Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives
Abstract
Featured Application
Abstract
1. Introduction
2. Characteristics of the Most Important SCP Producers
2.1. Saccharomyces cerevisiae
2.2. Kluyveromyces marxianus
2.3. Candida utilis
2.4. Yarrowia lipolytica
2.5. Fusarium venenatum
2.6. Aspergillus oryzae
2.7. Monascus purpureus
| [g/100 g] of SCP | ||||||
|---|---|---|---|---|---|---|
| Amino Acids | Candida utilis [58] | Fusarium venenatum [50] | Saccharomyces cerevisiae [59] | Aspergillus oryzae [51] | Yarrowia lipolytica [23] | Kluyveromyces marxianus [60] |
| Alanine | 7.59 | 2.41 | 6.89 | 2.37 | 8.00 | 3.63 |
| Arginine | 4.82 | 7.12 | 4.02 | 1.99 | 4.80 | 6.03 |
| Cystine | 10.16 | 2.11 | 1.24 | 0.28 | 1.10 | 1.11 |
| Glycine | 3.92 | 3.50 | 5.23 | 1.72 | 4.60 | 2.67 |
| Histidine | 3.78 | 7.22 | 2.78 | 0.75 | 2.60 | 2.66 |
| Isoleucine | 5.28 | 1.51 | 4.63 | 1.38 | 4.40 | 6.21 |
| Leucine | 6.73 | 1.90 | 8.75 | 2.46 | 6.80 | 9.57 |
| Lysine | 5.81 | 2.60 | 6.73 | 2.14 | 7.00 | 8.15 |
| Methionine | 1.67 | 4.21 | 3.12 | 0.57 | 1.20 | 2.48 |
| Phenylalanine | 4.62 | 3.01 | 5.57 | 1.42 | 4.00 | 5.61 |
| Threonine | 4.62 | 3.31 | 6.09 | 1.69 | 4.80 | 5.08 |
| Valine | 6.05 | 2.91 | 5.34 | 1.72 | 5.30 | 5.30 |
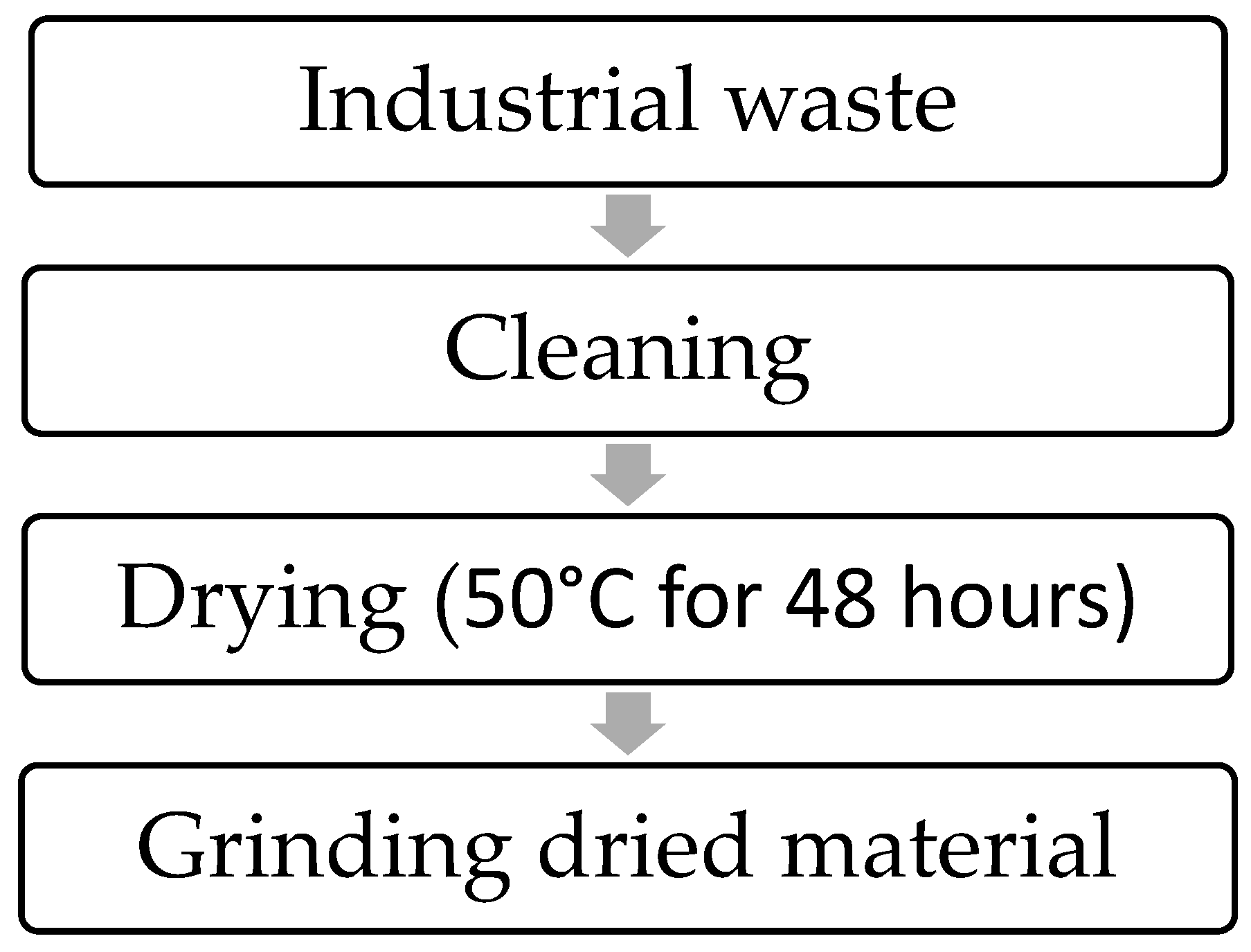
3. Bio-Utilization of Waste from the Agri-Food Industry during SCP Production
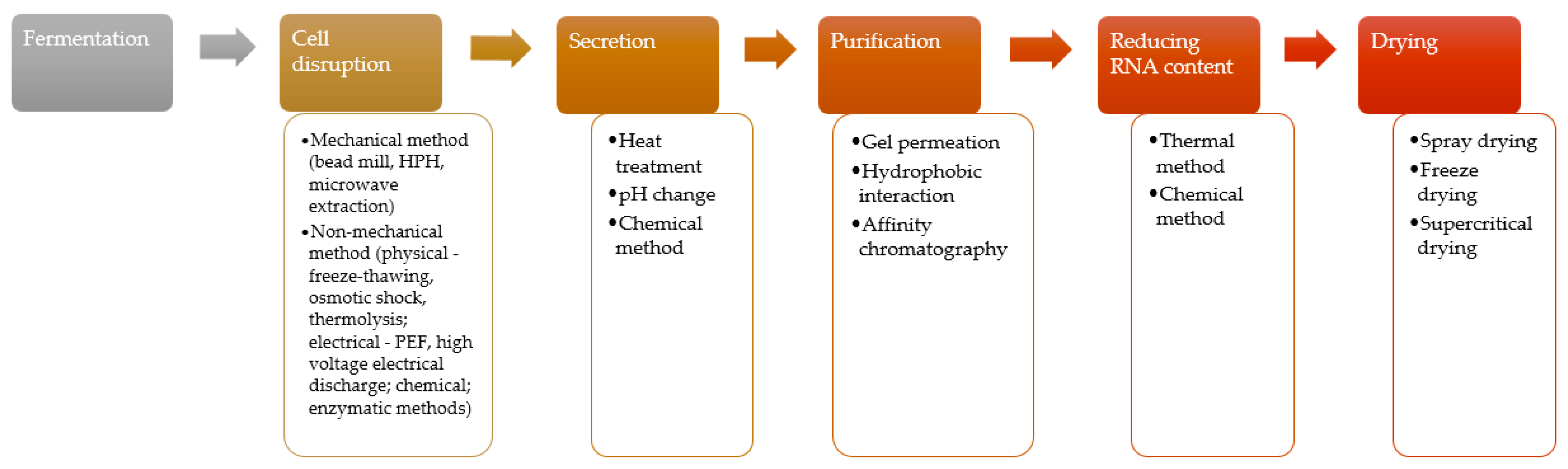
4. Preparation of Single-Cell Protein (SCP) for Use in Food
4.1. Cell Disruption
4.2. Protein Secretion
4.3. Protein Purification
4.4. Reducing the Nucleic Acid Content
4.5. Drying of Microbial Protein
5. Products Containing Single-Cell Protein (SCP) on the Global Market
6. Opportunities and Challenges for the Single-Cell Protein (SCP) Market
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cleland, J. World Population Growth; Past, Present and Future. Environ. Resour. Econ. 2013, 55, 543–554. [Google Scholar] [CrossRef]
- Röös, E.; Sundberg, C.; Tidåker, P.; Strid, I.; Hansson, P.A. Can Carbon Footprint Serve as an Indicator of the Environmental Impact of Meat Production? Ecol. Indic. 2013, 24, 573–581. [Google Scholar] [CrossRef]
- Mekonnen, M.M.; Neale, C.M.U.; Ray, C.; Erickson, G.E.; Hoekstra, A.Y. Water Productivity in Meat and Milk Production in the US from 1960 to 2016. Environ. Int. 2019, 132, 105084. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Trusinska, M.; Chraniuk, P.; Drudi, F.; Lukasiewicz, J.; Nguyen, N.P.; Przybyszewska, A.; Pobiega, K.; Tappi, S.; Tylewicz, U.; et al. Developments in Plant Proteins Production for Meat and Fish Analogues. Molecules 2023, 28, 2966. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Trusinska, M.; Chraniuk, P.; Piatkowska, J.; Pakulska, A.; Wisniewska, K.; Wierzbicka, A.; Rybak, K.; Pobiega, K. Plant-Based Fish Analogs—A Review. Appl. Sci. 2023, 13, 4509. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J. The Long-Term Health of Vegetarians and Vegans. Proc. Nutr. Soc. 2016, 75, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Greenebaum, J. Veganism, Identity and the Quest for Authenticity. Food Cult. Soc. 2012, 15, 129–144. [Google Scholar] [CrossRef]
- Iguacel, I.; Huybrechts, I.; Moreno, L.A.; Michels, N. Vegetarianism and Veganism Compared with Mental Health and Cognitive Outcomes: A Systematic Review and Meta-Analysis. Nutr. Rev. 2021, 79, 361–381. [Google Scholar] [CrossRef] [PubMed]
- Augustin, M.A.; Riley, M.; Stockmann, R.; Bennett, L.; Kahl, A.; Lockett, T.; Osmond, M.; Sanguansri, P.; Stonehouse, W.; Zajac, I.; et al. Role of Food Processing in Food and Nutrition Security. Trends Food Sci. Technol. 2016, 56, 115–125. [Google Scholar] [CrossRef]
- Kaur, N.; Singh, A. Role of Microorganisms in the Food Industry. In Food Microbial Sustainability; Springer: Singapore, 2023; pp. 1–23. ISBN 978-981-99-4784-3. [Google Scholar]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef] [PubMed]
- Kelechi, M. Ukaegbu-Obi Single Cell Protein: A Resort to Global Protein Challenge and Waste Management. J. Microbiol. Microb. Technol. 2016, 1, 251–262. [Google Scholar]
- Bratosin, B.C.; Darjan, S.; Vodnar, D.C. Single Cell Protein: A Potential Substitute in Human and Animal Nutrition. Sustainability 2021, 13, 9284. [Google Scholar] [CrossRef]
