Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects
Abstract
1. Introduction
2. Materials, Laboratory Equipment, and Methods
2.1. Materials and Laboratory Equipment
2.2. Preparing Fermented Cosmetic Raw Materials (FCRMs) from Dandelion Leaves, Roots, and Flowers
2.2.1. Raw Material Preparation
2.2.2. Inoculum Preparation
2.2.3. Co-Fermentation/Fermentation Process
2.3. Determination of Lactic Acid by GC-MS
2.4. Determination of Total Polyphenol Content Using the Folin–Ciocalteu Method
2.5. Determination of Antioxidant Activity: DPPH Assay
2.6. Determination of Antioxidant Activity: ORAC Assay
2.7. Lipophilicity Assessment
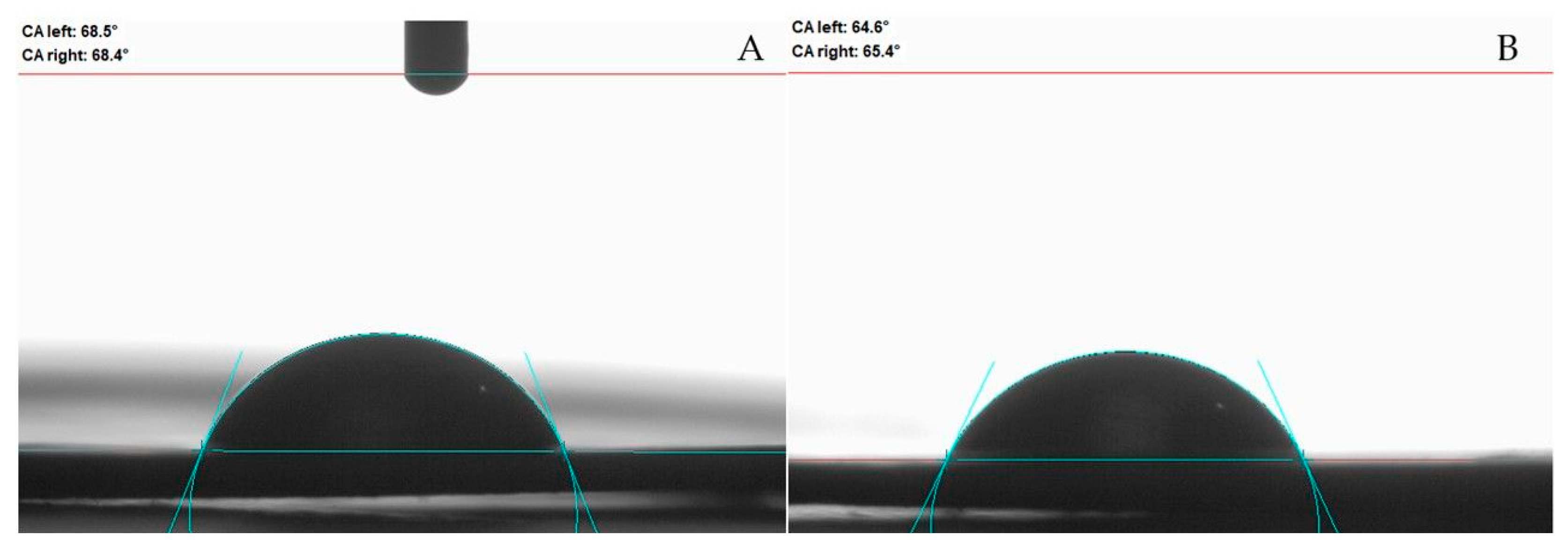
2.8. Wettability
2.9. Statistical Analysis
3. Results
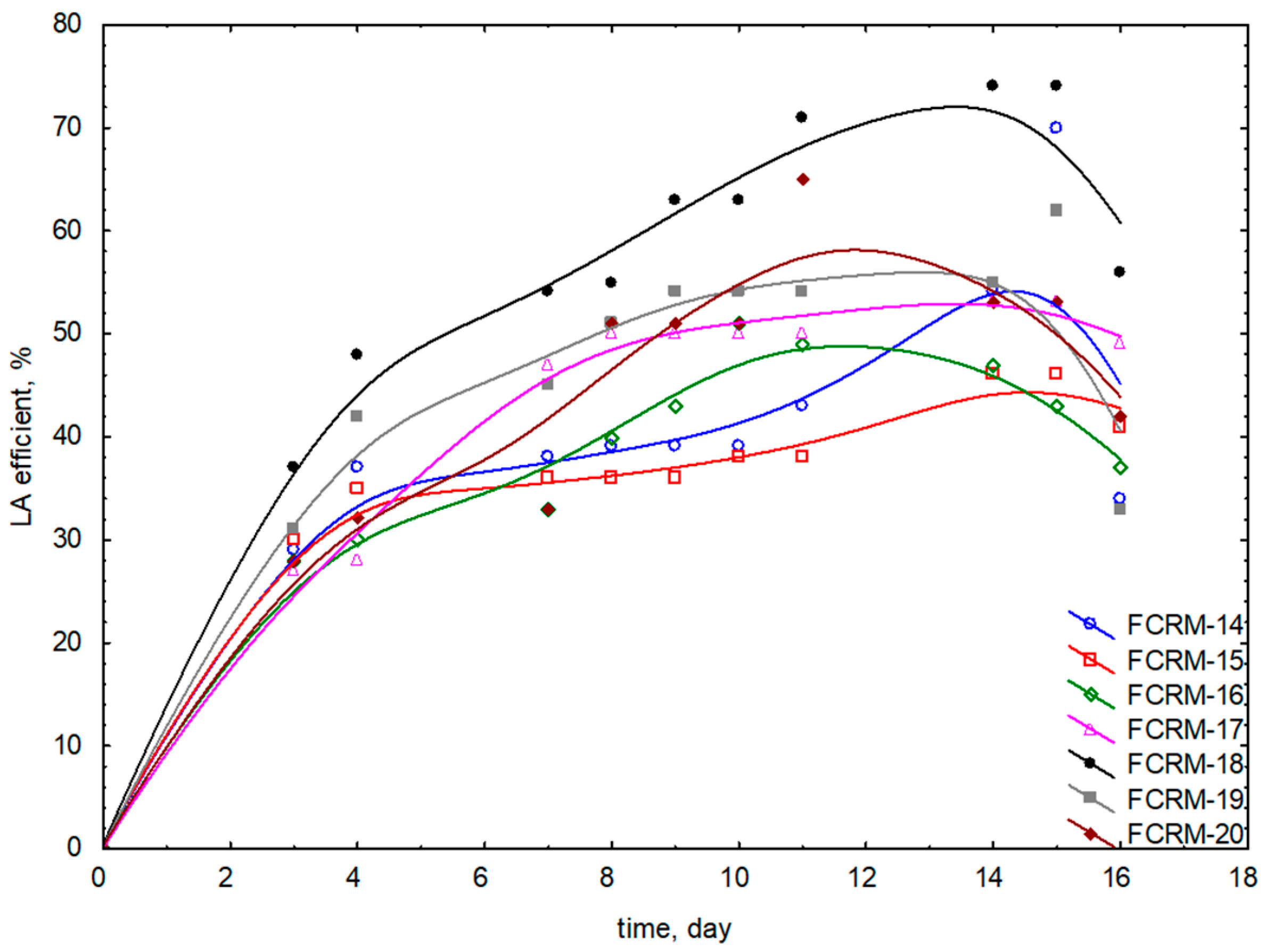
3.1. Methods for Obtaining Fermented Cosmetic Raw Materials and Evaluation of Lactic Acid Production During Dandelion Fermentation
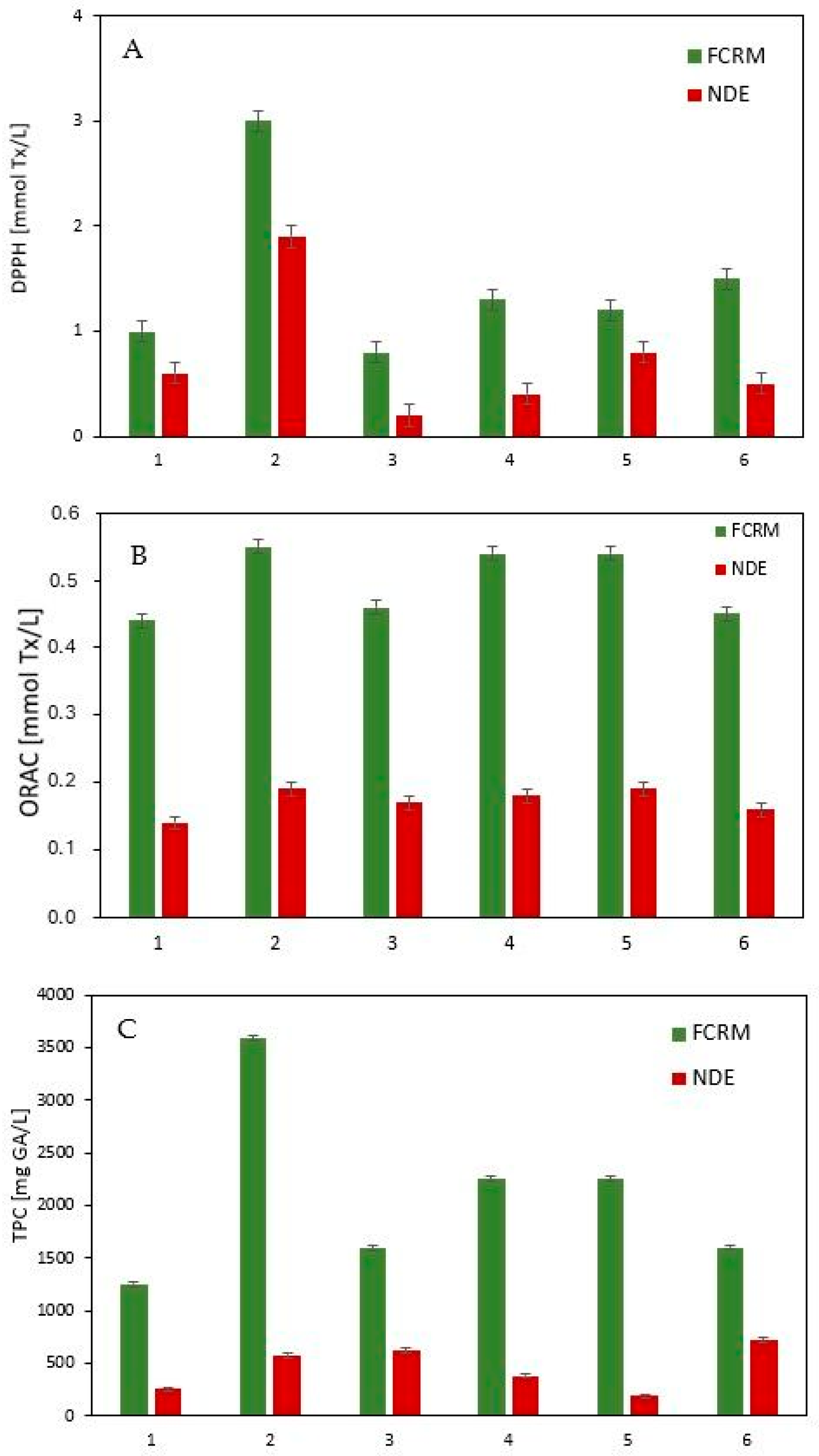
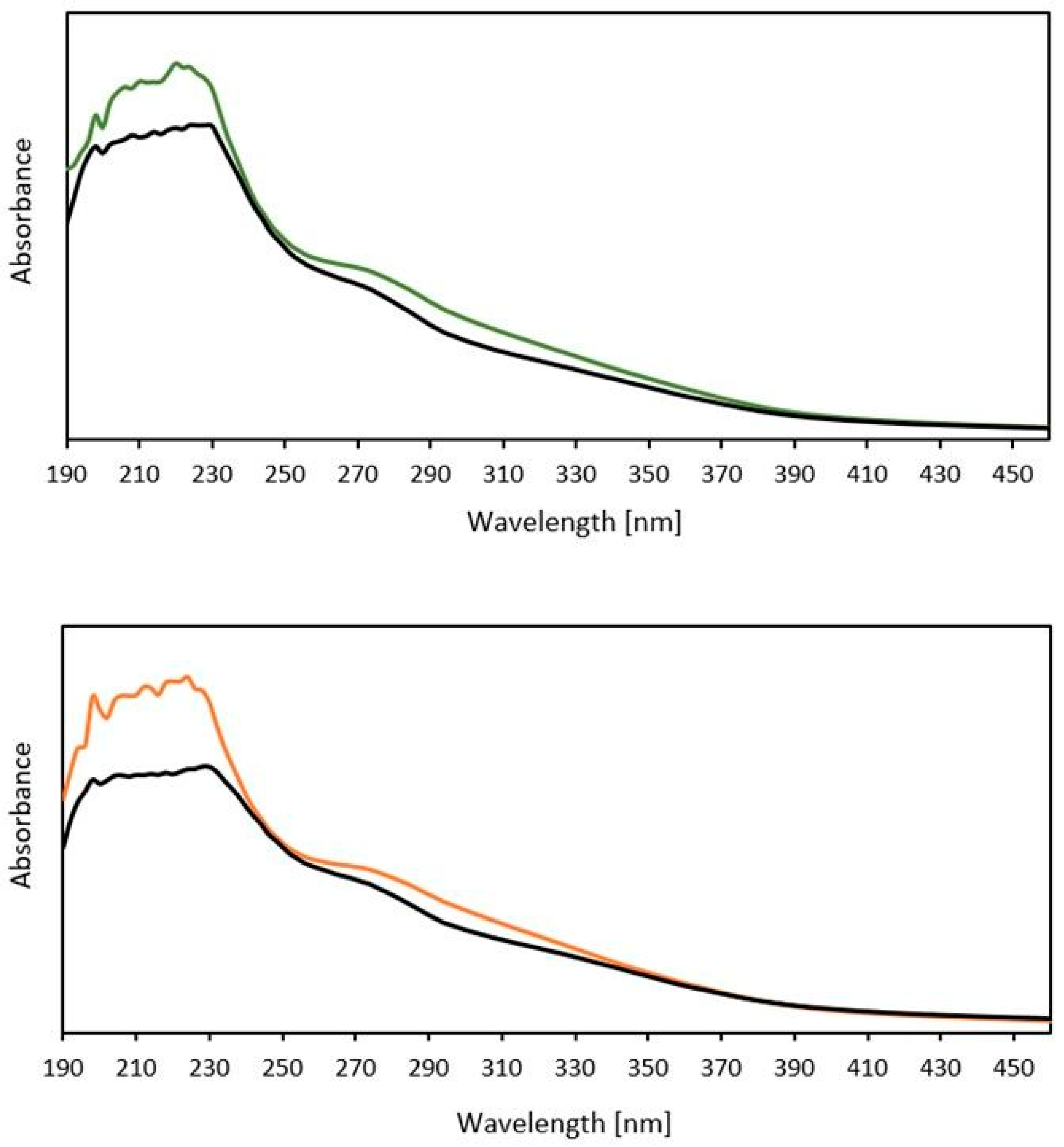
3.2. Antioxidant Activity and Total Polyphenol Content of Fermented Cosmetic Raw Materials
| Group | Count | Sum | Average | Variance |
|---|---|---|---|---|
| AA-DPPH (FCRM-1:FCRM-6) | 6 | 8.8 | 1.47 | 0.62 |
| AA-DPPH (NDE-1:NDE-6) | 6 | 4.4 | 0.733 | 0.37 |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between groups | 1.61 | 1 | 1.61 | 3.26 | 0.101 | 4.96 |
| Within groups | 4.95 | 10 | 0.49 | |||
| Total | 6.56 | 11 |
| Group | Count | Sum | Average | Variance |
|---|---|---|---|---|
| AA-ORAC (FCRM-1:FCRM-6) | 6 | 2.98 | 0.497 | 0.0027 |
| AA-ORAC (NDE-1:NDE-6) | 6 | 1.03 | 0.172 | 0.00038 |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between groups | 0.317 | 1 | 0.32 | 208 | 5.07∙10−8 | 4.96 |
| Within groups | 0.015 | 10 | 0.0015 | |||
| Total | 0.332 | 11 |
| Group | Count | Sum | Average | Variance |
|---|---|---|---|---|
| TPC (FCRM-1:FCRM-6) | 6 | 12,550 | 2092 | 697,642 |
| TPC (NDE-1:NDE-6) | 6 | 2738 | 456 | 46,504 |
| Source of Variation | SS | df | MS | F | p-Value | F Crit |
|---|---|---|---|---|---|---|
| Between groups | 8,022,945 | 1 | 8,022,945 | 21.6 | 0.000917 | 4.96 |
| Within groups | 3,720,733 | 10 | 372,073 | |||
| Total | 11,743,678 | 11 |
3.3. Lipophilicity Assessment
3.4. Wettability
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ikram, A.; Khan, R.; Kauser, S.; Khan, A.A.; Arshad, M.T.; Ahmad, M. Taraxacum (Dandelion). In Edible Flowers; Elsevier: Amsterdam, The Netherlands, 2024; pp. 281–300. ISBN 978-0-443-13769-3. [Google Scholar]
- Honek, A.; Martinkova, Z.; Saska, P. Post-Dispersal Predation of Taraxacum (Dandelion) Seed. J. Ecol. 2005, 93, 345–352. [Google Scholar] [CrossRef]
- McNeill, J.; Odell, E.A.; Consaul, L.L.; Katz, D.S. American Code and Later Lectotypifications of Linnaean Generic Names Dating from 1753: A Case Study of Discrepancies. TAXON 1987, 36, 350–401. [Google Scholar] [CrossRef]
- Martinez, M.; Poirrier, P.; Chamy, R.; Prüfer, D.; Schulze-Gronover, C.; Jorquera, L.; Ruiz, G. Taraxacum and Related Species—An Ethnopharmacological Review and Its Potential as a Commercial Medicinal Plant. J. Ethnopharmacol. 2015, 169, 244–262. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, B.; Vora, M.; Ulbricht, C.; Basch, E. Evidence-Based Systematic Review of Dandelion (Taraxacum) by Natural Standard Research Collaboration. J. Herb. Pharmacother. 2005, 5, 79–93. [Google Scholar] [CrossRef]
