Abstract
Ankle osteoarthritis (OA) is characterized by intraarticular, activity-related pain. mainly with weight-bearing activities. Several nonoperative therapies remain as the first choices, but there is still no consensus on which is the most effective. This study aimed to systematically synthesize the evidence regarding the effectiveness of PRP in the management of ankle OA. Searches were conducted on PubMed, Cochrane, CINAHL, and SPORTDiscus to find relevant articles from inception to December 2023. Details regarding study characteristics, PRP procedures, and outcomes were extracted. A quality assessment was developed according to Joanna Briggs Institute’s critical appraisal tool. A total of five studies met the inclusion criteria, with 184 patients receiving PRP therapy (mean age 55.7 years, and 51.1% were female). There were three case series, one prospective cohort, and one randomized controlled trial. Pain and function were the most frequently evaluated outcomes among the selected articles. They showed significant improvements in the short-term follow-ups, mostly in patients with II-III-stage ankle OA. There is currently insufficient evidence regarding the effectiveness and safety of PRPs in the treatment of ankle OA. A paucity of high-level research in the literature was also found.
1. Introduction
Osteoarthritis (OA) has been considered the most common form of joint disease worldwide, and its functional and clinical impairments place a major burden on both communities and healthcare systems. As a highly correlated condition with aging, its impact is expected to increase in a way that is directly proportional to global life expectancy. Additionally, the increasing appearance of OA risk factors, such as obesity, physical inactivity, and joint injury, is contributing to a propagation phenomenon [1].
Clinical evidence has suggested that the prevalence of ankle OA within the general population is significantly lower than OA in other joints [2]. With the hands, knees, and hips being the most common areas affected by OA [3], ankle OA in particular ranges from 9% to 15% of all OA cases in general adult populations [4]. However, ankle OA is more commonly found in the young, active population, predominantly in women, due to their tendency to experience repeated trauma throughout a longer lifetime period [5]. Quality of life and physical functioning in patients with ankle OA have been found to be equivalent to those perceived in patients with musculoskeletal comorbidities like hip OA, but also compared with systemic conditions, such as end-stage kidney disease or congestive heart failure [6].
Surgery methods like joint replacement, in contrast with their effect on hip/knee OA, are associated with important functional restrictions in ankle OA patients. Regarding nonoperative modalities, there is a wide variety of options, from pharmacological to physical therapies, but these are not without side effects or produce only short-term results over time [7,8]. Furthermore, a lack of evidence from high-quality studies has been suggested by recent systematic reviews on intra-articular injection therapy in large joint OA [9,10]. Recent advances in biological research have highlighted the importance of growth factors in cartilage pathology [11]. Platelet-rich plasma (PRP) arises as an effective treatment in the management of the symptoms of OA. However, despite its increasing popularity and potential biological benefits, the clinical improvements related to current PRP injectables are still uncertain [12]. The reasons for this include the lack of standardized protocols for preparation and administration, the existence of different types of PRP products, or the use of different activating methods for platelet stimulation, among others. Therefore, the effectiveness of PRP is still being debated, as the current literature reports contradictory results from different methodological approaches [11,12,13]. Despite all this incongruence, the use of PRP in treating ankle OA has become more prevalent in recent times, and several studies have demonstrated both positive and neutral outcomes. Due to these mixed results, we aimed to systematically synthesize the available scientific literature on PRP as a therapeutic intervention in managing ankle OA.
2. Materials and Methods
2.1. Design
The systematic review protocol was developed in accordance with the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines [14] and prospectively registered with PROSPERO (registration: CRD42018085261) [15].
2.2. Data Search
An electronic search was performed in mid-December 2023 on PubMed, Cochrane, CINAHL, and SPORTDiscus. Database searches were performed by two authors, with no limits, and checked by one of them (IM-P). The search strategy was based on both Medical Subject Heading (MeSH) and non-MeSH terms. The referred databases were queried based on three broad terms: (i) osteoarthritis, (ii) ankle, and (iii) platelet-rich plasma. All references from selected articles were manually reviewed for additional papers to maximize the search. Further information about the search strategies developed on databases is provided in Appendix A.
2.3. Study Selection and Eligibility Criteria
Studies were firstly screened by title and abstract by two independent evaluators (MO-C and IM-P). Potentially eligible full-text articles were obtained and assessed independently using the following inclusion criteria: (i) studies with human subjects; (ii) clinical studies (randomized/non-randomized clinical trials, comparative and observational studies, case reports); (iii) full-text articles published in English. Animal and preclinical studies, and papers not reporting follow-up, or involving patients with previous surgical procedures, were excluded. When the full-text version was not published in English, the paper was discarded. The same two evaluators independently reviewed the full text of the included studies, with a third author (AG-C) acting as the tiebreaker to resolve any disagreements.
2.4. Data Extraction
The Cochrane Collaboration data collection form for randomized and non-randomized controlled trials was employed to extract data for this systematic review [16]. Data extracted included sample characteristics, study design, level of evidence, type and characteristics of PRP intervention, and clinical and functional outcomes. Secondary outcomes, such as patient satisfaction, along with potential side effects, were also investigated. PRP therapy effectiveness was assessed through point measures and variance estimations of the selected outcomes. Reported details from every follow-up period were extracted and used in this analysis. Authors were contacted for additional data when necessary.
2.5. Quality Assessment
The standardized Joanna Briggs Institute (JBI) critical appraisal tool—JBI Meta-Analysis of Statistics Assessment and Review Instrument (JBI—MAStARI) [17]—was used for assessing risk of bias. The risk of bias tool assesses clear reporting of inclusion criteria, demographics, clinical information, incomplete outcome data, appropriate statistical analysis, and other sources of bias. Two reviewers (MO-C and IM-P) independently evaluated the quality of the selected studies. Discrepancies between them were discussed and reconsidered until consensus was reached. Again, when no consensus could be reached, a third reviewer was consulted (AG-C).
