Abstract
Concern for the future of the next generation leads to the search for alternative solutions for the proper management of materials considered as useless waste. This study fits into this research trend. Its aim is to demonstrate the potential of walnut husks as a source of compounds with antioxidant properties that can be used in non-food industries. Pressurized liquid extraction, i.e., one of the modern green extraction techniques used on an industrial scale, as well as conventional extraction in Soxhlet and maceration were applied to prepare the extracts. In order to assess in depth their antioxidant activity in relation to the content of characteristic components, various activity assessment methods were used in this research. The results proved that the husk components have such antioxidant properties that they can be of interest to the cosmetics and pharmaceutical industries regarding the management of this waste. The results confirmed the usefulness of assisted extraction in increasing the ecological and economic values of the proposed waste disposal. Moreover, they showed that juglonehas very weak antioxidant properties, and the antioxidant effect of the mixture containing husk extract and juglone solution is mainly additive.
Keywords:
walnut husks; antioxidant properties; juglone; wastes; green chemistry; phenolic compounds 1. Introduction
Walnut (Juglans regia L.) is a tree that can be found naturally in many parts of the world (including Asia, Europe and the Americas) [1,2]. It owes its popularity to tasty and healthy fruits rich in polyunsaturated fatty acids, including alpha-linolenic acid [3,4]. Additionally, walnuts are a source of easily digestible iron, magnesium, sodium and potassium compounds. It is estimated that world production of walnuts is approximately 2.1 million tons per year [5]. Most walnuts are harvested in China—approximately 1 million tons, and all production is used in the local market. The United States remains the second largest producer of walnuts—it produces over 660,000 tons. Poland is rated 30th in this ranking, with 6800 tons of production per year. Inedible walnut husks are mostly treated as useless agricultural and industrial waste, which is difficult to dispose of [6,7]. However, according to the literature, the walnut husks contain many valuable polyphenolic substances (including the most characteristic, juglone) with various properties, the most of which are inhibiting the development of various pathogenic bacteria and antifungal activity [8,9,10]. Therefore, after appropriate processing, these so far useless waste materials could be used as a source of substances sought by many non-food industries, including the cosmetics or pharmaceutical industries [11,12].
Nowadays, the use of natural and cheap waste materials has a double benefit for the environment: on the one hand, it allows for the effective use of waste, and on the other, it contributes to the creation of natural, safe products used in everyday life [13]. These activities are part of the so-called “circular economy model”, which is the European Union’s response to the threats that are becoming worse as the world’s population grows [14]. The basis of this model is the assumption that in order to limit the exploitation of natural resources and reduce waste generation, the value of raw materials and products should be maximized by leaving them in circulation for as long as possible. According to this model, the ability to properly process waste should be created so that it can be reused to produce new, high-value goods. At this point, it should be remembered that the history of humanity is, above all, the history of the relationship between people and the surrounding nature. Therefore, in parallel with the proper use of waste, the modern development of the world is based on technology using the principles of green chemistry, i.e., reducing the consumption of non-renewable raw materials, thus not polluting the environment [15,16]. This presented paper contributes to the development of these new trends.
Its main aim is to assess the antioxidant activity of extracts obtained from walnut husks. To prepare the extracts, in addition to the technique consistent with the principles of green chemistry—pressurized liquid extraction (PLE)—conventional extractions in the Soxhlet apparatus and maceration were applied. In each of them, methanol, ethyl acetate, acetone and chloroform were used as extraction solvents. The amount of polyphenolic compounds and juglone was determined in each of the obtained extracts.
Considering that antioxidant properties can be diverse (it is worth mentioning here the ability of antioxidants to prevent the formation of reactive species or to neutralize/scavenge free radicals), researchers use various methods to assess them. This presented paper uses some of the most frequently applied ones. These are colorimetric methods using the following: the radical cation of the 2,2′-Azino-Bis(3-ethylbenzoThiazoline-6-Sulfonic acid) diammonium salt; 2,2′-DiPhenyl-1-PicrylHydrazyl; β-carotene, as well as Ferric Ion Reducing Antioxidant Parameter and Cupric Reducing Antioxidant Capacity. Commonly used names for these methods are ABTS, DPPH, β-carotene, FRAP and CUPRAC, respectively. These are not only the most popular methods, but also the methods willingly used due to their sensitivity, simplicity of measurement, short experimental time and the employment of an inexpensive spectrophotometer, popular in many laboratories.
A characteristic component of walnut husks is juglone (5-hydroxy-1,4-naphthoquinone) [17]. This compound has documented antibacterial, antiviral and antifungal properties [8,9]. However, little is known about its antioxidant properties. In the literature, this information is not only rare and mostly based on speculation, but it is also contradictory. It is true that due to the presence of a hydroxyl group, this compound is classified as a phenolic compound generally known for its antioxidant activity. On this basis, the juglone molecule is assigned antioxidant properties. However, its structure also contains a quinone moiety, which is a redox factor and can produce reactive oxygen species [17]. It should also be remembered that the activity of a given compound generally may depend on the characteristics of the measurement system. With the above in mind, and in the absence of verifiable empirical data during these experiments, it was decided not only to measure the antioxidant properties of juglone but also to determine how different contents of this compound modify the antioxidant properties of the extract.
2. Materials and Methods
2.1. Plant Material and Chemicals
Walnut husks were collected from J. regia L. cultivars in September/October 2023. The husks (ca. 2 kg) were obtained from a20-years-old non-grafted tree growing in Lublin, Poland (DD: 51.25777184011066, 22.506641160041866). The husks were handpicked from the ground. The material was subjected to preliminary drying (temp. 105 °C),grinding and sieving (particle size < 0.5 mm). Thoroughly weighted portions of the samples were used for extractions.
