Tempeh and Fermentation—Innovative Substrates in a Classical Microbial Process
Abstract
1. Introduction
2. Methods
3. Characteristics of the Raw Materials Used for Tempeh Fermentation
- Legumes: broad beans (Vicia faba L.) [15,29,30,31,32], chickpeas (Cicer arietinum) [5,15,33,34], Jack bean (Canavalia ensiformis) [6,35,36,37,38,39,40], cowpea bean (Vigna unguiculata), Bambara groundnut (Vigna subterranea) [41], mung bean (Vigna radiata) [26,42], winged bean (Psophocarpus tetragonolobus) [23], common bean (Phaseolus vulgaris) [15,43,44,45,46,47], large pea (Lathyrus sativus) [48,49,50], lentils (Lens culinaris) [15], narrow-leaved lupin (Lupinus angustifolius) [51,52], tarwi (Lupinus mutabilis) [53], white mimosa (Leucaena leucocephala) [54];
- Plant additives: butterfly pea (Clitoria ternatea) flower petals [65];
- Other plants: wild turmeric (Curcuma aromatica) [66];
| Raw Material | Protein (% DM) | Fat (% DM) | Carbohydrates (% DM) | Fibre (% DM) | Bioactive Compounds | Health-Promoting Properties | Anti-Nutritional Factors |
|---|---|---|---|---|---|---|---|
| Adlay (Coix lacryma-jobi) | 20.0–31.7 [71] | 1.0–8.2 [72] | 56.0–75.0 [71] | 2.5–17.0 [72] | Polyphenols, flavonoids, coixol, phytosterols, saponins [71] | Hypolipidaemic, antidiabetic, antioxidant, prebiotic [71] | Phytic acid, saponins, trypsin inhibitors [73] |
| Broad beans (Vicia faba L.) | 26.0–33.0 [74] | <1.0 [74] | 45.7–70.1 [75] | 11.4–16.6 [74] | Polyphenols, flavonoids, L-DOPA, tannins, lectins [76] | Cholesterol lowering, heart support, antioxidant [77] | Phytic acid, trypsin inhibitors, saponins, vicine, convicine, lectins, tannins [74] |
| Chickpeas (Cicer arietinum) | 18.7–41.8 [78] | 2.7–6.5 cooked [79] | 60.0–65.0 [79] | 18.0–22.0 [80] | Polyphenols, sterols, carotenoids, tannins, isoflavones [80] | Cholesterol lowering, heart support, glycaemic control, antioxidant [81] | Phytates, lecithins, enzyme inhibitors, oligosaccharides [78] |
| Red algae (Porphyra sp.) | 33.7–41.8 [82] | Up to 1.2 [83] | 30.0–40.0 [84] | up to 48.0 [85] | Polysaccharides (porphyran), phycobiliproteins, polyphenols, flavonoids [84,86,87,88]. | Antioxidant [86], immune support [84], cholesterol lowering, heart support [89], antidiabetic and anti-inflammatory [84], improved iron bioavailability [90] | Phytic acid, saponins [91] |
| Eucheuma spinosum | 6.0–7.3 [92] | <0.1 [92] | 69.0–70.0 [92] | 15.0–20.0 [92] | Polyphenols, flavonoids, saponins, tannins, steroids, triterpenoids [93] | Antioxidant, antimicrobial, prebiotic, support for bone mineralisation [93] | Phytic acid, tannins [92] |
| Cowpea bean (Vigna unguiculata) | 17.5–32.5 [94] | 1.5 [95] | 62.1 [95] | 7.0–11.0 [96] | Polyphenols, flavonoids, saponins, tannins, phytosterols, L-DOPA [97] | Cholesterol lowering, glycaemic regulation, microbiome support, antioxidant [97] | Phytic acid, tannins, trypsin inhibitors [97] |
| Jack bean (Canavalia ensiformis) | 25.2 [98] | 5.2 [98] | 58.4 [98] | 7.1 [99] | Polyphenols, flavonoids, kaempferol glycosides, α-glucosidase inhibitors [100] | Antidiabetic [100], immunomodulatory [101] | Canavanine, trypsin inhibitors, lectins, oligosaccharides [99] |
| Bambara groundnut (Vigna subterranea) | 15.0–37.0 [102] | 1.3–7.4 [102] | 45.0–64.0 [102] | 3.7–6.4 [102] | Polyphenols, flavonoids, saponins, tannins, alkaloids, L-DOPA [103] | Cholesterol lowering, glycaemic regulation, satiety, antioxidant, anti-inflammatory [104] | Phytates, tannins, oxalates, trypsin inhibitors, lectins [104] |
| Mung bean (Vigna radiata) | 14.6–32.6 [105] | 1.2–1.9 [105] | 61.0–67.1 [105] | 5.5 [106] | Polyphenols, flavonoids, saponins, phenolic acids, alkaloids, bioactive peptides [107] | Cholesterol lowering, glycaemic regulation, antioxidant, anti-inflammatory, satiety [107] | Phytic acid, tannins, trypsin inhibitors, lectins [105] |
| Winged bean (Psophocarpus tetragonolobus) | 27.2–45.0 [108] | 15.2–23.4 [108] | 14.2–35.7 [108] | 1.6–26.2 [108] | Polyphenols, flavonoids, saponins, phytosterols, bioactive peptides [108] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant, anti-inflammatory [108] | Phytates, tannins, oxalates, trypsin inhibitors, haemagglutinins [109] |
| Common bean (Phaseolus vulgaris) | 20.0–27.0 [110] | 0.6–3.0 [111] | 58.0–70.0 [110] | 30.3–34.2 [112] | Polyphenols, anthocyanins, saponins, tannins, phytosterols [113] | Cholesterol lowering, heart support, glycaemic regulation, antioxidant [110] | Lectins, phytic acid, saponins, trypsin inhibitors, tannins [114] |
| Large peas (Lathyrus sativus) | 17.7–25.6 [115] 20.0–24.9 [116] 18.0–34.0 [117] | 1.7 [118] | 48.0–52.3 [118] | 1.1–1.7 [118] 4.0–7.0 [116] | Polyphenols, flavonoids, saponins, tannins, phytosterols, homoarginine, β-ODAP [118] | Antioxidant, hypolipidaemic, antidiabetic, anti-inflammatory [118] | β-ODAP, phytic acid, saponins, tannins [115,116] |
| Pearl barley (Hordeum vulgare) | 13.6 [119] | 2.8 [119] | 63.9 [119] | 4.7 [119] | β-Glucan, arabinoxylan, polyphenols, flavonoids, phytosterols [119] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant, anti-inflammatory [119]. | Phytic acid, saponins, tannins [119] |
| Rubber (Hevea brasiliensis) | 19.4–30.7 [120] 23.3 [121] 26.1 [122] 31.6 [123] | 42.5–54.2 [120] | 11.6–29.0 [120] | 5.9 [121] 43.0 [122] | Polyphenols, flavonoids [120] | Antioxidant, anti-inflammatory, metabolic, requires detoxification [31,120] | Tannins, saponins [121,123] |
| Wild turmeric (Curcuma aromatica) | 19.4 [124] | 2.5 [124] | 97.5 [124] | n.a. | Polyphenols (curcumin, demethoxycurcumin), flavonoids, terpenoids, essential oils [124,125] | Antioxidant, anti-inflammatory, anticancer, neuroprotective [124,126] | Saponins, tannins, alkaloids [124] |
| Flowers of butterfly pea (Clitoria ternatea) | 0.3 [127] | 2.5 [127] | 2.2 [127] | 2.1 [127] | Anthocyanins (300–500 mg/100 g), flavonoids (100–150 mg/100 g), phenolic acids, saponins, tannins, carotenoids, avenanthramides [127,128,129] | Potent antioxidant, anti-inflammatory, hypoglycaemic, neuroprotective, cardioprotective, anticancer, skin support [127,128] | Tannins, saponins, oxalates [127,128] |
| Lotus (Nelumbo nucifera) | 16.0–21.0 [130] | 2.4–3.0 [130] | 61.0–62.0 [130] | 2.8 [131] | Polysaccharides (porphyran), phycobiliproteins, polyphenols, flavonoids [132] | Antioxidant, immunomodulatory, prebiotic, antidiabetic [133] | Phytic acid, saponins [134,135] |
| Narrow-leaved lupin (Lupinus angustifolius) | 31.6–34.6 [136] 31.0–52.0 [137] | 6.0 [137] | <24.0 [138] | 37.5–40.2 [137] | Polyphenols, flavonoids, bioactive peptides, saponins, phytosterols [137] | Cholesterol lowering, glycaemic improvement, satiety, antioxidant, anti-inflammatory [138] | Alkaloids, phytic acid, trypsin inhibitors [137] |
| White mimosa (Leucaena leucocephala) | 26.6 [139] 31.1 [140] | 5.6 [140] 31.8 [139] | 18.6 [140] 15.3 [139] | 13.2 [140] 15.5 [139] | Polyphenols, flavonoids, saponins, tannins, phytosterols [141] | Antioxidant, hypolipidaemic, immunomodulatory [141,142] | Mimosine, tannins, saponins [141] |
| Brewer’s malt | 15.0–30.0 [143] 20.0 [144] | 3.0–13.9 [144] | n.a. | up to 80.0 [143] 70.0 [144] | Phenolic acids (ferulic, p-coumaric), flavonoids, antioxidant peptides, fibre, melanoidins, healthy fatty acids, and minerals [145,146] | Cholesterol lowering, glycaemic regulation, prebiotic, antioxidant [143] | Phytic acid, tannins, trypsin inhibitors [144] |
| Moringa oleifera (seeds) | 31.4 [147] 35.4 [148] | 36.7 [147] 43.6 [148] | 9.2 [148] 18.4 [147] | 4.7 [148] 7.3 [147] | Polyphenols, flavonoids, saponins, phytosterols, bioactive peptides [149,150] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [151] | Phytates, glucosinolates, tannins, trypsin inhibitors [150] |
