of four material factors on performance indicators. The four performance indicators were the setting time, flowability, bleeding rate, and compressive strength. The four material factors were the ratio of the zeolite powder to the GGBFS, the set retarder dosage, the alkali activator dosage, and the water–binder ratio. The one-way standard deviation method was used to assess the influence of each material factor on the performance indicators. Subsequently, Grey Relational Analysis (GRA) was used to evaluate the correlation between each performance indicator and the optimal mix ratio, aiming to determine the optimal mix ratio.
5.1. One-Way Standard Deviation Analysis
Performance data from single-factor tests at varying levels were used to calculate the standard deviations of the four performance indicators: setting time, flowability, bleeding rate, and compressive strength. Specifically, among the 18 ratio groups, Groups 1–6 were used for evaluating the effect of the zeolite powder–GGBFS ratio; Groups 4 and 7–10 were used for the CL dosage; Groups 8 and 11–14 were used for the NaOH dosage; and Groups 8 and 15–18 were used for the water–binder ratio. Standard deviation was used to assess the influence of different material factors on the performance indicators. The results are presented in Figure 9. The magnitude of the standard deviation provides an intuitive representation of the degree of impact, in contrast to the less intuitive nature of judging from the variance value [49,50]. When considering a single factor, it simplifies the analysis by avoiding the complexities of multi-factor analysis, allowing the research to remain focused.
where Xi and are the sample values and sample mean values of the performance indicators obtained by testing the single factor at different levels, respectively; n is the number of different factor levels.
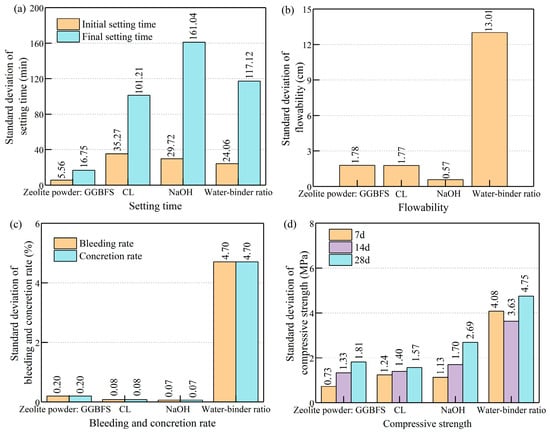
Figure 9.
Degree of influence of different factors on performance: (a) setting time; (b) flowability; (c) bleeding rate and concretion rate; (d) compressive strength.
As shown in Figure 9, the dosage of CL was the primary factor influencing the initial setting time of the slurry. The dosage of NaOH was a key factor in determining the final setting time. The water–binder ratio was the most significant parameter affecting flowability. The bleeding rate and concretion rate were primarily influenced by the water–binder ratio, followed by the dosage ratio of CL and zeolite powder to GGBFS. Regarding the compressive strength at 7, 14, and 28 days, the water–binder ratio was the most important factor, followed by the dosage of NaOH.
5.2. GRA-Based Evaluation of Impact Factors
Grey Relational Analysis (GRA) was used to assess the relevance of each performance indicator on the grouting material and to determine the optimal mix ratio [51,52]. It was found that, according to the relevant specifications [22,32,35] and field requirements [53,54] for the setting time, flowability, bleeding rate, and compressive strength, among the 18 ratio groups, the initial setting times of Groups 1 to 7, 11, and 14 did not meet the minimum standards. Groups 15 and 16 did not meet the minimum requirements for the initial setting time and flowability. The bleeding rate of Group 18 exceeded the upper limit, and the compressive strength of Group 17 did not meet the specified minimum standards. Five groups satisfied the specified criteria, and the GRA evaluated only these five groups. The following details were provided:
According to the stipulated specifications, the performance indicators (initial setting time, final setting time, flowability, bleeding rate, and compressive strength) of the selected ratio group (1–18) had to be met. This was achieved by constructing a comparative series Di(j), where j = 1, 2,…, m (m is the number of groups meeting the specification) and I = 1, 2,…, n (n is the number of control performance indicators).