Copper (II) chloride, iron (III) chloride hexahydrate, hydrochloric acid, ammonium acetate, ethanol, ethyl acetate, acetone, methanol, chloroform, sodium acetate, acetic acid and orthophosphoric acid were purchased from the Polish Chemical Plant POCh (Gliwice, Poland). 2,2′-Diphenyl-1-picrylhydrazyl (DPPH), potassium persulfate (di-potassium peroxdisulfate), 2,2-azinobis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS), acetonitrile for HPLC, linoleic acid, Tween 20, neocuproine (2,9-dimethyl-1,10-phenanthroline, Nc), β-carotene, 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), juglone (5-hydroxy-1,4-naphthoquinone) and 6-Hydroxy-2,5,7,8-tetramethychroman-2-carboxylic (Trolox) were purchased from Sigma Aldrich (Poznań, Poland). Water was purified on a Milli-Q system from Millipore (Millipore, Bedford, MA, USA).
2.2. Preparation of Extracts
2.2.1. Pressurized Liquid Extraction
PLE was performed on a Dionex ASE200 instrument (Dionex Corp., Sunnyvale, CA, USA). For this purpose, the plant material (1.0 g) was mixed with appropriately prepared sand to reduce the volume of the solvent used for extraction [18], placed in 22 mL extraction vessels made of stainless steel and extracted using a slightly modified procedure described in [19]. The following extraction conditions were used: extraction temperature 100 °C, extraction time 10 min, pressure 60 bar, volume of solvent rinsing the vessel equal to 100% of the volume of the extraction cell; purge time 60 s using nitrogen at a pressure of 10 bar. The system was washed with the extraction solvent between runs.
2.2.2. Extraction in the Soxhlet Apparatus
Exhaustive Soxhlet extraction was performed according to [20] using a 1 g portion of ground husks using either methanol or ethyl acetate or acetone or chloroform as the extraction solvent (70 mL). Each sample was extracted for 3 h. The extract was then cooled.
2.2.3. Maceration
Portions of 1 g of the material were immersed in methanol, ethyl acetate, acetone or chloroform (70 mL) [20]. The entire amount (tightly closed) was left for 72 h at room temperature. After this time, the extract was removed and filtered through Whatman no.4 paper.
2.3. Measurements of Antioxidant Properties
The antioxidant properties of the obtained extracts and juglone solutions were examined using various spectrophotometric methods. ABTS, DPPH, β-carotene bleaching assay, FRAP and CUPRAC methods were used. The spectrophotometric absorbance was measured by UV Probe-2550 Spectrophotometer (Shimadzu, Kyoto, Japan) with photometric range up to 5 Abs. Before measurements, the extracts were poured into 100 mL flasks, which were supplemented with the solvent used for extraction. The tests, unless otherwise stated, were carried out maintaining the same volume ratios, using 100 μL of extract in each test.
In order to standardize the presentation of the results, they were expressed in Trolox equivalents. For this purpose, calibration curves of the decrease in absorption (difference between initial absorptions and absorptions after the reaction) of Trolox as a function of its concentration in the range of 2.5–25 µg/mL were prepared in the ABTS and DPPH methods. In turn, in the FRAP and CUPRAC methods, calibration curves were prepared showing the dependence of Trolox absorbance as a function of its concentration in the same range, i.e., 2.5–25 µg/mL. Based on the obtained equations (see Table S1), the equivalent antioxidant capacity of Trolox was determined for a given percentage of scavenging or absorbance exhibited by the tested extracts.
2.3.1. ABTS Method
ABTS cation radical absorbance changes were monitored at 744 nm. The ABTS cationic radical (ABTS•+) was generated during a 16 h reaction of ABTS (5 mL of 7 mmol/L ABTS solution) with potassium persulfate (88 µL of potassium persulfate 140 mmol/L). After 16 h of incubation in the dark, the cation radical solution was diluted with methanol to obtain an absorbance of 0.7 ± 0.05 [21]. Amounts of 2900 µL of methanol solution of ABTS•+ and 100 µL of extract were used for measurements. To zero the spectrophotometer, 2900 μL of methanol and 100 μL of the appropriate extraction solvent (methanol or ethyl acetate or acetone or chloroform) were used. After 60 min reaction, % inhibition (% I) was calculated from the following equation:
where A60 and A0 are the absorbance values of ABTS•+ at the times 0 min and 60 min, respectively.
2.3.2. DPPH Method
The changes in the DPPH radical as a result of its reaction with the examined extracts were determined according to a slightly modified procedure described in [22]. For this purpose, 2900 µL of a methanolic solution of the DPPH radical with an absorbance of approximately 0.7 ± 0.05 was mixed with 100 µL of the examined extract. The absorbance changes at 516 nm were monitored during the 60 min reaction. To zero the spectrophotometer, 2900 μL of methanol and 100 μL of the appropriate extraction solvent (methanol or ethyl acetate or acetone or chloroform) were used. The percent inhibition I (%) was calculated from the following equation:
where A60 and A0 are the absorbance values of DPPH• at the times 0 min and 60 min, respectively.
2.3.3. FRAP Method
FRAP determination was performed using the Benzie and Strain method [23]. The reagent for measuring antioxidant properties using this method was freshly prepared by mixing the following solutions: FeCl3·6H2O (the final concentration of Fe(III) in the aqueous solution was 20 mM), TPTZ in 40 mM HCl (the final concentration of TPTZ was 10 mM) and 0.3 M CH3COOH/CH3COONa buffer solution with pH = 3.6. The volume ratio of the above solutions was 1:1:10. It was then poured into an optical glass cuvette and immediately placed in a spectrophotometer to measure the increase in absorbance at 593 nm. To zero the spectrophotometer, a mixture containing 2900 µL of FRAP reagent and 100 µL of extraction solvent (methanol or ethyl acetate or acetone or chloroform) was used. Antioxidant properties were expressed as absorbance values of the colored complex formed in the reaction after Fe(III) reduction by antioxidants.