- Narayana Saibaba, K. Applications of Microbes in Food Industry; Springer: Singapore, 2022; pp. 323–338. ISBN 978-981-16-2225-0. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Galili, G.; Amir, R. Fortifying Plants with the Essential Amino Acids Lysine and Methionine to Improve Nutritional Quality. Plant Biotechnol. J. 2013, 11, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Umesh, M.; Priyanka, K.; Thazeem, B.; Preethi, K. Production of Single Cell Protein and Polyhydroxyalkanoate from Carica Papaya Waste. Arab. J. Sci. Eng. 2017, 42, 2361–2369. [Google Scholar] [CrossRef]
- Carranza-Méndez, R.C.; Chávez-González, M.L.; Sepúlveda-Torre, L.; Aguilar, C.N.; Govea-Salas, M.; Ramos-González, R. Production of Single Cell Protein from Orange Peel Residues by Candida utilis. Biocatal. Agric. Biotechnol. 2022, 40, 102298. [Google Scholar] [CrossRef]
- Pereira, A.G.; Fraga-Corral, M.; Garcia-Oliveira, P.; Otero, P.; Soria-Lopez, A.; Cassani, L.; Cao, H.; Xiao, J.; Prieto, M.A.; Simal-Gandara, J. Single-Cell Proteins Obtained by Circular Economy Intended as a Feed Ingredient in Aquaculture. Foods 2022, 11, 2831. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, M.G. QuornTM Myco-Protein—Overview of a Successful Fungal Product. Mycologist 2004, 18, 17–20. [Google Scholar] [CrossRef]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Hilbert, F.; Lindqvist, R.; et al. Update of the List of QPS-Recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA 10: Suitability of Taxonomic Units Notified to EFSA until March 2019. EFSA J. 2019, 17, e05753. [Google Scholar] [CrossRef] [PubMed]
- Jach, M.E.; Serefko, A.; Ziaja, M.; Kieliszek, M. Yeast Protein as an Easily Accessible Food Source. Metabolites 2022, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of Cellular Disruption Processes, Chemical Composition, Functional Properties and Digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef] [PubMed]
- Dunuweera, A.N.; Nikagolla, D.N.; Ranganathan, K. Fruit Waste Substrates to Produce Single-Cell Proteins as Alternative Human Food Supplements and Animal Feeds Using Baker’s Yeast (Saccharomyces cerevisiae). J. Food Qual. 2021, 2021, 9932762. [Google Scholar] [CrossRef]
- Gervasi, T.; Pellizzeri, V.; Calabrese, G.; Di Bella, G.; Cicero, N.; Dugo, G. Production of Single Cell Protein (SCP) from Food and Agricultural Waste by Using Saccharomyces cerevisiae. Nat. Prod. Res. 2018, 32, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Razzaq, Z.U.; Khan, M.K.I.; Maan, A.A.; Rahman, S.U. Characterization of Single Cell Protein from Saccharomyces cerevisiae for Nutritional, Functional and Antioxidant Properties. J. Food Meas. Charact. 2020, 14, 2520–2528. [Google Scholar] [CrossRef]
- Ullah, M.; Rizwan, M.; Raza, A.; Xia, Y.; Han, J.; Ma, Y.; Chen, H. Snapshot of the Probiotic Potential of Kluveromyces marxianus DMKU-1042 Using a Comparative Probiogenomics Approach. Foods 2023, 12, 4329. [Google Scholar] [CrossRef] [PubMed]
- Koukoumaki, D.I.; Papanikolaou, S.; Ioannou, Z.; Gkatzionis, K.; Sarris, D. The Development of Novel Edible Films from Single-Cell Protein Produced by the Biotechnological Valorization of Cheese Whey. Appl. Microbiol. 2024, 4, 1030–1041. [Google Scholar] [CrossRef]
- Ruan, R.; Ioanna Koukoumaki, D.; Papanikolaou, S.; Ioannou, Z.; Mourtzinos, I.; Sarris, D. Single-Cell Protein and Ethanol Production of a Newly Isolated Kluyveromyces marxianus Strain through Cheese Whey Valorization. Foods 2024, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.A.P.; Lafia, A.T.; de Sousa, C.C.; Falleiros, L.N.S.S.; Guidini, C.Z.; Zotarelli, M.F. An Approach for Using Non-Purified β-Galactosidase: The Potential of β-Galactosidase in Kluyveromyces marxianus Cell Microparticles with Different Wall Materials. Food Bioprocess Technol. 2024, 1–16. [Google Scholar] [CrossRef]
- González-Orozco, B.D.; McGovern, C.J.; Barringer, S.A.; Simons, C.; Jiménez-Flores, R.; Alvarez, V.B. Development of Probiotic Yogurt Products Incorporated with Lactobacillus Kefiranofaciens OSU-BSGOA1 in Mono- and Co-Culture with Kluyveromyces marxianus. J. Dairy Sci. 2024, in press. [Google Scholar] [CrossRef] [PubMed]
- Centomo, A.M.; Diaz Vergara, L.I.; Rossi, Y.E.; Vanden Braber, N.L.; Bodoira, R.; Maestri, D.; Cavaglieri, L.R.; Montenegro, M.A. Co-Encapsulated of Potential Probiotic Yeast Kluyveromyces marxianus and Peanut Skin Polyphenolic Extract as a Functional Ingredient. Int. J. Food Sci. Technol. 2024, 59, 3918–3928. [Google Scholar] [CrossRef]
- Kieliszek, M.; Kot, A.M.; Bzducha-Wróbel, A.; BŁażejak, S.; Gientka, I.; Kurcz, A. Biotechnological Use of Candida Yeasts in the Food Industry: A Review. Fungal Biol. Rev. 2017, 31, 185–198. [Google Scholar] [CrossRef]
