Exploring the Potential of Desmodesmus sp. KNUA231 for Bioenergy and Biofertilizer Applications and Its Adaptability to Environmental Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification
2.1.1. Isolation and Molecular Identification
2.1.2. Morphological Identification
2.2. Growth Measurements
2.2.1. Growth Rate and Biomass Productivity
2.2.2. Carotenoid Analysis
2.3. pH and Salinity Stress Tolerance
2.4. Biochemical Composition
2.4.1. Carbohydrate, Protein and Lipid
2.4.2. Monosaccharide Analysis
2.4.3. Free Amino Acid Analysis
2.4.4. Fatty Acid Methyl Ester Analysis
2.5. Biodiesel Quality Assessment
2.6. Proximate Analysis and Ultimate Analysis
2.7. Nutrient and Metal Composition Analysis in Biofertilizer
3. Results and Discussion
3.1. Identification of KNUA231
3.1.1. Molecular Identification
3.1.2. Morphological Identification
3.2. Growth Measurements
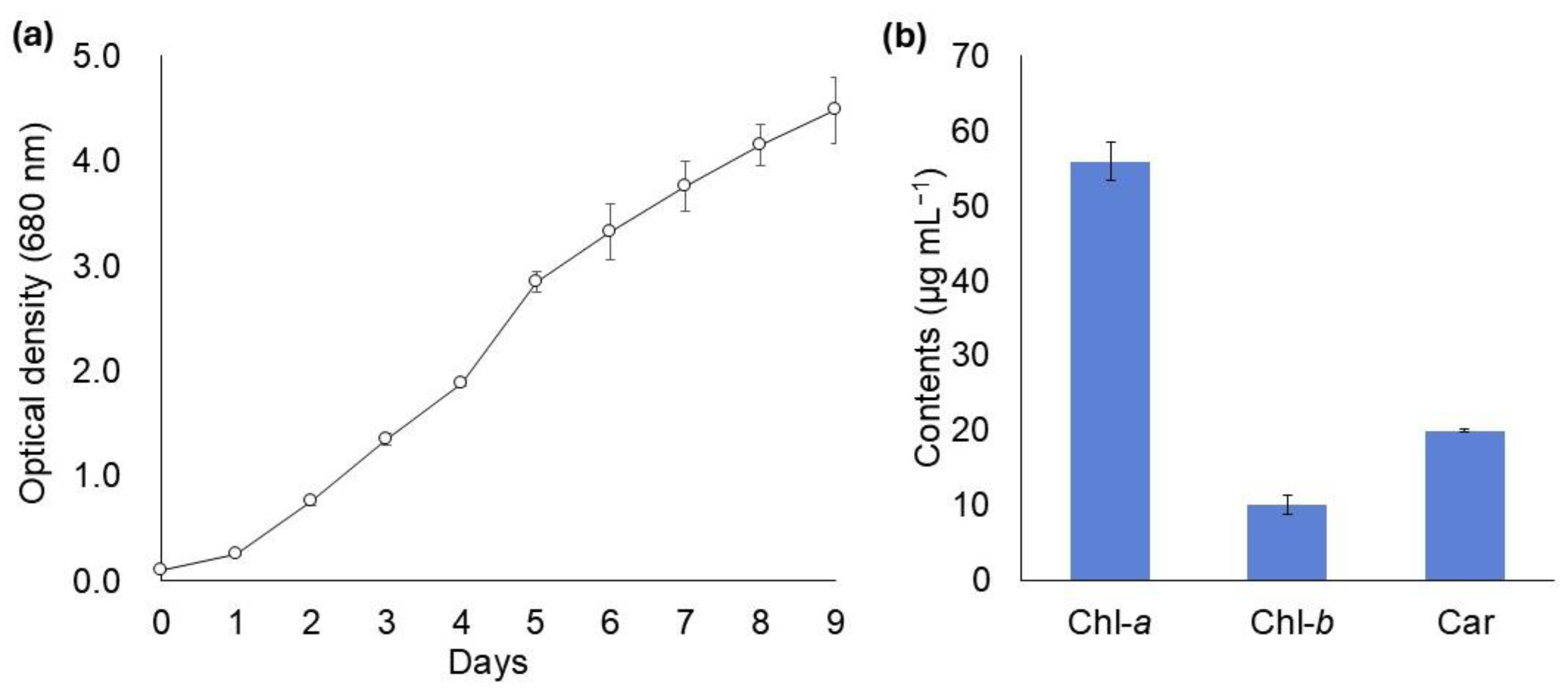
3.2.1. Growth Rate and Biomass Productivity
3.2.2. Carotenoid Analysis
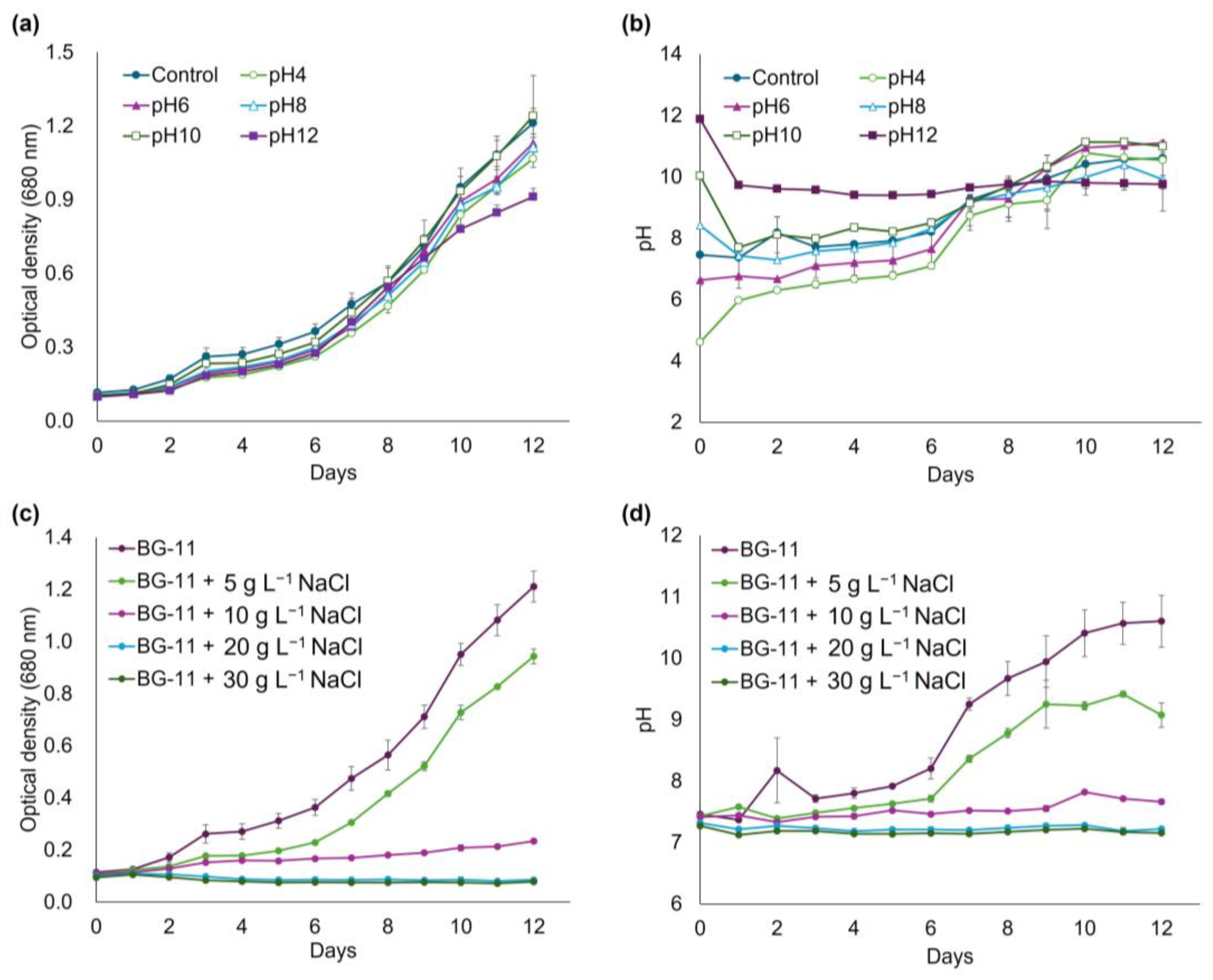
3.3. pH and Salinity Stress Tolerance
3.4. Biochemical Composition
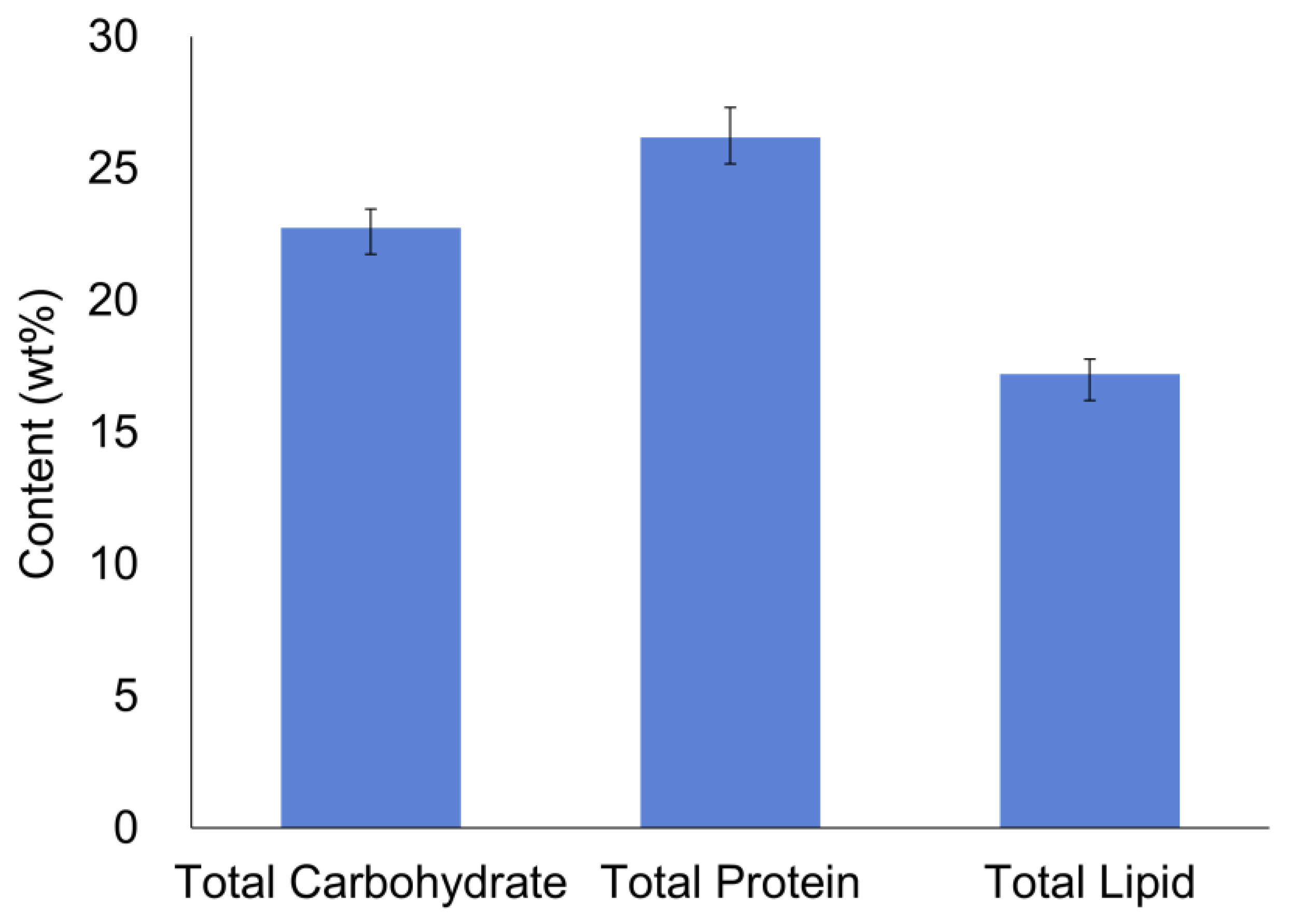
3.4.1. Carbohydrate, Protein and Lipid Contents
3.4.2. Monosaccharide Analysis
3.4.3. Free Amino Acid Analysis
3.4.4. Fatty Acid Methyl Ester Analysis
3.5. Biodiesel Quality
3.6. Proximate Analysis and Ultimate Analysis
3.7. Nutrient and Metal Composition Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeed, S.S.; Siraj, M.T. Global Renewable Energy Infrastructure: Pathways to Carbon Neutrality and Sustainability. Sol. Energy Sustain. Dev. 2024, 13, 183–203. [Google Scholar] [CrossRef]
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen Production through Renewable and Non-Renewable Energy Processes and Their Impact on Climate Change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Rosenzweig, C.; Elliott, J.; Deryng, D.; Ruane, A.C.; Müller, C.; Arneth, A.; Boote, K.J.; Folberth, C.; Glotter, M.; Khabarov, N.; et al. Assessing Agricultural Risks of Climate Change in the 21st Century in a Global Gridded Crop Model Intercomparison. Proc. Natl. Acad. Sci. USA 2014, 111, 3268–3273. [Google Scholar] [CrossRef]
- Demianenko, S.; Sas, I.; Fomichov, M. Global Warming and Its Impact on the Production Activities of Ukrainian Agricultural Enterprises. BIO Web Conf. 2024, 114, 01020. [Google Scholar] [CrossRef]
- Alvarez, A.L.; Weyers, S.L.; Goemann, H.M.; Peyton, B.M.; Gardner, R.D. Microalgae, Soil and Plants: A Critical Review of Microalgae as Renewable Resources for Agriculture. Algal Res. 2021, 54, 102200. [Google Scholar] [CrossRef]