2.6. Outcomes
Ankle OA pain severity and function were highlighted as the effectiveness outcomes of interest in our analysis. Pain severity and function on relevant tools were extracted from each study.
2.7. Strength-of-Evidence Assessment
The strength of evidence for PRP therapy for ankle OA was assessed by defining four levels of evidence, as defined by the Oxford Centre for Evidence-Based Medicine [18].
3. Results
3.1. Study Selection
A total of 511 citations were retrieved after the initial searches on databases, which also included articles obtained through manual search and reference lists. After removing duplicates, 422 articles were selected for screening, with 9 studies remaining for full-text assessment. After final screening, five studies fulfilled the eligibility criteria and were included in the final analysis. There were four uncontrolled trials, three of which were case series, one of which was a prospective cohort, and one randomized controlled trial. Further details of the selection process are provided in the PRISMA flowchart (Figure 1).
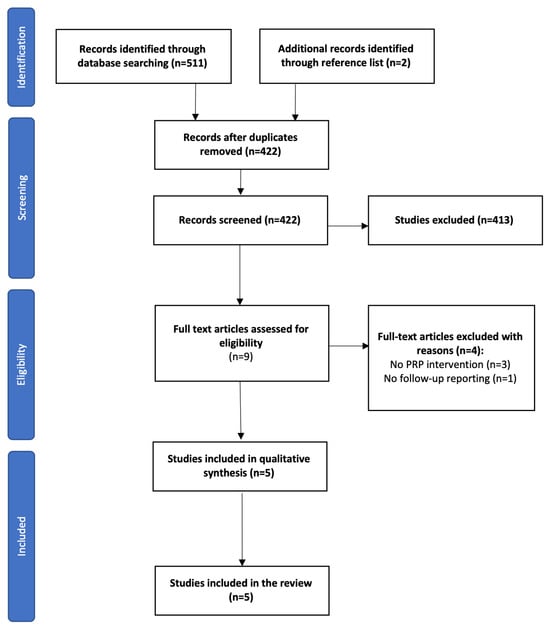
Figure 1.
PRISMA flow chart.
3.2. Patients and Study Characteristics
The mean age in the five studies was 55.7 years, with a range of 18–71. A total of 184 adult subjects with symptomatic ankle OA (184 ankles) were included, with 51.1% being female participants. The mean follow-up period was 10.4 months, ranging from 2 to 30. The severity of ankle OA was radiographically graded in accordance with Takakura or Kellgren–Lawrence classifications. The most prevalent ankle OA grades were reported to be grades III and IV, except for one study showing that most of its subjects had grade I [19]. Regarding study type, three articles were prospective case series, one study showed a retrospective design, and another one was a randomized clinical trial. Table 1 gathers the information about the characteristics of the included studies.
3.3. Risk of Bias Assessment
The mean number of accomplished domains in the JBI critical appraisal tool was 7.6/10 (range 6 to 9). There was a general accomplishment of the domains regarding bias in the diagnosis of the condition, reporting of clinical information, and appropriate statistical analysis. No studies reported details regarding the full inclusion of participants. The two reviewers agreed and reached a consensus on all the criteria. Table 2 includes the complete information about the risk of bias assessment.
3.4. PRP Procedures
All of the included studies described the followed PRP procedures, which were found to show great variability. In one study, the authors centrifuged the whole blood thrice [20], whereas in another study, the authors performed two centrifugations [21], the first at 800 g for 5 min, and the second at 1500 g for 8 min. Another two studies employed just one cycle at 1500 rpm for 5 and 8 min, respectively [19,22], whereas a fifth study used a range of 500–1200 rpm for 8 min [23]. After centrifugation, the final product was cryopreserved in two cases at different temperatures [20,21], whereas the other three cases employed no frozen phases [19,22,23].
The addition of an activator varied minimally among the studies. One study employed calcium chloride to activate PRP immediately before the injection [21], whereas the remaining four studies did not report the use of any activator substances.
The number of injections ranged from a single administration to four times during the therapy course, with frequencies ranging from once a week to once every two weeks. The post-treatment recommendations regarding medication allowance also differed, but there was a general consensus on not permitting heavy physical work right after the injection. Table 3 and Table 4 show further details about PRP preparation techniques and intervention procedures, respectively.
3.5. PRP Formulations
The diverse concentrations of PRP used were provided in all the included studies. Three studies reported low leukocyte concentrations in the final PRP product, being under 1000 cells/mL [20] or under 4% [23]. The two remaining studies reported not including white blood cells or red blood cells in any of the PRP samples [21,22].
3.6. PRP Effectiveness
Pain and function measures were included in all the selected studies. Pain was measured by using the Visual Analogue Scale (VAS) [19,20,21,23] and sub-scores from VAS Foot and Ankle (VAS-FA) [22], Ankle Osteoarthritis Score (AOS) [23], and Japanese Society for Surgery of the Foot (JSSF) [21]. Function was assessed with the Foot and Ankle Disability Index (FADI) [20], AOS [19], Ankle Activity Score (AAS) [19], Foot and Ankle Outcome Score (FAOS) [19], Single Leg Stance (SLS) test [23], and American Orthopaedic Foot and Ankle Score (AOFAS), and sub-scores from VAS-FA [22], AOFAS [19,23], and JSSF [21]. Secondary outcomes such as quality of life, satisfaction, achieved goals, and pathological condition of the foot and ankle were measured by using Short-Form 36 (SF-36) [19,22], the EuroQol Quality of Life questionnaire (EQ-5D-3L) [19], Goal Attainment Scaling (GAS) [19], the Self-Administered Foot Evaluation Questionnaire (SAFE-Q) [21], and a global satisfaction score [23].
