Microbiological and Sensory Quality of Artisanal Sour Cream
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Physicochemical Analysis
2.3. Microbiological Analysis
2.3.1. Isolation of Bacteria of the Salmonella Genus
2.3.2. Isolation of the Bacterial Species Listeria monocytogenes
2.3.3. Isolation of Coagulase-Positive Staphylococci/Staphylococcus aureus
2.3.4. Isolation of Enterobacteriaceae
2.3.5. Isolation of Escherichia coli
2.3.6. Yeast Isolation
2.3.7. Isolation of Sulphite-Reducing Clostridium spp.
2.4. Determination of Cream Colour
- -
- L*—lightness (lustre) of the cream, which can range from dark to light
- -
- a*—spectrum from green to red
- -
- b*—spectrum from blue to yellow
2.5. Sensory Analysis
2.6. Statistical Data Analysis
3. Results and Discussion
3.1. Physicochemical Composition of the Cream
Fatty Acid Composition
3.2. Microbiological Quality of the Cream
3.3. Sensory Analysis of Sour Cream Samples
3.3.1. Instrumental Colour
3.3.2. Cream Appearance
3.3.3. Odour Profile
3.3.4. Texture Properties
3.3.5. Taste Properties
3.3.6. Flavour Properties
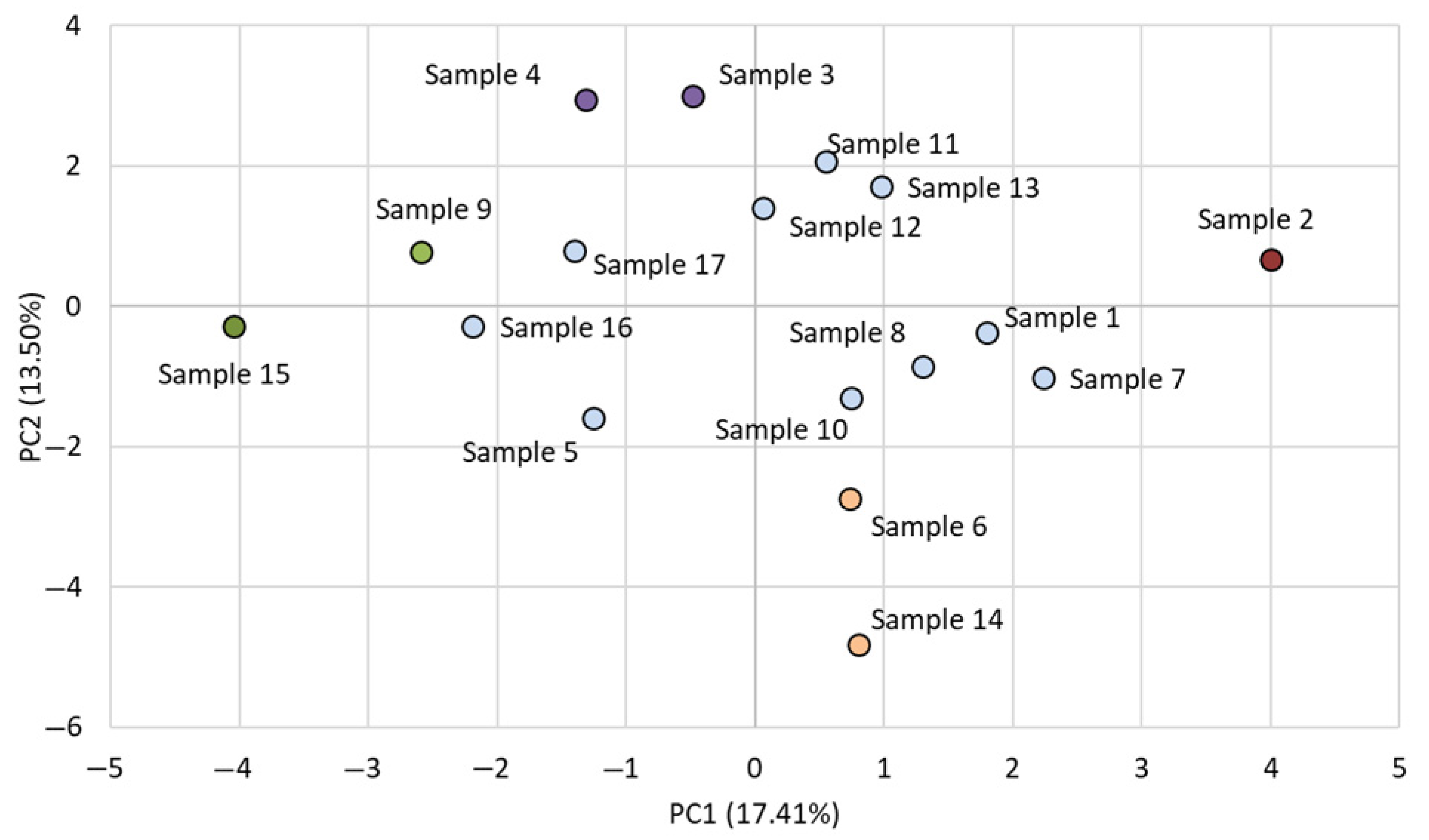
3.4. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- CXS 288-197; Codex Alimentarius. Standard for Cream and Prepared Creams. (FAO/WHO). Codex Alimentarius: Rome, Italy, 2022; Codex Standard 288. Available online: https://www.fao.org/fao-who-codexalimentarius/ (accessed on 1 February 2025).