- Miranda, A.M.; Hernandez-Tenorio, F.; Villalta, F.; Vargas, G.J.; Sáez, A.A. Advances in the Development of Biofertilizers and Biostimulants from Microalgae. Biology 2024, 13, 199. [Google Scholar] [CrossRef]
- Dorić, B. Economic Perspective of Renewable Energy Sources: Security of Supply, Innovations, and Challenges. Croat. Reg. Dev. J. 2024, 5, 59–74. [Google Scholar] [CrossRef]
- Ananthi, V.; Raja, R.; Carvalho, I.S.; Brindhadevi, K.; Pugazhendhi, A.; Arun, A. A Realistic Scenario on Microalgae Based Biodiesel Production: Third Generation Biofuel. Fuel 2021, 284, 118965. [Google Scholar] [CrossRef]
- Pramanik, A.; Sinha, A.; Chaubey, K.K.; Hariharan, S.; Dayal, D.; Bachheti, R.K.; Bachheti, A.; Chandel, A.K. Second-Generation Bio-Fuels: Strategies for Employing Degraded Land for Climate Change Mitigation Meeting United Nation-Sustainable Development Goals. Sustainability 2023, 15, 7578. [Google Scholar] [CrossRef]
- Gadhiya, S.; Shukla, A.; Modi, N. Third generation biodiesel: A potential sustainable energy source from microalgae. EPRA Int. J. Multidiscip. Res. (IJMR) 2020, 6, 144–149. [Google Scholar] [CrossRef]
- Silva, J.; Ferreira, A.C.; Teixeira, S.; Martins, L.; Ferreira, E.; Teixeira, J.C. Sawdust Drying Process in a Large-Scale Pellets Facility: An Energy and Exergy Analysis. Clean. Environ. Syst. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Ideris, F.; Zamri, M.F.M.A.; Shamsuddin, A.H.; Nomanbhay, S.; Kusumo, F.; Fattah, I.M.R.; Mahlia, T.M.I. Progress on Conventional and Advanced Techniques of In Situ Transesterification of Microalgae Lipids for Biodiesel Production. Energies 2022, 15, 7190. [Google Scholar] [CrossRef]
- Karpagam, R.; Jawaharraj, K.; Gnanam, R. Review on Integrated Biofuel Production from Microalgal Biomass through the Outset of Transesterification Route: A Cascade Approach for Sustainable Bioenergy. Sci. Total Environ. 2021, 766, 144236. [Google Scholar] [CrossRef] [PubMed]
- Alalawy, A.I.; Yang, Y.; Almutairi, F.M.; El Rabey, H.A.; Al-Duais, M.A.; Abomohra, A.; Salama, E.S. Freshwater Microalgae-Based Wastewater Treatment under Abiotic Stress. AIMS Envrion. Sci. 2023, 10, 504–515. [Google Scholar] [CrossRef]
- Ali, M.; Masood, A.; Saleem, M. Microalgae Cultivation in Wastewater for Simultaneous Nutrients Removal and Biomass Production. Int. J. Energy Environ. Eng. 2021, 12, 475–485. [Google Scholar] [CrossRef]