Overall, all of the studies showed significant results for pain and function measurements during and at the end of the different follow-up periods. However, one study divided the patients into two sub-groups regarding the stage of OA, and the results differed among them. The subjects suffering from early-stage OA had significantly better results in VAS and JSSF data in comparison with later-stage individuals (p = 0.02) [21]; concerning within-group evaluations, significant results were observed for VAS in the early-stage group (p = 0.01) and for JSSF in the late-stage group (p = 0.005) at 1-month follow-up; regarding the 3-month follow-up, the VAS and JSSF scores improved significantly in both early- and late-stage groups, although they did not persist over time. Another study did not show intra-group details from the different follow-ups, but these were significant between baseline and 26-week measurements in both groups (p < 0.001); nevertheless, no significant changes between groups were found [19].
Regarding secondary outcomes, one study showed significant improvements for SAFE-Q at 3 months through overall assessments, although these were not present when evaluating early- and late-stage subjects separately [21]. Another study divided subjects into two groups to determine the satisfaction with the results, and those in the satisfied group reported higher SF-36 scores than those belonging to the unsatisfied group (p = 0.003), in the same vein as that which occurred with the VAS-FA [22]. In this manner, Sun et al. also assessed patients’ satisfaction and found higher improvement rates in those who were more satisfied [23]. Repetto et al. also registered patients’ degree of conformity in their study and found that 80% of the subjects were satisfied and returned to their previous level of activity [20].
Table 5 shows further details about the studies’ reported outcomes.
3.7. Adverse Effects
Adverse effects were only observed in three studies, with rates ranging from 4 to 26%. The reported adverse events consisted of pain and swelling in the injection area [21,23], ipsilateral knee pain, and lower leg muscle soreness [19]. According to these authors, the symptoms resolved spontaneously 48 to 72 h later and were never the cause of withdrawal. Overall, the injection regimes were well tolerated, and no serious adverse events such as local/systemic infection or intra-articular hematoma were noted during treatment or follow-up.

Table 1.
Characteristics of the included studies.
Table 1.
Characteristics of the included studies.
| Author, Year | Geographic Location | Sample Size | Mean Age (Years) | Design | Previous Duration of Symptoms | Follow-Up Period | Measured Outcomes | Ankle OA Classifications | OCEBM Levels of Evidence | |
|---|---|---|---|---|---|---|---|---|---|---|
| Takakura (I, II, IIIA, IIIB, IV) | KLG (I, II, III, IV) | |||||||||
| Sun et al., 2021 [23] | Taiwan | 39 | 55.5 | Prospective study | >6 months | 6 months | VAS, AOS, AOFAS, SLS tests, analgesic intake, satisfaction | - | I and II: n = 28 III and IV: n = 11 | III |
| Paget et al., 2021 [19] | The Netherlands | 100 | 55.6 | Randomized controlled trial | >2 years | 26 weeks | VAS, AOS, FAOS, AOFAS, AAS, SF-36, GAS, EQ-5D-3L | I: n = 55 II: n = 16 III: n = 15 IV: n = 14 | III: n = 69 IV: n = 31 | II |
| Fukawa et al., 2017 [21] | Japan | 20 | 59.3 | Case series | >6 months | 24 weeks | VAS, JSSF scale, SAFE-Q | I: n = 0 II: n = 2 IIIA: n = 5 IIIB: n = 10 IV: n = 3 | - | IV |
| Repetto et al., 2017 [20] | Italy | 20 | 57.5 | Case series | >12 months | 12 to 30 months (mean 17.7) | VAS, FADI | - | I: n = 0 II: n = 0 III: n = 11 IV: n = 9 | IV |
| Angthong et al., 2013 [22] | Thailand | 12 (n = 5 with ankle OA) | 50.8 | Retrospective case series | >6 months | 2 to 22.3 months (mean 16) | VAS-FA, SF-36 | I: n = 2 II: n = 1 IIIA: n = 0 IIIB: n = 1 IV: n = 0 | - | IV |
Abbreviations: VAS, Visual Analogue Scale; VAS-FA, Visual Analogue Scale Foot and Ankle; JSSF, Japanese Society for Surgery of the Foot; SAFE-Q, Self-Administered Foot Evaluation Questionnaire; FADI, Foot and Ankle Disability Index; AOS, Ankle Osteoarthritis Score; AAS, Ankle Activity Score; FAOS, Foot and Ankle Outcome Score; SF-36, 36-Item Short-Form Health Survey; SLS, Single Leg Stance test; AOFAS, American Orthopaedic Foot and Ankle Score; GAS, Goal Attainment Scaling; EQ-5D-3L, EuroQol Quality of Life questionnaire; OCEBM, Oxford Centre for Evidence-Based Medicine.

Table 2.
Qualitative assessment according to Joanna Briggs Institute critical appraisal tool.
Table 2.
Qualitative assessment according to Joanna Briggs Institute critical appraisal tool.
| Authors, Year | Clear Inclusion Criteria | Standardized Measurements of the Condition | Valid Diagnostic Methods | Consecutive Inclusion of Participants | Complete Inclusion of Participants | Clear Reporting of Demographics | Clear Reporting of Clinical Information | Clear Reporting of Outcomes | Clear Reporting of Center Demographics | Appropriate Statistical Analysis |
|---|---|---|---|---|---|---|---|---|---|---|
| Sun et al., 2021 [23] | ||||||||||
| Paget et al., 2021 [19] | ||||||||||
| Fukawa et al., 2017 [21] | ||||||||||
| Repetto et al., 2017 [20] | ||||||||||
| Angthong et al., 2013 [22] |
Color coding: Gray: Yes; White: No; Black: Unclear.

