Featured Application
This study highlights the potential of pulsed electric field (PEF) technology as an innovative, non-thermal seed treatment method to improve tomato seed quality. By enhancing the germination performance, increasing the tolerance to abiotic stress, and reducing microbial contamination, the PEF treatment offers a sustainable and efficient approach for seed priming in commercial agriculture and seed processing industries.
Abstract
Seed quality is vital for agricultural productivity, as it directly influences the crop yield and resilience to environmental stressors. This study evaluated the effectiveness of a pulsed electric field (PEF) treatment in enhancing the tomato (Solanum lycopersicum) seed quality, seedling growth, and microbial safety. Tomato seeds were treated with PEFs at energy levels ranging from 1.07 to 17.28 J, and several parameters were assessed, including the germination rate, normal seedling development, tolerance to cold and salinity stress, electrical conductivity, and microbial inactivation. The highest germination rate (72.81%) was observed at 15.36 J on the seventh day of germination, whereas the highest normal seedling rate (94.62%) was recorded at 17.28 J (p ≤ 0.05). The germination under cold stress (5 days at 24 °C) was highest, with a 46.67% germination observed at both 1.92 and 10.88 J. PEF-treated seeds exposed to 100 and 200 mM of NaCl exhibited significantly improved germination compared to the controls (p ≤ 0.05). The electrical conductivity (EC) was more influenced by the incubation time than by the PEF intensity, as the EC of all samples showed a significant increase from 4 to 8 h. The samples treated with 17.28 J exhibited the highest germination rates under salt stress, reaching 62.00 ± 0.90% and 50.00 ± 0.60% under 100 mM and 200 mM of NaCl, respectively (p ≤ 0.05). The initial mean counts of the total mesophilic aerobic bacteria and the total mold and yeast—4.00 ± 0.03 and 3.06 ± 0.03 log cfu/g, respectively—were reduced to undetectable levels by the application of 17.28 J, with higher energy levels yielding greater inactivation. These findings demonstrate that the PEF is a promising technique for enhancing seed quality, promoting seedling vigor, and reducing microbial contamination, supporting its application in sustainable agriculture.
1. Introduction
The tomato (Solanum lycopersicum) is one of the most widely cultivated crops worldwide. In 2022, China led the global tomato production with 68,241,810 tons, followed by India with 20,694,000 tons, and Turkey with 13,000,000 tons [1]. Tomatoes are an important source of essential nutrients, including vitamins, carotenoids, and phenolic compounds, which contribute to various health benefits such as reduced risks of cancer, osteoporosis, and cardiovascular disease [2,3,4]. Whether consumed raw or cooked, tomatoes play a vital role in human nutrition.
The quality of tomato seeds is crucial for sustaining agricultural productivity, as it directly affects germination, seedling vigor, and the eventual crop yield. With the increase in agricultural production, the risk of seed contamination and infection has also risen, posing a significant threat to crop performance [5]. Chemical treatments, such as hydrogen peroxide, sorbic acid, peracetic acid, and sodium benzoate, are commonly used to manage seed-borne diseases; however, these methods raise concerns regarding environmental sustainability and safety [6]. In response to the growing demand for sustainable food systems, the agricultural and food industries are increasingly adopting environmentally friendly technologies that ensure both safety and the preservation of nutritional quality [7].
The pulsed electric field (PEF) treatment is an advanced non-thermal processing technology that uses short bursts of high-voltage electric pulses (typically 20–80 kV/cm for nanoseconds to microseconds) to inactivate microorganisms while preserving the nutritional and sensory properties of food. These high-intensity electric fields induce electroporation—the formation of transient or permanent pores in cell membranes—without causing significant temperature increases in the treated material. The PEF is valued for its ease of operation, automation potential, and short processing time, making it an attractive alternative to conventional thermal treatments. The technology has demonstrated effectiveness in microbial inactivation, shelf-life extension, and the preservation of bioactive compounds in various liquid foods [8,9]. It is widely used in food pasteurization to ensure microbial safety while maintaining quality [10,11].
Despite its proven application in liquid foods, the potential of PEFs to improve seed quality—particularly germination and viability—remains underexplored. The processing of solid agricultural materials such as seeds requires novel PEF system designs and treatment chamber configurations to accommodate their physical properties. Recent advancements by our research group have led to the development of a novel PEF system capable of treating dry products such as grains, seeds, and spices. This system has been successfully tested for seed surface disinfection [12,13,14,15] and for spice treatments aimed at mycotoxin reduction and Aspergillus spp. inactivation [16,17]. However, further studies are necessary to explore the potential of PEF technology to enhance the seed vigor and germination across different seed types. This study aims to evaluate the effects of the PEF treatment on tomato seed germination, stress tolerance, and microbial surface decontamination, contributing to the development of a more sustainable and efficient approach to seed treatments.
2. Materials and Methods
2.1. Tomato Seeds
Tomato seeds (Solanum lycopersicum var. lycopersicum) were obtained from local farmer (Okçular Village, Bolu, Türkiye) and stored in vacuum-sealed packaging at room temperature until subjected to the PEF treatment.
