Abstract
Apple by-products are a valuable raw material due to their high content of dietary fiber, minerals, and bioactive compounds, making them a promising functional ingredient in food products. The aim of this study was to evaluate the effect of adding a residue obtained from the isolation of starch from unripe apples of the Pyros and Oliwka varieties on the nutritional composition, mineral content, polyphenol and fiber levels, and color of wheat muffins. Additionally, the oxidative stability was analyzed. The results showed that the addition of the residue significantly increased the total, soluble, and insoluble fiber content, as well as the protein content. The polysaccharide fraction residue from unripe Oliwka apples had a stronger impact on enhancing the fiber content of the muffins. In contrast, muffins enriched with the polysaccharide fraction residue from unripe Pyros apples exhibited higher levels of calcium, potassium, and magnesium, while the Oliwka residue increased the contents of sodium, strontium, and iron. The addition of the polysaccharide fraction residue significantly increased the levels of chlorogenic acid, phloridzin, quercetin, and procyanidin B1. Color analysis revealed a darkening effect in the muffins after the addition of the residue, and the oxidative stability decreased with increasing levels of the polysaccharide fraction residue. This study demonstrated that apple residues obtained after starch isolation can effectively enrich muffins with nutrients and health-promoting compounds; however, their impact on oxidative stability requires further investigation.
1. Introduction
Apples are the most popular fruits within the Rosaceae family and represent one of the most important species in the global fruit industry. Their popularity is not only due to their climatic adaptability and long shelf life but also to their wide range of health-promoting properties [1,2,3]. Apples are a rich source of vitamins, such as vitamin C, B-group vitamins, and others, as well as minerals including potassium, calcium, magnesium, and iron. They also provide dietary fiber—particularly pectin—and polyphenols with strong antioxidant activity [1,4,5,6,7]. Poland, alongside China, the USA, India, and Turkey, is one of the largest apple producers in the world and the leading producer in Europe (www.agroindustry.pl (accessed on 18 February 2025). In 2023, global apple production reached 97.3 million tonnes, with Poland accounting for 3.9 Mt [8]. Over 20% of apples produced in Poland are exported, around 10% are consumed domestically, and nearly 70% are processed [9]. The significant share of apples directed to processing generates large amounts of by-products, which are only marginally utilized, while their disposal is costly and environmentally burdensome. This situation contradicts the objectives of the European Union’s policies—particularly those outlined in the European Green Deal and the principles of the Circular Economy, which promote waste reduction and efficient resource use through recycling and “zero waste” strategies [10]. Therefore, identifying alternative applications for apples in the food industry—especially those aligned with the concept of sustainable development—is highly justified.
An interesting solution could be the isolation of starch from unripe apples, whose functional properties have shown promise. This starch is characterized by a low tendency to retrogradation, favorable hydration properties, and the ability to form elastic-dominant gel networks [11]. The residue left after this process is rich in other polysaccharides, dietary fiber, and health-beneficial compounds and can be used as a functional concentrate in various food products—for example, in muffin production. The choice of muffins as a model food product is intentional: sweet baked goods remain highly popular among consumers. Their texture is desirable, they are convenient and easy to consume, and due to their relatively low moisture content, they have an extended shelf life [12].
The confectionery market in Poland exceeds EUR 4.4 billion annually (as of 2024), with bakery products accounting for approximately one-third of the industry and being the most frequently purchased items in hypermarkets [13]. The global cookie and biscuit market was valued at USD 101.3 billion in 2023, and it is projected to reach USD 158.5 billion by the end of 2032 [14]. Muffins represent a tasty and versatile snack that can be consumed in various forms [15,16]. Contemporary research increasingly focuses on identifying alternative sources of nutrients and health-promoting compounds, opening up new possibilities in the field of functional foods. One particularly interesting approach is the enhancement of the nutritional and health value of muffins by enriching them with waste material obtained from the isolation of polysaccharides (starch) from apples. Given the limited health benefits typically associated with this type of snack, enriching it with bioactive compounds—especially those derived from food industry by-products—seems highly justified and aligns well with the principles of sustainable development [17].
The aim of this study was to bake muffins enriched with polysaccharide fraction residue from unripe apples and to evaluate their nutritional value, mineral content, polyphenol profile, and oxidation stability. The total dietary fiber content was determined, along with its two fractions: water-soluble and insoluble fiber. Additionally, the color of the resulting products was analyzed.
2. Materials and Methods
2.1. Research Materials
2.1.1. Reagents and Raw Materials
All reagents were of analytical grade. Analytical reagents and chemicals were purchased from Sigma Aldrich (St. Louis, MO, USA), including xylanase (X2753 Sigma, St. Louis, MO, USA), with the exception of quercetin, phloridzin, catechin, procyanidin, and phenolic acids, which were purchased from Extrasynthese (Genay, France).
The raw materials for the muffin production were bought from a market in Krakow (Poland): wheat flour type 450 (PZZ, Kraków, Poland), baking powder (Dr. Oetker, Gdansk, Poland), sugar (Sodzucker Polska S.A., Wrocław, Poland), salt (CIECH, Kujawy, Poland), lemon peel (Dr. Oetker, Gdansk, Poland), vanilla sugar (Dr. Oetker, Gdansk, Poland), eggs (Biedronka. Kraków, Poland), yogurt (Bakoma S.A, Warszawa, Poland), and grape seed oil (Monini-Polska Sp.zoo, Poznan, Poland).
2.1.2. Materials
The detailed methodologies concerning the isolation of apple polysaccharide fraction residues and the muffin preparation protocol were explained in Gumul et al.’s manuscript [18].
For clarity and reference, a brief summary of the procedure is presented below:
Isolation of polysaccharide Fraction Residue from apples. Approximately 1 kg of apples (without stalks) from each selected variety was mixed with 1.5 L of distilled water and xylanase (X2753 Sigma, St. Louis, MO, USA) and then crushed using a Zelmer ZHM0861X mixer. The pulp was stirred using a mechanical mixer (RW 20, IKA, Königswinter, Germany) for 2 h, filtered through 315 μm gauze, and centrifuged for 15 min at 2260× g. The top layer of sludge was collected, air-dried, and ground using a Pulverisette 2 ball mill (Fritsch, Idar-Oberstein, Germany). The obtained material was labeled as apple polysaccharide sediment, derived from two unripe apple varieties—Pyros and Oliwka.
Preparation of muffins. The standard muffin recipe (Table 1) [18] was adapted by incorporating either 5% or 10% of the dried polysaccharide sediment into the flour component. All dry ingredients were mixed, followed by the addition of wet ingredients (egg, yogurt, oil), and baked at 190 °C for approximately 20 min using a Condo 20608 oven (MIWE, Arnstein, Germany).

Table 1.
Ingredients used for laboratory muffin preparation [18].
2.2. Methods
2.2.1. Analysis of Chemical Composition
Proximate Composition of Polysaccharide Sediment and Muffins and Caloric Value Calculation of Muffins
The protein content was analyzed according to the Kjeldahl method [19], which estimates total nitrogen levels in the sample. The process entailed sample digestion with concentrated sulfuric acid in the presence of a catalyst, followed by alkalization with sodium hydroxide and subsequent distillation of the liberated ammonia. The amount of ammonia, and thus nitrogen, was determined through titration. The protein content was then calculated using a nitrogen-to-protein conversion factor of N × 5.7 [19], reflecting the average nitrogen proportion in proteins typically found in cereal-based foods. Measurements were performed with a Kjeltec 2200 distillation unit (Foss, Hillerød, Denmark). The fat content was determined via the Soxhlet extraction method [20]. A weighed sample was enclosed in a porous cellulose thimble and placed in a Soxtec Avanti 2055 apparatus (Foss, Hillerød, Denmark), where it underwent solvent extraction using petroleum ether. After the solvent was evaporated, the remaining fat residue was weighed to determine the fat content. The ash content was measured by incinerating the samples in a muffle furnace at 550 °C, in accordance with AOAC method 920.183 [21]. During this process, all organic components were removed, and the residual mineral matter (ash) was weighed to express the mineral content as a percentage of the original sample mass. The available carbohydrate content was calculated by the difference method:
This estimation is based on the assumption that all components not directly measured in the sample are carbohydrates, offering a widely accepted and practical approach to determining the carbohydrate content.
The caloric value of the muffins was calculated in accordance with the methodology outlined in EU Regulation 1169/2011 [22]. The overall energy content was determined using the following formula:
Dietary Fiber Content of Polysaccharide Fraction Residue and Muffins
The total dietary fiber (TDF) content, along with its soluble (SDF) and insoluble (IDF) fractions, was determined using an enzymatic–gravimetric procedure described by AACC (2000) [23]. TDF was calculated as the combined amount of both fractions. To eliminate starch and proteins, ground samples were dispersed in water and subjected to sequential enzymatic hydrolysis with α-amylase, protease, and glucosidase. For TDF analysis, the resulting digest was treated with ethanol to precipitate soluble fibers. The precipitate was then filtered, washed with ethanol and acetone, dried, and weighed. In the determination of IDF and SDF, the undissolved material (IDF) was separated by filtration, rinsed with hot water, dried, and weighed. The soluble fraction (SDF) was obtained by precipitating the filtrate and washings with ethanol, followed by filtration, drying, and gravimetric measurement. All fiber values (TDF, IDF, and SDF) were corrected for residual protein, ash content, and blank sample contributions.