| Oats (Avena sativa) | 10.0–17.2 [151] 13.7 [152] | 2.1–10.3 [151] 7.6 [152] | 47.9–74.3 [151] 62.7 [152] | 2.1–15.4 [151] 10.1 [152] | β-Glucans, avenanthramides, polyphenols, flavonoids [152] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [152] | Phytic acid, saponins, β-glucan [152] |
| Rice (Oryza sativa) | 16.8–24.1 [153] 6.0–7.8 [154] 5.5 [155] | 1.6–2.8 [154] 0.8 [155] | 82.7–84.5 [154] | 2.1–2.7 [154] | Polyphenols, phytosterols, γ-oryzanol, tocopherols [156] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [156] | Phytic acid, tannins [156] |
| Basmati rice | 7.6–9.1 [157] | 1.6–2.4 [157] 3.0–3.5 [158] | 77.4–79.4 [157] | 1.0–1.8 [157] | γ-Oryzanol, tocopherols, phytosterols [158] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [158] | Phytic acid, tannins [157] |
| Lentils (Lens culinaris) | 20.5–26.0 [159] | 0.6–1.0 [159] | 63.7–69.8 [159] | 19.3–26.4 [159] | Polyphenols, flavonoids, saponins, phenolic acids, oligosaccharides [160] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [160] | Phytic acid, tannins, trypsin inhibitors, lectins [160] |
| Soybean (Glycine max) | 39.0 [14] 40.0 [161] | 17.0–20.0 [14] 20.0 [161] | 18.0 [14] 31.1 [5] | 5.1 [5] | Isoflavones, saponins, phytosterols, lecithins [161] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [161] | Phytates, trypsin inhibitors, saponins, lectins [161] |
| Sorghum (Sorghum bicolor) | 8.0–12.0 [160] | 1.5–3.5 [160] | 65.0–75.0 [160] | 6.0–10.0 [160] | Polyphenols, tannins, phytosterols, resistant starch [160] | Cholesterol lowering, glycaemic regulation, immune support, antioxidant [160] | Tannins, phytic acid, trypsin inhibitors [160] |
| Tarwi (Lupinus mutabilis) | 44.7 [162] | 15.4 [162] | n.a. | n.a. | Polyphenols, flavonoids, isoflavones, saponins [163] | Cholesterol lowering, glycaemic improvement, satiety, antioxidant, anti-inflammatory [163] | Quinolizidine alkaloids, phytic acid [163] |
| Linseed pomace | 21.3 [164] | 43.9 [164] | n.a. | 6.2 [164] | Lignans, polyphenols, flavonoids, plant mucilages [164] | Cholesterol lowering, glycaemic regulation, prebiotic, antioxidant [50] | Cyanogenic glycosides, phytic acid [50] |
| Rapeseed pomace | 38.1 [165] | 33.5 [165] | n.a. | 15.3 [165] | Polyphenols (mainly ferulic acid, hydroxycinnamic acids), flavonoids, glucosinolates, phytates, isothiocyanates, sinapic acid, tannins, and saponins [68] | Antioxidant and anti-inflammatory, support of lipid and carbohydrate metabolism, promotion of favourable gut microbiota [68] | Glucosinolates, phytates [68] |
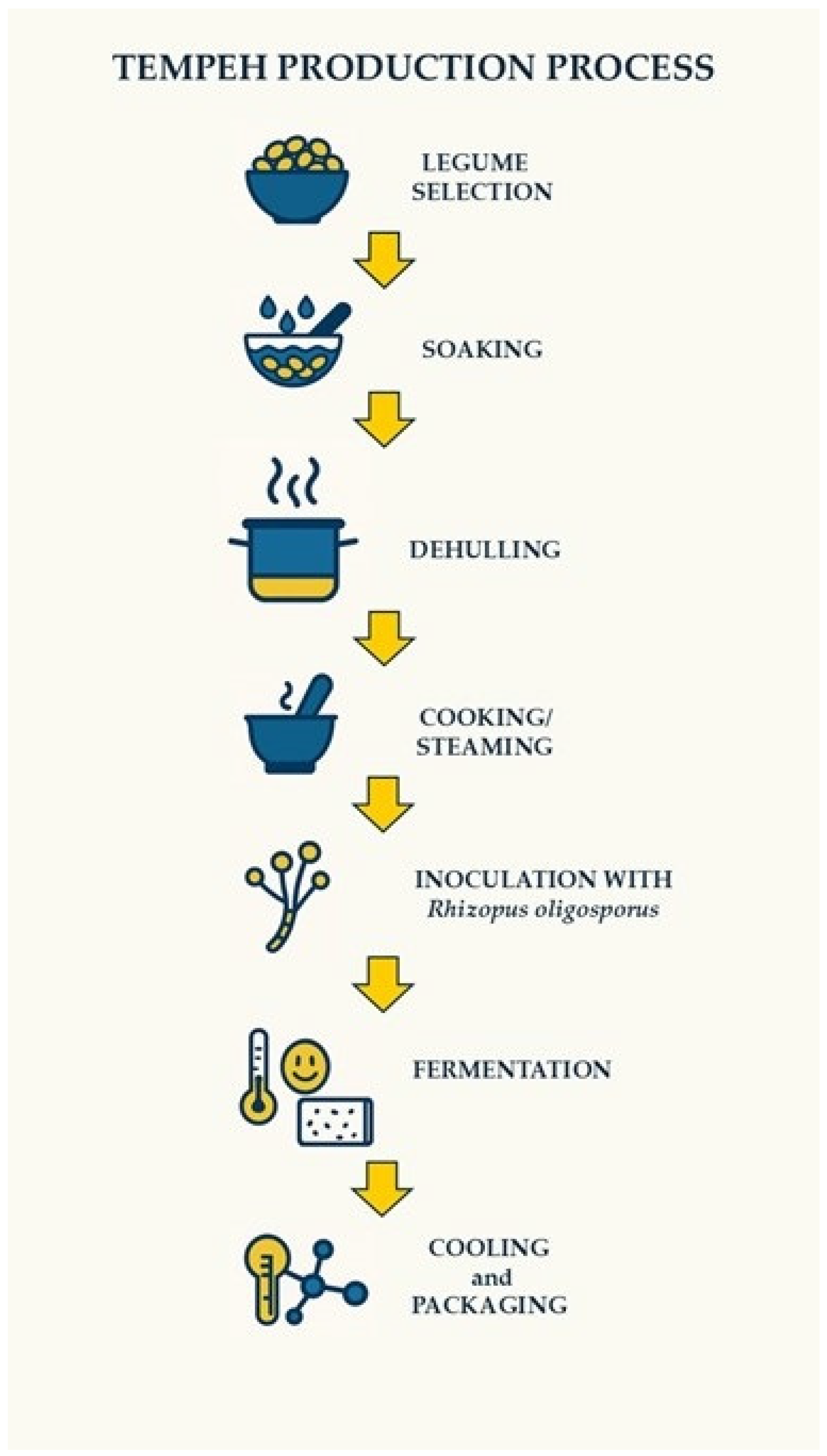
4. Technology for the Production of Tempeh
5. Chemical Composition of Tempeh
6. Content of Polyphenols, Isoflavones, and Antioxidant Activity
Reduction of Anti-Nutritional Factors Through Fermentation
7. Conclusions
8. Remarks on Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Kaur, S.; Das, M. Functional Foods: An Overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Kuligowski, M.; Pawłowska, K.; Jasińska-Kuligowska, I.; Nowak, J. Isoflavone composition, polyphenols content and antioxidative activity of soybean seeds during tempeh fermentation. CyTA J. Food 2017, 15, 27–33. [Google Scholar] [CrossRef]
- Martineau-Côté, D.; Achouri, A.; Karboune, S.; L’Hocine, L. Faba Bean: An Untapped Source of Quality Plant Proteins and Bioactives. Nutrients 2022, 14, 1541. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G. Soy-Based Tempeh as a Functional Food: Evidence for Human Health and Future Perspective. Front. Biosci. 2024, 16, 3. [Google Scholar] [CrossRef]
- Toor, B.S.; Kaur, A.; Kaur, J. Fermentation of legumes with Rhizopus oligosporus: Effect on physicochemical, functional and microstructural properties. Int. J. Food Sci. Technol. 2022, 57, 1763–1772. [Google Scholar] [CrossRef]
- Tsalissavrina, I.; Murdiati, A.; Raharjo, S.; Lestari, L.A. The Effects of Duration of Fermentation on Total Phenolic Content, Antioxidant Activity, and Isoflavones of The Germinated Jack Bean Tempeh (Canavalia ensiformis). Indones. J. Pharm. 2023, 34, 460–470. [Google Scholar] [CrossRef]
- Barus, T.; Titarsole, N.N.; Mulyono, N.; Prasasty, V.D. Tempeh Antioxidant Activity Using DPPH Method: Effects of Fermentation, Processing, and Microorganisms. J. Food Eng. Technol. 2019, 8, 75–80. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. A Super Soyfood from Indonesia. In The Book of Tempeh; Soyinfo Center: Lafayette, CA, USA, 2001. [Google Scholar]
- Chang, C.-T.; Hsu, C.-K.; Chou, S.-T.; Chen, Y.-C.; Huang, F.-S.; Chung, Y.-C. Effect of fermentation time on the antioxidant activities of tempeh prepared from fermented soybean using Rhizopus oligosporus. Int. J. Food Sci. Technol. 2009, 44, 799–806. [Google Scholar] [CrossRef]
- Omosebi, M.O.; Otunola, E.T. Preliminary studies on tempeh flour produced from three different Rhizopus species. Int. J. Biotechnol. Food Sci. 2013, 1, 90–96. [Google Scholar]
- Chong, B.C.W.; Panizzi, M.C.C.; Mandarino, J.M.G.; Silva, J.B.D.; Benedetti, S.; Ida, E.I. Contents and bioconversion of β glycoside isoflavones to aglycones in the processing conditions of soybean tempeh. Pesqui. Agropecu. Bras. 2016, 51, 271–279. [Google Scholar] [CrossRef]
- Hidayat, S.N.; Nuringtyas, T.R.; Triyana, K. Electronic Nose Coupled with Chemometrics for Monitoring of Tempeh Fermentation Process. In Proceedings of the 2018 4th International Conference on Science and Technology, ICST 2018, Yogyakarta, Indonesia, 7–8 August 2018; Institute of Electrical and Electronics Engineers: New York, NY, USA, 2018. [Google Scholar] [CrossRef]
- Tahir, A.; Anwar, M.; Mubeen, H.; Raza, S. Evaluation of Physicochemical and Nutritional Contents in Soybean Fermented Food Tempeh by Rhizopus oligosporus. J. Adv. Biol. Biotechnol. 2018, 17, 1–9. [Google Scholar] [CrossRef]
- Erkan, S.B.; Gürler, H.N.; Bilgin, D.G.; Germec, M.; Turhan, I. Production and characterization of tempehs from different sources of legume by Rhizopus oligosporus. LWT 2020, 119, 108880. [Google Scholar] [CrossRef]
- Pramudito, T.E.; Putri, E.G.A.; Paluphi, E.; Yogiara, Y. The effect of starter culture on bacterial profile in soybean tempeh. Food Res. 2021, 5, 380–389. [Google Scholar] [CrossRef]
- Rizal, S.; Kustyawati, M.E.; Murhadi; Hasanudin, U. The growth of yeast and fungi, the formation of β-glucan, and the antibacterial activities during soybean fermentation in producing tempeh. Int. J. Food Sci. 2021, 2021, 6676042. [Google Scholar] [CrossRef] [PubMed]
- Yudiono, K.; Ayu, W.C.; Susilowati, S. Antioxidant activity, total phenolic, and aflatoxin contamination in tempeh made from assorted soybeans (Glycine max L. Merrill). Food Res. 2021, 5, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Bintari, S.H.; Purnama, D.F.E.; Saputro, D.D.; Sunyoto, S.; Dewi, P.; Mubarok, I. Microbiological and Biochemical Tests on Tempe Production Using Tempe Mold Innovation. Biosaintifika 2022, 14, 245–253. [Google Scholar] [CrossRef]
- Chitisankul, W.T.; Shimada, K.; Tsukamoto, C. Antioxidative Capacity of Soyfoods and Soy Active Compounds. Pol. J. Food Nutr. Sci. 2022, 72, 101–108. [Google Scholar] [CrossRef]
- Dwiatmaka, Y.; Yuniarti, N.; Lukitaningsih, E.; Wahyuono, S. Fermentation of Soybean Seeds Using Rhizopus oligosporus for Tempeh Production and Standardization Based on Isoflavones Content. Int. J. Appl. Pharm. 2022, 14, 131–136. [Google Scholar] [CrossRef]