- Tanasa (Acretei), M.-V.; Negreanu-Pirjol, T.; Olariu, L.; Negreanu-Pirjol, B.-S.; Lepadatu, A.-C.; Anghel (Cireasa), L.; Rosoiu, N. Bioactive Compounds from Vegetal Organs of Taraxacum Species (Dandelion) with Biomedical Applications: A Review. Int. J. Mol. Sci. 2025, 26, 450. [Google Scholar] [CrossRef] [PubMed]
- Herrera Vielma, F.; Quiñones San Martin, M.; Muñoz-Carrasco, N.; Berrocal-Navarrete, F.; González, D.R.; Zúñiga-Hernández, J. The Role of Dandelion (Taraxacum) in Liver Health and Hepatoprotective Properties. Pharmaceuticals 2025, 18, 990. [Google Scholar] [CrossRef]
- Xie, P.; Huang, L.; Zhang, C.; Ding, S.; Deng, Y.; Wang, X. Skin-Care Effects of Dandelion Leaf Extract and Stem Extract: Antioxidant Properties, Tyrosinase Inhibitory and Molecular Docking Simulations. Ind. Crops Prod. 2018, 111, 238–246. [Google Scholar] [CrossRef]
- Law, S.; Lo, C.; Han, J.; Leung, A.W.; Xu, C. Traditional Chinese Herbal, “Dandelion” and Its Applications on Skin-Care. TIM 2021, 6, 152–157. [Google Scholar] [CrossRef]
- Behl, T.; Rocchetti, G.; Chadha, S.; Zengin, G.; Bungau, S.; Kumar, A.; Mehta, V.; Uddin, M.S.; Khullar, G.; Setia, D.; et al. Phytochemicals from Plant Foods as Potential Source of Antiviral Agents: An Overview. Pharmaceuticals 2021, 14, 381. [Google Scholar] [CrossRef]
- Wójciak, K.; Materska, M.; Pełka, A.; Michalska, A.; Małecka-Massalska, T.; Kačániová, M.; Čmiková, N.; Słowiński, M. Effect of the Addition of Dandelion (Taraxacum) on the Protein Profile, Antiradical Activity, and Microbiological Status of Raw-Ripening Pork Sausage. Molecules 2024, 29, 2249. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, X.; Song, H.; Zhang, Y. Dandelion (Taraxacum genus): A Review of Chemical Constituents and Pharmacological Effects. Molecules 2023, 28, 5022. [Google Scholar] [CrossRef] [PubMed]
- Pfingstgraf, I.O.; Taulescu, M.; Pop, R.M.; Orăsan, R.; Vlase, L.; Uifalean, A.; Todea, D.; Alexescu, T.; Toma, C.; Pârvu, A.E. Protective Effects of Taraxacum (Dandelion) Root Extract in Experimental Acute on Chronic Liver Failure. Antioxidants 2021, 10, 504. [Google Scholar] [CrossRef]
- Olas, B. New Perspectives on the Effect of Dandelion, Its Food Products and Other Preparations on the Cardiovascular System and Its Diseases. Nutrients 2022, 14, 1350. [Google Scholar] [CrossRef]
- Zhuang, X.; Shi, W.; Shen, T.; Cheng, X.; Wan, Q.; Fan, M.; Hu, D. Research Updates and Advances on Flavonoids Derived from Dandelion and Their Antioxidant Activities. Antioxidants 2024, 13, 1449. [Google Scholar] [CrossRef]
- Thilakarathna, S.; Rupasinghe, H. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Miean, K.H.; Mohamed, S. Flavonoid (Myricetin, Quercetin, Kaempferol, Luteolin, and Apigenin) Content of Edible Tropical Plants. J. Agric. Food Chem. 2001, 49, 3106–3112. [Google Scholar] [CrossRef]