To ensure the accuracy of the results and avoid the effects of significant differences between the data, the data were preprocessed by normalization, as shown in Equation (2).
According to the standard [22,32,52] and site requirements, the optimal range for the performance indicators is as follows: initial setting time of 45–120 min, final setting time of 150–600 min, flowability of 26–33 cm, bleeding rate of 0–5%, and compressive strength of 12–18 MPa.
The construction of the referenced series Z0(j) is shown in Equation (3).
The grey relational coefficient ξi is shown in Equation (4).
where ρ is the grey discrimination coefficient, taken as 0.5. In a large number of practical applications and studies, it is found that setting the discrimination coefficient to 0.5 often provides a more reasonable reflection of the true correlation between factors in most cases [55,56].
The degree of serial correlation between the series Di(j) and the reference series Zi was compared using the correlation ri, as shown in Equation (5). The correlation degree of each performance index of the grouting material was then determined.
where the closer the correlation ri is to 1, the better the series Di(j) is correlated to the performance metrics.
The indicator weights Wi, as shown in Equation (6), should be used. The optimal mix ratio group was then determined, as shown in Table 3.

Table 3.
Normalized weighted total scores for grouting materials.
As shown in Figure 10, the correlation of the alkali-activated GGBFS–zeolite powder slurry was investigated. The correlation of the compressive strength was found to be the highest and closest to 1, indicating that this performance indicator had the greatest correlation with optimum proportioning. Except for the compressive strength correlation, which exceeded 0.7, the correlations of the other four performance indicators were all greater than 0.5, and all were correlated with the optimal mix ratio. The weights of the performance indicators (initial setting time, final setting time, flowability, bleeding rate, and compressive strength) on the mix ratio were 19.71%, 19.72%, 19.98%, 19.67%, and 20.92%, respectively. The correlation of each performance index of the grouting material on the optimal mix ratio was, in descending order, as follows: compressive strength, flowability, final setting time, initial setting time, and bleeding rate.
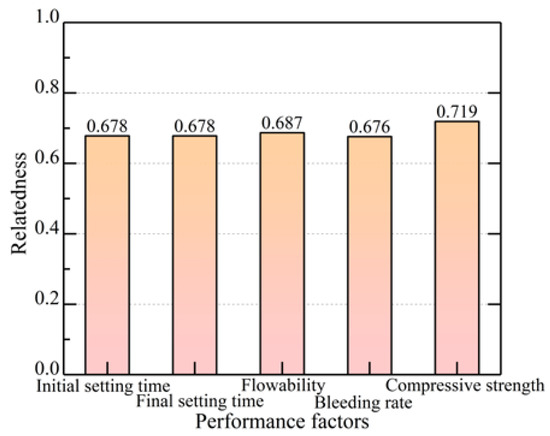
Figure 10.
Correlation of different performance indicators.
The priority given to compressive strength over the other factors is due to the primary objective of grouting being the enhancement of the load-bearing capacity of silty mudstone slopes. When the compressive strength is inadequate, the slopes may fail under loading even if the other parameters meet the requirements. The final setting time needs to be reasonably controlled to avoid slurry loss, but a slightly longer period of time is acceptable to meet the construction schedule and to achieve the required strength. Flowability determines whether the slurry can be injected smoothly, but focusing on it alone and ignoring strength is not effective for reinforcement. Similarly, the bleeding rate and initial setting time have an effect, but to a lesser extent than the strength when the basic properties and construction operations are ensured. Considering the relative importance of these factors, the weight of each performance indicator in determining the grouting effectiveness was comprehensively assessed. The optimum ratio was determined using the indicator weights from Group 10, with a zeolite powder:GGBFS:CL:NaOH ratio of 30:70:5:7 and a water–binder ratio of 0.6.
