Green Oxidation of Starch Using Ozone: A Comparative Study on Rheological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Starch Raw Material
2.2. Starch Modification Procedure
- Potato starch treated for 1 h, 2 h, 4 h, and 6 h, suspension concentration 20%, 15 min retention time (ozone dose of 0.11 ± 0.00; 0.22 ± 0.01; 0.43 ± 0.02 and 0.65 g ± 0.02 per 1 kg of starch, respectively).
- Pea starch treated for 1 h, 2 h, 4 h, and 6 h, suspension concentration 20%, 15 min retention time (ozone dose of 0.11 ± 0.00; 0.22 ± 0.01; 0.43 ± 0.02 and 0.65 g ± 0.02 per 1 kg of starch, respectively).
- Potato starch treated for 6 h, suspension concentration 20%, 1.5 min retention time (ozone dose of 6.48 g ± 0.24 g per 1 kg of starch).
- Pea starch treated for 6 h, suspension concentration 20%, 1.5 min retention time (ozone dose of 6.48 g ± 0.24 g per 1 kg of starch).
- Potato starch treated for 6 h, suspension concentration 30%, 1.5 min retention time (ozone dose of 4.32 g ± 0.16 g per 1 kg of starch).
2.3. Proximate Composition of Starch
2.4. Rheological Characterization of Starch
2.4.1. Pasting Characteristics
2.4.2. Texture Profile Analysis
2.4.3. Rheological Properties
2.5. Molecular Characterization of Starch Using SEC with Triple Detection
2.6. Statistical Analysis
3. Results
3.1. Proximate Composition of Starch
3.2. Rheological Studies of Potato Starch
3.2.1. Influence of Oxidation Time
3.2.2. Influence of Suspension Concentration
3.3. Rheological Studies of Pea Starch
3.3.1. Influence of Oxidation Time
3.3.2. Influence of Retention Volume
3.4. Molecular Mass Distribution and Hydrodynamic Parameters of Modified Starch Macromolecules
3.5. Principal Component Analysis
3.6. Research Limitations and Directions of Further Research
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Production, Structure, Physicochemical and Functional Properties of Maize, Cassava, Wheat, Potato and Rice Starches. Starch—Stärke 2015, 67, 14–29. [Google Scholar] [CrossRef]
- Takeda, Y.; Hizukuri, S.; Takeda, C.; Suzuki, A. Structures of Branched Molecules of Amyloses of Various Origins, and Molar Fractions of Branched and Unbranched Molecules. Carbohydr. Res. 1987, 165, 139–145. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Le Thanh-Blicharz, J.; Szwengiel, A. Insight into Rheological Properties and Structure of Native Waxy Starches: Cluster Analysis Grouping. Molecules 2024, 29, 2669. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Li, S.; Zhao, G.; Zhai, L.; Qin, P.; Yang, L. Starch Modification with Molecular Transformation, Physicochemical Characteristics, and Industrial Usability: A State-of-the-Art Review. Polymers 2023, 15, 2935. [Google Scholar] [CrossRef] [PubMed]
