Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review
Abstract
1. Introduction
2. Application of Polyphenols Recovered from By-Products
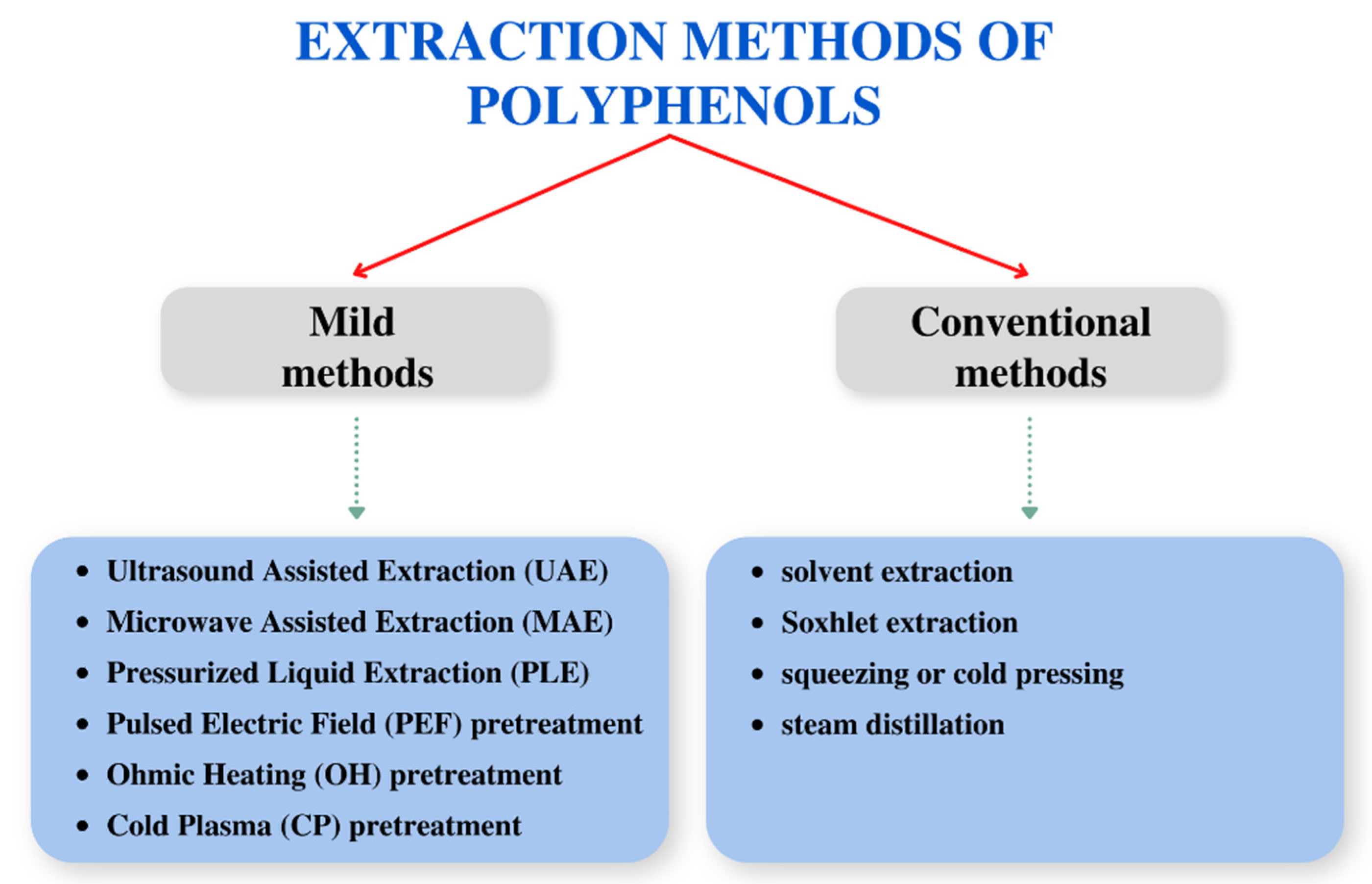
3. Different Extraction Methods of Polyphenols
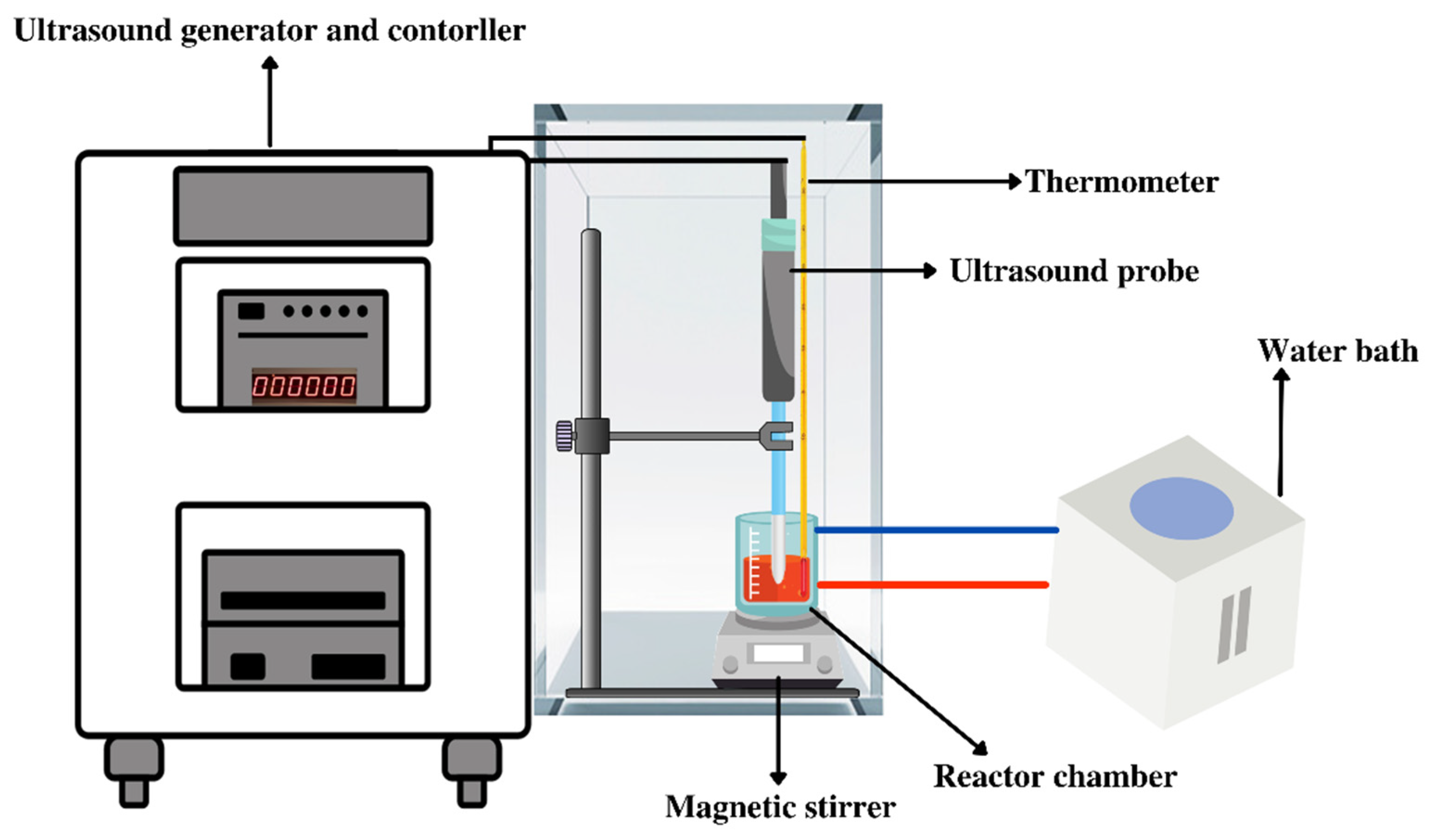
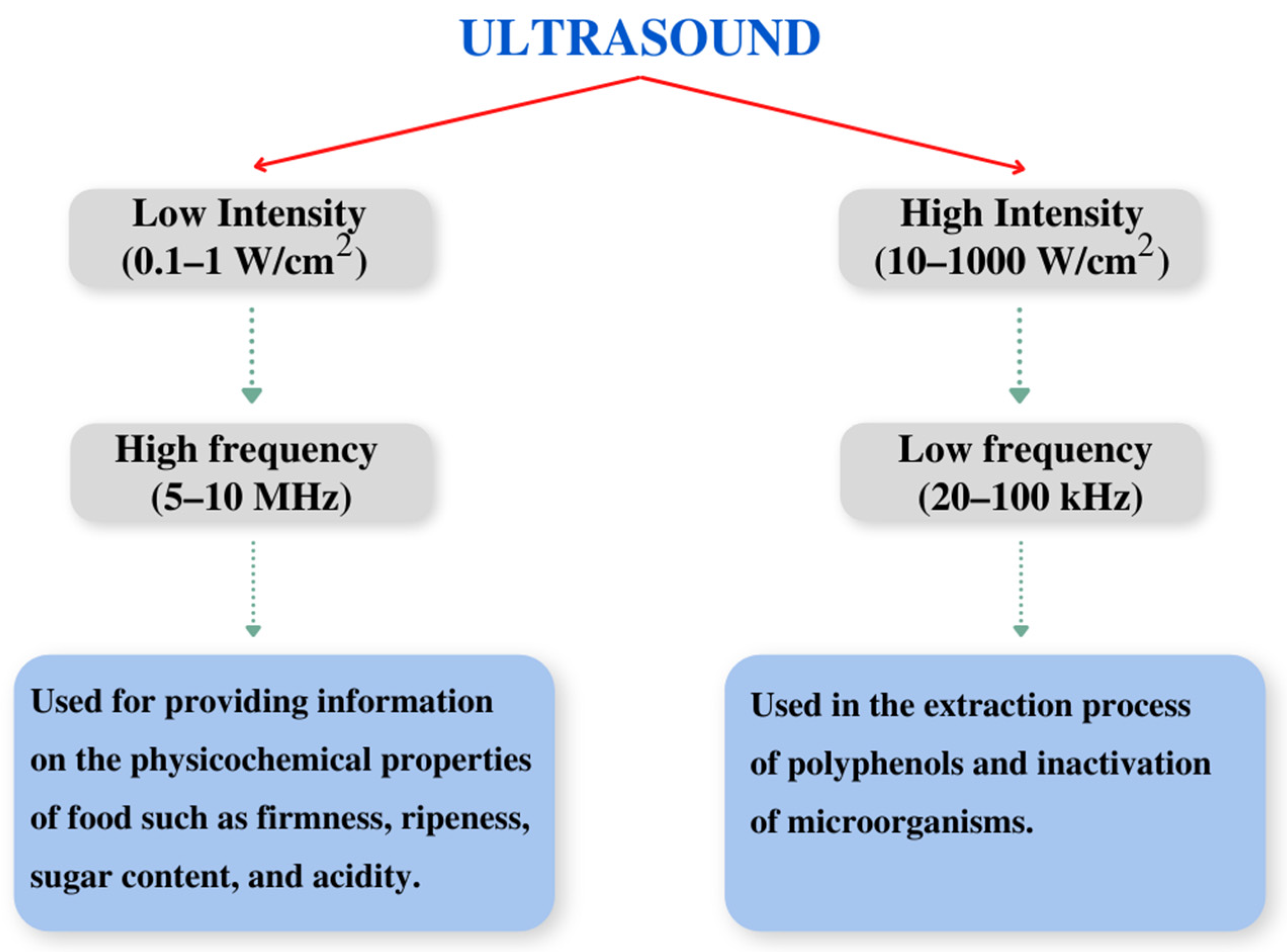
3.1. Ultrasound-Assisted Extraction (UAE)
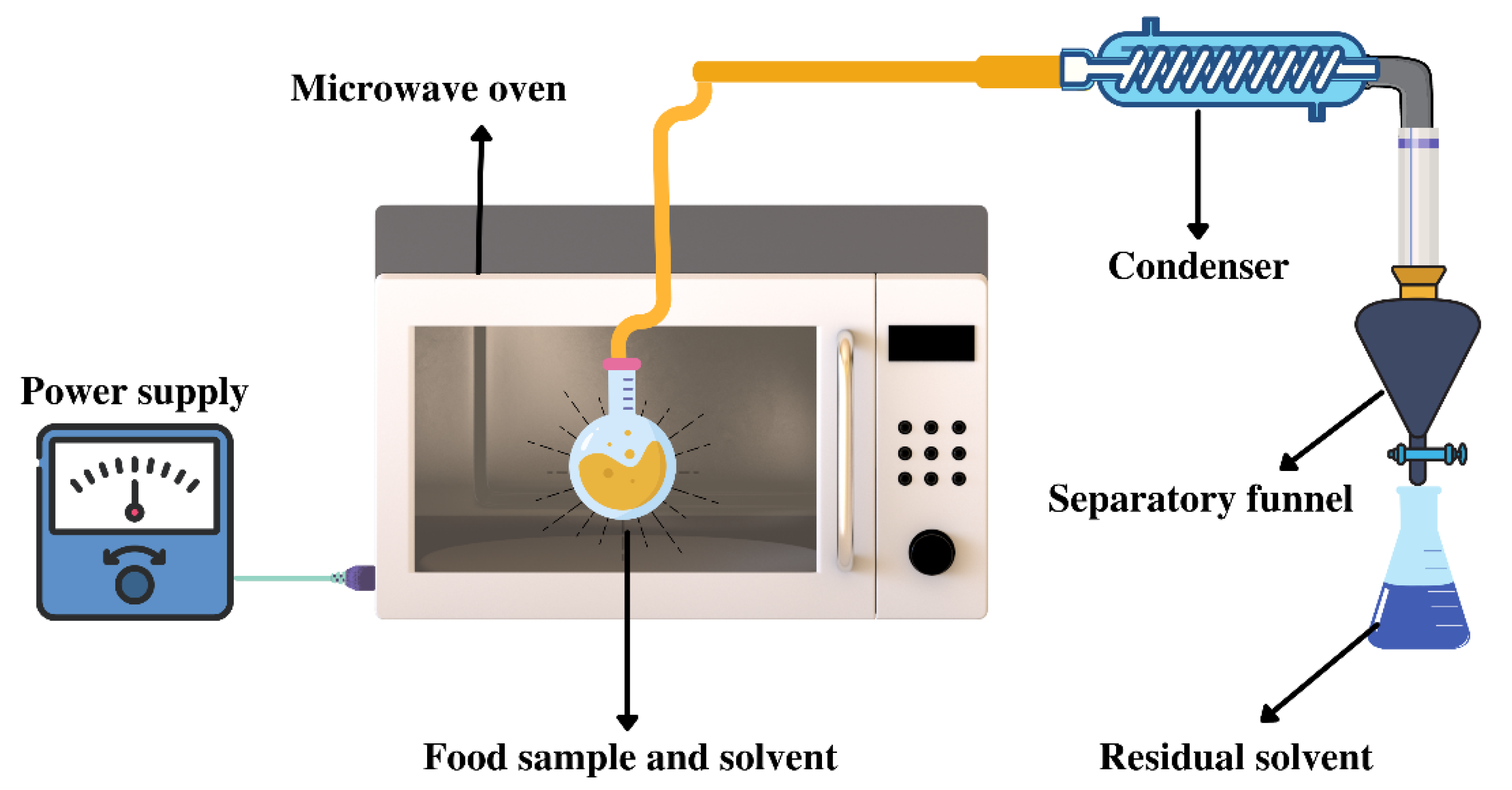
3.2. Microwave-Assisted Extraction (MAE)
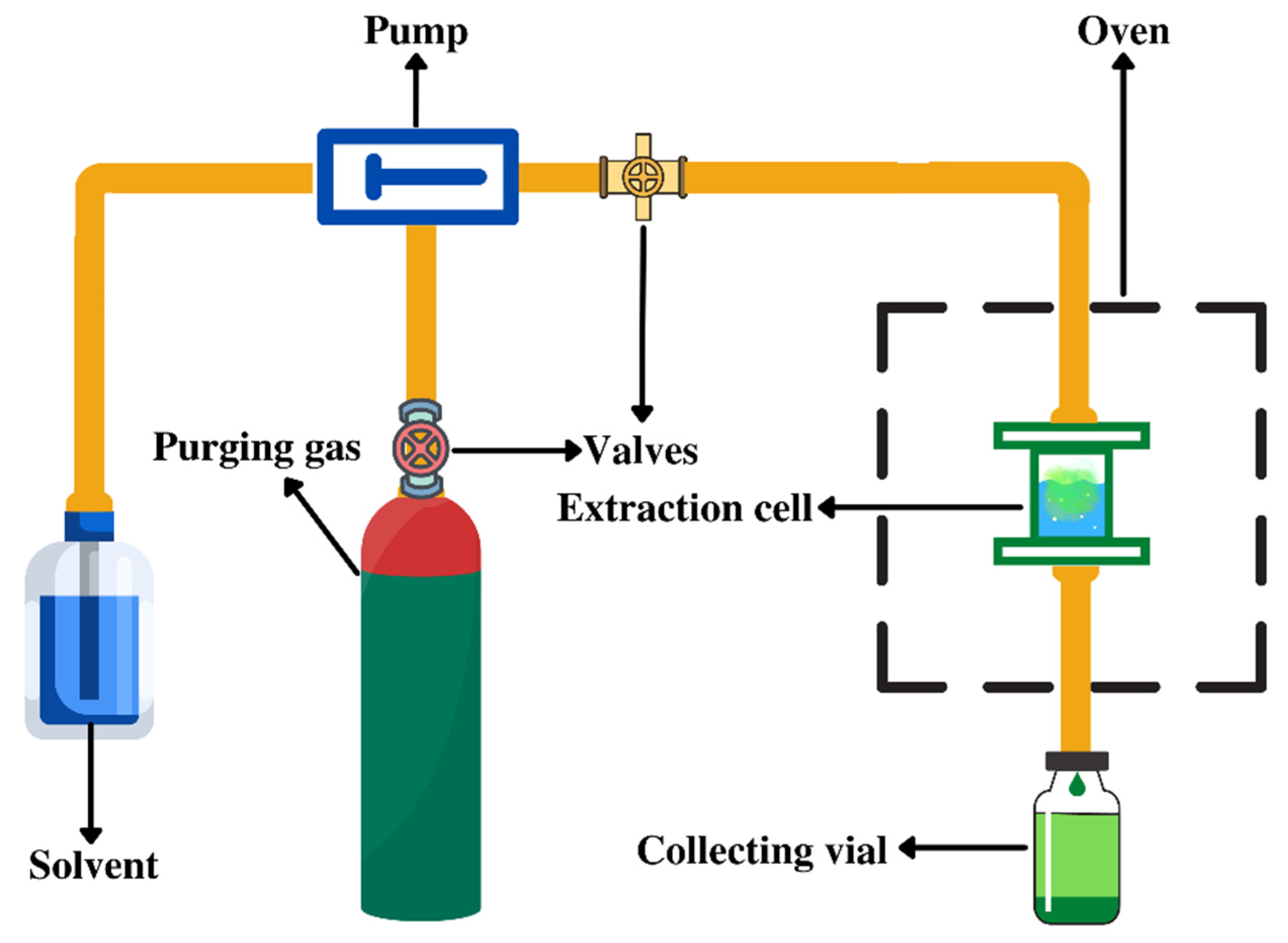
3.3. Pressurized Liquid Extraction (PLE)
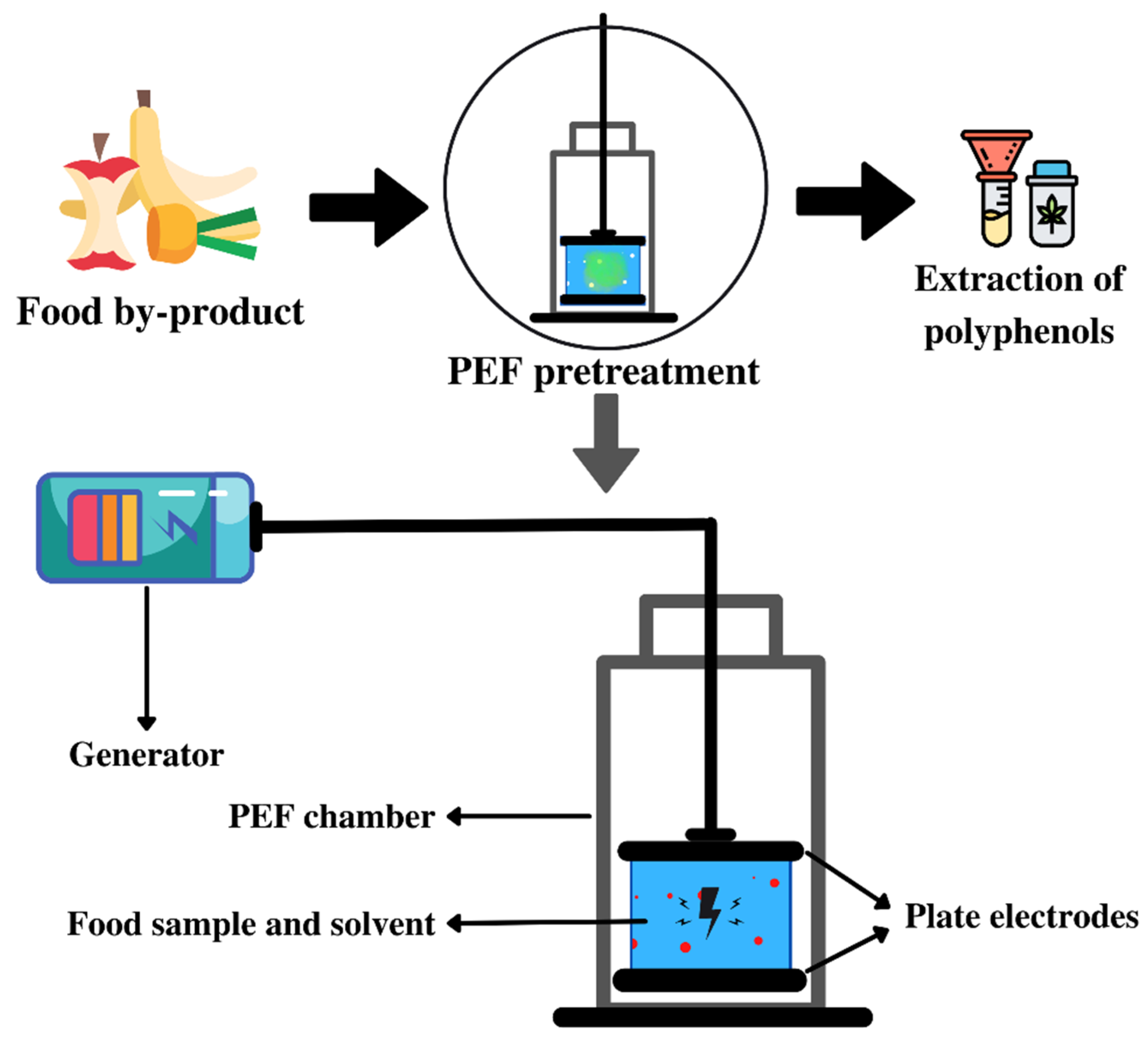
3.4. Pulsed Electric Field (PEF) Pretreatment
3.5. Ohmic Heating (OH) Pretreatment
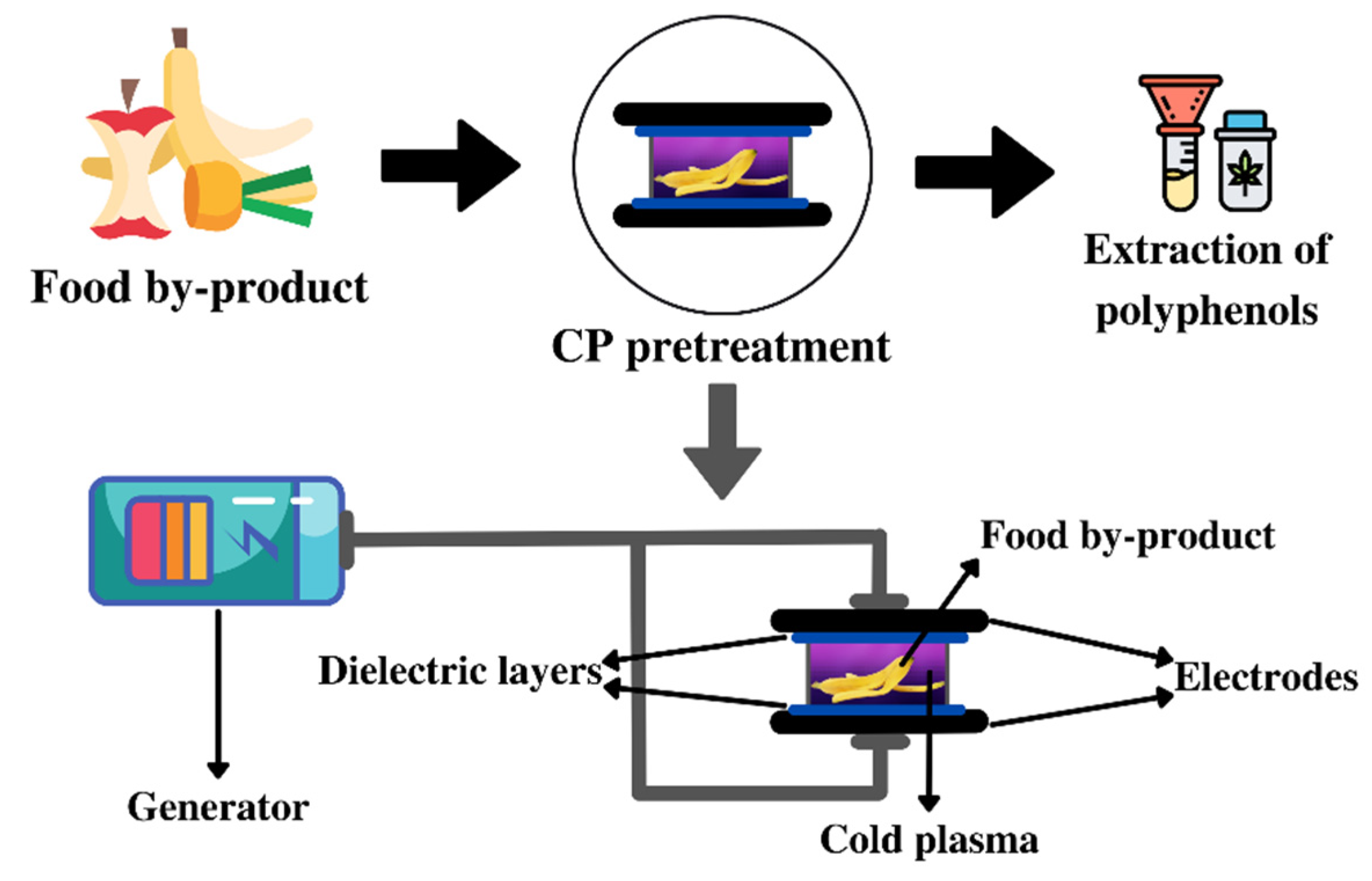
3.6. Cold Plasma (CP) Pretreatment
4. Control of Polyphenol Oxidase (PPO) Using Mild Technologies
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roda, A.; Lambri, M. Food uses of pineapple waste and by-products: A review. Int. J. Food Sci. Technol. 2019, 54, 1009–1017. [Google Scholar] [CrossRef]
- Zocca, F.; Lomolino, G.; Lante, A. Antibrowning potential of Brassicacaea processing water. Bioresour. Technol. 2010, 101, 3791–3795. [Google Scholar] [CrossRef] [PubMed]
- Tinello, F.; Lante, A. Valorisation of Ginger and Turmeric Peels as Source of Natural Antioxidants. Plant Foods Hum. Nutr. 2019, 74, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Lante, A.; Tinello, F. Citrus hydrosols as useful by-products for tyrosinase inhibition. Innov. Food Sci. Emerg. Technol. 2015, 27, 154–159. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Recent advances in controlling polyphenol oxidase activity of fruit and vegetable products. Innov. Food Sci. Emerg. Technol. 2018, 50, 73–83. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Mihaylova, D. Valorization of onion extracts as anti-browning agents. Food Sci. Appl. Biotechnol. 2020, 3, 16. [Google Scholar] [CrossRef]
- Zia, S.; Khan, M.R.; Shabbir, M.A.; Aslam Maan, A.; Khan, M.K.I.; Nadeem, M.; Khalil, A.A.; Din, A.; Aadil, R.M. An Inclusive Overview of Advanced Thermal and Nonthermal Extraction Techniques for Bioactive Compounds in Food and Food-related Matrices. Food Rev. Int. 2020, 1–31. [Google Scholar] [CrossRef]
- Ding, Y.; Morozova, K.; Scampicchio, M.; Ferrentino, G. Non-Extractable Polyphenols from Food by-Products: Current Knowledge on Recovery, Characterisation, and Potential Applications. Processes 2020, 8, 925. [Google Scholar] [CrossRef]
- Jeswani, H.K.; Figueroa-Torres, G.; Azapagic, A. The extent of food waste generation in the UK and its environmental impacts. Sustain. Prod. Consum. 2021, 26, 532–547. [Google Scholar] [CrossRef]
- Cisneros-Yupanqui, M.; Zagotto, A.; Alberton, A.; Lante, A.; Zagotto, G.; Ribaudo, G.; Rizzi, C. Study of the phenolic profile of a grape pomace powder and its impact on delaying corn oil oxidation. Nat. Prod. Res. 2020, 1–5. [Google Scholar] [CrossRef]
- Da Fonseca Machado, A.P.; Geraldi, M.V.; do Nascimento, R.D.P.; Moya, A.M.T.M.; Vezza, T.; Diez-Echave, P.; Gálvez, J.J.; Cazarin, C.B.B.; Maróstica Júnior, M.R. Polyphenols from food by-products: An alternative or complementary therapy to IBD conventional treatments. Food Res. Int. 2021, 140, 110018. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Popova, A.; Desseva, I.; Petkova, N.; Stoyanova, M.; Vrancheva, R.; Slavov, A.; Slavchev, A.; Lante, A. Comparative Study of Early-and Mid-Ripening Peach (Prunus persica L.) Varieties: Biological Activity, Macro-, and Micro-Nutrient Profile. Foods 2021, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, D.; Desseva, I.; Popova, A.; Dincheva, I.; Vrancheva, R.; Lante, A.; Krastanov, A. GC-MS metabolic profile and α-glucosidase-, α-amylase-, lipase-, and acetylcholinesterase-inhibitory activities of eight peach varieties. Molecules 2021, 26, 4183. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Yupanqui, M.; Chalova, V.I.; Kalaydzhiev, H.R.; Mihaylova, D.; Krastanov, A.I.; Lante, A. Preliminary Characterisation of Wastes Generated from the Rapeseed and Sunflower Protein Isolation Process and Their Valorisation in Delaying Oil Oxidation. Food Bioprocess Technol. 2021, 14, 1962–1971. [Google Scholar] [CrossRef]
- Zabihpour, T.; Ebrahimi, P.; Kartalaee, N.M.; Morakabati, N.; Dehghan, L.; Latifi, Z.; Shahidi, S.A. Determination of Total Phenolic and Flavonoid Contents, Antioxidant Activity and B Vitamins of Different Iranian Non-Alcoholic Beers. Int. J. Mod. Agric. 2021, 10, 308–318. [Google Scholar]