6. Conclusions
In this study, the engineering performance regarding the setting time, flowability, bleeding rate, concretion rate, and compressive strength of alkali-activated GGBFS–zeolite powder slurry with different mix ratios were measured. The surface morphology and chemical composition of slurry concretions was analyzed using SEM and XRD. Simultaneously, the influence of various material factors and performance indicators on the slurry was quantitatively analyzed using one-factor standard deviation and GRA. The main conclusions are as follows:
- (1)
- The setting time of alkali-activated GGBFS–zeolite powder slurry exhibits a positive correlation with the proportions of zeolite powder and CL and the water–binder ratio, while it is inversely related to the amount of NaOH. Notably, when the NaOH dosage is 1%, the slurry fails to set. With an increase in the dosage of zeolite powder and NaOH, the fluidity of the slurry is significantly enhanced. Conversely, a higher CL dosage and water–binder ratio lead to a decline in flowability and an increase in the slurry’s viscosity. This insight is crucial for maximizing the workability of the grouting material under varying conditions.
- (2)
- The compressive strength of the slurry stone bodies gradually decreases with an increasing zeolite powder dosage and water–binder ratio. Conversely, an increase in the CL dosage enhances the compressive strength of the stone bodies. At the same time, the impact of NaOH doping on the compressive strength of the stone bodies initially increases, followed by a subsequent decrease. At a 5% doping level, the compressive strength of the stone bodies reaches a maximum of 17.59 MPa after 28 days. The compressive strength of this grout is compatible with silty mudstone, making it suitable for reinforcing silty mudstone slopes without the risk of secondary cracking along the material interface.
- (3)
- In the case of alkali-activated GGBFS–zeolite powder slurry, the water–binder ratio is identified as a key factor influencing performance indices. The correlation between each performance index of the grouting material and the optimal mix ratio is, in descending order, as follows: compressive strength, flowability, final setting time, initial setting time and bleeding rate. The optimal mix ratio is zeolite powder:GGBFS:calcium lignosulphonate (CL):NaOH = 30:70:5:7, with a water–binder ratio of 0.6. This mix ratio not only meets the specification requirements but also provides a cost-effective and environmentally sustainable solution, utilizing widely available industrial byproducts.
Future efforts will focus on validating the optimal alkali-activated GGBFS–zeolite powder slurry ratio for practical use, alongside studies on shrinkage, durability, and adaptability to different construction environments. Additionally, life cycle assessments or CO2 emission comparisons with silicate cement will be conducted to evaluate the environmental sustainability of the slurry.
Author Contributions
Conceptualization, L.W., H.F. and Q.G.; data curation, G.Y. and J.L.; formal analysis, L.W. and J.L.; funding acquisition, H.F. and L.W.; investigation, J.S. and Y.L.; methodology, Q.G. and J.T.; project administration, Q.G.; resources, J.T.; validation, L.W. and G.Y.; visualization, G.Y.; writing—original draft, L.W. and J.L.; writing—review and editing, H.F., Q.G. and Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Nos. 52378440, 42477143); the Natural Science Foundation of Hunan Province (No. 2023JJ10045); the Key Science and Technology Program in the Transportation Industry (Nos. 2022-MS1-032, 2022-MS5-125); the Project Fund Practical Innovation and Entrepreneurship Enhancement Program of Changsha University of Science and Technology, China (No. CLSJCX23009); and the Guangxi KeyResearch and Development Program (No. AB23075184).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Acknowledgments
The authors gratefully acknowledge the financial support of Wenguang Wang and Ling Zeng for this study. And special thanks also go to Key Laboratory of Road Structure and Material Ministry of Communication.