2.2. Pilot-Scale Pulsed Electric Field System
The PEF system for seed treatment consists of a pulse generator, high-voltage (HV) power supply, HV switch, control PC, treatment chamber, and a conveyor. The treatment chamber is equipped with two parallel steel electrodes, commonly referred to as parallel plates. The gap between these plates is adjusted according to the size of the seeds being processed. Once the electric field parameters—frequency, intensity, and treatment time—are configured, the seeds are introduced into the treatment chamber and exposed to the electric field at the specified dose and duration as they pass between the parallel plates along the vertical axis, driven by gravity after the system is activated. The electrode spacing is optimized to ensure effective electric field exposure, while a UV lamp positioned between the electrodes ionizes the surrounding air [13,17,18].
In the pilot-scale PEF system, tomato seeds were treated with pulsed electric fields at frequencies of 110, 140, 160, and 180 Hz, with energy levels ranging from 1.07 to 17.28 J. The specific treatment durations and energy inputs are provided in Table 1.

Table 1.
PEF process parameters applied to tomato seeds.
2.3. Germination Test
Fifty control and PEF-treated tomato seeds were subjected to a germination test in accordance with the criteria established by the International Seed Testing Association [19]. The seeds were placed between two layers of blotting paper, covered with an additional layer, then rolled and positioned in germination cabinets. To determine the average germination rate, the seeds were incubated in the dark at 24 °C for a period of 2 to 7 days, with daily observations conducted. Germination was defined as the emergence of a radicle measuring at least 2 mm in length, and the germination rate (%) was calculated accordingly [18].
2.4. Normal Seedling Rate
At the end of the germination period, seedlings were classified as either normal (characterized by a well-developed root and shoot) or abnormal (exhibiting traits such as curling or a glassy appearance). The seedling rate—expressed as the percentage of normal seedlings—was calculated using the following formula:
For each treatment group, 30 seeds were planted under controlled conditions.
2.5. Cold Stress Test
Two blotting papers, each measuring 40 × 20 cm2, were used to cover 200 g of soil, which was then moistened with 35 mL of water. Tomato seeds were sown on the moistened soil and covered with two additional layers of blotting paper. The prepared samples were placed in a refrigerator bag and stored at 10 °C for 7 days and at 25 °C for 5 days, separately. The percentage of germinated seeds was then calculated [13].
2.6. Electrical Conductivity
The initial weights of 50 tomato seeds were recorded, and the seeds were placed in a 250 mL jar. Pure water with an electrical conductivity no higher than 5 μS/cm was added to the seeds. The jars were then placed in climate cabinets set at 20 °C, and the electrical conductivity was measured using a Sension 5 Model meter (HACH, Loveland, CO, USA) after 4, 8, and 24 h. The electrical conductivity of the tomato seeds was determined by substituting the measurement results into the following formula [18].
EC(µS/cmg) = (EC of the samples − EC of the water)/(Sample weight)
2.7. Salt Stress Test
Fifty tomato seeds were sown at a depth of approximately 4 cm. Two NaCl concentrations—100 mM (conductivity: 10.8 mS/cm) and 200 mM (conductivity: 19.8 mS/cm)—were applied as separate treatments. On the first day, each group was irrigated with 100 mL of the respective solution, followed by 50 mL of daily irrigation thereafter. Seed emergence was monitored and recorded daily [13,18].
2.8. Microbial Enumeration
Control and PEF-treated tomato seeds were homogenized with 0.1% peptone water at a ratio of 1:9 (v/v), and serial dilutions were prepared. Appropriate dilutions were inoculated onto plate count agar (PCA) medium for the total mesophilic aerobic bacterial count using the spread plate method and incubated at 35 ± 2 °C for 24 to 48 h. For total yeast and mold counts, appropriate dilutions were plated on potato dextrose agar (PDA) using the spread plate method and incubated at 22 ± 2 °C for 3 to 5 days. The results were expressed as log CFU/g [13,18].
2.9. Statistical Analysis
All statistical data analyses were performed using MINITAB software (Version 16.2.9, State College, PA, USA) at a significance level of p ≤ 0.05. Electric field strength, treatment time, and electric field energy were selected as explanatory variables. One-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison tests, was used to determine the significant differences for each of the selected response variables related to physical properties and microbial inactivation as a function of the 16 PEF energy levels, excluding control samples. All experiments were conducted in triplicate.
3. Results and Discussion
The germination rate of tomato seeds is a critical factor that influences the success of the crop establishment, yield potential, and resource efficiency. A high germination rate ensures a uniform seedling emergence, leading to synchronized plant growth and development, which is essential for optimizing productivity. It minimizes the need for overseeding, reducing input costs and maximizing the efficient use of water, nutrients, and space. Additionally, fast and consistent germination helps seedlings avoid stress and vulnerability to diseases or pests, contributing to healthier and more robust plants. Therefore, improving germination rates is vital for enhancing the overall performance and profitability of tomato cultivation [20,21]. The germination rate of tomato seeds increased with both the germination time and the energy applied. In the control samples, the germination rate rose from 0.00 ± 0.00% on the second day to 57.78 ± 1.92% on the seventh day. In comparison, the highest germination rate for PEF-treated tomato seeds increased from 3.33 ± 0.33% at 6.80 J on the second day to 72.81 ± 3.85% at 15.36 J on the seventh day (p ≤ 0.05). PEF treatments applied at 1.36, 3.21, 5.76, 6.48, and 15.36 J provided a significant increase in the germination rate of the tomato seeds at the seventh day (p ≤ 0.05) (Table 2).

Table 2.
The germination rate (%) of the control and the PEF-treated tomato seeds.