- Samaržija, D. Dairy Microbiology; Croatian Dairy Association: Zagreb, Croatia, 2021; pp. 2–21, 130–176, 187–193, 228–235, 240–259. [Google Scholar]
- Official Gazette. Rulebook on the Registration and Approval of Facilities and on the Registration of Food Business Operators. NN 129/2019. 2019. Available online: https://narodne-novine.nn.hr/clanci/sluzbeni/2019_12_123_2434.html (accessed on 1 February 2025).
- Kirin, S. Bjelovar homemade cream. Mljekarstvo 2009, 59, 343–348. [Google Scholar]
- Hergešić, B. Mikrobiolological quality of sour cream. Mljekarstvo 1965, 15, 73–76. [Google Scholar]
- Samaržija, D. Fermented Milks, Cream and Butter. [Online]. University of Zagreb, Faculty of Agriculture. 2011. Available online: https://urn.nsk.hr/urn:nbn:hr:204:303724 (accessed on 1 February 2025).
- Tesfaw, L.B.; Alemu, S.; Alemayehu, H.; Sisay, Z.; Negussie, H. Prevalence and antimicrobial resistance profile of Salmonella isolates from dairy products of Addis Ababa, Ethiopia. Afr. J. Microbiol. Res. 2013, 7, 5046–5050. [Google Scholar] [CrossRef]
- Bintsis, T. Foodborne pathogens. AIMS Microbiol. 2017, 3, 529–563. [Google Scholar] [CrossRef] [PubMed]
- HRN EN ISO 2450; Determination of Fat Content—Gravimetric Method (Reference Method). Croatian Standards Institute: Zagreb, Croatia, 2008.
- HRN EN ISO 8968-1:2014; Determination of Nitrogen Content—Part 1: Kjeldahl Principle and Crude Protein Calculation. Croatian Standards Institute: Zagreb, Croatia, 2014.
- HRN ISO 6731:2010; Milk, Cream and Evaporated Milk—Determination of Total Solids Content (Reference Method). Croatian Standards Institute: Zagreb, Croatia, 2017.
- HRN ISO 22662:2024; Milk and Milk Products—Determination of Lactose Content by High-Performance Liquid Chromatography (Reference Method). Croatian Standards Institute: Zagreb, Croatia, 2010.
- HRN ISO 15884:2002; Milk Fat—Preparation of Fatty Acid Methyl Esters. Croatian Standards Institute: Zagreb, Croatia, 2003.
- HRN ISO 15885:2002; Milk Fat—Determination of The Fatty Acid Composition by Gas-Liquid Chromathography. Croatian Standards Institute: Zagreb, Croatia, 2003.
- ISO/TS 22113; Milk and Milk Products—Determination of the Titratable Acidity of Milk Fat. Croatian Standards Institute: Zagreb, Croatia, 2012.
- HRN EN ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. Croatian Standards Institute: Zagreb, Croatia, 2017.
- HRN EN ISO 6579-1:2017/A1; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp.—Amendment 1 Broader Range of Incubation Temperatures, Amendment to the Status of Annex D, and Correction of the Composition of MSRV and SC. Croatian Standards Institute: Zagreb, Croatia, 2020.
- HRN EN ISO 11290-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Listeria Monocytogenes and Other Listeria spp.—Part 1: Detection Method. Croatian Standards Institute: Zagreb, Croatia, 2017.
- HRN EN ISO 6888-1:2021/A1:2023; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Coagulase-Positive Staphylococci (Staphylococcus aureus and Other Species)—Part 1: Method Using Baird-Parker Agar Medium. Croatian Standards Institute: Zagreb, Croatia, 2023.
- HRN EN ISO 21528-2:2017; Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 2: Colony-Count Technique. Croatian Standards Institute: Zagreb, Croatia, 2017.
- HRN ISO 16649-2:2001; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Beta-Glucuronidase-Positive Escherichia coli—Part 2: Colony-Count Technique at 44 Degrees C Using 5-Bromo-4-chloro-3-indolyl Beta-D-Glucuronide. Croatian Standards Institute: Zagreb, Croatia, 2001.
- HRN ISO 21527-1:2008; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Molds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95. Croatian Standards Institute: Zagreb, Croatia, 2012.
- HRN EN ISO 15213-1:2023; Microbiology of The Food Chain—Horizontal Method for the Detection and Enumeration of Clostridium spp.—Part 1: Enumeration of Sulfite-Reducing Clostridium spp. by Colony-Count Technique. Croatian Standards Institute: Zagreb, Croatia, 2023.
- CIE. Recommendations on Uniform Color Spaces, Color Difference Equations, Psychometric Color Terms (Supplement nº. 2 of Publication Commission Internationale de l’Eclairage (CIE) nº. 15 (E-1.3.1); Bureau Central de la CIE: Paris, France, 1978. [Google Scholar]
- HRN ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. Croatian Standards Institute: Zagreb, Croatia, 2008.
- ISO 22935-1; Milk and Milk Products—Sensory Analysis—Part 1: Recruitment, Selection, Training and Monitoring of Assessors. Croatian Standards Institute: Zagreb, Croatia, 2023.