- Zhang, C.; Li, S.; Ho, S.H. Converting Nitrogen and Phosphorus Wastewater into Bioenergy Using Microalgae-Bacteria Consortia: A Critical Review. Bioresour. Technol. 2021, 342, 126056. [Google Scholar] [CrossRef] [PubMed]
- Occhipinti, P.S.; Russo, N.; Foti, P.; Zingale, I.M.; Pino, A.; Romeo, F.V.; Randazzo, C.L.; Caggia, C. Current Challenges of Microalgae Applications: Exploiting the Potential of Non-Conventional Microalgae Species. J. Sci. Food Agric. 2024, 104, 3823–3833. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.; Ferreira, A.; Antunes, M.; Silva, J.L.; Mendes, S.; Gil, M.M.; Tecelão, C. Nannochloropsis Oceanica as a Sustainable Source of N-3 Polyunsaturated Fatty Acids for Enrichment of Hen Eggs. Appl. Sci. 2021, 11, 8747. [Google Scholar] [CrossRef]
- Sehl, A.; Caderby, E.; Bouhouda, S.; Rébeillé, F.; Griffiths, H.; Da Rocha Gomes, S. How Do Algae Oils Change the Omega-3 Polyunsaturated Fatty Acids Market? OCL Oilseeds Fats Crops Lipids 2022, 29, 20. [Google Scholar] [CrossRef]
- Kusmayadi, A.; Leong, Y.K.; Yen, H.W.; Huang, C.Y.; Chang, J.S. Microalgae as Sustainable Food and Feed Sources for Animals and Humans—Biotechnological and Environmental Aspects. Chemosphere 2021, 271, 129800. [Google Scholar] [CrossRef]
- Orozco Colonia, B.S.; Vinícius de Melo Pereira, G.; Soccol, C.R. Omega-3 Microbial Oils from Marine Thraustochytrids as a Sustainable and Technological Solution: A Review and Patent Landscape. Trends Food Sci. Technol. 2020, 99, 244–256. [Google Scholar] [CrossRef]
- Gonçalves, J.; Freitas, J.; Fernandes, I.; Silva, P. Microalgae as Biofertilizers: A Sustainable Way to Improve Soil Fertility and Plant Growth. Sustainability 2023, 15, 12413. [Google Scholar] [CrossRef]
- Aditi; Bhardwaj, R.; Yadav, A.; Swapnil, P.; Meena, M. Characterization of Microalgal β-Carotene and Astaxanthin: Exploring Their Health-Promoting Properties under the Effect of Salinity and Light Intensity. Biotechnol. Biofuels Bioprod. 2025, 18, 18. [Google Scholar] [CrossRef] [PubMed]
- Rusdianasari, R.; Arissetyadhi, I.; Kalsum, L.; Bow, Y.; Syarif, A.; Arifin, F. Characterization of Empty Fruit Bunch of Palm Oil as Co-Firing Biomass Feedstock. AJARCDE (Asian J. Appl. Res. Community Dev. Empower.) 2023, 7, 74–78. [Google Scholar] [CrossRef]
- Siddiki, S.Y.A.; Mofijur, M.; Kumar, P.S.; Ahmed, S.F.; Inayat, A.; Kusumo, F.; Badruddin, I.A.; Khan, T.M.Y.; Nghiem, L.D.; Ong, H.C.; et al. Microalgae Biomass as a Sustainable Source for Biofuel, Biochemical and Biobased Value-Added Products: An Integrated Biorefinery Concept. Fuel 2022, 307, 121782. [Google Scholar] [CrossRef]
