Antioxidant and Anti-Inflammatory Effects of Agarum cribrosum Extract and Its Fractions in LPS-Induced RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Preparation of the Extraction and Fractions
2.3. Total Phenolic Content
2.4. DPPH Radical Scavenging Activity
2.5. ABTS Radical Scavenging Assay
2.6. Ferric-Reducing Antioxidant Power Assay
2.7. Cell Culture
2.8. Cell Viability
2.9. Quantitative Real-Time PCR (qRT-PCR)
2.10. Western Blot Analysis
2.11. Statistical Analysis
3. Results
3.1. Extraction Yield and TPC
3.2. Total Antioxidant Capacity
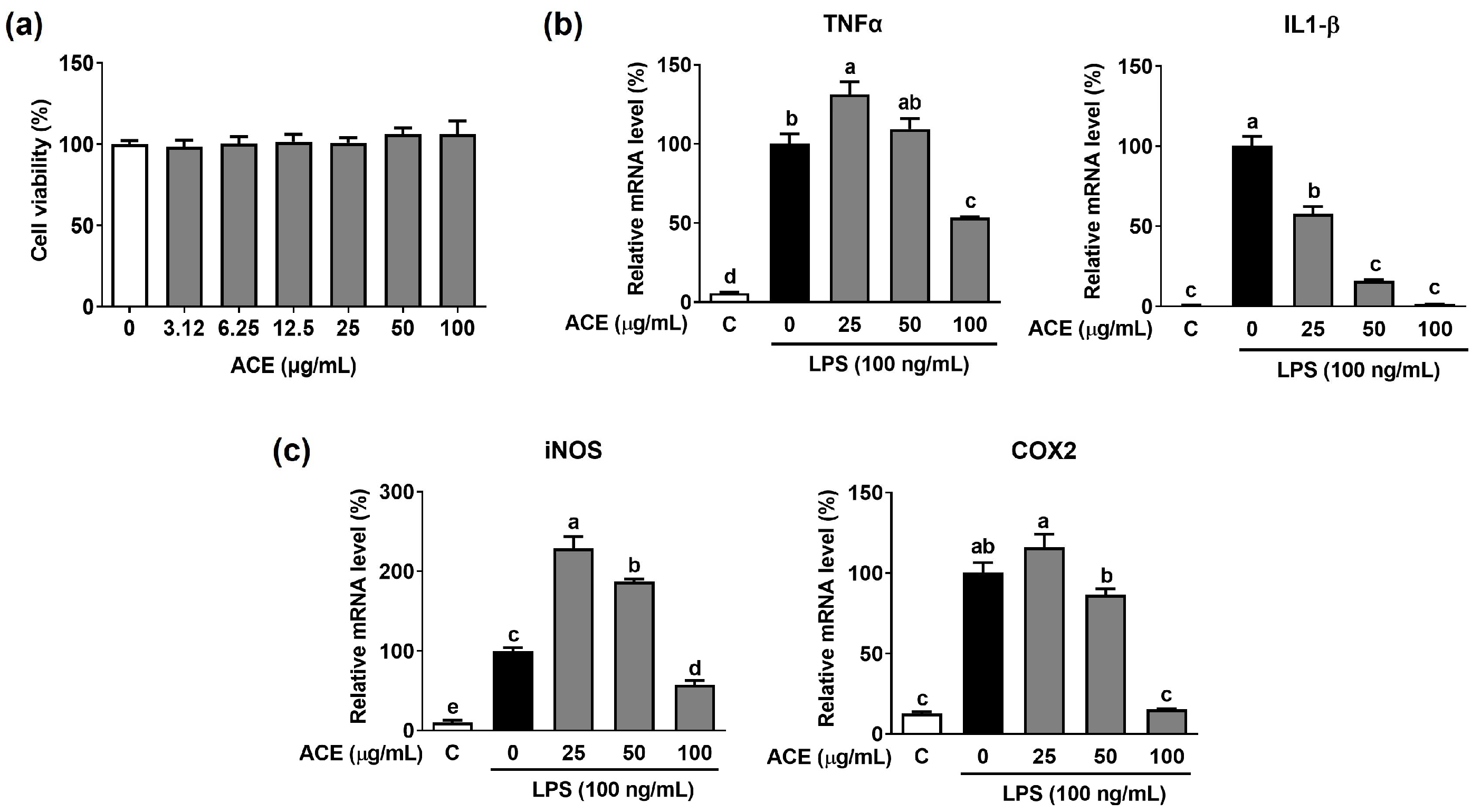
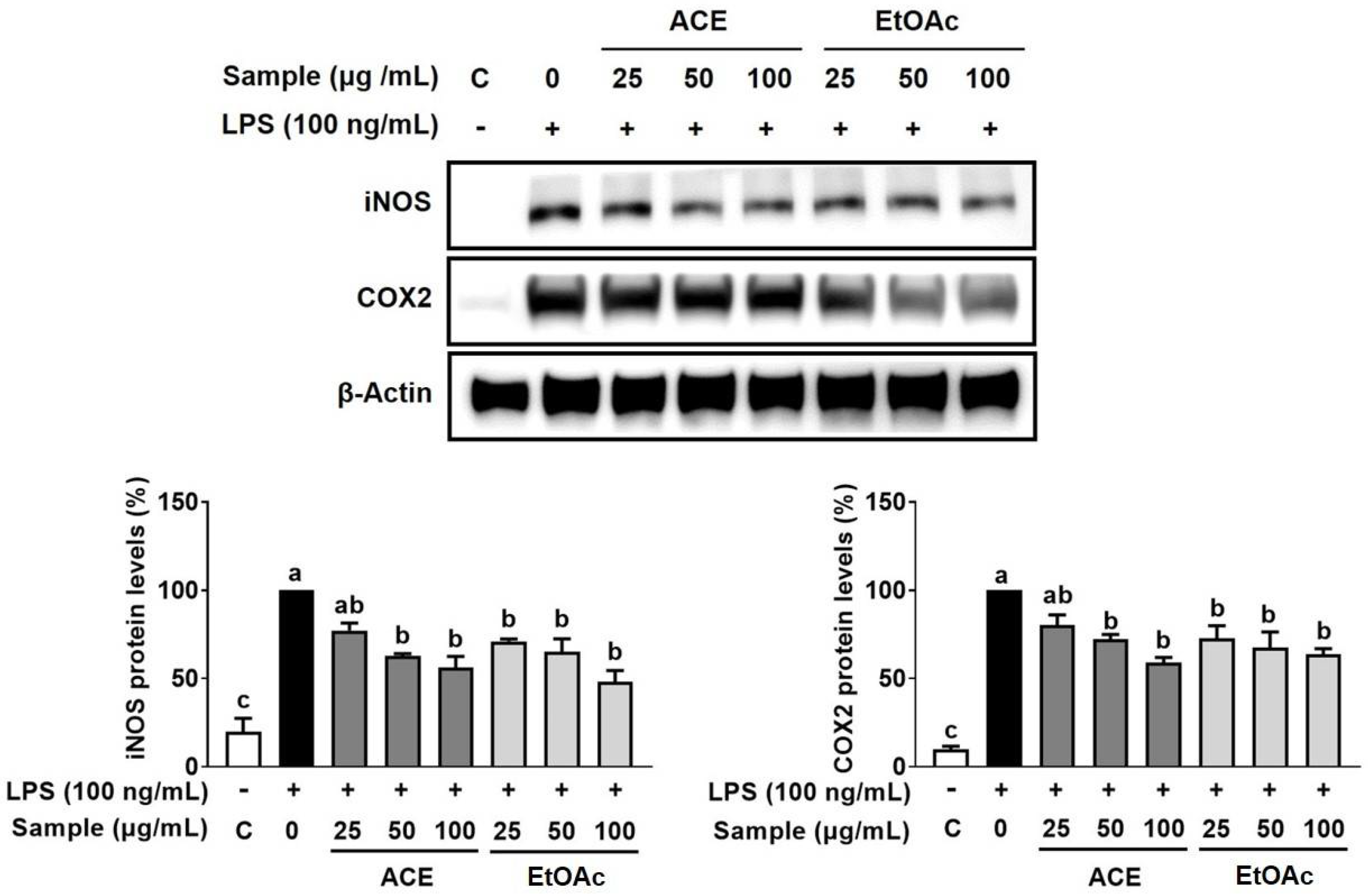
3.3. Inhibitory Effect of ACE on LPS-Stimulated Inflammation in Macrophages
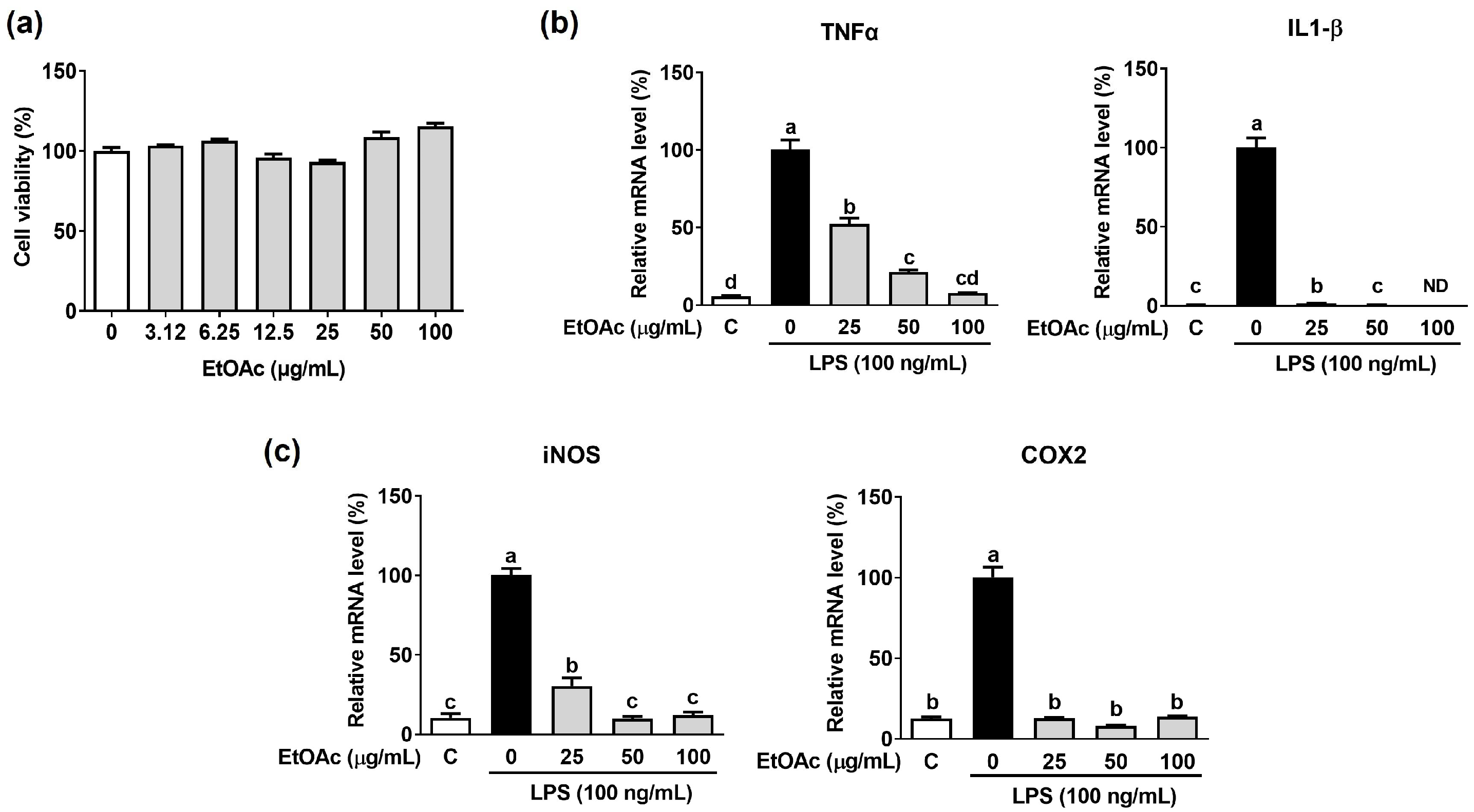
3.4. Effect of EtOAc Fraction from ACE on LPS-Induced Inflammation in Macrophages
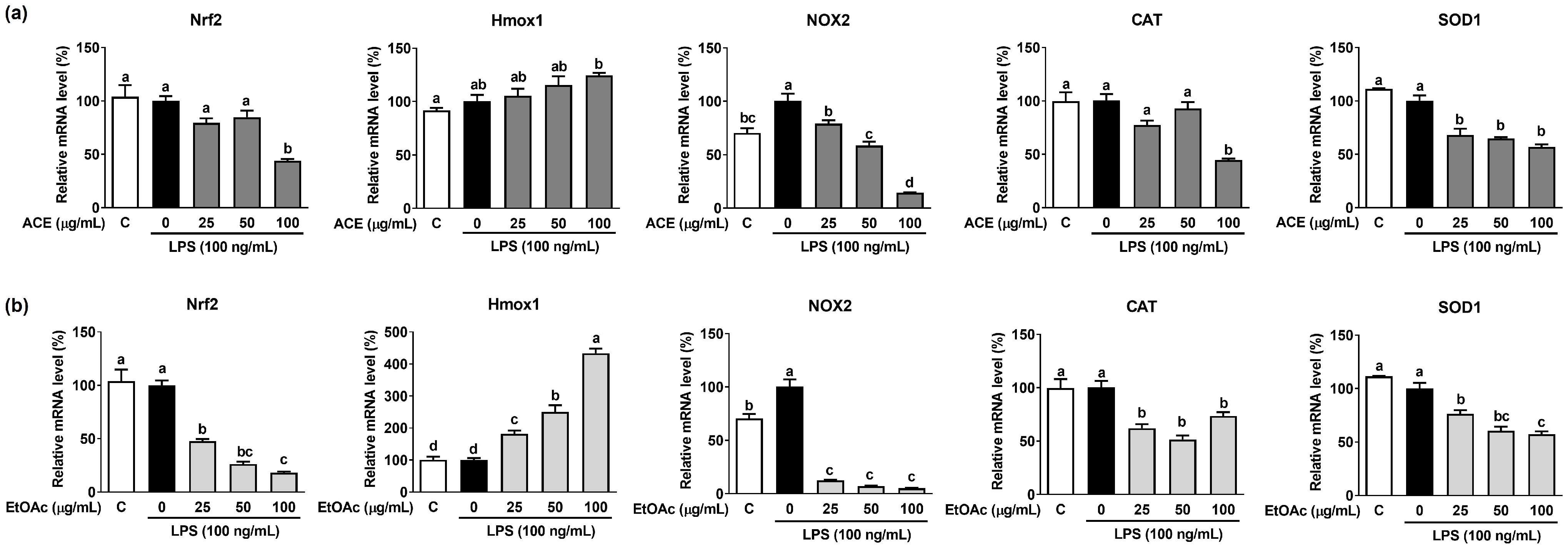
3.5. Effects of ACE and Its EtOAc Fraction on LPS-Induced Antioxidant Gene Expression in Macrophages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, inflammation, and oxidative stress: An integrative view in metabolism. Oxid. Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Qian, J.; Li, B.; Lu, M.; Le, G.; Xie, Y. Metabolomics based on 1H-NMR reveal the regulatory mechanisms of dietary methionine restriction on splenic metabolic dysfunction in obese mice. Foods 2021, 10, 2439. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-B.; Kang, H.; Li, Y.; Park, Y.-K.; Lee, J.