- Adewale, P.; Yancheshmeh, M.S.; Lam, E. Starch Modification for Non-Food, Industrial Applications: Market Intelligence and Critical Review. Carbohydr. Polym. 2022, 291, 119590. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch—Stärke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Ashogbon, A.O.; Akintayo, E.T. Recent Trend in the Physical and Chemical Modification of Starches from Different Botanical Sources: A Review. Starch—Stärke 2014, 66, 41–57. [Google Scholar] [CrossRef]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A Review of the Chemical Modification Techniques of Starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Błaszczak, W.; Lewandowicz, G. Light Microscopy as a Tool to Evaluate the Functionality of Starch in Food. Foods 2020, 9, 670. [Google Scholar] [CrossRef]
- Lewandowicz, G. Physical Modification of Starch—Really Physical? In Proceedings of the 13th International Conference on Polysaccharides-Glycoscience, Prague, Czech Republic, 8–10 November 2017; Rapkova, J., Sarka, E.R.C., Eds.; Czech Chemical Society: Prague, Czech Republic, 2017; pp. 9–14. [Google Scholar]
- Haq, F.; Yu, H.; Wang, L.; Teng, L.; Haroon, M.; Khan, R.U.; Mehmood, S.; Bilal-Ul-Amin; Ullah, R.S.; Khan, A.; et al. Advances in Chemical Modifications of Starches and Their Applications. Carbohydr. Res. 2019, 476, 12–35. [Google Scholar] [CrossRef]
- Xie, Q.; Fan, B.; Tao, R.; Wang, F.; Sun, Y. A Comprehensive Review of Starch: Structure, Properties, Chemical Modification, and Application in Food Preservation. Food Front. 2025, 6, 1645–1657. [Google Scholar] [CrossRef]
- Vanier, N.L.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Molecular Structure, Functionality and Applications of Oxidized Starches: A Review. Food Chem. 2017, 221, 1546–1559. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent Advances on the Preparation Conditions, Structural Characteristics, Physicochemical Properties, Functional Properties and Potential Applications of Dialdehyde Starch: A Review. Int. J. Biol. Macromol. 2024, 259, 129261. [Google Scholar] [CrossRef] [PubMed]