- Lo, D.; Romulo, A.; Lin, J.Y.; Wang, Y.T.; Wijaya, C.H.; Wu, M.C. Effect of Different Fermentation Conditions on Antioxidant Capacity and Isoflavones Content of Soy Tempeh. AIMS Agric. Food 2022, 7, 567–579. [Google Scholar] [CrossRef]
- Maitresya, L.B.; Surya, R. Development of Tempeh Made from Soybeans, Black-Eyed Beans, and Winged Beans. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: Bristol, UK, 2023; Volume 1200. [Google Scholar] [CrossRef]
- Rizal, S.; Kustyawati, M.E.; Suharyono, A.S.; Suyarto, V.A. Changes of Nutritional Composition of Tempeh during Fermentation with the Addition of Saccharomyces Cerevisiae. Biodiversitas 2022, 23, 1553–1559. [Google Scholar] [CrossRef]
- Yodsenee, K.; Suthirawut, S.; Pilasombut, K.; Urairong, H.; Rumjuankiat, K. In Vitro Study of Antioxidant and Antimicrobial Activities of Soybean Tempeh and Split Gill Fungus (Schizophyllum Commune) as Plant-Based Diets. Int. J. Agric. Technol. 2022, 18, 1339–1354. [Google Scholar]
- Herawati, H.; Kamsiati, E.; Afifah, D.N.; Kusumaningtyas, E.; Bachtiar, M.; Sunarmani; Agustinisari, I. Characteristics of GABA (Gamma Amino Butyric Acid), Antioxidant and Sensory Quality of Modified Tempeh. Int. J. Food Prop. 2023, 26, 3532–3543. [Google Scholar] [CrossRef]
- Liu, W.T.; Huang, C.L.; Liu, R.; Yang, T.C.; Lee, C.L.; Tsao, R.; Yang, W.J. Changes in Isoflavone Profile, Antioxidant Activity, and Phenolic Contents in Taiwanese and Canadian Soybeans during Tempeh Processing. LWT 2023, 186, 115207. [Google Scholar] [CrossRef]
- Syahputra, T.S.; Ihsan, N.; Kombo, K.O.; Faizah, K.; Wahyono; Widada, J.; Triyana, K. Integration of Low-Cost Multispectral Sensors and Electronic Nose for Enhanced Fermentation Monitoring in Tempeh Production. J. Food Meas. Charact. 2025, 19, 3687–3701. [Google Scholar] [CrossRef]
- Polanowska, K.; Szwengiel, A.; Kuligowski, M.; Nowak, J. Degradation of Pyrimidine Glycosides and L-DOPA in the Faba Bean by Rhizopus oligosporus. LWT 2020, 127, 109353. [Google Scholar] [CrossRef]
- Fernandez Castaneda, L.A.; Auer, J.; Leong, S.L.L.; Newson, W.R.; Passoth, V.; Langton, M.; Zamaratskaia, G. Optimizing Soaking and Boiling Time in the Development of Tempeh-Like Products from Faba Bean (Vicia faba L.). Fermentation 2024, 10, 807. [Google Scholar] [CrossRef]
- Fernandez Castaneda, L.A.; Saini, S.; Laaksonen, O.; Kårlund, A.; Leong, S.L.L.; Newson, W.R.; Zamaratskaia, G. Sensory and volatile compound profiles in tempeh-like products from faba bean and oats. Curr. Res. Food Sci. 2025, 10, 101029. [Google Scholar] [CrossRef]
- Thulesen, L.; Duque-Estrada, P.; Zhang, L.; Martin, M.S.; Aaslyng, M.D.; Petersen, I.L. Faba bean tempeh: The effects of fermentation and cooking on protein nutritional quality and sensory quality. Food Chem. Adv. 2025, 6, 100894. [Google Scholar] [CrossRef]
- Tan, Z.J.; Abu Bakar, M.F.; Lim, S.Y.; Sutimin, H. Nutritional composition and sensory evaluation of tempeh from different combinations of beans. Food Res. 2024, 8, 138–146. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, J.; Kim, S.H.; Moon, K.D. Development of chickpea tempeh using Rhizopus oryzae for dysphagia diet: Effect of fermentation time and heat treatment. Innov. Food Sci. Emerg. Technol. 2025, 100, 103940. [Google Scholar] [CrossRef]
- Andriati, N.; Anggrahini, S.; Setyaningsih, W.; Sofiana, I.; Pusparasi, D.A.; Mossberg, F. Physicochemical characterization of jack bean (Canavalia ensiformis) tempeh. Food Res. 2018, 2, 481–485. [Google Scholar] [CrossRef]
- Purwandari, F.A.; Fogliano, V.; Capuano, E. Tempeh fermentation improves the nutritional and functional characteristics of Jack beans (Canavalia ensiformis (L.) DC). Food Funct. 2024, 15, 3680–3691. [Google Scholar] [CrossRef]
- Rizal, S.; Kustyawati, M.E.; Sartika, D.; Sasriany, R.; Hidayat, R.; Suyantohadi, A. Innovation of tempeh from jack bean (Canavalia ensiformis) fermented with Mosaccha inoculum. Food Biosci. 2024, 62, 105564. [Google Scholar] [CrossRef]
- Yarlina, V.P.; Djali, M.; Andoyo, R.; Lani, M.N.; Rifqi, M. Effect of Soaking and Proteolytic Microorganisms Growth on the Protein and Amino Acid Content of Jack Bean Tempeh (Canavalia ensiformis). Processes 2023, 11, 1161. [Google Scholar] [CrossRef]
- Yarlina, V.P.; Nabilah, F.; Zaida; Nurhasanah, S.; Lani, M.N. Optimal Fermentation Time for Jack Bean (Canavalia ensiformis) Tempeh: A Comprehensive Pattern Analysis of Chemical and Enzyme Changes. Curr. Res. Nutr. Food Sci. 2024, 12, 1143–1153. [Google Scholar] [CrossRef]
- Agustia, F.C.; Winarsi, H.; Fitriani, A.; Latifasari, N. Impact of Packaging Variations on the Amino Acid Profile, Proximate Content, and Antinutritional Components of Tempeh from Jack Bean Sprouts. Prev. Nutr. Food Sci. 2025, 30, 56–67. [Google Scholar] [CrossRef]
- Amadi, E.N.; Uneze, R.; Barimalaa, I.S.; Achinewhu, S.C. Studies on the production of bambara groundnut (Vigna subterranea) tempe. Plant Foods Hum. Nutr. 1999, 53, 199–208. [Google Scholar] [CrossRef]
- Lakshmy, P.S.; Usha, V.; Sharon, C.L.; Aneena, E.R. Rice and Green Gram Based ‘Tempeh’—Development and In Vitro Mineral Bioavailability. J. Trop. Agric. 2015, 53, 166–172. [Google Scholar]
- Reyes-Bastidas, M.; Reyes-Fernández, E.Z.; López-Cervantes, J.; Milán-Carrillo, J.; Loarca-Piña, G.F.; Reyes-Moreno, C. Physicochemical, Nutritional and Antioxidant Properties of Tempeh Flour from Common Bean (Phaseolus vulgaris L.). Food Sci. Technol. Int. 2010, 16, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, M.; Gorzan, A.; Jasińska-Kuligowska, I.; Nowak, J. Ocena Wpływu Obróbki Hydrotermicznej i Czasu Fermentacji na Zawartość Polifenoli i Właściwości Antyoksydacyjne Produktów Tempeh. Zesz. Probl. Post. Nauk. Roln. 2014, 577, 73–82. [Google Scholar]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of Controlled Lactic Acid Fermentation on Selected Bioactive and Nutritional Parameters of Tempeh Obtained from Unhulled Common Bean (Phaseolus vulgaris) Seeds. J. Sci. Food Agric. 2014, 94, 359–366. [Google Scholar] [CrossRef]
- Vital, R.J.; Bassinello, P.Z.; Cruz, Q.A.; Carvalho, R.N.; De Paiva, J.C.M.; Colombo, A.O. Production, Quality, and Acceptance of Tempeh and White Bean Tempeh Burgers. Foods 2018, 7, 136. [Google Scholar] [CrossRef]
- Jaijaroensakundee, C.; Shen, J.; Zhou, Z.; Xiao, H. Exploring Black Bean Tempeh: A Novel Addition to Plant-Based Nutrition. Curr. Dev. Nutr. 2025, 9, 106688. [Google Scholar] [CrossRef]
- Stodolak, B.; Starzyńska-Janiszewska, A.; Mickowska, B. Effect of Flaxseed Oil-Cake Addition on the Nutritional Value of Grass Pea Tempeh. Food Sci. Technol. Res. 2013, 19, 1107–1114. [Google Scholar] [CrossRef][Green Version]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Wikiera, A. Proteolysis in Tempeh-Type Products Obtained with Rhizopus and Aspergillus Strains from Grass Pea (Lathyrus sativus) Seeds. Acta Sci. Pol. Technol. Aliment. 2015, 14, 125–132. [Google Scholar] [CrossRef]
- Stodolak, B.; Starzyńska-Janiszewska, A.; Wikiera, A. Wpływ Dodatku Wytłoków Lnianych na Potencjał Antyoksydacyjny Tempe z Nasion Lędźwianu. Żywność Nauka Technol. Jakość/Food Sci. Technol. Qual. 2015, 22, 96–105. [Google Scholar] [CrossRef]
- Signorini, C.; Carpen, A.; Coletto, L.; Borgonovo, G.; Galanti, E.; Capraro, J.; Scarafoni, A. Enhanced vitamin B12 production in an innovative lupin tempeh is due to synergic effects of Rhizopus and Propionibacterium in cofermentation. Int. J. Food Sci. Nutr. 2018, 69, 451–457. [Google Scholar] [CrossRef]
- Wolkers-Rooijackers, J.C.M.; Endika, M.F.; Smid, E.J. Enhancing vitamin B12 in lupin tempeh by in situ fortification. LWT 2018, 96, 513–518. [Google Scholar] [CrossRef]