- Albergamo, A.; Salvo, A.; Carabetta, S.; Arrigo, S.; Di Sanzo, R.; Costa, R.; Dugo, G.; Russo, M. Development of an antioxidant formula based on peanut by-products and effects on sensory properties and aroma stability of fortified peanut snacks during storage. J. Sci. Food Agric. 2021, 101, 638–647. [Google Scholar] [CrossRef]
- Boghori, P.; Latifi, Z.; Ebrahimi, P.; Mohamadi Kartalaei, N.; Dehghan, L. Effect of whey protein concentrate-Shiraz thyme (Zataria multiflora) essential oil coating on the shelf life of peanut. J. Adv. Pharm. Educ. Res. 2020, 10, 131–138. [Google Scholar]
- Cisneros-Yupanqui, M.; Lante, A. Tea from the Food Science Perspective: An Overview. Open Biotechnol. J. 2020, 14, 78–83. [Google Scholar] [CrossRef]
- Latifi, Z.; Biderooni, B.I.; Ebrahimi, P.; Moghadam, S.K.; Azadi, R.; Nasiraie, L.R. Effect of adding cinnamon and using spray drying method on antioxidant properties of instant green tea. Arch. Pharm. Pract. 2020, 11, 118–123. [Google Scholar]
- Prandi, B.; Ferri, M.; Monari, S.; Zurlini, C.; Cigognini, I.; Verstringe, S.; Schaller, D.; Walter, M.; Navarini, L.; Tassoni, A.; et al. Extraction and Chemical Characterization of Functional Phenols and Proteins from Coffee (Coffea arabica) by-Products. Biomolecules 2021, 11, 1571. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Lante, A. Polyphenols: A Comprehensive Review of their Nutritional Properties. Open Biotechnol. J. 2021, 15, 164–172. [Google Scholar] [CrossRef]
- Ebrahimi, P.; Shahidi, S.-A.; Bijad, M. A rapid voltammetric strategy for determination of ferulic acid using electrochemical nanostructure tool in food samples. J. Food Meas. Charact. 2020, 14, 3389–3396. [Google Scholar] [CrossRef]
- Mihaylova, D.; Popova, A.; Alexieva, I.; Krastanov, A.; Lante, A. Polyphenols as Suitable Control for Obesity and Diabetes. Open Biotechnol. J. 2018, 12, 219–228. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green non-conventional techniques for the extraction of polyphenols from agricultural food by-products: A review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Cano, A.; Andres, M.; Chiralt, A.; González-Martinez, C. Use of tannins to enhance the functional properties of protein based films. Food Hydrocoll. 2020, 100, 105443. [Google Scholar] [CrossRef]
- Weremfo, A.; Adulley, F.; Adarkwah-Yiadom, M. Simultaneous Optimization of Microwave-Assisted Extraction of Phenolic Compounds and Antioxidant Activity of Avocado (Persea americana Mill.) Seeds Using Response Surface Methodology. J. Anal. Methods Chem. 2020, 2020, 7541927. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC—Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Batista, J.D.F.; Dantas, A.M.; dos Santos Fonseca, J.V.; Madruga, M.S.; Fernandes, F.A.N.; Rodrigues, S.; da Silva Campelo Borges, G. Effects of cold plasma on avocado pulp (Persea americana Mill.): Chemical characteristics and bioactive compounds. J. Food Process. Preserv. 2021, 45, e15179. [Google Scholar] [CrossRef]
- Zderic, A.; Zondervan, E. Polyphenol extraction from fresh tea leaves by pulsed electric field: A study of mechanisms. Chem. Eng. Res. Des. 2016, 109, 586–592. [Google Scholar] [CrossRef]
- Alvarez-Rivera, G.; Bueno, M.; Ballesteros-Vivas, D.; Mendiola, J.A.; Ibañez, E. Pressurized Liquid Extraction. In Liquid-Phase Extraction; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–398. ISBN 9780128169117. [Google Scholar]
- Coelho, M.; Pereira, R.; Rodrigues, A.S.; Teixeira, J.A.; Pintado, M.E. Extraction of tomato by-products’ bioactive compounds using ohmic technology. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Moreira, S.A.; Alexandre, E.M.C.; Pintado, M.; Saraiva, J.A. Effect of emergent non-thermal extraction technologies on bioactive individual compounds profile from different plant materials. Food Res. Int. 2019, 115, 177–190. [Google Scholar] [CrossRef] [PubMed]
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant efficacy of litchi (Litchi chinensis Sonn.) pericarp extract in sheep meat nuggets. Antioxidants 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Yupanqui, M.; Lante, A.; Rizzi, C. Preliminary Characterization of a Functional Jam from Red Chicory by-Product. Open Biotechnol. J. 2021, 15, 183–189. [Google Scholar] [CrossRef]
- Jirasuteeruk, C.; Theerakulkait, C. Ultrasound-Assisted Extraction of Phenolic Compounds from Mango (Mangifera indica cv. Chok Anan) Peel and Its Inhibitory Effect on Enzymatic Browning of Potato Puree. Food Technol. Biotechnol. 2019, 57, 350–357. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, B.R.; Oliveira, M.B.P.P.; Barros, L.; Ferreira, I.C.F.R. Could fruits be a reliable source of food colorants? Pros and cons of these natural additives. Crit. Rev. Food Sci. Nutr. 2021, 61, 805–835. [Google Scholar] [CrossRef]