Determination of Polyphenols by UPLC of Polysaccharide Fraction Residue and Muffins
Extraction procedure: One gram of the sample was subjected to extraction using 10 cm3 of a solvent mixture composed of UPLC-grade methanol (30%), ascorbic acid (2.0 g/100 cm3), and acetic acid (1.0 cm3/100 cm3). The extraction process was carried out twice by sonicating the mixture (Sonic 6D, Polsonic, Warsaw, Poland) for 20 min with intermittent stirring. The resulting suspension was then centrifuged at 19,000× g for 10 min. The collected supernatant was filtered through a 0.20 µm hydrophilic PTFE membrane and used for further analysis [24].
Analysis: Polyphenolic compounds were identified and quantified using an Acquity UPLC system (Waters Corporation, Milford, MA, USA), equipped with a binary solvent manager (BSM) and a sample manager (SM), coupled to a photodiode array detector (PDA) and a quadrupole time-of-flight (Q-TOF) mass spectrometer (Waters, Manchester, UK). Separation was achieved on a BEH C18 UPLC column (2.1 × 100 mm, 1.7 µm; Waters) using a binary solvent system: 2% formic acid in water (solvent A) and acetonitrile (solvent B). The flow rate was maintained at 0.45 mL/min. The gradient program began with 99% A for 1 min, transitioning linearly to 75% B over 12 min. The column temperature was set to 30 °C, and the injection volume was 5 µL. Polyphenols were quantified using UV detection (PDA) with calibration curves from standards, while the Q-TOF mass spectrometer served exclusively for compound identification and confirmation.
Mass spectrometry conditions: The Q-TOF detector was operated with a capillary voltage of 2.5 kV and a cone voltage of 30 V. The source and desolvation temperatures were maintained at 130 °C and 350 °C, respectively. Nitrogen served as the desolvation gas at a flow rate of 300 L/h. Data acquisition was carried out in full-scan mode (m/z 100–1500), with a resolution of 5000 and mass accuracy tolerance of 0.001 Da. Internal calibration was achieved using leucine enkephalin introduced via the lockspray reference channel. Chromatographic peaks were analyzed based on base peak intensity (BPI), normalized to 12,400 cps (100%). Data acquisition and processing were conducted using MassLynx v. 4.1 software (Waters). Tandem MS/MS spectra were obtained by collision-induced dissociation (CID), with collision energies optimized individually for each analyte. UV detection was performed at the following wavelengths: 320 nm for phenolic acids, 360 nm for flavonols, 280 nm for flavan-3-ols, and 340 nm for flavanones.
Mineral Compounds Composition of Polysaccharide Fraction Residue and Muffins
The quantification of mineral elements was conducted using the ICP-OES method [25]. For this analysis, 0.2 g of each sample was subjected to high-pressure mineralization in 65% ultrapure nitric acid. The samples were carefully weighed and placed into Teflon vessels, followed by the addition of 8 mL of nitric acid. The vessels were then securely sealed. During the microwave-assisted digestion, one blank sample containing only 8 mL of nitric acid was processed alongside every batch of nine samples to serve as a reference.
The digestion process was carried out for one hour using the Ethos One microwave digestion system (Milestone). A temperature increase algorithm, specifically optimized for biological materials, was applied without exceeding a maximum of 200 °C. After digestion, the vessels were opened, and the digested samples were cooled to room temperature. The final volume of each sample was adjusted to 50 mL with deionized water.
The detection limits for the analyzed elements were below 0.01 mg/kg, with the instrument’s sensitivity set to detect concentrations greater than 1 ppb. Measurements were performed using the Thermo iCAP Dual 6500 ICP-OES spectrometer (Thermo Fisher Scientific, Bremen, Germany), which employs horizontal plasma and supports both radial and axial plasma views for enhanced sensitivity and precision.
Before analyzing each batch, the spectrometer was calibrated using certified reference standards (Merck, Darmstadt, Germany). A 3-point calibration curve was generated for each element, with corrections applied based on the blank sample readings. Internal standards (yttrium and ytterbium ions at concentrations of 2 mg/L and 5 mg/L, respectively) were used to improve the accuracy of the measurements and compensate for any matrix effects.
The results were corrected for the blank sample readings to ensure precision. This method provided reliable and reproducible data on the mineral composition of the analyzed samples.
2.2.2. Oxidative Stability of Polysaccharide Fraction Residue and Muffins
The oxidative stability was carried out in an 892 Rancimat apparatus (Metrohm, Herisau, Switzerland) using ISO 6886:1997 [26] utilizing a sample of 0.5 ± 0.01 g. All samples were analyzed at 120 °C, under a constant air flow (20 L/h). The induction times were printed automatically by apparatus software with an accuracy of 0.005.
2.2.3. Instrumental Color Analysis of Polysaccharide Fraction Residue and Muffins
Color Measurement: The color of the muffin samples was evaluated instrumentally using the CIE Lab* color space system [27]. Reflectance was measured with a spectrophotometer (Konica Minolta CM-3500d, Tokyo, Japan) following the guidelines of the CIE (International Commission on Illumination, 2004) [27], applying a 10° standard observer angle and a 30 mm aperture. Samples were placed in 55 mm diameter Petri dishes for analysis. The color parameters assessed included the following:
L* (lightness; 0 = black, 100 = white),
a* (negative = green, positive = red),
b* (negative = blue, positive = yellow).
Each measurement was performed in four replicates (n = 4) per sample.
Color Difference Calculation: Differences in color (ΔE00) between samples were determined using the CIEDE2000 formula, which accounts for perceptual non-uniformities in human color vision. This advanced method incorporates correction terms for lightness (ΔL′), chroma (ΔC′), and hue (ΔH′), as well as their interactions, providing a more accurate estimation of perceived color variation than the traditional ΔE*ab metric. This approach is especially effective when assessing subtle color shifts or samples with intense saturation [27].
where:
- ΔL′: Difference in lightness,
- ΔC′: Difference in chroma,
- ΔH′: Difference in hue,
- SL, SC, SH: Scaling functions for lightness, chroma, and hue, respectively,
- RT: A rotation factor that adjusts for the interaction between chroma and hue,
- kL, kC, kH: Parametric weights for lightness, chroma, and hue (typically set to 1 for standard conditions).
ΔE00 values are interpreted as follows:
- –1: Color difference is imperceptible to the human eye.
- 1–2: Slight difference, visible only under careful inspection.
- 2–3: Noticeable difference, detectable by most observers.
- >3: Clear and easily perceptible color difference.
The hue angle (Hue°) was calculated as arctangent (b/a*) and expressed in degrees, using the atan2 function to correctly determine the quadrant.
2.2.4. Statistical Analysis
The experimental data were subjected to univariate analysis of variance (ANOVA) followed by Duncan’s multiple range test at a significance level of 0.05, using Statistica v. 8.0 software (StatSoft Inc., Tulsa, OK, USA). For comparisons involving only two groups (Table 2 and Table 3), Student’s t-test was applied for parametric data and the Mann–Whitney U test for non-parametric data, following verification of data distribution with the Shapiro–Wilk test. All measurements were performed at least in duplicate. The results are presented as means with standard deviation. The correlation coefficient (r) was calculated using Microsoft Excel 2019 (Microsoft Corporation, Redmond, WA, USA).

Table 2.
Dietary fiber content, energy value, and the mineral compound profiles of polysaccharide fraction residue from unripe Pyros variety apples and polysaccharide fraction residue from unripe Oliwka variety apples.

Table 3.
Content of polyphenolic compounds in the polysaccharide fraction residue from unripe apples (mg/100 g DM).
3. Results and Discussion
3.1. Characteristic of Polysaccharide Fraction Residues from the Unripe Pyros and Oliwka Apple Varieties
3.1.1. Chemical Composition of Polysaccharide Fraction Residues from Unripe Apples
The residues remaining after starch isolation from unripe apples—i.e., polysaccharide fraction residues from the unripe Pyros and Oliwka apple varieties—differed in nutritional composition, which may be primarily due to varietal differences. The polysaccharide fraction residue from the unripe Oliwka apple variety showed a 13% higher protein content (23.46 g/100 g DM) than that from the Pyros variety (20.70 g/100 g DM), indicating a greater potential of the Oliwka variety as a protein-enriching ingredient. Nevertheless, both varieties exhibited high protein levels. The protein content in apples is 0.1–0.5% in fresh mass and 4–7% in dried apple pomace [28]. This can be explained by the fact that the starch isolation process likely removed a large portion of starch and water-soluble fiber, enriching the remaining residue in other components, including proteins. It is also worth noting that the Kjeldahl method used measures the total nitrogen content in the sample, without distinguishing its source. This means that the presence of nitrogenous compounds other than proteins, such as pectins containing amide groups, hemicelluloses, or arabinogalactans, as well as polyphenols such as phloridzin, quercetin derivatives, or catechins, may have contributed to an overestimation of the protein content.