- Hu, L.; Luo, Y.; Yang, J.; Cheng, C. Botanical Flavonoids: Efficacy, Absorption, Metabolism and Advanced Pharmaceutical Technology for Improving Bioavailability. Molecules 2025, 30, 1184. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, İ. Antioxidants: A Comprehensive Review. Arch. Toxicol. 2025, 99, 1893–1997. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, E.; Grygorcewicz, B.; Spietelun, M.; Olszewska, P.; Bobkowska, A.; Ryglewicz, J.; Nowak, A.; Muzykiewicz-Szymańska, A.; Kucharski, Ł.; Pełech, R. Potential Role of Bioactive Compounds: In Vitro Evaluation of the Antioxidant and Antimicrobial Activity of Fermented Milk Thistle. Appl. Sci. 2024, 14, 4287. [Google Scholar] [CrossRef]
- Azman, N.A.M.; Skowyra, M.; Muhammad, K.; Gallego, M.G.; Almajano, M.P. Evaluation of the Antioxidant Activity of Betula Pendula Leaves Extract and Its Effects on Model Foods. Pharm. Biol. 2017, 55, 912–919. [Google Scholar] [CrossRef]
- Zhu, Q.; Shi, G.; Gu, J.; Du, J.; Guo, J.; Wu, Y.; Yang, S.; Jiang, J. Impact of LA Bacterial Fermentation on the Chemical Composition, Antioxidant Capacities and Flavor Properties of Dandelion. Food Biosci. 2024, 62, 105313. [Google Scholar] [CrossRef]
- Liu, N.; Song, M.; Wang, N.; Wang, Y.; Wang, R.; An, X.; Qi, J. The Effects of Solid-State Fermentation on the Content, Composition and In Vitro Antioxidant Activity of Flavonoids from Dandelion. PLoS ONE 2020, 15, e0239076. [Google Scholar] [CrossRef]
- Majchrzak, W.; Śmigielski, K.; Motyl, I.; Oracz, J.; Skura, K.; Motyl, S. Kamchatka Berry (Lonicera Caerulea L.) Pomace Bioferment as an Innovative Cosmetic Raw Material. Appl. Sci. 2024, 14, 3218. [Google Scholar] [CrossRef]
- Krzyżostan, M.; Wawrzyńczak, A.; Nowak, I. Use of Waste from the Food Industry and Applications of the Fermentation Process to Create Sustainable Cosmetic Products: A Review. Sustainability 2024, 16, 2757. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Nizioł-Łukaszewska, Z.; Ziemlewska, A.; Zagórska-Dziok, M.; Mokrzyńska, A.; Wójciak, M.; Sowa, I. Apiaceae Bioferments Obtained by Fermentation with Kombucha as an Important Source of Active Substances for Skin Care. Molecules 2025, 30, 983. [Google Scholar] [CrossRef]
- Tian, X.; Liu, Y.; Feng, X.; Khaskheli, A.A.; Xiang, Y.; Huang, W. The Effects of Alcohol Fermentation on the Extraction of Antioxidant Compounds and Flavonoids of Pomelo Peel. LWT 2018, 89, 763–769. [Google Scholar] [CrossRef]
- Zhao, Y.-S.; Eweys, A.S.; Zhang, J.-Y.; Zhu, Y.; Bai, J.; Darwesh, O.M.; Zhang, H.-B.; Xiao, X. Fermentation Affects the Antioxidant Activity of Plant-Based Food Material through the Release and Production of Bioactive Components. Antioxidants 2021, 10, 2004. [Google Scholar] [CrossRef]
- Chai, W.Y.; Teo, K.T.K.; Tan, M.K.; Tham, H.J. Fermentation Process Control and Optimization. Chem. Eng. Technol. 2022, 45, 1731–1747. [Google Scholar] [CrossRef]
- Schmidt, F.R. Optimization and Scale up of Industrial Fermentation Processes. Appl. Microbiol. Biotechnol. 2005, 68, 425–435. [Google Scholar] [CrossRef]