- FAO; WHO. Compendium of Food Additive Specifications. Joint FAO/WHO Expert Committee on Food (JECFA), 86th Meeting June 2018; FAO JECFA Monographs 22; Food & Agriculture Organization: Rome, Italy, 2018; ISBN 9789251311004. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources added to Food (ANS); Mortensen, A.; Aguilar, F.; Crebelli, R.; Di Domenico, A.; Dusemund, B.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Re-Evaluation of Oxidised Starch (E 1404), Monostarch Phosphate (E 1410), Distarch Phosphate (E 1412), Phosphated Distarch Phosphate (E 1413), Acetylated Distarch Phosphate (E 1414), Acetylated Starch (E 1420), Acetylated Distarch Adipate (E 1422), Hydroxypropyl Starch (E 1440), Hydroxypropyl Distarch Phosphate (E 1442), Starch Sodium Octenyl Succinate (E 1450), Acetylated Oxidised Starch (E 1451) and Starch Aluminium Octenyl Succinate (E 1452) as Food Additives. EFSA J. 2017, 15, e04911. [Google Scholar] [CrossRef]
- Whistler, R.L.; Schweiger, R. Oxidation of Amylopectin with Hypochlorite at Different Hydrogen Ion Concentrations. J. Am. Chem. Soc. 1957, 79, 6460–6464. [Google Scholar] [CrossRef]
- Finch, C.A. Modified starches: Properties and uses Edited by O. B. Wurzburg, CRC Press, Boca Raton, Florida, 1986. pp. vi + 277, price £101.50. ISBN 0-8493-5964-3. Br. Polym. J. 1989, 21, 87–88. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Le Thanh-Blicharz, J.; Szwengiel, A. The Effect of Chemical Modification on the Rheological Properties and Structure of Food Grade Modified Starches. Processes 2022, 10, 938. [Google Scholar] [CrossRef]
- Łabanowska, M.; Bidzińska, E.; Pietrzyk, S.; Juszczak, L.; Fortuna, T.; Błoniarczyk, K. Influence of Copper Catalyst on the Mechanism of Carbohydrate Radicals Generation in Oxidized Potato Starch. Carbohydr. Polym. 2011, 85, 775–785. [Google Scholar] [CrossRef]
- Chan, H.T.; Leh, C.P.; Bhat, R.; Senan, C.; Williams, P.A.; Karim, A.A. Molecular Structure, Rheological and Thermal Characteristics of Ozone-Oxidized Starch. Food Chem. 2011, 126, 1019–1024. [Google Scholar] [CrossRef]
- Chan, H.T.; Bhat, R.; Karim, A.A. Physicochemical and Functional Properties of Ozone-Oxidized Starch. J. Agric. Food Chem. 2009, 57, 5965–5970. [Google Scholar] [CrossRef]
- Çatal, H.; Ibanoǧlu, Ş. Effect of Aqueous Ozonation on the Pasting, Flow and Gelatinization Properties of Wheat Starch. LWT—Food Sci. Technol. 2014, 59, 577–582. [Google Scholar] [CrossRef]
- Çatal, H.; Ibanoǧlu, Ş. Ozonation of Corn and Potato Starch in Aqueous Solution: Effects on the Thermal, Pasting and Structural Properties. Int. J. Food Sci. Technol. 2012, 47, 1958–1963. [Google Scholar] [CrossRef]