Table 3.
Platelet-rich plasma preparation protocols in the included studies.
Table 3.
Platelet-rich plasma preparation protocols in the included studies.
| Authors, Year | Extracted Volume of Blood (mL) | Centrifugation | Aliquots Obtained | Storage Temperature | Platelet Concentration | White/Red Cells Count | Activator | Source for Each Injection | PRP System |
|---|---|---|---|---|---|---|---|---|---|
| Sun et al., 2021 [23] | 7 | 1 centrifugation: 500 to 1200 rpm (8 min) | Units of 3 mL | NR | NR, but 2.4× greater than baseline † | Leukocytes: <3.7% † Erythrocytes: NR | NR | Fresh sample | NR |
| Paget et al., 2021 [19] | 15 | 1 centrifugation: 1500 rpm (5 min) † | Units of 2 mL | NR | NR, but probably >1× greater than baseline | Leukocytes: poor (NR) Erythrocytes: NR | None | Fresh sample | ACP |
| Fukawa et al., 2017 [21] | 200 | 2 centrifugations:
| 3 units of 2 mL each | −30 °C | 1310.4 ± 667 × 103/μL (5.1 ± 2.3 times higher than in whole blood) | Leukocytes: 0 Erythrocytes: 0 | 10% calcium chloride | Frozen sample | Fresh sample |
| Repetto et al., 2017 [20] | 450 | 3 centrifugations:
| 4 units of 3 mL each | −80 °C | 600,000 cells/μL (range 250,000 to 900,000) | Leukocytes: <1000 cells/μL Erythrocytes: NR | NR | Frozen sample | NR |
| Angthong et al., 2013 [22] | 9–10 | 1 centrifugation: 1500 rpm (5 min) | Units of 3 mL † | NR | NR, but 2–3× greater than average † | Leukocytes: 0 † Erythrocytes: 0 † | None † | Fresh sample | ACP |
Abbreviations: NR, not reported; rpm, revolutions per minute; g, gravitational force; ACP, autologous conditioned plasma. Symbols: †, information retrieved according to manufacturer’s non-published data.

Table 4.
Platelet-rich plasma interventions in the selected studies.
Table 4.
Platelet-rich plasma interventions in the selected studies.
| Authors, Year | Number of Injections | Volume Injected (mL) | Injected Sites | Sequence of Injections | Image Guidance | Post PRP Intervention | Follow-Up |
|---|---|---|---|---|---|---|---|
| Sun et al., 2021 [23] | 1 | 3 | NR | - | NR | NSAIDs, analgesics, chondroitin and glucosamine were not allowed during the study | Baseline, 1, 3, and 6 months after injection |
| Paget et al., 2021 [19] | 2 | 2 | Anteromedial/anterolateral approach | Once every 6 weeks | US | Heavy labour and repetitive stress were not allowed 48 h after injection. NSAID intakes during treatment were registered | Baseline, 6, 12, and 26 weeks after last injection |
| Fukawa et al., 2017 [21] | 3 | 2 | Anteromedial approach | Once every 2 weeks | US | Heavy labour and sport activities were not allowed 24 h after injection | 1 week before, 4, 12, and 24 weeks after last injection |
| Repetto et al., 2017 [20] | 4 | 3 | Anteromedial approach | Once a week | NR | Rest, paracetamol, ice, and avoiding unnecessary walking for 24 h. NSAIDs and heavy physical work not allowed during treatment | 17.7 ± 6.4 months (range 12 to 30) |
| Angthong et al., 2013 [22] | 1 | 3 | Perilesional area | - | US or fluoroscopy | High-impact activities were not allowed for 4 weeks after last injection. Additional medication for pain control was allowed, but not NSAIDs during 2 weeks after PRP treatment | 16 ± 6.76 months (range 2 to 22.3) |

Table 5.
Reported outcomes from included studies.
Table 5.
Reported outcomes from included studies.