- Parra-Gallardo, G.; Quimbiulco-Sánchez, K.; Salas-Sanjuán, M.d.C.; del Moral, F.; Valenzuela, J.L. Alternative Development and Processing of Fermented Beverage and Tempeh Using Green Beans from Four Genotypes of Lupinus mutabilis. Fermentation 2023, 9, 590. [Google Scholar] [CrossRef]
- Suryanti, V.; Marliyana, S.D.; Rohana, G.L.; Trisnawati, E.W.; Widiyanti, W. Bioactive compound contents and antioxidant activity of fermented lead tree (Leucaena leucocephala (Lmk.) de Wit) seeds. Molekul 2021, 16, 192–199. [Google Scholar] [CrossRef]
- Cai, S.; Gao, F.; Zhang, X.; Wang, O.; Wu, W.; Zhu, S.; Ji, B. Evaluation of γ-aminobutyric acid, phytate and antioxidant activity of tempeh-like fermented oats (Avena sativa L.) prepared with different filamentous fungi. J. Food Sci. Technol. 2014, 51, 2544–2551. [Google Scholar] [CrossRef]
- Zwinkels, J.; Wolkers-Rooijackers, J.; Smid, E.J. Solid-state fungal fermentation transforms low-quality plant-based foods into products with improved protein quality. LWT 2023, 184, 114979. [Google Scholar] [CrossRef]
- Ridhowati, S.; Lestari, S.D.; Wulandari, W.; Rinto, R. Lotus (Nelumbo nucifera) tempeh Indonesia as antioxidant and breast anticancer food-a preliminary study. Asian J. Plant Sci. 2020, 19, 406–411. [Google Scholar] [CrossRef]
- Ridhowati, S.; Nainggolan, K.; Sudirman, S. Optimalisasi Respons Surface Terhadap Profil Asam Tempe Lotus (Nelumbo nucifera) Rawa Perikanan. J. FishtecH 2023, 11, 107–115. [Google Scholar] [CrossRef]
- Ridhowati, S.; Herpandi, H.; Widiastuti, I. Profiles of volatile metabolite compounds of lotus tempeh: In-RSM-Boxbehken approach. Int. J. Second. Metab. 2025, 12, 355–367. [Google Scholar] [CrossRef]
- Nurhaeni; Darwis, D.; Satrimafitrah, P. Fermented Moringa oleifera seeds enhanced with Euchema cottonii as an alternative tempeh: Organoleptic analysis, protein, and fiber content. Rasayan J. Chem. 2021, 14, 155–160. [Google Scholar] [CrossRef]
- Pantaya, D.; Wulandari, S.; Yulinarsari, A.P.; Poernomo, H. Evaluation of Rubber Seed Meal (Hevea brasiliensis) by Fermentation Method Using Rhizopus oligosporus and Neurospora sitophila Fungi. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: Bristol, UK, 2023; Volume 1168. [Google Scholar] [CrossRef]
- Surya, R.; Megumi, E.H.; Rombot, O.; Nugroho, D.; Tedjakusuma, F. Supplementation of Red Alga (Porphyra) Improves Nutritional Profile, Protein Digestibility and Sensory Acceptance of Tempeh. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: Bristol, UK, 2024; Volume 1413. [Google Scholar] [CrossRef]
- Surya, R.; Tedjakusuma, F.; Rombot, O.; Nugroho, D. Supplementation of Dried Porphyra Alga (Nori) Improves Antioxidant Activity and Isoflavone Bioavailability of Tempeh. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: Bristol, UK, 2025; Volume 1445. [Google Scholar] [CrossRef]
- Dewi, E.N.; Susanto, E.; Purnamayati, L. Application of Eucheuma spinosum for Enhancing the Nutritional Value of Tempeh. Pertanika J. Trop. Agric. Sci. 2025, 48, 543–560. [Google Scholar] [CrossRef]
- Evangelista, P.; Surya, R. Nutritional Profile, Antioxidant Activities and Organoleptic Properties of Tempeh Fermented with Additional Butterfly Pea Flower Petals. In IOP Conference Series: Earth and Environmental Science; Institute of Physics: Bristol, UK, 2024; Volume 1324. [Google Scholar] [CrossRef]
- Lim, J.; Nguyen, T.T.H.; Pal, K.; Gil Kang, C.; Park, C.; Kim, S.W.; Kim, D. Phytochemical Properties and Functional Characteristics of Wild Turmeric (Curcuma aromatica) Fermented with Rhizopus oligosporus. Food Chem. X 2022, 13, 100198. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Camacho, C.E.; Mollea, C.; Mazzarino, I.; Ruggeri, B.; Bosco, F. Repurposing Tempeh Fermentation: A Promising Protein Source Using Food Residues and Edible Filamentous Fungi. Chem. Eng. Trans. 2022, 93, 37–42. [Google Scholar] [CrossRef]
- Lücke, F.K.; Fritz, V.; Tannhäuser, K.; Arya, A. Controlled Fermentation of Rapeseed Presscake by Rhizopus, and Its Effect on Some Components with Relevance to Human Nutrition. Food Res. Int. 2019, 120, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Gimbun, J.; Ali, S.; Kanwal, C.C.S.C.; Shah, L.A.; Hidayah, N.; Cheng, C.K.; Nurdin, S. Biodiesel production from rubber seed oil using activated cement clinker as catalyst. Procedia Eng. 2013, 53, 13–19. [Google Scholar] [CrossRef]
- Ahmad, J.; Yusup, S.; Bokhari, A.; Kamil, R.N.M. Study of fuel properties of rubber seed oil based biodiesel. Energy Convers. Manag. 2014, 78, 266–275. [Google Scholar] [CrossRef]
- Weng, W.F.; Peng, Y.; Pan, X.; Yan, J.; Li, X.D.; Liao, Z.Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; et al. Adlay, an ancient functional plant with nutritional quality, improves human health. Front. Nutr. 2022, 9, 1019375. [Google Scholar] [CrossRef]
- Ahmad, R.; Liaquat, M.; Sammi, S.; Al-Hawadi, J.S.; Jahangir, M.; Mumtaz, A.; Khan, I.; Okla, M.K.; Alaraidh, I.A.; AbdElgawad, H.; et al. Physicochemical and nutritional profiles of wild adlay (Coix lacryma-jobi Linn) accessions by GC, FTIR, and spectrophotometer. Food Chem. X 2024, 22, 101418. [Google Scholar] [CrossRef]
- Seyie, Z.; Saikia, K.; Saikia, C.K.; Handique, G.K.; Handique, A.K. Evaluation of Underutilized Cereal Crop Coix lacryma jobi (Job’s Tear) for Nutritive and Nutraceutical Values. Int. J. Agric. Environ. Sci. 2018, 5, 17–24. [Google Scholar]
- Labba, I.-C.M.; Frøkiær, H.; Sandberg, A.-S. Nutritional and antinutritional composition of fava bean (Vicia faba L., var. minor) cultivars. Food Res. Int. 2021, 140, 110038. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, Q.; Zhang, Y.; Chen, J.; Sun, Z.; Ren, C.; Zhang, Z.; Cheng, X.; Huang, Y. Nutritive value of faba bean (Vicia faba L.) as a feedstuff resource in livestock nutrition: A review. Food Sci. Nutr. 2021, 9, 5244–5262. [Google Scholar] [CrossRef]
- Yehmed, J.; Tlahig, S.; Mohamed, A.; Yahia, H.; Lachiheb, B.; Ben Yahia, L.; Loumerem, M. Nutritional and Phytochemical Profiling of Vicia faba L. var. Minor Seeds: A Multifaceted Exploration of Natural Antioxidants and Functional Food Potential. Appl. Biochem. Biotechnol. 2024, 196, 8471–8492. [Google Scholar] [CrossRef]
- Baranowska, A. Health-promoting properties of broad beans (Vicia faba L.) Właściwości Prozdrowotne Bobu (Vicia faba L.). Health Prob. Civ. 2024, 18, 481–490. [Google Scholar]
- Kumar, N.; Hong, S.; Zhu, Y.; Garay, A.; Yang, J.; Henderson, D.; Zhang, X.; Xu, Y.; Li, Y. Comprehensive review of chickpea (Cicer arietinum): Nutritional significance, health benefits, techno-functionalities, and food applications. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70152. [Google Scholar] [CrossRef]
- Kaur, R.; Prasad, K. Technological, Processing and Nutritional Aspects of Chickpea (Cicer arietinum)—A Review. Trends Food Sci. Technol. 2021, 109, 448–463. [Google Scholar] [CrossRef]
- Budryn, G.; Grzelczyk, J. Assessment of the Nutritional Value and Antioxidant Properties of Plant-Based Yogurt from Chickpeas. Appl. Sci. 2024, 14, 9228. [Google Scholar] [CrossRef]
- Akram, S.; Afzal, M.F.; Anwer, K.; Farman, L.; Zubair, M.; Kousar, S.; Iqbal, T.; Khalid, W.; Elawad, M.A.; Ahmed, A.; et al. Nutraceutical Properties, Biological Activities, and Industrial Applications of Chickpea Protein. Cogent Food Agric. 2024, 10, 2338653. [Google Scholar] [CrossRef]