- Castanha, N.; Miano, A.C.; Jones, O.G.; Reuhs, B.L.; Campanella, O.H.; Augusto, P.E.D. Starch Modification by Ozone: Correlating Molecular Structure and Gel Properties in Different Starch Sources. Food Hydrocoll. 2020, 108, 106027. [Google Scholar] [CrossRef]
- Kurdziel, M.; Pietrzyk, S.; Łabanowska, M.; Pająk, P.; Królikowska, K. The Effect of Ozonation of Dry and Moist Corn and Potato Starches with Different Amylose Contents on Their Structural and Functional Properties. Food Chem. 2025, 490, 145029. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.; Vanier, N.L.; Moomand, K.; Pinto, V.Z.; Colussi, R.; Da Rosa Zavareze, E.; Dias, A.R.G. Ozone Oxidation of Cassava Starch in Aqueous Solution at Different PH. Food Chem. 2014, 155, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, H.P.S.; Manthey, F.A.; Simsek, S. Ozone Gas Affects Physical and Chemical Properties of Wheat (Triticum aestivum L.) Starch. Carbohydr. Polym. 2012, 87, 1261–1268. [Google Scholar] [CrossRef]
- La Fuente, C.I.A.; de Souza, A.T.; Tadini, C.C.; Augusto, P.E.D. Ozonation of Cassava Starch to Produce Biodegradable Films. Int. J. Biol. Macromol. 2019, 141, 713–720. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Sui, C.; Wang, L. Recent Development in Ozone-Based Starch Modification: From Generation Methods to Film Applications. Int. J. Biol. Macromol. 2025, 309, 142780. [Google Scholar] [CrossRef]
- Teixeira, B.S.; del Mastro, N.L. Effects of Electron Beam Irradiation on Ozone-Modified Potato Starch Film. Radiat. Phys. Chem. 2023, 213, 111234. [Google Scholar] [CrossRef]
- Oladebeye, A.O.; Oshodi, A.A.; Amoo, I.A.; Karim, A.A. Gaseous Ozonation of Pigeon Pea, Lima Bean, and Jack Bean Starches: Functional, Thermal, and Molecular Properties. Starch—Stärke 2018, 70, 1700367. [Google Scholar] [CrossRef]
- PN-EN ISO 1666:2000; Starch—Determination of Moisture Content—Oven-Drying Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 2000.
- PN-EN ISO 3188:2000; Starches and Derived Products—Determination of Nitrogen Content by the Kjeldahl Method—Titrimetric Method. Polski Komitet Normalizacyjny: Warsaw, Poland, 2000.
- PN-EN ISO 10520:2002; Native Starch—Determination of Starch Content—Ewers Polarimetric Method Starch. Polski Komitet Normalizacyjny: Warsaw, Poland, 2002.
- PN-EN ISO 3593:2000; Starch—Determination of Ash. Polski Komitet Normalizacyjny: Warsaw, Poland, 2000.
- PN-EN ISO 11214:2001; Modified Starch—Determination of Carboxyl Group Content of Oxidized Starch. Polski Komitet Normalizacyjny: Warsaw, Poland, 2001.
