Abstract
In the presented work, an attempt was made to digest kefir enriched with microalgae additives from the species Arthrospira platensis and Chlorella pyrenoidosa in four concentrations—0.1, 0.5, 1, and 5%. The level of released protein, phosphorus, iron, iodine, and selected vitamins from the B group was analyzed, and their bioavailability was additionally estimated. The amount of iron released in these conditions increased significantly from 0.1% of the supplementation level. Higher values of iron were obtained for Chlorella, and in the case of protein, slightly higher values were noted for Spirulina. In turn, for vitamin B2, higher amounts were noted for Chlorella for doses of 1 and 5%. In the case of vitamin B12, significantly higher amounts were noted in the case of Spirulina supplementation. After in vitro digestion, an increase in the bioavailability of protein and phosphorus was observed with an increase in the dose of microalgae. The relative bioavailability of iron decreased with an increase in the dose of microalgae used, similarly to vitamin B12. Chlorella was characterized by higher iron bioavailability than Spirulina, and in the case of vitamin B2 only at the highest doses of 1 and 5% of the algal supplement. The tests carried out show that microalgae supplementation significantly increases the content of protein, phosphorus, iron, and vitamin B12 in the tested kefir.
1. Introduction
Diet plays a crucial role in health and well-being, leading to increased interest in functional foods. The definition of such foods includes, among others, milk and dairy products containing probiotic bacteria cultures and enriched with additional ingredients that significantly increase the nutritional properties of the final product [1,2]. Microalgae can become such an additive to improve the nutritional value. Over the last decade, we have observed a growing interest in food supplementation with microalgae and extracts or other ingredients derived from microalgae biomass. This is mainly due to the high content of protein and valuable vitamins and minerals. The most popular microalgae used in industry are Spirulina (Arthrospira platensis) and Chlorella (Chlorella pyrenoidosa), which are obtained in mass cultures in photobioreactors. Currently, due to their chemical composition, they play a key role in aquaculture, as a component of feed [3]. Fish fed with microalgae show faster growth and are characterized by an increased content of valuable nutrients in their meat [4]. In addition, microalgae are used in the production of cosmetics [5] and in the production of biofuels [6]. In the case of the food industry, microalgae are currently mainly used as a food dye—directly or extracted from them, e.g., chlorophylls and carotenoids [7,8]. These beneficial features of microalgae have not yet found their full application, and they can be relatively easily used as a valuable food additive [5]. Moreover, microalgae such as Spirulina have been historically consumed in the Americas (Mexico) and Africa (Chad) for a long time [9]. After drying, they can be taken in the form of a powder added to food and drinks or as tablets swallowed during a meal [10].
The group of numerous substances with bioactive potential, occurring in significant quantities in microalgae of the Arthrospira and Chlorella species, includes, among others, carotenoids, fatty acids, vitamins (mainly from the B group), micro- and macroelements (zinc, iodine, bromine, iron, copper, phosphorus, and magnesium) as well as high-value protein, which can constitute 60–70% of the dry mass [11]. The high protein content in microalgae is mainly due to proteins participating in photosynthesis. In cyanobacteria such as Arthrospira, it is mainly phycocyanin, which can constitute 15–20% of the dry mass of algae. This protein has a blue color and is responsible for the absorption of energy from orange and red light, which it then transfers to chlorophyll. Phycocyanin has strong antioxidant properties, inhibits the growth of cancer cells in vitro, and can bind metals, which is why it is often used as a component of various types of preparations in the cosmetic and pharmaceutical industries [12,13]. It is worth noting, however, that within the same species of microalgae, their chemical composition is not constant and depends on factors that determine growth in the culture, such as temperature, pH, lighting, mineral content in the medium, and carbon dioxide concentration [3]. Therefore, algal preparations obtained from the same species of microalgae, but from different manufacturers, will significantly differ in the content of nutrients, e.g., protein or iron.
The food industry has explored the use of microalgae in various products, such as bread [14] and cookies [15]. Research by Donato et al. (2019) [16] found that adding Spirulina to cookies increased their protein content; cookies with a 15% Spirulina addition had the highest protein and mineral levels.
Dairy products are a promising base for microalgae enrichment. Kefir is a valued dairy product due to its probiotic properties and rich nutritional composition. Its nutritional value and the bioavailability of its constituents are typically higher than that of milk, thanks to the fermentation process. However, the values reported in the literature may vary depending on the milk used for production (e.g., milk source, season, and feeding method), the specific starter cultures used, and the fermentation and processing conditions ([17,18]). Typically, the protein content in kefir based on cow’s milk ranges from 2.7 to 4 g/100 g, the fat content ranges from 0.5 (for skimmed milk) to 3.5 g/100 g, and carbohydrates range from 3 to 6 g/100 g. Furthermore, the phosphorus content is reported to be in the range of 90 to even 300 mg/100 g, riboflavin from 0.15 to 0.2 mg/100 g, and vitamin B12 from 0.2 to 0.5 µg/100 g (Rosa 2017 [18], Otles 2003 [19], La Torre 2024 [17], Yilmaz-Ersan 2024 [20]). All of these aforementioned components are characterized by high bioavailability. Iron, however, is present in small quantities—typically below 0.1 mg/100 g—and its bioavailability is also low due to interactions with milk proteins and calcium, a characteristic shared with other dairy products (Perales 2006 [21]). Kefir, being a fermented product, provides probiotic bacteria beneficial to the digestive system. Enriching such products with additional nutrients significantly improves their nutritional value [22,23]. Moreover, polysaccharides present in microalgae may exhibit prebiotic activity, positively affecting the intestinal microflora [24]. The complex chemical composition of microalgae may improve the quality of fermented dairy products. Their addition may stimulate the growth and survival of beneficial bacteria, such as Lactobacillus acidophilus and Lactobacillus bulgaricus, both during fermentation and storage [10]. In vitro studies on the digestibility and absorption of nutrients from kefir enriched with microalgae may be crucial to understanding how different processing methods affect their availability. During the digestion of algal proteins, active peptides are released, which can act as inhibitors of the angiotensin-converting enzyme ACE [25] or perform other therapeutic functions, e.g., antioxidant or antibacterial. Adding microalgae rich in bioactive compounds to fermented milk drinks could significantly enhance their properties, strengthening their position as functional food.
The enrichment of dairy products with algae, however, is associated with a number of limiting factors that pose a significant challenge. At least four key areas of such challenges can be distinguished: sensory properties, technological challenges, consumer attitudes, and safety issues [26,27]. Depending on the dose, the algal additive can significantly affect the product’s sensory properties. Spirulina or Chlorella often impart an intense taste and smell (“fishy” or “grassy” notes) and can alter the natural color of the dairy product (to green or bluish). Furthermore, they may change its texture by increasing graininess and viscosity. Conversely, many valuable nutrients from microalgae (vitamins, proteins, and unsaturated fatty acids) are sensitive to processing conditions (heat, light, and oxygen) encountered during food production. This sensitivity can lead to the loss of their nutritional or functional value. Moreover, microalgae components can interact with the dairy matrix constituents, thereby affecting product stability and structure. Microalgae are frequently associated with dietary supplements or health foods, yet their widespread introduction into everyday dairy products necessitates a shift in consumer perception. For many consumers, microalgae are perceived as an “unconventional” food, which can lead to distrust. In addition, some microalgae may carry the risk of accumulating heavy metals (e.g., cadmium, lead, and arsenic) or producing toxins (e.g., microcystin), which require rigorous quality controls, especially when cultivated in open systems. Finally, there is also a potential risk of allergic reactions to certain components of microalgae.