- Shahidi, S.-A.; Ebrahimi, P.; Zabihpour, T.; Raeisi, S.N. Electrochemical Analysis of Sunset Yellow Based on NiO-SWCNTs NC/IL Modified Carbon Paste Electrode in Food Samples. J. Food Biosci. Technol. 2021, 11, 11–22. [Google Scholar]
- Caleja, C.; Ribeiro, A.; Filomena Barreiro, M.; CFR Ferreira, I. Phenolic compounds as nutraceuticals or functional food ingredients. Curr. Pharm. Des. 2017, 23, 2787–2806. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, X.; Qin, Y.-R.; Zhang, Y.-H.; Wang, X.-P.; Wang, J.-Y.; Ning, Z.-X.; Ruan, Q.-J.; Zhang, Y.-S. Preparation and characterization of a novel colorimetric indicator film based on gelatin/polyvinyl alcohol incorporating mulberry anthocyanin extracts for monitoring fish freshness. Food Res. Int. 2019, 126, 108604. [Google Scholar] [CrossRef]
- Zhang, W.; Jiang, H.; Rhim, J.W.; Cao, J.; Jiang, W. Tea polyphenols (TP): A promising natural additive for the manufacture of multifunctional active food packaging films. Crit. Rev. Food Sci. Nutr. 2021, 1–14. [Google Scholar] [CrossRef]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Mukundan, U. Polyphenols: Methods of Extraction. Sci. Rev. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.V.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef] [PubMed]
- Tzima, K.; Brunton, N.P.; Lyng, J.G.; Frontuto, D.; Rai, D.K. The effect of Pulsed Electric Field as a pre-treatment step in Ultrasound Assisted Extraction of phenolic compounds from fresh rosemary and thyme by-products. Innov. Food Sci. Emerg. Technol. 2021, 69, 102644. [Google Scholar] [CrossRef]
- Hosseini, H.; Bolourian, S.; Yaghoubi Hamgini, E.; Ghanuni Mahababadi, E. Optimization of heat- and ultrasound-assisted extraction of polyphenols from dried rosemary leaves using response surface methodology. J. Food Process. Preserv. 2018, 42, e13778. [Google Scholar] [CrossRef]
- Barbosa-Cánovas, G.V.; Donsì, F.; Yildiz, S.; Candoğan, K.; Pokhrel, P.R.; Guadarrama-Lezama, A.Y. Nonthermal Processing Technologies for Stabilization and Enhancement of Bioactive Compounds in Foods. Food Eng. Rev. 2022, 14, 63–99. [Google Scholar] [CrossRef]
- Lante, A.; Friso, D. Oxidative stability and rheological properties of nanoemulsions with ultrasonic extracted green tea infusion. Food Res. Int. 2013, 54, 269–276. [Google Scholar] [CrossRef]
- Da Silva, H.R.P.; da Silva, C.; Bolanho, B.C. Ultrasonic-assisted extraction of betalains from red beet (Beta vulgaris L.). J. Food Process Eng. 2018, 41, e12833. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Canovas, G.V.; Orlien, V. Bioaccessibility of bioactive compounds from fruits and vegetables after thermal and nonthermal processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Tiwari, B.K.; Mason, T.J. Ultrasound processing of fluid foods. In Novel Thermal and Non-Thermal Technologies for Fluid Foods; Academic Press: San Diego, CA, USA, 2012; pp. 135–165. [Google Scholar]
- Wen, L.; Zhang, Z.; Rai, D.; Sun, D.W.; Tiwari, B.K. Ultrasound-assisted extraction (UAE) of bioactive compounds from coffee silverskin: Impact on phenolic content, antioxidant activity, and morphological characteristics. J. Food Process Eng. 2019, 42, e13191. [Google Scholar] [CrossRef]
- Zhou, T.; Xu, D.-P.; Lin, S.-J.; Li, Y.; Zheng, J.; Zhou, Y.; Zhang, J.-J.; Li, H.-B. Ultrasound-assisted extraction and identification of natural antioxidants from the fruit of Melastoma sanguineum Sims. Molecules 2017, 22, 306. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Khan, M.K. Applications of ultrasound in food technology: Processing, preservation and extraction. Ultrason. Sonochem. 2011, 18, 813–835. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, G.; Balzan, S.; Montemurro, F.; Fasolato, L.; Andrighetto, I.; Segato, S.; Novelli, E. Effect of ultrasound alone or ultrasound coupled with CO2 on the chemical composition, cheese-making properties and sensory traits of raw milk. Innov. Food Sci. Emerg. Technol. 2012, 16, 391–397. [Google Scholar] [CrossRef]
