Valorization of Grape Pomace Extract Through Dextran–Grape Conjugates: A Sustainable Approach for Cosmetic and Dermatological Applications
Abstract
1. Introduction
- the reuse of waste raw materials, thereby promoting a circular economy through waste reduction and repurposing;
- the application of rapid dynamic solid–liquid extraction (RSLDE, Naviglio®) to maximize the preservation of bioactive compounds [21];
- the grafting of polymers in an aqueous environment, using dextran—a natural, biocompatible, and biodegradable polysaccharide—due to its moisturizing, film-forming, and carrier properties. Its biocompatibility and chemical stability make it particularly suitable for various applications, protecting active ingredients from degradation [22,23,24,25,26].
2. Materials and Methods
2.1. Reagents
2.2. Extract Preparation Using a Naviglio Extractor®
2.3. Preparation and Characterization of the Polymeric Conjugates
2.3.1. Grafting Reaction
2.3.2. Total Polyphenol Content Quantification
2.3.3. DPPH Assay
2.3.4. In Vitro Diffusion Studies
2.3.5. Stability Studies
2.4. In Vitro Safety Assessment
2.4.1. Neutral Red Uptake Assay
2.4.2. Skin Sensitization Potential Test (h-CLAT)
2.4.3. Skin Irritation Test
- Mean tissue viability < 50%: IRRITANT
- Mean tissue viability > 50%: NON-IRRITATING.
2.5. In Vitro Efficacy Assessment
2.5.1. Experimental Conditions
2.5.2. DCFDA Cellular ROS Assay
2.5.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.5.4. Senescence-Associated β-Galactosidase Activity
2.5.5. Immunofluorescence
2.6. Statistical Analysis
3. Results
3.1. Characterization of the PLG–GRAPE Conjugates
3.1.1. Evaluation of the Total Polyphenol Content: Folin–Ciocalteu Assay
3.1.2. Determination of DPPH Radical Scavenging Activity
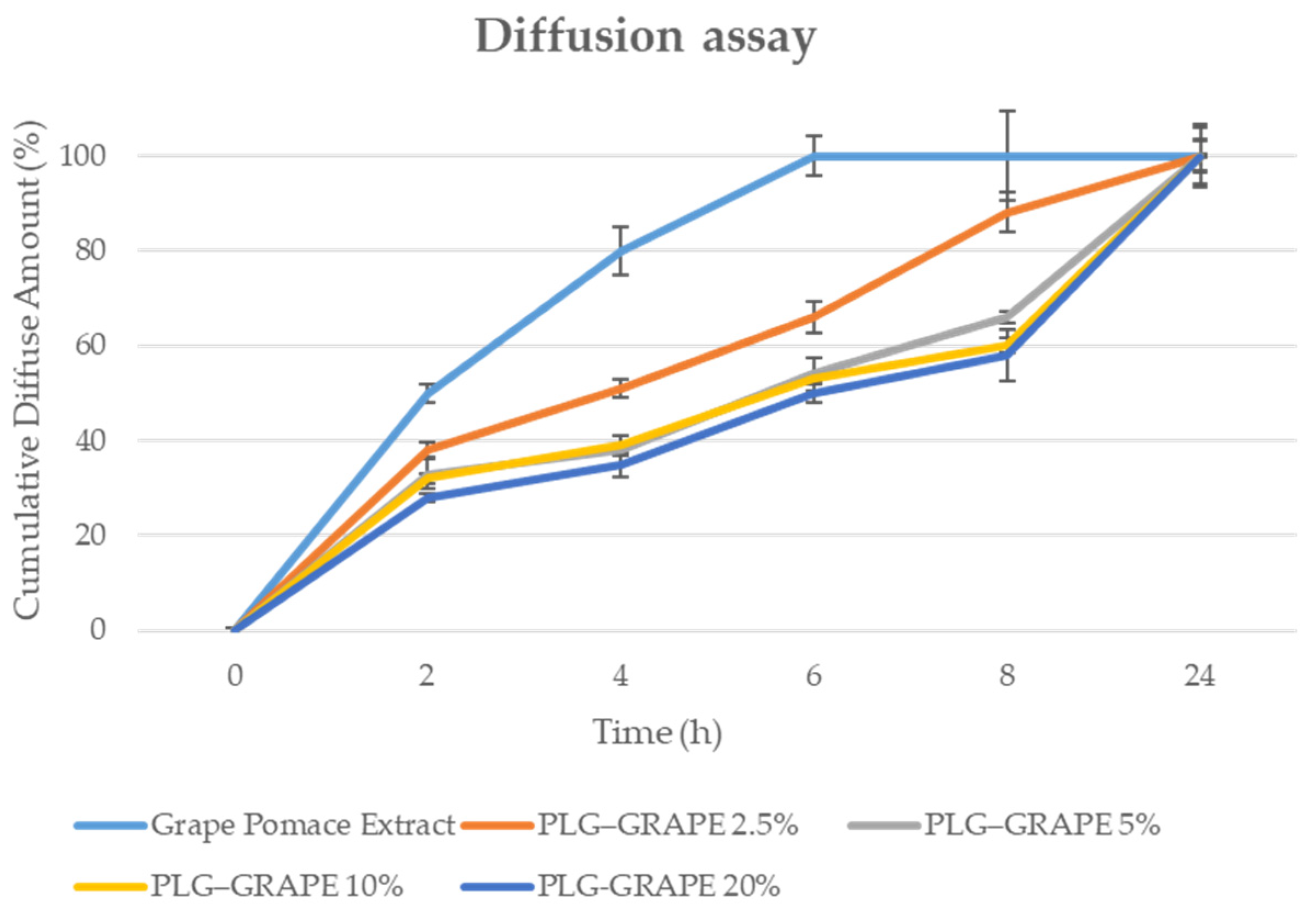
3.1.3. In Vitro Diffusion Studies by Vertical Franz Cells