- Ding, H.; Li, J.; Deng, F.; Huang, S.; Zhou, P.; Liu, X.; Li, Z.; Li, D. Ammonia Nitrogen Recovery from Biogas Slurry by SCP Production Using Candida utilis. J. Environ. Manag. 2023, 325, 116657. [Google Scholar] [CrossRef] [PubMed]
- Jalasutram, V.; Kataram, S.; Gandu, B.; Anupoju, G.R. Single Cell Protein Production from Digested and Undigested Poultry Litter by Candida utilis: Optimization of Process Parameters Using Response Surface Methodology. Clean. Technol. Environ. Policy 2013, 15, 265–273. [Google Scholar] [CrossRef]
- Kurcz, A.; Błażejak, S.; Kot, A.M.; Bzducha-Wróbel, A.; Kieliszek, M. Application of Industrial Wastes for the Production of Microbial Single-Cell Protein by Fodder Yeast Candida utilis. Waste Biomass Valorization 2018, 9, 57–64. [Google Scholar] [CrossRef]
- Zhou, X.; Hu, W.; Ran, C.; Chen, J.; Xie, T.; Zhang, Y. Nutrient Removal in Sludge Extracts and Protein Production by Cultivation of Candida utilis. J. Chem. Technol. Biotechnol. 2021, 96, 990–998. [Google Scholar] [CrossRef]
- Jach, M.E.; Malm, A. Yarrowia lipolytica as an Alternative and Valuable Source of Nutritional and Bioactive Compounds for Humans. Molecules 2022, 27, 2300. [Google Scholar] [CrossRef] [PubMed]
- Ben Tahar, I.; Kus-Liśkiewicz, M.; Lara, Y.; Javaux, E.; Fickers, P. Characterization of a Nontoxic Pyomelanin Pigment Produced by the Yeast Yarrowia lipolytica. Biotechnol. Prog. 2020, 36, e2912. [Google Scholar] [CrossRef] [PubMed]
- Petra de Oliveira Barros, V.; Macedo Silva, J.R.; Maciel Melo, V.M.; Terceiro, P.S.; Nunes de Oliveira, I.; Duarte de Freitas, J.; Francisco da Silva Moura, O.; Xavier de Araújo-Júnior, J.; Erlanny da Silva Rodrigues, E.; Maraschin, M.; et al. Biosurfactants Production by Marine Yeasts Isolated from Zoanthids and Characterization of an Emulsifier Produced by Yarrowia lipolytica LMS 24B. Chemosphere 2024, 355, 141807. [Google Scholar] [CrossRef] [PubMed]
- Kamzolova, S.V.; Samoilenko, V.A.; Lunina, J.N.; Morgunov, I.G. Large-Scale Production of Isocitric Acid Using Yarrowia lipolytica Yeast with Further Down-Stream Purification. BioTech 2023, 12, 22. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, J.N.; Fan, G.; Zhang, L.L.; Pan, S.Y. Isolation, Purification, and Mass Spectrometry Identification of the Enzyme Involved in Citrus Flavor (+)-Valencene Biotransformation to (+)-Nootkatone by Yarrowia lipolytica. J. Sci. Food Agric. 2023, 103, 4792–4802. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rojas, T.; Espinoza-Culupú, A.; Ramírez, P.; Iwai, L.K.; Montoni, F.; Macedo-Prada, D.; Sulca-López, M.; Durán, Y.; Farfán-López, M.; Herencia, J. Proteomic Study of Response to Copper, Cadmium, and Chrome Ion Stress in Yarrowia lipolytica Strains Isolated from Andean Mine Tailings in Peru. Microorganisms 2022, 10, 2002. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, J.A.; Johnson, R.I.; Finnigan, T.J.A.; Avery, S.V.; Dyer, P.S. The Biotechnology of Quorn Mycoprotein: Past, Present and Future Challenges. In Grand Challenges in Fungal Biotechnology; Grand Challenges in Biology and Biotechnology Series; Springer: Cham, Switzerland, 2020; pp. 59–79. [Google Scholar] [CrossRef]
- Ahmad, M.I.; Farooq, S.; Alhamoud, Y.; Li, C.; Zhang, H. A Review on Mycoprotein: History, Nutritional Composition, Production Methods, and Health Benefits. Trends Food Sci. Technol. 2022, 121, 14–29. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Hosseini, S.M.; Assarehzadegan, M.A.; Khosravi-Darani, K.; Hosseini, H. Safety Assays and Nutritional Values of Mycoprotein Produced by Fusarium venenatum IR372C from Date Waste as Substrate. J. Sci. Food Agric. 2020, 100, 4433–4441. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Pan, J.H.; Kim, D.; Heo, W.; Shin, E.C.; Kim, Y.J.; Shim, Y.Y.; Reaney, M.J.T.; Ko, S.G.; Hong, S.B.; et al. Mycoproteins and Their Health-Promoting Properties: Fusarium Species and Beyond. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13365. [Google Scholar] [CrossRef] [PubMed]
- Wiebe, M. Myco-Protein from Fusarium venenatum: A Well-Established Product for Human Consumption. Appl. Microbiol. Biotechnol. 2002, 58, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Fatemeh, S.; Reihani, S.; Khosravi-Darani, K. Mycoprotein Production from Date Waste Using Fusarium venenatum in a Submerged Culture. Appl. Food Biotechnol. 2018, 5, 243–352. [Google Scholar] [CrossRef]
- Karimi, S.; Soofiani, N.M.; Lundh, T.; Mahboubi, A.; Kiessling, A.; Taherzadeh, M.J. Evaluation of Filamentous Fungal Biomass Cultivated on Vinasse as an Alternative Nutrient Source of Fish Feed: Protein, Lipid, and Mineral Composition. Fermentation 2019, 5, 99. [Google Scholar] [CrossRef]