2.3.4. CUPRAC Method
Antioxidant properties in the CUPRAC method are determined by measuring the absorbance of the colored complex, formed as a result of the reduction of the copper-neocuproate complex (Cu(II)-Nc) to the copper form (Cu(I)-Nc), using the examined antioxidants [24]. The reaction solution is prepared from CuCl2 (final concentration of Cu(II) in the solution was 10 mM), neocuproine in ethanol final concentration 7.5 mM) and 1.0 M CH3COOH/CH3COONH4 buffer solution with pH = 7.0. The reagents are measured as follows: 740 µL of Cu(II) solution + 740 µL of Nc solution + 740 µL of buffer + 100 µL of tested extracts + 680 µL of water. The resulting mixture was shaken vigorously for 30 s and left in the dark for 60 min. Then, after placement in spectrophotometer, the absorbance was measured at a wavelength of 450 nm. To zero the spectrophotometer, a mixture containing all reagents and 100 µL of extraction solvent (methanol or ethyl acetate or acetone or chloroform) was used. Antioxidant properties were expressed as absorbance values of the colored complex formed in the reaction after Cu(II) reduction by antioxidants.
2.3.5. β-Carotene Bleaching Assay
Measurements of antioxidant properties using the β-carotene method were performed using a modified method described in [25]. An emulsion of β-carotene/linoleic acid in water was prepared by mixing 25 µL of linoleic acid, 185 µL of Tween 20 (200 mg) and 5 mL of β-carotene solution (containing 0.5 mg of β-carotene in 1 mL of chloroform). Chloroform was evaporated from the mixture using a vacuum evaporator, and the residue was dispersed in 100 mL of distilled water saturated with oxygen (saturation time 30 min, oxygen flow 100 mL/min). For measurement, 2900 µL of emulsion and 100 µL of extract were used, which were placed in a glass cuvette. The cuvette was tightly closed and mixed, and then (with the emulsion and antioxidant) placed in a water bath (45 °C). Changes in β-carotene absorbance were monitored at 470 nm. The first absorbance measurement (at 0 min) was performed immediately after mixing the ingredients. Subsequent readings were taken at constant time intervals (10 min) until the orange color of the control sample disappeared (approximately 180 min). A mixture containing 2900 µL of β-carotene emulsion and 100 µL of extraction solvent (methanol or ethyl acetate or acetone or chloroform) was used as a control sample. To zero the spectrophotometer, a mixture containing 100 µL of extraction solvent and 2900 µL of emulsion without β-carotene was used. The % inhibition of the sample (containing β-carotene and the tested extract) in relation to the control sample (containing only β-carotene only) was calculated according to the following formula:
where I(%)—inhibition percent, DRC—degradation rate of β-carotene in the control sample = {[ln (a/b)]/t}, DRS—degradation rate of β-carotene in the sample with antioxidant = {[ln (a/b)]/t}, a = absorbance at time = 0, b = absorbance at defined time (for example at 10, 20 …. to 180 min), t = time.
2.3.6. Determination of Antioxidant Properties of Juglone Alone and Its Mixtures with Extracts
The effect of juglone content on the antioxidant properties of the methanol extract obtained by maceration and in the Soxhlet apparatus was studied using the ABTS method at different volume ratios of reagents in the measuring system and at different concentrations of juglone within the range in which this compound occurs in walnut husks, according to the literature, i.e., 0.06 mg/mL or 0.2 mg/mL. The volumes of individual antioxidant and the ABTS cationic radical solutions used in one-component systems (containing extract or juglone solution) and two-component systems (containing a mixture of the extract and juglone solution) are presented in Table 1.

Table 1.
Volumes of solutions used for the determination of the antioxidant properties of methanolic extracts and juglone, and their binary mixtures.
2.4. Determination of Polyphenolic Compounds
The total polyphenol contents in the extracts was determined using the Folin–Ciocalteu method with some modification [26]. Briefly, a portion of 0.1 mL of extracts was mixed with 1.58 mL of water and 0.1 mL of the Folin–Ciocalteu reagent. Then, 0.3 mL of 20% w/v sodium carbonate aqueous solution was added to the mixture. After incubation (2 h, at room temperature), the absorbance was measured at 765 nm using the spectrophotometer. Calibration curves were made for gallic acid in different solvents for the concentration range of 2.5–500 mg/L. These data are shown in Table S2. The results were expressed as gallic acid equivalents (GAEs), micrograms per gram of dry plant material. The measurement for each extract was repeated three times.
2.5. HPLC Analysis of Juglone
Chromatographic measurements were performed using Varian ProStar model 210/215 with Pro Star 325 UV/VIS detector (Varian, Walnut Creek, CA, USA) and ODS columns (Microsorb MV100C18, Varian, Walnut Creek, CA, USA). All measurements were carried out using the 150 × 4.6 mm column. Samples were injected with the Rheodyne 7725 sample injector (Sigma-Aldrich, Poznań, Poland) equipped with a 20 μL loop. The HPLC measurements were carried out using the following conditions: gradient elution B (25–40 %) from 0 to 30 min. The solvents A and B were water (with phosphoric acid, 0.5% v/v) and acetonitrile, respectively. Detection was carried out at 420 nm. The calibration curve for juglone was constructed for the concentration range 0.001–0.05 mg/mL. Its characteristics are as follows: calibration curve equation: y = 8.95 × 108x − 5.77 × 104, standard error of the curve: Sy = 1.6 × 105, standard deviation of the slope coefficient: Sa = 2.1 × 1057, standard deviation of the intercept: Sb = 1.1 × 105, limit of quantification: LOQ = 0.001 mg/mL, coefficient of determination: R2 = 0.9989. The LOQ was assumed to be 10 × Sy/slope.
Analyses were performed for the extracts obtained without and after 40-fold concentration. For this purpose, 20 mL of each extract was evaporated to dryness using CentriVap Cold Trap Labconco (Labconco Corp., Kansas City, MO, USA) and the dry residue was dissolved in 0.5 mL of the mobile phase.