-Y. Fucoxanthin inhibits lipopolysaccharide-induced inflammation and oxidative stress by activating nuclear factor E2-related factor 2 via the phosphatidylinositol 3-kinase/AKT pathway in macrophages. Eur. J. Nutr. 2021, 60, 3315–3324. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ren, Y.; Chang, K.; Wu, W.; Griffiths, H.R.; Lu, S.; Gao, D. Adipose tissue macrophages as potential targets for obesity and metabolic diseases. Front. Immunol. 2023, 14, 1153915. [Google Scholar] [CrossRef] [PubMed]
- Rendra, E.; Riabov, V.; Mossel, D.M.; Sevastyanova, T.; Harmsen, M.C.; Kzhyshkowska, J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019, 224, 242–253. [Google Scholar] [CrossRef]
- Chai, J.; Luo, L.; Hou, F.; Fan, X.; Yu, J.; Ma, W.; Tang, W.; Yang, X.; Zhu, J.; Kang, W. Agmatine reduces lipopolysaccharide-mediated oxidant response via activating PI3K/Akt pathway and up-regulating Nrf2 and HO-1 expression in macrophages. PLoS ONE 2016, 11, e0163634. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M. An overview of potential seaweed-derived bioactive compounds for pharmaceutical applications. Mar. Drugs 2022, 20, 141. [Google Scholar] [CrossRef]
- Begum, R.; Howlader, S.; Mamun-Or-Rashid, A.; Rafiquzzaman, S.; Ashraf, G.M.; Albadrani, G.M.; Sayed, A.A.; Peluso, I.; Abdel-Daim, M.M.; Uddin, M.S. Antioxidant and signal-modulating effects of brown seaweed-derived compounds against oxidative stress-associated pathology. Oxid. Med. Cell. Longev. 2021, 2021, 9974890. [Google Scholar] [CrossRef]