The aim of the conducted research was to enrich kefir with microalgae additives of two species, Arthrospira platensis and Chlorella pyrenoidosa. The work focused on the analysis of the estimated quantity and bioavailability of selected nutrients released after in vitro digestion, which in the context of their potentially high content in the introduced algal additives could be a quality characteristic of the final product. For this purpose, the content and bioavailability of protein, phosphorus, iron, iodine, and vitamins B2 and B12 were analyzed.
2. Materials and Methods
2.1. Tested Material
For this study, commercially available kefir (a fermented milk product) was used. To this kefir, powdered microalgae, specifically Arthrospira platensis (Spirulina) and Chlorella pyrenoidosa (Chlorella), were added. The microalgae were purchased as a dietary supplement from a local health food store. The kefir, produced by the OSM Krasnystaw (Krasnystaw, Poland) company, had a manufacturer-declared composition of milk, milk proteins, live bacterial cultures, and kefir grains. The microalgae, supplied by the Green Essence (M-Internet, Pyrzyce, Poland) company, contained solely dried Spirulina or Chlorella, without any additional ingredients. The determined nutritional values of the tested materials are presented in Table 1, with the manufacturer’s declared values included in brackets. Immediately prior to the in vitro digestion experiment, the microalgae were incorporated into portions of kefir at concentrations of 0.1, 0.5, 1, and 5% (w/w). The product was mixed until it had a uniform color. The material prepared in this way was subjected to in vitro digestion to determine the amount of released vitamins, protein, phosphorus, iron, and iodine and to determine their bioavailability. In order to determine the total content of phosphorus, iron, and protein, the tested material was subjected to mineralization. For iodine determination, alkaline extraction with tetramethylammonium hydroxide (TMAH) was performed. The term “in vitro bioavailability” was defined as the ratio between the content of the tested compounds in the dialysate that crossed the pore barrier in the dialysis membrane and found themselves in the buffer solution during simulated in vitro digestion, relative to their total content in the starting material, expressed as % (w/w).

Table 1.
Determined nutritional value of the tested material; the values declared by the manufacturer are given in brackets; n.d.—no data.
2.2. In Vitro Digestion
In vitro digestion was performed using the method described in our previous work [14]. To 0.5 g of the homogenized research material sample, 0.2 mL of pepsin (6 mg/mL) (P6887, Sigma-Aldrich) was added. Subsequently, 0.5 M HCl was introduced to adjust the pH to 2. The solution was then diluted with deionized water to a total volume of 2 mL, mixed and placed in a water bath at 37 °C for 2 h (simulated stomach). Next, 0.5 mL of pancreatin solution (9 mg/mL) (P-7545, Sigma-Aldrich), along with bile, was added followed by sufficient 1 M NaHCO3 to adjust the pH to 7. The final volume was then brought to 4.5 mL with deionized water and the mixture was thoroughly agitated. The solution was transferred to dialysis bags (Sigma-Aldrich (St. Louis, MO, USA), cellulose membrane, 25 mm × 90 mm, MWCO 12000), which were placed in flasks containing 45.5 mL of 0.05 M succinate buffer, pH 6.1, in 0.1 M NaCl. The flasks were incubated on a shaker at 37 °C for 4 h, corresponding to digestion and absorption in the intestine. The dialysates were stored frozen (−20 °C) until further analysis. Each sample was performed in triplicate.
2.3. Mineralization
In order to determine the total content of phosphorus, iron, and nitrogen, kefir and microalgae samples were mineralized. For this purpose, approximately 250 mg of the tested sample was placed in a combustion flask, and 4 mL of concentrated sulfuric acid (VI) was added. The vessel was placed in a Hach Digesdahl Digestion Apparatus (Hach Comp., Loveland, CO, USA) and heated to 280 °C. During the first 4 min, the sample was carbonized, and then 10 mL of 30% H2O2 was gradually added using a capillary funnel. Mineralization was continued until a clear solution was obtained, which took about 20 min. After this time, the flask was cooled, and the contents were alkalized with 20 mL of 5 M NaOH and adjusted to pH 3.0. The solution was topped up with deionized water to a capacity of 100 mL. Mineralization of each sample was performed in triplicate.
2.4. Protein Analysis
The amount of peptides released after in vitro digestion was determined using the Lowry method [28]. For this purpose, 5 mL of freshly prepared Lowry reagent was added to 1 mL of diluted dialysate and left for 10 min. Then, 0.5 mL of 2 M Folin–Ciocalteu reagent was added, mixed, and placed in a water bath at 37 °C. After 30 min of incubation, the samples were removed, and the absorbance was read at 750 nm on a Specord 40 spectrophotometer (Analityk Jena GmbH, Jena, Germany) within the next 30 min. Based on the absorbance readings for the reference protein (casein), a standard curve was prepared, which was used to calculate the protein content in the dialysate samples.
The total protein content in the test material was measured indirectly based on the ammonium nitrogen content in the mineralized sample. For this purpose, 1 mL of 1% gum arabic and 1 mL of Nessler’s reagent (basic solution of potassium tetraiodomercurate (II)) were added to 6 mL of the standard or the sample (diluted with water). After 10 min, the absorbance of the samples and standards was read at λ = 430 nm on a Specord 40 spectrophotometer (Analityk Jena GmbH, Jena, Germany). The nitrogen content was calculated based on the standard curve prepared using NH4Cl solution calcined at 110 °C to a constant mass. The calculated amount of nitrogen was converted to protein content using a conversion factor of 6.25.
2.5. Phosphorus Analysis
Phosphorus was determined using the blue method [29]. For this purpose, 1 mL of molybdate reagent (2.5% NH4MoO4 in 1.5 M H2SO4) was added to 5 mL of standard or suitably diluted sample (dialysate from in vitro digestion or mineralized sample) and stirred. After 5 min, 1 mL of metol reagent (1% N-methylaminophenol in 5% anhydrous Na2SO3 solution) was added and stirred again. After 15 min, 3 mL of deionized water was added. Absorbance was read on a Specord 40 spectrophotometer (Analityk Jena GmbH, Jena, Germany) at λ = 660 nm up to 45 min after water addition. The concentration of orthophosphate ions (V) in the samples was calculated using a standard curve prepared using KH2PO4 solution.
2.6. Iron Analysis
Iron content was determined using the phenanthroline method [30]. For this purpose, 5 to 25 mL of sample or standard (FeCl3·6H2O) was added to carefully washed 50 mL volumetric flasks. Then, 2 mL of 10% hydroxylamine hydrochloride solution and 5 mL of 10% sodium citrate were added to each flask to reduce iron Fe3+ to Fe2+. After a minute, 5 mL of 0.25% 1,10-phenanthroline solution in 0.1 M HCl was added to the flasks, topped up with deionized water to the mark and mixed. After 10 min, the absorbance of standards and samples was measured using a Specord 40 spectrophotometer (Analityk Jena GmbH, Jena, Germany) at a wavelength of λ= 512 nm. The iron content was calculated using the standard curve.