- Uwineza, C.; Sar, T.; Mahboubi, A.; Taherzadeh, M.J. Evaluation of the Cultivation of Aspergillus oryzae on Organic Waste-Derived VFA Effluents and Its Potential Application as Alternative Sustainable Nutrient Source for Animal Feed. Sustainability 2021, 13, 12489. [Google Scholar] [CrossRef]
- Mahboubi, A.; Ferreira, J.A.; Taherzadeh, M.J.; Lennartsson, P.R. Production of Fungal Biomass for Feed, Fatty Acids, and Glycerol by Aspergillus oryzae from Fat-Rich Dairy Substrates. Fermentation 2017, 3, 48. [Google Scholar] [CrossRef]
- Parchami, M.; Mahboubi, A.; Agnihotri, S.; Taherzadeh, M.J. Biovalorization of Brewer’s Spent Grain as Single-Cell Protein through Coupling Organosolv Pretreatment and Fungal Cultivation. Waste Manag. 2023, 169, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Souza Filho, P.F.; Nair, R.B.; Andersson, D.; Lennartsson, P.R.; Taherzadeh, M.J. Vegan-Mycoprotein Concentrate from Pea-Processing Industry Byproduct Using Edible Filamentous Fungi. Fungal Biol. Biotechnol. 2018, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhu, X.; Shi, L.; Ni, C.; Hou, J.; Cheng, J. Enhancement of Soluble Protein, Polypeptide Production and Functional Properties of Heat-Denatured Soybean Meal by Fermentation of Monascus purpureus 04093. CyTA—J. Food 2019, 17, 1014–1022. [Google Scholar] [CrossRef]
- Yudiarti, T.; Sugiharto, S.; Isroli, I.; Widiastuti, E.; Wahyuni, H.I.; Sartono, T.A. Effect of Fermentation Using Chrysonillia Crassa and Monascus purpureus on Nutritional Quality, Antioxidant, and Antimicrobial Activities of Used Rice as a Poultry Feed Ingredient. J. Adv. Vet. Anim. Res. 2019, 6, 168. [Google Scholar] [CrossRef] [PubMed]
- Somda, M.K.; Nikiema, M.; Keita, I.; Mogmenga, I.; Kouhounde, S.H.S.; Dabire, Y.; Coulibaly, W.H.; Taale, E.; Traore, A.S.; Pr, O.; et al. Production of Single Cell Protein (SCP) and Essentials Amino Acids from Candida utilis FMJ12 by Solid State Fermentation Using Mango Waste Supplemented with Nitrogen Sources. Afr. J. Biotechnol. 2018, 17, 716–723. [Google Scholar] [CrossRef]
- Onofre, S.B.; Bertoldo, I.C.; Abatti, D.; Refosco, D. Chemical Composition of the Biomass of Saccharomyces cerevisiae—(Meyen Ex E. C. Hansen, 1883) Yeast Obtained from the Beer Manufacturing Process. Int. J. Environ. Agric. Biotechnol. 2017, 2, 558–562. [Google Scholar] [CrossRef]
- Marcišauskas, S.; Ji, B.; Nielsen, J. Reconstruction and Analysis of a Kluyveromyces marxianus Genome-Scale Metabolic Model. BMC Bioinform. 2019, 20, 551. [Google Scholar] [CrossRef]
- Yang, R.; Chen, Z.; Hu, P.; Zhang, S.; Luo, G. Two-Stage Fermentation Enhanced Single-Cell Protein Production by Yarrowia lipolytica from Food Waste. Bioresour. Technol. 2022, 361, 127677. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Jiang, Y.; Su, Y.; Zhang, Y. Upcycling Waste Organic Acids and Nitrogen into Single Cell Protein via Brewer’s Yeast. J. Clean. Prod. 2022, 369, 133279. [Google Scholar] [CrossRef]
- Dubey, P.; Tripathi, G.; Mir, S.S.; Yousuf, O. Current Scenario and Global Perspectives of Citrus Fruit Waste as a Valuable Resource for the Development of Food Packaging Film. Trends Food Sci. Technol. 2023, 141, 104190. [Google Scholar] [CrossRef]
- Rages, A.A.; Haider, M.M.; Aydin, M. Alkaline Hydrolysis of Olive Fruits Wastes for the Production of Single Cell Protein by Candida lipolytica. Biocatal. Agric. Biotechnol. 2021, 33, 101999. [Google Scholar] [CrossRef]
- Khan, M.K.I.; Asif, M.; Razzaq, Z.U.; Nazir, A.; Maan, A.A. Sustainable Food Industrial Waste Management through Single Cell Protein Production and Characterization of Protein Enriched Bread. Food Biosci. 2022, 46, 101406. [Google Scholar] [CrossRef]
- Saleem, M.; Saeed, M.T. Potential Application of Waste Fruit Peels (Orange, Yellow Lemon and Banana) as Wide Range Natural Antimicrobial Agent. J. King Saud. Univ. Sci. 2020, 32, 805–810. [Google Scholar] [CrossRef]
- Bacha, U.; Nasir, M.; Khalique, A.; Anjum, A.A.; Jabbar, M.A. Comparative Assessment of Various Agro-Industrial Wastes for Saccharomyces cerevisiae Biomass Production and Its Quality Evaluation as Single Cell Protein. J. Anim. Plant Sci. 2011, 21, 844–849. [Google Scholar]
- Mensah, J.K.M.; Twumasi, P. Use of Pineapple Waste for Single Cell Protein (SCP) Production and the Effect of Substrate Concentration on the Yield. J. Food Process Eng. 2017, 40, e12478. [Google Scholar] [CrossRef]