| Authors, Year | Sample Size | Outcomes | Follow-Ups (Months) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 0.5 | 1 | 1.5 | 3 | 6 | 6.5 | 17.7 (Mean) | |||
| Sun et al., 2021 [23] | 39 | VAS | 4.1 ± 1.7 | - | 2.2 ± 1.9 * | - | 1.7 ± 1.5 * | 1.8 ± 1.6 * | - | - |
| AOS | 4.3 ± 1.7 | - | 2.6 ± 2.0 * | - | 2.1 ± 1.7 * | 2.2 ± 1.7 * | - | - | ||
| AOFAS | 80.3 ± 8.9 | - | 87.2 ± 10.3 * | - | 91.6 ± 9.1 * | 89.7 ± 10.0 * | - | - | ||
| SLS test | 27.5 ± 33.5 | - | 41.4 ± 35.8 * | - | 43.7 ± 35.1 * | 42.8 ± 34.3 * | - | - | ||
| Analgesic (tablets/week) | 15.1 ± 6.2 | - | 6.3 ± 2.5 * | - | 5.7 ± 2.4 * | 6.5 ± 2.6 * | - | - | ||
| Satisfaction | - | - | 70.9 ± 21.2 | - | 71.7 ± 20.1 | 71.7 ± 21.2 | - | - | ||
| Paget et al., 2021 [19] | 100 | VAS | Intra-group changes in the different follow-ups in both groups: NR Intra-group changes between 26-week and baseline measurements in both groups: p < 0.001 Between-group changes in the different follow-ups in both groups: p > 0.05 | |||||||
| AOS | ||||||||||
| FAOS | ||||||||||
| AOFAS | ||||||||||
| AAS | ||||||||||
| SF-36 | ||||||||||
| GAS | ||||||||||
| EQ-5D-3L | ||||||||||
| Fukawa et al., 2017 [21] | 20 | Overall (n = 20) | VAS | 59.7 ± 15.3 | 39.7 ± 18.7 * | 33.9 ± 16.5 * | 42.4 ± 21.9 * | |||
| JSSF | 52.4 ± 13.9 | 70.7 ± 9.8 * | 69.2 ± 12.5 * | 65.5 ± 17.2 * | ||||||
| SAFE-Q | 46.7 ± 16.4 | 57.9 ± 16.9 | 61.8 ± 17 * | 56.1 ± 19.3 | ||||||
| Early-stage OA (n = 7) | VAS | 57.7 ± 16 | 22.9 ± 8.9 * | 25.9 ± 13.9 * | 43.7 ± 20.1 | |||||
| JSSF | 56.9 ± 8.8 | 72.3 ± 7.6 | 76.9 ± 6.9 * | 66.2 ± 19.7 | ||||||
| SAFE-Q | 56.9 ± 14.4 | 70.0 ± 13.2 | 75.3 ± 11.6 | 66.2 ± 18.8 | ||||||
| Late-stage OA (n = 13) | VAS | 60.7 ± 14.7 | 48.7 ± 16.0 | 38.2 ± 16.1 * | 40.7 ± 22.2 | |||||
| JSSF | 48.5 ± 14 | 69.8 ± 10.4 * | 65.1 ± 12.3 * | 65.0 ± 15 | ||||||
| SAFE-Q | 41.2 ± 14.3 | 51.4 ± 14.8 | 54.3 ± 14.2 | 52.8 ± 18.4 | ||||||
| Repetto et al., 2017 [20] | 20 | VAS | 7.8 ± 0.5 | - | - | - | - | - | 2.6 ± 2.2 * | |
| FADI | 59.2 ± 3.6 | - | - | - | - | - | 80.2 ± 17.3 * | |||
| Angthong et al., 2013 [22] | 5 | VAS-FA | 69.6 ± 18 | † | - | † | † | 84.5 ± 10.3 * | ||
| SF-36 | - | 68.0 ± 24.4 | ||||||||
Values are mean ± standard deviation or otherwise indicated. Abbreviations: VAS, Visual Analogue Scale; FADI, Foot Ankle Disability Index; VAS-FA, Visual Analogue Scale Foot and Ankle; SF-36, Short Form, 36-Item Survey; AOS, Ankle Osteoarthritis Score; AAS, Ankle Activity Score; FAOS, Foot and Ankle Outcome Score; SLS, Single Leg Stance test; AOFAS, American Orthopaedic Foot and Ankle Score; GAS, Goal Attainment Scaling; EQ-5D-3L, EuroQol Quality of Life questionnaire. Symbols: †, data not provided; *, significant within-group changes.
4. Discussion
PRP has gained popularity in the treatment of large joint OA due to its relative simplicity and acceptable cost when compared to other invasive procedures. In most cases, the promising results pertaining to this innovative approach derive from clinical studies with a limited scientific background [11,24,25]. Several animal studies have shown that intraarticular PRP injections can promote cartilage regeneration by enhancing chondrogenic differentiation, inhibiting chondral degeneration, and decreasing synovial inflammation. Thus, PRP is theorized to act in OA joints through cytokines, chemokines, and growth factors that inhibit the destruction of hyaline cartilage, or even regenerate it [24].
This work provides a comprehensive review of the effectiveness of PRP for the treatment of ankle OA. There have been only five published studies on using PRP for this condition. During the eligibility phase, two studies were excluded, since the subjects suffered from osteochondral lesions and were not strictly diagnosed with ankle OA. Another study was also excluded because the injection techniques did not consider the PRP modality.
The quality of the evidence of the selected studies was low, as the majority were level IV, whereas bias in the selection of participants in the studies was identified as the most common type of bias. The results of this systematic review thus provide level IV evidence that injection of PRP seems effective in short follow-ups in patients with different stages of ankle OA. These reported positive results should be considered in the context of the studies’ inherent methodological limitations, their variable reporting characteristics, and the natural course of ankle OA. Therefore, the generalization of these findings is limited by the weakness of the selected studies. Limited data are thus available to confirm that current PRP therapy is effective as postulated. Moreover, there is growing interest in the biological mechanisms behind PRP injections and, more importantly, in how to modulate these processes for beneficial functional and clinical effects.
Despite the small number of included studies, our review has confirmed the wide variation in reported preparation procedures for PRP, as the scientific literature reflects [25,26,27]. Each factor pertaining to the preparation procedure may represent a source of variation that can influence the effectiveness of the final product. Platelet viability and the number of released growth factors and cytokines are dependent on the length of time for each spin cycle and the centrifugal acceleration parameters. Given these already known data, the standardization of platelet-obtaining methodology is essential to ensure a comparable grade of growth factor release. All the included studies in this review employed a different type of PRP based on the preparation method (single, double, or triple centrifugation) and cellular component (the concentration of platelets and the presence of red cells). One of the selected studies chose to include red cells in the PRP [20], while the other two studies elected to exclude them [21,22]. Furthermore, it is important to highlight the variation in the platelet concentration, as it is presumably a critical aspect of the regeneration process. The number of injections and the site of administration varied among the included investigations as well. Angthong et al. and Sun et al. treated their patients with only one injection of PRP [22,23], whereas Paget et al. employed two applications, with one injection every 6 weeks [19]. The other proposals included at least three injections administered once every week/two weeks [20,21]. All the included studies reported intraarticular administration, except for the study by Angthong et al., who injected the perilesional area under US/fluoroscopy guidance [22]. However, the severity of ankle OA did not significantly differ among the papers. This variability is supposed to lead to different biological and physiological processes and probably, with this, to differences in efficacy. Different therapeutic protocols promote further confounding factors and contribute to establishing a direct and clear correlation between PRP and its benefits. There is therefore no solid evidence to draw conclusions about the preparation method, procedure, dosimetry, or frequency of PRP treatment. Future investigations should focus on determining optimal protocols for PRP therapy to shed light on it.