The ash content, which indicates the mineral content, was also higher in Oliwka (1.55 g/100 g DM) than in Pyros (1.46 g/100 g DM), although the difference was only 6%. These differences may be due to both the genetic potential of the varieties for mineral accumulation and the type of soil [29]. The polysaccharide fraction residue from the unripe Oliwka apple variety also showed an 8% higher fat content (4.88 g/100 g DM) than Pyros (4.51 g/100 g DM) (Figure 1). However, both residues had a higher fat content than typically found in apple pomace (from 0.26 to 3.12 per 100 g DM) [28,30,31]. The fat content in apples mainly results from the presence of lipids in cell membranes and cuticle layers [32,33], and the Oliwka variety may have thicker cell walls and more lipid membranes than Pyros. Additionally, the starch isolation process may have removed a substantial portion of starch and fiber, thereby increasing the relative proportion of fat in the residue. The presence of certain polyphenols and waxes may also have influenced the fat analysis by being detected as lipid substances. Combined with natural varietal differences in metabolism, this could have led to the increased synthesis of fatty acids and lipids. This is related to the methodology, in which the extraction solvent (petroleum ether), being non-polar, will also extract other fat-soluble compounds, including lipophilic polyphenols (e.g., quercetin aglycones, methylated flavonoids) as well as naturally occurring epicuticular waxes on apples, the amount of which depends mainly on the cultivar.
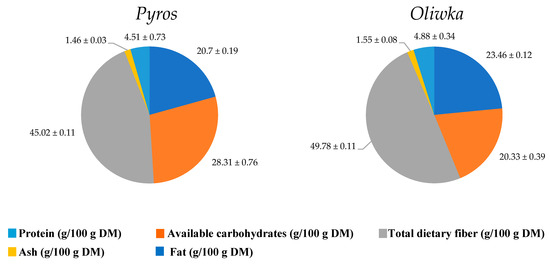
Figure 1.
Chemical composition (g/100 g DM) of polysaccharide fraction residue from unripe Pyros variety apples and polysaccharide fraction residue from unripe Oliwka variety apples (energy value: Pyros—326.67 kcal/100 g; Oliwka—318.64 kcal/100 g).
An opposite trend was observed in the case of carbohydrates, which were among the three dominant fractions in both samples. Their content was 39% higher in the polysaccharide fraction residue from unripe Pyros variety apples (28.31 g/100 g DM) compared to Oliwka (20.33 g/100 g DM) (Figure 1). This may be attributed to varietal differences, including differences in enzymatic activity and tissue structure. It may also be linked to starch hydrolysis, which is the main process responsible for increasing the sugar content during fruit ripening [34]. During apple development, starch is gradually hydrolyzed into fructose, glucose, and sucrose, leading to an increase in the total carbohydrate content and the sweetness of the fruit [35,36].
At the same time, the polysaccharide fraction residue from unripe Oliwka variety apples exhibited a higher total dietary fiber content as compared to Pyros, amounting to a 10.57% difference. The greatest difference was observed in the insoluble fiber fraction, which was 11.08% higher in Oliwka compared to Pyros. The soluble fiber fraction was also higher in Oliwka, although the difference was smaller—8.63% (10.20 vs. 9.39 g/100 g DM in Pyros) (Table 2).
These findings confirm that the process of starch isolation from unripe apples results in by-products with varied nutritional compositions, which may have both technological and dietary implications. The polysaccharide fraction residue from unripe Pyros variety apples, due to its higher content of available carbohydrates, may have greater potential as a source of easily digestible energy. On the other hand, the polysaccharide fraction residue from unripe Oliwka variety apples, owing to its higher fiber content, may be more suitable for applications requiring increased fiber fractions—for example, in the production of foods with a lower glycemic index or products aimed at supporting digestion.
The polysaccharide fraction residues resulting from starch isolation from unripe Oliwka and Pyros apple varieties exhibited significantly higher levels of mineral elements compared to typical values reported for apple pomace [37], including that from unripe apples [38]. The Pyros sludge was characterized by a higher content of most mineral elements compared to the polysaccharide fraction residue from unripe Oliwka variety apples (Table 2), which may result from both genetic factors and environmental conditions such as temperature and sunlight duration, known to significantly influence mineral accumulation.
Potassium was the dominant element in the obtained production residues, which is consistent with the literature data indicating that potassium is also the most abundant mineral in apples (over 40% of ash content) and apple pomace [37,39,40]. The Oliwka residue showed higher levels of potassium and sodium, with amounts 15% and 5% greater, respectively, than in Pyros (1464.50 mg/100 g DM and 156.46 mg/100 g DM in Oliwka; 1271.90 mg/100 g DM and 148.68 mg/100 g DM in Pyros) (Table 2).
Calcium and magnesium, also among the dominant minerals in apple pomace, were present in higher amounts in Pyros, with concentrations 32% and 12% greater than in Oliwka (619.79 mg/100 g DM and 27.45 mg/100 g DM in Pyros; 470.37 and 24.60 mg/100 g DM in Oliwka), indicating more effective accumulation of these elements by the Pyros variety (Table 2).
Similarly, higher values were noted for aluminum and iron, with Pyros showing 10% and 8% more, respectively (94.49 and 115.57 mg/100 g DM in Pyros; 85.81 and 107.04 mg/100 g DM in Oliwka). Barium and copper were also found in greater concentrations in Pyros, by 3% and 8%, respectively (2.49 and 3.69 mg/100 g DM in Pyros; 2.42 and 3.42 mg/100 g DM in Oliwka) (Table 2).
The most pronounced difference was observed in manganese, whose content was 48% higher in Pyros (17.27 mg/100 g DM than in Oliwka (11.70 mg/100 g DM)). In addition, selenium was detected only in the Pyros sample (0.90 mg/100 g DM). Neither of the residues contained detectable levels of silver, cadmium, chromium, lithium, nickel, lead, zinc, arsenic, or antimony (Table 2).
The differences in mineral composition may result from both varietal differences in element accumulation and climatic and soil conditions [41]. Moreover, lower temperatures can reduce water and mineral uptake, negatively affecting mineral accumulation in fruit [38]. For this reason, growing conditions may be just as important for the chemical composition of apples as the varietal characteristics themselves.
3.1.2. Quality and Quantity Profiles of Polyphenols of Polysaccharide Fraction Residues from Unripe Apples
The polyphenol content in the polysaccharide residue from unripe apples of the Oliwka and Pyros varieties ranged from 470 to 584 mg/100 g dry matter (Table 3) and was several times higher than that found in pomace from ripe apples, suggesting their potentially greater biological and antioxidant value. For comparison, in the study by Leyva-Corral et al. [42], the total content of identified polyphenols in ripe apple pomace was 114.54 mg/100 g DM, while in the study by Ćetković et al. [43], the concentration was even lower, ranging from 69.2 to 147.4 mg/100 g DM. Fruit mass increases significantly during ripening due to the accumulation of water, sugars, and minerals, which leads to a decrease in the relative concentration of polyphenols in the apple. Additionally, as apples ripen, the activity of oxidative enzymes, such as polyphenol oxidase and peroxidase, increases, causing the degradation of polyphenols [42,44,45,46].