Conflicts of Interest
Authors Jianping Song and Youjun Li were employed by the company Guangxi Transportation Science and Technology Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GGBFS | Ground granulated blast furnace slag |
| CL | Calcium lignosulphonate |
| GP | Glass powder |
| FA | Fly ash |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| GRA | Grey Relational Analysis |
| C-S-H | Cement Silicate Hydrogel |
| C-(A)-S-H | Calcium Sulfoaluminate Hydrates |
References
- Fu, H.Y.; Jiang, H.B.; Qiu, X.; Ji, Y.P.; Chen, W.; Zeng, L. Seepage characteristics of a fractured silty mudstone underdifferent confining pressures and temperatures. J. Cent. South Univ. 2020, 27, 1907–1916. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers and geopolymeric materials. J. Therm. Anal. 1989, 35, 429–441. [Google Scholar] [CrossRef]
- Matsimbe, J.; Dinka, M.; Olukanni, D.; Musonda, I. Geopolymer: A Systematic Review of Methodologies. Materials 2022, 15, 6852. [Google Scholar] [CrossRef]
- Khan, M.N.N.; Kuri, J.C.; Sarker, P.K. Effect of waste glass powder as a partial precursor in ambient cured alkali activated fly ash and fly ash-GGBFS mortars. J. Build. Eng. 2021, 34, 101934. [Google Scholar] [CrossRef]
- Abed, M.H.; Abbas, I.S.; Hamed, M.; Canakci, H. Rheological, fresh, and mechanical properties of mechanochemically activated geopolymer grout: A comparative study with conventionally activated geopolymer grout. Constr. Build. Mater. 2022, 322, 126338. [Google Scholar] [CrossRef]
- Zhang, K.; Yang, Y.; Li, H. Cementitious properties of coal-based metakaolin prepared from coal gangue via Fe3O4-Assisted microwave activation. J. Clean. Prod. 2024, 448, 141277. [Google Scholar] [CrossRef]
- Hamada, H.M.; Abed, F.; Katman, H.Y.B.; Humada, A.M.; Al Jawahery, M.S.; Majdi, A.; Yousif, S.T.; Thomas, B.S. Effect of silica fume on the properties of sustainable cement concrete. J. Mater. Res. Technol. 2023, 24, 8887–8908. [Google Scholar] [CrossRef]
- Pan, S.; Li, J.; Gong, H.; Zhu, Z.; Xu, S.; Jiang, C.; Cai, W. Resource Disposal and Products of Fly Ash from Domestic Waste Incineration in Zhejiang Province, China: Migration and Change of Hazardous Heavy Metals. Sustainability 2024, 16, 302. [Google Scholar] [CrossRef]
- Teixeira, E.R.; Camoes, A.; Branco, F.G.; Aguiar, J.B.; Fangueiro, R. Recycling of biomass and coal fly ash as cement replacement material and its effect on hydration and carbonation of concrete. Waste Manag. 2019, 94, 39–48. [Google Scholar] [CrossRef]
- Loke, C.K.; Lehane, B.; Aslani, F.; Majhi, S.; Mukherjee, A. Non-Destructive Evaluation of Mortar with Ground Granulated Blast Furnace Slag Blended Cement Using Ultrasonic Pulse Velocity. Materials 2022, 15, 6957. [Google Scholar] [CrossRef]
- Sitarz, M.; Hager, I.; Choińska, M. Evolution of Mechanical Properties with Time of Fly-Ash-Based Geopolymer Mortars under the Effect of Granulated Ground Blast Furnace Slag Addition. Energies 2020, 13, 1135. [Google Scholar] [CrossRef]
- Xiang, J.C.; He, Y.; Liu, L.P.; Zheng, H.; Cui, X.M. Exothermic behavior and drying shrinkage of alkali-activated slag concrete by low temperature-preparation method. Constr. Build. Mater. 2020, 262, 120056. [Google Scholar] [CrossRef]
- Kouchachvili, L.; Bardy, D.A.; Djebbar, R.; Hogg, L.E. Natural zeolites as host matrices for the development of low-cost and stable thermochemical energy storage materials. J. Porous Mater. 2023, 30, 163–173. [Google Scholar] [CrossRef]