- Yun, H.S.; Lee, J.H.; Choo, Y.S.; Pak, J.H.; Kim, H.S.; Kim, Y.S.; Yoon, H.S. Environmental Factors Associated with the Eukaryotic Microbial Diversity of Ulleungdo Volcanic Island in South Korea. Microbiology 2022, 91, 801–817. [Google Scholar] [CrossRef]
- Jung, S.W.; Oh, Y.S.; Rho, H.S.; Choi, C.G. Subtidal Marine Algal Community and Endangered Species in Dokdo and Ulleungdo, Two Oceanic Islands in the East Sea of Korea. Ocean. Sci. J. 2020, 55, 537–547. [Google Scholar] [CrossRef]
- Chen, W.; Gao, L.; Song, L.; Sommerfeld, M.; Hu, Q. An Improved Phenol-Sulfuric Acid Method for the Quantitative Measurement of Total Carbohydrates in Algal Biomass. Algal Res. 2023, 70, 102986. [Google Scholar] [CrossRef]
- Bakhsh, A.; Park, J.; Baritugo, K.A.; Kim, B.; Sil Moon, S.; Rahman, A.; Park, S. A Holistic Approach toward Development of Plant-Based Meat Alternatives through Incorporation of Novel Microalgae-Based Ingredients. Front. Nutr. 2023, 10, 1110613. [Google Scholar] [CrossRef]
- Anschau, A.; Caruso, C.S.; Kuhn, R.C.; Franco, T.T. Validation of the Sulfo-Phosphovanillin (SPV) Method for the Determination of Lipid Content in Oleaginous Microorganisms. Braz. J. Chem. Eng. 2017, 34, 19–27. [Google Scholar] [CrossRef]
- Niemi, C.; Lage, S.; Gentili, F.G. Comparisons of Analysis of Fatty Acid Methyl Ester (FAME) of Microalgae by Chromatographic Techniques. Algal Res. 2019, 39, 101449. [Google Scholar] [CrossRef]
- Moirangthem, K.; Baxter, D. Alternative Fuels for Marine and Inland Waterways; European Commission: Petten, The Netherlands, 2016. [Google Scholar]
- Al-Hakkani, M.F. Guideline of Inductively Coupled Plasma Mass Spectrometry “ICP–MS”: Fundamentals, Practices, Determination of the Limits, Quality Control, and Method Validation Parameters. SN Appl. Sci. 2019, 1, 791. [Google Scholar] [CrossRef]
- Demura, M.; Noma, S.; Hayashi, N. Species and Fatty Acid Diversity of Desmodesmus (Chlorophyta) in a Local Japanese Area and Identification of New Docosahexaenoic Acid-Producing Species. Biomass 2021, 1, 105–118. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, X.; Melkonian, M.; Wang, S.; Sahu, S.K. Comparative Chloroplast Genome Analysis of Two Desmodesmus Species Reveals Genome Diversity within Scenedesmaceae (Sphaeropleales, Chlorophyceae). Protist 2024, 175, 126073. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.C.; Lee, N.-J.; Jeon, G.Y.; Lee, O.-M.; Yang, E.C. Phylogeny of <I>Desmodesmus</I> (Scenedesmaceae, Chlorophyceae) in Korea Based on Multigene Data Analysis. Environ. Biol. Res. 2023, 41, 345–363. [Google Scholar] [CrossRef]
