Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Seed Oil Extraction
2.3. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
2.4. Measurement of Radical Scavenging Activity
2.5. Molecular Docking
2.6. Molecular Property and Bioactivity Prediction
2.7. Statistical Analysis
3. Results and Discussion
3.1. Extraction of The Seed Oil of Prunus mahaleb L. Using Different Solvents
3.2. Composition of the Seed Oil
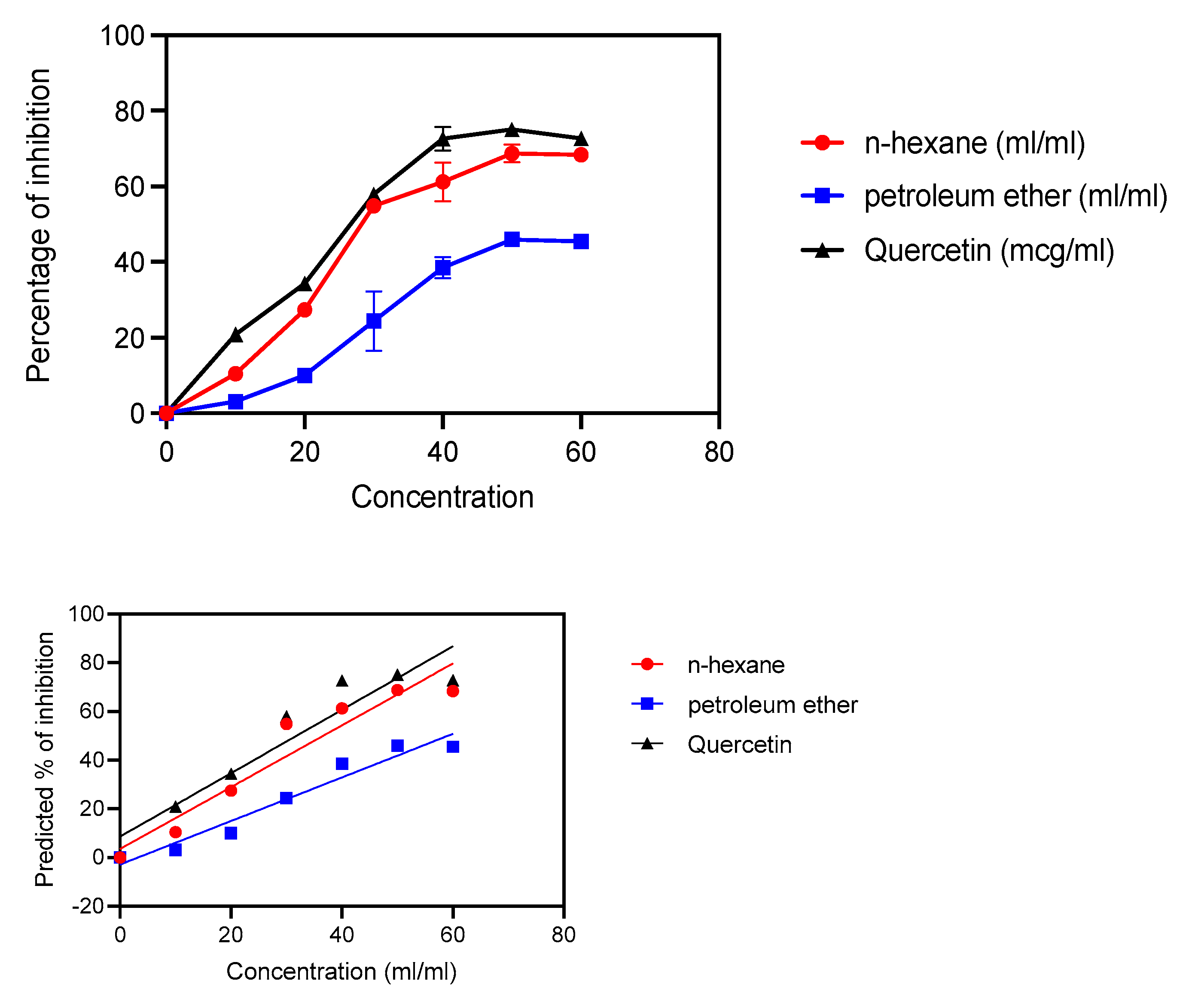
3.3. In Vitro Antioxidant Activity (DPPH Free Radical Scavenging Activity)
3.4. In Silico Investigations (Molecular Docking)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive oxygen species—Sources, functions, oxidative damage. Pol. Merkur. Lek. 2020, 48, 124–127. [Google Scholar]
- Li, R.; Jia, Z.; Trush, M.A. Defining ROS in Biology and Medicine. React. Oxyg. Species 2016, 1, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Ray, P.D.; Huang, B.W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS Generation and Antioxidant Defense Systems in Normal and Malignant Cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irazabal, M.V.; Torres, V.E. Reactive oxygen species and redox signaling in chronic kidney disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Lian, G. ROS and diseases: Role in metabolism and energy supply. Mol. Cell. Biochem. 2019, 467, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Alfadda, A.A.; Sallam, R.M. Reactive Oxygen Species in Health and Disease. J. Biomed. Biotechnol. 2012, 2012, 936486. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Physiol. 2018, 9, 477. [Google Scholar] [CrossRef] [Green Version]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Halliwell, B. (Ed.) How to characterize an antioxidant: An update. In Biochemical Society Symposium; Portland Press: South Portland, UK, 1995. [Google Scholar]
- Shi, H.; Noguchi, N.; Niki, E. Comparative study on dynamics of antioxidative action of α-tocopheryl hydroquinone, ubiquinol, and α-tocopherol against lipid peroxidation. Free. Radic. Biol. Med. 1999, 27, 334–346. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ROS) and response of antioxidants as ROS-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Tavassolifar, M.J.; Vodjgani, M.; Salehi, Z.; Izad, M. The Influence of Reactive Oxygen Species in the Immune System and Pathogenesis of Multiple Sclerosis. Autoimmune Dis. 2020, 2020, 5793817. [Google Scholar] [CrossRef] [PubMed]
- Chun, O.K.; Kim, D.-O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, S.C.; Moldão-Martins, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sources to Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikora, E.; Cieślik, E.; Topolska, K. The sources of natural antioxidants. Acta Sci. Pol. Technol. Aliment. 2008, 7, 5–17. [Google Scholar]
- Arnao, M.B.; Cano, A.; Acosta, M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001, 73, 239–244. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Sousa, M.J.; Ferreira, I.C.F.R. Comparative study of lipophilic and hydrophilic antioxidants from in vivo and in vitro grown coriandrum sativum. Plant Foods Hum. Nutr. 2011, 66, 181–186. [Google Scholar] [CrossRef]
- Saini, R.K.; Prasad, P.; Sreedhar, R.V.; Naidu, K.A.; Shang, X.; Keum, Y.-S. Omega−3 Polyunsaturated Fatty Acids (PUFAs): Emerging Plant and Microbial Sources, Oxidative Stability, Bioavailability, and Health Benefits—A Review. Antioxidants 2021, 10, 1627. [Google Scholar] [CrossRef]
- Oppedisano, F.; Macrì, R.; Gliozzi, M.; Musolino, V.; Carresi, C.; Maiuolo, J.; Bosco, F.; Nucera, S.; Zito, M.C.; Guarnieri, L.; et al. The Anti-inflammatory and antioxidant properties of n-3 PUFAs: Their role in cardiovascular protection. Biomedicines 2020, 8, 306. [Google Scholar] [CrossRef] [PubMed]
- Öz, M.; Ucak, İ.; Nayik, G.A. Chapter 10—PUFA and MUFA. In Nutraceuticals and Health Care; Kour, J., Nayik, G.A., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 199–215. [Google Scholar]