- Ismail, M.M.; El Zokm, G.M.; Lopez, J.M.M. Nutritional, bioactive compounds content, and antioxidant activity of brown seaweeds from the Red Sea. Front. Nutr. 2023, 10, 1210934. [Google Scholar] [CrossRef]
- Dutot, M.; Olivier, E.; Fouyet, S.; Magny, R.; Hammad, K.; Roulland, E.; Rat, P.; Fagon, R. In Vitro chemopreventive potential of phlorotannins-rich extract from brown algae by inhibition of benzo [a] pyrene-induced P2x7 activation and toxic effects. Mar. Drugs 2021, 19, 34. [Google Scholar] [CrossRef]
- Lomartire, S.; Gonçalves, A.M. Marine Macroalgae Polyphenols as Potential Neuroprotective Antioxidants in Neurodegenerative Diseases. Mar. Drugs 2023, 21, 261. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.-K.; Lee, B.; Kwon, M.; Yoon, N.; Shin, T.; Kim, N.-G.; Choi, J.-S.; Kim, H.-R. Phlorofucofuroeckol B suppresses inflammatory responses by down-regulating nuclear factor κB activation via Akt, ERK, and JNK in LPS-stimulated microglial cells. Int. Immunopharmacol. 2015, 28, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, Y.; Katsuzaki, H.; Imai, K.; Amano, H. The anti-allergic and anti-inflammatory effects of phlorotannins from the edible brown algae, Ecklonia sp. and Eisenia sp. Nat. Prod. Commun. 2021, 16, 1934578X211060924. [Google Scholar]
- Boo, G.H.; Lindstrom, S.C.; Klochkova, N.G.; Yotsukura, N.; Yang, E.C.; Kim, H.G.; Waaland, J.R.; Cho, G.Y.; Miller, K.A.; Boo, S.M. Taxonomy and biogeography of Agarum and Thalassiophyllum (Laminariales, Phaeophyceae) based on sequences of nuclear, mitochondrial, and plastid markers. Taxon 2011, 60, 831–840. [Google Scholar] [CrossRef]