While germination is an important criterion in terms of seed strength, the normal seedling ratio of germinated seeds is also important. The normal seedling rate is a crucial parameter for evaluating the quality and viability of tomato seeds, as it directly impacts the successful establishment of healthy plants. A higher rate of normal seedlings indicates that the majority of seeds can develop into robust, well-structured plants capable of achieving optimal growth and productivity. This is particularly important in tomato cultivation, where uniformity in seedling vigor can lead to synchronized flowering, fruiting, and harvest times, thus improving the overall crop management and yield. Additionally, a higher normal seedling rate reduces the need for reseeding and minimizes input costs, ensuring the efficient use of resources such as water, nutrients, and space. Therefore, maintaining a high normal seedling rate is vital for maximizing the economic and agronomic success of tomato production [22,23,24]. The normal seedling rate of the tomato seeds was 73.20 ± 3.67%, and this number ranged from 80.43 ± 1.74% for 1.07 J to 94.62 ± 0.45% for 17.28 J, revealing a significant increase by all PEF treatments (p ≤ 0.05) (Table 3).

Table 3.
The normal seedling rate (%) of the control and the PEF-treated tomato seed samples.
In practical terms, such increases in seed performance can have meaningful impacts on the crop establishment and overall productivity, especially under suboptimal conditions such as salinity or cold stress. Improved germination ensures a more uniform and timely emergence of seedlings, which is essential for synchronized growth and effective crop management in mechanized agricultural systems.
From a grower’s perspective, a 10–15% increase in germination and seedling vigor can reduce the need for replanting, lower input costs (such as seeds, water, and fertilizers), and enhance the use efficiency of the available arable land. This is particularly critical in stress-prone environments, where abiotic factors often compromise the crop stand and reduce the yield potential. Moreover, early seedling vigor is often associated with an improved nutrient and water uptake efficiency, which can influence downstream developmental stages, including the flowering, fruit set, and final yield.
Germination under cold stress is a critical factor for the tomato seed performance, as it determines the seed’s ability to establish in suboptimal growing conditions. Cold stress can significantly slow down or inhibit germination, leading to poor crop establishment and uneven growth. For tomato seeds, which are often sensitive to low temperatures, the ability to germinate efficiently under cold stress conditions is essential for early-season planting or cultivation in cooler climates. An enhanced cold tolerance during germination not only improves the seedling vigor and uniformity but also reduces the risk of seed decay and disease, which are more prevalent in slower-germinating seeds. Furthermore, successful germination under cold stress can extend the growing season, increase the yield potential, and offer growers greater flexibility in planting schedules [25,26,27].
Tomato seeds were kept in two different environmental conditions, 7 days at 10 °C and 5 days at 24 °C, to determine their germination ability under cold stress. Both the control and PEF-treated samples showed no germination for 7 days at 10 °C; however all samples showed germination for 5 days at 24 °C. While the germination rate for the control samples was 22.22 ± 1.92%, the germination rate for the PEF-treated samples ranged from 12.22 ± 1.92% to 46.67 ± 3.33%. The germination rate of the PEF-treated samples by 1.92, 5.76, 10.88, 15.36, and 17.28 J was significantly higher than that of the control samples (p ≤ 0.05) (Table 4).

Table 4.
The germination rate (%) of the control and the PEF-treated tomato seeds under the cold stress.
Electrical conductivity (EC) is a critical parameter in assessing the quality and viability of tomato seeds, as it reflects the integrity of seed membranes. High EC values indicate an increased leakage of electrolytes, typically resulting from damaged or deteriorated seed membranes. This leakage is associated with reduced seed vigor, lower germination rates, and a decreased seedling growth potential. Therefore, monitoring EC provides valuable insights into the physiological state of tomato seeds, enabling the early detection of deterioration and supporting the better management of seed storage and handling practices [18,28].
The EC values of the control tomato seed samples measured at 4, 8, and 24 h after the PEF treatment were 75.29 ± 1.88, 77.16 ± 2.38, and 86.04 ± 5.12 µS/cm·g, respectively. The EC of PEF-treated tomato seeds did not exhibit a consistent increasing or decreasing trend with rising energy levels, but a general increase was observed over time following the treatment. The lowest EC values among PEF-treated seeds were recorded at 3.21 J as 59.67 ± 4.21, 60.67 ± 4.13, and 65.38 ± 5.41 µS/cm·g at 4, 8, and 24 h, respectively. In contrast, the highest EC values were observed at 2.16 J as 81.34 ± 4.25, 84.51 ± 4.22, and 92.72 ± 9.51 µS/cm·g at the same time points. As the measurement time increased, the EC values of both the control and PEF-treated samples significantly rose (p ≤ 0.05). Although some PEF-treated samples exhibited EC values close to those of the control, these differences were not statistically significant after 24 h (Table 5).

Table 5.
The electrical conductivity of the control and the PEF-treated tomato seeds.
The salt stress tolerance in tomato seeds refers to the ability of seeds to germinate and grow under saline conditions, which is a crucial trait for ensuring crop productivity in areas affected by soil salinity. High salt concentrations can disrupt the water uptake, induce osmotic stress, and cause ion toxicity, leading to reduced seed germination, poor seedling development, and an overall plant growth inhibition. Tomato seed varieties with higher salt tolerances can better maintain their cellular homeostasis, osmotic balance, and ion compartmentalization under saline stress. The identification and cultivation of salt-tolerant tomato varieties is essential for sustainable agriculture in salt-affected regions. This can be achieved through genetic breeding, seed priming techniques, and the use of biostimulants or treatments like PEF to enhance the seed vigor and resilience [24,28,29].