- Latreille, J.; Kesse-Guyot, E.; Malvy, D.; Andreeva, V.; Galan, P.; Tschachler, E.; Hercberg, S.; Guinot, C.; Ezzedine, K. Dietary monounsaturated fatty acids intake and risk of skin photoaging. PLoS ONE 2012, 7, e44490. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, B.; Kapoor, D.; Gautam, S.; Singh, R.; Bhardwaj, S. Dietary polyunsaturated fatty acids (PUFAs): Uses and potential health benefits. Curr. Nutr. Rep. 2021, 10, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Telle-Hansen, V.H.; Gaundal, L.; Myhrstad, M.C. Polyunsaturated fatty acids and glycemic control in type 2 diabetes. Nutrients 2019, 11, 1067. [Google Scholar] [CrossRef] [Green Version]
- Wall, R.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Fatty acids from fish: The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr. Rev. 2010, 68, 280–289. [Google Scholar] [CrossRef] [PubMed]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Polyunsaturated fatty acids and inflammation. IUBMB Life 2015, 67, 659–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, metabolism and functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Minihane, A.M.; Lovegrove, J.A. 5—Health benefits of polyunsaturated fatty acids (PUFAs). In Improving the Fat Content of Foods; Williams, C., Buttriss, J., Eds.; Woodhead Publishing: Sawston, UK, 2006; pp. 107–140. [Google Scholar]
- López-Miranda, J.; Pérez-Martínez, P.; Pérez-Jiménez, F. Health benefits of monounsaturated fatty acids. In Improving the Fat Content of Foods; Elsevier: Amsterdam, The Netherlands, 2006; pp. 71–106. [Google Scholar]
- Wong, E.; Yan, H.; Li, M.; Lie, P.; Mruk, D.; Cheng, C. 11.09—Cell Junctions in the Testis as Targets for Toxicants. In Comprehensive Toxicology, 2nd ed.; McQueen, C.A., Ed. Elsevier: Oxford, UK, 2010; pp. 167–188. [Google Scholar]
- Huang, L.; Zhu, X.; Zhou, S.; Cheng, Z.; Shi, K.; Zhang, C.; Shao, H. Phthalic acid esters: Natural sources and biological activities. Toxins 2021, 13, 495. [Google Scholar] [CrossRef]
- Roy, R.N. Bioactive natural derivatives of phthalate ester. Crit. Rev. Biotechnol. 2020, 40, 913–929. [Google Scholar] [CrossRef]
- Qian, Z.-J.; Kang, K.-H.; Kim, S.-K. Isolation and antioxidant activity evaluation of two new phthalate derivatives from seahorse, Hippocampus Kuda Bleeler. Biotechnol. Bioprocess Eng. 2012, 17, 1031–1040. [Google Scholar] [CrossRef]
- Kiros, T.; Eswaramoorthy, R.; Melaku, Y.; Dekebo, A. In Vitro Antibacterial and Antioxidant Activities and Molecular Docking Analysis of Phytochemicals from Cadia purpurea Roots. J. Trop. Med. 2022, 2022, 4190166. [Google Scholar] [CrossRef]
- Berg, M.V.D.; Sanderson, T.; Kurihara, N.; Katayama, A. Role of metabolism in the endocrine-disrupting effects of chemicals in aquatic and terrestrial systems. Pure Appl. Chem. 2003, 75, 1917–1932. [Google Scholar] [CrossRef]
- Smith, A.D.; Tennyson, A.G.; Smith, R.C. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Dhara, O.; Azmeera, T.; Eanti, A.; Chakrabarti, P.P. Garden cress oil as a vegan source of PUFA: Achieving through optimized supercritical carbon dioxide extraction. Innov. Food Sci. Emerg. Technol. 2023, 84, 103283. [Google Scholar] [CrossRef]
- Czerwonka, M.; Białek, A. Fatty Acid Composition of Pseudocereals and Seeds Used as Functional Food Ingredients. Life 2023, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Sbihi, H.M.; Nehdi, I.A.; Al-Resayes, S.I. Characterization of White Mahlab (Prunus mahaleb L.) Seed Oil: A Rich Source of α-Eleostearic Acid. J. Food Sci. 2014, 79, C795–C801. [Google Scholar] [CrossRef]
- Francenia Santos Sánchez, N.; Salas-Coronado, R.; Villanueva-Cañongo, C.; Hernández-Carlos, B. Antioxidant compounds and their antioxidant mechanism. Antioxidants 2019, 10, 1–29. [Google Scholar]
- Alma, M.H.; Karaogul, E.; Ertas, M.; Altuntas, E.; Karaman, S.; Diraz, E. Chemical composition of seed oil from turkish Prunus mahaleb L. Anal. Chem. Lett. 2012, 2, 182–185. [Google Scholar] [CrossRef]
- Farag, M.A.; Khattab, A.R.; Shamma, S.; Afifi, S.M. Profiling of primary metabolites and volatile determinants in mahlab cherry (Prunus mahaleb L.) seeds in the context of its different varieties and roasting as analyzed using chemometric tools. Foods 2021, 10, 728. [Google Scholar] [CrossRef]
- Mariod, A.A.; Aseel, K.M.; Mustafa, A.A.; Abdel-Wahab, S.I. Characterization of the seed oil and meal from Monechma ciliatum and Prunus mahaleb seeds. J. Am. Oil Chem. Soc. 2009, 86, 749–755. [Google Scholar] [CrossRef]