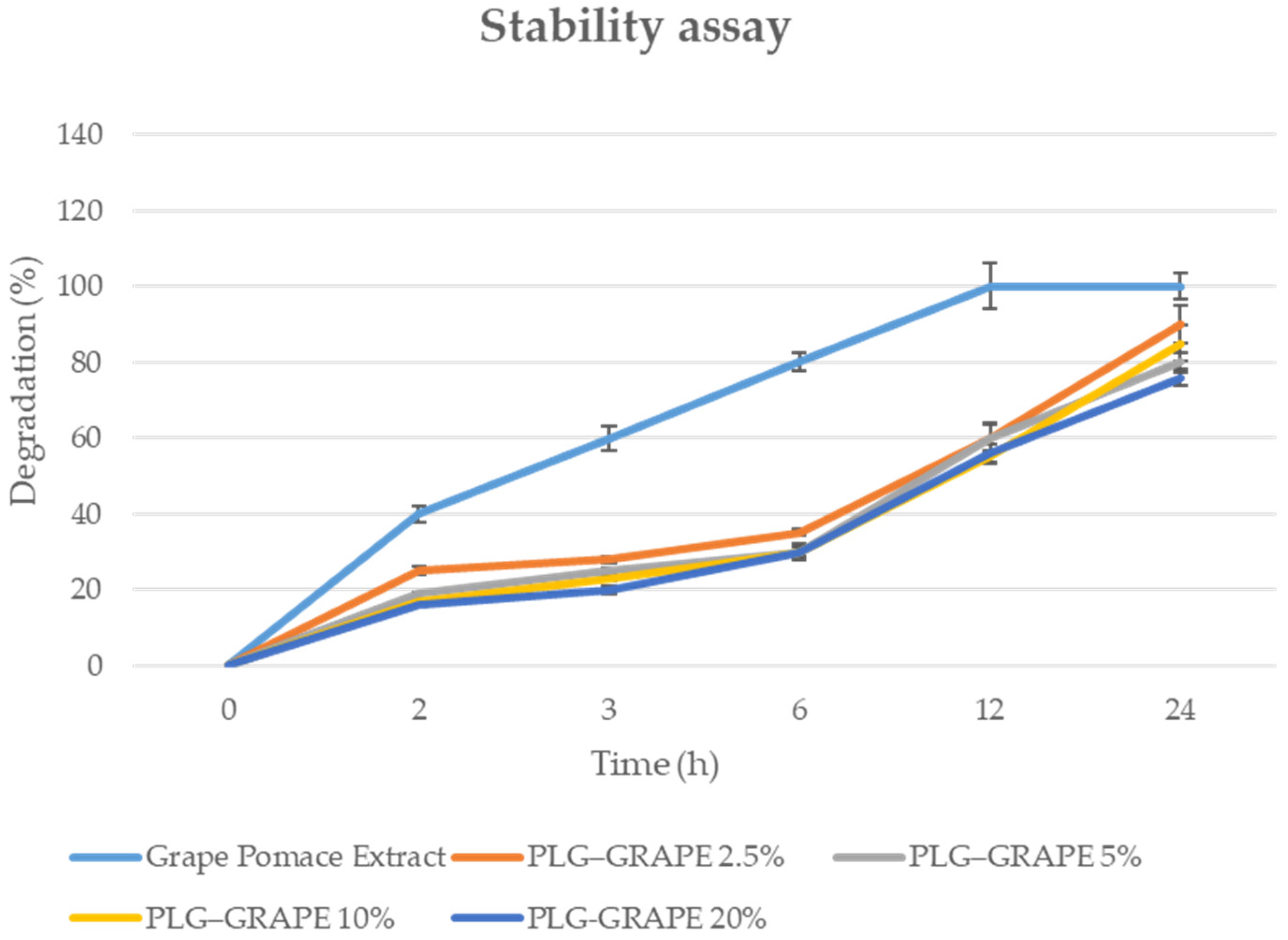
3.1.4. Stability Studies
3.2. Safety Assessment
3.2.1. Cytotoxicity Studies
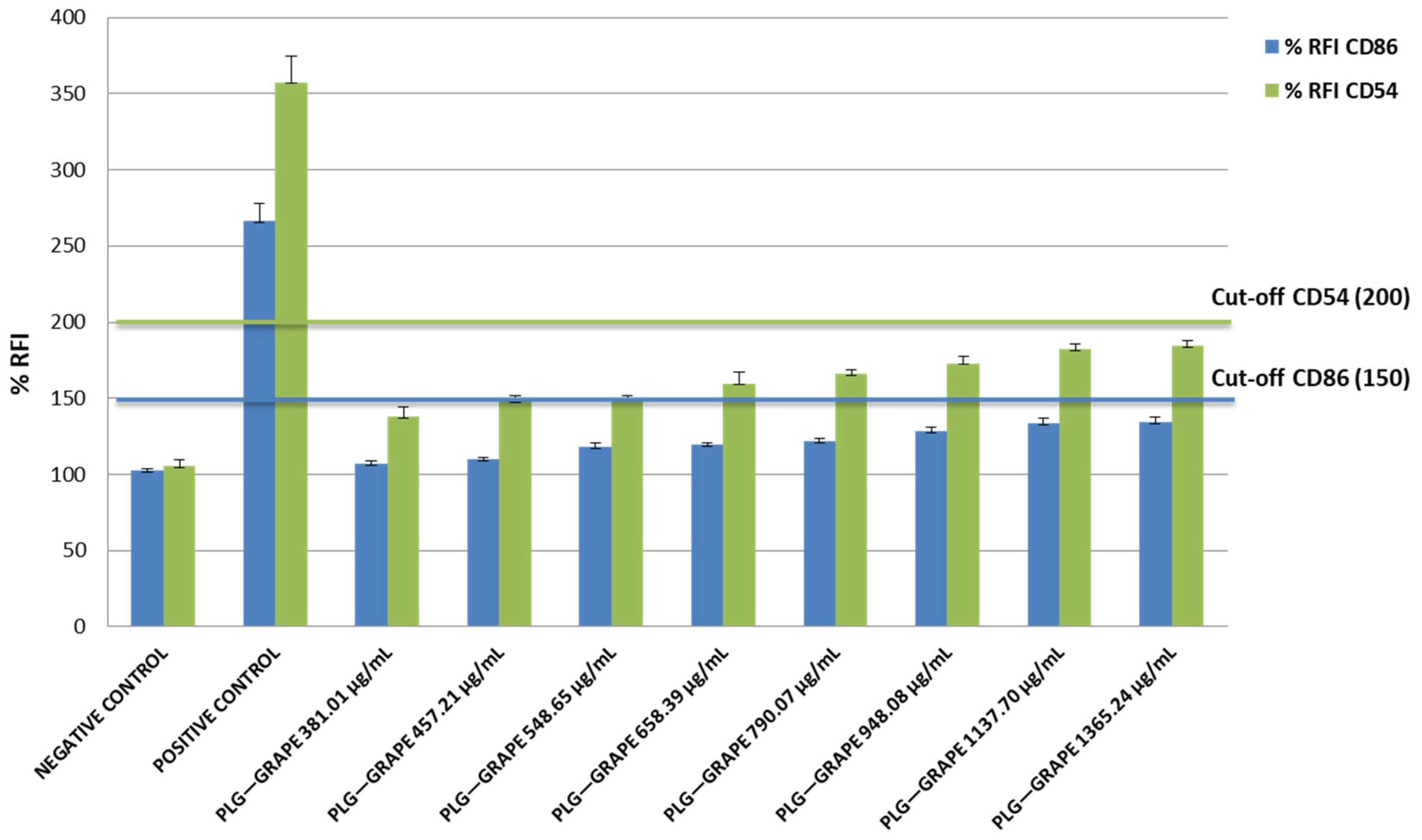
3.2.2. Skin Sensitization Potential Test (h-CLAT)
3.2.3. Skin Irritation Test
3.3. Efficacy Assessment
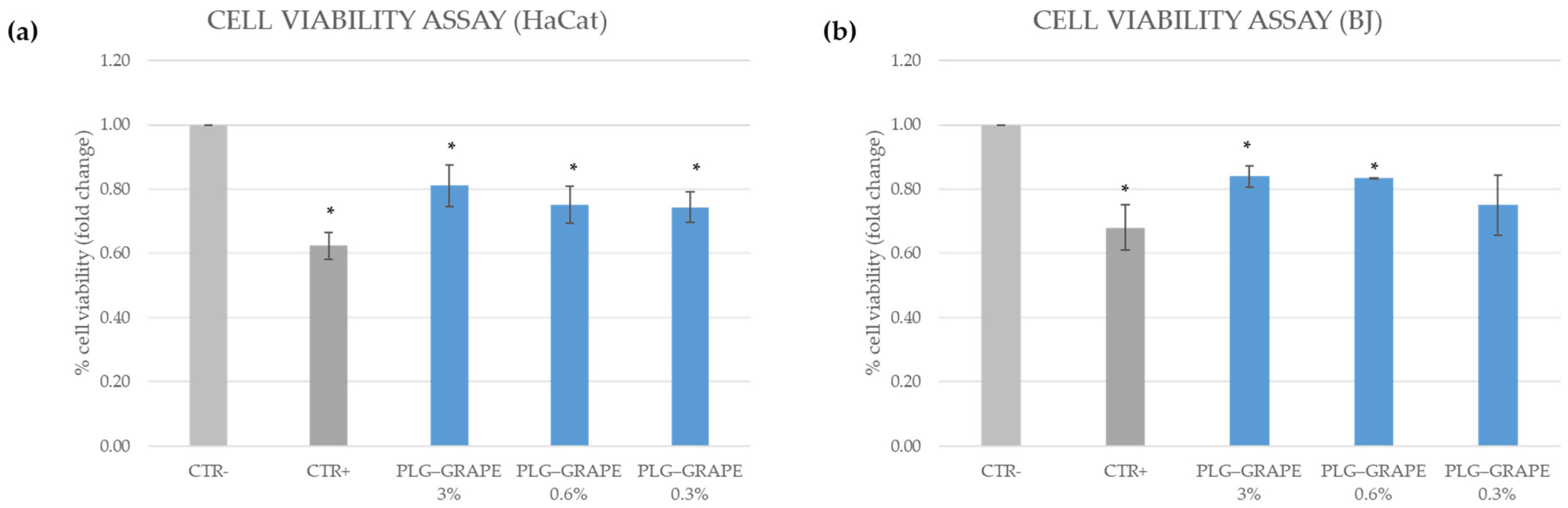
3.3.1. Cell Viability Assay
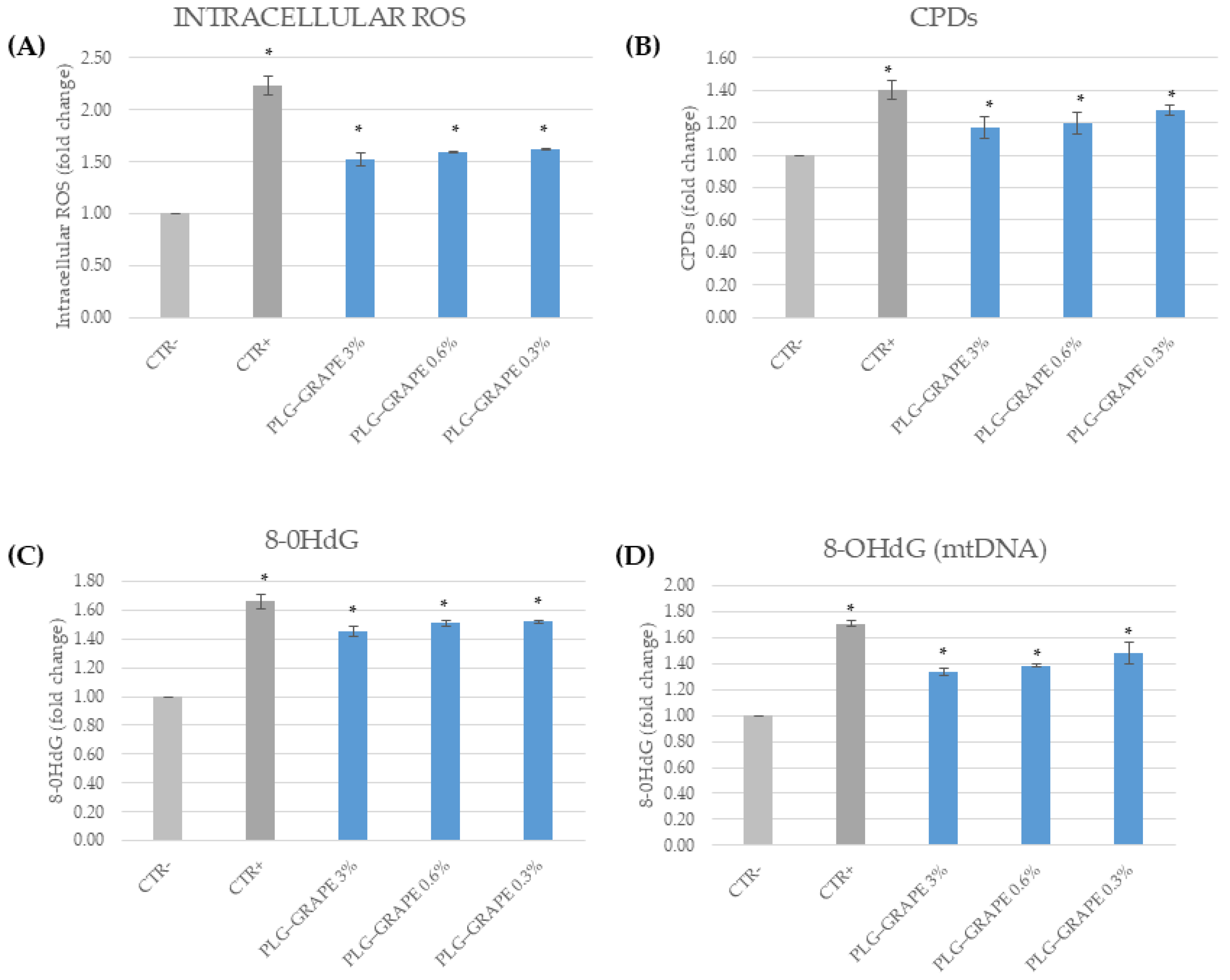
3.3.2. Antioxidant Effect
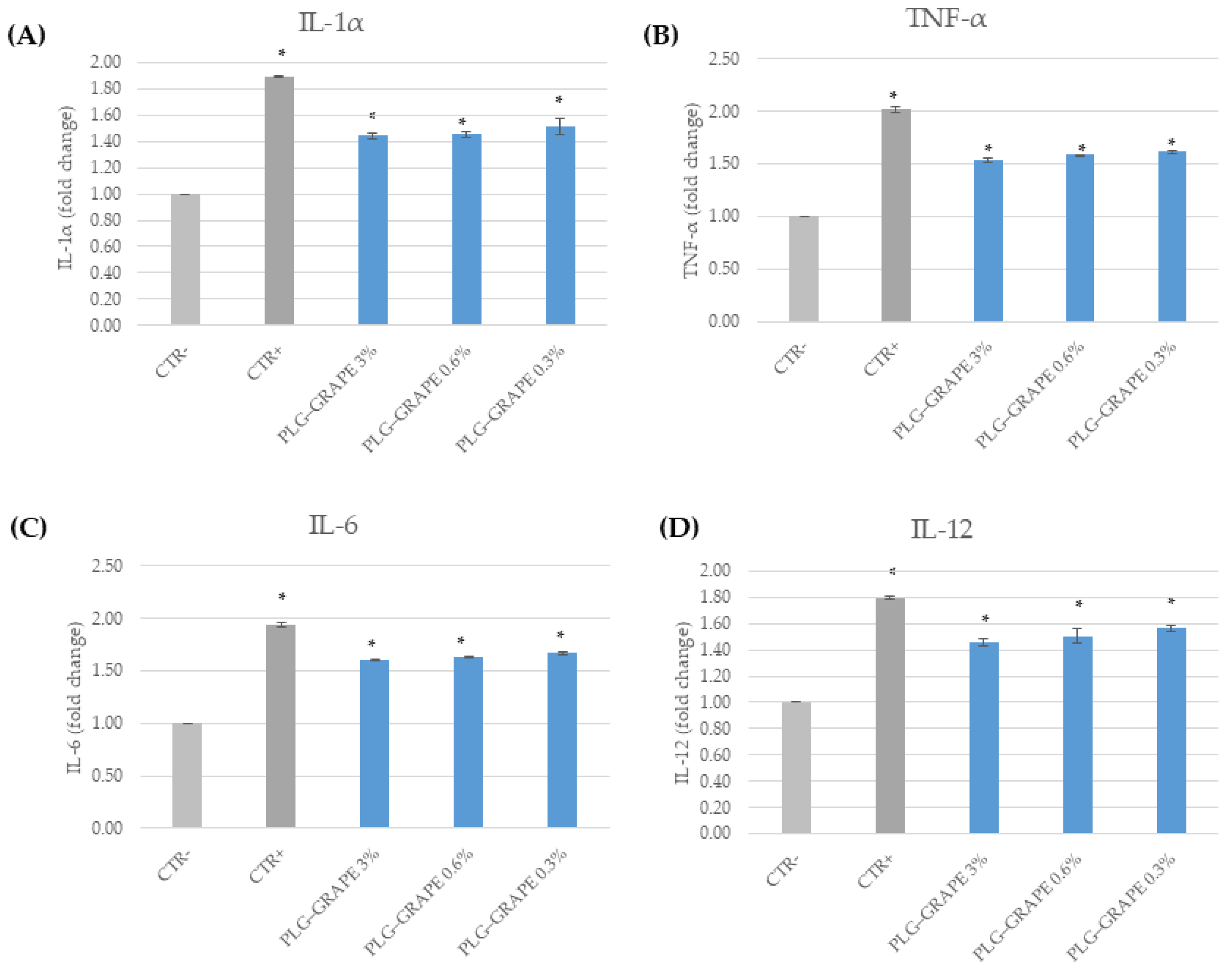
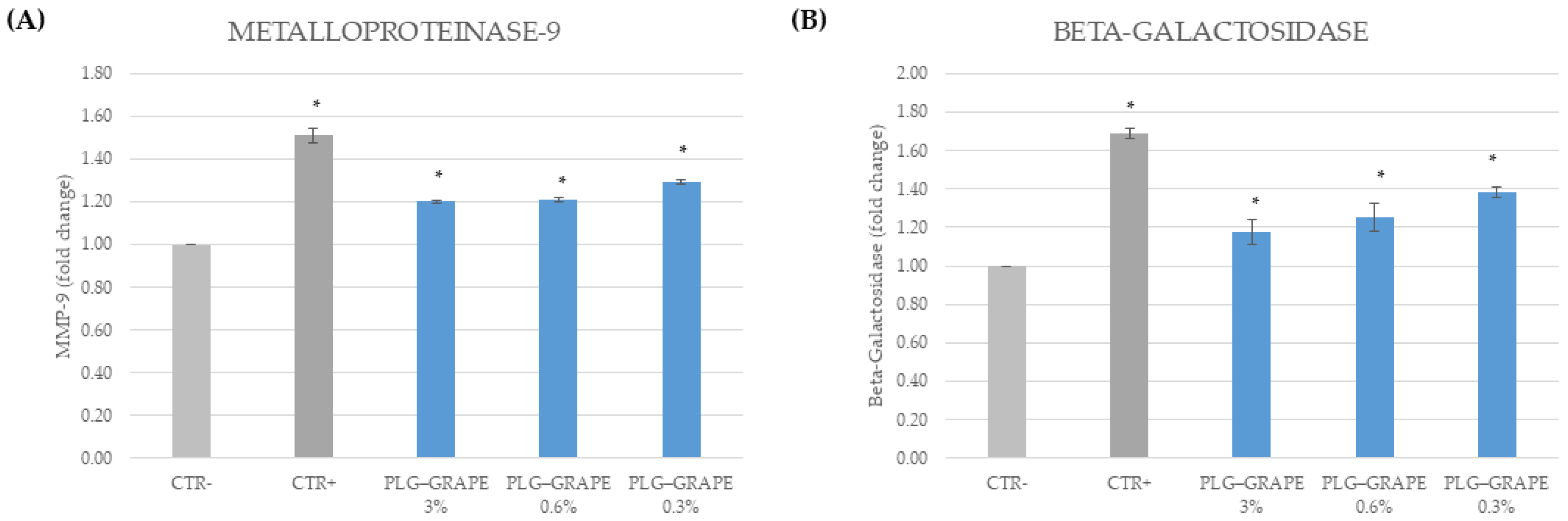
3.3.3. Anti-Inflammatory Efficacy
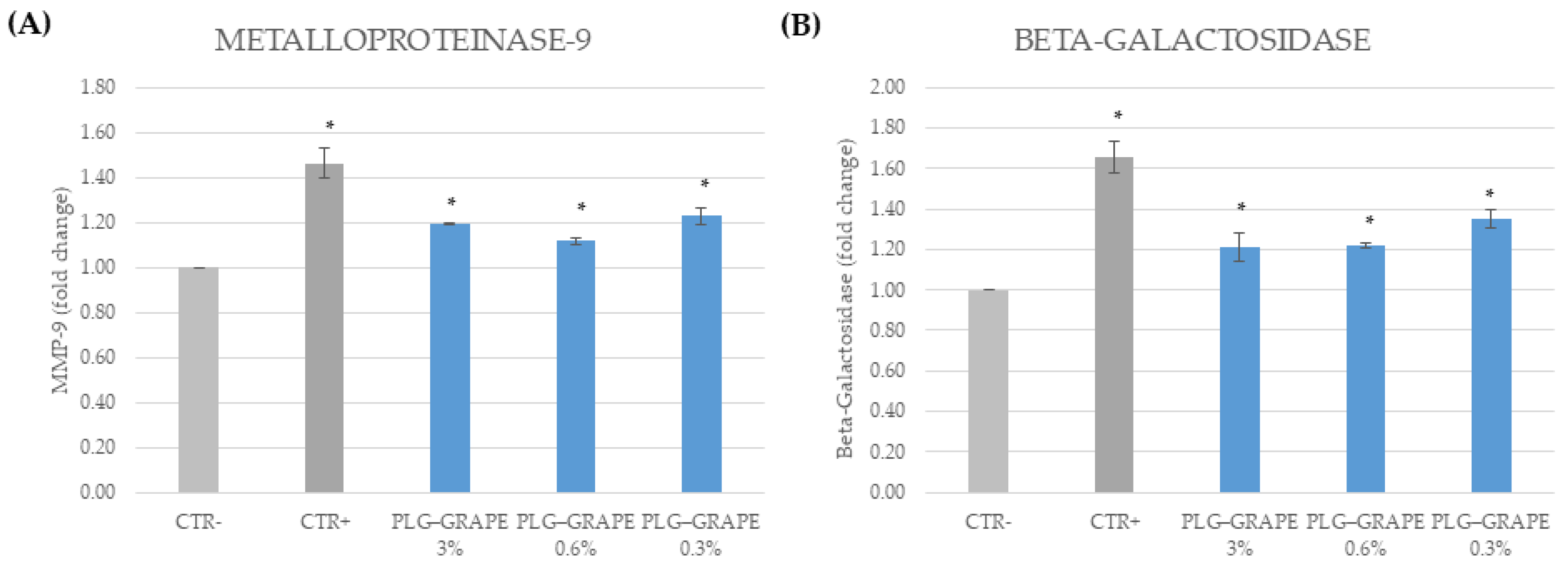
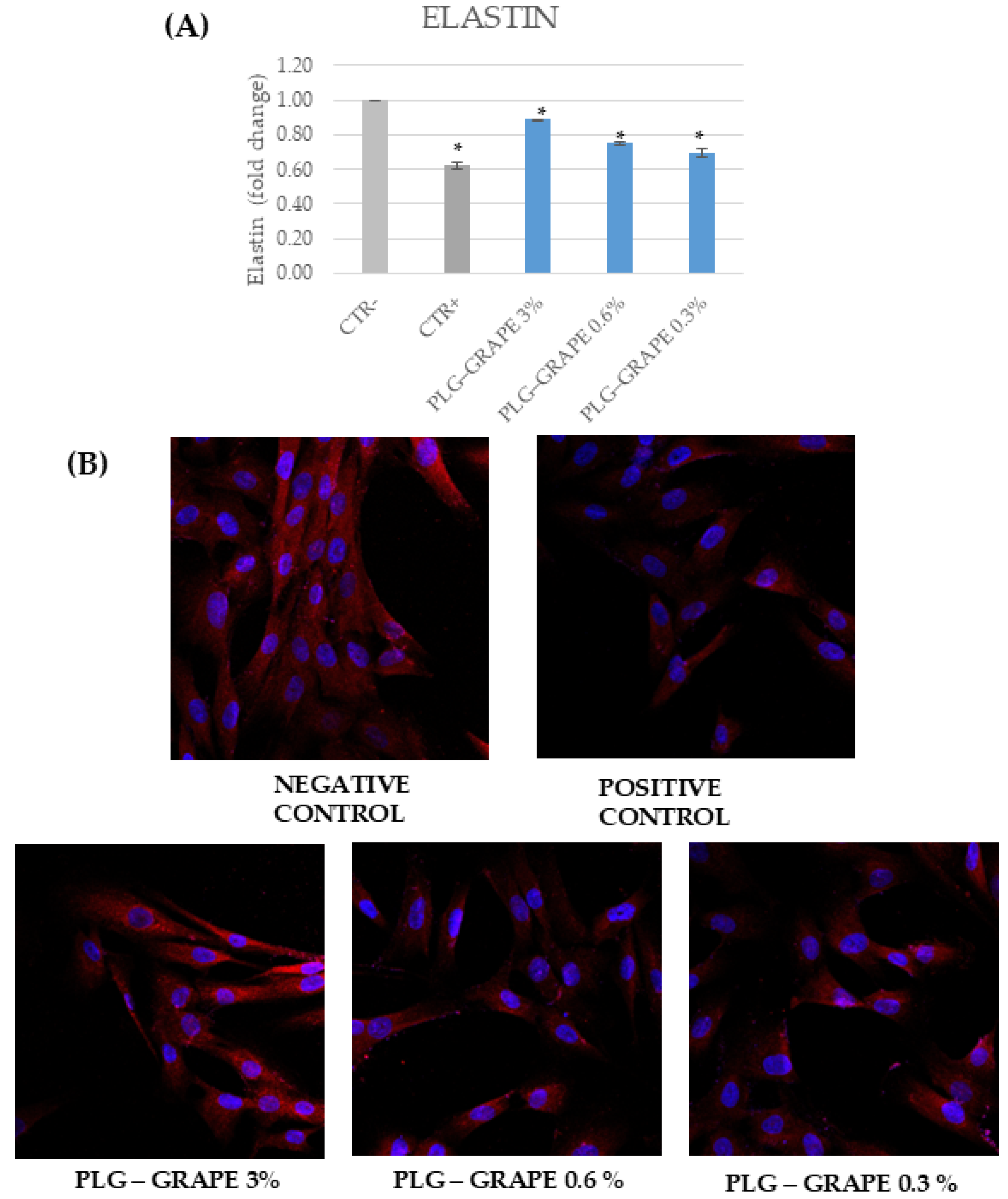
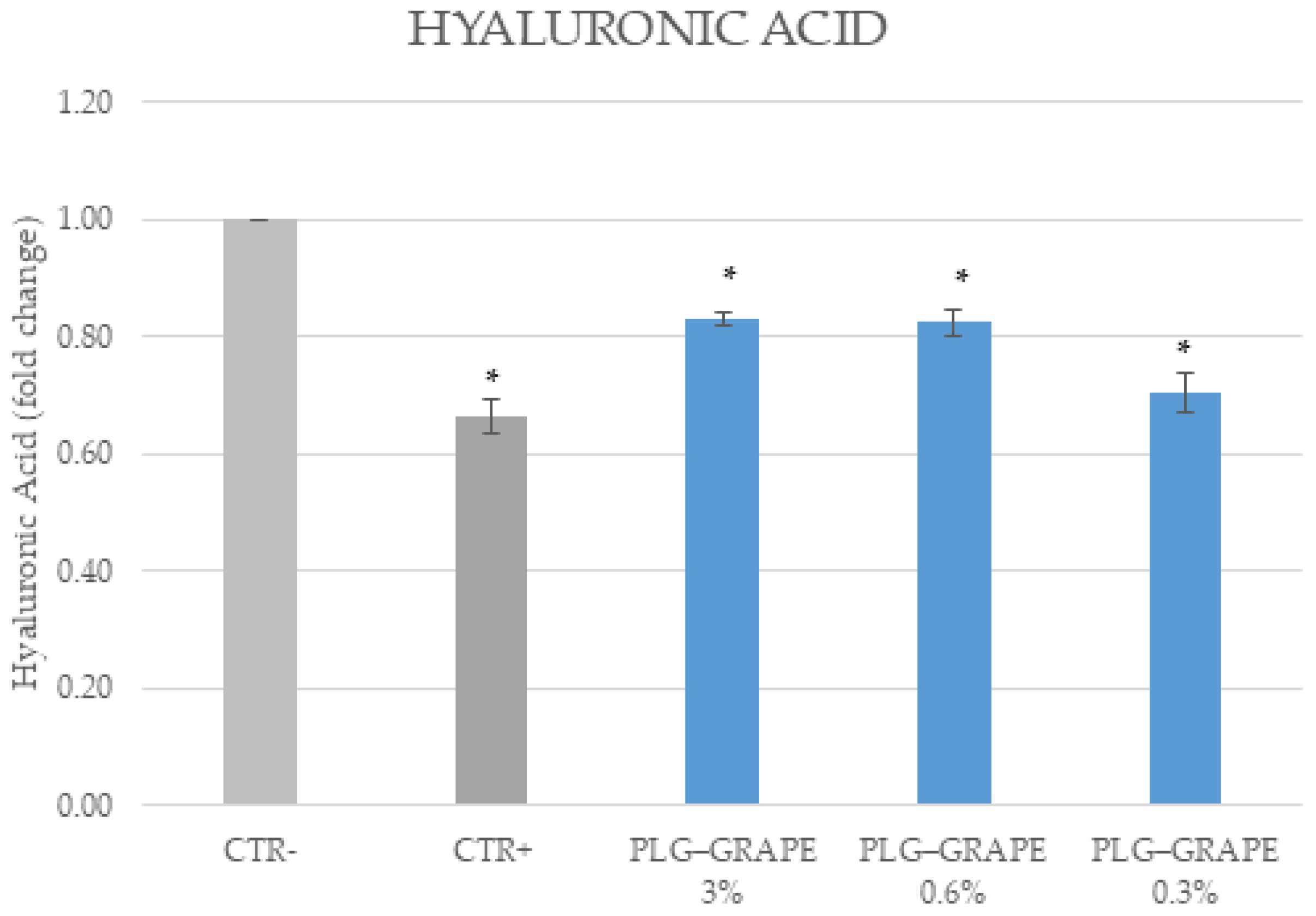
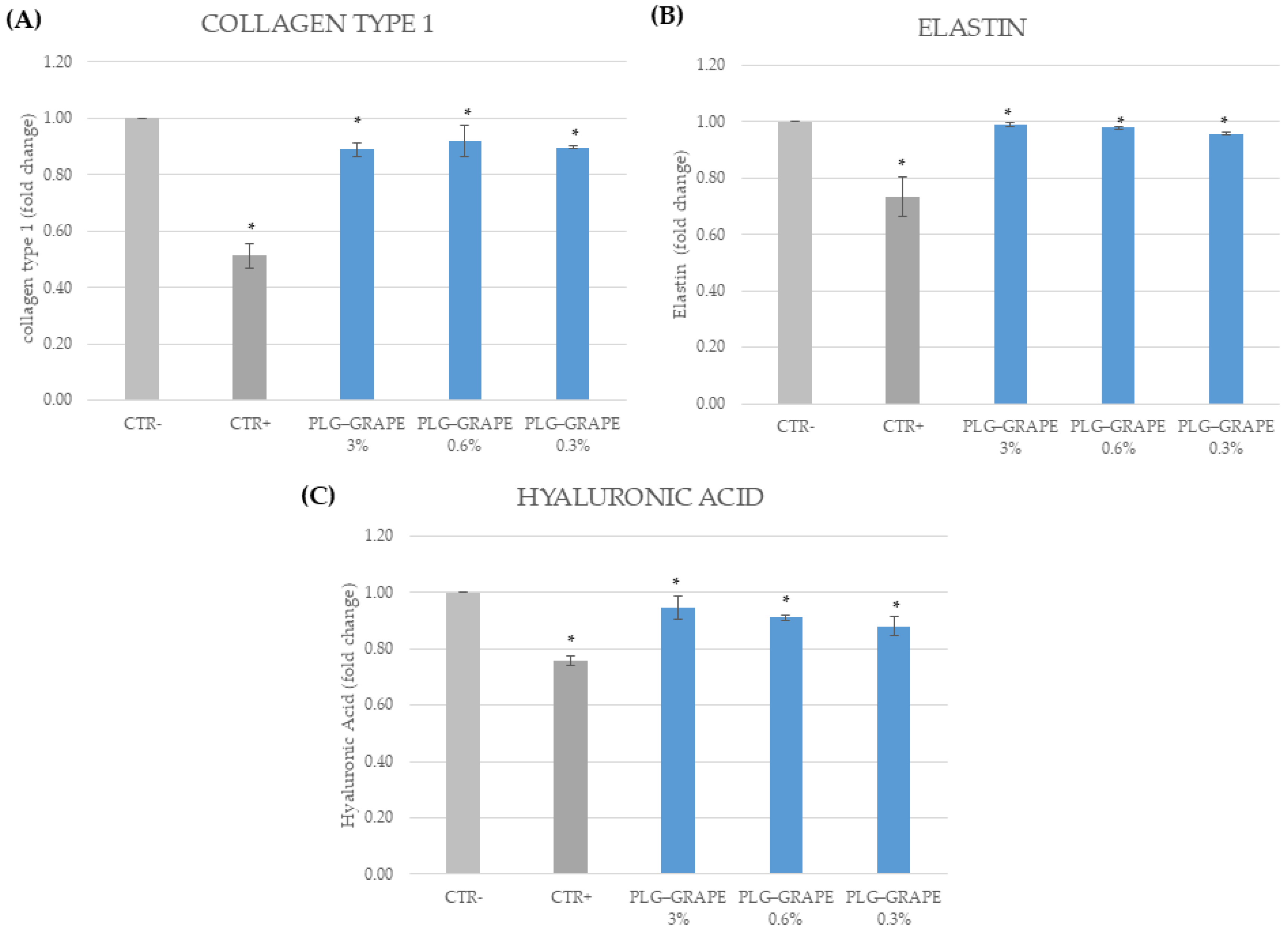
3.3.4. Anti-Age Effect
Results on BJ-Cells
Results on Full Thickness Skin Tissues
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Omran, B.A.; Baek, K.-H. Valorization of Agro-Industrial Biowaste to Green Nanomaterials for Wastewater Treatment: Approaching Green Chemistry and Circular Economy Principles. J. Environ. Manag. 2022, 311, 114806. [Google Scholar] [CrossRef]