Under the 100 mM NaCl salt stress, the germination rate of both untreated and PEF-treated tomato seeds steadily increased from day 7 to day 12, regardless of the applied energy level. In control seeds, the germination progressed from 0.00 ± 0.00% on day 7 to 30.00 ± 0.60% on day 12. Among the treated groups, the earliest notable germination (23.33 ± 0.60%) was observed on day 7 at 1.07 J. The highest overall germination rate (62.00 ± 0.90%) by day 12 was achieved at 17.28 J, followed closely by 6.48 J (61.00 ± 0.46%) and 1.07 J (60.30 ± 1.90%). Energy levels between 1.07 J and 17.28 J generally produced superior germination performances compared to the control, particularly from day 10 onward. Statistically significant improvements (p ≤ 0.05) in germination were observed at 1.07, 1.36, 1.92, 3.21, 4.08, 5.35, 5.76, 6.48, 10.88, 15.36, and 17.28 J treatments by day 12. These results suggest that moderate- to high-energy PEF treatments can effectively mitigate the salt-stress-induced germination inhibition in tomato seeds (Table 6).

Table 6.
The germination rate (%) of the control and PEF-treated tomato seed samples under the 100 mM NaCl salt stress.
Under the 200 mM NaCl salt stress, the germination of untreated tomato seeds remained limited, increasing only from 0.00 ± 0.00% on day 7 to 13.30 ± 0.00% by day 12. In contrast, the PEF treatment enhanced the germination across various energy levels, with the earliest notable germination on day 7 observed at 3.21 J (3.30 ± 0.40%). By day 12, the highest germination rate (53.03 ± 0.00%) was recorded at 10.80 J, followed by 17.28 J (50.00 ± 0.60%) and 15.36 J (43.30 ± 0.00%). Energy levels between 3.21 J and 17.28 J were particularly effective in improving the germination under high salt stress. Treatments at 1.07, 1.36, 1.92, 2.16, 3.21, 4.08, 6.48, 6.80, 8.55, 9.60, 10.80, 15.36, and 17.28 J yielded significantly higher germination rates than the control by day 12 (p ≤ 0.05), suggesting that PEFs can alleviate the salt-induced germination inhibition in tomato seeds at moderate- to high-energy applications (Table 7).

Table 7.
The germination rate (%) of the control and the PEF-treated tomato seed samples under the 200 mM NaCl salt stress.
The presence of a high number of total bacteria on tomato seeds may indicate contamination that could negatively impact seed health. Some bacteria can cause seed rot, reduce germination rates, or even transmit seed-borne diseases, which may affect young seedlings and lower the overall crop yield. Fungal contamination, particularly by molds and yeasts, can damage seeds by colonizing their surfaces, leading to decay or reduced seedling vigor. Fungal infections may weaken the seed coat, resulting in improper or delayed germination. Elevated levels of mold and yeast can also create an inhospitable environment for seed growth by consuming nutrients or producing toxic metabolites that inhibit sprouting [30]. Therefore, the inactivation of the endogenous microflora is crucial, as both bacteria and molds can adversely affect the germination process. The mean initial counts of TMAB and TMY in tomato seeds were 4.00 ± 0.03 and 3.06 ± 0.03 log CFU/g, respectively. Both TMAB and TMY counts were reduced to non-detectable levels following the application of 17.28 kV/cm. All PEF treatments resulted in significant reductions in the initial mean counts of TMAB and TMY (p ≤ 0.05) (Table 8).

Table 8.
Inactivation of natural microflora in control and PEF-treated tomato seeds.
Seedling development and root formation are critical stages in the early growth of plants, laying the foundation for overall health, productivity, and resilience. A strong, healthy seedling with a well-developed root system is essential for efficient nutrient and water uptake, which directly influences the plant’s growth rate, vigor, and ability to withstand environmental stressors, such as drought, pests, and disease. Proper root formation ensures a firm anchorage in the soil, reducing the risk of lodging or uprooting under adverse conditions. Additionally, the root system plays a key role in supporting the above-ground development by facilitating the absorption of essential nutrients necessary for photosynthesis and overall plant metabolism. An early and robust seedling growth increases the likelihood of higher yields, enabling the plant to reach its full genetic potential in terms of both productivity and quality [31].
The seedling development and root formation in both control and PEF-treated tomato seeds revealed that the number of seedlings was higher in the PEF-treated group, despite the same number of seeds being planted. The PEF-treated seedlings also exhibited a stronger growth, with thicker stems compared to the control samples (Figure 1). Additionally, they showed a more robust root formation than the control group (Figure 2). Germination studies under salt stress indicated that the number of germinated seedlings was higher in the PEF-treated group under both 100 mM and 200 mM salt stress conditions. Furthermore, PEF-treated seeds produced taller seedlings with a stronger structural development (Figure 3 and Figure 4).