- Özgül-Yücel, S. Determination of conjugated linolenic acid content of selected oil seeds grown in Turkey. J. Am. Oil Chem. Soc. 2005, 82, 893–897. [Google Scholar] [CrossRef]
- Hrotkó, K. Potentials in Prunus mahaleb L. for cherry rootstock breeding. Sci. Hortic. 2016, 205, 70–78. [Google Scholar] [CrossRef]
- Guest, E.; Townsend, C. Flora of Iraq; Ministry of Agriculture of the Republic of Iraq: Baghdad, Iraq, 1966; Volume 3. [Google Scholar]
- Chen, X.; Shen, X.; Jiang, D. Complete chloroplast genome sequence of Prunus mahaleb. Mitochondrial DNA Part B 2019, 4, 2204–2205. [Google Scholar] [CrossRef] [Green Version]
- Blando, F.; Albano, C.; Liu, Y.; Nicoletti, I.; Corradini, D.; Tommasi, N.; Gerardi, C.; Mita, G.; Kitts, D.D. Polyphenolic composition and antioxidant activity of the under-utilised Prunus mahaleb L. fruit. J. Sci. Food Agric. 2016, 96, 2641–2649. [Google Scholar] [CrossRef] [PubMed]
- Abdelgadir, A.A.; Ahmed, M.E.O.; Ahmed, E.M. Traditional Uses of Herbal Medicines in Khartoum and Gezira state (Central Sudan). Arab. J. Med. Aromat. Plants 2021, 7, 29–73. [Google Scholar]
- Orlando, G.; Chiavaroli, A.; Adorisio, S.; Delfino, D.V.; Brunetti, L.; Recinella, L.; Leone, S.; Zengin, G.; Acquaviva, A.; Angelini, P.; et al. Unravelling the phytochemical composition and the pharmacological properties of an optimized extract from the fruit from Prunus mahaleb L.: From traditional liqueur market to the pharmacy shelf. Molecules 2021, 26, 4422. [Google Scholar] [CrossRef] [PubMed]
- Al-Said, M.S.; Hifnawy, M.S. Dihydrocoumarin and certain other coumarins from Prunus mahaleb seeds. J. Nat. Prod. 1986, 49, 721. [Google Scholar] [CrossRef]
- Ieri, F.; Pinelli, P.; Romani, A. Simultaneous determination of anthocyanins, coumarins and phenolic acids in fruits, kernels and liqueur of Prunus mahaleb L. Food Chem. 2012, 135, 2157–2162. [Google Scholar] [CrossRef] [Green Version]
- Sbihi, H.M.; Nehdi, I.A.; El Blidi, L.; Rashid, U.; Al-Resayes, S.I. Lipase/enzyme catalyzed biodiesel production from Prunus mahaleb: A comparative study with base catalyzed biodiesel production. Ind. Crop. Prod. 2015, 76, 1049–1054. [Google Scholar] [CrossRef]
- Bodily, H.L. Official Methods of Analysis of the Association of Official Agricultural Chemists; American Public Health Association: Washington, DC, USA, 1956. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Keneni, Y.G.; Bahiru, L.A.; Marchetti, J.M. Effects of Different Extraction Solvents on Oil Extracted from Jatropha Seeds and the Potential of Seed Residues as a Heat Provider. Bio. Energy Res. 2021, 14, 1207–1222. [Google Scholar] [CrossRef]
- Chin, F.S.; Chong, K.P.; Markus, A.; Wong, N.K. Tea polyphenols and alkaloids content using Soxhlet and direct extraction method. World J. Agric. Sci. 2013, 9, 266–270. [Google Scholar] [CrossRef]
- Okeleye, A.A.; Betiku, E. Kariya (Hildegardia barteri) seed oil extraction: Comparative evaluation of solvents, modeling, and optimization techniques. Chem. Eng. Commun. 2019, 206, 1181–1198. [Google Scholar] [CrossRef]
- Zuo, H.; Yang, F.-Q.; Huang, W.-H.; Xia, Z.-N. Preparative gas chromatography and its applications. J. Chromatogr. Sci. 2013, 51, 704–715. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Moreno, C. Review: Methods Used to Evaluate the Free Radical Scavenging Activity in Foods and Biological Systems. Food Sci. Technol. Int. 2002, 8, 121–137. [Google Scholar] [CrossRef]
- Meng, X.-Y.; Zhang, H.-X.; Mezei, M.; Cui, M. Molecular Docking: A Powerful Approach for Structure-Based Drug Discovery. Curr. Comput. Aided-Drug Des. 2011, 7, 146–157. [Google Scholar] [CrossRef]
- De Brito, M.A. Pharmacokinetic study with computational tools in the medicinal chemistry course. Braz. J. Pharm. Sci. 2011, 47, 797–805. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Manochai, B.; Paisooksantivatana, Y.; Choi, H.; Hong, J.H. Variation in DPPH scavenging activity and major volatile oil components of cassumunar ginger, Zingiber montanum (Koenig), in response to water deficit and light intensity. Sci. Hortic. 2010, 126, 462–466. [Google Scholar] [CrossRef]