The distribution of polyphenolic compound groups in the polysaccharide residue from unripe apples was as follows: flavan-3-ols accounted for approximately 46%, phenolic acids 37%, flavonols 6%, and dihydrochalcones 10% (Figure 2). These values differ significantly from the polyphenol composition found in pomace from ripe apples, as reported by Persic et al. [47]. In those pomace samples, flavonols ranged from 18% to 60%, phenolic acids from 2% to 21%, dihydrochalcones from 1% to 18%, and flavan-3-ols from 25% to 70%. It is worth emphasizing that Akiyama et al. [48] demonstrated that unripe apples contain up to ten times more polyphenols than ripe fruits, making them a particularly valuable source of bioactive compounds. Similar results were also reported by Burda et al. [44]. In the present study, the chlorogenic acid content in the polysaccharide residue from unripe apples ranged from 162 to 197 mg/100 g DM (Table 3), whereas in pomace from ripe apples, the concentration was significantly lower, within the range of 1.43–41.55 mg/100 g DM [42,43,45]. The fact that chlorogenic acid was the dominant phenolic acid in both ripe apple pomace and unripe apple residue confirms its key role in the phenolic profile of these raw materials. Notably, the contents of catechins, procyanidins, and phloridzin in the unripe apple residue were much higher than those reported in ripe apple pomace. According to Ćetković et al. [43], the catechin content in ripe apple pomace ranged from 1.7 to 12.7 mg/100 g DM., while Kammerer et al. [45] reported 0.24 mg/100 g DM, and Rabetafika et al. [46] found values between 0.94 and 1.4 mg/100 g DM. Similar differences were observed for procyanidin B2: Ćetković et al. [43] reported 2.3 to 10 mg/100 g DM, Kammerer et al. [45] reported 0.93 mg/100 g DM, and Rabetafika et al. [46] recorded values between 9.3 and 16 mg/100 g DM [43,46,49]. It is also noteworthy that the phloridzin content in ripe apple pomace, according to Ćetković et al. [43], ranged from 0.7 to 8.5 mg/100 g DM., while Kammerer et al. [45] reported a value of 4.04 mg/100 g DM. Based on the obtained results, it can be concluded that polysaccharide residues from unripe apples are an exceptional source of bioactive phenolic compounds, surpassing ripe apple pomace in this regard. In our previous study [18] on the polysaccharide fraction residue after starch isolation from unripe apples, a high concentration of polyphenolic compounds in this material was also observed, based on spectrophotometric determinations of total polyphenols and total phenolic acids. Similarly to the findings of the present study, it was shown that the polysaccharide fraction residues from the unripe apple variety Oliwka contained higher levels of these compounds compared to the Pyros variety (Table 3) [18]. The high polyphenol content in the polysaccharide fraction residue from unripe apples significantly contributed to the notable antioxidant activities of this material, as demonstrated in our previous publication [18]. The high content of polyphenols, including procyanidins, catechins, chlorogenic acid, and phloridzin, highlights their potential biological and functional value in food and pharmaceutical applications.
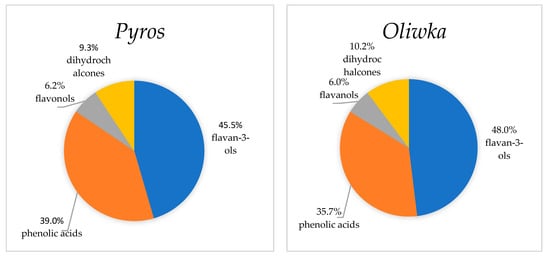
Figure 2.
Distribution of polyphenol compounds in the polysaccharide fraction residue from unripe apples.
3.1.3. Oxidation Stability of Polysaccharide Fraction Residues from Unripe Apples
The values for the polysaccharide fraction residue from unripe Pyros variety apples (51.87 h) and the polysaccharide fraction residue from unripe Oliwka variety apples (50.32 h) indicate that both samples exhibit relatively high oxidative stability (Figure 3). This could suggest that these samples have a higher level of antioxidants or are more resistant to oxidative degradation. Previous studies by researchers such as Frankel [50] have noted that the presence of natural antioxidants in plant oils can significantly enhance oxidative stability. Studies on unripe apples indicate that differences in the content of mineral elements and phenolic compounds may result from both genetic factors and environmental conditions. In particular, temperature and duration of sunlight exposure have a significant impact on the synthesis of secondary metabolites and the accumulation of mineral components [35,36,51,52].
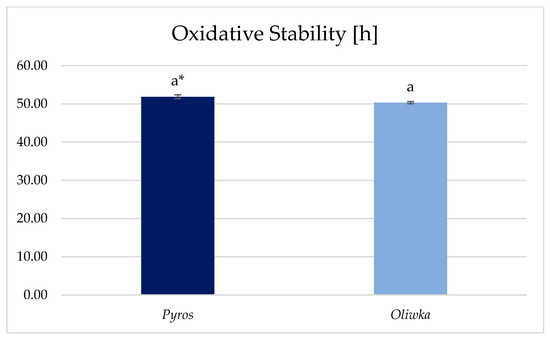
Figure 3.
Oxidative stability of polysaccharide fraction residue from unripe apples. *—different letters indicate significant differences between individual samples (p ≤ 0.05).
3.1.4. Color Parameters of Polysaccharide Fraction Residues from Unripe Apples
The polysaccharide fraction residue from unripe Pyros variety apples and polysaccharide fraction residue from unripe Oliwka variety apples, which served as the base materials for enrichment—not subjected to thermal treatment—exhibited similar color parameters L*, a*, and b* (68.50, 3.23, and 12.07, respectively, for Pyros and 68.56, 3.21, and 12.12, respectively, for Oliwka). The ΔE00 values were not analyzed, as these materials functioned solely as base substrates in the preparation of the additives (Table 4, Figure 4).

Table 4.
The color parameters of polysaccharide fraction residue from unripe apples.

Figure 4.
Visual representation of polysaccharide fraction residue from unripe apples. * The hue angle (Hue°) of the polysaccharide fraction residue was 75.02° for the Pyros variety and 75.17° for the Oliwka variety. ** The displayed colors are computer-generated visualizations based on the measured CIE L, a*, and b* values and do not represent actual photographs of the samples.
3.2. Characteristics of Muffins Enriched with Polysaccharide Fraction Residue from Unripe Pyros and Oliwka Apples
3.2.1. Chemical Composition of Muffins Enriched with Polysaccharide Fraction Residue from Unripe Pyros and Oliwka Apples
The control sample (control muffin) and those enriched with polysaccharide fraction residue from unripe Pyros (P) and Oliwka (O) apples showed distinct differences in chemical composition, depending on the type and level of additive used. The protein content increased in the enriched samples at a 10% addition level of the polysaccharide fraction residue (p < 0.05). The addition of 10% polysaccharide fraction residue from unripe Pyros and Oliwka apple varieties increased the protein content by 8% compared to the control (p < 0.05), highlighting the potential of these varieties to enhance the nutritional value of products. The carbohydrate content in the samples enriched with the Pyros residue decreased slightly—by 3% at a 5% addition level and by 4% at 10%—in comparison to the control. In the case of Oliwka, the changes were also minor—approximately 2% at 5% addition and 5% at 10% addition (Table 5). This slight decrease in the measured carbohydrate content may be attributed to sugar caramelization at high baking temperatures and partially to Maillard reactions between sugars and amino acids [53]. The fat content remained relatively stable—with an approximate 2% increase in muffins enriched with Pyros and a slight decrease in samples with Oliwka compared to the control (Table 5).

Table 5.
Chemical composition (g/100 g DM) of muffins with polysaccharide fraction residue from unripe apples and their energy value (kcal/100 g).
The ash content, which reflects the mineral composition, showed a slight increase in the samples enriched with the residues compared to the control (1.24 g/100 g DM). It reached 1.24 and 1.31 g/100 g DM in samples with 5% and 10% Pyros, respectively, and 1.19 and 1.29 g/100 g DM in samples with 5% and 10% Oliwka, respectively (Table 5).
The caloric value of muffins containing polysaccharide fraction residue from unripe apples ranged from 475.65 ± 0.08 kcal (5% Oliwka) to 481.03 ± 0.18 kcal (control) (Table 5). The addition of these residues did not significantly affect the energy value of the products, indicating their potential use in food products without notably altering their caloric content. These values are typical for many carbohydrate-rich food products such as pasta or bread. For comparison, dry wheat pasta provides approximately 348 kcal per 100 g [54], while higher-fat foods, such as nuts, may deliver over 600 kcal per 100 g. According to Carson [55], the energy value of apple pomace ranged from 184.71 to 217.81 kcal/100 g (DWB). The significantly higher energy content of the polysaccharide fraction residue from unripe Oliwka and Pyros apple varieties—approximately twice that of drum-dried apple pomace—may be attributed to several factors. Firstly, the apple pomace samples contained 47.11–52.55 g/100 g DM insoluble fiber, which lowers energy value, as dietary fiber is not fully digestible. Additionally, the Oliwka and Pyros residues showed a higher fat content (4.51 g/100 g DM—Figure 1), which increases caloric value compared to standard apple pomace. Furthermore, partial removal of the fiber and starch during the starch isolation process may have resulted in a higher proportion of more energy-dense components.