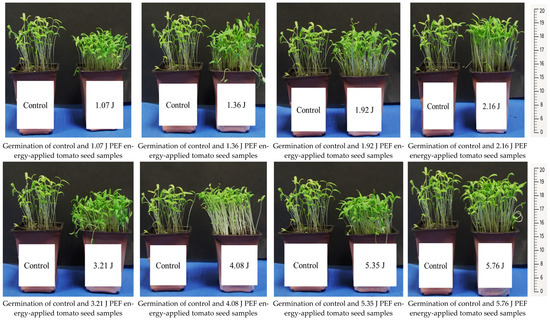
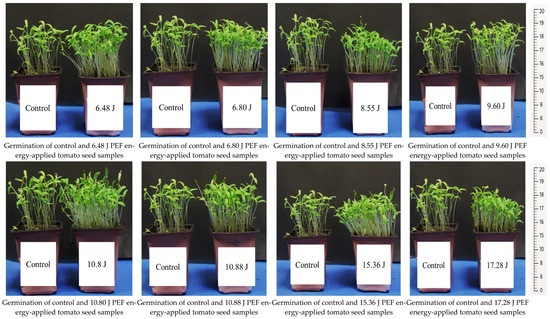
Figure 1.
Seedling development in control and PEF-treated tomato seeds.
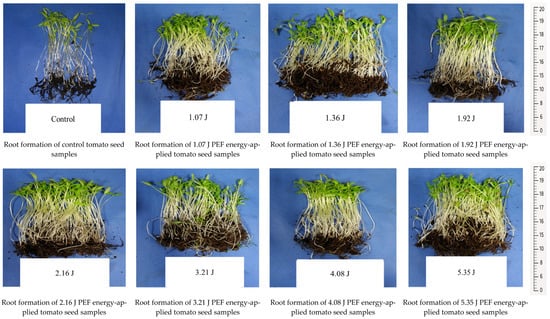

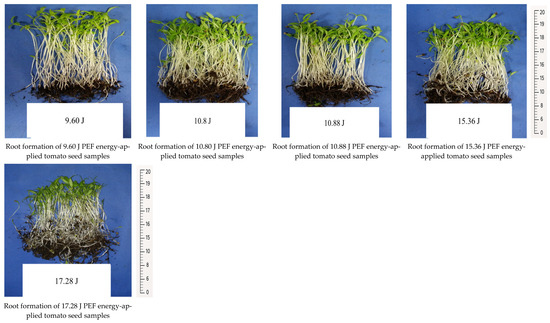
Figure 2.
Root development in control and PEF-treated tomato seeds.
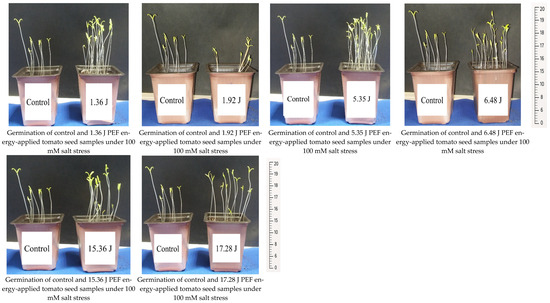
Figure 3.
Seedling formation of PEF-treated and control tomato seeds under 100 mM salt stress.

Figure 4.
Seedling formation of PEF-treated and control tomato seeds under 200 mM salt stress.
The inactivation mechanism of the PEF treatment is explained by thermal effects, electroporation, and the formation of reactive oxygen species (ROS). The high-intensity PEF application leads to a temperature increase in and around the cell, which can cause the denaturation of cellular proteins and the disruption of the cell membrane structure. Electroporation occurs when the permeability of the cell membrane increases due to the application of an electric field. During this process, temporary pores form in the membrane, and if the electric field is strong enough, the damage becomes permanent, ultimately resulting in cell death [32,33]. Additionally, ROS may be generated under high electric fields, and these free radicals can damage the cell membrane and internal components, contributing further to cell death. The effectiveness of this inactivation mechanism depends on several factors, including the frequency, duration, energy density, waveform, and pulse width of the PEF application [34,35].
Tomato seeds exposed to pulsed electromagnetic fields with pulse energies of 35–80 J/pulse for 10 to 15 min demonstrated enhanced plant growth [36]. Similarly, tomato seeds subjected to alternating current (AC) electric fields of 4–12 kV/cm and AC magnetic fields of 3–1000 G for 15–60 s germinated 1.1–2.8 times faster than control seeds. However, the application of a 12 kV electric field for 60 s had an inhibitory effect. It was also observed that germination rates increased by 1.1 to 2.8 times following the electric field treatment [37]. Another study reported that tomato seeds treated with an electric field of 20 kV/cm for 20 s achieved a 100% germination rate, compared to 76% in untreated seeds [38].
The disruption of the cell membrane by PEFs leads to an imbalance in the membrane’s transport system, resulting in increased permeability. This elevated permeability enhances the nutrient and water uptake from the soil. Permeability is typically assessed by measuring changes in EC. An increase in the EC of the surrounding medium reflects the electrolyte leakage from seeds into water, indicating membrane damage [39]. In corn grains, a significant increase in EC was observed over time, suggesting that cell damage may be more time-dependent, rather than being directly caused by the PEF treatment. Similar results were reported for cucumber [13], corn [12], and wheat grains [18], where EC increases were primarily attributed to time rather than the PEF application itself.