- Petkova, N.; Ivanov, I.; Mihaylova, D.; Lante, A. Effect of pressure liquid extraction and ultrasonic irradiation frequency on inulin, phenolic content and antioxidant activity in burdock (Arctium lappa L.) roots. Acta Sci. Pol. Hortorum Cultus 2020, 19, 125–133. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Martiny, T.R.; Dotto, G.L.; Vanga, S.K.; Parrine, D.; Gariepy, Y.; Lefsrud, M.; Raghavan, V. Eco-friendly extraction for the recovery of bioactive compounds from Brazilian olive leaves. Sustain. Mater. Technol. 2021, 28, 3–8. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Ostojić, J.; Miletić, V.; Djaković, S.; Bosiljkov, T.; Zorić, Z.; Ježek, D.; Rimac Brnčić, S.; Brnčić, M. Application of high hydrostatic pressure and ultrasound-assisted extractions as a novel approach for pectin and polyphenols recovery from tomato peel waste. Innov. Food Sci. Emerg. Technol. 2020, 64, 102424. [Google Scholar] [CrossRef]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a sustainable ultrasound-assisted extraction method for the recovery of polyphenols from lemon by-products: Comparison with hot water and organic solvent extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef]
- Kaur, B.; Panesar, P.S.; Anal, A.K. Standardization of ultrasound assisted extraction for the recovery of phenolic compounds from mango peels. J. Food Sci. Technol. 2021. [Google Scholar] [CrossRef]
- Nutter, J.; Fernandez, M.V.; Jagus, R.J.; Agüero, M.V. Development of an aqueous ultrasound-assisted extraction process of bioactive compounds from beet leaves: A proposal for reducing losses and increasing biomass utilization. J. Sci. Food Agric. 2020, 101, 1989–1997. [Google Scholar] [CrossRef]
- Motikar, P.D.; More, P.R.; Arya, S.S. A novel, green environment-friendly cloud point extraction of polyphenols from pomegranate peels: A comparative assessment with ultrasound and microwave-assisted extraction. Sep. Sci. Technol. 2020, 56, 1014–1025. [Google Scholar] [CrossRef]
- Vural, N.; Algan Cavuldak, Ö.; Anlı, R.E. Multi response optimisation of polyphenol extraction conditions from grape seeds by using ultrasound assisted extraction (UAE). Sep. Sci. Technol. 2018, 53, 1540–1551. [Google Scholar] [CrossRef]
- Da Rosa, G.S.; Vanga, S.K.; Gariepy, Y.; Raghavan, V. Comparison of microwave, ultrasonic and conventional techniques for extraction of bioactive compounds from olive leaves (Olea europaea L.). Innov. Food Sci. Emerg. Technol. 2019, 58, 102234. [Google Scholar] [CrossRef]
- Rodsamran, P.; Sothornvit, R. Extraction of phenolic compounds from lime peel waste using ultrasonic-assisted and microwave-assisted extractions. Food Biosci. 2019, 28, 66–73. [Google Scholar] [CrossRef]
- Parniakov, O.; Apicella, E.; Koubaa, M.; Barba, F.J.; Grimi, N.; Lebovka, N.; Pataro, G.; Ferrari, G.; Vorobiev, E. Ultrasound-assisted green solvent extraction of high-added value compounds from microalgae Nannochloropsis spp. Bioresour. Technol. 2015, 198, 262–267. [Google Scholar] [CrossRef]
- Bengardino, M.B.; Fernandez, M.V.; Nutter, J.; Jagus, R.J.; Agüero, M.V. Recovery of bioactive compounds from beet leaves through simultaneous extraction: Modelling and process optimization. Food Bioprod. Process. 2019, 118, 227–236. [Google Scholar] [CrossRef]
- Gadkari, P.V.; Balarman, M.; Kadimi, U.S. Polyphenols from fresh frozen tea leaves (Camellia assamica L.) by supercritical carbon dioxide extraction with ethanol entrainer-application of response surface methodology. J. Food Sci. Technol. 2015, 52, 720–730. [Google Scholar] [CrossRef][Green Version]
- Nüchter, M.; Ondruschka, B.; Bonrath, W.; Gum, A. Microwave assisted synthesis-a critical technology overview. Green Chem. 2004, 6, 128–141. [Google Scholar] [CrossRef]
- Chuyen, H.V.; Nguyen, M.H.; Roach, P.D.; Golding, J.B.; Parks, S.E. Microwave-assisted extraction and ultrasound-assisted extraction for recovering carotenoids from Gac peel and their effects on antioxidant capacity of the extracts. Food Sci. Nutr. 2018, 6, 189–196. [Google Scholar] [CrossRef]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar] [CrossRef]
- Suriapparao, D.V.; Vinu, R. Resource recovery from synthetic polymers via microwave pyrolysis using different susceptors. J. Anal. Appl. Pyrolysis 2015, 113, 701–712. [Google Scholar] [CrossRef]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Wai-Lee Kung, F. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45. [Google Scholar] [CrossRef]
- Ekezie, F.-G.C.; Sun, D.-W.; Cheng, J.-H. Acceleration of microwave-assisted extraction processes of food components by integrating technologies and applying emerging solvents: A review of latest developments. Trends Food Sci. Technol. 2017, 67, 160–172. [Google Scholar] [CrossRef]
- Bai, X.L.; Yue, T.L.; Yuan, Y.H.; Zhang, H.W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Pettinato, M.; Perego, P. Polyphenols from apple skins: A study on microwave-assisted extraction optimization and exhausted solid characterization. Sep. Purif. Technol. 2020, 240, 116640. [Google Scholar] [CrossRef]