- Kucharska, E.; Zagórska-Dziok, M.; Bilewicz, P.; Kowalczyk, S.; Jurkiewicz, M.; Wachura, D.; Miądlicki, P.; Pełech, R. Application of Response Surface Methodology for Fermented Plant Extract from Syzygium aromaticum L. (Myrtaceae): Optimisation of Antioxidant Activity, Total Polyphenol Content, and LA Efficiency. Appl. Sci. 2024, 14, 4763. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The Efficient Clade: LA Bacteria for Industrial Chemical Production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Ismaïl, R.; Aviat, F.; Michel, V.; Le Bayon, I.; Gay-Perret, P.; Kutnik, M.; Fédérighi, M. Methods for Recovering Microorganisms from Solid Surfaces Used in the Food Industry: A Review of the Literature. Int. J. Environ. Res. Public Health 2013, 10, 6169–6183. [Google Scholar] [CrossRef] [PubMed]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef]
- Essmat, R.A.; Altalla, N.; Amen, R.A. Fermentation-Derived Compounds and Their Impact on Skin Health and Dermatology: A Review. Innov. Med. Omics 2024, 2, 19. [Google Scholar] [CrossRef]
- Zapaśnik, A.; Sokołowska, B.; Bryła, M. Role of LA Bacteria in Food Preservation and Safety. Foods 2022, 11, 1283. [Google Scholar] [CrossRef]
- García-Cano, I.; Rocha-Mendoza, D.; Kosmerl, E.; Zhang, L.; Jiménez-Flores, R. Technically Relevant Enzymes and Proteins Produced by LAB Suitable for Industrial and Biological Activity. Appl. Microbiol. Biotechnol. 2020, 104, 1401–1422. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, N. Microbial Lipase Mediated by Health Beneficial Modification of Cholesterol and Flavors in Food Products: A Review. Recent Pat. Biotechnol. 2018, 12, 81–91. [Google Scholar] [CrossRef]
- Kucharska, E.; Zagórska-Dziok, M.; Bilewicz, P.; Kowalczyk, S.; Pełech, R. Use of Syzygium aromaticum L. Fermented Plant Extract to Enhance Antioxidant Potential: Fermentation Kinetics. Appl. Sci. 2024, 14, 4900. [Google Scholar] [CrossRef]
- El Ghallab, Y.; Al Jahid, A.; Jamal Eddine, J.; Ait Haj Said, A.; Zarayby, L.; Derfoufi, S. Syzygium aromaticum L.: Phytochemical Investigation and Comparison of the Scavenging Activity of Essential Oil, Extracts and Eugenol. Adv. Tradit. Med. 2020, 20, 153–158. [Google Scholar] [CrossRef]
- Kucharska, E.; Sarpong, R.; Bobkowska, A.; Ryglewicz, J.; Nowak, A.; Kucharski, Ł.; Muzykiewicz-Szymańska, A.; Duchnik, W.; Pełech, R. Use of Silybum marianum Extract and Bio-Ferment for Biodegradable Cosmetic Formulations to Enhance Antioxidant Potential and Effect of the Type of Vehicle on the Percutaneous Absorption and Skin Retention of Silybin and Taxifolin. Appl. Sci. 2024, 14, 169. [Google Scholar] [CrossRef]
- Bamidele, M.O.; Bamikale, M.B.; Cárdenas-Hernández, E.; Bamidele, M.A.; Castillo-Olvera, G.; Sandoval-Cortes, J.; Aguilar, C.N. Bioengineering in Solid-State Fermentation for next Sustainable Food Bioprocessing. Next Sustain. 2025, 6, 100105. [Google Scholar] [CrossRef]
- Brandão, M.; Marques, D.J.; Sousa, S.; Mateus, M.; Pinheiro, H.M.; Da Fonseca, M.M.R.; Pires, C.; Nunes, M.L.; Marques, A.; Cesário, M.T. LA Bacteria and Yeast Fermentation to Improve the Nutritional Value of Ulva Rigida. Mar. Drugs 2025, 23, 106. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Sadova, N.; Dornmayr, M.; Schwarzinger, B.; Neuhauser, C.; Stadlbauer, V.; Wallner, M.; Woischitzschläger, J.; Müller, A.; Tona, R.; et al. Combined Acid Hydrolysis and Fermentation Improves Bioactivity of Citrus Flavonoids In Vitro and In Vivo. Commun. Biol. 2023, 6, 1083. [Google Scholar] [CrossRef] [PubMed]