Dietary fiber is the main component of the dry matter in apple pomace (5). The control samples (control muffin) and those enriched with residues from Pyros (P) and Oliwka (O) varieties showed significant differences in dietary fiber content, both in the insoluble and soluble fractions. Enrichment with the residues resulted in a systematic increase in the total dietary fiber content, indicating a beneficial effect of these additives on the fiber profile of the products (Table 6). Moreover, in line with other studies, this may also improve technological process efficiency and increase crumb moisture [56]. The insoluble fiber fraction increased significantly in all enriched samples compared to the control (0.94 g/100 g DM) with just a 5% addition ensuring at least a 2-fold increase in this fraction. This component consists mainly of cellulose, which supports intestinal peristalsis and lowers postprandial blood glucose levels [57,58], and lignins, which bind fatty acids [6]. For the water-soluble fiber fraction, an increase was also observed with the rising addition level. In muffins enriched with Pyros, the soluble fiber content nearly doubled compared to the control (0.98 g/100 g DM for 10% MSP), while in Oliwka-enriched samples, the effect was more pronounced, reaching a maximum of 1.13 at the highest addition level (Table 6). The soluble fiber fraction from apples consists mainly of pectins, which bind heavy metal ions, exhibit prebiotic properties, and help reduce blood cholesterol and postprandial glucose levels [5]. The total dietary fiber content in the enriched samples increased proportionally with the level of residue addition, reaching more than double the control value (e.g., 3.80 for 10% Oliwka vs. 1.46 g/100 g DM in the control), with Oliwka exerting a greater impact on the total fiber content than Pyros (Table 6). The obtained results indicate that enrichment with residues from starch isolation may be an effective strategy for increasing the dietary fiber content, both in soluble and insoluble forms, which can positively affect the health-promoting and functional properties of food products.

Table 6.
Content of dietary fiber in muffins with polysaccharide fraction residue from unripe apples.
The control muffins and those enriched with polysaccharide fraction residue from unripe Pyros variety and Oliwka variety apples differed significantly in terms of mineral composition, depending on the type and amount of additive used (Table 7). Calcium reached the highest concentrations in muffins enriched with 5% Pyros and 10% Oliwka, exceeding the control by 16% and 15%, respectively. The lowest calcium content was recorded in muffins with 5% Oliwka addition. Sodium and potassium followed similar trends. Sodium reached its highest concentration in muffins with 10% Oliwka and 10% Pyros additions, exceeding the control by 13% and 12% (Table 7). The potassium content was highest in muffins with 10% Pyros and 10% Oliwka, exceeding the control by 14% (Table 7). The lowest sodium and potassium contents were recorded in muffins with 5% Oliwka addition. Magnesium, manganese, and iron also showed higher concentrations in enriched muffins. Magnesium and manganese reached their maximum values in muffins with 10% Pyros and 10% Oliwka additions. The magnesium content exceeded the control by over 15% (25.31 mg/100 g DM and 25.14 mg/100 g DM vs. 21.90 mg/100 g DM), while the manganese content was nearly twice as high (12.98 mg/100 g DM and 12.63 mg/100 g DM vs. 6.89 mg/100 g DM) (Table 7). The iron content was highest in muffins enriched with 10% Oliwka, exceeding the control by 102% (4.54 mg/100 g DM vs. 2.25 mg/100 g DM) (Table 7). The lowest concentrations of these elements were found in muffins with 5% Oliwka addition. Copper and strontium also showed notably higher values in enriched muffins. Copper reached its maximum concentration in muffins with 5% Pyros and 10% Oliwka, exceeding the control by approximately 38% (Table 7). Strontium was highest in muffins with 10% Oliwka, exceeding the control by 43% (2.81 mg/100 g DM vs. 1.96 mg/100 g DM). Selenium was detected only in muffins enriched with polysaccharide fraction residue from unripe Pyros apples, reaching its highest value in the sample with 10% addition (0.18 mg/100 g DM). In contrast, selenium was not detected in either the control or in muffins enriched with Oliwka. Elements such as silver, cadmium, chromium, lithium, nickel, lead, arsenic, antimony, and zinc were not detected in any of the analyzed samples (Table 7).

Table 7.
Profiles of the analyzed mineral compounds in muffins with polysaccharide fraction residue from unripe apples.
In summary, muffins enriched with polysaccharide fraction residue from unripe Oliwka variety apples were characterized by higher contents of sodium, strontium, and iron, making them suitable for products aimed at enrichment with these elements. On the other hand, muffins with the addition of polysaccharide fraction residue from unripe Pyros variety apples provided more calcium, potassium, magnesium, manganese, and selenium (Table 7), indicating their potential use in functional food products designed to support mineral balance in the body. The observed increase in elemental contents in the muffins was consistent with their proportions in the raw materials, confirming the effectiveness of mineral enrichment using polysaccharide fraction residue from unripe apples of the Pyros and Oliwka varieties.
3.2.2. Profile of Polyphenolic Compounds of Muffins Enriched with Polysaccharide Fraction Residue from Unripe Pyros and Oliwka Apples
In the control sample of wheat muffins, the presence of phenolic acid derivatives was noted, which may originate from the wheat flour used in baking, namely, ferulic acid, caffeic acid, chlorogenic acid, and p-coumaric acid (Table 8). These findings are consistent with reports from other authors who confirmed that wheat flour is a source of the aforementioned acids (ferulic acid 15–37 µg/g DM; p-coumaric 0.2–17.0 µg/g DM; caffeic 0.2–37 µg/g DM; chlorogenic acid 10–35 µg/g DM) [59,60,61]. The control sample also showed high levels of procyanidins, catechin, and gallic acid (Table 8), which likely originate from the grape seed oil used in muffin preparation [62]. Additionally, this oil contains significant amounts of caffeic and p-coumaric acids (according to the same authors). Replacing wheat flour with apple polysaccharide residue, which does not contain ferulic acid, resulted in a decrease in the ferulic acid content in the muffins enriched with these residues compared to the control. In contrast, the significant increase in chlorogenic acid observed in the muffins with apple residues (up to a 65-fold increase) was due to the use of this material in the formulation (Table 8). As for dihydrochalcones, they were detected only in muffins enriched with apple polysaccharide residue and were absent in the control sample. This is related to the use of this additive, as dihydrochalcones are polyphenolic markers specific to apples. An increasing trend was also observed for flavan-3-ols, such as catechin and procyanidin, which was associated both with the added material (especially at the 10% level) and with the oil used during baking (Table 8). Moreover, the increase in chlorogenic acid in the muffins was greater than expected based on the amount of added material, which may be explained by the thermal degradation of quercetin derivatives, especially quercetin rutinoside, leading to the formation of phenolic acids [59,63,64]. Considering that the apple polysaccharide residue contains a high amount of quercetin derivatives (Table 8), their thermal degradation may partially contribute to the increased content of this phenolic acid in muffins containing the residue. According to Rohn et al. [65], the thermal stability of quercetin derivatives is highest for 3-O-galactoside and 3-O-rutinoside and lowest for 3-O-glucoside and 3-O-rhamnoside. In a study by Mildner-Szkudlarz et al. [66], it was found that galactoside moieties in quercetin exhibit the highest thermal stability. Quercetin-3-O-galactoside, identified in muffins prepared with cranberry pomace, showed approximately 22% higher recovery than quercetin-3-O-glucoside. In the case of caffeic and p-coumaric acids, a decrease was noted due to thermal decarboxylation, which leads to the formation of compounds such as 4-vinylguaiacol [67]. This phenomenon was observed in muffins with apple residue compared to the control, despite the fact that these acids originate from both the oil and the apple-derived additive. The content of procyanidins and catechin increased in muffins with unripe apple residue compared to the control, which is related to their high concentration in both the residue and the oil used for baking. However, this increase was not proportional to the level of addition, which may be due to the fact that flavan-3-ols can undergo oxidation, isomerization, epimerization, and degradation during baking, as well as complexation with polysaccharides and, notably, gluten proteins present in wheat flour [62,68,69].

Table 8.
Content of polyphenolic compounds (mg/100 g DM) in muffins with polysaccharide fraction residue from unripe apples.
When comparing the results presented in this study regarding the sum of the individual polyphenol contents determined by UPLC in muffins enriched with the polysaccharide fraction residue from unripe apples (Table 8) with our previous research, in which the polyphenol content was determined spectrophotometrically using both the Folin–Ciocalteu reagent and without it in the same products [18], a similar trend in the concentration of these bioactive compounds can be observed. Muffins containing the polysaccharide fraction residue from the unripe apple variety Oliwka exhibited significantly higher levels of these compounds using all the aforementioned methods compared to muffins containing the Pyros variety. It should be emphasized that muffins enriched with polysaccharide fraction residue from both unripe apple varieties contained considerably more polyphenols than the control muffins (Table 8).
Our previous assumptions presented in another publication [18] regarding the degradation of quercetin derivatives leading to the formation of phenolic acids were confirmed in this study. This was particularly evident in the case of chlorogenic acid—the dominant phenolic acid—whose content increased significantly, even up to 65-fold, in muffins with the polysaccharide fraction residue from unripe apples compared to the control muffins. Such a remarkable increase in the concentration of individual polyphenolic compounds in muffins with the polysaccharide fraction residue from unripe apples contributed to the high antioxidant activity determined by three different methods [18], among which the DPPH assay was the least conclusive, as previously explained [18].