2.7. Analysis of Iodine
In order to evaluate the content of iodine in samples after in vitro digestion, the alkaline extraction of samples with tetramethylammonium hydroxide (TMAH) PN-EN 15111-2008 [31] was conducted. The 0.5 mL of sample was mixed with 9.5 mL of deionized water and 1 mL of 25% TMAH (Sigma-Aldrich, St. Louis, MO, USA) into 30 mL Falcon tubes. After mixing, the samples were incubated for 3 h at 90 °C. After incubation, samples were cooled to a room temperature (20 °C) and filled to 30 mL with deionized water. After mixing, the samples were centrifuged for 15 min at 4500 rpm. The measurements of iodine content using an ICP-MS/MS triple quadruple spectrometer (iCAP TQ ICP-MS ThermoFisher Scientific, Bremen, Germany) were conducted in the supernatant. Measurement of I127 was conducted in the S-SQ-KED mode using a tellurium solution as an internal standard introduced on-line to the ICP-MS/MS spectrometer [32].
2.8. Vitamin B2 Analysis
Vitamin B2 determination was performed as described in the work of Starzyńska-Janiszewska et al. (2016) [33]. Separation of riboflavin (B2) was performed by reversed-phase high-performance liquid chromatography (Luna C18 column, 250 mm × 4 mm i.d. 5 µm, Phenomenex, Torrance, CA, USA) isocratically using a mobile phase consisting of a mixture of methanol and 0.05 M sodium acetate (30:70 v/v) at a flow rate of 1 mL·min−1. The fluorimetric detector RF2000 Dionex (Dionex, Sunnyvale, CA, USA) was set to an excitation wavelength of 422 nm and an emission wavelength of 533 nm for vitamin B2 analyses.
2.9. Vitamin B12 Analysis
To determine native (non-fortified) vitamin B12, protein-bound forms were released enzymatically in the presence of cyanide. Precisely 1.00 g of homogenized kefir sample was weighed into a 50 mL polypropylene centrifuge tube. Subsequently, 20 mL of deionized water and 250 μL of freshly prepared 1% sodium cyanide (NaCN) solution were added. The mixture was vortexed, and the pH was adjusted to 4.5 using hydrochloric acid. Following thorough mixing, 300 mg of taka-diastase (Aspergillus oryzae, Fluka cat. no. 86250) was added to facilitate the release of protein-bound vitamin B12. The samples were incubated in the dark at 37 °C for 60 min with intermittent shaking. After incubation, the volume was adjusted to 40 mL with deionized water. The mixture was then heated in a water bath at 95 °C for 30 min with vigorous shaking at least five times throughout the heating process. Centrifuge tubes were tightly sealed during heating to prevent evaporation. Afterward, the extracts were rapidly cooled to below 30 °C and centrifuged at 6000× g for 10 min. The clear supernatant was transferred to sterile 1.5 or 2.0 mL reaction vials and, if necessary, further diluted with sterile deionized water prior to analysis.
Vitamin B12 was determined using the VitaFast® Vitamin B12 Biopharm test Cat. No. R2103 (Biopharm, Darmstadt, Germany) on an Elisa Sunrise™ microplate reader (Tecan, Männedorf, Switzerland). VitaFast® Vitamin B12 (Cyanocobalamin) (Biopharm, Darmstadt, Germany) is a microbiological test using the microplate method for the quantitative determination of total vitamin B12 (native and added) in food samples and pharmaceutical products. The microbiological test used complies with international standards.
Vitamin B12 extracted from the sample was diluted. Then, the culture medium and diluted extract or dialysate from in vitro digestion were added to the microplate wells coated with Lactobacillus delbrueckii subspecies lactis (leichmannii). The growth of Lactobacillus delbrueckii depends on the amount of vitamin B12 supplied with the sample. The bacteria grow until the entire supply of vitamin B12 is used up. Incubation takes place in the dark at 37 °C for 44–48 h. The dependence of the metabolism and growth of the bacteria on the vitamin B12 extracted from the sample is revealed as turbidity and is read from the standard curve. The measurement is performed on Elisa Sunrise™ a microplate reader (Tecan, Männedorf, Switzerland) at a wavelength of 620 nm.
2.10. Statistical Analyses
The experimental data were subjected to one-way analysis of variance (ANOVA) to detect significant differences between means expressed as mean ± standard deviation (SD). Differences between means were verified by the LSD test at p ≤ 0.05 using Statgraphic Centurion 18 (version 18.1.13) software (Statgraphics Technologies, Inc., The Plains, Virginia, NV, USA).
3. Results
3.1. Protein
As the concentration of microalgae increases, there is a general trend of an increase in the amount of protein released, with the most significant increase occurring at 5% (Figure 1). At lower concentrations (0.1–1%), Spirulina appears to release slightly more protein compared to Chlorella. At the highest concentration (5%), the amount of protein released from both microalgae is very high and similar. These data suggest that higher concentrations of Spirulina and Chlorella lead to significant protein release under the conditions tested.
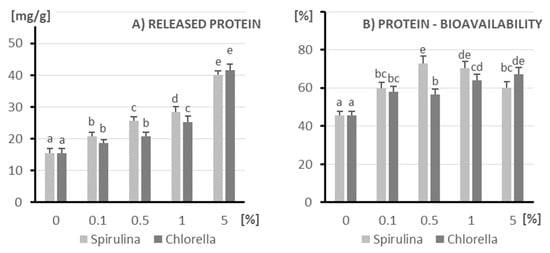
Figure 1.
The amount of released protein (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
Protein bioavailability from both microalgae generally increases with increasing concentration in the range from 0% to 0.5% or 1% and then stabilizes or decreases slightly at the highest concentration (5%). At 0.5% concentration, Spirulina shows significantly higher protein bioavailability compared to Chlorella. At the remaining concentrations (0.1%, 1%, and 5%), the protein bioavailability from both microalgae is relatively high and similar to each other. These data suggest that microalgae, especially Spirulina at 0.5%, may be a good source of bioavailable protein. Higher concentrations also provide good protein bioavailability, although Spirulina does not show a further increase after 0.5%.
3.2. Phosphorus
Numerous researchers [10,34] indicate that products containing microalgae are characterized by high content and high absorption of phosphorus, which was confirmed in this study (Figure 2).
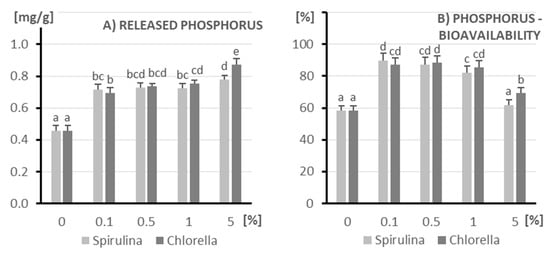
Figure 2.