- Phasanasophon, K.; Kim, S.M. Antioxidant and cosmeceutical activities of Agarum cribrosum phlorotannin extracted by ultrasound treatment. Nat. Prod. Commun. 2018, 13, 1934578X1801300513. [Google Scholar] [CrossRef]
- Phasanasophon, K.; Kim, S.M. Anti-inflammatory activity of the phlorotannin trifuhalol A using LPS-stimulated RAW264. 7 cells through NF-κB and MAPK main signaling pathways. Nat. Prod. Commun. 2019, 14, 1934578X19849798. [Google Scholar]
- Baek, S.H.; Cao, L.; Jeong, S.J.; Kim, H.-R.; Nam, T.J.; Lee, S.G. The comparison of total phenolics, total antioxidant, and anti-tyrosinase activities of Korean Sargassum species. J. Food Qual. 2021, 2021, 6640789. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Ma, L.; Li, J.; Shahzad, N.; Kim, C.K. Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids. Sci. Rep. 2020, 10, 2611. [Google Scholar] [CrossRef]
- Mao, X.-Y.; Cheng, X.; Wang, X.; Wu, S.-J. Free-radical-scavenging and anti-inflammatory effect of yak milk casein before and after enzymatic hydrolysis. Food Chem. 2011, 126, 484–490. [Google Scholar] [CrossRef]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- Cotas, J.; Leandro, A.; Monteiro, P.; Pacheco, D.; Figueirinha, A.; Gonçalves, A.M.; da Silva, G.J.; Pereira, L. Seaweed phenolics: From extraction to applications. Mar. Drugs 2020, 18, 384. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.L.; Lee, D.-J.; Lee, H.-S.; Lee, Y.-J.; You, S.G. LPS-induced NO inhibition and antioxidant activities of ethanol extracts and their solvent partitioned fractions from four brown seaweeds. Ocean Sci. J. 2013, 48, 349–359. [Google Scholar] [CrossRef]