Furthermore, a very strong correlation was found between the content of polyphenols, flavonoids, and phenolic acids (determined spectrophotometrically) and the antioxidant activity of the muffins [18]. This strong association between phenolic acids (particularly chlorogenic acid) and flavonoids in muffins with the polysaccharide fraction residue from unripe apples was further confirmed by correlation analysis between chlorogenic acid (determined by UPLC) and ABTS (r = 0.860) and FRAP (r = 0.948), as well as between the flavonoid content (determined by UPLC; procyanidin B1, catechin, quercetin 3-O-galactoside, quercetin glucoside, phloridzin) and ABTS (r = 0.912) and FRAP (r = 0.995).
According to several authors [70,71,72], phenolic acids—especially chlorogenic acid—exhibit antioxidant activity comparable to that of flavonoids. It is also worth noting that this phenolic acid has been shown to exert protective effects against degenerative diseases and coronary heart disease and possesses anticarcinogenic, antiviral, and antibacterial properties, as well as the ability to lower blood pressure [73,74]. Therefore, its high content in muffins with the polysaccharide fraction residue from unripe apples represents a significant achievement of the present study.
These findings confirm that the polysaccharide fraction residue from unripe apples is a concentrated source of polyphenols, and its inclusion in muffins may ensure a high potential for health-promoting effects.
3.2.3. Oxidative Stability of Muffins Enriched with Polysaccharide Fraction Residue from Unripe Pyros and Oliwka Apples
The samples with 5% added percentages of residues show notably lower oxidative stability values than the raw material. For instance, the 5% polysaccharide fraction residue from unripe Pyros variety apple (6.02 h) and 5% polysaccharide fraction residue from unripe Oliwka variety apple (4.62 h) values are considerably lower than the raw material (Table 9), indicating that at these concentrations, the added substances do not provide sufficient protection against oxidation. This is contrary to what might be expected, as the addition of antioxidants is generally thought to improve oxidative stability as discussed by Frankel and Huang [75].

Table 9.
Oxidative stability of muffins enriched with polysaccharide fraction residue derived from unripe apple starch isolation.
The 10% addition further decrease in stability, with samples with Oliwka showing the lowest value (3.83 h). This raises questions about the effectiveness of these specific additives at higher concentrations, which may promote oxidation instead of preventing it, possibly due to pro-oxidant effects or negative interactions with the oil matrix. The control sample (7.52 h) (Table 9) demonstrates a moderate level of oxidative stability, which might be expected if it lacks added antioxidants. This finding aligns with the literature indicating that samples without protective additives tend to have limited stability over time [76]. These results highlight the variability in oxidative stability among different samples and the importance of selecting appropriate antioxidants or preservatives. The findings suggest that simply adding more antioxidants may not yield better results and that the type of antioxidant used is critical. Previous research [77] emphasizes the importance of understanding the mechanism of oxidation and the role of specific antioxidants in enhancing stability. In our previous study [18] on muffins enriched with residues, we measured the tocopherol content and found that it was significantly higher in the control muffins compared to those containing the polysaccharide residue. This likely explains the markedly better oxidative stability observed in the control samples. Therefore, it can be assumed that the tocopherol content, which decreased drastically upon the addition of the polysaccharide residue into muffins, plays a major role in determining oxidative stability. Further studies could investigate the chemical composition of the polysaccharide fraction residue from unripe Pyros variety apples and the polysaccharide fraction residue from unripe Oliwka variety apples to identify other specific phytochemicals contributing to their high oxidative stability. Additionally, exploring the interactions between different concentrations of Pyros and Oliwka bakery products could provide insight into optimizing formulations for better oxidative stability.
3.2.4. The Color Parameters of Muffins Enriched with Polysaccharide Fraction Residue from Unripe Pyros and Oliwka Apples
The control sample (76.04) showed the highest lightness (L*) and intense red and yellow hues (a* = 6.24; b* = 19.35), clearly distinguishing it from the enriched samples. The addition of apple polysaccharide isolation residues (polysaccharide fraction residue from unripe Pyros variety and Oliwka variety apples) significantly affected the color parameters of the muffins, as measured in the CIE Lab* system. This was confirmed by ΔE00 values exceeding 9.0, indicating clearly perceptible color differences compared to the control (Table 10). According to Saunders [78], a ΔE00 value above 3.5 already signifies strongly noticeable color differences. Samples with increasing levels of polysaccharide fraction residue from unripe Pyros variety and Oliwka variety apples showed a decrease in lightness (L*) and an increase in the intensity of red (a*) and yellow (b*) hues (Table 10). The highest color intensity was observed in the sample with 10% Pyros addition, which was the darkest and warmest in tone. This sample exhibited the greatest perceptual color difference, with a ΔE00 value of 13.70. In contrast, muffins with Oliwka residue additions (5% MSO and 10% MSO) showed higher lightness compared to corresponding samples with Pyros residue (Table 10; Figure 5). The darkening of the final products was associated with the presence of sugars and proteins, which can participate in Maillard reactions and caramelization during baking and originated from the raw material [79].

Table 10.
The color parameters of muffins enriched with polysaccharide fraction residue derived from unripe apples.

Figure 5.
Visual representation of colors based on CIE lab values of muffins enriched with polysaccharide fraction residue derived from unripe apples. * The hue angle (Hue°) values for the muffin samples were as follows: K—72.13°, 5% MSP—70.72°, 10% MSP—70.98°, 5% MSO—73.95°, 10% MSO—72.34°. ** The displayed colors are computer-generated visualizations based on the measured CIE L, a*, and b* values and do not represent actual photographs of the samples.
4. Conclusions
It was demonstrated that the polysaccharide fraction residues obtained from the isolation of the starch from unripe Pyros variety and Oliwka variety apples differed in terms of nutritional composition. Nevertheless, both raw materials exhibited favorable nutritional properties and moderate caloric values. Both polysaccharide fraction residues were characterized by a high polyphenol content, several times greater than that found in pomace from ripe apples, suggesting enhanced nutritional and health-promoting potential.
Muffins enriched with polysaccharide fraction residue from unripe Pyros and Oliwka apple varieties showed an 8% increase in protein content at a 10% inclusion level. At the same time, the carbohydrate content slightly decreased, while the fat and mineral contents remained relatively stable, with minor differences between the varieties. The addition of polysaccharide fraction residues had no significant effect on the caloric value of the muffins, but it notably increased the insoluble dietary fiber fraction, positively affecting the dietary value of the final product.
The addition of polysaccharide fraction residue from unripe Pyros and Oliwka apple varieties significantly increased the content of chlorogenic acid (up to 65-fold), dihydrochalcones, quercetin derivatives, and procyanidin B1 (up to 15-fold) compared to control muffins, thus greatly enhancing their health-promoting properties. While the polysaccharide fraction residues exhibited high oxidative stability on their own, their incorporation into muffins reduced oxidative stability, particularly at the 10% supplementation level.
The addition of polysaccharide fraction residues induced pronounced color differences in the muffins compared to the control. The 10% addition of Pyros residue resulted in the darkest and warmest color tone, whereas the Oliwka residue maintained a lighter appearance.
This study, together with the previous studies on this topic presented in the publication by Gumul et al. [18], demonstrates the possibility of utilizing polysaccharide-rich apple residues—by-products of starch extraction from unripe apples—as functional ingredients in bakery products. The application of these natural polysaccharide residues, especially those derived from unripe apples of the Oliwka variety, in muffins allows for the development of fiber-enriched products with improved antioxidant potential as well as more favorable quality and physical characteristics of the final products. This research supports circular economy strategies by reducing food processing waste and promoting the sustainable production of functional foods.