The amount of released phosphorus (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
As the concentration of microalgae increases, there is a general trend of increasing the amount of released phosphorus, with the most significant increase occurring at the highest concentration (5%). At concentrations from 0.1% to 1%, the amount of released phosphorus from both microalgae remains fairly stable, higher than in the control. At the highest concentration (5%), Chlorella releases slightly more phosphorus compared to Spirulina. These data suggest that higher concentrations of Spirulina and Chlorella lead to greater release of phosphorus under the conditions studied, and the addition of these microalgae, even at low concentrations, increases the amount of released phosphorus compared to the control.
Both Spirulina and Chlorella show a similar trend in phosphorus bioavailability depending on the algal additive concentration. Addition of Spirulina and Chlorella to the tested matrix significantly increases phosphorus bioavailability at low concentrations (0.1% and 0.5%). At 1% concentration, phosphorus bioavailability is still high, although slightly lower than at 0.1 and 0.5%. The highest algal additive concentration tested (5%) leads to a decrease in phosphorus bioavailability to levels similar to the control. These data suggest that low concentrations of Spirulina and Chlorella can effectively increase phosphorus bioavailability. Increasing the concentration above a certain optimum (approximately 0.1–0.5%) does not provide further benefits and may even lead to a decrease in phosphorus bioavailability.
3.3. Iron
More than 20 years ago, studies using Caco-2 cell models [35] showed that Spirulina may be a better source of iron than beef. Our results on iron release confirm this (Figure 3). As the concentration of microalgae (both Spirulina and Chlorella) increases, the amount of released iron systematically increases. At all tested concentrations above 0%, Chlorella releases slightly more iron compared to Spirulina. The highest amount of released iron is observed at the highest concentration (5%) for both microalgae. From samples containing Spirulina, about 0.768 µg of iron/g is released, and from Chlorella significantly more—about 0.896 µg/g.
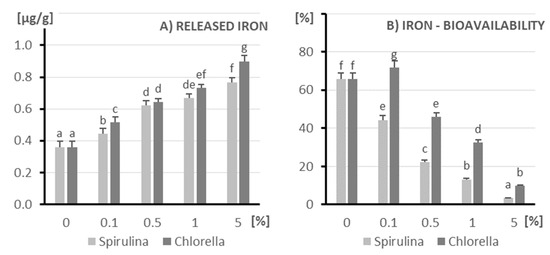
Figure 3.
The amount of released iron (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
Only Chlorella demonstrates good iron bioavailability at low concentrations (0.1%). As the concentration of the microalgae increases (from 0.5% to 5%), the iron bioavailability from both sources significantly decreases. Chlorella tends to exhibit higher iron bioavailability compared to Spirulina, but at the highest concentration (5%), the iron bioavailability from both is very poor.
3.4. Iodine
The amount of released iodine from Spirulina and Chlorella in the tested conditions is low and stable regardless of the applied concentration of microalgae (ranging from 0% to 5%) (Figure 4). There is no significant effect of the type of microalgae or its concentration on the amount of released iodine. These results suggest that in the tested conditions Spirulina and Chlorella are not a significant source of released iodine, and their addition in different concentrations does not increase its availability in the measured environment.
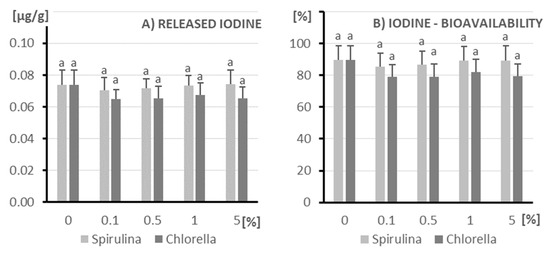
Figure 4.
The amount of released iodine (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
Both Spirulina (light grey bars) and Chlorella (dark grey bars) samples show very high (80% and higher) iodine bioavailability. Values for both microalgae range from about 80% to 90% at all concentrations. There are no statistically significant differences in iodine bioavailability between Spirulina and Chlorella, nor between different concentrations of these microalgae supplements. Unfortunately, there is no difference between the control samples either.
3.5. Vitamin B2
Figure 5 presents the effect of Spirulina (light bars) and Chlorella (dark bars) supplementation in kefir (0.1–5%) on the amount of released vitamin B2 (panel A) and its bioavailability after simulated gastrointestinal digestion (panel B). A dose-dependent increase in the released riboflavin content was observed for both microalgae. Chlorella, in particular, led to a significantly higher release of vitamin B2 at 5% inclusion (approx. 1.48 µg/g) compared to Spirulina at the same concentration (approx. 1.25 µg/g). These results suggest that both algae species can act as sources of riboflavin in fermented milk products, with Chlorella showing higher release efficiency, possibly due to its higher intracellular vitamin B2 content.
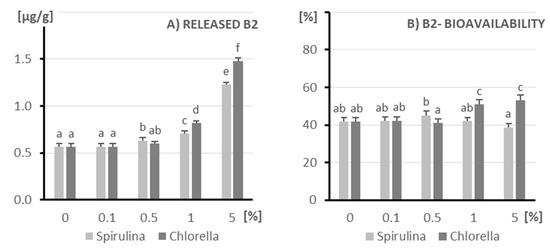
Figure 5.
The amount of released vitamin B2 (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
Additionally, panel B shows the relationship between algae concentration and bioavailability. Control and low-dose samples (0–0.1%) demonstrated comparable bioavailability (~45–50%) to the samples with an increasing concentration of microalgae (1–5%) in vitamin B2 bioaccessibility.
3.6. Vitamin B12
In order to assess the effect of microalgae addition on the content and bioavailability of vitamin B12, samples enriched with Spirulina and Chlorella at concentrations of 0–5% were analyzed (Figure 6). Panel A shows the amount of released vitamin B12 (ng/g), while panel B shows the relative bioavailability (%) of this vitamin after in vitro digestion simulation. The introduction of Spirulina and Chlorella extract to kefir samples led to a significant (p < 0.05) increase in the content of released vitamin B12. The highest level was noted in the sample with 5% Spirulina addition (5.01 ± 0.27 ng/g), which had a significantly higher level in relation to all other variants. Spirulina also significantly increased the amount of vitamin B12, but these values were slightly lower than Chlorella at the highest level (5%) of algae addition (4.24 ± 0.23 ng/g). Despite the increased total B12 content, a clear decrease in its bioavailability was observed with increasing microalgae content (Figure 1B). For samples with 5% addition of both Spirulina and Chlorella, the bioavailability dropped below 10%, indicating a potential interference of the algal matrix in the digestion and absorption processes. The highest bioavailability (~45–50%) was recorded in the control samples and those with the lowest level of addition (0.1%).
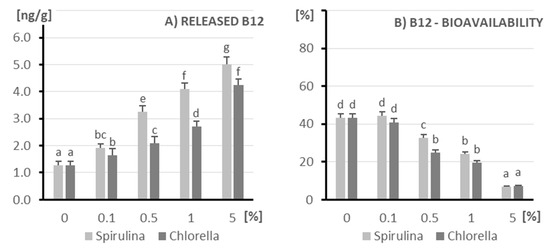
Figure 6.
The amount of released vitamin B12 (A) and its bioavailability (B) after in vitro digestion of kefir enriched with different doses of algae (X axis; doses from 0 to 5%): on the left (light columns), Spirulina; on the right (dark columns), Chlorella. The columns represent different doses of microalgae, and columns marked with different letters differ significantly (p ≤ 0.05, test LSD).