- Pham, T.-N.; Cazier, E.A.; Gormally, E.; Lawrence, P. Valorization of Biomass Polyphenols as Potential Tyrosinase Inhibitors. Drug Discov. Today 2024, 29, 103843. [Google Scholar] [CrossRef]
- López-Astorga, M.; Molina-Domínguez, C.C.; Ovando-Martínez, M.; Leon-Bejarano, M. Orujo de Uva: Más Que Un Residuo, Una Fuente de Compuestos Bioactivos. Epistemus 2023, 16. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and Antimicrobial Effects of Grape Pomace Extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape Pomace as a Sustainable Source of Bioactive Compounds: Extraction, Characterization, and Biotechnological Applications of Phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Europe, P.M.C. Europepmc.org. Available online: https://europepmc.org/article/PMC/4200838 (accessed on 10 February 2025).
- Soceanu, A.; Dobrinas, S.; Sirbu, A.; Manea, N.; Popescu, V. Economic Aspects of Waste Recovery in the Wine Industry. A Multidisciplinary Approach. Sci. Total Environ. 2021, 759, 143543. [Google Scholar] [CrossRef]
- Agati, G.; Brunetti, C.; Di Ferdinando, M.; Ferrini, F.; Pollastri, S.; Tattini, M. Functional Roles of Flavonoids in Photoprotection: New Evidence, Lessons from the Past. Plant Physiol. Biochem. 2013, 72, 35–45. [Google Scholar] [CrossRef]
- Alexis, A.F.; Grimes, P.; Boyd, C.; Downie, J.; Drinkwater, A.; Garcia, J.K.; Gallagher, C.J. Racial and Ethnic Differences in Self-Assessed Facial Aging in Women. Dermatol. Surg. 2019, 45, 1635–1648. [Google Scholar] [CrossRef] [PubMed]
- Arct, J.; Pytkowska, K. Flavonoids as Components of Biologically Active Cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, C.; Li, Y.; Yuan, K.; Zhang, W.; Cai, D.; Peng, Z.; Hu, Y.; Sun, J.; Bai, W. Bioactivity and Application of Anthocyanins in Skin Protection and Cosmetics: An Extension as a Functional Pigment. Phytochem. Rev. 2023, 22, 1441–1467. [Google Scholar] [CrossRef]
- Khan, A.Q.; Agha, M.V.; Sheikhan, K.S.A.M.; Younis, S.M.; Tamimi, M.A.; Alam, M.; Ahmad, A.; Uddin, S.; Buddenkotte, J.; Steinhoff, M. Targeting Deregulated Oxidative Stress in Skin Inflammatory Diseases: An Update on Clinical Importance. Biomed. Pharmacother. 2022, 154, 113601. [Google Scholar] [CrossRef]
- Quan, T. Human Skin Aging and the Anti-Aging Properties of Retinol. Biomolecules 2023, 13, 1614. [Google Scholar] [CrossRef] [PubMed]
- Rojo, L.E.; Roopchand, D.; Graf, B.; Raskin, I. Role of Anthocyanins in Skin Aging and UV Induced Skin Damage. ResearchGate. Available online: https://www.researchgate.net/publication/281031602_Role_of_Anthocyanins_in_Skin_Aging_and_UV_Induced_Skin_Damage (accessed on 31 May 2023).