- SAS Institute. SAS Studio, Enterprise Edition, Release: 3.81; SAS Institute: Cary, NC, USA, 2022. [Google Scholar]
- Konjačić, M.; Marković, T.; Ivanković, A.; Stručić, D. Effect of year and season on the chemical composition and hygienic quality of organic cow’s milk. Stockbreeding: J. Anim. Improv. 2015, 69, 45–53. [Google Scholar]
- Lukač, J.; Samaržija, D. Quality of home-made dairy products on Zagreb green-market. Mljekarstvo 1990, 40, 209–215. [Google Scholar]
- Barbir, T.; Vulić, A.; Pleadin, J. Fats and fatty acids in food of animal origin. Vet. Pract. 2014, 45, 97–110. [Google Scholar]
- Izsó, T.; Kasza, G.Y.; Somogyı, L. Differences between fat-related characteristics of sour cream and sour cream analogues. Acta Aliment. 2020, 49, 390–397. [Google Scholar] [CrossRef]
- Mieželienė, A.; Alenčikienė, G.; Zaborskienė, G.; Garmienė, G. Effect of fat origin on sensory and physicochemical characteristics of sour cream. Milchwissenschaft 2012, 67, 64–67. [Google Scholar]
- Palmquist, D.L.; Beaulieu, A.D.; Barbano, D.M. Feed and Animal Factors Influencing Milk Fat Composition. J. Dairy Sci. 1993, 76, 1753–1771. [Google Scholar] [CrossRef] [PubMed]
- Samaržija, D.; Podoreški, M.; Sikora, S.; Skelin, A.; Pogačić, T. Spoilage microorganisms in milk and dairy products. Mljekarstvo 2007, 57, 251–273. [Google Scholar]
- Markov, K.; Frece, J.; Čvek, D.; Delaš, F. Listeria monocytogenes and other contaminants in fresh cheese and cream from Zagreb city area domestic production. Mljekarstvo 2009, 59, 225–231. [Google Scholar]
- Shepard, L.; Miracle, R.E.; Leksrisompong, P.; Drake, M.A. Relating sensory and chemical properties of sour cream to consumer acceptance. J. Dairy Sci. 2013, 96, 5435–5454. [Google Scholar] [CrossRef] [PubMed]
- European Union. Cream. Modern and Innovative Online-Based Know-How on European Dairy Processing (Milk-ed). 2022. Available online: https://milk-ed.eu/wp-content/uploads/2022/05/Cream_HR.pdf (accessed on 1 February 2025).