- Akmarov, K.A.; Lapshov, S.N.; Sherstobitova, A.S.; Yas’kov, A.D. Optical Properties of Aqueous Solutions of Dimethyl Sulfoxide and Application of Refractometry for Monitoring Their Composition. J. Appl. Spectrosc. 2013, 80, 610–614. [Google Scholar] [CrossRef]
- Chakraborty, S.; Sahoo, B.; Teraoka, I.; Gross, R.A. Solution Properties of Starch Nanoparticles in Water and DMSO as Studied by Dynamic Light Scattering. Carbohydr. Polym. 2005, 60, 475–481. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Wronkowska, M.; Sadowska, J.; Soral-Śmietana, M.; Błaszczak, W.; Walkowski, A. Influence of Potato Starch Oxidation on Texture, and Rheological Behaviour of Some Sweet Desserts. Pol. J. Food Nutr. Sci. 2003, 53, 31–36. [Google Scholar]
- Kedziora, P.; Le Thanh, J.; Lewandowicz, G.; Prochaska, K. An Attempt to Application of Continuous Recycle Membrane Reactor for Hydrolysis of Oxidised Derivatives of Potato Starch. J. Memb. Sci. 2006, 282, 14–20. [Google Scholar] [CrossRef]
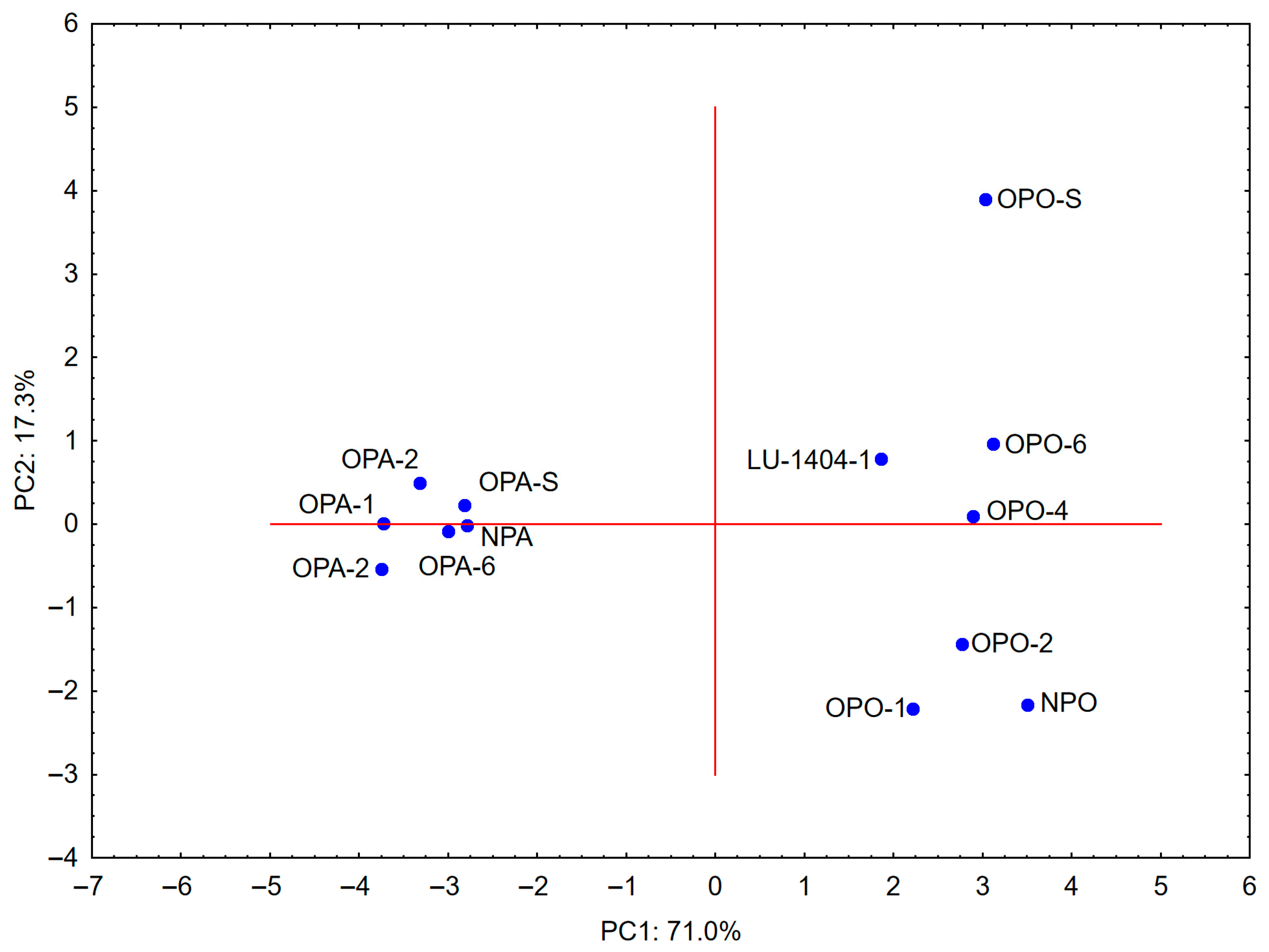
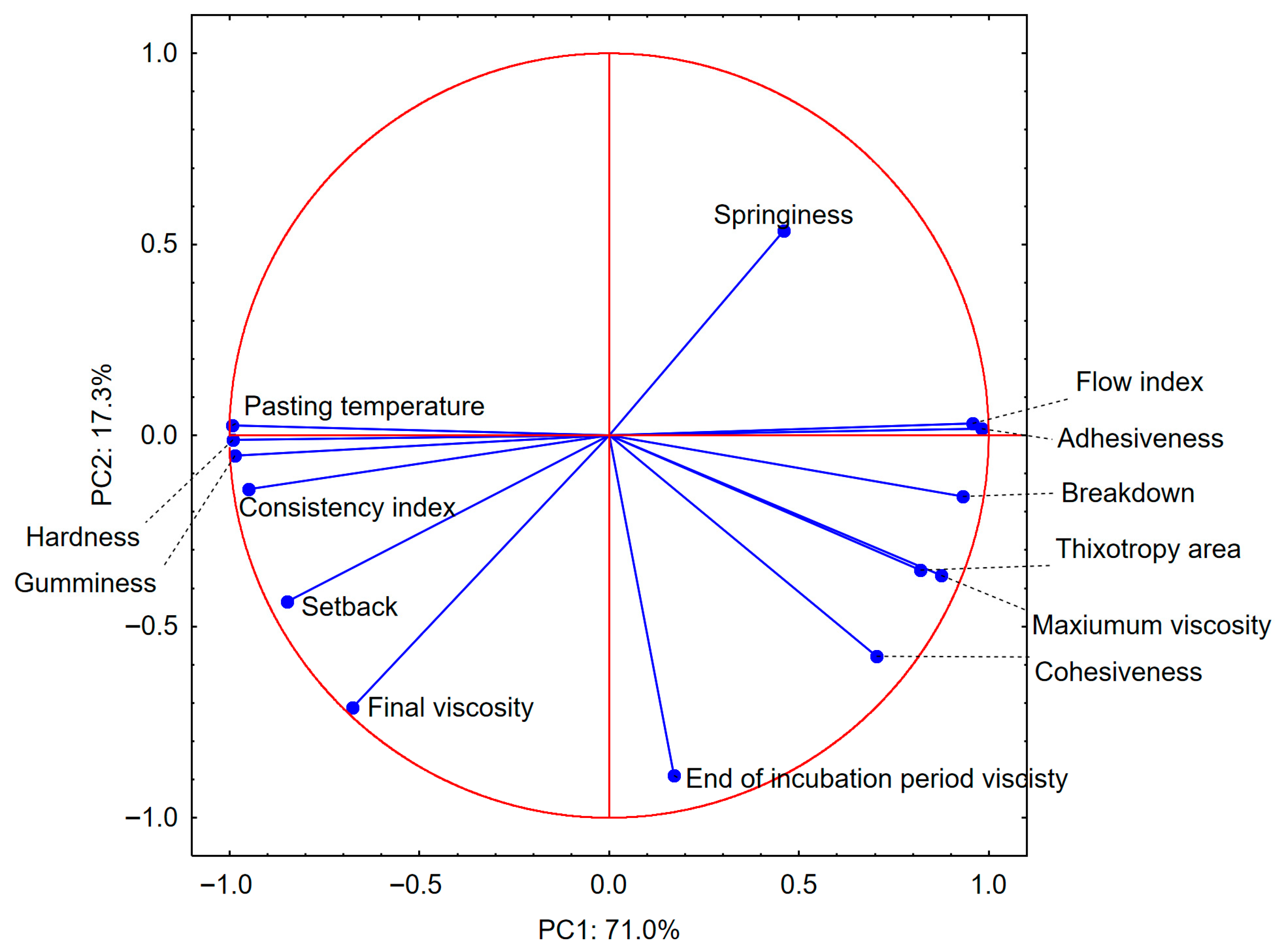
| Parameter | Potato Starch | Pea Starch |
|---|---|---|
| Moisture [%] | 19.1 ± 0.3 b | 12.0 ± 0.2 a |
| Protein [%] | 0.17 ± 0.01 a | 0.88 ± 0.05 b |
| Starch [%] | 96.9 ± 0.2 b | 97.8 ± 0.1 a |
| Ash [%] | 0.38 ± 0.03 b | 0.10 ± 0.03 a |
| Carboxyl groups [%] (after most extensive ozonation) | 0.33 ± 0.01 b | 0.11 ± 0.00 a |
| Starch | Pasting Temperature (°C) | Maximum Viscosity (BU) | Breakdown (BU) | Setback (BU) | End of Holding Period Viscosity (BU) | Final Viscosity (BU) |
|---|---|---|---|---|---|---|
| Native | 61.9 | 1879 | 1303 | 506 | 570 | 1082 |
| Oxidized 1 h | 63.1 | 1366 | 841 | 624 | 525 | 1151 |
| Oxidized 2 h | 62.5 | 1435 | 962 | 547 | 473 | 1021 |
| Oxidized 4 h | 62.5 | 1292 | 963 | 393 | 328 | 724 |
| Oxidized 6 h | 63.2 | 1176 | 933 | 284 | 243 | 528 |
| Commercial | 63.8 | 525 | 221 | 171 | 304 | 475 |
| Starch | Hardness (N) | Adhesiveness (N∙s) | Cohesiveness (-) | Springiness (-) | Gumminess (N) |
|---|---|---|---|---|---|
| Native | 0.45 ± 0.02 a | −0.10 ± 0.02 ab | 0.73 ± 0.00 d | 0.97 ± 0.00 cd | 0.33 ± 0.02 bc |
| Oxidized 1 h | 0.58 ± 0.01 b | −0.40 ± 0.03 d | 0.68 ± 0.01 ab | 0.94 ± 0.00 a | 0.39 ± 0.00 c |
| Oxidized 2 h | 0.43 ± 0.01 a | −0.14 ± 0.01 bc | 0.72 ± 0.00 cd | 0.96 ± 0.00 bc | 0.31 ± 0.01 ab |
| Oxidized 4 h | 0.43 ± 0.00 a | −0.08 ± 0.01 ab | 0.70 ± 0.00 bc | 0.97 ± 0.00 cd | 0.30 ± 0.00 ab |