2.6. Statistical Analysis
Extraction with each technique and solvent was repeated three times. The antioxidant measurements are presented as mean values from five independent measurements ± standard deviation (SD). The one-way analysis of variance (ANOVA) and Fisher coefficient (F) value were used to assess the influence of experimental factors on the activity. If the calculated value of F (Fcal) exceeds the tabular value F (Ftab), this indicates a statistically significant influence of the given parameter. To determine the significance of each Fisher coefficient, the p-values were used. The values were considered to be significantly different when the results of the compared parameters differed at the p = 0.05 significance level. The statistical analysis was performed using Excel (Microsoft Excel 2010).
3. Results and Discussion
3.1. Antioxidant Properties of Extracts Obtained from Walnut Husks
The antioxidant properties of walnut husk extracts determined using the ABTS, DPPH and β-carotene methods are presented in Figure 1 whereas using FRAP and CUPRAC methods are shown in Figure 2. As can be seen from the presented data, the tested extracts exhibit different antioxidant properties depending on the research method and extraction techniques used, as well as the type of extraction solvent. These relationships are known from the literature [27]. As shown previously [9], higher temperatures enhance the extraction yield of compounds and increase the biological activity of extracts. Changing the type of extractant results in a variation of the extraction selectivity and likely modifies the biological activity. When comparing the antioxidant properties of the extracts, it can be noticed, however, that the higher the extraction temperature, the greater the antioxidant properties [19]. This fact is rarely raised in the literature because an increase in temperature generally favors the thermal degradation of compounds, resulting in a decrease in antioxidant properties.
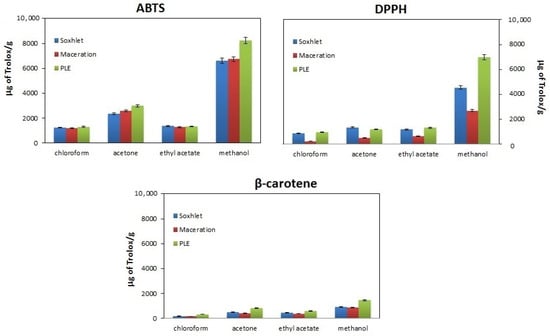
Figure 1.
Comparison of the antioxidant properties of walnut husk extracts obtained by extraction in the Soxhlet apparatus (blue bars), maceration (red bars) and pressurized liquid extraction (PLE) (green bars) using different extractants (chloroform, acetone, ethyl acetate and methanol) and methods for assessing antioxidant properties (ABTS, DPPH and β-carotene).
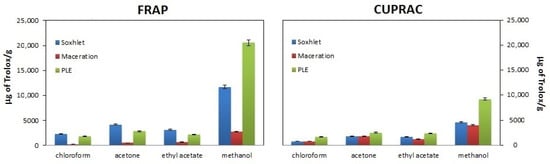
Figure 2.
Comparison of the antioxidant properties of walnut husk extracts obtained by extraction in the Soxhlet apparatus (blue bars), maceration (red bars) and pressurized liquid extraction (PLE)(green bars) using different extractants (chloroform, acetone, ethyl acetate and methanol) and FRAP and CUPRAC methods for assessing antioxidant properties.
According to the presented data, the highest antioxidant properties are obtained for extracts obtained using PLE, and the weakest for maceration. This fact is known from the literature because the PLE technique is characterized by a higher analyte mass transfer rate compared to classical approaches [28]. As for the influence of the method of assessing antioxidant properties, as can be seen in Figure 1, the weakest antioxidant properties (lowest % inhibition values, lowest bars) are demonstrated bythe DPPH method, while the ABTS and β-carotene methods are more sensitive, resulting inhigher % inhibition values (higher bars).
Differences in antioxidant properties between methods result from the fact that a different type of antioxidant activity is determined in them. In the ABTS and DPPH methods, the ability of the sample to neutralize colored radicals (ABTS cation radical and DPPH radical, respectively) is measured, while in the β-carotene method, antioxidant properties are demonstrated by substances that have the ability to delay the oxidation of the standard molecule (β-carotene) [29] Hence, there are differences in the percentage of inhibition obtained between the β-carotene method and the two methods mentioned above. In turn, the differences in antioxidant properties between the DPPH and ABTS methods result from differences in the availability of unpaired electrons in radicals. In the former, the unpaired electron is less accessible due to spherical hindrance, hence the lower inhibition percent in the DPPH method [30].
Figure 2 shows the antioxidant properties, expressed as the absorbance value obtained for the examined extracts, assessed using the FRAP and CUPRAC methods. As can be seen from the presented data, the values obtained for the FRAP method exceed those obtained using the CUPRAC method. Most likely, despite the same nature of the measurement (both methods determine the ability to reduce metal ions, Fe3+ and Cu2+, respectively), they are not the same. They differ in the reaction environment (FRAP method pH = 3.6 and CUPRAC method pH = 7), which may result in a different oxidative response of the antioxidants present in the sample and thus may influence the reduction reaction. The copper reaction (CUPRAC method) is more specific for antioxidants [31]. Moreover, in FRAP, lower pH levels reduce the ionization potential, which drives electron transfer, while increasing the redox potential [29].
Regardless of the isolation method used and the method of testing antioxidant properties, the highest antioxidant properties are demonstrated by methanol extracts and the weakest by chloroform extracts. It should be clarified here that when assessing antioxidant properties, the solvent has two functions. On the one hand, as a reaction medium, it determines the effective isolation of compounds, and on the other hand, it participates in the neutralization reaction of reactive species. Taking the above into account, and additionally remembering that, according to the literature, walnut shells are a rich source of polyphenolic compounds [8,9,32], in the next stage of the research, the total content of phenolic compounds was determined in each extract. These data are presented in Table 2.

Table 2.
The total phenolics amount, expressed in μg per 1 g of dry plant material, in extracts obtained by extraction in the Soxhlet apparatus, maceration and PLE using different extractants (chloroform, acetone, ethyl acetate and methanol). Various superscripts in the row indicate data for which the difference in phenolics amounts is not statistically significantly (Fcal < Ftab, p > 0.05, see Table S1).