- Zheng, H.; Zhao, Y.; Guo, L. A bioactive substance derived from brown seaweeds: Phlorotannins. Mar. Drugs 2022, 20, 742. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A review of extraction methods, structural characteristics, bioactivities, bioavailability, and future trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Phang, S.J.; Teh, H.X.; Looi, M.L.; Arumugam, B.; Fauzi, M.B.; Kuppusamy, U.R. Phlorotannins from brown algae: A review on their antioxidant mechanisms and applications in oxidative stress-mediated diseases. J. Appl. Phycol. 2023, 35, 867–892. [Google Scholar] [CrossRef]
- Wang, T.; Jónsdóttir, R.S.; Liu, H.; Gu, L.; Kristinsson, H.G.; Raghavan, S.; Ólafsdóttir, G.N. Antioxidant capacities of phlorotannins extracted from the brown algae Fucus vesiculosus. J. Agric. Food Chem. 2012, 60, 5874–5883. [Google Scholar] [CrossRef]
- Zou, Y.; Qian, Z.-J.; Li, Y.; Kim, M.-M.; Lee, S.-H.; Kim, S.-K. Antioxidant effects of phlorotannins isolated from Ishige okamurae in free radical mediated oxidative systems. J. Agric. Food Chem. 2008, 56, 7001–7009. [Google Scholar] [CrossRef]
- Pradhan, B.; Ki, J.S. Antioxidant and chemotherapeutic efficacies of seaweed-derived phlorotannins in cancer treatment: A review regarding novel anticancer drugs. Phytother. Res. 2023, 37, 2067–2091. [Google Scholar] [CrossRef]