- Guan, B.; Sun, Y.; Liu, X.; Zhong, C.; Li, D.; Shan, X.; Hui, X.; Lu, C.; Huo, Y.; Sun, R.; et al. Comparative Evaluation of Amino Acid Profiles, Fatty Acid Compositions, and Nutritional Value of Two Varieties of Head Water Porphyra yezoensis: “Jianghaida No. 1” and “Sutong No.1”. Food Chem. X 2024, 22, 100825. [Google Scholar] [CrossRef] [PubMed]
- Arakaki, N.; Flores Ramos, L.; Oscanoa Huaynate, A.I.; Ruíz Soto, A.; Ramírez, M.E. Biochemical and Nutritional Characterization of Edible Seaweeds from the Peruvian Coast. Plants 2023, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Jiang, H.; Fu, L.; Ci, F.; Mao, X. Porphyran and Oligo-Porphyran Originating from Red Algae Porphyra: Preparation, Biological Activities, and Potential Applications. Food Chem. 2021, 349, 129188. [Google Scholar] [CrossRef]
- Cian, R.E.; Fajardo, M.A.; Alaiz, M.; Vioque, J.; González, R.J.; Drago, S.R. Chemical Composition, Nutritional and Antioxidant Properties of the Red Edible Seaweed Porphyra columbina. Int. J. Food Sci. Nutr. 2014, 65, 299–305. [Google Scholar] [CrossRef]
- Venkatraman, K.L.; Mehta, A. Health Benefits and Pharmacological Effects of Porphyra Species. Plant Foods Hum. Nutr. 2019, 74, 10–17. [Google Scholar] [CrossRef]
- Park, S.K.; Kang, J.Y.; Kim, J.M.; Kim, M.J.; Lee, H.L.; Moon, J.H.; Jeong, H.R.; Kim, H.-J.; Chung, M.-Y.; Heo, H.J. Porphyra tenera Protects against PM2.5-Induced Cognitive Dysfunction with the Regulation of Gut Function. Mar. Drugs 2022, 20, 439. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Nieto, G. Bioaccessibility, Digestibility and Nutritional Properties of Algae and Cyanophyceae as Basis of Their Potential as Functional Food Ingredients. Appl. Food Res. 2024, 4, 100247. [Google Scholar] [CrossRef]
- Aziz, E.; Batool, R.; Khan, M.U.; Rauf, A.; Akhtar, W.; Heydari, M.; Rehman, S.; Shahzad, T.; Malik, A.; Mosavat, S.H.; et al. An Overview on Red Algae Bioactive Compounds and Their Pharmaceutical Applications. J. Complement. Integr. Med. 2020, 17, 1–21. [Google Scholar] [CrossRef]
- García-Casal, M.N.; Ramírez, J.; Leets, I.; Pereira, A.C.; Quiroga, M.F. Antioxidant Capacity, Polyphenol Content and Iron Bioavailability from Algae (Ulva sp., Sargassum sp. and Porphyra sp.) in Human Subjects. Br. J. Nutr. 2009, 101, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Pise, N.M.; Sabale, A.B. Biochemical Composition of Seaweeds along Central West Coast of India. Pharmacogn. J. 2010, 2, 148–150. [Google Scholar] [CrossRef]
- Diharmi, A.; Fardiaz, D.; Andarwulan, N. Chemical and Minerals Composition of Dried Seaweed Eucheuma spinosum Collected from Indonesia Coastal Sea Regions. Int. J. Oceans Oceanogr. 2019, 13, 65–71. [Google Scholar]
- Damongilala, L.J.; Wewengkang, D.S.; Losung, F.; Tallei, T.E. Phytochemical and Antioxidant Activities of Eucheuma spinosum as Natural Functional Food from North Sulawesi Waters, Indonesia. Pak. J. Biol. Sci. 2021, 24, 132–138. [Google Scholar] [CrossRef]
- Boukar, O.; Massawe, F.; Muranaka, S.; Franco, J.; Maziya-Dixon, B.; Singh, B.; Fatokun, C. Evaluation of Cowpea Germplasm Lines for Protein and Mineral Concentrations in Grains. Plant Genet. Resour. 2011, 9, 515–522. [Google Scholar] [CrossRef]
- Amadou, I.; Sankhon, A.; Souley, R.A.; Harou, L.I. Nutritional Physical and Functional Properties of Wild Cowpea (Vigna vexillata (L.)) Grown in the Sahel Region. Am. J. Food Nutr. 2024, 12, 107–113. [Google Scholar] [CrossRef]
- Bai, Z.; Huang, X.; Meng, J.; Kan, L.; Nie, S. A Comparative Study on Nutritive Peculiarities of 24 Chinese Cowpea Cultivars. Food Chem. Toxicol. 2020, 146, 111841. [Google Scholar] [CrossRef]
- Sardar, H.; Hadi, F.; Alam, W.; Halawani, I.F.; Alzahrani, F.M.; Saleem, R.A.; Cerqua, I.; Khan, H.; Capasso, R. Unveiling the Therapeutic and Nutritious Potential of Vigna unguiculata in Line with Its Phytochemistry. Heliyon 2024, 10, e37911. [Google Scholar] [CrossRef]
- Okomoda, V.T.; Tiamiyu, L.O.; Uma, S.G. Effects of Hydrothermal Processing on Nutritional Value of Canavalia ensiformis and Its Utilization by Clarias gariepinus (Burchell, 1822) Fingerlings. Aquac. Rep. 2016, 3, 214–219. [Google Scholar] [CrossRef]
- Solomon, S.G.; Okomoda, V.T.; Oguche, O. Nutritional Value of Raw Canavalia ensiformis and Its Utilization as Partial Replacement for Soybean Meal in the Diet of Clarias gariepinus (Burchell, 1822) Fingerlings. Food Sci. Nutr. 2017, 6, 207–213. [Google Scholar] [CrossRef]
- Sutedja, A.M.; Yanase, E.; Batubara, I.; Fardiaz, D.; Lioe, H.N. Identification and Characterization of α-Glucosidase Inhibition Flavonol Glycosides from Jack Bean (Canavalia ensiformis (L.) DC). Molecules 2020, 25, 2481. [Google Scholar] [CrossRef]
- Madani, A.M.A.; Muhlisin, M.; Kurniawati, A.; Baskara, A.P.; Al Anas, M. Dietary jack bean (Canavalia ensiformis L.) supplementation enhanced intestinal health by modulating intestinal integrity and immune responses of broiler chickens. Heliyon 2024, 10, e034389. [Google Scholar] [CrossRef] [PubMed]
- Musah, M.; Azeh, Y.; Mathew, J.T.; Nwakife, C.N.; Mohammed, A.I.; Saidu, F. Nutritional Evaluation of Bambara Groundnut (Vigna subterranea (L.) Verdc) From Lapai, Nigeria. Afr. J. Agric. Food Sci. 2021, 4, 32–39. [Google Scholar] [CrossRef]
- Sarki, A.A.; Shoge, M.O.; Tamasi, A.A.; Ozioko, E.N.; Aliyu, M.O.; Adegboyega, T.T. Antimicrobial and Phytochemical Evaluation of Vigna subterranean (L.) Verdc. (Bambara groundnut). J. Underutil. Legum. 2021, 7, 22–28. [Google Scholar]
- Okafor, J.N.C.; Jideani, V.A.; Meyer, M.; Le Roes-Hill, M. Bioactive Components in Bambara Groundnut (Vigna subterranea (L.) Verdc) A Potential. Source Nutraceutical Ingredients. Heliyon 2022, 8, e09024. [Google Scholar] [CrossRef] [PubMed]
- Shahrajabian, M.H.; Sun, W.; Cheng, Q. A Short Review of Health Benefits and Nutritional Values of Mung Bean in Sustainable Agriculture. Pol. J. Agron. 2019, 37, 31–36. [Google Scholar]
- Anyiam, P.N.; Phongthai, S.; Sai-Ut, S.; Kingwascharapong, P.; Jung, Y.H.; Zhang, W.; Rawdkuen, S. Nutritional Components and Digestibility Profiles of Some Potential Plant-Based Protein Sources. Foods 2025, 14, 1769. [Google Scholar] [CrossRef]
- Karami, Z.; Changsiripun, C.; Duangmal, K.; Chotechuang, N. Health Benefits and Challenges of Mung Bean Bioactive Compounds: A Systematic Review of In Vivo Evidence for Functional Food Applications. Food Rev. Int. 2025, 41, 1681–1708. [Google Scholar] [CrossRef]
- Bepary, R.H.; Roy, A.; Pathak, K.; Deka, S.C. Biochemical Composition, Bioactivity, Processing, and Food Applications of Winged Bean (Psophocarpus tetragonolobus): A Review. Legum. Sci. 2023, 5, e187. [Google Scholar] [CrossRef]
- Alalade, J.A.; Akinlade, J.A.; Aderinola, O.A.; Fajemisin, A.N.; Muraina, T.O.; Amoo, T.A. Proximate, Mineral and Anti-Nutrient Contents in Psophocarpus tetragonolobus (L) DC. (Winged Bean) Leaves. Br. J. Pharm. Res. 2016, 10, 1–7. [Google Scholar] [CrossRef]
- Singh, A.; Gupta, A.; Sharma, S. Handbook of Cereals, Pulses, Roots, and Tubers; CRC Press: Boca Raton, FL, USA, 2021. [Google Scholar]
- Shimelis, E.A.; Rakshit, S.K. Proximate composition and physico-chemical properties of improved dry bean (Phaseolus vulgaris L.) varieties grown in Ethiopia. LWT 2005, 38, 331–338. [Google Scholar] [CrossRef]
- Brigide, P.; Canniatti-Brazaca, S.G.; Silva, M.O. Nutritional Characteristics of Biofortified Common Beans. Food Sci. Technol. Camp. 2014, 34, 493–500. [Google Scholar] [CrossRef]
- Mani, N.; Beatrice, A.D.; Priyadarshini, D.R. Phytochemical Analysis, In vitro Anti-oxidant, Anti-diabetic and Anti-inflammatory Activity of Red Kidney Bean (Phaseolus vulgaris L.). Int. J. Health Allied Sci. 2024, 13, 76–90. [Google Scholar] [CrossRef]