The obtained results are consistent with previous reports indicating that microalgae can be a source of the bioactive form of vitamin B12, but the presence of pseudoforms (e.g., biologically inactive B12 analogues) may affect their bioavailability [36,37,38]. According to Watanabe et al. (2014) [38], Chlorella contains significant amounts of the methylcobalamin form, but its absorption may be limited due to the structural components of the cell wall, which are not digestible. In turn, Spirulina contains mainly B12 analogues, which explains the lower bioavailability despite the presence of a higher amount of total cyanocobalamin [39]. This phenomenon suggests that despite the potential fortification of food with vitamin B12 using microalgae, a detailed assessment of the bioavailability and chemical forms of this vitamin present in the final product is necessary.
4. Discussion
We have shown that the addition of Spirulina and Chlorella generally improves the protein bioavailability of kefir compared to the control (without added algae). At a concentration of 0.5%, Spirulina showed particularly high protein bioavailability. At higher concentrations, the protein bioavailability of both algae was still good. Research indicates that protein from Spirulina and Chlorella is generally well digested and absorbed [22,23], although this may vary depending on the method of processing the algae (e.g., breaking down the cell walls may improve bioavailability). Microalgae such as Arthrospira and Chlorella can increase the amount of protein released in food products. For example, Fradique et al. (2010) [40] observed an increase in the amount of protein released from pasta enriched with these algae. Rodríguez et al. (2013) [41] indicated that Spirulina protein has a better availability than Chlorella protein due to the lack of cellulose in its cell wall. Similar results were reported for fermented dairy products. In the study by Silva et al. (2019) [42], the addition of dried Spirulina to yogurt increased the protein content. Atallah et al. (2020) [43] also observed an increase in the protein content of yogurt from 5.4% to 6.4% after the addition of 1% Spirulina. Spirulina was also added to buttermilk [44], where it also increased the protein content and other nutrients. Our results confirm that the addition of Spirulina or Chlorella can effectively improve the bioavailability of protein in kefir, especially at lower microalgae concentrations.
There may be interactions between milk proteins (e.g., casein) and algal proteins in the product, which may affect the digestibility and absorption of individual protein fractions, but the overall bioavailability remains high. However, it should be remembered that protein availability can be modified by the presence of antinutritional components such as polyphenols and polysaccharides. Oxidized phenols can react with proteins to form insoluble complexes that are more difficult to enzymatically digest [5,9]. On the other hand, the fermentation process of kefir may partially break down proteins, potentially making them easier to digest and absorb, which could work synergistically with algal protein.
Our results indicate improved protein bioavailability after adding Spirulina and Chlorella to kefir, which is consistent with the general knowledge of good-quality protein in these algae. Interactions with milk proteins do not seem to significantly reduce overall bioavailability, and the fermentation process of kefir may even aid in protein digestion.
Unfortunately, there are no direct studies on the effect of Spirulina and Chlorella on the bioavailability of iron in kefir. According to the literature, iron absorption may increase with the addition of microalgae, mainly due to phycocyanin, which forms complexes with iron [45]. On the other hand, calcium and casein in dairy products are known inhibitors of non-heme iron absorption (which is the form of iron found in plants and microalgae) [46]. This could partially explain the decrease in iron bioavailability at higher microalgae concentrations, where the greater amount of algal biomass introduces more iron, but at the same time, other components may impede its absorption in the presence of kefir components. Some studies suggest that algal components like some proteins and phenolic molecules may bind iron, reducing its solubility [47,48]. The cellular structure of Spirulina and Chlorella, as well as the presence of fiber and other compounds, may affect the release and solubility of iron in the digestive tract, which is crucial for its absorption. Higher concentrations of algae may create a more complex matrix, potentially hindering iron availability. On the other hand, fermentation processes (as in the case of kefir) have the potential to modify food components and affect mineral bioavailability, but the specific effects of Spirulina and Chlorella in kefir require further study.
In their work, Isani et al. (2022) [47] draw attention to the significant variability in iron content in Spirulina supplements, which can reach up to tenfold. They emphasize that the concentration of iron in the microalgae culture medium affects the fluctuations in the amount of this element in the obtained biomass. This fact has been well demonstrated in the work of Kougia et al. (2023) [49]; moreover, they showed that increased iron supply by Spirulina may result in a decrease in the content of other nutrients, e.g., lipids and phycocyanin. In turn, the research of Rutar et al. (2022) [50] showed that in Spirulina supplements the predominant proportion of iron (82–92%) occurs in the form of Fe3+ ions, and only a small amount is in the form of more absorbable Fe2+ ions. This finding may explain the observed low bioavailability of iron from microalgae supplements.
Our results suggest that a decrease in iron bioavailability at higher concentrations of algae in kefir may be related to the inhibitory effects of milk components and the potential properties of the algae matrix that impede iron release. Further studies are needed, specifically for kefir with the addition of these microalgae to better understand these interactions and potentially optimize iron’s bioavailability.
Our results indicate a very low iodine content in the microalgae material, despite the values declared by the seller on the packaging. The amount of iodine in algae strictly depends on the cultivation conditions, which indicates a low content of this element in the cultivation medium. Conclusion: The declarations on the label should not always be trusted; the content of bioactive ingredients should be verified in the context of this product segment, especially such specific ones as iodine. The introduced microalgae were probably grown under strictly controlled conditions, in bioreactors with a medium of a defined composition, and not in semi-open tanks with iodine-rich seawater. The discrepancy between the declared and analyzed iodine content of microalgae supplements may result from multiple factors beyond the composition of the growth medium. It is hypothesized that some technological process (e.g., high-temperature drying) or degradation of iodine over time—especially in the form of volatile iodine compounds or due to light and storage conditions—may also contribute to this variability in the final product.
Our findings about vitamin B2 are in line with earlier reports confirming that Chlorella and Spirulina are rich in B-complex vitamins, including riboflavin [5,51,52]. Safi et al. [52] report riboflavin contents of 3–6 mg/100 g in dried Chlorella biomass, slightly higher than values observed for Spirulina. However, bioavailability remains a limiting factor. Chlorella’s rigid cell wall, composed largely of cellulose, impairs nutrient release during digestion unless cell disruption technologies (e.g., high-pressure homogenization or enzymatic lysis) are applied [3,53].
Additionally, Spirulina tends to have more digestible cell walls but contains a lower intrinsic amount of B2, which may explain its lower efficacy at higher doses despite better digestibility. Moreover, previous studies have highlighted that while microalgae fortification may increase the nutritional value of fermented products, it also introduces structural complexity that can hinder nutrient release [54,55]. Therefore, although algae are promising functional ingredients, optimizing processing methods to enhance micronutrient bioaccessibility is crucial for their effective use in dairy matrices.
Riboflavin is a B vitamin involved in various metabolic processes in the human body, including glucose metabolism, neurotransmission, gene replication, fetal tissue development, and corticosteroid biosynthesis [56]. It is sensitive to the effects of temperature, light, and other factors, and therefore to the technological processes to which dairy products are subjected. During processing, it can partially degrade to as much as 50% of the initial level in the raw material [57]. Therefore, some products are fortified with B vitamins to regain their original level, and also due to the fact that a significant part of the population is deficient in riboflavin [58,59].