- Gorabi, A.M.; Kiaie, N.; Khosrojerdi, A.; Jamialahmadi, T.; Al-Rasadi, K.; Johnston, T.P.; Sahebkar, A. Implications for the role of lipopolysaccharide in the development of atherosclerosis. Trends Cardiovasc. Med. 2022, 32, 525–533. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef]
- Shiau, D.-J.; Kuo, W.-T.; Davuluri, G.V.N.; Shieh, C.-C.; Tsai, P.-J.; Chen, C.-C.; Lin, Y.-S.; Wu, Y.-Z.; Hsiao, Y.-P.; Chang, C.-P. Hepatocellular carcinoma-derived high mobility group box 1 triggers M2 macrophage polarization via a TLR2/NOX2/autophagy axis. Sci. Rep. 2020, 10, 13582. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-J.; Hyun, C.-G. Acenocoumarol Exerts Anti-Inflammatory Activity via the Suppression of NF-κB and MAPK Pathways in RAW 264.7 Cells. Molecules 2023, 28, 2075. [Google Scholar] [CrossRef] [PubMed]
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The nitration of proteins, lipids and DNA by peroxynitrite derivatives-chemistry involved and biological relevance. Stresses 2022, 2, 53–64. [Google Scholar] [CrossRef]
| Extract/Fractions | Extraction Yield | TPC (mg PGE/g) | ABTS (mg VCE/g) | DPPH (mg VCE/g) | FRAP (mM FSE/g) | |
|---|---|---|---|---|---|---|
| % (w/w) | g | |||||
| ACE | 48.57 | 44.00 | 144.62 ± 10.33 c | 744.48 ± 9.62 c | 18.70 ± 0.24 b | 0.26 ± 0.01 b |
| Hex | 0.67 | 0.20 | 17.04 ± 0.37 e | 9.38 ± 0.78 e | 1.97 ± 0.19 e | 0.02 ± 0.00 d |
| CHCl3 | 2.00 | 0.60 | 47.80 ± 3.10 d | 110.48 ± 6.60 d | 5.02 ± 0.08 d | 0.09 ± 0.02 c |
| EtOAc | 3.00 | 0.90 | 320.97 ± 3.41 a | 1144.48 ± 25.76 a | 37.63 ± 0.93 a | 0.4a1 ± 0.05 a |