- Suhajda, Á.; Hegóczki, J.; Janzsó, B.; Pais, I.; Vereczkey, G. Preparation of Selenium Yeasts I. Preparation of Selenium-Enriched Saccharomyces cerevisiae. J. Trace Elem. Med. Biol. 2000, 14, 43–47. [Google Scholar] [CrossRef]
- Al-Mudhafar, A.; Al-Mudhaffar, A.H.; Al-Mudhaffar, A.A.; Al-Mudhaffar, A.M. The Use of Sugar Cane Waste (Molasses) in the Production of LA by L. Paracasei CAU 9836 and Its Identification by Infrared Spectrum (FT.IR). Al-Qadisiyah J. Agric. Sci. 2023, 13, 123–132. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Shah, N.P. Probiotics—From Metchnikoff to Bioactives. Int. Dairy J. 2008, 18, 714–728. [Google Scholar] [CrossRef]
- Zeng, X.; Cheng, S.; Li, H.; Yu, H.; Cui, Y.; Fang, Y.; Yang, S.; Feng, Y. Design, Synthesis, and Activity Evaluation of Novel Multitargeted l -tryptophan Derivatives with Powerful Antioxidant Activity against Alzheimer’s Disease. Arch. der Pharm. 2024, 357, 2300603. [Google Scholar] [CrossRef]
- Muzykiewicz-Szymańska, A.; Kucharska, E.; Pełech, R.; Nowak, A.; Jakubczyk, K.; Kucharski, Ł. The Optimisation of Ultrasound-Assisted Extraction for the Polyphenols Content and Antioxidant Activity on Sanguisorba officinalis L. Aerial Parts Using Response Surface Methodology. Appl. Sci. 2024, 14, 9579. [Google Scholar] [CrossRef]
- Anjani, Q.K.; Nainggolan, A.D.C.; Li, H.; Miatmoko, A.; Larrañeta, E.; Donnelly, R.F. Parafilm® M and Strat-M® as Skin Simulants in In Vitro Permeation of Dissolving Microarray Patches Loaded with Proteins. Int. J. Pharm. 2024, 655, 124071. [Google Scholar] [CrossRef]
- Michels, M.; Jesus, G.F.A.; Abatti, M.R.; Córneo, E.; Cucker, L.; De Medeiros Borges, H.; Da Silva Matos, N.; Rocha, L.B.; Dias, R.; Simon, C.S.; et al. Effects of Different Probiotic Strains B. lactis, L. rhamnosus and L. reuteri on Brain-Intestinal Axis Immunomodulation in an Endotoxin-Induced Inflammation. Mol. Neurobiol. 2022, 59, 5168–5178. [Google Scholar] [CrossRef]
- Jurkowski, A.; Kozioł, J.J.; Gronczewska, E. A Method to Increase the Survival of Probiotic Bacteria Lactobacillus brevis at a Lowered pH. Biol. Lett. 2019, 54, 13–20. [Google Scholar] [CrossRef]
- Gao, H.; Li, X.; Chen, X.; Hai, D.; Wei, C.; Zhang, L.; Li, P. The Functional Roles of Lactobacillus acidophilus in Different Physiological and Pathological Processes. J. Microbiol. Biotechnol. 2022, 32, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Narendranath, N.V.; Thomas, K.C.; Ingledew, W.M. Acetic Acid and LA Inhibition of Growth of Saccharomyces Cerevisiae by Different Mechanisms. J. Am. Soc. Brew. Chem. 2001, 59, 187–194. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, X.; Gong, X.; Tao, T.; Wang, Z.; Zhang, J.; Zhu, Y. Recovery and Utilization of Waste Filtrate from Industrial Biological Fermentation: Development and Metabolite Profile of the Bacillus cereus Liquid Bio-Fertilizer. J. Environ. Manag. 2023, 346, 118945. [Google Scholar] [CrossRef]