White and red cells are questionable PRP components, as significant cell death and proinflammatory mediator production have been related to their inclusion [28]. On one hand, white cells are considered sources of cytokines and enzymes that may lead to infection processes [29]. On the other hand, PRP obtention practices generate shear forces that can cause damage to red cells. This can disintegrate the red cell membrane and involve the secretion of noxious hemoglobin, equivalent to plasma-free hemoglobin, iron, and hemin [30]. In both cases, the released molecules can activate inflammatory pathways and oxidative stress, which can lead to microcirculatory dysfunction and tissue damage, through a major cytotoxic effect. For these reasons, clinicians should consider using white- and red-cell-free formulations of PRP when administering it at any site.
The follow-up period was generally short, ranging from 2 to 28 weeks in four studies [19,21,22,23]; the remaining study widely ranged from 12 to 30 months (mean 17.7) [20], which hinders the interpretation of the results in terms of their correlation to precise follow-up evaluations. Therefore, accurate long-term assessments for PRP intervention in ankle OA patients were not performed. This lack limits the generalizability of the conclusions from this review and corresponds to a certain degree of measurement bias. Additionally, the sample sizes were generally small in all the studies. Thus, the effectiveness of PRP treatment cannot be documented with the required accuracy. Mimicking the diversity of published trials in terms of consensus over preparation protocols and clinical interventions would be of great value.
Due to the invasive nature of PRP administration, patient safety is an important aspect to be considered. Musculoskeletal PRP applications have been related to infection in the treated area [30]. No systemic reactions or serious adverse events following PRP applications were described in the selected studies. Three studies in this review reported local adverse reactions characterized by pain and swelling in the injection area [19,21,23]. These complications have been previously demonstrated to correlate with the number of injections. Overall, in light of these data, it seems that ankle PRP therapy for ankle OA is a reasonably safe procedure.
Limitations
The qualitative results of this study are limited by several factors. The study quality and the small number of studies are major limitations. The existing research is limited by its observational nature and the sample sizes. Furthermore, there is variability in the employed PRP procedures, PRP formulations, injection techniques, and data reporting. Different PRP kits were used among the selected studies, with different preparation protocols that may have changed the final composition of the PRP delivered. These confounding parameters, along with the duration and number of centrifugations, the use of frozen phase, and the type of activation substance, all contribute to reducing the homogeneity of the included studies. Accordingly, and given the growing interest in PRP use, it may be plausible that a certain number of investigations that might be in progress, or recently concluded but not yet published, may have been omitted. In addition, the research diversity and a lack of reported data during the experiment in certain investigations limited the comparability among studies. High variability in the PRP activation protocol and volume of injections was identified, thus modifying the studied biological activity. This heterogeneity has been previously reported in PRP reviews and necessarily limits comparison between studies. On the other hand, since non-English published research was excluded, the risk of selection and publication bias is present.
5. Conclusions
In conclusion, due to the lack of high-level, appropriately powered investigations and the significant variability in the reporting characteristics, the therapeutic potential of PRP injections in ankle OA remains unclear. Therefore, there is currently insufficient evidence regarding the effectiveness and safety of PRP injections in the treatment of ankle OA, and without further standardization, these questions will remain open.
Author Contributions
Conceptualization, I.M.-P.; methodology, I.M.-P. and M.O.-C.; validation, A.G.-C.; formal analysis, I.M.-P. and M.O.-C.; investigation, I.M.-P. and M.O.-C.; resources, I.M.-P. and M.O.-C.; data curation, I.M.-P. and M.O.-C.; writing—original draft preparation, I.M.-P. and M.O.-C.; writing—review and editing, A.G.-C.; visualization, A.G.-C.; supervision, A.G.-C.; project administration, I.M.-P. and M.O.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
The authors would like to thank Sofia Sanz-De-Diego, Raquel Navarro-Ger, and Pablo Mar-tin-Garcia for their support, and for their thoughtful and helpful comments and suggestions.
Conflicts of Interest
The authors declare no conflicts of interest.