- Al-Farsi, M.; Al Bakir, A.; Al Marzouqi, H.; Thomas, R. Production of Single Cell Protein from Date Waste. Mater. Res. Proc. 2019, 11, 303–312. [Google Scholar] [CrossRef]
- García Martínez, J.B.; Egbejimba, J.; Throup, J.; Matassa, S.; Pearce, J.M.; Denkenberger, D.C. Potential of Microbial Protein from Hydrogen for Preventing Mass Starvation in Catastrophic Scenarios. Sustain. Prod. Consum. 2021, 25, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Pikaar, I.; Matassa, S.; Rabaey, K.; Bodirsky, B.L.; Popp, A.; Herrero, M.; Verstraete, W. Microbes and the Next Nitrogen Revolution. Environ. Sci. Technol. 2017, 51, 7297–7303. [Google Scholar] [CrossRef] [PubMed]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid State Fermentation of Food Waste Mixtures for Single Cell Protein, Aroma Volatiles and Fat Production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Muniz, C.E.S.; Santiago, Â.M.; Gusmão, T.A.S.; Oliveira, H.M.L.; Conrado, L.d.S.; Gusmão, R.P.D. Solid-State Fermentation for Single-Cell Protein Enrichment of Guava and Cashew by-Products and Inclusion on Cereal Bars. Biocatal. Agric. Biotechnol. 2020, 25, 101576. [Google Scholar] [CrossRef]
- Shahzad, H.M.A.; Asim, Z.; Mahmoud, K.A.; Abdelhadi, O.M.A.; Almomani, F.; Rasool, K. Optimizing Cultural Conditions and Pretreatment for High-Value Single-Cell Protein from Vegetable Waste. Process Saf. Environ. 2024, 189, 685–692. [Google Scholar] [CrossRef]
- Goldberg, I. The SCP Product; Springer: Berlin/Heidelberg, Germany, 1985; pp. 129–152. ISBN 978-3-642-46540-6. [Google Scholar]
- Łebkowska, M.; Załęska-Radziwiłł, M. Usable Products from Sewage and Solid Waste. Arch. Environ. Prot. 2011, 37, 15–19. [Google Scholar]
- Pacheco, M.T.B.; Caballero-Cordoba1, M.; Sgarbieri1, V.C. Composition and Nutritive Value of Yeast Biomass and Yeast Protein Concentrates. J. Nutr. Sci. Vitaminol. 1997, 43, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Hurraß, J.; Teubel, R.; Fischer, G.; Heinzow, B.; Wiesmüller, G.A. What Effect Do Mycotoxins, Cell Wall Components, Enzymes and Other Mold Components and Metabolites Have on Our Health? Allergo J. Int. 2024, 33, 124–132. [Google Scholar] [CrossRef]
- Cardoso Alves, S.; Díaz-Ruiz, E.; Lisboa, B.; Sharma, M.; Mussatto, S.I.; Thakur, V.K.; Kalaskar, D.M.; Gupta, V.K.; Chandel, A.K. Microbial Meat: A Sustainable Vegan Protein Source Produced from Agri-Waste to Feed the World. Food Res. Int. 2023, 166, 112596. [Google Scholar] [CrossRef] [PubMed]
- García-Garibay, M.; Gómez-Ruiz, L.; Cruz-Guerrero, A.E.; Bárzana, E. Single Cell Protein | Yeasts and Bacteria. In Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Robinson, R.K., Eds.; Academic Press: Cambridge, MA, USA, 2014; pp. 431–438. ISBN 9780123847331. [Google Scholar]
- Hadi, J.; Brightwell, G. Safety of Alternative Proteins: Technological, Environmental and Regulatory Aspects of Cultured Meat, Plant-Based Meat, Insect Protein and Single-Cell Protein. Foods 2021, 10, 1226. [Google Scholar] [CrossRef] [PubMed]
- Gautério, G.V.; da Silva, R.M.; Karraz, F.C.; Coelho, M.A.Z.; Ribeiro, B.D.; Lemes, A.C. Cell Disruption and Permeabilization Methods for Obtaining Yeast Bioproducts. Clean. Chem. Eng. 2023, 6, 100112. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of Protein-Rich Extracts from Spent Brewer’s Yeast (Saccharomyces cerevisiae): An Overview. Biomass Convers. Biorefin. 2022, 1, 1–23. [Google Scholar] [CrossRef]
- Chew, K.W.; Chia, S.R.; Lee, S.Y.; Zhu, L.; Show, P.L. Enhanced Microalgal Protein Extraction and Purification Using Sustainable Microwave-Assisted Multiphase Partitioning Technique. Chem. Eng. J. 2019, 367, 1–8. [Google Scholar] [CrossRef]
- Gomes, T.A.; Zanette, C.M.; Spier, M.R. An Overview of Cell Disruption Methods for Intracellular Biomolecules Recovery. Prep. Biochem. Biotechnol. 2020, 50, 635–654. [Google Scholar] [CrossRef] [PubMed]
- Phong, W.N.; Show, P.L.; Ling, T.C.; Juan, J.C.; Ng, E.P.; Chang, J.S. Mild Cell Disruption Methods for Bio-Functional Proteins Recovery from Microalgae—Recent Developments and Future Perspectives. Algal Res. 2018, 31, 506–516. [Google Scholar] [CrossRef]
- Coustets, M.; Joubert-Durigneux, V.; Hérault, J.; Schoefs, B.; Blanckaert, V.; Garnier, J.P.; Teissié, J. Optimization of Protein Electroextraction from Microalgae by a Flow Process. Bioelectrochemistry 2015, 103, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.M.; Cebrián, G.; Álvarez, I.; Raso, J. Release of Mannoproteins during Saccharomyces cerevisiae Autolysis Induced by Pulsed Electric Field. Front. Microbiol. 2016, 7, 216549. [Google Scholar] [CrossRef] [PubMed]