- Jervis, S.M.; Gerard, P.; Drake, S.; Lopetcharat, K.; Drake, M.A. The perception of creaminess in sour cream. J. Sens. Stud. 2014, 29, 248–257. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021, 12, 612285. [Google Scholar] [CrossRef] [PubMed]
- Wilbey, R.A. Microbiology of cream and butter. In Dairy Microbiology Handbook: The Microbiology of Milk and Milk Products; Wiley-Interscience: Hoboken, NJ, USA, 2002; pp. 123–174. [Google Scholar]
- Li, D.; Cui, Y.; Wu, X.; Li, J.; Min, F.; Zhao, T.; Zhang, J.; Zhang, J. Graduate Student Literature Review: Network of flavor compounds formation and influence factors in yogurt. J. Dairy Sci. 2024, 107, 8874–8886. [Google Scholar] [CrossRef] [PubMed]
- Bandić, L.M.; Oštarić, F.; Vinceković, M.; Mikulec, N. Biochemistry of aroma compounds in cheese. Mljekarstvo 2023, 73, 211–224. [Google Scholar] [CrossRef]
- Tian, H.; Xiong, J.; Yu, H.; Chen, C.; Lou, X. Flavor optimization in dairy fermentation: From strain screening and metabolic diversity to aroma regulation. Trends Food Sci. Technol. 2023, 141, 104194. [Google Scholar] [CrossRef]
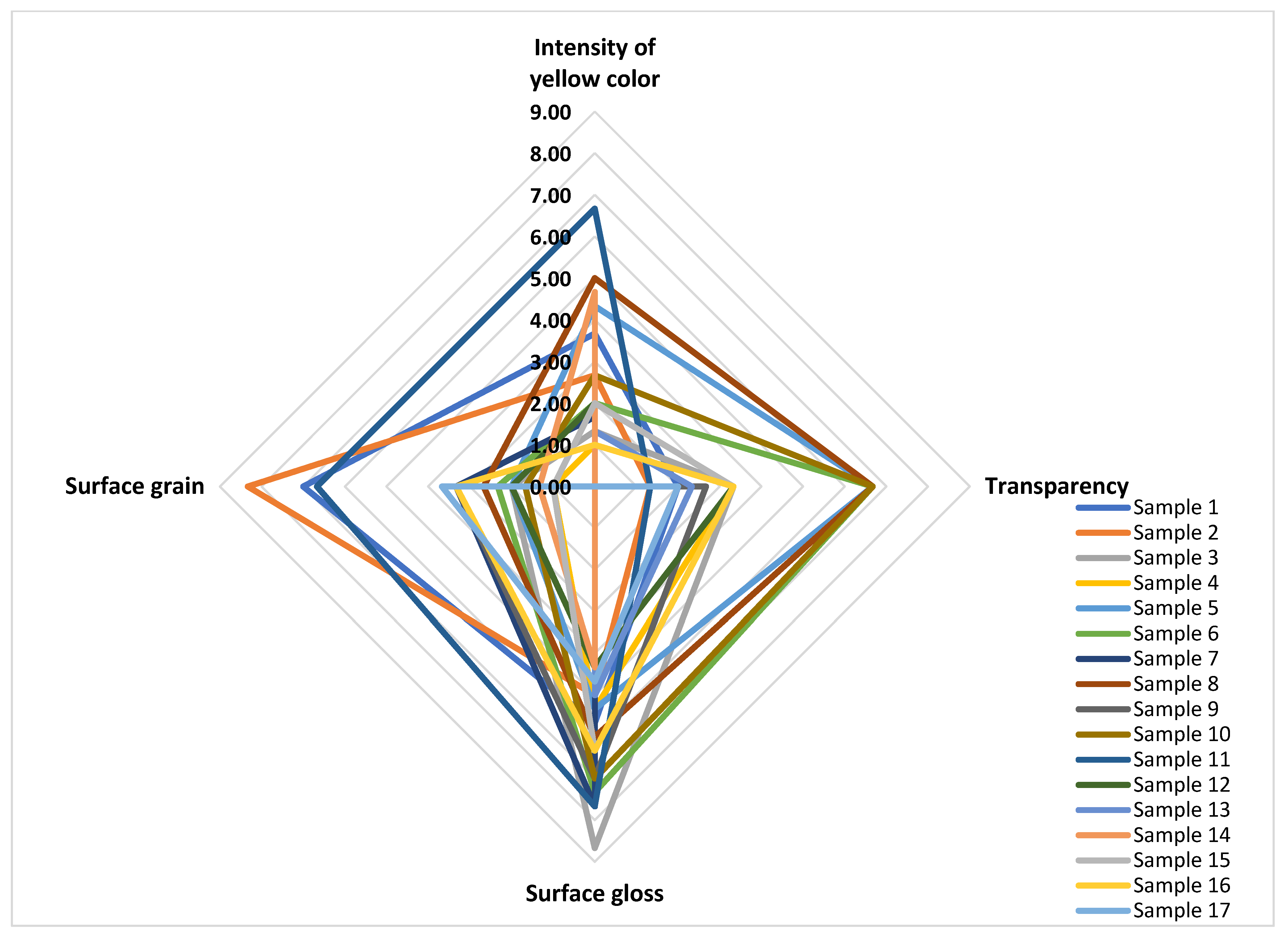
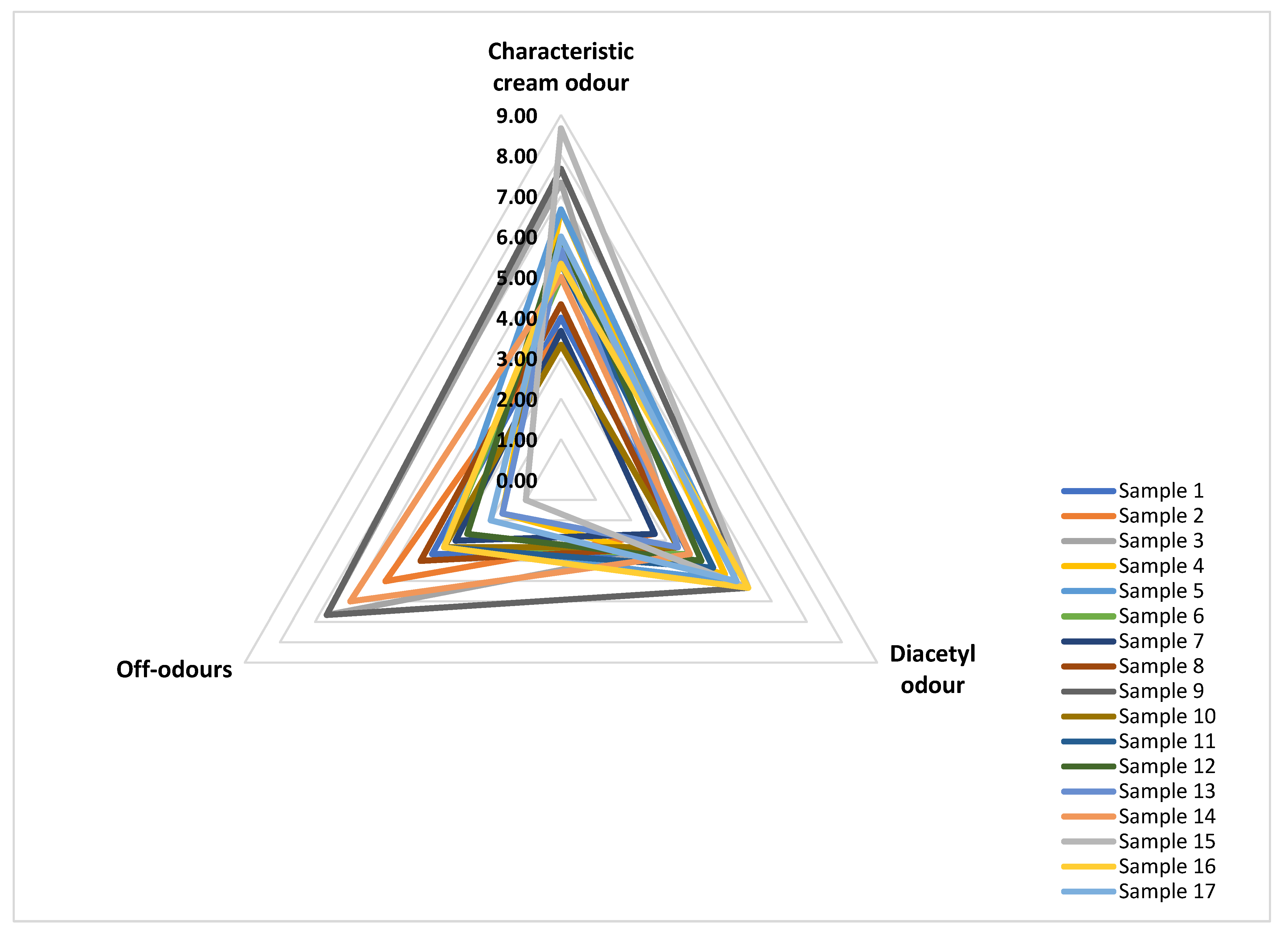
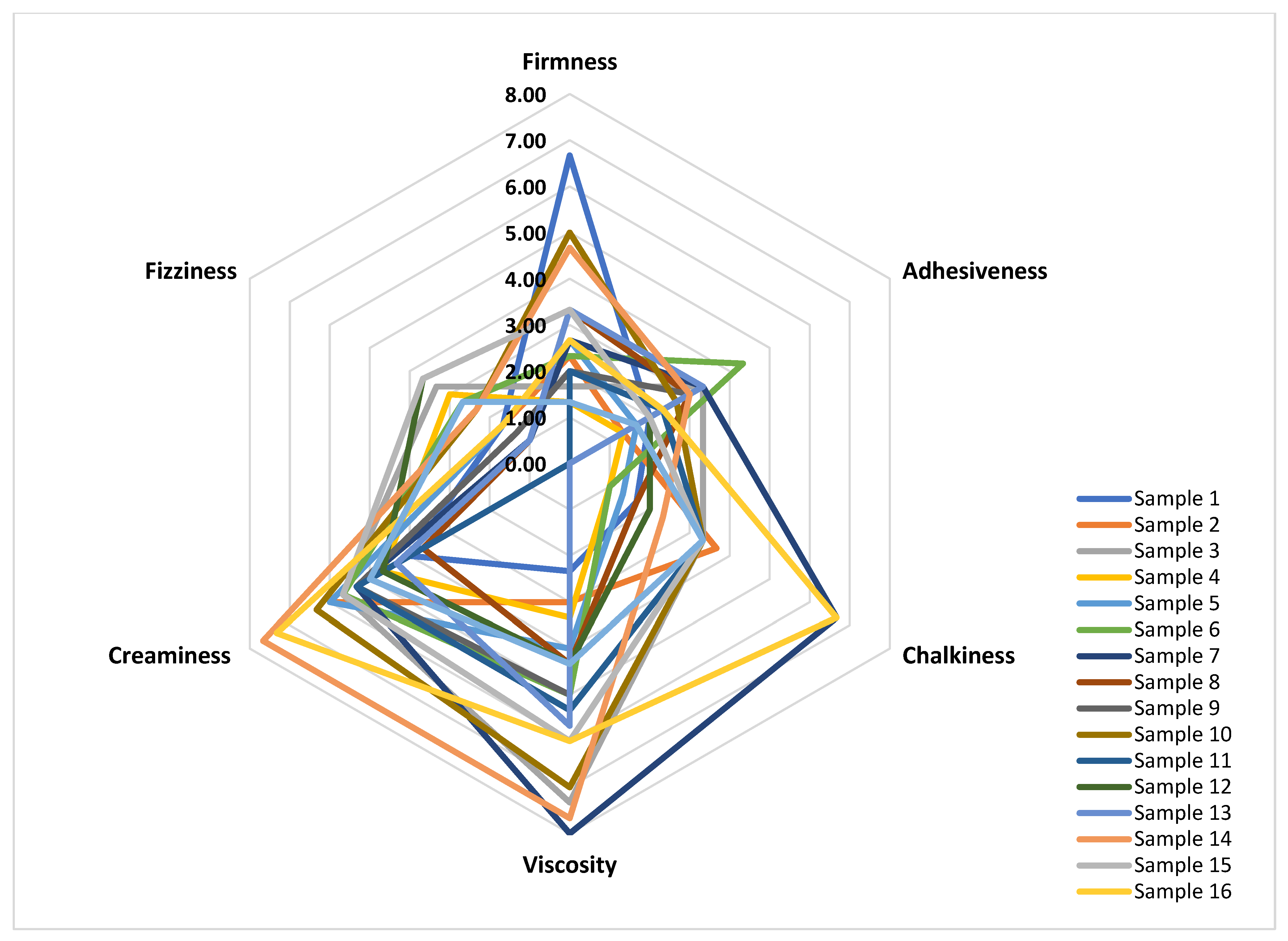


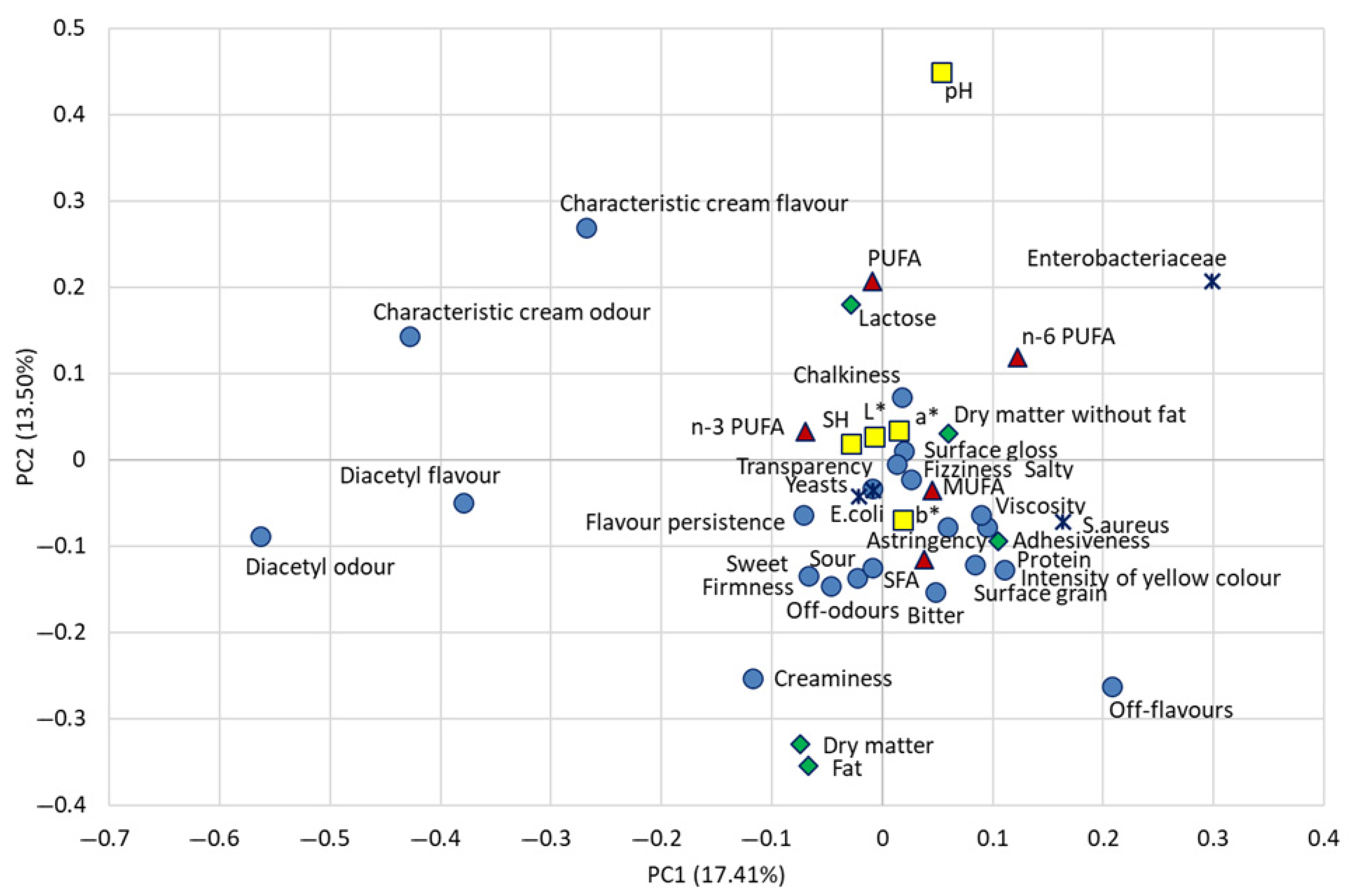
| Sensory Attribute | Definition |
|---|---|
| Appearance | |
| Intensity of yellow colour | Intensity of yellow colour (white to yellow) |
| Transparency | Degree of transparency (opaque to fully transparent) |
| Surface gloss | Intensity of surface gloss (dull to glossy) |
| Surface grain | Visibility of surface grain (smooth to grainy) |
| Odour | |
| Characteristic cream odour | Intensity of typical cream odour (absent to intense) |
| Diacetyl odour | Intensity of buttery (diacetyl) odour (absent to intense) |
| Off-odours | Intensity of off-odours (absent to intense) |
| Texture | |
| Firmness | Intensity of firmness (soft to firm) |