The simplest option is to introduce purified riboflavin into the raw material or semi-finished product, but the more beneficial option from the point of view of bioavailability is the enrichment variant using natural ingredients. Therefore, attempts were made to select strains of microorganisms (Lactobacillus fermentum) used in the fermentation process to increase the pool of riboflavin in yogurts or bread [60]. However, comparing the effects of riboflavin enrichment obtained in the above work with the results included in this study, our functional dairy product offers higher values of released vitamin B2 with an acceptable, relatively high bioavailability of this component (45–53%).
A microbiological test for vitamin B12 content was used in the study. It is worth noting that despite the approval of the test by AOAC, it is not absolutely specific for cyanocobalamin and may show interactions with other metabolic derivatives of corrinoids [61]. Therefore, the above analyses should be treated as semi-quantitative; nevertheless, they allow for the recording of a certain trend of increasing vitamin B12 content with the increasing amount of microalgae addition, especially with a 5% share of microalgae in kefir. Of course, it should be remembered that the in vitro method used here is a mathematical indicator of digestibility, and perhaps a better model would be to use Caco-2 or HT-29 cell lines to study the absorption of this component [62], or final confirmation in the form of clinical tests and measurement of homocysteine or methylmalonic acid concentration in blood [63]. In a paper summarizing the estimated bioavailability of vitamin B12 in the available literature (several hundred articles were analyzed), significant ranges of this parameter were noted in the range of 11–84% [61]. In some in vitro studies and clinical tests from the early 1990s, it was found that cobalamin or vitamin B12 derivatives are not absorbed and metabolized, either due to specific interactions or the inactive form of certain cyanocobalamin analogues present in this material [64]. In 2015, an article was published indicating the presence of the active form of vitamin B12 in Chlorella, which means that the first non-animal source of this ingredient was noted at that time [65]. In conclusion, further studies using more selective corrinoid assay techniques are necessary to confirm the observations regarding vitamin B12 reported in this work.
5. Conclusions
The results of the presented studies indicate that enriching functional milk drinks with microalgae (Spirulina and Chlorella) can improve their composition in terms of protein, phosphorus, and vitamins B2 and B12, as well as potentially increasing the absorption of some of these ingredients. Nevertheless, the bioavailability of iron and iodine turned out to be problematic.
The obtained results constitute valuable information on the impact of kefir supplementation with Spirulina and Chlorella on the bioavailability of the tested nutrients. Due to the limited number of specific literature data regarding algae supplementation of dairy products, further research is necessary, which should include in vivo studies (on animals or people) to more accurately assess iron and protein absorption, analysis of specific interactions between dairy ingredients and algae biomass, and the impact of various algae processing methods on the bioavailability of nutrients in supplemented food.
Our analyses indicate that enriching kefir with higher doses of microalgae is not always justified because it does not lead to a significant increase in the bioavailability of some tested compounds (iron, vitamins B2, and B12). However, the use of additives in low concentrations (in our research, 0.1–0.5%) can provide a positive effect in the form of increasing the amount of released nutrients, without significant deterioration of the product characteristics.
Our results are a promising starting point for further research on the potential of enriching dairy products with Spirulina and Chlorella as a source of valuable nutrients, with particular emphasis on optimization of methods that improve the bioavailability of iron and vitamins from group B at higher algae concentrations.
Author Contributions
Conceptualization, Ł.B.; methodology, Ł.B., R.D. and S.S.; software, Ł.B., R.D. and S.S.; validation Ł.B., R.D. and S.S.; formal analysis, Ł.B. and R.D.; investigation, Ł.B. and R.D.; resources, Ł.B. and R.D.; data curation, Ł.B., R.D. and S.S.; writing—original draft preparation, Ł.B. and R.D.; writing—review and editing, Ł.B.; visualization, Ł.B.; supervision, Ł.B.; project administration, Ł.B. and R.D.; funding acquisition, Ł.B. and R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SUP-RIM “Research network of natural science universities for the development of the Polish dairy sector—research project” (MEIN/2023/DPI/2872).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article.
Acknowledgments
The authors want to thank the whole team from Department of Biotechnology and General Food Technology URK for technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanguigni, V.; Manco, M.; Sorge, R.; Gnessi, L.; Francomano, D. Natural Antioxidant Ice Cream Acutely Reduces Oxidative Stress and Improves Vascular Function and Physical Performance in Healthy Individuals. Nutrition 2017, 33, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Turgut, T.; Cakmakci, S. Investigation of the Possible Use of Probiotics in Ice Cream Manufacture. Int. J. Dairy Technol. 2009, 62, 444–451. [Google Scholar] [CrossRef]
- Becker, E.W. Microalgae for Human and Animal Nutrition. In Handbook of Microalgal Culture; Amos, R., Qiang, H., Eds.; Wiley: Hoboken, NJ, USA, 2013; pp. 461–503. [Google Scholar]
- Dohaish, E.; Dhahri, M.A.; Omar, H. Potential application of the blue-green alga (Spirulina platensis) as a supplement in the diet of nile tilapia (Oreochromis niloticus). Appl. Ecol. Environ. Res. 2018, 16, 7883–7902. [Google Scholar] [CrossRef]
- Gouveia, L.; Marques, A.E.; Sousa, J.M.; Moura, P.; Bandarra, N.M. Microalgae—Source of Natural Bioactive Molecules as Functional Ingredients. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 21–37. [Google Scholar] [CrossRef]
- Voloshin, R.A.; Rodionova, M.V.; Zharmukhamedov, S.K.; Nejat Veziroglu, T.; Allakhverdiev, S.I. Review: Biofuel Production from Plant and Algal Biomass. Int. J. Hydrogen Energy 2016, 41, 17257–17273. [Google Scholar] [CrossRef]