- Han, C.; Hua, W.; Li, J.; Qiao, Y.; Yao, L.; Hao, W.; Li, R.; Fan, M.; De Jaeger, G.; Yang, W.; et al. TOR Promotes Guard Cell Starch Degradation by Regulating the Activity of β-AMYLASE1 in Arabidopsis. Plant Cell 2022, 34, 1038–1053. [Google Scholar] [CrossRef] [PubMed]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Iwamoto, H.; Soccol, C.R.; Molina-Aulestia, D.T.; Cardoso, J.; de Melo Pereira, G.V.; de Souza Vandenberghe, L.P.; Manzoki, M.C.; Ambati, R.R.; Ravishankar, G.A.; de Carvalho, J.C. Lutein from Microalgae: An Industrial Perspective of Its Production, Downstream Processing, and Market. Fermentation 2024, 10, 106. [Google Scholar] [CrossRef]
- Ahn, Y.J.; Kim, H. Lutein as a Modulator of Oxidative Stress-Mediated Inflammatory Diseases. Antioxidants 2021, 10, 1448. [Google Scholar] [CrossRef]
- Desjardins, S.M.; Laamanen, C.A.; Basiliko, N.; Scott, J.A. Selection and Re-Acclimation of Bioprospected Acid-Tolerant Green Microalgae Suitable for Growth at Low PH. Extremophiles 2021, 25, 129–141. [Google Scholar] [CrossRef]
- Ji, F.; Hao, R.; Liu, Y.; Li, G.; Zhou, Y.; Dong, R. Isolation of a Novel Microalgae Strain Desmodesmus Sp. and Optimization of Environmental Factors for Its Biomass Production. Bioresour. Technol. 2013, 148, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Azevedo, J.L.T. Estimating the Higher Heating Value of Biomass Fuels from Basic Analysis Data. Biomass Bioenergy 2005, 28, 499–507. [Google Scholar] [CrossRef]
- Maksimuk, Y.; Antonava, Z.; Krouk, V.; Korsakova, A.; Kursevich, V. Prediction of Higher Heating Value (HHV) Based on the Structural Composition for Biomass. Fuel 2021, 299, 120860. [Google Scholar] [CrossRef]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Pereira, A.S.A.d.P.; Castro, J.d.S.; Ribeiro, V.J.; Calijuri, M.L. Organomineral Fertilizers Pastilles from Microalgae Grown in Wastewater: Ammonia Volatilization and Plant Growth. Sci. Total Environ. 2021, 779, 146205. [Google Scholar] [CrossRef]
- Hodaifa, G.; Martínez, M.E.; Sánchez, S. Influence of PH on the Culture of Scenedesmus Obliquus in Olive-Mill Wastewater. Biotechnol. Bioprocess. Eng. 2009, 14, 854–860. [Google Scholar] [CrossRef]
- Amini, G.; Najafpour, G.; Mohammadi, M. Optimal Cultivation of Scenedesmus sp. Microalgae in a Bubble Column. Photobioreactor; National Institute of Science Communication and Policy Research (NIScPR): New Delhi, India, 2015; Volume 22, pp. 20–25. [Google Scholar]