| BuOH | 6.66 | 2.00 | 157.10 ± 2.23 c | 763.33 ± 39.01 c | 19.27 ± 0.44 b | 0.25 ± 0.01 b |
| H2O | 59.29 | 17.80 | 173.66 ± 2.69 b | 822.86 ± 27.67 b | 15.57 ± 0.80 c | 0.29 ± 0.01 b |
| Antioxidant Activity | TPC | ABTS Assay | DPPH Assay | FRAP Assay |
|---|---|---|---|---|
| TPC | 1 | 0.94 (p < 0.01) | 0.98 (p < 0.01) | 0.96 (p < 0.01) |
| ABTS assay | 1 | 0.90 (p < 0.01) | 0.97 (p < 0.01) | |
| DPPH assay | 1 | 0.92 (p < 0.01) | ||
| FRAP assay | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-B.; Lee, H.; Vance, T.; Lee, S.G. Antioxidant and Anti-Inflammatory Effects of Agarum cribrosum Extract and Its Fractions in LPS-Induced RAW 264.7 Macrophages. Appl. Sci. 2023, 13, 10048. https://doi.org/10.3390/app131810048
Kim M-B, Lee H, Vance T, Lee SG. Antioxidant and Anti-Inflammatory Effects of Agarum cribrosum Extract and Its Fractions in LPS-Induced RAW 264.7 Macrophages. Applied Sciences. 2023; 13(18):10048. https://doi.org/10.3390/app131810048
Chicago/Turabian StyleKim, Mi-Bo, Hyeju Lee, Terrence Vance, and Sang Gil Lee. 2023. "Antioxidant and Anti-Inflammatory Effects of Agarum cribrosum Extract and Its Fractions in LPS-Induced RAW 264.7 Macrophages" Applied Sciences 13, no. 18: 10048. https://doi.org/10.3390/app131810048
APA StyleKim, M.-B., Lee, H., Vance, T., & Lee, S. G. (2023). Antioxidant and Anti-Inflammatory Effects of Agarum cribrosum Extract and Its Fractions in LPS-Induced RAW 264.7 Macrophages. Applied Sciences, 13(18), 10048. https://doi.org/10.3390/app131810048