- Restuccia, D.; Sicari, V.; Pellicanò, T.M.; Spizzirri, U.G.; Loizzo, M.R. The impact of cultivar on polyphenol and biogenic amine profiles in Calabrian red grapes during winemaking. Food Res. Int. 2017, 102, 303–312. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Matei, C.; Deaconu, M.; Stanciuc, A.-M.; Trifan, A.; Gaspar-Pintiliescu, A.; Berger, D. Polyphenols Extract from Grape Pomace. Characterization and Valorisation through Encapsulation into Mesoporous Silica-Type Matrices. Food Chem. Toxicol. 2019, 133, 110787. [Google Scholar] [CrossRef]
- Burin, V.M.; Ferreira-Lima, N.E.; Panceri, C.P.; Bordignon-Luiz, M.T. Bioactive Compounds and Antioxidant Activity of Vitis Vinifera and Vitis Labrusca Grapes: Evaluation of Different Extraction Methods. Microchem. J. 2014, 114, 155–163. [Google Scholar] [CrossRef]
- Millinia, B.L.; Mashithah, D.; Nawatila, R.; Kartini, K. Microencapsulation of Roselle (Hibiscus sabdariffa L.) Anthocyanins: Effects of Maltodextrin and Trehalose Matrix on Selected Physicochemical Properties and Antioxidant Activities of Spray-Dried Powder. Future Foods 2024, 9, 100300. [Google Scholar] [CrossRef]
- Parisi, O.; Malivindi, R.; Amone, F.; Ruffo, M.; Malanchin, R.; Carlomagno, F.; Piangiolino, C.; Nobile, V.; Pezzi, V.; Scrivano, L.; et al. Safety and Efficacy of Dextran-Rosmarinic Acid Conjugates as Innovative Polymeric Antioxidants in Skin Whitening: What Is the Evidence? Cosmetics 2017, 4, 28. [Google Scholar] [CrossRef]
- Gallo, M.; Formato, A.; Giacco, R.; Riccardi, G.; Luongo, D.; Formato, G.; Amoresano, A.; Naviglio, D. Corrigendum to “Mathematical Optimization of the Green Extraction of Polyphenols from Grape Peels through a Cyclic Pressurization Process”. Heliyon 2019, 5, e01593. [Google Scholar] [CrossRef]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and Physical Modifications of Starch for Renewable Polymeric Materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Dahiya, D.; Nigam, P.S. Dextran of Diverse Molecular-Configurations Used as a Blood-Plasma Substitute, Drug-Delivery Vehicle and Food Additive Biosynthesized by Leuconostoc, Lactobacillus and Weissella. Appl. Sci. 2023, 13, 12526. [Google Scholar] [CrossRef]
- Sleightholm, R.; Watley, D.; Wahlmeier, S.; Patel, A.; Foster, J.M. The Efficacy of Dextran-40 as a Venous Thromboembolism Prophylaxis Strategy in Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy. Am. Surg. 2017, 83, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Huang, G.; Huang, H. Preparation and Application of Dextran and Its Derivatives as Carriers. Int. J. Biol. Macromol. 2019, 145, 827–834. [Google Scholar] [CrossRef]
- Moreno-Mendieta, S.; Guillén, D.; Hernández-Pando, R.; Sánchez, S.; Rodríguez-Sanoja, R. Potential of Glucans as Vaccine Adjuvants: A Review of the α-Glucans Case. Carbohydr. Polym. 2017, 165, 103–114. [Google Scholar] [CrossRef]
- Puoci, F.; Iemma, F.; Pezzi, V.; Picci, N.; Cirillo, G.; Curcio, M.; Parisi, O.I. Cosmetic, Pharmaceutical or Nutraceutical Formulations Containing Antioxidant Molecules and Polymeric Materials. Patent EP2535087A2, 19 December 2012. [Google Scholar]
- Pagliarulo, C.; De Vito, V.; Picariello, G.; Colicchio, R.; Pastore, G.; Salvatore, P.; Volpe, M.G. Inhibitory Effect of Pomegranate (Punica granatum L.) Polyphenol Extracts on the Bacterial Growth and Survival of Clinical Isolates of Pathogenic Staphylococcus aureus and Escherichia coli. Food Chem. 2016, 190, 824–831. [Google Scholar] [CrossRef]
- Puoci, F.; Morelli, C.; Cirillo, G.; Curcio, M.; Parisi, O.I.; Maris, P.; Sisci, D.; Picci, N. Anticancer Activity of a Quercetin-Based Polymer Towards HeLa Cancer Cells. Anticancer Research. Available online: https://www.semanticscholar.org/paper/Anticancer-activity-of-a-quercetin-based-polymer-Puoci-Morelli/a84e3b46520410aece13910b9e997c5dba91fb93 (accessed on 10 February 2025).
- Ng, S.-F.; Rouse, J.; Sanderson, D.; Eccleston, G. A Comparative Study of Transmembrane Diffusion and Permeation of Ibuprofen across Synthetic Membranes Using Franz Diffusion Cells. Pharmaceutics 2010, 2, 209–223. [Google Scholar] [CrossRef] [PubMed]
- Parisi, O.; Scrivano, L.; Amone, F.; Malivindi, R.; Ruffo, M.; Vattimo, A.F.M.; Pezzi, V.; Puoci, F. Interconnected PolymerS TeChnology (IPSTiC): An Effective Approach for the Modulation of 5α-Reductase Activity in Hair Loss Conditions. J. Funct. Biomater. 2018, 9, 44. [Google Scholar] [CrossRef]
- Siewert, M.; Dressman, J.; Brown, C.K.; Shah, V.P.; Aiache, J.-M.; Aoyagi, N.; Bashaw, D.; Brown, C.; Brown, W.; Burgess, D.; et al. FIP/AAPS Guidelines to Dissolution/in Vitro Release Testing of Novel/Special Dosage Forms. AAPS PharmSciTech 2003, 4, 43–52. [Google Scholar] [CrossRef]
- Shah, V.P.; Elkins, J.S.; Williams, R.L. Evaluation of the Test System Used for in Vitro Release of Drugs for Topical Dermatological Drug Products. Pharm. Dev. Technol. 1999, 4, 377–385. [Google Scholar] [CrossRef]
- Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Marques, M.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and Transdermal Drug Products. Dissolution Technol. 2010, 17, 12–25. [Google Scholar] [CrossRef]
- Millipore, M. Substituting Strat-M® Membrane for Human Skin in Evaluating Effect of Encapsulation on Diffusion of Sunscreen Formulations; Application Note [Online]; EMD Millipore: Billerica, MA, USA, 2013. [Google Scholar]
- Zanta, C.L.P.S.; Martínez-Huitle, C.A. Degradation of 2-Hydroxybenzoic Acid by Advanced Oxidation Processes. Braz. J. Chem. Eng. 2009, 26, 503–513. [Google Scholar] [CrossRef]
- Legrini, O.; Oliveros, E.; Braun, A.M. Photochemical Processes for Water Treatment-Legrini. Chem. Rev. 1993, 93, 671–698. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference materials. ISO: Geneva, Switzerland, 2021.