- Shimamura, T.; Sumikura, Y.; Yamazaki, T.; Tada, A.; Kashiwagi, T.; Ishikawa, H.; Matsui, T.; Sugimoto, N.; Akiyama, H.; Ukeda, H. Applicability of the dpph assay for evaluating the antioxidant capacity of food additives-inter-laboratory evaluation study. Anal. Sci. 2014, 30, 717–721. [Google Scholar] [CrossRef] [Green Version]
- Marvin. 5.5. 1.0 Program, Chemaxon; ChemAxon Ltd.: Budapest, Hungary, 2011. [Google Scholar]
- ChemAxon. MarvinSketch, Version 20.16.; ChemAxon Ltd.: Budapest, Hungary, 2020. [Google Scholar]
- Berman, H.; Henrick, K.; Nakamura, H. Announcing the worldwide Protein Data Bank. Nat. Struct. Mol. Biol. 2003, 10, 980. [Google Scholar] [CrossRef]
- Lountos, G.T.; Jiang, R.; Wellborn, W.B.; Thaler, T.L.; Bommarius, A.S.; Orville, A.M. The Crystal Structure of NAD(P)H Oxidase from Lactobacillus sanfranciscensis: Insights into the Conversion of O2 into Two Water Molecules by the Flavoenzyme, Biochemistry 2006, 45, 9648–9659. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The structure of human 5-lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knez, D.; Colettis, N.; Iacovino, L.G.; Sova, M.; Pišlar, A.; Konc, J.; Lešnik, S.; Higgs, J.; Kamecki, F.; Mangialavori, I.C.; et al. Stereoselective Activity of 1-Propargyl-4-styrylpiperidine-like Analogues That Can Discriminate between Monoamine Oxidase Isoforms A and B. J. Med. Chem. 2020, 63, 1361–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.H.; Valente, M.W.N.; Halpern, O.S.; Jusuf, S.; Khan, J.A.; Locke, G.A.; Duke, G.J.; Liu, X.; Duclos, F.J.; Wexler, R.R.; et al. Small molecule and macrocyclic pyrazole derived inhibitors of myeloperoxidase (MPO). Bioorganic Med. Chem. Lett. 2021, 42, 128010. [Google Scholar] [CrossRef]
- Vasu, D.; Li, H.; Hardy, C.D.; Poulos, T.L.; Silverman, R.B. 2-Aminopyridines with a shortened amino sidechain as potent, selective, and highly permeable human neuronal nitric oxide synthase inhibitors. Bioorganic Med. Chem. 2022, 69, 116878. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco, A.B.; Hpc, L. Introduction to AutoDock and AutoDock Tools; Louisiana State University: Baton Rouge, LA, USA, 2012. [Google Scholar]
- Valdés-Tresanco, M.S.; Valiente, P.A.; Moreno, E. AMDock: A versatile graphical tool for assisting molecular docking with Autodock Vina and Autodock4. Biol. Direct 2020, 15, 12. [Google Scholar] [CrossRef]
- Jagadish, P.; Soni, N.; Verma, A. Design, synthesis, and in vitro antioxidant activity of 1, 3, 5-trisubstituted-2-pyrazolines derivatives. J. Chem. 2013, 2013, 765768. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.; Sirajuddin, M.; Ahmad, S.; Tirmizi, S.A.; Ali, M.I.; Hameed, A. Synthesis, spectral characterization and in vitro antibacterial evaluation and Petra/Osiris/Molinspiration analyses of new Palladium(II) iodide complexes with thioamides. Alex. J. Med. 2016, 52, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, R. Theoretical Studies on the Molecular Properties, Toxicity, and Biological Efficacy of 21 New Chemical Entities. ACS Omega 2021, 6, 24891–24901. [Google Scholar] [CrossRef] [PubMed]
- Kandi, S.; Charles, A.L. Statistical comparative study between the conventional DPPH spectrophotometric and dropping DPPH analytical method without spectrophotometer: Evaluation for the advancement of antioxidant activity analysis. Food Chem. 2019, 287, 338–345. [Google Scholar] [CrossRef]
- Chen, Z.; Bertin, R.; Froldi, G. EC50 estimation of antioxidant activity in DPPH assay using several statistical programs. Food Chem. 2013, 138, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Straccia, M.C.; Siano, F.; Coppola, R.; La Cara, F.; Volpe, M.G. Extraction and characterization of vegetable oils from cherry seed by different extraction processes. Chem. Eng. Trans. 2012, 27, 391–396. [Google Scholar]
- Shivani, P.; Khushbu, P.; Faldu, N.; Thakkar, V.; Shubramanian, R.B. Extraction and analysis of Jatropha curcas L. seed oil. Afr. J. Biotechnol. 2011, 10, 18210–18213. [Google Scholar]
- Santos SB, D.; Martins, M.A.; Caneschi, A.L.; Aguilar PR, M.; Coimbra JS, D.R. Kinetics and thermodynamics of oil extraction from Jatropha curcas L. using ethanol as a solvent. Int. J. Chem. Eng. 2015, 2015, 871236. [Google Scholar] [CrossRef] [Green Version]