- Tesson, S.V.M. Physiological Responses to PH in the Freshwater Microalga Limnomonas Gaiensis. J. Basic. Microbiol. 2023, 63, 944–956. [Google Scholar] [CrossRef]
- Chowdury, K.H.; Nahar, N.; Deb, U.K. The Growth Factors Involved in Microalgae Cultivation for Biofuel Production: A Review. Comput. Water Energy Environ. Eng. 2020, 09, 185–215. [Google Scholar] [CrossRef]
- Austin, C.; Biotechnol Bioeng, J. Citation: Filali R, Tian H, Michiels E and Taidi B. Evaluation of the Growth Performance of Microalgae Based On; Austin Publishing Group: Austin, TX, USA, 2021; Volume 8, p. 1109. [Google Scholar]
- Esteves, A.F.; Soares, S.M.; Salgado, E.M.; Boaventura, R.A.R.; Pires, J.C.M. Microalgal Growth in Aquaculture Effluent: Coupling Biomass Valorisation with Nutrients Removal. Appl. Sci. 2022, 12, 2608. [Google Scholar] [CrossRef]
- Ma, M.; Hu, Q. Microalgae as Feed Sources and Feed Additives for Sustainable Aquaculture: Prospects and Challenges. Rev. Aquac. 2024, 16, 818–835. [Google Scholar] [CrossRef]
- Lin, J.; Yan, H.; Zhao, L.; Li, Y.; Nahidian, B.; Zhu, M.; Hu, Q.; Han, D. Interaction between the Cell Walls of Microalgal Host and Fungal Carbohydrate-Activate Enzymes Is Essential for the Pathogenic Parasitism Process. Envrion. Microbiol. 2021, 23, 5114–5130. [Google Scholar] [CrossRef] [PubMed]
- Çelekli, A.; Özbal, B.; Bozkurt, H. Challenges in Functional Food Products with the Incorporation of Some Microalgae. Foods 2024, 13, 725. [Google Scholar] [CrossRef] [PubMed]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Moldovan, O.L.; Rusu, A.; Tanase, C.; Vari, C.E. Glutamate—A Multifaceted Molecule: Endogenous Neurotransmitter, Controversial Food Additive, Design Compound for Anti-Cancer Drugs. A Critical Appraisal. Food Chem. Toxicol. 2021, 153, 112290. [Google Scholar] [CrossRef]
- Ghosh, U.K.; Islam, M.N.; Siddiqui, M.N.; Cao, X.; Khan, M.A.R. Proline, a Multifaceted Signalling Molecule in Plant Responses to Abiotic Stress: Understanding the Physiological Mechanisms. Plant Biol. 2022, 24, 227–239. [Google Scholar] [CrossRef]
- Hou, D.; Tang, J.; Feng, Q.; Niu, Z.; Shen, Q.; Wang, L.; Zhou, S. Gamma-Aminobutyric Acid (GABA): A Comprehensive Review of Dietary Sources, Enrichment Technologies, Processing Effects, Health Benefits, and Its Applications. Crit. Rev. Food Sci. Nutr. 2023, 64, 8852–8874. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhang, X. Metabolic Engineering of Microorganisms for L-Alanine Production. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab057. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.Q.; Wang, L.R.; Zhang, Z.X.; Sun, X.M.; Huang, H. Stresses as First-Line Tools for Enhancing Lipid and Carotenoid Production in Microalgae. Front. Bioeng. Biotechnol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Conde, T.A.; Neves, B.F.; Couto, D.; Melo, T.; Neves, B.; Costa, M.; Silva, J.; Domingues, P.; Domingues, M.R. Microalgae as Sustainable Bio-Factories of Healthy Lipids: Evaluating Fatty Acid Content and Antioxidant Activity. Mar. Drugs 2021, 19, 357. [Google Scholar] [CrossRef]
- Bayu, A.B.; Beyan, S.M.; Amibo, T.A.; Mekonnen, D.T. Production of Fuel Briquette from Solid Waste Biomass Using Natural Resin as a Binder. Environ. Health Eng. Manag. 2022, 9, 321–328. [Google Scholar] [CrossRef]
- Ismaila, A.; Nasiru, R.; Kaisan, M.U.; Garba, N.N. Determination of energy content of plant biomass for domestic and small-scale industrial heating applications. Fudma J. Sci. 2024, 8, 362–368. [Google Scholar] [CrossRef]
- Huygens, D.; Delgado Sancho, L.; Saveyn, H.G.M.; Tonini, D.; Eder, P. Technical Proposals for Selected New Fertilising Materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009): Process and Quality Criteria, and Assessment of Environmental and Market Impacts for Precipitated Phosphate Salts & Derivates, Thermal Oxidation Materials & Derivates and Pyrolysis & Gasification Materials; Publications Office of the European Union: Luxembourg, 2019. [Google Scholar]
| Marker Gene | Length (bp) | Closest Match (Accession No.) | Query Cover | Identification |
|---|---|---|---|---|
| ITS | 653 | Desmodesmus sp. KNUA024 (MT603589) | 100% | 100% |
| 18S rRNA | 407 | Desmodesmus sp. (OM893304) | 100% | 99.51% |
| tufA | 920 | Desmodesmus spinosus (MN218421) | 94% | 96.08% |
| Amino Acids | Content (µg mL−1) | |
|---|---|---|
| Essential | Threonine | 1.802 |
| Valine | 1.607 | |
| Methionine | 0.230 | |
| Isoleucine | 0.819 | |
| Leucine | 1.138 | |
| Phenylalanine | 0.925 | |
| Lysine | 4.219 | |
| Histidine | 0.309 | |
| Conditionally essential | Arginine | 2.371 |
| Glycine | 2.272 | |
| Tyrosine | 0.665 | |
| Proline | 2.576 | |
| Non-essential | Aspartic Acid | 0.480 |
| Serine | 1.490 | |
| Glutamic acid | 41.567 | |
| Alanine | 20.280 | |
| Gamma-Aminobutyric Acid (GABA) | 2.344 | |
| (%) | Desmodesmus sp. KNUA231 |
|---|---|
| C16:0 | 22.54 |
| C16:1 | 2.40 |
| C16:2 (ω6) | 2.40 |
| C16:3 (ω3) | 1.88 |
| C16:4 (ω3) | 16.38 |
| C18:0 | 0.78 |
| C18:1 | 9.12 |