Salinity is a major factor reducing crop yields, as high salt concentrations cause hyperosmotic stress and ion imbalance [40], necessitating mechanisms for maintaining homeostasis, detoxification, and growth to develop salt tolerance. Salinity stress adversely affects plant growth by inducing osmotic stress and ionic toxicity, which hinder photosynthesis, nutrient uptake, and the overall development [41]. However, salinity tolerance remains a complex and not fully understood process. The PEF treatment may enhance the salt tolerance under 100 mM and 200 mM NaCl stress conditions, possibly by activating one or more of the plant’s stress tolerance mechanisms. An increased salt tolerance following PEF treatments has also been reported in cucumber seeds [13], corn [12], and wheat grains [18].
The natural microflora of seeds, including bacteria, can influence the seed germination and seedling growth. While various chemicals, such as calcium cyanamide (CaCN₂), are used to disinfect seeds and improve productivity, chemical treatments have drawbacks, including adverse effects on worker safety and the environment [42,43]. As a more sustainable alternative, the PEF treatment has shown promise for seed surface disinfection. For example, a PEF application resulted in a 63.33% reduction in Aspergillus parasiticus in corn seeds [12], a 64.37% reduction in red pepper flakes [16], and a 60% reduction in sesame seeds [17]. Significant reductions in the endogenous microflora of wheat grains [18,44], cucumber seeds [13], and corn grains [12] were also achieved at higher energy levels. Similarly, the substantial inactivation of the total aerobic mesophilic bacteria (TAMB) and total mold and yeast (TMY) was observed in cabbage, lettuce, garden rocket, wheat [15,18], cucumber [13], and corn [12] under high-energy PEF treatments. Additionally, PEFs have been effective in inactivating seed-borne pathogens, such as Drechslera graminea, Xanthomonas campestris pv. campestris, Fusarium graminearum, and Alternaria brassicae, on red cabbage seeds with an increased treatment time and frequency [14].
4. Conclusions
The transition toward sustainable agriculture requires minimizing the use of chemical agents that contribute to environmental degradation and health concerns. Pulsed electric field (PEF) technology, a non-chemical approach, emerges as a promising solution for eco-friendly seed priming. In the present study, the PEF application markedly improved both the germination and seedling development in tomato seeds under various environmental conditions.
The most rapid and pronounced germination response was observed on the seventh day following the exposure to 15.36 J of PEFs, reaching 72.81%, while the highest proportion of morphologically normal seedlings was noted at 17.28 J, achieving a rate of 94.62% (p ≤ 0.05). Cold stress experiments (5 days at 24 °C) revealed that treatments with 1.92 and 10.88 J produced the highest germination rates, each at 46.67%. In saline conditions, the 17.28 J treatment yielded the most favorable outcomes, with germination reaching 62.00 ± 0.90% under 100 mM of NaCl and 50.00 ± 0.60% under 200 mM of NaCl, which are both statistically superior to the untreated controls (p ≤ 0.05). Electrical conductivity (EC) measurements indicated a significant rise from 4 to 8 h of incubation across all samples, suggesting that the duration of the incubation had a more pronounced impact on ion leakage than the PEF dose itself. Additionally, microbial assessments showed that the PEF treatment at 17.28 J effectively eliminated detectable levels of total mesophilic aerobic bacteria and fungal contaminants, enhancing seed hygiene.
The physiological enhancements observed may stem from electroporation-induced changes, such as the increased membrane permeability, improved water and oxygen uptake, and activation of metabolic enzymes, all contributing to a faster and more uniform germination. To build on these findings, future studies should prioritize large-scale field evaluations to assess the consistency of PEF effects under variable agronomic conditions. Furthermore, molecular and biochemical investigations are needed to clarify the pathways through which the PEF enhances the seed vigor and stress tolerance. Evaluating impacts on crop yield, quality, and soil health will be essential to confirm its viability as a replacement for traditional chemical-based seed treatments.
Author Contributions
G.A.E.: Conceptualization, data curation, data analyses, modeling, optimization, writing—original draft; B.Y.: Conducting test and analysis, writing first draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by TUBITAK, (Project no: 217O068).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
Data will be available upon request.
Conflicts of Interest
The authors have declared no conflicts of interest for this article.