- Mandal, V.; Mohan, Y.; Hemalatha, S. Microwave Assisted Extraction—An Innovative and Promising Extraction Tool for Medicinal Plant Research. Pharmacogn. Rev. 2007, 1, 7–18. [Google Scholar]
- Xiao, W.; Han, L.; Shi, B. Microwave-assisted extraction of flavonoids from Radix Astragali. Sep. Purif. Technol. 2008, 62, 614–618. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, J.; Wang, X.; Zhao, B.; Wu, Y.; Yao, J. A comparison study on microwave-assisted extraction of Artemisia sphaerocephala polysaccharides with conventional method: Molecule structure and antioxidant activities evaluation. Int. J. Biol. Macromol. 2009, 45, 483–492. [Google Scholar] [CrossRef]
- Sun, Y.; Liao, X.; Wang, Z.; Hu, X.; Chen, F. Optimization of microwave-assisted extraction of anthocyanins in red raspberries and identification of anthocyanin of extracts using high-performance liquid chromatography-mass spectrometry. Eur. Food Res. Technol. 2007, 225, 511–523. [Google Scholar] [CrossRef]
- Zhou, H.-Y.; Liu, C.-Z. Microwave-assisted extraction of solanesol from tobacco leaves. J. Chromatogr. A 2006, 1129, 135–139. [Google Scholar] [CrossRef]
- Mandal, V.; Mandal, S.C. Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem. Eng. J. 2010, 50, 63–70. [Google Scholar] [CrossRef]
- Lu, Y.; Yue, X.-F.; Zhang, Z.-Q.; Li, X.-X.; Wang, K. Analysis of Rodgersia aesculifolia Batal. Rhizomes by Microwave-Assisted Solvent Extraction and GC-MS. Chromatographia 2007, 66, 443–446. [Google Scholar] [CrossRef]
- Lianfu, Z.; Zelong, L. Optimization and comparison of ultrasound/microwave assisted extraction (UMAE) and ultrasonic assisted extraction (UAE) of lycopene from tomatoes. Ultrason. Sonochem. 2008, 15, 731–737. [Google Scholar] [CrossRef]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Kashif, M.; Khan, I.; Ansar, M.; Nazir, A.; Maan, A. Sustainable dehydration of onion slices through novel microwave hydro-diffusion gravity technique. Innov. Food Sci. Emerg. Technol. 2016, 33, 327–332. [Google Scholar] [CrossRef]
- Mihaylova, D.S.; Lante, A.; Tinello, F.; Krastanov, A.I. Study on the antioxidant and antimicrobial activities of Allium ursinum L. pressurised-liquid extract. Nat. Prod. Res. 2014, 28, 2000–2005. [Google Scholar] [CrossRef]
- Mihaylova, D.; Lante, A.; Krastanov, A.I. A study on the antioxidant and antimicrobial activities of pressurized-liquid extracts of Clinopodium vulgare and Sideritis scardica. Agro Food Ind. Hi Tech. 2014, 25, 55–58. [Google Scholar]
- Pagano, I.; Piccinelli, A.L.; Celano, R.; Campone, L.; Gazzerro, P.; Russo, M.; Rastrelli, L. Pressurized hot water extraction of bioactive compounds from artichoke by-products. Electrophoresis 2018, 39, 1899–1907. [Google Scholar] [CrossRef]
- Mazzutti, S.; Rodrigues, L.G.G.; Mezzomo, N.; Venturi, V.; Ferreira, S.R.S. Integrated green-based processes using supercritical CO2 and pressurized ethanol applied to recover antioxidant compouds from cocoa (Theobroma cacao) bean hulls. J. Supercrit. Fluids 2018, 135, 52–59. [Google Scholar] [CrossRef]
- García, P.; Fredes, C.; Cea, I.; Lozano-Sánchez, J.; Leyva-Jiménez, F.J.; Robert, P.; Vergara, C.; Jimenez, P. Recovery of Bioactive Compounds from Pomegranate (Punica granatum L.) Peel Using Pressurized Liquid Extraction. Foods 2021, 10, 203. [Google Scholar] [CrossRef]
- Espada-Bellido, E.; Ferreiro-González, M.; Barbero, G.F.; Carrera, C.; Palma, M.; Barroso, C.G. Alternative Extraction Method of Bioactive Compounds from Mulberry (Morus nigra L.) Pulp Using Pressurized-Liquid Extraction. Food Anal. Methods 2018, 11, 2384–2395. [Google Scholar] [CrossRef]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- Luthria, D.L. Influence of experimental conditions on the extraction of phenolic compounds from parsley (Petroselinum crispum) flakes using a pressurized liquid extractor. Food Chem. 2008, 107, 745–752. [Google Scholar] [CrossRef]
- Pereira, D.T.V.; Tarone, A.G.; Cazarin, C.B.B.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from grape marc. J. Food Eng. 2019, 240, 105–113. [Google Scholar] [CrossRef]
- Haberl, S.; Miklavčič, D.; Serša, G.; Frey, W.; Rubinsky, B. Cell membrane electroporation-Part 2: The applications. IEEE Electr. Insul. Mag. 2013, 29, 29–37. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation of cells and tissues. IEEE Trans. Plasma Sci. 2000, 28, 24–33. [Google Scholar] [CrossRef]
- Soliva-Fortuny, R.; Balasa, A.; Knorr, D.; Martín-Belloso, O. Effects of pulsed electric fields on bioactive compounds in foods: A review. Trends Food Sci. Technol. 2009, 20, 544–556. [Google Scholar] [CrossRef]