- Parasuraman, S.; Sujithra, J.; Syamittra, B.; Yeng, W.Y.; Ping, W.Y.; Muralidharan, S.; Raj, P.V.; Dhanaraj, S.A. Evaluation of sub-chronic toxic effects of petroleum ether, a laboratory solvent in Sprague-Dawley rats. J. Basic Clin. Pharm. 2014, 5, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Johansson, A.; Laakso, P.; Kallio, H. Characterization of seed oils of wild, edible Finnish berries. Z. Lebensm. Forsch. A 1997, 204, 300–307. [Google Scholar] [CrossRef]
- Siejak, P.; Smułek, W.; Nowak-Karnowska, J.; Dembska, A.; Neunert, G.; Polewski, K. Bird Cherry (Prunus padus) Fruit Extracts Inhibit Lipid Peroxidation in PC Liposomes: Spectroscopic, HPLC, and GC–MS Studies. Appl. Sci. 2022, 12, 7820. [Google Scholar] [CrossRef]
- Mead, H.M.; El-Shafiey, S.N.; Sabry, H.M. Chemical constituents and ovicidal effects of mahlab, Prunus mahaleb L. kernels oil on cotton leafworm, Spodoptera littoralis (Boisd.) eggs. J. Plant Prot. Res. 2016, 56. [Google Scholar] [CrossRef] [Green Version]
- Rijai, H.R.; Fakhrudin, N.; Wahyuono, S. Isolation and identification of DPPH radical (2, 2-diphenyl-1-pikrylhidrazyl) scavenging active compound in ethyl acetat fraction of Piper acre Blume. Maj. Obat Tradis. 2019, 24, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Valantina, R.S.; Neelamegam, P. Selective ABTS and DPPH-radical scavenging activity of peroxide from vegetable oils. Int. Food Res. J. 2015, 22, 289. [Google Scholar]
- Adejoh, I.P.; Barnabas, A.; Chiadikaobi, O.S. Comparative anti-radical activity of five indigenous herbal plants and their polyherbal extract. Int. J. Biochem. Res. Rev. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhou, K.; Ge, X.; Xu, N.; Wang, X.; He, Q.; Zhang, C.; Jun, C.; Li, Q. Effects of Extraction Technique on the Content and Antioxidant Activity of Flavonoids from Gossypium Hirsutum linn. Flowers. Molecules 2022, 27, 5627. [Google Scholar] [CrossRef]
- Abu, F.; Mat Taib, C.N.; Mohd Moklas, M.A.; Mohd Akhir, S. Antioxidant properties of crude extract, partition extract, and fermented medium of Dendrobium sabin flower. Evid. -Based Complement. Altern. Med. 2017, 2017, 2907219. [Google Scholar] [CrossRef] [Green Version]
- Vorobyova, V.I.; Skiba, M.I.; Shakun, A.S.; Nahirniak, S.V. Relationship between the inhibition and antioxidant properties of the plant and biomass wastes extracts-A Review. Int. J. Corros. Scale Inhib. 2019, 8, 150–178. [Google Scholar]
- Begum, R.; Thota, S.; Abdulkadir, A.; Kaur, G.; Bagam, P.; Batra, S. NADPH oxidase family proteins: Signaling dynamics to disease management. Cell. Mol. Immunol. 2022, 19, 660–686. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Haque, M.M. Nitric oxide synthase enzymology in the 20 years after the Nobel Prize. Br. J. Pharmacol. 2019, 176, 177–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suh, L.Y.; Babu, D.; Tonoyan, L.; Reiz, B.; Whittal, R.; Tabatabaei-Dakhili, S.A.; Morgan, A.G.; Velazquez-Martinea, C.A. Myeloperoxidase-mediated oxidation of edaravone produces an apparent non-toxic free radical metabolite and modulates hydrogen peroxide-mediated cytotoxicity in HL-60 cells. Free. Radic. Biol. Med. 2019, 143, 422–432. [Google Scholar] [CrossRef]
- Elsayed, F.F.; Elshenawy, W.M.; Khalifa, E.M.; Rizq, M.R.; Abdelaziz, R.R. Ameliorative effect of flavocoxid on cyclophosphamide-induced cardio and neurotoxicity via targeting the GM-CSF/NF-κB signaling pathway. Environ. Sci. Pollut. Res. 2022, 29, 69635–69651. [Google Scholar] [CrossRef]
- Ghosh, S.; Dutta, N.; Banerjee, P.; Gajbhiye, R.L.; Sareng, H.R.; Kapse, P.; Pal, S.; Burdely, L.; Mandal, N.C.; Ravichandiran, V.; et al. Induction of monoamine oxidase A-mediated oxidative stress and impairment of NRF2-antioxidant defence response by polyphenol-rich fraction of Bergenia ligulata sensitizes prostate cancer cells in vitro and in vivo. Free. Radic. Biol. Med. 2021, 172, 136–151. [Google Scholar] [CrossRef]
- Kuppusamy, P.; Zweier, J.L. Characterization of free radical generation by xanthine oxidase: Evidence for hydroxyl radical generation. J. Biol. Chem. 1989, 264, 9880–9884. [Google Scholar] [CrossRef]