References
- Uzundumlu, A.S.; Pınar, V.; Tosun, N.E.; Kumbasaroğlu, H. Global Pistachio Production Forecasts for 2020–2025. KSU J. Agric. Nat. 2024, 27, 1105–1115. [Google Scholar] [CrossRef]
- Bhowmik, D.; Kumar, K.P.S.; Paswan, S.; Srivastava, S. Tomato-A Natural Medicine and Its Health Benefits. J. Pharmacogn. Phytochem. 2012, 1, 33–43. [Google Scholar]
- Collins, E.J.; Bowyer, C.; Tsouza, A.; Chopra, M. Tomatoes: An Extensive Review of the Associated Health Impacts of Tomatoes and Factors That Can Affect Their Cultivation. Biology 2022, 11, 239. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.-P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef]
- Kim, H.-S.; Ko, M.-J.; Park, C.-H.; Chung, M.-S. Application of Pulsed Electric Field as a Pre-Treatment for Subcritical Water Extraction of Quercetin from Onion Skin. Foods 2022, 11, 1069. [Google Scholar] [CrossRef]
- Fidan, H.; Ulusoy, D.; Albezirgan, H.N. Exploring Effective Strategies for ToBRFV Management in Tomato Production: Insights into Seed Transmission Dynamics and Innovative Control Approaches. Agriculture 2024, 14, 108. [Google Scholar] [CrossRef]
- Arshad, R.N.; Abdul-Malek, Z.; Roobab, U.; Munir, M.A.; Naderipour, A.; Qureshi, M.I.; El-Din Bekhit, A.; Liu, Z.-W.; Aadil, R.M. Pulsed Electric Field: A Potential Alternative towards a Sustainable Food Processing. Trends Food Sci. Technol. 2021, 111, 43–54. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Irfan, S.; Lorenzo, J.M.; Shafique, B.; Kanwal, R.; Pateiro, M.; Arshad, R.N.; Wang, L.; Nayik, G.A.; Roobab, U.; et al. Sonication, a Potential Technique for Extraction of Phytoconstituents: A Systematic Review. Processes 2021, 9, 1406. [Google Scholar] [CrossRef]
- Xi, J.; Li, Z.; Fan, Y. Recent Advances in Continuous Extraction of Bioactive Ingredients from Food-Processing Wastes by Pulsed Electric Fields. Crit. Rev. Food Sci. Nutr. 2021, 61, 1738–1750. [Google Scholar] [CrossRef]
- Araújo, A.; Barbosa, C.; Alves, M.R.; Romão, A.; Fernandes, P. Implications of Pulsed Electric Field Pre-Treatment on Goat Milk Pasteurization. Foods 2023, 12, 3913. [Google Scholar] [CrossRef]
- Charles-Rodríguez, A.V.; Nevárez-Moorillón, G.V.; Zhang, Q.H.; Ortega-Rivas, E. Comparison of Thermal Processing and Pulsed Electric Fields Treatment in Pasteurization of Apple Juice. Food Bioprod. Process. 2007, 85, 93–97. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Atmaca, B.; Uzuner, S. Corn Processing by Pulsed Electric Fields with Respect to Microbial Inactivation and Improvement of Seed Vigour. Comput. Electron. Agric. 2024, 219, 108830. [Google Scholar] [CrossRef]
- Atmaca, B.; Evrendilek, G.A.; Bulut, N.; Uzuner, S. Unrevealing the Impact of Pulsed Electric Fields (PEF) on Cucumber Seed Vigour and Surface Disinfection. EuroBiotech J. 2021, 5, 180–193. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Karatas, B.; Uzuner, S.; Tanasov, I. Design and Effectiveness of Pulsed Electric Fields towards Seed Disinfection. J. Sci. Food Agric. 2019, 99, 3475–3480. [Google Scholar] [CrossRef]
- Evrendilek, G.A.; Tanasov, I. Configuring Pulsed Electric Fields to Treat Seeds: An Innovative Method of Seed Disinfection. Seed Sci. Technol. 2017, 45, 72–80. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Bulut, N.; Atmaca, B.; Uzuner, S. Prediction of Aspergillus Parasiticus Inhibition and Aflatoxin Mitigation in Red Pepper Flakes Treated by Pulsed Electric Field Treatment Using Machine Learning and Neural Networks. Food Res. Int. 2022, 162, 111954. [Google Scholar] [CrossRef]
- Bulut, N.; Atmaca, B.; Akdemir Evrendilek, G.; Uzuner, S. Potential of Pulsed Electric Field to Control Aspergillus Parasiticus, Aflatoxin and Mutagenicity Levels: Sesame Seed Quality. J. Food Saf. 2020, 40, e12855. [Google Scholar] [CrossRef]
- Akdemir Evrendilek, G.; Atmaca, B.; Bulut, N.; Uzuner, S. Development of Pulsed Electric Fields Treatment Unit to Treat Wheat Grains: Improvement of Seed Vigour and Stress Tolerance. Comput. Electron. Agric. 2021, 185, 106129. [Google Scholar] [CrossRef]
- International Seed Testing Association. International Rules for Seed Testing. Seed Sci. Technol. 1999; Wallisellen, Switzerland. Available online: https://www.seedtest.org/en/publications/international-rules-seed-testing.html (accessed on 31 December 2024).