- Yarmush, M.L.; Golberg, A.; Serša, G.; Kotnik, T.; Miklavčič, D. Electroporation-Based Technologies for Medicine: Principles, Applications, and Challenges. Annu. Rev. Biomed. Eng. 2014, 16, 295–320. [Google Scholar] [CrossRef]
- Rifna, E.J.; Misra, N.N.; Dwivedi, M. Recent advances in extraction technologies for recovery of bioactive compounds derived from fruit and vegetable waste peels: A review. Crit. Rev. Food Sci. Nutr. 2021, 1–34. [Google Scholar] [CrossRef]
- Luengo, E.; Franco, E.; Ballesteros, F.; Álvarez, I.; Raso, J. Winery Trial on Application of Pulsed Electric Fields for Improving Vinification of Garnacha Grapes. Food Bioprocess Technol. 2014, 7, 1457–1464. [Google Scholar] [CrossRef]
- Rtolas, E.P.; Saldaña, G.; Alvarez, I.; Raso, J. Effect of Pulsed Electric Field Processing of Red Grapes on Wine Chromatic and Phenolic Characteristics during Aging in Oak Barrels. J. Agric. Food Chem. 2010, 58, 2351–2357. [Google Scholar] [CrossRef]
- Peiró, S.; Luengo, E.; Segovia, F.; Raso, J.; Almajano, M.P. Improving Polyphenol Extraction from Lemon Residues by Pulsed Electric Fields. Waste Biomass Valoriz. 2019, 10, 889–897. [Google Scholar] [CrossRef]
- Rajha, H.N.; Abi-Khattar, A.M.; El Kantar, S.; Boussetta, N.; Lebovka, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Comparison of aqueous extraction efficiency and biological activities of polyphenols from pomegranate peels assisted by infrared, ultrasound, pulsed electric fields and high-voltage electrical discharges. Innov. Food Sci. Emerg. Technol. 2019, 58, 102212. [Google Scholar] [CrossRef]
- Parniakov, O.; Barba, F.J.; Grimi, N.; Lebovka, N.; Vorobiev, E. Extraction assisted by pulsed electric energy as a potential tool for green and sustainable recovery of nutritionally valuable compounds from mango peels. Food Chem. 2016, 192, 842–848. [Google Scholar] [CrossRef]
- Segovia, F.J.; Luengo, E.; Corral-Pérez, J.J.; Raso, J.; Almajano, M.P. Improvements in the aqueous extraction of polyphenols from borage (Borago officinalis L.) leaves by pulsed electric fields: Pulsed electric fields (PEF) applications. Ind. Crop. Prod. 2015, 65, 390–396. [Google Scholar] [CrossRef]
- El Kantar, S.; Boussetta, N.; Lebovka, N.; Foucart, F.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Pulsed electric field treatment of citrus fruits: Improvement of juice and polyphenols extraction. Innov. Food Sci. Emerg. Technol. 2018, 46, 153–161. [Google Scholar] [CrossRef]
- Sarkis, J.R.; Boussetta, N.; Blouet, C.; Tessaro, I.C.; Marczak, L.D.F.; Vorobiev, E. Effect of pulsed electric fields and high voltage electrical discharges on polyphenol and protein extraction from sesame cake. Innov. Food Sci. Emerg. Technol. 2015, 29, 170–177. [Google Scholar] [CrossRef]
- Fincan, M. Extractability of phenolics from spearmint treated with pulsed electric field. J. Food Eng. 2015, 162, 31–37. [Google Scholar] [CrossRef]
- Jan, B.; Shams, R.; Rizvi, Q.E.H.; Manzoor, A. Ohmic heating technology for food processing: A review of recent developments. J. Postharvest Technol. 2021, 9, 20–34. [Google Scholar]
- Kumar, T. A review on ohmic heating technology: Principle, applications and scope. Int. J. Agric. Environ. Biotechnol. 2018, 11, 679–687. [Google Scholar] [CrossRef]
- Wu, D.; Forghani, F.; Banan-Mwine Daliri, E.; Li, J.; Liao, X.; Liu, D.; Ye, X.; Chen, S.; Ding, T. Microbial response to some nonthermal physical technologies. Trends Food Sci. Technol. 2019, 95, 107–117. [Google Scholar] [CrossRef]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A.; Cappato, L.P.; da Silva Ferreira, M.V.; da Silva Rocha, R.; da Cruz, A.G. Ohmic heating for the dairy industry: A potential technology to develop probiotic dairy foods in association with modifications of whey protein structure. Curr. Opin. Food Sci. 2018, 22, 95–101. [Google Scholar] [CrossRef]
- Khajehei, F.; Niakousari, M.; Damyeh, M.S.; Merkt, N.; Claupein, W.; Graeff-Hoenninger, S. Impact of Ohmic-Assisted Decoction on Bioactive Components Extracted from Yacon (Smallanthus sonchifolius Poepp.) Leaves: Comparison with Conventional Decoction. Molecules 2017, 22, 2043. [Google Scholar] [CrossRef]
- Kutlu, N.; Isci, A.; Sakiyan, O.; Yilmaz, A.E. Effect of ohmic heating on ultrasound extraction of phenolic compounds from cornelian cherry (Cornus mas). J. Food Process. Preserv. 2021, 45, e15818. [Google Scholar] [CrossRef]
- Pereira, R.N.; Coelho, M.I.; Genisheva, Z.; Fernandes, J.M.; Vicente, A.A.; Pintado, M.E.; Teixeira, E.J.A. Using Ohmic Heating effect on grape skins as a pretreatment for anthocyanins extraction. Food Bioprod. Process. 2020, 124, 320–328. [Google Scholar] [CrossRef]