- Kontos, H.A.; Wei, E.P.; Ellis, E.F.; Jenkins, L.W.; Povlishock, J.T.; Rowe, G.T.; Hess, M.L. Appearance of superoxide anion radical in cerebral extracellular space during increased prostaglandin synthesis in cats. Circ. Res. 1985, 57, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Di Meo, S.; Venditti, P. Evolution of the Knowledge of Free Radicals and Other Oxidants. Oxidative Med. Cell. Longev. 2020, 2020, 9829176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Limongelli, V. Ligand binding free energy and kinetics calculation in 2020. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1455. [Google Scholar] [CrossRef]
- VGoncharov, N.; VAvdonin, P.; DNadeev, A.; LZharkikh, I.; OJenkins, R. Reactive oxygen species in pathogenesis of atherosclerosis. Curr. Pharm. Des. 2015, 21, 1134–1146. [Google Scholar] [CrossRef] [Green Version]
- Abeyrathne, E.D.N.S.; Nam, K.; Ahn, D.U. Analytical methods for lipid oxidation and antioxidant capacity in food systems. Antioxidants 2021, 10, 1587. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.L. Small molecules: The missing link in the central dogma. Nat. Chem. Biol. 2005, 1, 64–66. [Google Scholar] [CrossRef]
- Protti, Í.F.; Rodrigues, D.R.; Fonseca, S.K.; Alves, R.J.; de Oliveira, R.B.; Maltarollo, V.G. Do Drug-likeness Rules Apply to Oral Prodrugs? ChemMedChem 2021, 16, 1446–1456. [Google Scholar] [CrossRef]
- Flores-Holguín, N.; Frau, J.; Glossman-Mitnik, D. Chemical-reactivity properties, drug likeness, and bioactivity scores of Seragamides A–F anticancer marine peptides: Conceptual density functional theory viewpoint. Computation 2019, 7, 52. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A. Lead-and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Van de Waterbeemd, H. In Silico Models to Predict Oral Absorption. In Comprehensive Medicinal Chemistry II; Taylor, J.B., Triggle, D.J., Eds.; Elsevier: Oxford, UK, 2007; pp. 669–697. [Google Scholar]
- Ghannay, S.; Bakari, S.; Msaddek, M.; Vidal, S.; Kadri, A.; Aouadi, K. Design, synthesis, molecular properties and in vitro antioxidant and antibacterial potential of novel enantiopure isoxazolidine derivatives. Arab. J. Chem. 2020, 13, 2121–2131. [Google Scholar] [CrossRef]
- Unnisa, A.; Abouzied, A.S.; Anupama, B.; Fatima, S.B.; Lakshmi, K.N.V.C.; Unissa, R. Design, synthesis, characterization, computational study and in-vitro antioxidant and anti-inflammatory activities of few novel 6-aryl substituted pyrimidine azo dyes. Arab. J. Chem. 2020, 13, 8638–8649. [Google Scholar] [CrossRef]
- Kuchana, M.; Bethapudi, D.R.; Ediga, R.K.; Sisapuram, Y. Synthesis, in-vitro antioxidant activity and in-silico prediction of drug-likeness properties of a novel compound: 4-(3, 5-Di-tert-butyl-4-hydroxybenzylidene)-3-methylisoxazol-5 (4H)-one. J. Appl. Pharm. Sci. 2019, 9, 105–110. [Google Scholar]
- Ramírez, A.; Vázquez-Sánchez, A.Y.; Carrión-Robalino, N.; Camacho, J. Ion Channels and Oxidative Stress as a Potential Link for the Diagnosis or Treatment of Liver Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 3928714. [Google Scholar] [CrossRef] [Green Version]
- Breznik, M.; Ge, Y.; Bluck, J.P.; Briem, H.; Hahn, D.F.; Christ, C.D.; Mortier, J.; Mobley, D.L.; Meier, K. Prioritizing Small Sets of Molecules for Synthesis through in-silico Tools: A Comparison of Common Ranking Methods. ChemMedChem 2023, 18, e202200425. [Google Scholar] [CrossRef] [PubMed]

| # | Methods Used in the Study | Other Research Methods | |
|---|---|---|---|
| Advantages | Disadvantages | ||
| 1 | Soxhlet method: To extract seed oil using n-hexane and petroleum ether as solvents |
| |
| 2 | GC-MS |
|
|
| 3 | DPPH radical scavenging activity |
|
|
| 4 | Molecular docking with AutoDock 4 |
|
|
| 5 | Molecular property and bioactivity prediction in silico |
|
|
| Protein | PDB ID | Resolution Å | Grid Box Center | Grid Box Size | Ref. |
|---|---|---|---|---|---|
| NADPH oxidase | 2cdu | 1.8 | x center = 6.9 | x-dimension = 54 | [14] |
| y center = −1.5 | y-dimension =58 | ||||
| z center = 2.8 | z-dimension = 46 | ||||