- Ganeva, V.; Angelova, B.; Galutzov, B.; Goltsev, V.; Zhiponova, M. Extraction of Proteins and Other Intracellular Bioactive Compounds From Baker’s Yeasts by Pulsed Electric Field Treatment. Front. Bioeng. Biotechnol. 2020, 8, 552335. [Google Scholar] [CrossRef] [PubMed]
- Roselló-Soto, E.; Barba, F.J.; Parniakov, O.; Galanakis, C.M.; Lebovka, N.; Grimi, N.; Vorobiev, E. High Voltage Electrical Discharges, Pulsed Electric Field, and Ultrasound Assisted Extraction of Protein and Phenolic Compounds from Olive Kernel. Food Bioprocess Technol. 2015, 8, 885–894. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction Assisted by Pulsed Electric Energy as a Potential Tool for Green and Sustainable Recovery of Nutritionally Valuable Compounds from Mango Peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef] [PubMed]
- Sarkis, J.R.; Boussetta, N.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Application of Pulsed Electric Fields and High Voltage Electrical Discharges for Oil Extraction from Sesame Seeds. J. Food Eng. 2015, 153, 20–27. [Google Scholar] [CrossRef]
- Boussetta, N.; Grimi, N.; Vorobiev, E. Pulsed Electrical Technologies Assisted Polyphenols Extraction from Agricultural Plants and Bioresources: A Review. Int. J. Food Process. Technol. 2015, 2, 1–10. [Google Scholar] [CrossRef]
- Zhang, T.; Lei, J.; Yang, H.; Xu, K.; Wang, R.; Zhang, Z. An Improved Method for Whole Protein Extraction from Yeast Saccharomyces cerevisiae. Yeast 2011, 28, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Ge, L.; Wang, X.T.; Tan, S.N.; Tsai, H.H.; Yong, J.W.H.; Hua, L. A Novel Method of Protein Extraction from Yeast Using Ionic Liquid Solution. Talanta 2010, 81, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Phong, W.N.; Show, P.L.; Le, C.F.; Tao, Y.; Chang, J.S.; Ling, T.C. Improving Cell Disruption Efficiency to Facilitate Protein Release from Microalgae Using Chemical and Mechanical Integrated Method. Biochem. Eng. J. 2018, 135, 83–90. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast Cell Disruption Strategies for Recovery of Intracellular Bio-Active Compounds—A Review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Alavijeh, R.S.; Karimi, K.; Wijffels, R.H.; van den Berg, C.; Eppink, M. Combined Bead Milling and Enzymatic Hydrolysis for Efficient Fractionation of Lipids, Proteins, and Carbohydrates of Chlorella vulgaris Microalgae. Bioresour. Technol. 2020, 309, 123321. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.M.; Hosano, N.; Hosano, H. Recovering Microalgal Bioresources: A Review of Cell Disruption Methods and Extraction Technologies. Molecules 2022, 27, 2786. [Google Scholar] [CrossRef] [PubMed]
- Awad, D.; Brueck, T. Optimization of Protein Isolation by Proteomic Qualification from Cutaneotrichosporon Oleaginosus. Anal. Bioanal. Chem. 2020, 412, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Tovar Jiménez, X.; Arana Cuenca, A.; Téllez Jurado, A.; Abreu Corona, A.; Muro Urista, C.R. Traditional Methods for Whey Protein Isolation and Concentration: Effects on Nutritional Properties and Biological Activity. J. Mex. Chem. Soc. 2012, 56, 369–377. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.J. Comparison of Various Industrially Applicable Disruption Methods to Produce Yeast Extract Using Spent Yeast from Top-Fermenting Beer Production: Influence on Amino Acid and Protein Content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.J. Yeast Extract Production Using Spent Yeast from Beer Manufacture: Influence of Industrially Applicable Disruption Methods on Selected Substance Groups with Biotechnological Relevance. Eur. Food Res. Technol. 2019, 245, 1169–1182. [Google Scholar] [CrossRef]
- Yadav, J.S.S.; Bezawada, J.; Ajila, C.M.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Mixed Culture of Kluyveromyces marxianus and Candida Krusei for Single-Cell Protein Production and Organic Load Removal from Whey. Bioresour. Technol. 2014, 164, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Sun, Y.; Meng, D.; Zhou, Z.; Zhang, Y.; Yang, R. Yeast Proteins: The Novel and Sustainable Alternative Protein in Food Applications. Trends Food Sci. Technol. 2023, 135, 190–201. [Google Scholar] [CrossRef]
- Bonnaillie, L.M.; Qi, P.; Wickham, E.; Tomasula, P.M. Enrichment and Purification of Casein Glycomacropeptide from Whey Protein Isolate Using Supercritical Carbon Dioxide Processing and Membrane Ultrafiltration. Foods 2014, 3, 94–109. [Google Scholar] [CrossRef] [PubMed]