- de Boer, L. Biotechnological Production of Colorants. Adv. Biochem. Eng. Biotechnol. 2013, 143, 51–89. [Google Scholar] [CrossRef]
- Sun, H.; Wang, Y.; He, Y.; Liu, B.; Mou, H.; Chen, F.; Yang, S. Microalgae-Derived Pigments for the Food Industry. Mar. Drugs 2023, 21, 82. [Google Scholar] [CrossRef]
- Andrade, L.M.; Andrade, C.J.; Dias, M.; Nascimento, C.A.; Mendes, M.A. Chlorella and Spirulina Microalgae as Sources of Functional Foods, Nutraceuticals, and Food Supplements; an Overview. MOJ Food Process. Technol. 2018, 6, 45–58. [Google Scholar] [CrossRef]
- Pehlivanov, I.; Gentscheva, G.; Nikolova, K.; Andonova, V. Some Applications of Arthrospira platensis and Algae in Pharmaceutical and Food Technologies. Biointerface Res. Appl. Chem. 2024, 14, 32. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed Proteins: Biochemical, Nutritional Aspects and Potential Uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Nuhu, A.A. Spirulina (Arthrospira): An Important Source of Nutritional and Medicinal Compounds. J. Mar. Sci. 2013, 2013, 325636. [Google Scholar] [CrossRef]
- Manirafasha, E.; Ndikubwimana, T.; Zeng, X.; Lu, Y.; Jing, K. Phycobiliprotein: Potential Microalgae Derived Pharmaceutical and Biological Reagent. Biochem. Eng. J. 2016, 109, 282–296. [Google Scholar] [CrossRef]
- Byczyński, Ł.; Duliński, R. The effect of the addition of algae to rye bread on the content and in vitro availability of selected micro- and macroelements. Food Sci. Technol. Qual. 2022, 29, 86–98. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae Biomass as an Alternative Ingredient in Cookies: Sensory, Physical and Chemical Properties, Antioxidant Activity and In Vitro Digestibility. Algal. Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Donato, N.R.; de Melo Queiroz, A.J.; Feitosa, R.M.; de Figueirêdo, R.M.F.; dos Santos Moreira, I.; de Lima, J.F. Production of Cookies Enriched With Spirulina platensis Biomass. J. Agric. Stud. 2019, 7, 323–342. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Cione, E.; Fazio, A. Comparing Nutritional Values and Bioactivity of Kefir from Different Types of Animal Milk. Molecules 2024, 29, 2710. [Google Scholar] [CrossRef]
- Rosa, D.D.; Dias, M.M.S.; Grześkowiak, Ł.M.; Reis, S.A.; Conceição, L.L.; Peluzio, M.D.C.G. Milk Kefir: Nutritional, Microbiological and Health Benefits. Nutr. Res. Rev. 2017, 30, 82–96. [Google Scholar] [CrossRef]
- Otles, S. Ozlem Cagindi Kefir: A Probiotic Dairy-Composition, Nutritional and Therapeutic Aspects. Pakistan J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Yilmaz-Ersan, L.; Ozcan, T.; Usta-Gorgun, B.; Ciniviz, M.; Keser, G.; Bengu, I.; Keser, R.A. Bioaccessibility and Antioxidant Capacity of Kefir-Based Smoothies Fortified with Kale and Spinach after in Vitro Gastrointestinal Digestion. Food Sci. Nutr. 2024, 12, 2153–2165. [Google Scholar] [CrossRef]
- Perales, S.; Barberá, R.; Lagarda, M.J.; Farré, R. Fortification of Milk with Calcium: Effect on Calcium Bioavailability and Interactions with Iron and Zinc. J. Agric. Food Chem. 2006, 54, 4901–4906. [Google Scholar] [CrossRef]
- Szmejda, K.; Duliński, R.; Byczyński, Ł.; Karbowski, A.; Florczyk, T.; Żyła, K. Analysis of the Selected Antioxidant Compounds n Ice Cream Supplemented with Spirulina (Arthrospira platensis) Extract. Biotechnol. Food Sci. 2018, 82, 41–48. [Google Scholar] [CrossRef]
- Bosnea, L.; Terpou, A.; Pappa, E.; Kondyli, E.; Mataragas, M.; Markou, G.; Katsaros, G. Incorporation of Spirulina platensis on Traditional Greek Soft Cheese with Respect to Its Nutritional and Sensory Perspectives. In Proceedings of the 1st International Electronic Conference on Food Science and Functional Foods, Online, 10–25 November 2020; MDPI: Basel, Switzerland, 2020; p. 99. [Google Scholar]
- Lv, K.; Yuan, Q.; Li, H.; Li, T.; Ma, H.; Gao, C.; Zhang, S.; Liu, Y.; Zhao, L. Chlorella pyrenoidosa Polysaccharides as a Prebiotic to Modulate Gut Microbiota: Physicochemical Properties and Fermentation Characteristics In Vitro. Foods 2022, 11, 725. [Google Scholar] [CrossRef]
- Ovando, C.A.; Carvalho, J.C.D.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional Properties and Health Benefits of Bioactive Peptides Derived from Spirulina: A Review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Hosseinkhani, N.; McCauley, J.I.; Ralph, P.J. Key Challenges for the Commercial Expansion of Ingredients from Algae into Human Food Products. Algal Res. 2022, 64, 102696. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]
- Fiske, C.H.; Subbarow, Y. The Colorimetric Determination of Phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Pyenson, H.; Tracy, P.H. A 1,10—Phenanthroline Method for the Determination of Iron in Powdered Milk. J. Dairy Sci. 1945, 28, 401–412. [Google Scholar] [CrossRef]
- PN-EN 15111—2008; Foodstuffs—Determination of Trace Elements—Determination of Iodine by ICP-MS (Inductively Coupled Plasma Mass Spectrometry). Polish Committee of Standardization: Warsaw, Poland, 2007. (In Polish)
- Smoleń, S.; Kowalska, I.; Halka, M.; Ledwozyw-Smoleń, I.; Grzanka, M.; Skoczylas, Ł.; Czernicka, M.; Pitala, J. Selected Aspects of Iodate and Iodosalicylate Metabolism in Lettuce Including the Activity of Vanadium Dependent Haloperoxidases as Affected by Exogenous Vanadium. Agronomy 2020, 10, 1. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Duliński, R.; Baczkowicz, M.; Mickowska, B.; Wikiera, A.; Byczyński, L. Effect of Solid-State Fermentation Tempe Type on Antioxidant and Nutritional Parameters of Buckwheat Groats as Compared with Hydrothermal Processing. J. Food Process Preserv. 2016, 40, 298–305. [Google Scholar] [CrossRef]
- Gentscheva, G.; Nikolova, K.; Panayotova, V.; Peycheva, K.; Makedonski, L.; Slavov, P.; Radusheva, P.; Petrova, P.; Yotkovska, I. Application of Arthrospira platensis for Medicinal Purposes and the Food Industry: A Review of the Literature. Life 2023, 13, 845. [Google Scholar] [CrossRef] [PubMed]
- Puyfoulhoux, G.; Rouanet, J.M.; Besançon, P.; Baroux, B.; Baccou, J.C.; Caporiccio, B. Iron Availability from Iron-Fortified Spirulina by an In Vitro Digestion/Caco-2 Cell Culture Model. J. Agric. Food Chem. 2001, 49, 1625–1629. [Google Scholar] [CrossRef]
- Allen, L.H. Bioavailability of Vitamin B12. Int. J. Vitam. Nutr. Res. 2010, 80, 330–335. [Google Scholar] [CrossRef]