| 5-Lipoxygenase | 3o8y | 2.39 | x center = −4.3 | x-dimension = 72 | [15] |
| y center = 15.7 | y-dimension = 46 | ||||
| z center = 5.9 | z-dimension = 52 | ||||
| Monoamine oxidase B | 6rkb | 2.3 | x center = 55 | x-dimension = 56 | [16] |
| y center = 155.2 | y-dimension = 68 | ||||
| z center = 30.3 | z-dimension = 56 | ||||
| Myeloperoxidase | 7lae | 2.97 | x center = −9.3 | x-dimension = 84 | [17] |
| y center = 21.5 | y-dimension = 66 | ||||
| z center = −20.6 | z-dimension = 54 | ||||
| Nitric oxide synthase | 7tsh | 2.15 | x center = 51.6 | x-dimension = 70 | [18] |
| y center = 31.6 | y-dimension = 84 | ||||
| z center = −188.3 | z-dimension = 76 |
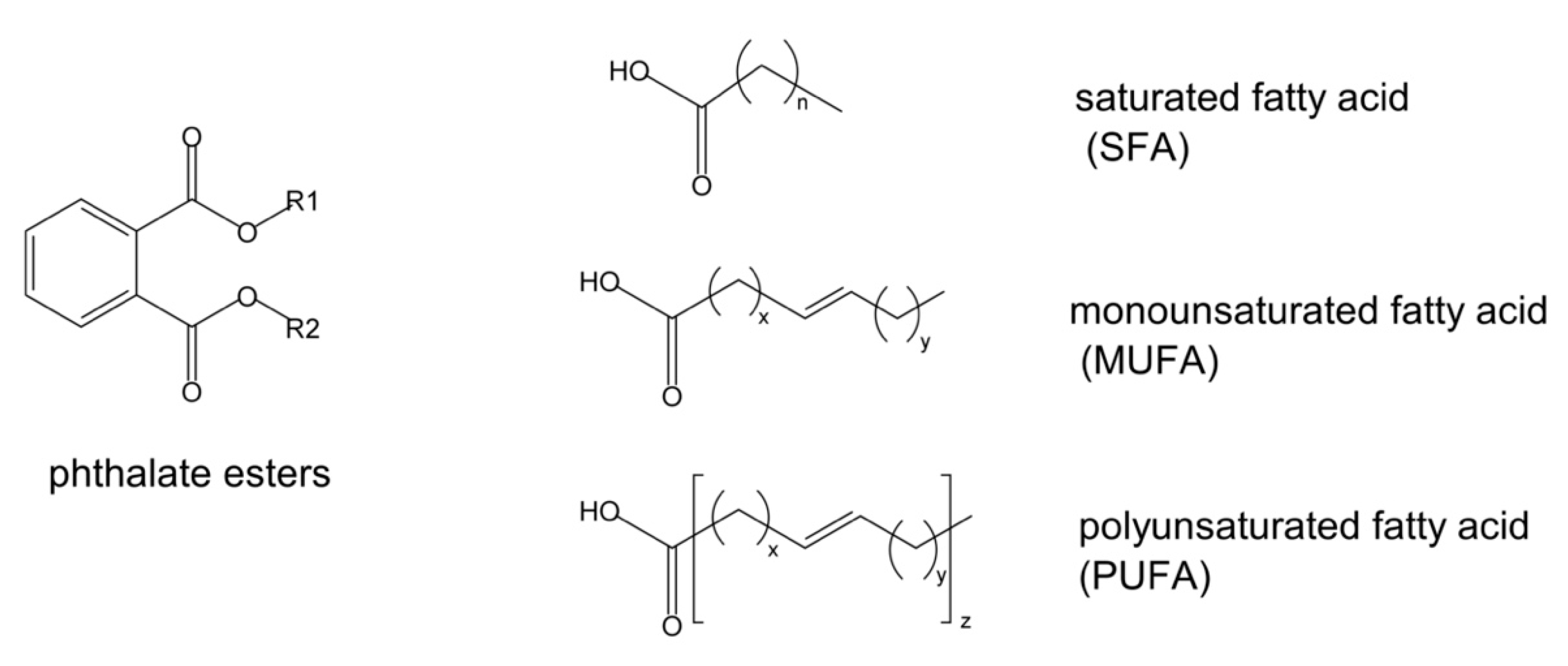
| Comp. | IUPAC Name | Common Name | MW (g/mol) | RT (min) | Percentage PMSOE (%) | % of Similarity by NIST.20 | m/z Values | |
|---|---|---|---|---|---|---|---|---|
| n-hexane | PE ** | |||||||
| C01 p * | 9,11,13-octadecatrienoic acid | α -eleosteric acid | 278 | 16.09 | 0.19 | 0.18 | 90 | 292, 261, 232, 135, 92, 59 |
| C02 p | 9,12-Octadecadienoic acid | Linoleic acid | 280 | 13.72 | 25.63 | 24.47 | 96 | 294, 263, 137, 97, 59, 57 |
| C03 p | 6,9,11-octadecatrienoic acid | X | 278 | 15.56 | 34.00 | 32.04 | 91 | 292, 177, 137, 71, 66 |
| C04 m * | 9-eicosenoic acid | Gadelaidic acid | 310 | 16.52 | 0.13 | 0.12 | 91 | 324, 293, 250, 208, 127, 85 |
| C05 m | 9-heptadecenoic acid | Margaroleic acid | 268 | 12.54 | 0.04 | 0.03 | 90 | 282, 251, 125, 85, 59 |
| C06 m | 9-Octadecenoic acid | Oleic acid | 282 | 13.85 | 32.11 | 37.91 | 96 | 296, 265, 99, 59, 41 |
| C07 m | 11-hexadecenoic acid | Palmitvaccenic acid | 254 | 11.45 | 0.17 | 0.19 | 94 | 268, 237, 83, 59, 41 |
| C08 m | 9-Hexadecenoic acid | Palmitiolic acid | 254 | 12.00 | 0.25 | X | 91 | 268, 237, 111, 71, 59, 41 |
| C13 s * | Hexadecanoic acid | Palmitic acid | 256 | 11.70 | 3.10 | 3.00 | 97 | 270, 239, 57, 43, 29 |
| C14 s | Octadecanoic acid | Stearic acid | 284 | 14.14 | 1.96 | 1.45 | 97 | 298, 267, 224, 59, 57, 43 |
| C15 s | Eicosanoic acid | Arachidic acid | 312 | 16.93 | 0.28 | 0.27 | 95 | 326, 295, 59, 57, 43, 29 |
| C16 s | Docosanoic acid | Behenic acid | 340 | 19.85 | 0.08 | 0.06 | 93 | 354, 323, 71, 59,57, 43, 29 |
| C17 s | Heptadecanoic acid | Margaric acid | 270 | 12.85 | 0.04 | 0.04 | 91 | 284, 253, 59, 57, 43, 29 |
| C18 s | Tetracosanoic acid | Lignoceric acid | 368 | 22.74 | 0.06 | 0.05 | 91 | 382, 351, 71, 59, 57, 43 |
| PUFA | 59.82 | 56.69 | ||||||
| MUFA | 32.70 | 38.25 | ||||||
| SFA | 5.62 | 4.87 | ||||||
| Common Name | Percentage of Fatty Acid Composition % | ||||
|---|---|---|---|---|---|
| Egypt (Maceration) | Iraq (Soxhlet) | Sudan, (Soxhlet) | Syria (Soxhlet) | Turkey (Cold Press) | |
| Lauric acid | - | - | < 0.1 pe * | - | - |
| Myristic acid | - | - | < 0.1 pe | 0.04 n ± 0.01 | 0.1 c * |
| Palmitic acid | 2.74 pe | 3.1 n *, 3.0 pe | 5.7 pe ± 0.02 | 3.84 n ± 0.05 | 5.6 c |
| Palmitiolic acid | 0.17 pe | 0.25 n | - | 0.23 n ± 0.01 | 0.5 c |
| Palmitvaccenic acid | - | 0.17 n, 0.19 pe | - | - | - |
| Margaric acid | 0.06 pe | 0.04 n, 0.04 pe | - | - | - |
| Margaroleic acid | 0.06 pe | 0.04 n, 0.03 pe | - | - | - |
| Stearic acid | 1.73 pe | 1.96 n, 1.45 pe | 1.3 pe ± 0.3 | 1.88 n ± 0.04 | 2.2 c |
| Oleic acid | 28.71 pe | 32.11 n, 37.91 pe | 45 pe ± 0.5 | 29.83 n ± 0.5 | 35.8 c |
| Cis-vaccenic acid | - | - | - | 0.67 n ± 0.04 | - |
| Linoleic acid | 24.35 pe | 25.63 n, 24.47 pe | 47 pe ± 0.5 | 21.68 n ± 0.4 | 24.9 c |
| Linolelaidic acid | - | - | - | - | 22.6 c |
| α-Linoleic acid | - | - | - | - | 3 c |
| 6,9,11-octadecatrienoic acid | - | 34.00 n, 32.04 pe | - | - | - |
| α-eleosteric acid | - | 0.19 n, 0.18 pe | - | 40.71 n ± 0.8 | - |
| α-Linolenic acid | 0.37 pe | - | 0.1 pe ± 0.02 | - | - |
| Arachidic acid | 0.73 pe | 0.28 n, 0.27 pe | - | 0.33 n ± 0.01 | 0.5 c |
| Gadelaidic acid | - | 0.13 n, 0.12 pe | - | - | - |
| Gadoleic acid | 0.41 pe | - | - | 0.43 n ± 0.03 | 0.3 c |
| Eicosadienoic acid | - | - | - | 0.29 n ± 0.01 | 0.3 c |
| Timnodonic acid | 33.07 pe | - | - | - | - |
| Behenic acid | 0.72 pe | 0.08 n, 0.06 pe | - | 0.4 n ± 0.01 | 0.3 c |
| Erucic acid | 6.74 pe | - | - | - | - |