- Marson, G.V.; Lacour, S.; Hubinger, M.D.; Belleville, M.P. Serial Fractionation of Spent Brewer’s Yeast Protein Hydrolysate by Ultrafiltration: A Peptide-Rich Product with Low RNA Content. J. Food Eng. 2022, 312, 110737. [Google Scholar] [CrossRef]
- Marson, G.V.; Belleville, M.P.; Lacour, S.; Hubinger, M.D. Membrane Fractionation of Protein Hydrolysates from By-Products: Recovery of Valuable Compounds from Spent Yeasts. Membranes 2020, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Nadar, C.G.; Fletcher, A.; Moreira, B.R.d.A.; Hine, D.; Yadav, S. Waste to Protein: A Systematic Review of a Century of Advancement in Microbial Fermentation of Agro-Industrial Byproducts. Compr. Rev. Food Sci. Food Saf. 2024, 23, e13375. [Google Scholar] [CrossRef] [PubMed]
- Ritala, A.; Häkkinen, S.T.; Toivari, M.; Wiebe, M.G. Single Cell Protein-State-of-the-Art, Industrial Landscape and Patents 2001–2016. Front. Microbiol. 2017, 8, 300587. [Google Scholar] [CrossRef] [PubMed]
- Trinci, A.P.J. Myco-Protein: A Twenty-Year Overnight Success Story. Mycol. Res. 1992, 96, 1–13. [Google Scholar] [CrossRef]
- Maul, S.B.; Sinskey, A.J.; Tannenbaum, S.R. New Process for Reducing the Nucleic Acid Content of Yeast. Nature 1970, 228, 181. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, R.; Enriquez, A. Nucleic Acid Reduction in Yeast. Appl. Microbiol. Biotechnol. 1988, 29, 208–210. [Google Scholar] [CrossRef]
- Zee, J.A.; Simard, R.E. Simple Process for the Reduction in the Nucleic Acid Content in Yeast. Appl. Microbiol. 1975, 29, 62. [Google Scholar] [CrossRef] [PubMed]
- Jankojć, A.; Lesiów, T.; Biazik, E. QUORNTM Meat Substitutes On Polish Market. Part 1. Eng. Sci. Technol. 2016, 3, 36–50. [Google Scholar]
- Kurek, M.A.; Onopiuk, A.; Pogorzelska-Nowicka, E.; Szpicer, A.; Zalewska, M.; Półtorak, A. Novel Protein Sources for Applications in Meat-Alternative Products—Insight and Challenges. Foods 2022, 11, 957. [Google Scholar] [CrossRef] [PubMed]
- Sadler, M.J. Meat Alternatives—Market Developments and Health Benefits. Trends Food Sci. Technol. 2004, 15, 250–260. [Google Scholar] [CrossRef]
- Gottardi, D.; Siroli, L.; Vannini, L.; Patrignani, F.; Lanciotti, R. Recovery and Valorization of Agri-Food Wastes and by-Products Using the Non-Conventional Yeast Yarrowia lipolytica. Trends Food Sci. Technol. 2021, 115, 74–86. [Google Scholar] [CrossRef]
- Elghandour, M.M.Y.; Tan, Z.L.; Abu Hafsa, S.H.; Adegbeye, M.J.; Greiner, R.; Ugbogu, E.A.; Cedillo Monroy, J.; Salem, A.Z.M. Saccharomyces cerevisiae as a Probiotic Feed Additive to Non and Pseudo-Ruminant Feeding: A Review. J. Appl. Microbiol. 2020, 128, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Rout, S.; Sowmya, R.S.; Srivastav, P.P. A Review on Development of Plant-Based Meat Analogues as Future Sustainable Food. Int. J. Food Sci. Technol. 2024, 59, 481–487. [Google Scholar] [CrossRef]
- Quorn Vegetarian Chicken Pieces|Quorn. Available online: https://www.quorn.co.uk/products/chicken-style-pieces (accessed on 26 May 2024).
- Quorn Vegan Fishless Fingers|Quorn. Available online: https://www.quorn.co.uk/products/vegan-fishless-fingers (accessed on 26 May 2024).
- Ekstrakt z Drożdży Vegemite 220 g—J. Cook. Available online: https://jcook.pl/ekstrakt-z-drozdzy-vegemite-220g (accessed on 26 May 2024).
- Marmite Yeast Extract—125 g e. Available online: https://pl.openfoodfacts.org/product/50184385/marmite-yeast-extract (accessed on 26 May 2024).
- Marmite Yeast Extract Dynamite Chilli Limited Edition 250 g BritishShopInWarsaw. Available online: https://britishshop.pl/pl/p/Marmite-Yeast-Extract-Dynamite-Chilli-Limited-Edition-250g/6589 (accessed on 26 May 2024).
- Marmite Flatbreads 140 g BritishShopInWarsaw. Available online: https://britishshop.pl/pl/p/Marmite-Flatbreads-140g/6141 (accessed on 26 May 2024).
- Marmite Crunchy Peanut Butter 225 g BritishShopInWarsaw. Available online: https://britishshop.pl/pl/p/Marmite-Crunchy-Peanut-Butter-225g/5920 (accessed on 26 May 2024).
- Yeast Protein 500 g—ALLNUTRITION. 49 Zł. Przebadane|Allnutrition.Pl. Available online: https://allnutrition.pl/ALLNUTRITION_Yeast_Protein-opis41988.html (accessed on 26 May 2024).
- Enkioo Białko Drożdżowe, Wegańskie 700 g|Produkty Enkioo Suplementy Diety\Białko Dla Sportowców\Odżywki Białkowe. Available online: https://enkioo.com/pl/products/enkioo-bialko-drozdzowe-weganskie-700g-10.html?country=1143020003&utm_source=iai_ads&utm_medium=google_shopping&gad_source=1&gclid=CjwKCAiA0PuuBhBsEiwAS7fsNZHLEStUkauNtB6qEg2x8ynN2XhAQrDe04LB1LsOACYDgk6__j_8fhoCDUYQAvD_BwE (accessed on 26 May 2024).