| Adhesiveness | Degree of adhesiveness in mouth (non-adhesive to highly adhesive) |
| Chalkiness | Perception of chalky mouthfeel (absent to pronounced) |
| Viscosity | Intensity of viscosity (liquid to thick) |
| Creaminess | Intensity of creaminess (watery to very creamy) |
| Fizziness | Presence of fizziness or effervescence (absent to strong) |
| Taste | |
| Sweet | Intensity of sweet taste (not sweet to very sweet) |
| Salty | Intensity of salty taste (not salty to very salty) |
| Sour | Intensity of sour taste (not sour to very sour) |
| Bitter | Intensity of bitter taste (not bitter to very bitter) |
| Astringency | Intensity of astringency (none to strong) |
| Flavour | |
| Characteristic cream flavour | Intensity of typical cream flavour (absent to strong) |
| Diacetyl flavour | Intensity of buttery (diacetyl) flavour (absent to strong) |
| Off-flavours | Intensity of undesirable flavours (none to strong) |
| Flavour persistence | Duration of flavour after swallowing (short to long) |
| Sample | Milk Fat (%) | Protein (%) | TS (%) | SNF (%) | Lactose (%) | pH | °SH |
|---|---|---|---|---|---|---|---|
| 1 | 27.74 | 2.93 | 33.7 | 6.3 | 3.03 | 4.48 | 8.40 |
| 2 | 18.61 | 4.77 | 25.08 | 5.55 | 1.70 | 4.53 | 7.84 |
| 3 | 19.35 | 2.92 | 26.76 | 8.02 | 4.49 | 4.70 | 7.72 |
| 4 | 23.16 | 3.13 | 29.93 | 7.42 | 3.64 | 4.67 | 5.60 |
| 5 | 33.30 | 2.89 | 38.33 | 4.78 | 2.14 | 4.37 | 12.00 |
| 6 | 39.80 | 2.72 | 44.3 | 4.75 | 1.78 | 4.11 | 5.24 |
| 7 | 30.80 | 3.52 | 36.89 | 6.51 | 2.57 | 4.30 | 5.24 |
| 8 | 36.52 | 2.96 | 42.25 | 7.16 | 2.77 | 4.58 | 5.68 |
| 9 | 25.28 | 3.25 | 31.70 | 6.71 | 3.17 | 4.35 | 9.12 |
| 10 | 33.99 | 3.05 | 40.41 | 7.42 | 3.37 | 4.25 | 3.92 |
| 11 | 15.36 | 3.23 | 23.11 | 8.02 | 4.52 | 4.71 | 14.24 |
| 12 | 25.92 | 3.51 | 32.80 | 6.58 | 3.37 | 4.59 | 9.20 |
| 13 | 26.30 | 3.19 | 31.41 | 4.34 | 1.92 | 4.31 | 11.48 |
| 14 | 35.89 | 4.54 | 42.74 | 6.99 | 2.31 | 4.09 | 9.36 |
| 15 | 29.92 | 3.29 | 35.99 | 5.78 | 2.78 | 4.11 | 14.84 |