- Klongklaew, A.; Banwo, K.; Soodsawaeng, P.; Christopher, A.; Khanongnuch, C.; Sarkar, D.; Shetty, K. LA Bacteria Based Fermentation Strategy to Improve Phenolic Bioactive-Linked Functional Qualities of Select Chickpea (Cicer arietinum L.) Varieties. NFS J. 2022, 27, 36–46. [Google Scholar] [CrossRef]
- Lisov, N.; Čakar, U.; Milenković, D.; Čebela, M.; Vuković, G.; Despotović, S.; Petrović, A. The Influence of Cabernet Sauvignon Ripeness, Healthy State and Maceration Time on Wine and Fermented Pomace Phenolic Profile. Fermentation 2023, 9, 695. [Google Scholar] [CrossRef]
- Algiert-Zielińska, B.; Mucha, P.; Rotsztejn, H. Lactic and Lactobionic Acids as Typically Moisturizing Compounds. Int. J. Dermatol. 2019, 58, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Harding, C.R.; Watkinson, A.; Rawlings, A.V.; Scott, I.R. Dry Skin, Moisturization and Corneodesmolysis. Int. J. Cosmet. Sci. 2000, 22, 21–52. [Google Scholar] [CrossRef]
- Huang, H.-C.; Lee, I.J.; Huang, C.; Chang, T.-M. LA Bacteria and LA for Skin Health and Melanogenesis Inhibition. Curr. Pharm. Biotechnol. 2020, 21, 566–577. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef]
- Yang, F.; Chen, C.; Ni, D.; Yang, Y.; Tian, J.; Li, Y.; Chen, S.; Ye, X.; Wang, L. Effects of Fermentation on Bioactivity and the Composition of Polyphenols Contained in Polyphenol-Rich Foods: A Review. Foods 2023, 12, 3315. [Google Scholar] [CrossRef]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Lys, I.M. The Role of Lactic Fermentation in Ensuring the Safety and Extending the Shelf Life of African Indigenous Vegetables and Its Economic Potential. Appl. Res. 2025, 4, e202400131. [Google Scholar] [CrossRef]
- Tang, J.; Wang, X.C.; Hu, Y.; Zhang, Y.; Li, Y. Effect of pH on LA Production from Acidogenic Fermentation of Food Waste with Different Types of Inocula. Bioresour. Technol. 2017, 224, 544–552. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Zagórska-Dziok, M.; Nowak, A.; Muzykiewicz-Szymańska, A.; Wójciak, M.; Sowa, I.; Szczepanek, D.; Nizioł-Łukaszewska, Z. Enhancing the Cosmetic Potential of Aloe Vera Gel by Kombucha-Mediated Fermentation: Phytochemical Analysis and Evaluation of Antioxidant, Anti-Aging and Moisturizing Properties. Molecules 2025, 30, 3192. [Google Scholar] [CrossRef]
- Couceiro, B.; Hameed, H.; Vieira, A.C.F.; Singh, S.K.; Dua, K.; Veiga, F.; Pires, P.C.; Ferreira, L.; Paiva-Santos, A.C. Promoting Health and Sustainability: Exploring Safer Alternatives in Cosmetics and Regulatory Perspectives. Sustain. Chem. Pharm. 2025, 43, 101901. [Google Scholar] [CrossRef]
- Bom, S.; Fitas, M.; Martins, A.M.; Pinto, P.; Ribeiro, H.M.; Marto, J. Replacing Synthetic Ingredients by Sustainable Natural Alternatives: A Case Study Using Topical O/W Emulsions. Molecules 2020, 25, 4887. [Google Scholar] [CrossRef] [PubMed]
- Maaloul, S.; Ghzaiel, I.; Mahmoudi, M.; Mighri, H.; Pires, V.; Vejux, A.; Martine, L.; De Barros, J.-P.P.; Prost-Camus, E.; Boughalleb, F.; et al. Characterization of Silybum marianum and Silybum eburneum Seed Oils: Phytochemical Profiles and Antioxidant Properties Supporting Important Nutritional Interests. PLoS ONE 2024, 19, e0304021. [Google Scholar] [CrossRef] [PubMed]