| Lignoceric acid | 0.14 pe | 0.06 n, 0.05 pe | - | - | 0.7 c |
| SFA | 6.12 | 5.62 n, 4.87 pe | 7.2 | 6.49 ± 0.12 | 9.4 |
| MUFA | 36.09 | 32.7 n, 38.25 pe | 45 ± 0.5 | 31.16 ± 0.58 | 36.6 |
| PUFA | 57.79 | 59.82 n, 56.69 pe | 47.1 ± 0.5 | 62.68 ± 1.21 | 52.1 |
| Comp. | IUPAC Name | Common Name | MW (g/mole) | Retention Time (min) | Percentage (%) | % of Similarity by NIST.20 | m/z Values |
|---|---|---|---|---|---|---|---|
| C09 | 1,2-diethyl benzene-1,2-dicarboxylate | Diethyl Phthalate | 222 | 8.69 | 0.26 | 89 | 222, 177, 150, 132, 76 |
| C10 | 1-(2-ethylhexyl) 2-methyl benzene-1,2-dicarboxylate | 1-2-ethylhexyl2-methylphthalate | 292 | 12.68 | 0.07 | 87 | 292, 150, 92, 76 |
| C11 | 1,2-bis(2-ethylhexyl) benzene-1,2-dicarboxylate | Etalon | 390 | 19.76 | 0.43 | 97 | 390, 261, 132, 76, 29 |
| C12 | 1,2-bis-2methylpropyl benzene-1,2-dicarboxylate | Diisobutyl pthalate | 278 | 10.91 | 0.33 | 94 | 278, 205, 132, 76, 29 |
| Compound | Chemical Formula | ΔG (kcal/mol) | ||||
|---|---|---|---|---|---|---|
| NOX | 5−LOX | MAOB | MPO | NOS | ||
| C01 | C18H30O2 | −5.78 | −4.79 | −6.90 | −5.34 | −4.99 |
| C02 | C18H32O2 | −4.99 | −3.81 | −6.71 | −7.17 | −4.24 |
| C03 | C18H30O2 | −5.74 | −5.81 | −7.18 | −6.60 | −6.89 |
| C04 | C20H38O2 | −6.54 | −4.58 | −4.39 | −7.04 | −5.85 |
| C05 | C17H32O2 | −4.79 | −5.96 | −7.55 | −5.53 | −5.78 |
| C06 | C18H34O2 | −4.75 | −5.87 | −7.05 | −6.17 | −5.30 |
| C07 | C16H30O2 | −6.16 | −4.84 | −6.84 | −5.31 | −5.40 |
| C08 | C16H30O2 | −5.35 | −5.10 | −7.13 | −6.06 | −5.77 |
| C09 | C12H14O4 | −5.52 | −6.30 | −6.03 | −6.40 | −5.77 |
| C10 | C17H24O4 | −7.27 | −7.50 | −8.52 | −8.60 | −7.98 |
| C11 | C24H38O4 | −6.35 | −4.74 | −5.09 | −4.56 | −7.21 |
| C12 | C16H22O4 | −5.87 | −6.91 | −6.48 | −6.05 | −7.50 |
| Quercetin | C15H10O7 | −6.87 | −7.81 | −8.65 | −8.38 | −6.59 |
| Compound | milogP | MW | HB Acceptor | HB Donor | Violations | RB | TPSA |
|---|---|---|---|---|---|---|---|
| C01 | 6.60 | 278.44 | 2 | 1 | 1 | 13 | 37.30 |
| C02 | 6.86 | 280.45 | 2 | 1 | 1 | 14 | 37.30 |
| C03 | 6.37 | 278.44 | 2 | 1 | 1 | 13 | 37.30 |
| C04 | 8.47 | 310.52 | 2 | 1 | 1 | 17 | 37.30 |
| C05 | 7.08 | 268.44 | 2 | 1 | 1 | 14 | 37.30 |
| C06 | 7.58 | 282.47 | 2 | 1 | 1 | 15 | 37.30 |
| C07 | 6.57 | 254.41 | 2 | 1 | 1 | 13 | 37.30 |
| C08 | 6.57 | 254.41 | 2 | 1 | 1 | 13 | 37.30 |
| C09 | 2.31 | 222.24 | 4 | 0 | 0 | 6 | 52.61 |
| C10 | 4.75 | 292.38 | 4 | 0 | 0 | 10 | 52.61 |
| C11 | 7.94 | 390.56 | 4 | 0 | 1 | 16 | 52.61 |
| C12 | 3.80 | 278.35 | 4 | 0 | 0 | 8 | 52.61 |
| Quercetin | 1.68 | 302.24 | 7 | 5 | 0 | 1 | 131.35 |
| Compound | GPCR Ligand | Ion Channel Modulator | Kinase Inhibitor | Nuclear Receptor Ligand | Protease Inhibitor | Enzyme Inhibitor |
|---|---|---|---|---|---|---|
| C01 | 0.2 | 0.1 | −0.2 | 0.3 | 0.1 | 0.3 |
| C02 | 0.3 | 0.2 | −0.2 | 0.3 | 0.1 | 0.4 |
| C03 | 0.3 | 0.2 | −0.1 | 0.4 | 0.1 | 0.4 |
| C04 | 0.2 | 0.1 | −0.1 | 0.3 | 0.1 | 0.3 |
| C05 | 0.1 | 0.1 | −0.3 | 0.2 | 0.0 | 0.3 |
| C06 | 0.2 | 0.1 | −0.2 | 0.2 | 0.1 | 0.3 |
| C07 | 0.1 | 0.1 | −0.4 | 0.1 | 0.0 | 0.3 |
| C08 | 0.1 | 0.1 | −0.4 | 0.1 | 0.0 | 0.3 |
| C09 | −0.6 | −0.2 | −0.7 | −0.5 | −0.7 | −0.3 |
| C10 | −0.1 | −0.1 | −0.3 | 0.0 | −0.1 | −0.1 |
| C11 | 0.0 | 0.0 | −0.1 | 0.1 | 0.0 | 0.0 |
| C12 | −0.2 | −0.1 | −0.3 | −0.1 | −0.2 | −0.1 |
| Quercetin | −0.1 | −0.2 | 0.3 | 0.4 | −0.3 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, Z.N.; Azeez, H.A.; Salih, T. Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies. Appl. Sci. 2023, 13, 7430. https://doi.org/10.3390/app13137430
Hussein ZN, Azeez HA, Salih T. Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies. Applied Sciences. 2023; 13(13):7430. https://doi.org/10.3390/app13137430
Chicago/Turabian StyleHussein, Zhawen Noori, Hoshyar Abdullah Azeez, and Twana Salih. 2023. "Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies" Applied Sciences 13, no. 13: 7430. https://doi.org/10.3390/app13137430
APA StyleHussein, Z. N., Azeez, H. A., & Salih, T. (2023). Antioxidant Activity of the Prunus mahaleb Seed Oil Extracts Using n-Hexane and Petroleum Ether Solvents: In Silico and In Vitro Studies. Applied Sciences, 13(13), 7430. https://doi.org/10.3390/app13137430