Author Contributions
Conceptualization, D.G.; methodology, D.G., M.K., E.I. and I.J.; software, D.G.; validation, D.G. and M.K.; formal analysis, D.G. and M.K.; investigation, D.G., M.K., E.I. and I.J.; resources, D.G.; data curation, D.G.; writing—original draft preparation, D.G., M.K. and E.I.; writing—review and editing, D.G. and M.K.; visualization, D.G. and M.K.; supervision, D.G.; project administration, D.G.; funding acquisition, D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
This work was supported by funding from the Polish Ministry of Science and Higher Education.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| K | Control muffins |
| 5% MSP | Muffins with 5% share of polysaccharide fraction residue from Pyros variety |
| 10% MSP | Muffins with 10% share of polysaccharide fraction residue from Pyros variety |
| 5% MSO | Muffins with 5% share of polysaccharide fraction residue from Oliwka variety |
| 10% MSO | Muffins with 10% share of polysaccharide fraction residue from Oliwka variety |
| TDF | Total dietary fiber |
| SDF | Soluble dietary fiber |
| IDF | Insoluble dietary fiber |
| TPC | Total polyphenol content |
| TEAC | Trolox equivalents antioxidant capacity |
| DM | Dry matter |
| %RSA | Radical scavenging activity (percentage) |
References
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef]
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Khan, M.A.; Rahman, A.A.; Islam, S.; Khandokhar, P.; Parvin, S.; Islam, M.B.; Hossain, M.; Rashid, M. and Sadik, G. An overview of apple varieties and the importance of apple consumption for health. J. Food Sci. Nutr. 2023, 11, 123–135. [Google Scholar]
- Hyson, D.A. A comprehensive review of apples and apple components and their relationship to human health. Adv. Nutr. Int. Rev. J. 2011, 2, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, G.; Turco, I.; Bacchetti, T. Apple as a source of dietary phytonutrients: Bioavailability and evidence of protective effects against human cardiovascular disease. Food Nutr. Sci. 2014, 5, 1234–1246. [Google Scholar] [CrossRef]
- Gazalli, H.; Malik, A.H.; Sofi, A.H.; Wani, S.A.; Pal, M.A.; Mir, A.; Ashraf, H. Nutritional value and physiological effect of apple pomace. Int. J. Food Nutr. Saf. 2014, 5, 11–15. [Google Scholar]
- Skinner, R.C.; Gigliotti, J.C.; Ku, K.-M.; Tou, J.C. A comprehensive analysis of the composition, health benefits, and safety of apple pomace. Nutr. Rev. 2018, 76, 893–909. [Google Scholar] [CrossRef]
- FAOSTAT. Apple Production in 2023; from Pick Lists: Crops/World Regions/Production Quantity. UN Food and Agriculture Organization, Statistics Division, 2025. Available online: https://www.fao.org/faostat (accessed on 18 February 2025).
- Pogoda, M. Przetwórstwo Owoców i Warzyw w Polsce—Uwarunkowania i Perspektywy; Kancelaria Senatu: Warsaw, Poland, 2020; ISBN 978-83-65711-75-5. [Google Scholar]
- European Parliament and Council. Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives (Waste Framework Directive); Official Journal of the European Union L312; European Parliament and Council: Strasbourg, France, 2008; pp. 3–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32008L0098 (accessed on 23 April 2025).
- Gumul, D.; Korus, J.; Orczykowska, M.; Rosicka-Kaczmarek, J.; Oracz, J.; Areczuk, A. Starch from unripe apples (Malus domestica Borkh) as an alternative for application in the food industry. Molecules 2024, 29, 1707. [Google Scholar] [CrossRef] [PubMed]
- Olawoye, B.; Fagbohun, O.F.; Popoola-Akinola, O.; Adetola, D.B.; Gbadamosi, S.O.; Akanbi, C.T. That we may eat and be healthy: A case of slowly digestible cookies from cardaba banana starch. Appl. Food Res. 2023, 3, 100342. [Google Scholar] [CrossRef]
- Jurkitewicz, K. Rynek Słodyczy w Polsce; HURT & DETAL: Warsaw, Poland, 2024. [Google Scholar]
- Transparency Market Research (n.d.). Biscuits Market: Overview, Trends, and Forecast 2023–2033. Available online: https://www.transparencymarketresearch.com/biscuits-market.html (accessed on 23 April 2025).
- Heo, Y.; Kim, M.; Lee, J.; Moon, B. Muffins Enriched with Dietary Fiber from Kimchi By-Product: Baking Properties, Physical–Chemical Properties, and Consumer Acceptance. Food Sci. Nutr. 2019, 7, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Heras, M.; Gómez, I.; de Pablos-Alcalde, S.; González-Sanjosé, M.L. Application of the Just-About-Right Scales in the Development of New Healthy Whole-Wheat Muffins by the Addition of a Product Obtained from White and Red Grape Pomace. Foods 2019, 8, 419. [Google Scholar] [CrossRef]
- Baranda, V.; del Cerro, L.; Izquierdo, V.; Paz, F.; Rodríguez, A.; Martínez, V.; Olt, V.; Báez, J.; Medrano, A.; Fernández-Fernández, A.M. Muffins with Tannat Grape Pomace: A Sus-tainable Approach to Value-Added Foods. Biol. Life Sci. Forum 2024, 40, 11. [Google Scholar] [CrossRef]
- Gumul, D.; Kowalski, S.; Mikulec, A. Quality and Content of Bioactive Compounds in Muffins with Residue After Isolation of Starch from Unripe Apples (Malus domestica Borkh). Molecules 2025, 30, 2189. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000; Method 920.87. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2000; Method 953.38. [Google Scholar]
- AOAC International. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Regulation (EU) No 1169/2011 on the Provision of Food Information to Consumers. Retrieved 24 February 2023. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2011:304:0018:0063:en:PDF (accessed on 24 April 2025).
- AACC International. Approved Methods of the AACC, 10th ed.; American Association of Cereal Chemists: St. Paul, MN, USA, 2000. [Google Scholar]
- Oszmiański, J.; Kolniak-Ostek, J.; Wojdyło, A. Application of Ultra Performance Liquid Chromatography-Photodiode Detector-Quadrupole/Time of Flight-Mass Spectrometry (UPLC-PDA-Q/TOF-MS) Method for the Characterization of Phenolic Compounds of Lepidium sativum L. sprouts. Eur. Food Res. Technol. 2013, 236, 699–706. [Google Scholar] [CrossRef]
- Cassap, M. Using ICP-OES for Accurate Monitoring of Metallic Contaminants in Water According to U.S. EPA Method 200.7. Spectroscopy 2010, 25, 64–69. [Google Scholar]
- ISO 6886:1997; Animal and Vegetable Fats and Oils—Determination of Oxidation Stability (Accelerated Oxidation Test). International Organization for Standardization: Geneva, Switzerland, 1997.
- CIE. Colorimetry, 3rd ed.; International Commission on Illumination: Vienna, Austria, 2004. [Google Scholar]
- Gazalli, H.; Malik, A.H.; Jalal, H.; Afshan, S.; Mir, A. Proximate composition of carrot powder and apple pomace powder. Int. J. Food Saf. 2013, 3, 25–28. [Google Scholar]
- He, J.; Ren, Y.; Zhu, C.; Jiang, D.; Sun, Y.; Yu, M. Characteristics of cadmium accumulation and tolerance in apple plants grown in different soils. Front. Plant Sci. 2023, 14, 1122334. [Google Scholar]
- Pieszka, M.; Gogol, P.; Pietras, M.; Pieszka, M. Valuable components of dried pomaces of chokeberry, black currant, strawberry, apple, and carrot as a source of natural antioxidants and nutraceuticals in the animal diet. Ann. Anim. Sci. 2015, 15, 475–491. [Google Scholar] [CrossRef]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Veraverbeke, E.A.; Verboven, P.; Van Oostveldt, P.; Nicolaï, B.M. Prediction of moisture loss across the cuticle of apple (Malus sylvestris subsp. mitis (Wallr.)) during storage: Part 2. Model simulations and practical applications. Postharvest Biol. Technol. 2003, 30, 75–88. [Google Scholar]
- Wolfe, K.L.; Liu, R.H. Apple peels as a value-added food ingredient. J. Agric. Food Chem. 2003, 51, 1676–1683. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.Q.; Chen, J.; Zheng, H.Y.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of the composition of sugar and malic acid in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Janssen, B.J.; Thodey, K.; Schaffer, R.J.; Alba, R.; Balakrishnan, L.; Bishop, R.; Bowen, J.H.; Crowhurst, R.N.; Gleave, A.P.; Ledger, S.; et al. Global gene expression analysis of apple fruit development from the floral bud to ripe fruit. BMC Plant Biol. 2008, 8, 16. [Google Scholar] [CrossRef]
- Mureşan, E.A.; Muste, S.; Vlaic, R.A.; Mureşan, C.C.; Cerbu, C.G.; Mureşan, V. The dynamics of starch and total sugars during fruit development for Ionathan, Starkrimson and Golden Delicious apple. Bull. UASVM Food Sci. Technol. 2015, 72, 120–126. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Goldstein, H. Improving policing: A problem-oriented approach. Crime Delinq. 1979, 25, 236–258. [Google Scholar] [CrossRef]
- Geleta, B.T.; Lee, J.-C.; Heo, J.-Y. Antioxidant activity and mineral content in unripe fruits of 10 apple cultivars growing in the northern part of Korea. Horticulturae 2023, 9, 114. [Google Scholar] [CrossRef]
- Mattick, L.R.; Moyer, J.C. Composition of grape pomace from white pressings. J. Food Sci. 1983, 48, 398–399. [Google Scholar]
- Gebhardt, S.E.; Matthews, R.H.; Pounds, J. Composition of Foods: Baked Products: Raw, Processed, Prepared (Agriculture Handbook No. 8–10); U.S. Department of Agriculture, Science and Education Administration: Washington, DC, USA, 1982.