- Hu, H.B.; He, Z.H.; Fan, K.J.; Shibro, T.G.; Liu, B.J.; Shi, J.Y. Properties enhancement of recycled coarse aggregates by pre-coating/pre-soaking with zeolite powder/calcium hydroxide. Constr. Build. Mater. 2021, 286, 122888. [Google Scholar] [CrossRef]
- Mijailović, N.R.; Nedić Vasiljević, B.; Ranković, M.; Milanović, V.; Uskoković-Marković, S. Environmental and Pharmacokinetic Aspects of Zeolite/Pharmaceuticals Systems—Two Facets of Adsorption Ability. Catalysts 2022, 12, 837. [Google Scholar] [CrossRef]
- Rahman, S.K.; Al-Ameri, R. Marine Geopolymer Concrete-A Hybrid Curable Self-Compacting Sustainable Concrete for Marine Applications. Appl. Sci. 2022, 12, 3116. [Google Scholar] [CrossRef]
- Ni, Z.L.; Wang, S.Y.; Zheng, X.C.; Qi, C.H. Application of geopolymer in synchronous grouting for reusing of the shield muck in silty clay layer. Constr. Build. Mater. 2024, 419, 135345. [Google Scholar] [CrossRef]
- Le, H.; Sun, S.; Kulatilake, P.H.; Wei, J. Effect of grout on mechanical properties and cracking behavior of rock-like specimens containing a single flaw under unconfined compressive. Int. J. Geomech. 2018, 18, 04018129. [Google Scholar] [CrossRef]
- Li, Q.T.; Li, Z.G.; Yuan, G.L. Effects of elevated temperatures on properties of concrete containing ground granulated blast furnace slag as cementitious material. Constr. Build. Mater. 2012, 35, 687–692. [Google Scholar] [CrossRef]
- Feng, B.; Zhang, Y.; Li, W.; Che, G.; Liu, C.; Jv, A.; Lu, J.; Sun, W.; Guan, R. Efficient photocatalytic removal of ciprofloxacin through in-situ growth of g-C3N4 on the surface of clinoptilolite. J. Phys. Chem. Solids 2024, 192, 112077. [Google Scholar] [CrossRef]
- Qu, J.S.; Li, H.Q.; Li, S.P.; Hou, X.J.; Chang, R.Q.; Zhang, Y.F. Coal gasification slag-derived highly reactive silica for high modulus sodium silicate synthesis: Process and mechanism. Chem. Eng. J. 2024, 479, 1385–8947. [Google Scholar] [CrossRef]
- GB 175-2023; Common Portland Cement; State Administration for Market Regulation; National Standardization Administration. Standards Press of China: Beijing, China, 2023.
- Yu, H.; Yi, Y.L.; Unluer, C. Heat of hydration, bleeding, viscosity, setting of Ca(OH)2-GGBS and MgO-GGBS grouts. Constr. Build. Mater. 2021, 270, 121839. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Awed, A.M.; Xie, J.; Gu, F. Performance evaluation of high early strength micro-expansion geopolymer grout potentially used for sustainable road infrastructure. Transp. Geotech. 2024, 49, 101400. [Google Scholar] [CrossRef]
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement; General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China. Standards Press of China: Beijing, China, 2011.
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar; General Administration of Quality Supervision; Inspection and Quarantine of the People’s Republic of China; Standardization Administration of the People’s Republic of China. Standards Press of China: Beijing, China, 2005.
- JTG 3420-2020; Testing Methods of Cement and Concrete for Highway Engineering; Ministry of Transport of the People’s Republic of China. China Communication Press: Beijing, China, 2020.
- GB/T 17671-2021; Test Method of Cement Mortar Strength; State Administration for Market Regulation; Standardization Administration of the People’s Republic of China. Standards Press of China: Beijing, China, 2021.