- Sillman, J.; Uusitalo, V.; Ruuskanen, V.; Ojala, L.; Kahiluoto, H.; Soukka, R.; Ahola, J. A Life Cycle Environmental Sustainability Analysis of Microbial Protein Production via Power-to-Food Approaches. Int. J. Life Cycle Assess. 2020, 25, 2190–2203. [Google Scholar] [CrossRef]
- Kalmykova, Y.; Sadagopan, M.; Rosado, L. Circular Economy—From Review of Theories and Practices to Development of Implementation Tools. Resour. Conserv. Recycl. 2018, 135, 190–201. [Google Scholar] [CrossRef]
- Koukoumaki, D.I.; Tsouko, E.; Papanikolaou, S.; Ioannou, Z.; Diamantopoulou, P.; Sarris, D. Recent Advances in the Production of Single Cell Protein from Renewable Resources and Applications. Carbon. Resour. Convers. 2024, 7, 100195. [Google Scholar] [CrossRef]
- Aidoo, R.; Kwofie, E.M.; Adewale, P.; Lam, E.; Ngadi, M. Overview of Single Cell Protein: Production Pathway, Sustainability Outlook, and Digital Twin Potentials. Trends Food Sci. Technol. 2023, 138, 577–598. [Google Scholar] [CrossRef]
- Khoshnevisan, B.; Tabatabaei, M.; Tsapekos, P.; Rafiee, S.; Aghbashlo, M.; Lindeneg, S.; Angelidaki, I. Environmental Life Cycle Assessment of Different Biorefinery Platforms Valorizing Municipal Solid Waste to Bioenergy, Microbial Protein, Lactic and Succinic Acid. Renew. Sustain. Energy Rev. 2020, 117, 109493. [Google Scholar] [CrossRef]
- Lappi, J.; Silventoinen-Veijalainen, P.; Vanhatalo, S.; Rosa-Sibakov, N.; Sozer, N. The Nutritional Quality of Animal-Alternative Processed Foods Based on Plant or Microbial Proteins and the Role of the Food Matrix. Trends Food Sci. Technol. 2022, 129, 144–154. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and Adequacy of the Vegan Diet. A Systematic Review of the Evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef] [PubMed]
- Sharif, M.; Zafar, M.H.; Aqib, A.I.; Saeed, M.; Farag, M.R.; Alagawany, M. Single Cell Protein: Sources, Mechanism of Production, Nutritional Value and Its Uses in Aquaculture Nutrition. Aquaculture 2021, 531, 735885. [Google Scholar] [CrossRef]
- Rzymski, P.; Niedzielski, P.; Kaczmarek, N.; Jurczak, T.; Klimaszyk, P. The Multidisciplinary Approach to Safety and Toxicity Assessment of Microalgae-Based Food Supplements Following Clinical Cases of Poisoning. Harmful Algae 2015, 46, 34–42. [Google Scholar] [CrossRef]
- Jones, S.W.; Karpol, A.; Friedman, S.; Maru, B.T.; Tracy, B.P. Recent Advances in Single Cell Protein Use as a Feed Ingredient in Aquaculture. Curr. Opin. Biotechnol. 2020, 61, 189–197. [Google Scholar] [CrossRef] [PubMed]
| Component | Biomass (%) [65,76] | Extracted Protein (%) [77] |
|---|---|---|
| Protein | 30–70 | 76 |
| Lysine | 35–60 | 8.78 |
| Methionine | 6.5–7.8 | 1.82 |
| Lipids | 1.5–1.8 | 6.30 |
| Carbohydrates | 34.88 | 8.78 |
| Nucle acids | 9.7 | 2.2 |
| Ash | 7.85 | 6.1 |
| Type of Product | Name of the Product | Producer | Microorganisms | Ingredients | Nutritional Value | References |
|---|---|---|---|---|---|---|
| Meat substitute | Crispy nuggets | Quorn | F. venenatum | Fusarium venenatu, soy sauce, pea fiber | 190 kcal/100 g | [120] |
| Meat substitute | Vegetarian chicken pieces | Quorn | F. venenatum | Mycoprotein (95%), hydrated free-range egg white, natural flavouring, firming agents: calcium chloride, calcium acetate | 97 kcal/100 g | [121] |
| Fish substitute | Vegan fishless sticks | Quorn | F. venenatum | Rice flakes, wheat flour, mycoprotein (12%), water, natural flavouring, rapeseed oil, stabiliser: methylcellulose; yeast, salt, paprika, colouring: paprika extract | 214 kcal/100 g | [122] |
| Paste | Paste with yeast extract | Vegemite (Mondelez) | S. cerevisiae | yeast extract (from barley, spelt), salt, malt extract (from barley), flavour enhancer (potassium chloride), colour (e150c), spice extract (contains seler), niacin, thiamin, riboflavin, folic acid | 186 kcal/100 g | [123] |
| Paste | Yeast extract paste | Marmite | S. cerevisiae | Yeast extract (contains barley), salt, vegetable juice from concentrate, vitamins (thiamin, riboflavin, niacin, vitamin b12, folic acid), natural flavourings (contains celery) | 250 kcal/100 g | [124] |
| Paste | Yeast extract dynamite | Marmite | S. cerevisiae | Yeast extract (contains barley, wheat, oat, rye), salt, vegetable juice concentrate, vitamins (thiamin, riboflavin, niacin, vitamin b12 and folic acid), natural chilli flavouring, natural flavouring (contains celery) | 279 kcal/100 g | [125] |
| Snacks | Flatbreads | Marmite | S. cerevisiae | fortified wheat flour (wheat flour, calcium carbonate, iron, niacin, thiamin), marmite (18%) (yeast extract (contains barley, wheat, oats, rye), salt, vegetable juice concentrate, vitamins (thiamin, riboflavin, niacin, vitamin b12, folic acid), natural flavouring (contains celery)), cheddar cheese (milk) (16%), rapeseed oil, raising agent: ammonium bicarbonate | 429 kcal/100 g | [126] |
| Paste | Crunchy peanut butter | Marmite | S. cerevisiae | peanuts (87%), yeast extract powder (9.5%), peanut oil, tocopherol extract (antioxidant), vitamins (thiamin, riboflavin, niacin, vitamin b12 and folic acid). may contain other nuts | 574 kcal/100 g | [127] |
| Protein supplement | Yeast protein | Allnutrition | S. cerevisiae | Saccharomyces cerevisiae yeast protein, reduced fat cocoa, flavouring, salt, thickeners: cellulose gum, guar gum, xanthan gum, sweeteners: steviol glycosides from stevia, acesulfame k, sucralose | 360 kcal/100 g | [128] |
| Food supplement | Yeast protein, vegan | Enkioo | S. cerevisiae | Yeast protein (derived from the yeast Saccharomyces cerevisiae) 100% | 411 kcal/100 g | [129] |
| S Strengths | W Weaknesses | O Opportunities | T Threats |
|---|---|---|---|
|
|
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pobiega, K.; Sękul, J.; Pakulska, A.; Latoszewska, M.; Michońska, A.; Korzeniowska, Z.; Macherzyńska, Z.; Pląder, M.; Duda, W.; Szafraniuk, J.; et al. Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives. Appl. Sci. 2024, 14, 6259. https://doi.org/10.3390/app14146259
Pobiega K, Sękul J, Pakulska A, Latoszewska M, Michońska A, Korzeniowska Z, Macherzyńska Z, Pląder M, Duda W, Szafraniuk J, et al. Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives. Applied Sciences. 2024; 14(14):6259. https://doi.org/10.3390/app14146259
Chicago/Turabian StylePobiega, Katarzyna, Joanna Sękul, Anna Pakulska, Małgorzata Latoszewska, Aleksandra Michońska, Zuzanna Korzeniowska, Zuzanna Macherzyńska, Michał Pląder, Wiktoria Duda, Jakub Szafraniuk, and et al. 2024. "Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives" Applied Sciences 14, no. 14: 6259. https://doi.org/10.3390/app14146259
APA StylePobiega, K., Sękul, J., Pakulska, A., Latoszewska, M., Michońska, A., Korzeniowska, Z., Macherzyńska, Z., Pląder, M., Duda, W., Szafraniuk, J., Kufel, A., Dominiak, Ł., Lis, Z., Kłusek, E., Kozicka, E., Wierzbicka, A., Trusińska, M., Rybak, K., Kot, A. M., & Nowacka, M. (2024). Fungal Proteins: Sources, Production and Purification Methods, Industrial Applications, and Future Perspectives. Applied Sciences, 14(14), 6259. https://doi.org/10.3390/app14146259