- Zagrodzki, P.; Krośniak, M.; Pazdan, K.; Piotrowicz, J.; Gąstoł, M. Stężenie wybranych składników mineralnych w sokach jabłkowych z uprawy konwencjonalnej i ekologicznej’. Bromatol. Chem. Toksykol. 2012, 45, 722–728. [Google Scholar]
- Leyva-Corral, J.; Quintero-Ramos, A.; Camacho-Dávila, A.; Zazueta-Morales, J.d.J.; Aguilar-Palazuelos, E.; Ruiz-Gutiérrez, M.G.; Meléndez-Pizarro, C.O.; Ruiz-Anchondo, T.d.J. Polyphenolic compound stability and antioxidant capacity of apple pomace in an extruded cereal. LWT Food Sci. Technol. 2016, 65, 228–236. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of polyphenolic content and in vitro antiradical characteristics of apple pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Burda, S.; Oleszek, W.; Lee, C.Y. Phenolic compounds and their changes during maturation and cold storage. J. Agric. Food Chem. 1990, 38, 945–948. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Rabetafika, H.N.; Bchir, B.; Blecker, C.; Richel, A. Fractionation of apple by-products as source of new ingredients: Current situation and perspectives. Trends Food Sci. Technol. 2014, 40, 99–114. [Google Scholar] [CrossRef]
- Persic, M.; Mikulic-Petkovsek, M.; Slatnar, A.; Veberic, R. Chemical composition of apple fruit, juice and pomace and the correlation between phenolic content, enzymatic activity and browning. LWT Food Sci. Technol. 2017, 82, 23–31. [Google Scholar] [CrossRef]
- Akiyama, H.; Sato, Y.; Watanabe, T.; Nagaoka, M.H.; Yoshioka, Y.; Shoji, T.; Kanda, T.; Yamada, K.; Totsuka, M.; Teshima, R.; et al. Dietary unripe apple polyphenol inhibits the development of food allergies in murine models. FEBS Lett. 2005, 579, 4485–4491. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M. High-performance liquid chromatography with diode-array detection for the determination of phenolic compounds in peel and pulp from different apple varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Frankel, E.N. In search of better methods to evaluate natural antioxidants and oxidative stability in food lipids. Trends Food Sci. Technol. 1993, 4, 220–225. [Google Scholar] [CrossRef]
- Shamloo, M.; Babawale, E.A.; Furtado, A.; Henry, R.J.; Eck, P.K.; Jones, P.J.H. Effects of genotype and temperature on accumulation of plant secondary metabolites in Canadian and Australian wheat grown under controlled environments. Sci. Rep. 2017, 7, 9133. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.-H.; Jeong, M.-C.; Kwon, S.-I.; Lee, J. Metabolite profiling and antioxidant activity of 10 new early- to mid-season apple cultivars and 14 traditional cultivars. Antioxidants 2020, 9, 443. [Google Scholar] [CrossRef]
- Kowalski, S.; Łukasiewicz, M. Wpływ warunków wypieku kruchych ciastek na powstawanie wybranych pochodnych furanowych. Żywność. Nauka. Technologia. Jakość 2014, 21, 187–198. [Google Scholar]
- USDA (n.d.) Dry Whole Wheat Pasta—Nutritional Information. FatSecret. Available online: https://www.fatsecret.com/calories-nutrition/usda/dry-whole-wheat-pasta?portionamount=100.000&portionid=62521 (accessed on 5 June 2025).
- Carson, K.J. Utilization of Apple Pomace in Food Products: Functional and Nutritional Properties; University of Tennessee: Knoxville, TN, USA, 1990; Available online: https://trace.tennessee.edu/utk_gradthes/7140 (accessed on 5 June 2025).
- Tańska, M.; Rotkiewicz, D. The possibility of using apple pomace for bread production. Bromatol. Chem. Toksykol. 2011, 44, 847–853. Available online: https://ptfarm.pl/pub/File/bromatologia_2011/3/847-853.pdf (accessed on 17 April 2025).
- Nawirska, A.; Kwaśniewska, M. Frakcje błonnika w wytłokach z owoców. Acta Sci. Pol. Technol. Aliment. 2004, 3, 13–20. [Google Scholar]
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Kołodziejczyk, K.; Sójka, M. Effects of dietary addition of a low-pectin apple fibre preparation on rats. Pol. J. Food Nutr. Sci. 2014, 64, 193–199. [Google Scholar] [CrossRef]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Rupasinghe, H.; Wang, L.; Huber, G.; Pitts, N. Effect of baking on dietary fibre and phenolics of muffins incorporated with apple skin powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Wang, L.; Yao, Y.; He, Z.; Wang, D.; Liu, A.; Zhang, Y. Determination of phenolic acid concentrations in wheat flours produced at different extraction rates. J. Cereal Sci. 2013, 57, 67–72. [Google Scholar] [CrossRef]
- Di Stefano, V.; Buzzanca, C.; Melilli, M.G.; Indelicato, S.; Mauro, M.; Vazzana, M.; Arizza, V.; Lucarini, M.; Durazzo, A.; Bongiorno, D. Polyphenol characterization and antioxidant activity of grape seeds and skins from Sicily: A preliminary study. Sustainability 2022, 14, 6702. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Foss, F.W.; Schug, K.A. Thermally accelerated oxidative degradation of quercetin using continuous flow kinetic electrospray-ion trap-time of flight mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1513–1522. [Google Scholar] [CrossRef]
- Rohn, S.; Buchner, N.; Driemel, G.; Rauser, M.; Kroh, L.W. Thermal degradation of onion quercetin glucosides under roasting conditions. J. Agric. Food Chem. 2007, 55, 1568–1573. [Google Scholar] [CrossRef] [PubMed]
- Mildner-Szkudlarz, S.; Bajerska, J.; Górnaś, P.; Segliņa, D.; Pilarska, A.; Jesionowski, T. Physical and Bioactive Properties of Muffins Enriched with Raspberry and Cranberry Pomace Powder: A Promising Application of Fruit By-Products Rich in Biocompounds. Plant Foods Hum. Nutr. 2016, 71, 165–173. [Google Scholar] [CrossRef]
- Maillard, M.-N.; Berset, C. Evolution of antioxidant activity during kilning: Role of insoluble bound phenolic acids of barley and malt. J. Agric. Food Chem. 1995, 43, 1789–1793. [Google Scholar] [CrossRef]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef]
- Sivam, A.S.; Sun-Waterhouse, D.; Quek, S.; Perera, C.O. Properties of bread dough with added fiber polysaccharides and phenolic antioxidants: A review. J. Food Sci. 2010, 75, R163–R174. [Google Scholar] [CrossRef]
- von Gadow, A.; Joubert, E.; Hansmann, C.F. Comparison of the Antioxidant Activity of Aspalathin with That of Other Plant Phenols of Rooibos Tea (Aspalathus linearis), α-Tocopherol, BHT and BHA. J. Agric. Food Chem. 1997, 45, 632–638. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Stöckmann, H.; Schwarz, K.; Heinonen, I.M.; Hopia, A.I. Antioxidant Activity and Partitioning of Phenolic Acids in Bulk and Emulsified Methyl Linoleate. J. Agric. Food Chem. 1999, 47, 3036–3043. [Google Scholar] [CrossRef]
- Szwajgier, D.; Pielecki, J.; Targoński, Z. Antioxidant Activities of Cinnamic and Benzoic Acid Derivatives. Acta Sci. Pol. Technol. Aliment. 2005, 4, 129–142. [Google Scholar]
- Nogueira, T.; Lago, C.L.D. Determination of Caffeine in Coffee Products by Dynamic Complexation with 3,4-Dimethoxycinnamate and Separation by CZE. Electrophoresis 2007, 28, 3570–3574. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chikama, A.; Mori, K.; Watanabe, T.; Shioya, Y.; Katsuragi, Y.; Tokimitsu, I. Hydroxyquinone-Free Coffee: A Double-Blind, Randomized Controlled Dose-Response Study of Blood Pressure. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 408–414. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W. Improving the oxidative stability of polyunsaturated vegetable oils by blending with high-oleic sunflower oil. J. Am. Oil Chem. Soc. 1994, 71, 255–259. [Google Scholar] [CrossRef]
- Maszewska, M.; Florowska, A.; Dłużewska, E.; Wroniak, M.; Marciniak-Lukasiak, K.; Żbikowska, A. Oxidative stability of selected edible oils. Molecules 2018, 17, 1746. [Google Scholar] [CrossRef] [PubMed]
- Decker, E.A. Strategies for manipulating the prooxidative/antioxidative balance of foods to maximize oxidative stability. Trends Food Sci. Technol. 1998, 9, 241–248. [Google Scholar] [CrossRef]
- Saunders, D. Colour, Colour Measurement and Colour Change; Routledge: Oxfordshire, UK, 2023; ISBN 978-1032501710. [Google Scholar]
- Lei, H.; Ruan, R.; Fulcher, R.G.; van Lengerich, B. Color development in an extrusion-cooked model system. Int. J. Agric. Biol. Eng. 2008, 1, 55–63. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).