| Oxidized 6 h | 0.41 ± 0.00 a | −0.04 ± 0.02 a | 0.70 ± 0.02 bc | 0.98 ± 0.01 d | 0.29 ± 0.01 a |
| Commercial | 0.57 ± 0.07 b | −0.21 ± 0.07 c | 0.67 ± 0.00 a | 0.95 ± 0.00 ab | 0.38 ± 0.05 c |
| Starch | Consistency Index (Pa∙sn) | Flow Index (-) | Thixotropy (Pa∙s−1) |
|---|---|---|---|
| Native | 9.2 ± 0.1 b | 0.656 ± 0.001 bc | 14,440 ± 710 c |
| Oxidized 1 h | 11.6 ± 1.3 b | 0.581 ± 0.006 b | 14,209 ± 9010 bc |
| Oxidized 2 h | 9.1 ± 0.1 b | 0.609 ± 0.011 bc | 10,110 ± 6492 abc |
| Oxidized 4 h | 5.0 ±1.4 a | 0.654 ± 0.052 bc | 1546 ± 1550 ab |
| Oxidized 6 h | 3.2 ± 0.5 a | 0.689 ± 0.020 c | 1359 ± 775 ab |
| Commercial | 10.3 ± 1.4 b | 0.453 ± 0.073 a | 9491 ± 2191 bc |
| Starch | Pasting Temperature (°C) | Maximum Viscosity (BU) | Breakdown (BU) | Setback (BU) | End of Holding Period Viscosity (BU) | Final Viscosity (BU) |
|---|---|---|---|---|---|---|
| Reference | 61.9 | 1879 | 1303 | 506 | 570 | 1082 |
| Slow retention | 63.2 | 1176 | 933 | 284 | 243 | 528 |
| Fast retention | 62.6 | 940 | 875 | 1 | 65 | 67 |
| Starch | Hardness (N) | Adhesiveness (N∙s) | Cohesiveness (-) | Springiness (-) | Gumminess (N) |
|---|---|---|---|---|---|
| Reference | 0.45 ± 0.02 b | −0.10 ± 0.02 b | 0.73 ± 0.00 c | 0.97 ± 0.00 a | 0.33 ± 0.02 c |
| Slow retention | 0.41 ± 0.03 b | −0.04 ± 0.02 a | 0.70 ± 0.02 b | 0.98 ± 0.01 a | 0.29 ± 0.01 b |
| Fast retention | 0.37 ± 0.01 a | 0.00 ± 0.00 a | 0.42 ± 0.00 a | 1.00 ± 0.00 b | 0.15 ± 0.00 a |
| Starch | Consistency Index (Pa∙sn) | Flow Index (-) | Thixotropy (Pa∙s−1) |
|---|---|---|---|
| Reference | 9.2 ± 0.1 b | 0.656 ± 0.001 a | 14,440 ± 710 b |
| Slow retention | 3.2 ± 0.5 a | 0.689 ± 0.020 a | 1359 ± 775 a |
| Fast retention | 0.8 ± 0.1 a | 0.653 ± 0.026 a | 753 ± 637 a |
| Suspension Concentration | Pasting Temperature (°C) | Maximum Viscosity (BU) | Breakdown (BU) | Setback (BU) | End of Holding Period Viscosity (BU) | Final Viscosity (BU) |
|---|---|---|---|---|---|---|
| 20% | 62.6 | 940 | 875 | 1 | 65 | 67 |
| 30% | 62.2 | 1456 | 1345 | 85 | 108 | 194 |
| Starch | Hardness (N) | Adhesiveness (N∙s) | Cohesiveness (-) | Springiness (-) | Gumminess (N) |
|---|---|---|---|---|---|
| 20% | 0.37 ± 0.01 a | 0.00 ± 0.00 a | 0.42 ± 0.00 a | 1.00 ± 0.00 a | 0.15 ± 0.00 a |
| 30% | 0.42 ± 0.00 b | 0.00 ± 0.00 a | 0.71 ± 0.02 b | 1.00 ± 0.02 a | 0.30 ± 0.01 b |
| Starch | Consistency Index (Pa∙sn) | Flow Index (-) | Thixotropy (Pa∙s−1) |
|---|---|---|---|