- Alvarado-Ramos, K.; Bravo-Nunez, Á.; Halimi, C.; Maillot, M.; Icard-Vernière, C.; Forti, C.; Preite, C.; Ferrari, L.; Sala, T.; Losa, A.; et al. Improving the Antinutritional Profiles of Common Beans (Phaseolus vulgaris L.) Moderately Impacts Carotenoid Bioaccessibility But Not Mineral Solubility. Sci. Rep. 2024, 14, 11908. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Nutritional characteristics of seed proteins in 15 Lathyrus species (Fabaceae) from Southern Spain. LWT Food Sci. Technol. 2011, 44, 1059–1064. [Google Scholar] [CrossRef]
- Aletor, O.; Onyemem, C.E.; Aletor, V.A. Nutrient Constituents, Functional Attributes and In Vitro Protein Digestibility of the Seeds of the Lathyrus Plant. Trans. Ecol. Environ. 2011, 152, 145–155. [Google Scholar]
- Gonçalves, L.; Rubiales, D.; Bronze, M.R.; Vaz Patto, M.C. Grass Pea (Lathyrus sativus L.)—A Sustainable and Resilient Answer to Climate Challenges. Agronomy 2022, 12, 1324. [Google Scholar] [CrossRef]
- Ramya, K.R.; Tripathi, K.; Pandey, A.; Barpete, S.; Gore, P.G.; Raina, A.P.; Khawar, K.M.; Swain, N.; Sarker, A. Rediscovering the Potential of Multifaceted Orphan Legume Grasspea—A Sustainable Resource with High Nutritional Values. Front. Nutr. 2022, 8, 826208. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Bashir, S.; Saeed, K.; Alsulami, T.; Rafique, H.; Mukonzo, E.K.L. Phytochemical and Structural Portrayal of Barley and Pearl Millet Through FTIR and SEM. Food Sci. Nutr. 2025, 13, e70120. [Google Scholar] [CrossRef]
- Agbai, C.M.; Olawuni, I.A.; Ofoedu, C.E.; Ibeabuchi, C.J.; Okpala, C.O.R.; Shorstkii, I.; Korzeniowska, M. Changes in Anti-Nutrient, Phytochemical, and Micronutrient Contents of Different Processed Rubber (Hevea brasiliensis) Seed Meals. PeerJ 2021, 9, e11327. [Google Scholar] [CrossRef]
- Udo, M.D.; Ekpo, U.; Ahamefule, F.O. Effects of Processing on the Nutrient Composition of Rubber Seed Meal. J. Saudi Soc. Agric. Sci. 2018, 17, 297–301. [Google Scholar] [CrossRef]
- Hossain, M.E.; Karim, M.H.; Alam, S.; Nath, S.K. Nutritive Value of Rubber Seed (Hevea brasiliensis). Online J. Anim. Feed Res. 2015, 5, 18–21. [Google Scholar]
- Soualiho, B.; Yao, J.-C.N.; John, K.K.; Losseyni, K.; Augustin, A.A. Evaluation of the Chemical and Biological Properties of Oil Extracted from Detoxified Rubber Tree (Hevea brasiliensis) Kernels. Am. J. BioSci. 2022, 10, 220–229. [Google Scholar] [CrossRef]
- Umar, N.M.; Parumasivam, T.; Aminu, N.; Toh, S.-M. Phytochemical and Pharmacological Properties of Curcuma aromatica Salisb (Wild Turmeric). J. Appl. Pharm. Sci. 2020, 10, 180–194. [Google Scholar] [CrossRef]
- Albaqami, J.J.; Hamdi, H.; Narayanankutty, A.; Visakh, N.U.; Sasidharan, A.; Kuttithodi, A.M.; Famurewa, A.C.; Pathrose, B. Chemical Composition and Biological Activities of the Leaf Essential Oils of Curcuma longa, Curcuma aromatica and Curcuma angustifolia. Antibiotics 2022, 11, 1547. [Google Scholar] [CrossRef]
- Pintatum, A.; Maneerat, W.; Logie, E.; Tuenter, E.; Sakavitsi, M.E.; Pieters, L.; Vanden Bergh, W.; Sripisut, T.; Deachathai, S.; Laphookhieo, S. In Vitro Anti-Inflammatory, Anti-Oxidant, and Cytotoxic Activities of Four Curcuma Species and the Isolation of Compounds from Curcuma aromatica Rhizome. Biomolecules 2020, 10, 799. [Google Scholar] [CrossRef]
- Hasanah, N.N.; Mohamad Azman, E.; Rozzamri, A.; Zainal Abedin, N.H.; Ismail-Fitry, M.R. A Systematic Review of Butterfly Pea Flower (Clitoria ternatea L.): Extraction and Application as a Food Freshness pH-Indicator for Polymer-Based Intelligent Packaging. Polymers 2023, 15, 2541. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Kumar, V.; Kumar, N.S.; Heinrich, M. The Ayurvedic Medicine Clitoria ternatea—From Traditional Use to Scientific Assessment. J. Ethnopharmacol. 2008, 120, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Kazuma, K.; Noda, N.; Suzuki, M. Malonylated Flavonol Glycosides from the Petals of Clitoria ternatea. Phytochemistry 2003, 62, 229–237. [Google Scholar] [CrossRef]
- Bangar, S.P.; Dunno, K.; Kumar, M.; Mostafa, H.; Maqsood, S. A Comprehensive Review on Lotus Seeds (Nelumbo nucifera Gaertn.): Nutritional Composition, Health-Related Bioactive Properties, and Industrial Applications. J. Funct. Foods 2022, 89, 104973. [Google Scholar] [CrossRef]
- Purintraphiban, S.; Xia, Y. Effects of Germination on Chemical and Functional Properties of Lotus Seeds. Food Sci. 2012, 33, 91–98. [Google Scholar]
- Awal, M.R.; Rahmatullah, S.M.; Nasrin, S. Nutrient Composition of Lotus (Nelumbo nucifera) Fruits. Asian Australas. J. Biosci. Biotechnol. 2020, 5, 115–120. [Google Scholar] [CrossRef]
- Dai, G.; Wang, J.; Zheng, J.; Xia, C.; Wang, Y.; Duan, B. Bioactive Polysaccharides from Lotus as Potent Food Supplements: A Review of Their Preparation, Structures, Biological Features and Application Prospects. Front. Nutr. 2023, 10, 1171004. [Google Scholar] [CrossRef]
- Lim, S.-H.; Kim, S.-H.; Park, J.-J.; Park, Y.-S.; Dhungana, S.K.; Kim, I.-D.; Shin, D.-H. Quality Characteristics and Antioxidant Activities of Lotus (Nelumbo nucifera Gaertn.) Sprouts Grown Under Different Conditions. Korean J. Plant Res. 2020, 33, 666–674. [Google Scholar]
- Liu, X.; Dong, W.; Yi, Y.; Wang, L.; Hou, W.; Ai, Y.; Wang, H.; Min, T. Comparison of Nutritional Quality and Functional Active Substances in Different Parts of Eight Lotus Seed Cultivars. Foods 2024, 13, 2335. [Google Scholar] [CrossRef] [PubMed]
- Adomas, B.; Piotrowicz-Cieślak, A.; Kowalik, K. Wartość biologiczna białka nasion łubinu. Post. Nauk Rol. 2005, 52, 55–63. [Google Scholar]
- Lemus-Conejo, A.; Rivero-Pino, F.; Montserrat-de la Paz, S.; Millan-Linares, M.C. Nutritional Composition and Biological Activity of Narrow-Leafed Lupins (Lupinus angustifolius L.) Hydrolysates and Seeds. Food Chem. 2023, 420, 136104. [Google Scholar] [CrossRef]
- Castillo, R.F.; García Pérez, R.; González Díaz, A.; Liñán González, A. Therapeutic Applications and Effects of Lupinus angustifolius (Blue Lupin) and Its Components: A Systematic Review and Meta-Analysis. Foods 2023, 12, 2749. [Google Scholar] [CrossRef]
- Hernández-Santos, B.; Quijano-Jerónimo, O.; Rodríguez-Miranda, J. Physical, Chemical, Tecno-Functional, and Thermal Properties of Leucaena leucocephala Seed. Food Sci. Technol. 2022, 42, e74921. [Google Scholar] [CrossRef]
- De Angelis, A.; Gasco, L.; Parisi, G.; Danieli, P.P. A Multipurpose Leguminous Plant for the Mediterranean Countries: Leucaena leucocephala as an Alternative Protein Source: A Review. Animals 2021, 11, 2230. [Google Scholar] [CrossRef]
- Aquino-González, L.V.; Noyola-Altamirano, B.; Méndez-Lagunas, L.L.; Rodríguez-Ramírez, J.; Sandoval-Torres, S.; Bernal, L.G.B. Potential of Leucaena leucocephala and Leucaena esculenta Seeds in Human Nutrition: Composition, Techno-Functional Properties, Toxicology and Pretreatment Technologies. Legum. Res. 2023, 46, 1261–1270. [Google Scholar] [CrossRef]
- Balderas-León, I.; Baigts-Allende, D.; Cardador-Martínez, A. Antioxidant, Angiotensin-Converting Enzyme, and α-Amylase Inhibitory Activities of Protein Hydrolysates of Leucaena leucocephala Seeds. CyTA J. Food 2021, 19, 349–359. [Google Scholar] [CrossRef]
- Shi, P. Unlocking the Potential of Brewer’s Spent Grains: A Mini Review on Health and Nutritional Benefits. Nov. Tech. Nutr. Food Sci. 2024, 7, 766–768. [Google Scholar] [CrossRef]