Data presented in Table 2 reveals that the most effective isolation technique of phenolics is PLE. This technique has repeatedly demonstrated its great separation potential resulting from short exposure of the sample to high temperature [28]. Among the solvents, the most effective in terms of isolating phenolic compounds was the most polar of the solvents used, methanol. Combining the data collected in Table 2 with the relationships presented in Figure 1 and Figure 2, it is not surprising that methanolic PLE extracts, being the most abundant in polyphenols, have the highest antioxidant properties in each of the tested methods.
According to the literature, the assessment of the antioxidant properties of compounds is influenced not only by their chemical structure, identified with the ability to donate hydrogen with electrons, chelation of metals and delocalization of an unpaired electron in the aromatic structure, but also the type of solvent in which the antioxidant properties are determined. Methods for measuring the antioxidant properties of substances are based on two main reaction mechanisms: the single electron transfer (SET) mechanism and the hydrogen transfer (HAT) mechanism [25,31]. Both mechanisms may occur simultaneously, and the dominance of one of them is determined by, among others, type of antioxidant properties, partition coefficient and type of solvent. In methods where the SET mechanism dominates (DPPH, ABTS, CUPRAC and FRAP), neutralization of reactive species takes place as a result of ionization/dissociation of the antioxidant, which is promoted by a solvent with a high dielectric constant (ε). Therefore, it is not surprising that in the mentioned methods, the highest antioxidant properties are exhibited by methanol and acetone extracts (ε = 32.6 and ε = 20.7, respectively) [33,34]. In turn, the low antioxidant properties of chloroform extracts can be explained, on the one hand, by the low content of polyphenols (see Table 2), and, on the other hand, by the low dielectric constant and difficult dissociation/ionization of the antioxidant (ε = 4.8). In the β-carotene method, the dominant mechanism is the HAT mechanism, or more precisely, its variant PCET (Proton Couple Electron Transfer), in which the neutralization of peroxyl radicals (LOO•) occurs as a result of the formation of a transition complex between the radical and the antioxidant, and then the separation of hydrogen from the antioxidant as a result of the homolytic dissociation of the molecular bond. Hydrogen transport is favored in a solvent characterized by a high value β, which expresses the hydrogen acceptor capacity of the solvent. Among the solvents used, chloroform has the lowest value of this parameter (β = 0.02), while for the other solvents, it ranges from 0.41–0.5 [35], hence the better antioxidant properties of the extracts obtained in these solvents.
3.2. The Influence of Juglone on the Antioxidant Properties of Selected Extracts Obtained from Walnut Husks
As mentioned in the Introduction, juglone is one of the characteristic compounds of walnut found, among others, in husks. The content of this compound is typically determined using chromatographic methods. However, to our surprise, chromatographic measurements performed on the obtained extracts showed the absence of juglone (see Figure S1 in the Supplementary Materials). While looking for the reasons for this situation, we found literature data revealing that this compound is not only thermally unstable, but also susceptible to the negative influence of air, water or bacteria [32,36]. Thus, the reasons for the absence of this compound in extracts can be attributed to the late harvest of walnut husks (late September and October). However, as mentioned, juglone has documented antibacterial, antiviral and antifungal properties. Taking into account the above and the fact that juglone has an -OH group in its structure with potential antioxidant properties (see Figure 3A), it was decided to check whether juglone also has antioxidant properties and whether its addition to the extracts changes their antioxidant properties.
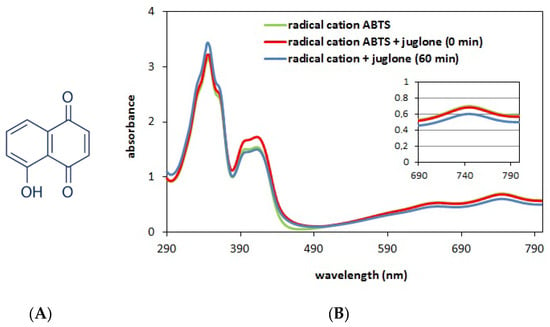
Figure 3.
Structural formula of juglone (A). Spectra of the cation radical solution (green curve, Ao = 0.7) and the solution containing the cation radical ABTS (2900 μL, A0 = 0.7) and juglone (100 μL, c = 0.2 mg/mL); red curve—spectrum obtained at 0 min of reaction (immediately after mixing the ingredients) and blue curve—spectrum obtained after 60 min of reaction (B).
In this series of studies, the antioxidant properties of juglone, methanol extracts (obtained in the Soxhlet apparatus and by maceration) and their binary mixtures were assessed using the ABTS method. Experiments were performed for two concentrations of juglone(c = 0.06 mg/mL and 0.2 mg/mL), which were mixed in different volume ratios with methanol extracts obtained from walnut husks. The concentrations of juglone selected for the experiment were based on literature data [37]. Since juglone solutions are characterized by intense color, it was first decided to check whether their addition would not affect the spectrum of the radical and disturb the measurement. For this purpose, the spectra of pure cationic radical (green curve) and solutions of cationic radical with juglone were compared (immediately after mixing—red curve and after 60 min of reaction—blue curve). The obtained results are presented in Figure 3B.
Analyzing the data presented in Figure 3B in the context of the position and intensity of the absorption bands before and after adding juglone to the cationic radical solution, it can be concluded that the position of the bands in the absorption spectrum of cationic radicals does not change. As for changes in the bands’ intensity, these are observed in the range from 290 to 450 nm. However, this range is not important for antioxidant tests. This occurs at a wavelength of 744 nm, but even here, after adding juglone (red curve), there are no changes in the assessed feature. Therefore, it can be concluded that the addition of colored juglone solution does not affect the quality of the measurement. However, after a 60 min reaction of this compound with a cationic radical, the intensity decreases slightly (blue curve). This observation indicates very low antioxidant properties of juglone (high concentration of juglone and a small decrease in the band at 744 nm). This is also confirmed by the results presented in Figure 4.