| 20% | 0.8 ± 0.1 a | 0.653 ± 0.026 a | 753 ± 637 a |
| 30% | 2.4 ± 0.5 b | 0.604 ± 0.030 a | 1490 ± 173 a |
| Starch | Pasting Temperature (°C) | Maximum Viscosity (BU) | Breakdown (BU) | Setback (BU) | End of Holding Period Viscosity (BU) | Final Viscosity (BU) |
|---|---|---|---|---|---|---|
| Native | 75.8 | 396 | 0 | 792 | 396 | 1194 |
| Oxidized 1 h | 75.8 | 378 | 0 | 904 | 378 | 1287 |
| Oxidized 2 h | 76.0 | 356 | 0 | 884 | 356 | 1245 |
| Oxidized 4 h | 75.9 | 299 | 0 | 909 | 299 | 1212 |
| Oxidized 6 h | 75.8 | 263 | 0 | 918 | 263 | 1186 |
| Starch | Hardness (N) | Adhesiveness (N∙s) | Cohesiveness (-) | Springiness (-) | Gumminess (N) |
|---|---|---|---|---|---|
| Native | 2.96 ± 1.11 a | −9.16 ± 0.91 a | 0.50 ± 0.05 a | 0.97 ± 0.02 a | 1.44 ± 0.40 a |
| Oxidized 1 h | 3.61 ± 0.57 a | −14.67 ± 11.21 a | 0.49 ± 0.03 a | 0.97 ± 0.02 a | 1.76 ± 0.38 a |
| Oxidized 2 h | 3.38 ± 0.14 a | −14.86 ± 3.97 a | 0.52 ± 0.02 a | 0.93 ± 0.07 a | 1.76 ± 0.02 a |
| Oxidized 4 h | 3.48 ± 1.05 a | −11.07 ± 10.42 a | 0.53 ± 0.03 a | 0.97 ± 0.01 a | 1.85 ± 0.73 a |
| Oxidized 6 h | 3.11 ± 0.91 a | −13.12 ± 8.64 a | 0.51 ± 0.03 a | 0.93 ± 0.07 a | 1.58 ± 0.37 a |
| Starch | Consistency Index (Pa∙sn) | Flow Index (-) | Thixotropy (Pa∙s−1) |
|---|---|---|---|
| Native | 44.9 ± 2.9 c | 0.263 ± 0.016 a | −5080 ± 4197 ab |
| Oxidized 1 h | 46.0 ± 1.3 c | 0.271 ± 0.029 a | −12,651 ± 4029 bc |
| Oxidized 2 h | 40.1 ± 0.9 bc | 0.288 ± 0.004 ab | −13,236 ± 1846 bc |
| Oxidized 4 h | 37.0 ± 1.6 ab | 0.326 ± 0.013 bc | −16,505 ± 5112 c |
| Oxidized 6 h | 30.8 ± 4.1 a | 0.343 ± 0.033 c | −2206 ± 818 a |
| Starch | Pasting Temperature (°C) | Maximum Viscosity (BU) | Breakdown (BU) | Setback (BU) | End of Holding Period Viscosity (BU) | Final Viscosity (BU) |
|---|---|---|---|---|---|---|
| Reference | 75.8 | 396 | 0 | 792 | 396 | 1194 |
| Slow retention | 75.8 | 263 | 0 | 918 | 263 | 1186 |
| Fast retention | 75.8 | 140 | 0 | 1061 | 140 | 1201 |
| Starch | Hardness (N) | Adhesiveness (N∙s) | Cohesiveness (-) | Springiness (-) | Gumminess (N) |
|---|---|---|---|---|---|
| Reference | 2.96 ± 1.11 a | −9.16 ± 0.91 a | 0.50 ± 0.05 a | 0.97 ± 0.02 a | 1.44 ± 0.40 a |
| Slow retention | 3.11 ± 0.91 a | −13.12 ± 8.64 a | 0.51 ± 0.03 a | 0.93 ± 0.07 a | 1.58 ± 0.37 a |
| Fast retention | 2.62 ± 1.20 a | −11.35 ± 1.44 a | 0.50 ± 0.05 a | 0.92 ± 0.03 a | 1.32 ± 0.38 a |
| Starch | Consistency Index (Pa∙sn) | Flow Index (-) | Thixotropy (Pa∙s−1) |
|---|---|---|---|