| 16 | 29.41 | 3.19 | 36.19 | 7.19 | 3.59 | 4.36 | 9.04 |
| 17 | 24.42 | 3.09 | 31.38 | 7.18 | 3.87 | 4.35 | 20.92 |
| Min | 15.36 | 2.72 | 23.11 | 4.34 | 1.70 | 4.09 | 3.92 |
| Max | 39.80 | 4.77 | 44.30 | 8.02 | 4.52 | 4.71 | 20.92 |
| Mean | 27.99 | 3.30 | 34.29 | 6.51 | 3.00 | 4.40 | 9.40 |
| SD | 6.73 | 0.55 | 6.19 | 1.12 | 0.87 | 0.20 | 4.30 |
| CV | 24.06 | 16.68 | 18.06 | 17.22 | 29.09 | 4.62 | 45.76 |
| C4:0 | C6:0 | C8:0 | C10:0 | C12:0 | C14:0 | C15:0 | C16:0 | C18:0 | SFA (%) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.21 | 2.34 | 1.32 | 2.82 | 3.15 | 10.73 | 1.10 | 26.85 | 10.94 | 64.56 |
| 2 | 3.58 | 2.26 | 1.37 | 3.31 | 3.77 | 12.31 | 0.00 | 32.18 | 7.99 | 68.09 |
| 3 | 4.09 | 2.31 | 1.33 | 2.93 | 3.34 | 11.27 | 1.19 | 28.11 | 11.28 | 66.80 |
| 4 | 3.45 | 2.46 | 2.43 | 2.58 | 2.87 | 10.24 | 2.02 | 29.45 | 11.17 | 67.44 |
| 5 | 3.94 | 2.21 | 1.21 | 2.51 | 2.85 | 11.05 | 1.17 | 29.62 | 10.14 | 65.48 |
| 6 | 3.87 | 1.96 | 1.04 | 2.13 | 2.40 | 9.34 | 1.06 | 28.48 | 10.91 | 62.20 |
| 7 | 4.49 | 2.37 | 1.34 | 3.09 | 3.64 | 11.94 | 1.62 | 29.45 | 10.04 | 69.20 |
| 8 | 5.42 | 2.09 | 0.95 | 1.62 | 1.67 | 6.91 | 0.81 | 26.65 | 12.81 | 60.35 |
| 9 | 4.59 | 2.49 | 1.39 | 2.88 | 3.16 | 11.39 | 1.18 | 27.42 | 11.53 | 67.18 |
| 10 | 5.55 | 2.73 | 1.52 | 3.11 | 3.52 | 11.07 | 1.03 | 31.64 | 10.78 | 72.47 |
| 11 | 4.92 | 2.37 | 1.27 | 2.62 | 2.93 | 9.66 | 1.10 | 26.84 | 10.59 | 64.42 |
| 12 | 4.20 | 1.96 | 1.10 | 2.12 | 2.40 | 8.19 | 1.06 | 24.17 | 8.92 | 58.03 |
| 13 | 3.61 | 1.87 | 1.02 | 2.18 | 2.61 | 9.66 | 1.44 | 28.44 | 10.35 | 62.58 |
| 14 | 4.90 | 2.49 | 1.30 | 2.58 | 2.82 | 10.32 | 1.71 | 28.02 | 9.90 | 66.12 |
| 15 | 3.84 | 2.28 | 1.34 | 3.07 | 3.58 | 11.60 | 1.16 | 31.18 | 9.36 | 68.31 |
| 16 | 3.73 | 2.27 | 1.37 | 3.26 | 3.83 | 12.08 | 1.11 | 31.45 | 10.47 | 70.67 |
| 17 | 3.88 | 2.22 | 1.23 | 2.67 | 3.08 | 11.53 | 1.18 | 31.58 | 10.41 | 68.95 |
| Sample | MUFA | PUFA | |||
|---|---|---|---|---|---|