- Jiang, J.; Wang, F.; Wang, L.; Zhang, J.; Wang, L. Experimental investigation on effects of polyaniline-modified-lignin on cement-based materials: Strength, hydration and dispersion. Constr. Build. Mater. 2023, 394, 131853. [Google Scholar] [CrossRef]
- Magina, S.; Barros-Timmons, A.; Evtuguin, D.V. Synthesis of Lignosulfonate-Based Dispersants for Application in Concrete Formulations. Materials 2021, 14, 7388. [Google Scholar] [CrossRef]
- de Gasperi, J.; Dörr, G.; Melchiors, E.F.; Longhi, M.A.; de Matos, P.R.; Rodríguez, E.D. Effect of activator type and concentration, water-to-solid ratio, and time on the flowability of metakaolin-based geopolymer pastes. J. Mater. Civ. Eng. 2022, 34, 04022205. [Google Scholar] [CrossRef]
- GB/T 50448-2015; Technical Code for Application of Cementitious Grout; Ministry of Housing and Urban-Rural Development of the People’s Republic of China; General Administration of Quality Supervision, Inspection and Quarantine of the People’s Republic of China. China Architecture & Building Press: Beijing, China, 2015.
- Topçu, I.B.; Atesin, Ö. Effect of high dosage lignosulphonate and naphthalene sulphonate based plasticizer usage on micro concrete properties. Constr. Build. Mater. 2016, 120, 189–197. [Google Scholar] [CrossRef]
- Liu, C.H.; Li, Z.Y.; Bezuijen, A.; Chen, L.H.; Cachim, P. Optimizing the shield tunnel backfilling grouts with supplementary cementitious materials by response surface methodology. Constr. Build. Mater. 2024, 421, 135575. [Google Scholar] [CrossRef]
- DL/T 5148-2021; Technical Specification for Cement Grouting of Hydraulic Structures; Ministry of Water Resources of the People’s Republic of China. China Water & Power Press: Beijing, China, 2021.
- Kantarci, F.; Maras, M.M. Fabrication of Novel Geopolymer Grout as Repairing Material for Application in Damaged RC Beams. Int. J. Civ. Eng. 2022, 20, 461–474. [Google Scholar] [CrossRef]
- Touloumet, Q.; Postole, G.; Massin, L.; Lorentz, C.; Auroux, A. Investigation of the impact of zeolite shaping and salt deposition on the characteristics and performance of composite thermochemical heat storage systems. J. Mater. Chem. A 2023, 11, 2737–2753. [Google Scholar] [CrossRef]
- Liang, G.W.; Yao, W.; She, A.M. Rheology and microstructure of lithium slag/metakaolin geopolymer pastes: Insights from particle packing and water dynamic evolution. J. Build. Eng. 2024, 95, 110261. [Google Scholar] [CrossRef]
- Mahfoud, E.; Ndiaye, K.; Maherzi, W.; Aggoun, S.; Abriak, N.E.; Benzerzour, M. Thermodynamic modeling of one-part-geopolymer binders based on fly ash and micronized dredged sediments. Constr. Build. Mater. 2024, 452, 138906. [Google Scholar] [CrossRef]
- Huang, D.; Chen, H.; Zou, Y.; Yuan, Q.; Peng, H. Influence of raw material properties on microscopic and mechanical characteristics of alkali-activated materials. Case Stud. Constr. Mater. 2024, 20, e03319. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.A.E.M.; Salem, H.A. Effect of water addition, plasticizer and alkaline solution constitution on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 121, 694–703. [Google Scholar] [CrossRef]
- Wan, Q.; Zhang, Y.M.; Zhang, R.B. The effect of pore behavior and gel structure on the mechanical property at different initial water content. Constr. Build. Mater. 2021, 309, 125146. [Google Scholar] [CrossRef]