| Reference | 44.9 ± 2.9 b | 0.263 ± 0.016 a | −5080 ± 4198 a |
| Slow retention | 30.8 ± 4.1 a | 0.343 ± 0.033 b | −2206 ± 818 a |
| Fast retention | 25.9 ± 2.9 a | 0.395 ± 0.011 b | −1742 ± 255 a |
| Starch | Mn (Da) | Mw (Da) | Mz (Da) | Mw/Mn | Rh (nm) | Rg (nm) |
|---|---|---|---|---|---|---|
| Native | 2.05 × 107 ± 2.19 × 106 c | 4.09 × 107 ± 3.70 × 106 b | 6.25 × 107 ± 7.49 × 106 b | 2.0 ± 0.2 a | 99 ± 5 b | 172 ± 12 c |
| Oxidized 2 h | 1.34 × 107 ± 1.22 × 106 b | 2.63 × 107 ± 1.85 × 106 a | 3.97 × 107 ± 1.62 × 106 a | 2.0 ± 0.1 a | 95 ± 1 b | 149 ± 2 b |
| Oxidized 4 h | 1.04 × 107 ± 7.56 × 105 b | 2.33 × 107 ± 2.12 × 106 a | 3.65 × 107 ± 7.91 × 105 a | 2.2 ± 0.0 a | 90 ± 6 b | 136 ± 4 b |
| Oxidized 6 h (FR) | 6.05 × 105 ± 6.47 × 104 a | 7.19 × 106 ± 1.64 × 105 a | 3.36 × 107 ± 2.48 × 106 a | 12.0 ± 1.3 b | 42 ± 5 a | 92 ± 7 a |
| Starch | Mn (Da) | Mw (Da) | Mz (Da) | Mw/Mn | Rh (nm) | Rg (nm) |
|---|---|---|---|---|---|---|
| Native | 2.10 × 107 ± 3.07 × 105 d | 6.64 × 107 ± 1.98 × 106 c | 1.23 × 108 ± 1.35 × 106 c | 3.2 ± 0.0 a | 83 ± 3 b | 159 ± 2 c |
| Oxidized 2 h | 1.80 × 107 ± 1.57 × 106 c | 6.19 × 107 ± 1.32 × 106 c | 1.20 × 108 ± 4.22 × 106 c | 3.5 ± 0.4 a | 91 ± 5 b | 149 ± 10 bc |
| Oxidized 4 h | 8.89 × 106 ± 4.26 × 105 b | 4.90 × 107 ± 2.57 × 106 b | 1.03 × 108 ± 2.60 × 106 b | 5.5 ± 0.5 b | 54 ± 5 a | 138 ± 10 b |
| Oxidized 6 h (FR) | 1.44 × 106 ± 1.31 × 105 a | 1.56 × 107 ± 1.20 × 106 a | 1.67 × 107 ± 2.48 × 106 a | 10.9 ± 0.8 c | 49 ± 4 a | 92 ± 5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Thanh-Blicharz, J.; Lewandowicz, J.; Zielonka, R.; Szwengiel, A. Green Oxidation of Starch Using Ozone: A Comparative Study on Rheological Properties. Appl. Sci. 2025, 15, 10924. https://doi.org/10.3390/app152010924
Le Thanh-Blicharz J, Lewandowicz J, Zielonka R, Szwengiel A. Green Oxidation of Starch Using Ozone: A Comparative Study on Rheological Properties. Applied Sciences. 2025; 15(20):10924. https://doi.org/10.3390/app152010924
Chicago/Turabian StyleLe Thanh-Blicharz, Joanna, Jacek Lewandowicz, Roman Zielonka, and Artur Szwengiel. 2025. "Green Oxidation of Starch Using Ozone: A Comparative Study on Rheological Properties" Applied Sciences 15, no. 20: 10924. https://doi.org/10.3390/app152010924
APA StyleLe Thanh-Blicharz, J., Lewandowicz, J., Zielonka, R., & Szwengiel, A. (2025). Green Oxidation of Starch Using Ozone: A Comparative Study on Rheological Properties. Applied Sciences, 15(20), 10924. https://doi.org/10.3390/app152010924