| C14:1 | C16:1 | C18:1n9 | C18:2n6 | C18:3n3 | |
| 1 | 0.77 | 1.54 | 25.07 | 2.23 | 0.71 |
| 2 | 0.88 | 1.56 | 20.14 | 2.33 | 0.78 |
| 3 | 0.89 | 1.48 | 23.60 | 1.98 | 0.50 |
| 4 | 1.58 | 1.78 | 20.81 | 2.54 | 0.14 |
| 5 | 1.02 | 1.62 | 24.56 | 1.69 | 0.31 |
| 6 | 0.75 | 1.71 | 28.09 | 2.03 | 0.44 |
| 7 | 1.06 | 1.96 | 21.75 | 1.66 | 1.32 |
| 8 | 0.54 | 2.18 | 33.89 | 2.07 | 0.23 |
| 9 | 0.77 | 1.14 | 23.51 | 1.94 | 0.42 |
| 10 | 0.93 | 1.50 | 20.60 | 2.75 | 0.00 |
| 11 | 0.81 | 1.44 | 21.94 | 1.85 | 0.69 |
| 12 | 0.00 | 1.52 | 18.80 | 2.01 | 1.07 |
| 13 | 0.86 | 0.10 | 26.32 | 1.89 | 0.47 |
| 14 | 0.80 | 1.65 | 22.62 | 0.59 | 1.24 |
| 15 | 0.94 | 1.64 | 21.79 | 2.06 | 0.53 |
| 16 | 0.77 | 1.40 | 20.56 | 2.09 | 0.38 |
| 17 | 1.02 | 1.31 | 22.86 | 2.00 | 0.00 |
| Sample | Yeast cfu/mL | B. spp. cfu/mL | L. mon. cfu/mL | St. aur. cfu/mL | Ent. cfu/mL | Sal. cfu/mL | E. coli cfu/mL | Cl. spp. cfu/mL |
|---|---|---|---|---|---|---|---|---|
| 1 | 72,727 | + | − | 13,636 | 973 | − | 250 | <10 |
| 2 | 54,545 | <10 | − | <100 | 80,000 | − | 3909 | <10 |
| 3 | 50,000 | <10 | − | <100 | 6364 | − | 1364 | <10 |
| 4 | 90,000 | <10 | − | <100 | 20,000 | − | 9091 | <10 |
| 5 | 86,364 | <10 | − | <100 | 15,636 | − | 11,364 | <10 |
| 6 | 104,545 | <10 | − | 3636 | 30,000 | − | 12,727 | <10 |
| 7 | 145,455 | <10 | − | 27,273 | 13,636 | − | 35 | <10 |
| 8 | 109,091 | <10 | − | <100 | 1445 | − | 180 | <10 |
| 9 | 90,909 | <10 | − | <100 | 718 | − | 409 | <10 |
| 10 | 50,000 | + | − | 3455 | 18,182 | − | 3636 | <10 |
| 11 | 55,455 | <10 | − | <100 | 16,364 | − | 4545 | <10 |
| 12 | 172,727 | + | − | <100 | 19,091 | − | 5455 | <10 |
| 13 | 70,909 | <10 | − | 2273 | 59,091 | − | 2727 | <10 |
| 14 | 54,545 | <10 | − | <100 | 973 | − | 255 | <10 |
| 15 | 107,273 | <10 | − | <100 | 636 | − | 150 | <10 |
| 16 | 81,818 | <10 | − | <100 | 10,090 | − | 4773 | <10 |
| 17 | 163,636 | + | − | 1909 | 3636 | − | 955 | <10 |
| Colour Parameter | |||
|---|---|---|---|