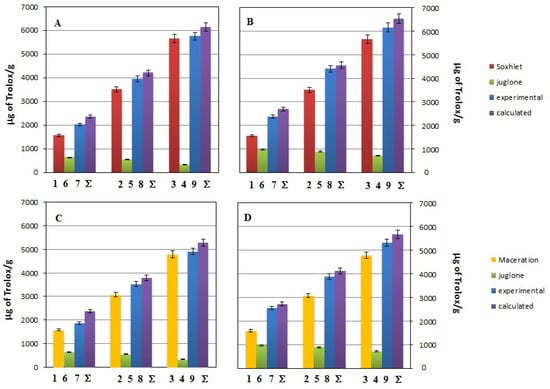
Figure 4.
Changes of the antioxidant activity estimated by the ABTS assay for systems containing different volumes of methanolic Soxhlet extract (red bars) or methanolic macerate (yellow bars), and juglone solution (green bars) at concentration of 0.06 mg/mL (data in (A,C)) or 0.2 mg/mL (data in (B,D)). Violet bars labeled as “Σ” correspond to expected values of antioxidant activity for given system. The bar numbers correspond to the samples’ numbers from Table 2.
Figure 4 shows the antioxidant activity, expressed as the percentage of ABTS cation radical inhibition, for methanolic extracts obtained by Soxhlet (red bars) or maceration (yellow bars) and juglone solutions (green bars), and for binary mixtures composed of the extract from the Soxhlet and juglone or the macerate and juglone (blue bars) differing in the amount of individual components in the measuring system and juglone concentration (0.06 mg/mL for data in Figure 4A,C or 0.2 mg/mL for data in Figure 4B,D). The bar numbers correspond to the samples’ numbers from Table 1:
- bar number 7 reflects the antioxidant activity of sample 7 from Table 1 composed of 20 µL of methanolic extract (obtained by extraction in Soxhlet or by maceration, Figure 4A,B or Figure 4C,D, respectively) and 80 µL of juglone solution (initial concentration c = 0.02 mg/mL or c = 0.6 mg/mL, Figure 4A,C or Figure 4B,D, respectively).
In these experiments, all the samples introduced to the measuring systems were 100 µL. The set of bars in Figure 4 also contains the expected antioxidant activities constructed by adding up the experimental activity data for each examined antioxidant (violet bars labeled “Σ”), e.g., bar labeled Σ on Figure 4A represents the anticipated inhibition percent of the mixture containing 20 µL of extract obtained by extraction in Soxhlet and 80 µL of juglone solution (initial concentration 0.02 mg/mL) assuming antioxidant effect additivity. For clarification, for this mixture, the calculated inhibition percentage is 28.71%, as shown by the bar marked Σ. This value was obtained after summing the % inhibition determined in systems containing the following: 20 µL of the extract obtained using the Soxhlet extraction method, i.e., 19.62% (value for bar no. 1) and 80 µL of juglone solution (initial concentration 0.02 mg/mL), i.e., 9.09% (value for bar no. 6). Whereas “bar no. 7” is the experimental value (Ie), the actual value obtained for a mixture containing 20 µL of the extract obtained by Soxhlet extraction and 80 µL of juglone solution (initial concentration 0.02 mg/mL) is 24.96%.
Comparing the data presented by the green bars in Figure 4A,B for juglone concentrations of 0.06 mg/mL and 0.2 mg/mL, respectively, it can be concluded that a more than threefold increase in concentration does not translate toa threefold increase in antioxidant properties. It seems that the -OH group in the molecule is somehow blocked by the quinone group and hence results invery low antioxidant properties. However, it seems more important to compare the % inhibition obtained for binary mixtures (experimental value, blue bars) with the % inhibition, which is the sum of the % inhibition of the components forming a given mixture (violet bars). Analysis of the results shows that the expected antioxidant properties of the binary mixture are in almost every case the same as determined experimentally. The significance of the difference between the experimental and expected antioxidant activity of the binary mixtures was estimated based on the F and p values given in Table 3.

Table 3.
Statistical significance (F and p values) of the difference between the experimental inhibition percent (Ie) and calculated inhibition percent (Ic) for the binary mixtures of juglone and walnut husk methanolic extract at three different volume ratios and with the difference (Ie-Ic) and with the observed effect and (the resultant) antioxidant effect of the antioxidants in the mixture (Fcrit = 7.71).
The data in the table present the results of the statistical analysis obtained for the differences between the experimentally determined inhibition percentage (Ie) and the theoretical inhibition percentages (Ic), and the sum of the percentage inhibition determined for individual antioxidant systems. The table contains data obtained for % inhibition determined at various volume ratios of antioxidants in their mixture. In the interpretation of the results, aimed at determining the net antioxidant effect of the mixture, it was assumed that the lack of a significant difference between the Ie and Ic values indicates the additive antioxidant effect of the mixture containing a given extract and juglone (Fcal < Fcrit). In turn, statistically significant differences (Fcal > Fcrit) indicate an antagonistic effect at a negative value of the difference between Ie and Ic.
According to the data presented in Table 3, juglone only in a few cases significantly affects the antioxidant properties of the tested extracts, causing an antagonistic antioxidant effect of the mixture. Fcrit < Fcal is observed only in systems where juglone is added in the form of 80 μL portions. In other cases, an additive effect of the mixture is observed, which proves that the components of the mixture are neutral towards each other in terms of antioxidant activity.
4. Conclusions
This paper presents and discusses the antioxidant properties of extracts obtained from walnut husks as well as the influence of juglone on the properties. The obtained results indicate the following:
- the examined extracts exhibit antioxidant properties, and their value depends not only on the technique used to obtain the extract, but also on the type of extracting solvent. In this context, the highest antioxidant properties demonstrate extracts obtained using PLE. In turn, methanol turned out to be the best solvent for the isolation of active compounds;
- juglone is characterized by very weak antioxidant properties;
- the antioxidant effect of the mixture containing walnut husks, methanolic extract and juglone solution is mostly additive. Antagonism is observed in the systems with high juglone content.