- Merten, D.; Erman, L.; Marabelli, G.P.; Leners, B.; Ney, Y.; Nasim, M.J.; Jacob, C.; Tchoumtchoua, J.; Cajot, S.; Bohn, T. Potential Health Effects of Brewers’ Spent Grain as a Functional Food Ingredient Assessed by Markers of Oxidative Stress and Inflammation Following Gastro-Intestinal Digestion and in a Cell Model of the Small Intestine. Food Funct. 2022, 13, 5327–5342. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ Spent Grain; Bioactivity of Phenolic Component, Its Role in Animal Nutrition and Potential for Incorporation in Functional Foods: A Review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Leone, A.; Spada, A.; Battezzati, A.; Schiraldi, A.; Aristil, J.; Bertoli, S. Moringa oleifera Seeds and Oil: Characteristics and Uses for Human Health. Int. J. Mol. Sci. 2016, 17, 2141. [Google Scholar] [CrossRef]
- Compaore, W.R.; Nikiema, P.A.; Bassole, H.I.N.; Savadogo, A.; Mouecoucou, J.; Hounhouigan, D.J.; Traore, S.A. Chemical Composition and Antioxidative Properties of Seeds of Moringa oleifera and Pulps of Parkia biglobosa and Adansonia digitata Commonly Used in Food Fortification in Burkina Faso. Curr. Res. J. Biol. Sci. 2011, 3, 64–72. [Google Scholar]
- Divya, S.; Pandey, V.K.; Dixit, R.; Rustagi, S.; Suthar, T.; Atuahene, D.; Nagy, V.; Ungai, D.; Ahmed, A.E.M.; Kovács, B.; et al. Exploring the Phytochemical, Pharmacological and Nutritional Properties of Moringa oleifera: A Comprehensive Review. Nutrients 2024, 16, 3423. [Google Scholar] [CrossRef]
- Jegede, E.R.; Ayeni, G.; Abaniwo, M.R.; Olutoye, A.F.; Audu, A.G.; Haruna, A.; Oluwole, S.O. Evaluation of Nutritional and Therapeutic Effects of Defatted Moringa oleifera Seeds in Protein Energy Malnourished Rats. Direct Res. J. Agric. Food Sci. 2025, 13, 79–89. [Google Scholar] [CrossRef]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Amare, E. Nutritional and Phytochemical Composition and Associated Health Benefits of Oat (Avena sativa) Grains and Oat-Based Fermented Food Products. Sci. World J. 2023, 2023, 2730175. [Google Scholar] [CrossRef]
- Singh, S.; Bisla, G. Nutritional and Lipid Composition of Avena sativa, Hordeum vulgare and Echinochloa frumentacea. World J. Adv. Res. Rev. 2021, 11, 23–27. [Google Scholar] [CrossRef]
- Peng, B.; Jin, K.-X.; Luo, D.-Y.; Tian, X.-Y.; Sun, Y.-F.; Huang, X.-H.; Pang, R.-H.; Wang, Q.-X.; Zhou, W.; Yuan, H.-Y. The Nutritional Components of Rice Are Closely Related to Grain Quality Traits in Rice. J. Biol. Life Sci. 2020, 11, 239–262. [Google Scholar] [CrossRef]
- Alege, G.O.; Animasaun, D.A.; Emmanuel, V.A. Evaluation of Proximate, Mineral and Protein Profile of Five Accessions of Oryza sativa (L.) (Rice). FUW Trends Sci. Technol. J. 2016, 1, 522–526. [Google Scholar]
- Dias, L.G.; Hacke, A.; Souza, E.d.S.; Nath, S.; Canesin, M.R.; Vilella, O.V.; Geloneze, B.; Pallone, J.A.L.; Cazarin, C.B.B.; Blakeslee, J.J.; et al. Comparison of Chemical and Nutritional Compositions between Aromatic and Non-Aromatic Rice from Brazil and Effect of Planting Time on Bioactive Compounds. J. Food Compos. Anal. 2022, 111, 104608. [Google Scholar] [CrossRef]
- Ajmera, I. Is Basmati Rice Healthy? Nutrients and More. 2020. Available online: https://www.healthline.com/nutrition/is-basmati-rice-healthy (accessed on 21 March 2025).
- Bhattacharjee, P.; Singhal, R.S.; Kulkarni, P.R. Basmati Rice: A Review. Int. J. Food Sci. Technol. 2002, 37, 1–12. [Google Scholar] [CrossRef]
- Zubair, M.; Anwar, F.; Ashraf, M.; Uddin, M.K. Characterization of High-Value Bioactives in Some Selected Varieties of Pakistani Rice (Oryza sativa L.). Int. J. Mol. Sci. 2012, 13, 4608–4622. [Google Scholar] [CrossRef] [PubMed]
- Liberal, A.; Almeida, D.; Fernandes, A.; Pereira, C.; Ferreira, I.C.F.R.; Vivar-Quintana, A.M.; Barros, L. Nutritional, Chemical and Antioxidant Evaluation of Armuña Lentil (Lens culinaris spp): Influence of Season and Soil. Food Chem. 2023, 411, 135491. [Google Scholar] [CrossRef]
- Sharmila, S.; Vijayakumar, T.P. Sorghum bicolor: Nutritional Powerhouse and Processing Innovations—A Comprehensive Review. Int. J. Creat. Res. Thoughts (IJCRT) 2025, 13, 960–978. [Google Scholar]
- Wilk, M. Soja źródłem cennych składników żywieniowych. Żywność Nauka Technol. Jakość/Food Sci. Technol. Qual. 2017, 24, 16–25. [Google Scholar] [CrossRef]
- Czubinski, J.; Grygier, A.; Siger, A. Lupinus mutabilis Seed Composition and Its Comparison with Other Lupin Species. J. Food Compos. Anal. 2021, 99, 103875. [Google Scholar] [CrossRef]
- Barba-Ostria, C.; Guamán-Bautista, J.; Tosi-Vélez, A.A.; Puente-Pineda, J.A.; Cedeño-Zambrano, M.A.; Teran, E.; Guamán, L.P. Recent Advances in the Therapeutic Potential of Bioactive Molecules from Plants of Andean Origin. Nutrients 2025, 17, 1749. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, C.; Rubilar, M.; Jara, C.; Verdugo, M.; Sineiro, J.; Shene, C. Flaxseed and Flaxseed Cake as a Source of Compounds for Food Industry. J. Soil. Sci. Plant Nutr. 2010, 10, 454–463. [Google Scholar] [CrossRef]
- Xiong, C.; Zou, X.; Phan, C.W.; Huang, W.; Zhu, Y. Enhancing the Potential of Rapeseed Cake as Protein-Source Food by Gamma Irradiation. Biosci. Rep. 2024, 44, BSR20231807. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Some Physico-Chemical Properties of Moringa oleifera Seed Oil Extracted Using Solvent and Aqueous Enzymatic Methods. Food Chem. 2005, 93, 253–263. [Google Scholar] [CrossRef]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Bhatia, S.; Sharma, A.; Sharma, K.; Kavale, M.; Chaugule, B.; Dhalwal, K.; Mahadik, K. Novel Algal Polysaccharides from Marine Source: Porphyran. Pharmacogn. Rev. 2008, 2, 271–280. [Google Scholar]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef]
- Hu, G.X.; Lin, H.; Lian, Q.Q.; Zhou, S.H.; Guo, J.; Zhou, H.Y.; Chu, Y.; Ge, R.S. Curcumin as a Potent and Selective Inhibitor of 11β-Hydroxysteroid Dehydrogenase 1: Improving Lipid Profiles in High-Fat-Diet-Treated Rats. PLoS ONE 2013, 8, e49976. [Google Scholar] [CrossRef]
- Malairaj, S.; Veeraperumal, S.; Yao, W.; Subramanian, M.; Tan, K.; Zhong, S.; Cheong, K.L. Porphyran from Porphyra haitanensis Enhances Intestinal Barrier Function and Regulates Gut Microbiota Composition. Mar. Drugs 2023, 21, 265. [Google Scholar] [CrossRef] [PubMed]
- Pereira, D.T.; García-García, P.; Korbee, N.; Vega, J.; Señoráns, F.J.; Figueroa, F.L. Optimizing the Extraction of Bioactive Compounds from Porphyra linearis (Rhodophyta): Evaluating Alkaline and Enzymatic Hydrolysis for Nutraceutical Applications. Mar. Drugs 2024, 22, 284. [Google Scholar] [CrossRef]
- Maestrini, M.; Tava, A.; Mancini, S.; Tedesco, D.; Perrucci, S. In Vitro Anthelmintic Activity of Saponins from Medicago spp. Against Sheep Gastrointestinal Nematodes. Molecules 2020, 25, 242. [Google Scholar] [CrossRef]
- Szymańska, R.; Kruk, J. Fitosterole—Występowanie i Znaczenie dla Człowieka. Kosmos 2007, 56, 107–114. [Google Scholar]
- Obrzut, O.; Gostyńska-Stawna, A.; Krajka-Kuźniak, V. Dwie Twarze Fitosteroli—Korzyści i Potencjalne Zagrożenia/Two Faces of Phytosterols—Benefits and Potential Threats. Farmacja Współczesna 2024, 17, 257–266. [Google Scholar]
- Wang, X.C.; Shen, X.Y.; Chen, L.; Wei, R.; Wei, M.Y.; Gu, C.H.; Pan, B. Preparation, Characterization, and Anticancer Effects of an Inclusion Complex of Coixol with β-Cyclodextrin Polymers. Pharm. Biol. 2024, 62, e2294331. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-Y.; Lu, Y.-N.; Shen, X.-Y.; Quan, Y.-Z.; Lu, J.-M.; Jin, G.-N.; Liu, Y.-M.; Zhang, S.-H.; Xu, G.-H.; Xu, X.; et al. Coixol Mitigates Toxoplasma gondii Infection-Induced Liver Injury by Inhibiting the Toxoplasma gondii HSP70/TLR4/NF-κB Signaling Pathway in Hepatic Macrophages. J. Ethnopharmacol. 2024, 335, 118694. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, B.; Yao, Z. Porphyran, Porphyran Oligosaccharides and Porphyranase: Source, Structure, Preparation Methods and Applications. Algal Res. 2023, 73, 103167. [Google Scholar] [CrossRef]