| Sample | L* | a* | b* |
| 1 | 97.34 | −2.02 | 17.29 |
| 2 | 99.62 | −2.72 | 17.15 |
| 3 | 102.33 | −1.88 | 14.3 |
| 4 | 98.29 | −1.97 | 9.73 |
| 5 | 102.29 | −1.14 | 16.81 |
| 6 | 99.53 | −1.06 | 15.26 |
| 7 | 101.12 | −1.72 | 15.83 |
| 8 | 97.63 | −1.99 | 24.14 |
| 9 | 98.24 | −1.86 | 8.69 |
| 10 | 99.1 | −0.94 | 15.32 |
| 11 | 99.6 | −2.44 | 13.08 |
| 12 | 96.98 | −1.84 | 16.57 |
| 13 | 96.72 | −1.76 | 13.02 |
| 14 | 99.11 | −3.26 | 23.47 |
| 15 | 101.57 | −3.07 | 18.78 |
| 16 | 99.94 | −1.13 | 14.71 |
| 17 | 99.52 | −1.93 | 11.57 |
| Min | 96.72 | −3.26 | 8.69 |
| Max | 102.33 | −0.94 | 24.14 |
| Mean | 99.35 | −1.93 | 15.63 |
| SD | 1.73 | 0.66 | 4.08 |
| CV | 1.75 | 34.49 | 26.13 |
| L* | a* | b* | p-Value | |||
|---|---|---|---|---|---|---|
| a* | −0.49 | 0.0174 | ||||
| b* | 0.31 | 0.43 | 0.1511 | 0.042 | ||
| Milk fat | 0.62 | −0.02 | 0.74 | 0.0017 | 0.909 | 0.00005 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bendelja Ljoljić, D.; Boroša, M.; Kos, I.; Cvetnić, L.; Vnučec, I.; Hulak, N.; Radeljević, B.; Jaki Tkalec, V. Microbiological and Sensory Quality of Artisanal Sour Cream. Appl. Sci. 2025, 15, 8234. https://doi.org/10.3390/app15158234
Bendelja Ljoljić D, Boroša M, Kos I, Cvetnić L, Vnučec I, Hulak N, Radeljević B, Jaki Tkalec V. Microbiological and Sensory Quality of Artisanal Sour Cream. Applied Sciences. 2025; 15(15):8234. https://doi.org/10.3390/app15158234
Chicago/Turabian StyleBendelja Ljoljić, Darija, Melita Boroša, Ivica Kos, Luka Cvetnić, Ivan Vnučec, Nataša Hulak, Biljana Radeljević, and Vesna Jaki Tkalec. 2025. "Microbiological and Sensory Quality of Artisanal Sour Cream" Applied Sciences 15, no. 15: 8234. https://doi.org/10.3390/app15158234
APA StyleBendelja Ljoljić, D., Boroša, M., Kos, I., Cvetnić, L., Vnučec, I., Hulak, N., Radeljević, B., & Jaki Tkalec, V. (2025). Microbiological and Sensory Quality of Artisanal Sour Cream. Applied Sciences, 15(15), 8234. https://doi.org/10.3390/app15158234