- Parisi, O.I.; Aiello, D.; Casula, M.F.; Puoci, F.; Malivindi, R.; Scrivano, L.; Testa, F. Mesoporous Nanocrystalline TiO2 Loaded with Ferulic Acid for Sunscreen and Photo-Protection: Safety and Efficacy Assessment. RSC Adv. 2016, 6, 83767–83775. [Google Scholar] [CrossRef]
- Draft proposal for a new test guideline. In vitro Skin Sensitization: Human Cell Line Activation Test (h-CLAT). In Oecd Guideline for the Testing of Chemicals; OECD: Paris, France, 2024.
- Patitucci, F.; Motta, M.F.; Dattilo, M.; Malivindi, R.; Leonetti, A.E.; Pezzi, G.; Prete, S.; Mileti, O.; Gabriele, D.; Parisi, O.I.; et al. 3D-Printed Alginate/Pectin-Based Patches Loaded with Olive Leaf Extracts for Wound Healing Applications: Development, Characterization and in Vitro Evaluation of Biological Properties. Pharmaceutics 2024, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- OECD 439: In vitro Skin Irritation: Reconstructed Human Epidermis Test Method. In OECD Guidelines for the Testing of Chemicals; OECD: Paris, France, 2013.
- ISO 10993-10:2010; Biological Evaluation of Medical Devices—Part 10: Tests for Irritation and Skin Sensitization. ISO: Geneva, Switzerland, 2010.
- Circular Economy in Europe—Developing the Knowledge Base. Europa.eu. Available online: https://www.eea.europa.eu/en/analysis/publications/circular-economy-in-europe (accessed on 18 January 2016).
- Di Prima, G.; Belfiore, E.; Migliore, M.; Scarpaci, A.G.; Angellotti, G.; Restivo, I.; Allegra, M.; Arizza, V.; De Caro, V. Green Extraction of Polyphenols from Waste Bentonite to Produce Functional Antioxidant Excipients for Cosmetic and Pharmaceutical Purposes: A Waste-to-Market Approach. Antioxidants 2022, 11, 2493. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s Principle and Presentation of an Innovative Solid–Liquid Extraction Technology: Extractor Navi-glio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Ferri, M.; Lima, V.; Zappi, A.; Fernando, A.L.; Melucci, D.; Tassoni, A. Phytochemicals Recovery from Grape Pomace: Extraction Improvement and Chemometric Study. Foods 2023, 12, 959. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chedea, V.S.; Macovei, Ș.O.; Bocșan, I.C.; Măgureanu, D.C.; Levai, A.M.; Buzoianu, A.D.; Pop, R.M. Grape Pomace Polyphenols as a Source of Compounds for Management of Oxidative Stress and Inflammation—A Possible Alternative for Non-Steroidal Anti-Inflammatory Drugs? Mol./Mol. Online/Mol. Annu. 2022, 27, 6826. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kyparos, A.; Nikolaidis, M.G.; Stagos, D.; Aligiannis, N.; Halabalaki, M.; Chronis, K.; Goutzourelas, N.; Skaltsounis, L.; Kouretas, D. The Antioxidant Effects of a Polyphenol-Rich Grape Pomace Extractin VitroDo Not Correspondin VivoUsing Exercise as an Oxidant Stimulus. Oxidative Med. Cell. Longev. 2012, 2012, 1–14. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary Polyphenols, Oxidative Stress and Antioxidant and Anti-Inflammatory Effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Gromkowska-Kępka, K.J.; Puścion-Jakubik, A.; Markiewicz-Żukowska, R.; Socha, K. The Impact of Ultraviolet Radiation on Skin Photoaging—Review of in Vitro Studies. J. Cosmet. Dermatol. 2021, 20, 3427–3431. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.J.; Navarro, C.; Durán, P.; Galan-Freyle, N.J.; Parra Hernández, L.A.; Pacheco-Londoño, L.C.; Castelanich, D.; Bermúdez, V.; Chacin, M. Antioxidants in Photoaging: From Molecular Insights to Clinical Applications. Int. J. Mol. Sci. 2024, 25, 2403. [Google Scholar] [CrossRef] [PubMed]
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef] [PubMed]


| Sample | Total Polyphenol Content | Dpph Inhibition (%) |
|---|---|---|
| (mg eq CA/g) | ||
| Grape pomace extract | 18.00 ± 0.6 | 65.0 ± 0.8 |
| Blank | 0.05 ± 0.4 | 0.2 ± 0.9 |
| PLG–GRAPE 2.5% | 17.91 ± 1.0 | 64.1 ± 0.6 |
| PLG–GRAPE 5% | 17.30 ± 0.7 | 64.9 ± 0.4 |
| PLG–GRAPE 10% | 18.11 ± 0.5 | 63.7 ± 0.7 |
| PLG–GRAPE 20% | 17.90 ± 1.1 | 65.2 ± 0.4 |
| Sample | % Cell Viability (Mean Value) ± ST.DEV | Results |
|---|---|---|
| Negative Control | 100% | |
| Positive Control (SDS 5%) | 7.23% ± 3.24% | Irritating |
| Tested Product (PLG–GRAPE) | 87.53% ± 4.33% | Non-Irritating |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Motta, M.F.; Vattimo, A.F.; Amone, F.; Malivindi, R.; Parisi, O.I.; Puoci, F. Valorization of Grape Pomace Extract Through Dextran–Grape Conjugates: A Sustainable Approach for Cosmetic and Dermatological Applications. Appl. Sci. 2025, 15, 3220. https://doi.org/10.3390/app15063220
Motta MF, Vattimo AF, Amone F, Malivindi R, Parisi OI, Puoci F. Valorization of Grape Pomace Extract Through Dextran–Grape Conjugates: A Sustainable Approach for Cosmetic and Dermatological Applications. Applied Sciences. 2025; 15(6):3220. https://doi.org/10.3390/app15063220
Chicago/Turabian StyleMotta, Marisa Francesca, Anna Francesca Vattimo, Fabio Amone, Rocco Malivindi, Ortensia Ilaria Parisi, and Francesco Puoci. 2025. "Valorization of Grape Pomace Extract Through Dextran–Grape Conjugates: A Sustainable Approach for Cosmetic and Dermatological Applications" Applied Sciences 15, no. 6: 3220. https://doi.org/10.3390/app15063220
APA StyleMotta, M. F., Vattimo, A. F., Amone, F., Malivindi, R., Parisi, O. I., & Puoci, F. (2025). Valorization of Grape Pomace Extract Through Dextran–Grape Conjugates: A Sustainable Approach for Cosmetic and Dermatological Applications. Applied Sciences, 15(6), 3220. https://doi.org/10.3390/app15063220