Appendix A. Search Strategies
PUBMED AND MEDLINE SEARCH STRATEGIES
- “Osteoarthritis”[Mesh]
- “Osteoarthritis/etiology”[Mesh] OR “Osteoarthritis/microbiology”[Mesh] OR “Osteoarthritis/pathology”[Mesh] OR “Osteoarthritis/rehabilitation”[Mesh] OR “Osteoarthritis/therapy”[Mesh]
- “Ankle osteoarthritis”
- #1 OR #2 OR #3
- “Ankle”[Mesh]
- “Ankle Joint”[Mesh]
- “Ankle Injuries”[Mesh]
- #5 OR #6 OR #7
- “Platelet-Rich Plasma”[Mesh]
- “Injections, Intra-Articular”[Mesh]
- “PRP”[mp]
- #9 OR #10 OR #11
- #4 AND #8 AND #12
((“Osteoarthritis”[Mesh]) OR (“Osteoarthritis/etiology”[Mesh] OR “Osteoarthritis/microbiology”[Mesh] OR “Osteoarthritis/pathology”[Mesh] OR “Osteoarthritis/rehabilitation”[Mesh] OR “Osteoarthritis/therapy”[Mesh]) OR (“Ankle osteoarthritis”)) AND ((“Ankle”[Mesh]) OR (“Ankle Joint”[Mesh]) OR (“Ankle Injuries”[Mesh])) AND ((“Platelet-Rich Plasma”[Mesh]) OR (“Injections, Intra-Articular”[Mesh]) OR (“PRP”[mp]))
SPORT DISCUS SEARCH STRATEGY
- DE “Osteoarthritis”
- “Ankle osteoarthritis”
- DE “Arthritis”
- #1 OR #2 OR #3
- DE “ANKLE”
- DE “ANKLE injuries”
- DE “ANKLE injury treatment”
- DE “ANKLEBONE”
- DE “ANKLEBONE injuries”
- #5 OR #6 OR #7 OR #8 OR #9
- DE “PLATELET-derived growth factor”
- DE “PLATELET-rich plasma”
- “PRP”
- DE “Injections”
- #11 OR #12 OR #13 OR #14
- #4 AND #10 AND #15
CINAHL SEARCH STRATEGY
- MH “Osteoarthritis”
- MH “Arthritis”
- “Ankle osteoarthritis”
- #1 OR #2 OR #3
- MH “Ankle”
- MH “Talus”
- MH “Ankle Joint”
- MH “Ankle Injuries”
- #5 OR #6 OR #7 OR #8
- MH “Platelet-Rich Plasma”
- MH “Platelet-Derived Growth Factor”
- “PRP”
- MH “Injections, Intraarticular”
- #10 OR #11 OR #12 OR #13
- #4 AND #9 AND #14
COCHRANE SEARCH STRATEGY
- #1.
- MeSH descriptor: [Osteoarthritis] explode all trees
- #2.
- MeSH descriptor: [Ankle Joint] explode all trees
- #3.
- MeSH descriptor: [Ankle] explode all trees
- #4.
- MeSH descriptor: [Platelet-Rich Plasma] explode all trees
- #5.
- MeSH descriptor: [Platelet-Derived Growth Factor] explode all trees
- #6.
- MeSH descriptor: [Injections, Intra-Articular] explode all trees
- #7.
- “PRP”
- #8.
- #1 AND (#2 OR #3) AND (#4 OR #5 OR
References
- Anderson, D.D.; Wilken, J.; Ledoux, W.; Lenz, A.L.; Easley, M.E.; de César Netto, C. Ankle osteoarthritis: Toward new understanding and opportunities for prevention and intervention. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2024; ahead of print. [Google Scholar] [CrossRef]
- Lawrence, R.C.; Felson, D.T.; Helmick, C.G.; Arnold, L.M.; Choi, H.; Deyo, R.A.; Gabriel, S.; Hirsch, R.; Hochberg, M.C.; Hunder, G.G.; et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008, 58, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.; Marshall, M.; Rathod, T.; Bowen, C.J.; Menz, H.B.; Roddy, E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS ONE 2018, 13, e0193662. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, C.L.; Salamon, M.L.; Blanchard, G.M.; Huff, T.; Hayes, A.; Buckwalter, J.A.; Amendola, A. Epidemiology of ankle arthritis: Report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop. J. 2005, 25, 44–46. [Google Scholar] [PubMed]
- Saltzman, C.L.; Zimmerman, M.B.; O’Rourke, M.; Brown, T.D.; Buckwalter, J.A.; Johnston, R. Impact of comorbidities on the measurement of health in patients with ankle osteoarthritis. J. Bone Jt. Surg. Am. 2006, 88, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Bloch, B.; Srinivasan, S.; Mangwani, J. Current Concepts in the Management of Ankle Osteoarthritis: A Systematic Review. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2015, 54, 932–939. [Google Scholar] [CrossRef]
- Herrera-Pérez, M.; Valderrabano, V.; Godoy-Santos, A.L.; de César Netto, C.; González-Martín, D.; Tejero, S. Ankle osteoarthritis: Comprehensive review and treatment algorithm proposal. EFORT Open Rev. 2022, 7, 448–459. [Google Scholar] [CrossRef]
- Sambe, H.G.; Yasir, M.; Man, R.K.; Gogikar, A.; Nanda, A.; Janga, L.S.N.; Hamid, P. Comparing Intra-articular Platelet-Rich Plasma with Hyaluronic Acid for the Treatment of Hip Osteoarthritis: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e47919. [Google Scholar] [CrossRef]
- De Marziani, L.; Sangiorgio, A.; Bensa, A.; Boffa, A.; Andriolo, L.; Filardo, G. Intra-articular injections in sport-active patients with degenerative cartilage lesions or osteoarthritis of the knee: A systematic review. J. Exp. Orthop. 2023, 10, 112. [Google Scholar] [CrossRef]
- Tang, X.; Muhammad, H.; McLean, C.; Miotla-Zarebska, J.; Fleming, J.; Didangelos, A.; Önnerfjord, P.; Leask, A.; Saklatvala, J.; Vincent, T.L. Connective tissue growth factor contributes to joint homeostasis and osteoarthritis severity by controlling the matrix sequestration and activation of latent TGFβ. Ann. Rheum. Dis. 2018, 77, 1372–1380. [Google Scholar] [CrossRef]
- Vannabouathong, C.; Del Fabbro, G.; Sales, B.; Smith, C.; Li, C.S.; Yardley, D.; Bhandari, M.; Petrisor, B.A. on behalf of the EnCORE Research Group. Intra-articular Injections in the Treatment of Symptoms from Ankle Arthritis: A Systematic Review. Foot Ankle Int. 2018, 39, 1141–1150. [Google Scholar] [CrossRef]
- Schneider, N.; Sinnott, M.; Patel, N.; Joseph, R. The Use of Platelet-Rich Plasma and Stem Cell Injections in Musculoskeletal Injuries. Cureus 2024, 16, e59970. [Google Scholar] [CrossRef] [PubMed]
- Thu, A.C. The use of platelet-rich plasma in management of musculoskeletal pain: A narrative review. J. Yeungnam Med. Sci. 2022, 39, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. J. Clin. Epidemiol. 2009, 62, e1–e34. [Google Scholar] [CrossRef] [PubMed]
- Medina-Porqueres, I.; Sanz-De Diego, S.; Martin-Garcia, P.; Reyes-Eldblom, M.; Reyes-Eldblom, R. Efficacy of Platelet Rich Plasma in the Treatment of Ankle Joint Osteoarthritis. PROSPERO CRD42018085261. 2018. Available online: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42018085261 (accessed on 27 September 2024).