| C18:2 | 9.17 |
| C18:3 (ω3) | 29.56 |
| C18:4 (ω3) | 5.78 |
| Saturated fatty acid | 19.99 |
| Monounsaturated Fatty Acids | 11.81 |
| Polyunsaturated Fatty Acids | 68.31 |
| Desmodesmus sp. KNUA231 | EN14214 | ASTM D6751 | |
|---|---|---|---|
| Saponification value (mg KOH g−1) | 167.29 | ||
| Iodine value (g I2 100 g−1 fat) | 169.04 | ≤120 | |
| Cetane number | 38.19 | ≥51 | ≥45 |
| Degree of unsaturation | 148.4 | ||
| Cold filter plugging point (°C) | −9.4 | −20~0 | |
| Oxidation stability (110 °C, h) | 5.0 | ≥6 | ≥3 |
| Kinematic viscosity (mm2 s−1) | 3.28 | 3.5 | 1.9~6.0 |
| Density (15 °C) (g cm−3) | 0.89 | 0.872~0.878 |
| (%) | Desmodesmus sp. KNUA231 |
|---|---|
| Proximate analysis (wt%) | |
| Moisture | 4.04 ± 0.51 |
| Volatile matter | 89.56 ± 1.11 |
| Ash | 6.40 ± 1.40 |
| Ultimate analysis (wt%) | |
| Carbon (C) | 53.14 ± 0.16 |
| Hydrogen (H) | 7.67 ± 0.16 |
| Oxygen (O) | 25.16 ± 0.01 |
| Nitrogen (N) | 8.80 ± 0.04 |
| Sulfur (S) | 0.30 ± 0.05 |
| CV * (MJ kg−1) | 25.49 ± 0.28 |
| Element | Concentration (mg kg−1) | |
|---|---|---|
| Macronutrient | K | 8495.826 |
| Ca | 1558.945 | |
| Mg | 2148.444 | |
| Na | 1519.323 | |
| S | 5595.413 | |
| P | 5877.109 | |
| Si | 74.623 | |
| Micronutrient | Zn | 55.919 |
| Cu | 20.072 | |
| Mn | 407.678 | |
| Fe | 1972.932 | |
| B | N.D. * | |
| Mo | N.D. | |
| Metal | As | 0.482 |
| Cd | 0.02 | |
| Pb | 0.366 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.-S.; Do, J.-M.; Noh, H.-S.; Yoon, H.-S. Exploring the Potential of Desmodesmus sp. KNUA231 for Bioenergy and Biofertilizer Applications and Its Adaptability to Environmental Stress. Appl. Sci. 2025, 15, 5097. https://doi.org/10.3390/app15095097
Shin Y-S, Do J-M, Noh H-S, Yoon H-S. Exploring the Potential of Desmodesmus sp. KNUA231 for Bioenergy and Biofertilizer Applications and Its Adaptability to Environmental Stress. Applied Sciences. 2025; 15(9):5097. https://doi.org/10.3390/app15095097
Chicago/Turabian StyleShin, Yeon-Su, Jeong-Mi Do, Hae-Seo Noh, and Ho-Sung Yoon. 2025. "Exploring the Potential of Desmodesmus sp. KNUA231 for Bioenergy and Biofertilizer Applications and Its Adaptability to Environmental Stress" Applied Sciences 15, no. 9: 5097. https://doi.org/10.3390/app15095097
APA StyleShin, Y.-S., Do, J.-M., Noh, H.-S., & Yoon, H.-S. (2025). Exploring the Potential of Desmodesmus sp. KNUA231 for Bioenergy and Biofertilizer Applications and Its Adaptability to Environmental Stress. Applied Sciences, 15(9), 5097. https://doi.org/10.3390/app15095097