- Watanabe, F.; Katsura, H.; Takenaka, S.; Fujita, T.; Abe, K.; Tamura, Y.; Nakatsuka, T.; Nakano, Y. Pseudovitamin B12 Is the Predominant Cobamide of an Algal Health Food, Spirulina Tablets. J. Agric. Food Chem. 1999, 47, 4736–4741. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-Containing Plant Food Sources for Vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The Antioxidant, Immunomodulatory, and Anti-Inflammatory Activities of Spirulina: An Overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Fradique, M.; Batista, A.P.; Nunes, M.C.; Gouveia, L.; Bandarra, N.M.; Raymundo, A. Incorporation of Chlorella Vulgaris and Spirulina Maxima Biomass in Pasta Products. Part 1: Preparation and Evaluation. J. Sci. Food Agric. 2010, 90, 1656–1664. [Google Scholar] [CrossRef]
- Rodríguez De Marco, E.; Steffolani, M.E.; Martínez, C.S.; León, A.E. Effects of Spirulina Biomass on the Technological and Nutritional Quality of Bread Wheat Pasta. LWT-Food Sci. Technol. 2014, 58, 102–108. [Google Scholar] [CrossRef]
- da Silva, S.C.; Fernandes, I.P.; Barros, L.; Fernandes, Â.; José Alves, M.; Calhelha, R.C.; Pereira, C.; Barreira, J.C.M.; Manrique, Y.; Colla, E.; et al. Spray-Dried Spirulina platensis as an Effective Ingredient to Improve Yogurt Formulations: Testing Different Encapsulating Solutions. J. Funct. Foods 2019, 60, 103427. [Google Scholar] [CrossRef]
- Atallah, A.A.; Morsy, O.M.; Gemiel, D.G. Characterization of Functional Low-Fat Yogurt Enriched with Whey Protein Concentrate, Ca-Caseinate and Spirulina. Int. J. Food Prop. 2020, 23, 1678–1691. [Google Scholar] [CrossRef]
- Vlasenko, I.; Bandura, V.; Semko, T.; Fialkovska, L.; Ivanishcheva, O.; Palamarchuk, V. Innovative Approaches to the Development of a New Sour Milk Product. Potravin. Slovak J. Food Sci. 2021, 15, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarczyk, E.; Łukasiak, J.; Szczurowski, K.; Prusakowski, M. Preparation of Iron(II) and Selenium(IV) Dietary Supplements by Their Biotransformation in Arthrospira Sp. Food Sci. Technol. Qual. 2024, 31, 199–210. [Google Scholar] [CrossRef]
- Piskin, E.; Cianciosi, D.; Gulec, S.; Tomas, M.; Capanoglu, E. Iron Absorption: Factors, Limitations, and Improvement Methods. ACS Omega 2022, 7, 20441–20456. [Google Scholar] [CrossRef]
- Isani, G.; Ferlizza, E.; Bertocchi, M.; Dalmonte, T.; Menotta, S.; Fedrizzi, G.; Andreani, G. Iron Content, Iron Speciation and Phycocyanin in Commercial Samples of Arthrospira Spp. Int. J. Mol. Sci. 2022, 23, 13949. [Google Scholar] [CrossRef]
- Abo-Shady, A.M.; Gheda, S.F.; Ismail, G.A.; Cotas, J.; Pereira, L.; Abdel-Karim, O.H. Antioxidant and Antidiabetic Activity of Algae. Life 2023, 13, 460. [Google Scholar] [CrossRef]
- Kougia, E.; Ioannou, E.; Roussis, V.; Tzovenis, I.; Chentir, I.; Markou, G. Iron (Fe) Biofortification of Arthrospira platensis: Effects on Growth, Biochemical Composition and in Vitro Iron Bioaccessibility. Algal Res. 2023, 70, 103016. [Google Scholar] [CrossRef]
- Rutar, J.M.; Hudobivnik, M.J.; Nečemer, M.; Mikuš, K.V.; Arčon, I.; Ogrinc, N. Nutritional Quality and Safety of the Spirulina Dietary Supplements Sold on the Slovenian Market. Foods 2022, 11, 849. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of Microalgal Biomass Profiles as Novel Functional Ingredient for Food Products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef]
- Safi, C.; Zebib, B.; Merah, O.; Pontalier, P.Y.; Vaca-Garcia, C. Morphology, Composition, Production, Processing and Applications of Chlorella Vulgaris: A Review. Renew. Sustain. Energy Rev. 2014, 35, 265–278. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffrey, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional Properties of Microalgae for Mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of Interest as Food Source: Biochemical Composition and Digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Hernández, H.; Nunes, M.C.; Prista, C.; Raymundo, A. Innovative and Healthier Dairy Products through the Addition of Microalgae: A Review. Foods 2022, 11, 755. [Google Scholar] [CrossRef] [PubMed]
- Wolak, N.; Zawrotniak, M.; Gogol, M.; Kozik, A.; Rapala-Kozik, M. Vitamins B1, B2, B3 and B9—Occurrence, Biosynthesis Pathways and Functions in Human Nutrition. Mini-Rev. Med. Chem. 2016, 17, 1075–1111. [Google Scholar] [CrossRef] [PubMed]
- Ottaway, P.B. Stability of Vitamins during Food Processing and Storage. In Chemical Deterioration and Physical Instability of Food and Beverages; Skibsted, L.H., Risbo, J., Andersen, M.L., Eds.; Woodhead Publishing: Sawston, UK, 2010; pp. 539–560. ISBN 9781845694951. [Google Scholar]
- Olive Li, Y.; Dueik González, V.P.; Diosady, L.L. Microencapsulation of Vitamins, Minerals, and Nutraceuticals for Food Applications. In Microencapsulation in the Food Industry: A Practical Implementation Guide; Academic Press: Cambridge, MA, USA, 2014; pp. 501–522. [Google Scholar] [CrossRef]
- Schwechheimer, S.K.; Park, E.Y.; Revuelta, J.L.; Becker, J.; Wittmann, C. Biotechnology of Riboflavin. Appl. Microbiol. Biotechnol. 2016, 100, 2107–2119. [Google Scholar] [CrossRef]
- Russo, P.; Capozzi, V.; Arena, M.P.; Spadaccino, G.; Dueñas, M.T.; López, P.; Fiocco, D.; Spano, G. Riboflavin-Overproducing Strains of Lactobacillus Fermentum for Riboflavin-Enriched Bread. Appl. Microbiol. Biotechnol. 2014, 98, 3691–3700. [Google Scholar] [CrossRef]
- Doets, E.L.; In’t Veld, P.H.; Szczecińska, A.; Dhonukshe-Rutten, R.A.M.; Cavelaars, A.E.J.M.; Van ’t Veer, P.; Brzozowska, A.; De Groot, L.C.P.G.M. Systematic Review on Daily Vitamin B12 Losses and Bioavailability for Deriving Recommendations on Vitamin B12 Intake with the Factorial Approach. Ann. Nutr. Metab. 2013, 62, 311–322. [Google Scholar] [CrossRef]
- Duliński, R.; Byczyński, Ł.; Karbowski, A. The Effect of Arthrospira platensis (Spirulina) Addition on the Content of Selected Mineral Elements, Carotenes, and Antioxidant Potential in Alginate Gel Beads. Int. J. Food Eng. 2020, 16, 20190053. [Google Scholar] [CrossRef]
- Wang, Z.J.; Shi, H.L.; Wang, P. The Online Morphology Control and Dynamic Studies on Improving Vitamin B12 Production by Pseudomonas Denitrificans with Online Capacitance and Specific Oxygen Consumption Rate. Appl. Biochem. Biotechnol. 2016, 179, 1115–1127. [Google Scholar] [CrossRef]
- van den Berg, H.; Brandsen, L.; Sinkeldam, B.J. Vitamin B-12 Content and Bioavailability of Spirulina and Nori in Rats. J. Nutr. Biochem. 1991, 2, 314–318. [Google Scholar] [CrossRef]
- Merchant, R.E.; Phillips, T.W.; Udani, J. Nutritional Supplementation with Chlorella Pyrenoidosa Lowers Serum Methylmalonic Acid in Vegans and Vegetarians with a Suspected Vitamin B12 Deficiency. J. Med. Food 2015, 18, 1357–1362. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).