- Ranal, M.A.; Santana, D.G.D. How and Why to Measure the Germination Process? Braz. J. Bot. 2006, 29, 1–11. [Google Scholar] [CrossRef]
- Soltani, E.; Ghaderi-Far, F.; Baskin, C.C.; Baskin, J.M. Problems with Using Mean Germination Time to Calculate Rate of Seed Germination. Aust. J. Bot. 2015, 63, 631–635. [Google Scholar] [CrossRef]
- Johnson, J.D.; Cline, M.L. Seedling Quality of Southern Pines. In Forest Regeneration Manual; Duryea, M.L., Dougherty, P.M., Eds.; Springer: Dordrecht, The Netherlands, 1991; pp. 143–159. ISBN 978-94-011-3800-0. [Google Scholar]
- Ozden, E.; Ozdamar, C.; Demir, I. Radicle Emergence Test Estimates Predictions of Percentage Normal Seedlings in Standard Germination Tests of Aubergine (Solanum Melongena L.) Seed Lots. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 177–182. [Google Scholar] [CrossRef]
- Silva, V.N.; Cicero, S.M. Image Seedling Analysis to Evaluate Tomato Seed Physiological Potential. Rev. Ciênc. Agron. 2014, 45, 327–334. [Google Scholar] [CrossRef]
- Foolad, M.R.; Lin, G.Y.; Chen, F.Q. Comparison of QTLs for Seed Germination under Non-Stress, Cold Stress and Salt Stress in Tomato. Plant Breed. 1999, 118, 167–173. [Google Scholar] [CrossRef]
- Zabihi-E-Mahmoodabad, R.; Jamaati, S.; Khayatnezhad, M.; Gholamin, R. Effect of Cold Stress on Germination and Growth of Wheat Cultivars. Adv. Environ. Biol. 2011, 5, 94–97. [Google Scholar]
- Zhang, Y.; Dai, T.; Liu, Y.; Wang, J.; Wang, Q.; Zhu, W. Effect of Exogenous Glycine Betaine on the Germination of Tomato Seeds under Cold Stress. Int. J. Mol. Sci. 2022, 23, 10474. [Google Scholar] [CrossRef]
- Marin, M.; Laverack, G.; Powell, A.A.; Matthews, S. Potential of the Electrical Conductivity of Seed Soak Water and Early Counts of Radicle Emergence to Assess Seed Quality in Some Native Species. Seed Sci. Technol. 2018, 46, 71–86. [Google Scholar] [CrossRef]
- Li, Y. Physiological Responses of Tomato Seedlings (Lycopersicon Esculentum) to Salt Stress. Modern Appl. Sci. 2009, 3, 171–176. [Google Scholar] [CrossRef]
- Kozlowski, T.T. Germination Control Metabolism, and Pathology; Elsevier: Amsterdam, The Netherlands, 2012; ISBN 978-0-323-14948-8. [Google Scholar]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant Root Growth, Architecture and Function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Simonis, P.; Kersulis, S.; Stankevich, V.; Sinkevic, K.; Striguniene, K.; Ragoza, G.; Stirke, A. Pulsed Electric Field Effects on Inactivation of Microorganisms in Acid Whey. Int. J. Food Microbiol. 2019, 291, 128–134. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Gullon, P.; Hesari, J.; Gullón, B.; Alirezalu, K.; Lorenzo, J. Quality Aspects and Safety of Pulsed Electric Field (PEF) Processing on Dairy Products: A Comprehensive Review. Food Rev. Int. 2022, 38, 96–117. [Google Scholar] [CrossRef]
- Wang, M.-S.; Wang, L.-H.; Bekhit, A.E.-D.A.; Yang, J.; Hou, Z.-P.; Wang, Y.-Z.; Dai, Q.-Z.; Zeng, X.-A. A Review of Sublethal Effects of Pulsed Electric Field on Cells in Food Processing. J. Food Eng. 2018, 223, 32–41. [Google Scholar] [CrossRef]
- Wouters, P.C.; Alvarez, I.; Raso, J. Critical Factors Determining Inactivation Kinetics by Pulsed Electric Field Food Processing. Trends Food Sci. Technol. 2001, 12, 112–121. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Katsenios, N.; Karkanis, A.; Papastylianou, P.; Triantafyllidis, V.; Travlos, I.; Bilalis, D.J. Effects of Presowing Pulsed Electromagnetic Treatment of Tomato Seed on Growth, Yield, and Lycopene Content. Sci. World J. 2014, 2014, 369745. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-D.; Chung, H.-S. Acceleration of Germination of Tomato Seed by Applying AC Electric and Magnetic Fields. J. Electrost. 2000, 48, 103–114. [Google Scholar] [CrossRef]
- Patwardhan, M.S.; Gandhare, W.Z. High Voltage Electric Field Effects on the Germination Rate of Tomato Seeds. Acta Agrophys. 2013, 20, 403–413. [Google Scholar]
- Berzosa, A.; Marín-Sánchez, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Pulsed Electric Field Technology for the Extraction of Glutathione from Saccharomyces Cerevisiae. Foods 2024, 13, 1916. [Google Scholar] [CrossRef]
- Granella, S.J.; Christ, D.; Werncke, I.; Bechlin, T.R.; Machado Coelho, S.R. Effect of Drying and Ozonation Process on Naturally Contaminated Wheat Seeds. J. Cereal Sci. 2018, 80, 205–211. [Google Scholar] [CrossRef]
- Safdar, H.; Amin, A.; Shafiq, Y.; Ali, A.; Yasin, R.; Sarwar, M.I. Abbas Shoukat, Maqsood Ul Hussan, Muhammad Ishtiaq Sarwar. A Review: Impact of Salinity on Plant Growth. Nat. Sci. 2019, 1, 34–40. [Google Scholar] [CrossRef]
- Chen, L.; Xie, X.; Kang, H.; Liu, R.; Shi, Y.; Li, L.; Xie, J.; Li, B.; Chai, A. Efficiency of Calcium Cyanamide on the Control of Tomato Soil-Borne Disease and Their Impacts on the Soil Microbial Community. Appl. Soil Ecol. 2022, 176, 104522. [Google Scholar] [CrossRef]
- Ding, L.-G.; Li, G.; Le, G.-M.; Gu, B.; Cao, X.-X. Seed populatıon ın large solar energetıc partıcle events and the twın-cme scenarıo. Astrophys. J. 2015, 812, 171. [Google Scholar] [CrossRef]
- Ahmed, Z.; Manzoor, M.F.; Ahmad, N.; Zeng, X.-A.; Din, Z.U.; Roobab, U.; Qayum, A.; Siddique, R.; Siddeeg, A.; Rahaman, A. Impact of Pulsed Electric Field Treatments on the Growth Parameters of Wheat Seeds and Nutritional Properties of Their Wheat Plantlets Juice. Food Sci. Nutr. 2020, 8, 2490–2500. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).