- Cochrane Effective Practice and Organisation of Care (EPOC). EPOC Resources for Review Authors. 2017. Available online: https://epoc.cochrane.org/resources/epoc-resources-review-authors (accessed on 28 October 2021).
- Munn, Z.; Barker, T.H.; Moola, S.; Tufanaru, C.; Stern, C.; McArthur, A.; Stephenson, M.; Aromataris, E. Methodological quality of case series studies: An introduction to the JBI critical appraisal tool. JBI Evid. Synth. 2020, 18, 2127–2133. [Google Scholar] [CrossRef]
- Oxford Centre for Evidence-Based Medicine—Levels of Evidence (March 2009); CEBM: Oxford, UK, 2009; Available online: https://www.cebm.net/2009/06/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed on 10 May 2020).
- Paget, L.D.A.; Reurink, G.; de Vos, R.-J.; Weir, A.; Moen, M.H.; Bierma-Zeinstra, S.M.A.; Stufkens, S.A.S.; Kerkhoffs, G.M.M.J.; Tol, J.L.; for the PRIMA Study Group. Effect of Platelet-Rich Plasma Injections vs Placebo on Ankle Symptoms and Function in Patients with Ankle Osteoarthritis: A Randomized Clinical Trial. JAMA 2021, 326, 1595–1605. [Google Scholar] [CrossRef]
- Repetto, I.; Biti, B.; Cerruti, P.; Trentini, R.; Felli, L. Conservative Treatment of Ankle Osteoarthritis: Can Platelet-Rich Plasma Effectively Postpone Surgery? J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2017, 56, 362–365. [Google Scholar] [CrossRef]
- Fukawa, T.; Yamaguchi, S.; Akatsu, Y.; Yamamoto, Y.; Akagi, R.; Sasho, T. Safety and Efficacy of Intra-articular Injection of Platelet-Rich Plasma in Patients with Ankle Osteoarthritis. Foot Ankle Int. 2017, 38, 596–604. [Google Scholar] [CrossRef]
- Angthong, C.; Khadsongkram, A.; Angthong, W. Outcomes and quality of life after platelet-rich plasma therapy in patients with recalcitrant hindfoot and ankle diseases: A preliminary report of 12 patients. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2013, 52, 475–480. [Google Scholar] [CrossRef]
- Sun, S.-F.; Hsu, C.-W.; Lin, G.-C.; Lin, H.-S.; Chou, Y.-J.; Wu, S.-Y.; Huang, H.-Y. Efficacy and Safety of a Single Intra-articular Injection of Platelet-rich Plasma on Pain and Physical Function in Patients with Ankle Osteoarthritis-A Prospective Study. J. Foot Ankle Surg. Off. Publ. Am. Coll. Foot Ankle Surg. 2021, 60, 676–682. [Google Scholar] [CrossRef]
- Bennell, K.L.; Hunter, D.J.; Paterson, K.L. Platelet-Rich Plasma for the Management of Hip and Knee Osteoarthritis. Curr. Rheumatol. Rep. 2017, 19, 24. [Google Scholar] [CrossRef]
- Lai, L.P.; Stitik, T.P.; Foye, P.M.; Georgy, J.S.; Patibanda, V.; Chen, B. Use of Platelet-Rich Plasma in Intra-Articular Knee Injections for Osteoarthritis: A Systematic Review. PM&R 2015, 7, 637–648. [Google Scholar] [CrossRef]
- Taylor, D.W.; Petrera, M.; Hendry, M.; Theodoropoulos, J.S. A systematic review of the use of platelet-rich plasma in sports medicine as a new treatment for tendon and ligament injuries. Clin. J. Sport. Med. Off. J. Can. Acad. Sport. Med. 2011, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.E.; Keaney, T.C. Systematic review of platelet-rich plasma (PRP) preparation and composition for the treatment of androgenetic alopecia. J. Cosmet. Dermatol. 2018, 17, 666–671. [Google Scholar] [CrossRef]
- Chen, X.; Jones, I.A.; Togashi, R.; Vagngsness, C.T. Use of Platelet-Rich Plasma for the Improvement of Pain and Function in Rotator Cuff Tears: A Systematic Review and Meta-analysis with Bias Assessment. Am. J. Sports Med. 2020, 48, 2028–2041. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The Effect of Platelet-Rich Plasma Formulations and Blood Products on Human Synoviocytes. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef]
- Filardo, G.; Kon, E.; Pereira Ruiz, M.T.; Vaccaro, F.; Guitaldi, R.; Di Martino, A.; Cenacchi, A.; Fornasari, P.M.; Marcacci, M. Platelet-rich plasma intra-articular injections for cartilage degeneration and osteoarthritis: Single- versus double-spinning approach. Knee Surg. Sports Traumatol. Arthrosc. Off. J. ESSKA 2012, 20, 2082–2091. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).