- Harahap, I.A.; Suliburska, J.; Karaca, A.C.; Capanoglu, E.; Esatbeyoglu, T. Fermented Soy Products: A Review of Bioactives for Health from Fermentation to Functionality. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70080. [Google Scholar] [CrossRef]
- Kuligowski, M.; Nowak, J. Możliwości modelowania cech funkcjonalnych żywności wytworzonej z nasion roślin strączkowych poprzez zastosowanie fermentacji typu tempeh. Biotechnologia 2007, 4, 113–124. [Google Scholar]
- Astuti, M.; Meliala, A.; Dalais, F.S.; Wahlqvist, M.L. Tempe, a Nutritious and Healthy Food from Indonesia. Asia Pac. J. Clin. Nutr. 2000, 9, 322–325. [Google Scholar] [CrossRef] [PubMed]
- Adamska, I.; Felisiak, K.; Kiliański, K. The Effect of Selected Seasonings on the Antioxidant Activity and Sensory Values of Tempeh. Żywność Nauka Technol. Jakość/Food Sci. Technol. Qual. 2024, 31, 95–112. [Google Scholar] [CrossRef]
- Astawan, M.; Prayudani, A.P.G.; Hadiningtias, P.; Wresdiyati, T.; Febrinda, A.E. Effect of Tea Extract (Camellia sinensis) on Shelf Life and Intrinsic Quality of Tempeh. Canrea J. Food Technol. Nutr. Culin. J. 2024, 7, 1–14. [Google Scholar] [CrossRef]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Hutkins, R. Health Benefits of Fermented Foods: Microbiota and Beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef]
- Villacrés, E.; Álvarez, J.; Rosell, C. Effects of Two Debittering Processes on the Alkaloid Content and Quality Characteristics of Lupin (Lupinus mutabilis Sweet). J. Sci. Food Agric. 2020, 100, 2166–2175. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Industrialization of Indigenous Fermented Foods; Marcel, D., Ed.; CRC Press: Boca Raton, FL, USA, 2004; ISBN 0824747844. [Google Scholar]
| Type of Tempeh | Protein [% DM] | Fat [% DM] | Carbohydrates [% DM] | Fibre [% DM] |
|---|---|---|---|---|
| Tempeh with adlay (Coix lacryma-jobi) | n.a. | n.a. | n.a. | n.a. |
| Tempeh from broad beans (Vicia faba L.) | 17.8 [15] 38.3 [32] | n.a. | 16.72 [15] | n.a. |
| Chickpea tempeh (Cicer arietinum) | 26.5 [5] 31.1 [33] 47.1 [15] | 6.26 [5] 9.88 [33] | 16.57 [33] 26.31 [15] 62.66 [5] | 2.95 [5] 4.40 [33] |
| Tempeh from Porphyra sp. | 17.6–18.5 [62] | 6.81–7.22 [62] | 11.58–12.15 [62] | n.a. |
| Tempeh from Eucheuma spinosum | 11.6–16.7 [64] | 4.34–6.61 [64] | 8.04–10.12 [64] | 14.40–17.99 [64] |
| Tempeh of cowpea bean (Vigna unguiculata) | n.a. | n.a. | n.a. | n.a. |
| Jack bean tempeh (Canavalia ensiformis) | 9.6 [35] 15.8 [37] 31.6 [36] 31.8 [40] 41.0 [39] | 1.6 [35] 4.1–4.5 [40] 6.2 [36] 10.9 [37] | 12.5 [37] 25.1–26.0 [35] 57-1–57.9 [40] | 16.6 (total) [36] |
| Tempeh from Bambara groundnut (Vigna subterranea) | 22.6–23.4 [41] | 1.4 [41] | 12.8–13.0 [41] | n.a. |
| Mung bean tempeh (Vigna radiata) | n.a. | n.a. | n.a. | n.a. |
| Tempeh of winged bean (Psophocarpus tetragonolobus) | 15.3–17.4 [23] | 7.7–9.4 [23] | n.a. | n.a. |
| Tempeh of common bean (Phaseolus vulgaris) | 16.3 [15] 21.4 [43] 23.3 [46] | 1.0 [43] 1.3 [46] | 27.0 [15] 55.5 [46] 68.4 [43] | 17.5 [46] |
| Tempeh of large peas (Lathyrus sativus) | 29.7 [48] 41.1 [49] | 19.4 [48] | n.a. | n.a. |
| Pearl barley (Hordeum vulgare) tempeh | 7.8 [56] | n.a. | n.a. | n.a. |
| Tempeh rubber (Hevea brasiliensis) | 20.6 [61] | n.a. | n.a. | n.a. |
| Wild turmeric tempeh (Curcuma aromatica) | n.a. | n.a. | n.a. | n.a. |
| Tempeh topped with butterfly pea flowers (Clitoria ternatea) | 15.6–17.1 [65] | 9.3–11.8 [65] | 12.1–12.9 [65] | n.a. |
| Lotus tempeh (Nelumbo nucifera) | 9.4 [59] 38.4 [58] | 5.8 [59] 5.6–8.3 [58] | 19.5 [59] 53.6–65.6 [58] | n.a. |
| Tempeh of the narrow-leafed lupin (Lupinus angustifolius) | n.a. | n.a. | n.a. | n.a. |
| Tempeh from white mimosa (Leucaena leucocephala) | n.a. | n.a. | n.a. | n.a. |
| Brewer’s mill tempeh | 5.0–7.9 [67] | n.a. | n.a. | n.a. |
| Moringa oleifera tempeh (seeds) | 7.6–20.6 [60] | n.a. | n.a. | 26.0–28.0 [60] |
| Tempeh from oats (Avena sativa) | n.a. | n.a. | n.a. | n.a. |
| Tempeh from rice (Oryza sativa) | n.a. | n.a. | n.a. | n.a. |
| Basmati rice tempeh | 7.6–9.5 [56] | n.a. | n.a. | n.a. |
| Lentil tempeh (Lens culinaris) | 15.1–46.2 [15] | n.a. | 16.1-35–7 [15] | n.a. |
| Tempeh from soya (Glycine max) | 14.6–17.4 [24] 16.5 [23] 16.8–18.6 [19] 25.0 [15] 37.4 [14] 44.7 [5] 44.3–44.9 [11] | 5.0–10.8 [23] 8.2–11.8 [24] 16.5–17.1 [11] 17.3 [14] 23.2 [5] | 7.3–12.0 [24] 9.9–18.4 [23] 27.1 [5] 32.6–33.6 [11] | 4.2 [5] |
| Tempeh of sorghum (Sorghum bicolor) | n.a. | n.a. | n.a. | n.a. |
| Tempeh from tarwi (Lupinus mutabilis) | 2.8–32.5 [53] | n.a. | n.a. | n.a. |
| Tempeh with linseed pomace added | n.a. | n.a. | n.a. | n.a. |
| Tempeh from rapeseed pomace | 36.5 [68] | 14.6 [68] | 12.0 [68] |
| Raw Material of Tempeh | Polyphenols [mg GAE/g] | DPPH [% Inhibition] or [ppm] |
|---|---|---|
| Adlay (Coix lacryma-jobi) | 58.2 [26] | 647.0 ppm [26] |
| Broad bean (Vicia faba L.) | 4.8 [15] | n.a. |
| Chickpea (Cicer arietinum) | 4.3 [15] | 6.4–14.7% [34] |
| Seaweed (Porphyra sp.) | n.a. | 85.0% [63] |
| Seaweed (Eucheuma spinosum) | n.a. | n.a. |
| Cowpea bean (Vigna unguiculata) | n.a. | n.a. |
| Jack bean (Canavalia ensiformis) | 4.0–10.7 [6] 12.0 [36] | 2.5–3.6 [35] 69.7% [37] 457.0–3436.6 ppm [6] |
| Tempeh from Bambara groundnut (Vigna subterranea) | n.a. | n.a. |
| Mung bean (Vigna radiata) | 137.5 [26] | 128.0 ppm [26] |
| Winged bean (Psophocarpus tetragonolobus) | n.a. | 39.1–51.8% [23] |
| Common bean (Phaseolus vulgaris) | 2.1–3.6 [15] | 43.0 [43] |
| Large peas (Lathyrus sativus) | 2.1 [50] | n.a. |
| Pearl barley (Hordeum vulgare) | n.a. | n.a. |
| Rubber (Hevea brasiliensis) | n.a. | n.a. |
| Wild turmeric (Curcuma aromatica) | 99.7 [66] | n.a. |
| With butterfly pea flowers (Clitoria ternatea) | n.a. | 91.5% [65] |
| With lotus flowers (Nelumbo nucifera) | n.a. | 72.6% [57] |
| Narrow-leafed lupin (Lupinus angustifolius) | n.a. | n.a. |
| White mimosa (Leucaena leucocephala) | n.a. | n.a. |
| Brewers’ spent grains | n.a. | n.a. |
| Seeds (Moringa oleifera) | n.a. | n.a. |
| Oats (Avena sativa) | 0.8–1.8 [55] | n.a. |
| Rice (Oryza sativa) | n.a. | n.a. |
| Basmati rice (Oryza sativa) | n.a. | n.a. |
| Lentils (Lens culinaris) | 2.7–4.3 [15] | n.a. |
| Soya beans (Glycine max) | 6.1 [15] | 45.1 [23] 50.0–84.0 [7] |
| Sorghum (Sorghum bicolor) | 64.2 [26] | 457.0 ppm [26] |
| Tarwi (Lupinus mutabilis) | n.a. | n.a. |
| Linseed oil-press cake | 3.9 [50] | n.a. |
| Rapeseed oil-press cake | n.a. | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, K.; Pejcz, E.; Harasym, J. Tempeh and Fermentation—Innovative Substrates in a Classical Microbial Process. Appl. Sci. 2025, 15, 8888. https://doi.org/10.3390/app15168888
Górska K, Pejcz E, Harasym J. Tempeh and Fermentation—Innovative Substrates in a Classical Microbial Process. Applied Sciences. 2025; 15(16):8888. https://doi.org/10.3390/app15168888
Chicago/Turabian StyleGórska, Katarzyna, Ewa Pejcz, and Joanna Harasym. 2025. "Tempeh and Fermentation—Innovative Substrates in a Classical Microbial Process" Applied Sciences 15, no. 16: 8888. https://doi.org/10.3390/app15168888
APA StyleGórska, K., Pejcz, E., & Harasym, J. (2025). Tempeh and Fermentation—Innovative Substrates in a Classical Microbial Process. Applied Sciences, 15(16), 8888. https://doi.org/10.3390/app15168888