- Coelho, M.I.; Pereira, R.N.C.; Teixeira, J.A.; Pintado, M.E. Valorization of tomato wastes: Influence of ohmic heating process on polyphenols extraction time. Food Bioprod. Process. 2019, 117, 329–339. [Google Scholar] [CrossRef]
- Jesus, M.S.; Ballesteros, L.F.; Pereira, R.N.; Genisheva, Z.; Carvalho, A.C.; Pereira-Wilson, C.; Teixeira, J.A.; Domingues, L. Ohmic heating polyphenolic extracts from vine pruning residue with enhanced biological activity. Food Chem. 2020, 316, 126298. [Google Scholar] [CrossRef]
- Moongngarm, A.; Sriharboot, N.; Loypimai, P.; Moontree, T. Ohmic heating-assisted water extraction of steviol glycosides and phytochemicals from Stevia rebaudiana leaves. LWT 2022, 154, 112798. [Google Scholar] [CrossRef]
- Muhammad, A.I.; Xiang, Q.; Liao, X.; Liu, D.; Ding, T. Understanding the Impact of Nonthermal Plasma on Food Constituents and Microstructure—A Review. Food Bioprocess Technol. 2018, 11, 463–486. [Google Scholar] [CrossRef]
- Keshavarzi, M.; Najafi, G.; Gavlighi, H.A.; Seyfi, P.; Ghomi, H. Enhancement of polyphenolic content extraction rate with maximal antioxidant activity from green tea leaves by cold plasma. J. Food Sci. 2020, 85, 3415–3422. [Google Scholar] [CrossRef]
- Amini, M.; Ghoranneviss, M. Black and Green Tea Decontamination by Cold Plasma. Res. J. Microbiol. 2016, 11, 42–46. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, R.; Gan, Z.; Shao, T.; Zhang, X.; He, M.; Sun, A. Effect of cold plasma on blueberry juice quality. Food Chem. 2019, 290, 79–86. [Google Scholar] [CrossRef]
- Sharma, R.R.; Reddy, S.V.R.; Sethi, S. Cold Plasma Technology for Surface Disinfection of Fruits and Vegetables. In Postharvest Disinfection of Fruits and Vegetables; Academic Press: London, UK, 2018; pp. 197–209. [Google Scholar]
- Tappi, S.; Berardinelli, A.; Ragni, L.; Dalla Rosa, M.; Guarnieri, A.; Rocculi, P. Atmospheric gas plasma treatment of fresh-cut apples. Innov. Food Sci. Emerg. Technol. 2014, 21, 114–122. [Google Scholar] [CrossRef]
- Grzegorzewski, F.; Ehlbeck, J.; Schlüter, O.; Kroh, L.W.; Rohn, S. Treating lamb’s lettuce with a cold plasma—Influence of atmospheric pressure Ar plasma immanent species on the phenolic profile of Valerianella locusta. LWT 2011, 44, 2285–2289. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Development of cold plasma pretreatment for improving phenolics extractability from tomato pomace. Innov. Food Sci. Emerg. Technol. 2020, 65, 102445. [Google Scholar] [CrossRef]
- Bao, Y.; Reddivari, L.; Huang, J.Y. Enhancement of phenolic compounds extraction from grape pomace by high voltage atmospheric cold plasma. LWT 2020, 133, 109970. [Google Scholar] [CrossRef]
- Lante, A.; Nardi, T.; Zocca, F.; Giacomini, A.; Corich, V. Evaluation of Red Chicory Extract as a Natural Antioxidant by Pure Lipid Oxidation and Yeast Oxidative Stress Response as Model Systems. J. Agric. Food Chem. 2011, 59, 5318–5324. [Google Scholar] [CrossRef]
- Tinello, F.; Mihaylova, D.; Lante, A. Effect of Dipping Pre-treatment with Unripe Grape Juice on Dried “Golden Delicious” Apple Slices. Food Bioprocess Technol. 2018, 11, 2275–2285. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Nicoletto, M. UV-A light treatment for controlling enzymatic browning of fresh-cut fruits. Innov. Food Sci. Emerg. Technol. 2016, 34, 141–147. [Google Scholar] [CrossRef]
- Paixão, L.M.N.; Fonteles, T.V.; Oliveira, V.S.; Fernandes, F.A.N.; Rodrigues, S. Cold Plasma Effects on Functional Compounds of Siriguela Juice. Food Bioprocess Technol. 2019, 12, 110–121. [Google Scholar] [CrossRef]
- Khani, M.R.; Shokri, B.; Khajeh, K. Studying the performance of dielectric barrier discharge and gliding arc plasma reactors in tomato peroxidase inactivation. J. Food Eng. 2017, 197, 107–112. [Google Scholar] [CrossRef]
- Tinello, F.; Lante, A. Evaluation of antibrowning and antioxidant activities in unripe grapes recovered during bunch thinning. Aust. J. Grape Wine Res. 2017, 23, 33–41. [Google Scholar] [CrossRef]
- Lante, A.; Tinello, F.; Lomolino, G. The use of polyphenol oxidase activity to identify a potential raisin variety. Food Biotechnol. 2016, 30, 98–109. [Google Scholar] [CrossRef]
- Han, Y.; Cheng, J.H.; Sun, D.W. Activities and conformation changes of food enzymes induced by cold plasma: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 794–811. [Google Scholar] [CrossRef]
- De Castro, D.R.G.; Mar, J.M.; da Silva, L.S.; da Silva, K.A.; Sanches, E.A.; de Araújo Bezerra, J.; Rodrigues, S.; Fernandes, F.A.N.; Campelo, P.H. Dielectric barrier atmospheric cold plasma applied on camu-camu juice processing: Effect of the excitation frequency. Food Res. Int. 2020, 131, 109044. [Google Scholar] [CrossRef]