In light of the results presented in this work, due to the rich content of phenols and strong antioxidant activity, walnut husks area good candidate for use by many industries, including the food, nutraceutical, cosmetic and pharmaceutical industries. Moreover, in order to increase the ecological and economic values of the proposed waste disposal, the PLE technique, considered as a green extraction technique, should be used.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app14072972/s1, Figure S1: Exemplary chromatograms of juglone standard (green color) and methanolic extracts obtained in the Soxhlet apparatus before concentration (blue color) and after concentrations (red color). Table S1. Equations of Trolox calibration curves in the concentration range of 2.5–25 µg/mL obtained using various methods for assessing antioxidant and solvents; Table S2. Equations of gallic acid calibration curves in the concentration range of 2.5–500 µg/mL obtained using various solvents; Table S3. F values and p values obtained during variance analysis for the data concerning total phenolics amount, expressed in extraction in the Soxhlet apparatus, maceration and PLE using different extractants (chloroform, acetone, ethyl acetate and methanol). Data from Table 2 Bold values indicate systems where the results are statistically insignificant (F < Ftab, p > 0.05; Ftab = 7.71).
Author Contributions
Conceptualization and methodology, M.O.-T.; writing—original draft preparation, M.O.-T.; writing—review and editing, D.W.; supervision, D.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Acknowledgments
The authors would like to thank the Institute of Chemical Sciences of the Maria Curie-Skłodowska University in Lublin for creating the research infrastructure, without which this research would not be possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- de Rigo, D.; Enescu, C.M.; Houston Durrant, T.; Tinner, W.; Caudullo, G. Juglans regia in Europe: Distribution, habitat, usage and threats. In European Atlas of Forest Tree Species; San-Miguel-Ayanz, J., de Rigo, D., Caudullo, G., Houston Durrant, T., Mauri, A., Eds.; Publications Office of the EU: Luxembourg, 2016; p. e01977c. [Google Scholar]
- Gauthier, M.M.; Jacobs, D.F. Walnut (Juglans spp.) ecophysiology in response to environmental stresses and potential acclimation to climate change. Ann. For. Sci. 2011, 68, 1277–1290. [Google Scholar] [CrossRef]
- Ozcan, M.M. Some nutritional characteristics of fruit and oil of walnut (Juglans regia L.) growing in Turkey. Iran. J. Chem. Chem. Eng. 2009, 28, 57–62. [Google Scholar] [CrossRef]
- Pei, Q.; Liu, Y.; Peng, S. Fatty acid profiling in kernels coupled with chemometric analyses as a feasible strategy for the discrimination of different walnuts. Foods 2022, 11, 500. [Google Scholar] [CrossRef] [PubMed]
- World Walnut Production by Country. Available online: https://www.atlasbig.com/pl/kraje-wedlug-produkcji-orzecha-wloskiego (accessed on 10 March 2024).
- Romano, R.; Aiello, A.; Meca, G.; De Luca, L.; Pizzolongo, F. Recovery of bioactive compounds from walnut (Juglans regia L.) green husk by supercritical carbon dioxide extraction. Int. J. Food Sci. Technol. 2021, 56, 4658–4669. [Google Scholar] [CrossRef]
- Czubaszek, R.; Wysocka-Czubaszek, A.; Tyborowski, R. Methane production potential from apple pomace, cabbage, leaves, pumpkin residue and walnut husks. Appl. Sci. 2022, 12, 6128. [Google Scholar] [CrossRef]
- Arasoglu, T.; Mansuroglu, B.; Derman, S.; Gumus, B.; Kocyigit, B.; Acar, T.; Kocacaliskan, I. Enhancement of antifungal activity of juglone (5-Hydroxy-1,4-naphthoquinone) using a poly(d,l-lactic-co-glycolic acid) (PLGA) nanoparticle system. J. Agric. Food Chem. 2016, 64, 7087–7094. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Garbaczewska, S.; Cieniecka-Roslonkiewicz, A.; Dawidowicz, A.L.; Typek, R.; Kielczewska, A. Influence of the extraction conditions on the antifungal properties of walnut green husk isolates. Anal. Lett. 2020, 53, 1970–1981. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Estanqueiro, M.; Oliveira, M.B.; Sousa Lobo, J.M. Main benefits and applicability of plant extracts in skin care products. Cosmetics 2015, 2, 48–65. [Google Scholar] [CrossRef]
- Fordos, S.; Abid, N.; Gulzar, M.; Pasha, I.; Oz, F.; Shahid, A.; Khan, M.K.I.; Khaneghah, A.M.; Aadil, R.M. Recent development in the application of walnut processing by-products (walnut shell and walnut husk). Biomass Convers. Biorefinery 2023, 13, 14389–14411. [Google Scholar] [CrossRef]
- Das, O.; Babu, K.; Shanmugam, V.; Sykam, K.; Tebyetekerwa, M.; Neisiany, R.E.; Försth, M.; Sas, G.; Gonzalez-Libreros, J.; Capezza, A.J.; et al. Natural and industrial wastes for sustainable and renewable polymer composites. Renew. Sustain. Energy Rev. 2022, 158, 112054. [Google Scholar] [CrossRef]
- Pomoni, D.I.; Koukou, M.K.; Vrachopoulos, M.G.; Vasiliadis, L. Circular economy: A multilevel approach for natural resources and wastes under an agri-food perspective. Water-Energy Nexus 2024, 7, 103–123. [Google Scholar] [CrossRef]