- Herman, A.; Herman, A.P. Biological Activity of Fermented Plant Extracts for Potential Dermal Applications. Pharmaceutics 2023, 15, 2775. [Google Scholar] [CrossRef] [PubMed]
- Wiechers, J.W.; Watkinson, A.C.; Cross, S.E.; Roberts, M.S. Predicting Skin Penetration of Actives from Complex Cosmetic Formulations: An Evaluation of Inter Formulation and Inter Active Effects during Formulation Optimization for Transdermal Delivery. Int. J. Cosmet. Sci. 2012, 34, 525–535. [Google Scholar] [CrossRef]
- Kalin, M.; Polajnar, M. The Correlation between the Surface Energy, the Contact Angle and the Spreading Parameter, and Their Relevance for the Wetting Behaviour of DLC with Lubricating Oils. Tribol. Int. 2013, 66, 225–233. [Google Scholar] [CrossRef]
- Hmingthansanga, V.; Singh, N.; Banerjee, S.; Manickam, S.; Velayutham, R.; Natesan, S. Improved Topical Drug Delivery: Role of Permeation Enhancers and Advanced Approaches. Pharmaceutics 2022, 14, 2818. [Google Scholar] [CrossRef]
- Pullar, J.; Carr, A.; Vissers, M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
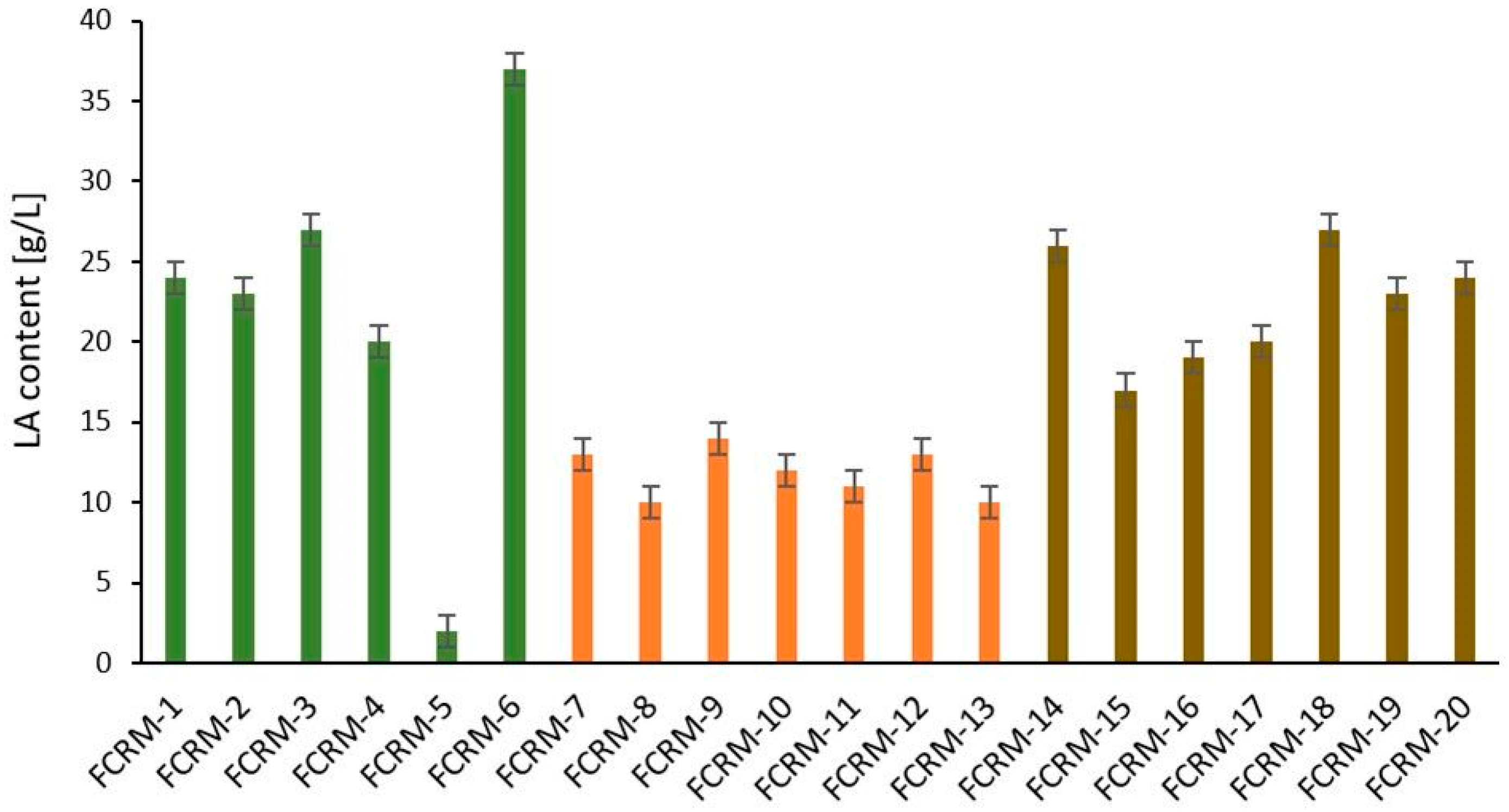
| Name FCRM | Plant Part | Molasses Type and Content [g (wt%)] | Water [g (wt%)] | Plant Material [g (wt%)] | LAB Strain [g (wt%)] | Yeast Strain [g (wt%)] | LAB/Yeast Ratio | Mineral Salts [g (wt%)] | Lipase [g (wt%)] | Fermentation Temp [°C] | Time [Days] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 FCRM-1 | leaf | Beet and cane 2.5/2.5 (2.8/2.8) | 75 (83) | 0.05 (0.06) | 9 (10) | 1 (1.1) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 7 |
| 1 FCRM-2 | leaf | Beet and cane 2.5/2.5 (2.7/2.7) | 75 (82) | 2 (2.2) | 9 (10) | 1 (1.1) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 7 |
| 1 FCRM-3 | leaf | Beet and cane 2.5/2.5 (3.1/3.1) | 75 (92) | 0.25 (0.31) | 0.9 (0.9) | 0.1 (0.1) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 3 |
| 1 FCRM-4 | leaf | Beet and cane 2.5/2.5 (2.4/2.4) | 75 (71) | 0.25 (0.24) | 22.5 (21) | 2.5 (2.4) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 5 |
| 1 FCRM-5 | leaf | Beet and cane 0.25/0.25 (0.2/0.2) | 75 (74) | 0.25 (0.25) | 22.5 (22) | 2.5 (2.5) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 4 |
| 1 FCRM-6 | leaf | Beet and cane 7.5/7.5 (6.5/6.5) | 75 (65) | 0.25 (0.22) | 22.5 (20) | 2.5 (2.2) | 9:1 | - | 0.001 (0.01) | 29.5–31.5 | 6 |
| 2 FCRM-7 | roots | Cane 6 (12) | 30 (60) | 0.1 (0.2) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 3 FCRM-8 | roots | Cane 6 (12) | 30 (60) | 0.2 (0.4) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 4 FCRM-9 | roots | Cane 6 (12) | 30 (60) | 0.3 (0.6) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 5 FCRM-10 | roots | Cane 6 (12) | 30 (60) | 0.4 (0.8) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 6 FCRM-11 | roots | Cane 6 (12) | 30 (60) | 0.5 (1) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 7 FCRM-12 | roots | Cane 6 (12) | 30 (60) | 1 (1.9) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 8 FCRM-13 | roots | Cane 6 (12) | 30 (60) | 1.5 (2.9) | 10 (20) | - | - | 2/1/1 (4/2/2) | 0.07 (0.14) | 37.5 ± 0.5 | 10 |