- Bauchy, M.; Qomi, M.J.A.; Ulm, F.J.; Pellenq, R.J.M. Order and disorder in calcium-silicate-hydrate. J. Chem. Phys. 2014, 140, 214503. [Google Scholar] [CrossRef] [PubMed]
- Ruengsillapanun, K.; Udtaranakron, T.; Pulngern, T.; Tangchirapat, W.; Jaturapitakkul, C. Mechanical properties, shrinkage, and heat evolution of alkali activated fly ash concrete. Constr. Build. Mater. 2021, 299, 123954. [Google Scholar] [CrossRef]
- Zhou, C.S.; Tang, Q.; Lu, L. Al Content Effect on Microstructure and Strength in Calcium Aluminosilicate Hydrate Chain Integration. Strength Mater. 2022, 54, 929–941. [Google Scholar] [CrossRef]
- Li, J.Q.; Zhang, W.X.; Garbev, K.; Monteiro, P.J.M. Coordination environment of Si in calcium silicate hydrates, silicate minerals, and blast furnace slags: A XANES database. Cem. Concr. Res. 2021, 143, 106376. [Google Scholar] [CrossRef]
- Liu, M.F.; Zhu, J.L.; Zhang, C.Y.; He, P.; Chen, D.X.; Zhong, G.J.; Liu, Q.; Hu, W.Y.; Chen, Y.Z.; Zhu, J.Y. Effect of calcium lignosulfonate on surface modification and bioleaching of chalcopyrite. Biochem. Eng. J. 2024, 207, 109329. [Google Scholar] [CrossRef]
- Rambo, D.A.S.; Ukrainczyk, N.; Silva, F.D.; Koenders, E.; Toledo, R.D.; Gomes, O.D.M. Calcium-aluminate mortars at high temperatures: Overcoming adverse conversion effects using clinker aggregates. Cem. Concr. Compos. 2019, 96, 212–224. [Google Scholar] [CrossRef]
- Abdrakhmanov, V.I.; Dobrotin, S.A.; Kosyreva, O.N.; Logutov, V.I. Statistical Evaluation of the Standard Deviation of Chromatographic Retention Time in the Capillary Column Temperature Programming Mode. J. Anal. Chem. 2021, 76, 641–652. [Google Scholar] [CrossRef]
- Wu, P.; Zhang, Q.; Wang, G.; Yang, F.; Xue, F. Dynamic feature selection combining standard deviation and interaction information. Int. J. Mach. Learn. Cybern. 2023, 14, 1407–1426. [Google Scholar] [CrossRef]
- Zhang, S.; Qiao, W.; Wu, Y.; Fan, Z.; Zhang, L. Multi-Response Optimization of Ultrafine Cement-Based Slurry Using the Taguchi-Grey Relational Analysis Method. Materials 2021, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Abid, N.; Ikram, M.; Wu, J.Z.; Ferasso, M. Towards environmental sustainability: Exploring the nexus among ISO 14001, governance indicators and green economy in Pakistan. Sustain. Prod. Consum. 2021, 27, 653–666. [Google Scholar] [CrossRef]
- Yu, G.; Fu, H.; Gao, Q.; Zeng, L.; Chen, J.; Ma, C. Development and Application of Flue Gas Desulfurized Gypsum and Blast-Furnace-Slag-Based Grouting Material for Cracked Silty Mudstone. Materials 2024, 17, 5975. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.W.; Fu, H.Y.; Zeng, L.; Liu, J.; Qiu, X.Y. Damage constitutive model for soft rocks and its experimental verification on silty mudstone considering cyclic rock–water interactions. Bull. Eng. Geol. Environ. 2024, 83, 254. [Google Scholar] [CrossRef]
- Liao, Y.; Zheng, W.; Long, J.; Xie, X.; Hu, J. Optimization of Backrest Skeleton of Carbon Fiber Reinforced Plastic Car Seat Based on Grey Euclidean Relational Analysis Method. Int. J. Automot. Technol. 2023, 24, 1189–1203. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, S.; Chang, J.; Hou, H.; Chen, C.; Cheng, F.; He, N.; Wu, X. Using Taguchi grey relational analysis to optimize the dimensional parameters of a Coercivity detection probe. Sādhanā 2023, 48, 249. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).