- de Marco, B.A.; Saú Rechelo, B.; Tótoli, E.G.; Kogawa, A.C.; Salgado, H.R.N. Evolution of green chemistry and its multidimensional impacts: A review. Saudi Pharm. J. 2019, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.N.; Zhang, D.; Miller, S.J.; Rossen, K.; Chirik, P.J.; Kozlowski, M.C.; Zimmerman, J.B.; Brooks, B.W.; Savage, P.E.; Allen, D.T.; et al. Green Chemistry: A Framework for a Sustainable Future. Org. Process. Res. Dev. 2021, 25, 1455–1459. [Google Scholar] [CrossRef]
- Strugstad, M.P.; Despotovski, S. A summary of extraction, synthesis, properties, and potential uses of juglone: A literature review. J. Ecosyst. Manag. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in Oxidative Stress and Cell Signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Typek, R.; Dawidowicz, A.L. Chlorogenic acid stability in pressurized liquid extraction conditions. J. AOAC Int. 2015, 98, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Wianowska, D.; Olszowy-Tomczyk, M.; Garbaczewska, S. A Central Composite Design in increasing the quercetin content in the aqueous onion waste isolates with antifungal and antioxidant properties. Eur. Food Res. Technol. 2022, 248, 497. [Google Scholar] [CrossRef]
- Olszowy-Tomczyk, M.; Garbaczewska, S.; Wianowska, D. Correlation study of biological activity with quercetin and phenolics content in onion extracts. Molecules 2022, 27, 8164. [Google Scholar] [CrossRef] [PubMed]
- Hussen, E.M.; Endalew, S.A. In vitro antioxidant and free-radical scavenging activities of polar leaf extracts of Vernonia amygdalina. BMC Complement Med. Ther. 2023, 23, 146. [Google Scholar] [CrossRef] [PubMed]
- Garcia, E.J.; Oldoni, T.; de Alencar, S.M.; Reis, A.; Loguercio, A.D.; Grande, R.H.M. Antioxidant activity by DPPH assay of potential solutions to be applied on bleached teeth. Braz. Dent. J. 2012, 23, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, M.; Kapusta, K.; Kołodziejczyk, W.; Saloni, J.; Zbikowska, B.; Hill, G.A.; Sroka, Z. Antioxidant activity of selected phenolic acids–Ferric Reducing Antioxidant Power Assay and QSAR analysis of the structural features. Molecules 2020, 25, 3088. [Google Scholar] [CrossRef] [PubMed]
- Özyürek, M.; Güçlü, K.; Tütem, E.; Başkan, K.S.; Erçağ, E.; Çelik, S.E.; Baki, S.; Yildis, L.; Karaman, S.; Apak, R. A comprehensive review of CUPRAC methodology. Anal. Methods 2011, 3, 2439–2453. [Google Scholar] [CrossRef]
- El-Zahar, K.M.; Al-Jamaan, M.E.; Al-Mutairi, F.R.; Al-Hudiab, A.M.; Al-Einzi, M.S.; Mohamed, A.A.Z. Antioxidant, Antibacterial, and Antifungal Activities of the Ethanolic Extract Obtained from Berberis vulgaris Roots and Leaves. Molecules 2022, 27, 6114. [Google Scholar] [CrossRef] [PubMed]
- Choma, I.M.; Olszowy, M.; Studziński, M.; Gnat, S. Determination of chlorogenic acid, polyphenols and antioxidants in green coffee by thin-layer chromatography, effect-directed analysis and dot blot—Comparison to HPLC and spectrophotometry methods. J. Sep. Sci. 2019, 42, 1542–1549. [Google Scholar] [CrossRef] [PubMed]
- Ghazzawi, H.A.; Al-Sayyed, H.F.; Al-Kurd, R.A.; Mwalla, M.M.; Arafat, T.A.; AbdelQader, S.M. Effect of different extraction solvents on the antioxidant content and capacity of nine seasonal fruits. Clin. Nutr. Open Sci. 2021, 38, 33–42. [Google Scholar] [CrossRef]
- Barp, L.; Višnjevec, A.M.; Moret, S. Pressurized Liquid Extraction: A powerful tool to implement extraction and purification of food contaminants. Foods 2023, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, M.C.; Orellana Palacios, J.C.; Hesami, G.; Jafarzadeh, S.; Lorenzo, J.M.; Domínguez, R.; Moreno, A.; Hadidi, M. Spectrophotometric methods for measurement of antioxidant activity in food and pharmaceuticals. Antioxidants 2022, 11, 2213. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Olszowy, M.; Jóźwik-Dolęba, M. Importance of solvent association in the estimation of antioxidant properties of phenolic compounds by DPPH method. Food Sci. Technol. 2015, 52, 4523–4529. [Google Scholar] [CrossRef] [PubMed]
- Kotha, R.R.; Tareq, F.S.; Yildiz, E.; Luthria, D.L. Oxidative stress and antioxidants—A critical review on in vitro antioxidant assays. Antioxidants 2022, 1, 2388. [Google Scholar] [CrossRef] [PubMed]
- Masek, A.; Latos-Brozio, M.; Chrzescijanska, E.; Podsedek, A. Polyphenolic profile and antioxidant activity of Juglans regia L. Leaves and husk extracts. Forests 2019, 10, 988. [Google Scholar] [CrossRef]
- Shcherbakov, V.V.; Artemkina, Y.M.; Akimova, I.A.; Artemkina, I.M. Dielectric characteristics, electrical conductivity and solvation of ions in electrolyte solutions. Materials 2021, 14, 5617. [Google Scholar] [CrossRef]
- Ahmad, I.; Sabah, A.; Anwar, Z.; Arif, A.; Arslan, A.; Qadeer, K. Effect of solvent polarity on the extraction of components of pharmaceutical plastic containers. Pak. J. Pharm. Sci. 2017, 30, 247–252. [Google Scholar] [PubMed]
- Waghorne, W.E. A study of Kamlet–Taft β and π* scales of solvent basicity and polarity/polarizability using computationally derived molecular properties. J. Solut. Chem. 2020, 49, 466–485. [Google Scholar] [CrossRef]
- Islam, M.A.K.M.; Widhalm, J.R. Agricultural uses of juglone: Opportunities and challenges. Agronomy 2020, 10, 1500. [Google Scholar] [CrossRef]
- Shi, B.; Zhang, W.; Li, X.; Pan, X. Seasonal variation of phenolic profiles and antioxidant activity of walnut (Juglans sigillata Dode) green husks. Int. J. Food Prop. 2017, 20, 2635–2646. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).