| 3 FCRM-14 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (0.6/0.3/0.3) | 0.02 (0.006) | 37.5 ± 0.5 | 15 |
| 2 FCRM-15 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 14 |
| 5 FCRM-16 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 10 |
| 4 FCRM-17 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 14 |
| 6 FCRM-18 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 15 |
| 7 FCRM-19 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 15 |
| 8 FCRM-20 | flowers | Cane 18 (5.42) | 300 (90) | 12 (3.6) | 10 (3) | - | - | 2/1/1 (4/2/2) | 0.02 (0.006) | 37.5 ± 0.5 | 11 |
| Name FCRM | AA-DPPH [mmol Tx/L] | AA-ORAC [mmol Tx/L] | TPC [mg GA/L] |
|---|---|---|---|
| FCRM-1/NDE-1 | 1.0 ± 0.1/0.6 ± 0.1 | 0.44 ± 0.01/0.14 ± 0.01 | 1251 ± 12/252 ± 8 |
| FCRM-2/NDE-2 | 3.0 ± 0.1/1.9 ± 0.1 | 0.55 ± 0.02/0.19 ± 0.01 | 3589 ± 25/576 ± 10 |
| FCRM-3/NDE-3 | 0.8 ± 0.1/0.2 ± 0.1 | 0.46 ± 0.01/0.17 ± 0.01 | 1595 ± 11/621 ± 13 |
| FCRM-4/NDE-4 | 1.3 ± 0.1/0.4 ± 0.1 | 0.54 ± 0.02/0.18 ± 0.01 | 2256 ± 32/381 ± 12 |
| FCRM-5/NDE-5 | 1.2 ± 0.1/0.8 ± 0.1 | 0.54 ± 0.02/0.19 ± 0.01 | 2256 ± 32/186 ± 6 |
| FCRM-6/NDE-6 | 1.5 ± 0.1/0.5 ± 0.1 | 0.45 ± 0.01/0.16 ± 0.01 | 1603 ± 30/722 ± 14 |
| Name FCRM | AA-DPPH [mmol Tx/L] | AA-ORAC [mmol Tx/L] | TPC [mg GA/L] |
|---|---|---|---|
| FCRM-7 | 1.7 ± 0.1 | 0.49 ± 0.01 | 90 ± 1 |
| FCRM-8 | 1.9 ± 0.1 | 0.51 ± 0.01 | 114 ± 1 |
| FCRM-9 | 1.6 ± 0.1 | 0.50 ± 0.01 | 106 ± 1 |
| FCRM-10 | 1.2 ± 0.1 | 0.48 ± 0.01 | 106 ± 1 |
| FCRM-11 | 1.2 ± 0.1 | 0.49 ± 0.01 | 102 ± 2 |
| FCRM-12 | 1.2 ± 0.1 | 0.49 ± 0.01 | 109 ± 2 |
| FCRM-13 | 1.3 ± 0.1 | 0.50 ± 0.01 | 111 ± 2 |
| Name FCRM | AA-DPPH [mmol Tx/L] | AA-ORAC [mmol Tx/L] | TPC [mg GA/L] |
|---|---|---|---|
| FCRM-14 | 1.1 ± 0.1 | 0.49 ± 0.01 | 2380 ± 12 |
| FCRM-15 | 1.3 ± 0.1 | 0.49 ± 0.01 | 2419 ± 11 |
| FCRM-16 | 1.2 ± 0.1 | 0.49 ± 0.01 | 2397 ± 12 |
| FCRM-17 | 1.6 ± 0.1 | 0.50 ± 0.01 | 2555 ± 10 |
| FCRM-18 | 1.5 ± 0.1 | 0.49 ± 0.01 | 2511 ± 12 |
| FCRM-19 | 1.6 ± 0.1 | 0.50 ± 0.01 | 2536 ± 13 |
| FCRM-20 | 1.2 ± 0.1 | 0.49 ± 0.01 | 2380 ± 11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, E.; Wachura, D.; Elchiev, I.; Bilewicz, P.; Gąsiorowski, M.; Pełech, R. Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects. Appl. Sci. 2025, 15, 9021. https://doi.org/10.3390/app15169021
Kucharska E, Wachura D, Elchiev I, Bilewicz P, Gąsiorowski M, Pełech R. Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects. Applied Sciences. 2025; 15(16):9021. https://doi.org/10.3390/app15169021
Chicago/Turabian StyleKucharska, Edyta, Dominika Wachura, Iskenderbek Elchiev, Paweł Bilewicz, Marek Gąsiorowski, and Robert Pełech. 2025. "Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects" Applied Sciences 15, no. 16: 9021. https://doi.org/10.3390/app15169021
APA StyleKucharska, E., Wachura, D., Elchiev, I., Bilewicz, P., Gąsiorowski, M., & Pełech, R. (2025). Co-Fermentation of Dandelion Leaves (Taraxaci folium) as a Strategy for Increasing the Antioxidant Activity of Fermented Cosmetic Raw Materials—Current Progress and Prospects. Applied Sciences, 15(16), 9021. https://doi.org/10.3390/app15169021