- Xu, B.; Chen, J.; Chitrakar, B.; Li, H.; Wang, J.; Wei, B.; Zhou, C.; Ma, H. Effects of flat sweep frequency and pulsed ultrasound on the activity, conformation and microstructure of mushroom polyphenol oxidase. Ultrason. Sonochem. J. 2022, 82, 105908. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, F.; Wang, D.; Zhao, M.; Cui, N.; Gao, G.; Guo, J.; Zhang, Q. Optimization of the process parameters of ultrasound on inhibition of polyphenol oxidase activity in whole potato tuber by response surface methodology. LWT 2021, 144, 111232. [Google Scholar] [CrossRef]
- Li, X.; Xu, X.; Wang, L.; Regenstein, J.M. Effect of ohmic heating on physicochemical properties and the key enzymes of water chestnut juice. J. Food Process. Preserv. 2019, 43, e13919. [Google Scholar] [CrossRef]
- Kanjanapongkul, K.; Baibua, V. Effects of ohmic pasteurization of coconut water on polyphenol oxidase and peroxidase inactivation and pink discoloration prevention. J. Food Eng. 2021, 292, 110268. [Google Scholar] [CrossRef]
- Manzoor, M.F.; Ahmed, Z.; Ahmad, N.; Karrar, E.; Rehman, A.; Aadil, R.M.; Al-Farga, A.; Iqbal, M.W.; Rahaman, A.; Zeng, X.-A. Probing the combined impact of pulsed electric field and ultra-sonication on the quality of spinach juice. J. Food Process. Preserv. 2021, 45, e15475. [Google Scholar] [CrossRef]
- Bußler, S.; Ehlbeck, J.; Schlüter, O.K. Pre-drying treatment of plant related tissues using plasma processed air: Impact on enzyme activity and quality attributes of cut apple and potato. Innov. Food Sci. Emerg. Technol. 2017, 40, 78–86. [Google Scholar] [CrossRef]
- Zhou, L.; Tey, C.Y.; Bingol, G.; Balaban, M.O.; Cai, S. Effect of different microwave power levels on inactivation of PPO and PME and also on quality changes of peach puree. Curr. Res. Food Sci. 2022, 5, 41–48. [Google Scholar] [CrossRef]


| Food By-Product | Solvent | Solid/Solvent Ratio (w/v) | Power (W) (Based on Amplitude) | Frequency (kHz) | Extraction Time (min) | TPC 1 in Final Extract (mg GAE 2/g) | Reference |
|---|---|---|---|---|---|---|---|
| Coffee silverskin | Methanol (80%) | 1:50 | NR 3 | 20 | 10 | 8.94 ± 0.01 | [52] |
| Brazilian olive leaves | Water | 0.5:25 | 247.5 | 20 | 29 | 80.51 ± 1.52 | [57] |
| Potato peels | Methanol (80%) | 1:10 | 100 | 33 | 900 | 4.24 ± 0.01 | [58] |
| Tomato peel | Ethanol (70%) | 1:50 | 380 | 30 | 15 | 36.43 ± 0.1 | [59] |
| Lemon wastes | Water | 1:100 | 250 | 43 ± 2 | 45 | 18.10 ± 0.24 | [60] |
| Mango peels | Ethanol (50%) | 1:30 | NR | NR | 10 | 35.5 | [61] |
| Mango peels | Water | 1:6 | 160 | 50 | 15 | 9.72 | [35] |
| Beet leaves | Water | 1:20 | 90 | 20 | 16 | 14.9 | [62] |
| Pomegranate peels | Ethanol (70%) | 1:70 | 140 | 40 | 30 | 69.89 ± 0.45 | [63] |
| Grape seeds | Ethanol (61.76%) | 1:30 | 250 | 28 | 20 | 25.96 ± 0.70 | [64] |
| Olive leaves | Water | 05:25 | 112.5 | 20 | 25 | 79.77 | [65] |
| Lime peel | Ethanol (55%) | 1.5:30 | NR | NR | 4 | 54 ± 0.2 | [66] |
| Food By-Product | Solvent | Solid/Solvent Ratio (w/v) | Power (W) | Temperature (°C) | Time (s) | TPC 1 in Final Extract (mg GAE 2/g) | Reference |
|---|---|---|---|---|---|---|---|
| Lime peel | Ethanol (55%) | 1.5:30 | 140 | Lower than 60 | 45 | 35 ± 0.5 | [66] |
| Apple pomace | Ethanol (62.1%) | 1:22.9 | 650.4 | 70 | 53.7 | ≈0.62 | [77] |
| Avocado seeds | Ethanol (60%) | 1:20 | 400 | NR 3 | 180 | 82.36 ± 1.05 | [26] |
| Pomegranate peels | Water | 5:100 | 600 | NR | 60 | 87.81 ± 0.83 | [63] |
| Blueberry leaves | Ethanol (30%) + citric acid (1.5 M) | 0.5:80 | 142.1 | NR | 1440 | 128.760 ± 1.2961 | [75] |
| Brazilian olive leaves | Water | 0.5:25 | 1000 | 86 | 180 | 104.22 ± 0.61 | [57] |
| Apple skins | Ethanol (68%) | 2:20 | NR | 150 | 5400 | 50.4 ± 3.4 | [78] |
| Olive leaves | Ethanol (70%) | 0.5:25 | 1000 | 65 | 300 | 157.62 | [65] |
| Food By-Product | Solvent | Pressure (MPa) | Temperature (°C) | Time (min) | TPC 1 in Final Extract (mg GAE 2/g) | Reference |
|---|---|---|---|---|---|---|
| Burdock roots | Ethanol (70%) | NR 3 | NR | 105 | 12.13 ± 0.34 | [56] |
| Cocoa bean hulls | Ethanol (70%) | 10 | 70 | 20 | 9.6 ± 0.3 | [92] |
| Pomegranate peel | Ethanol (77%) | 10.34 | 200 | 20 | 17 ± 3.6 | [93] |
| Mulberry pulp | Methanol (74.6%) | 10.13 | 99.4 | 10 | 2.18 | [94] |
| Jabuticaba skin | Ethanol (99.5%) | 5 | 120 | 15 | 18.7 ± 0.4 | [95] |
| Parsley flakes | Ethanol (50%) | 6.9 | 40 | 5 | 22.9 | [96] |
| Grape marc | Ethanol (50%) | 10 | 100 | 40 | 65.68 ± 2.24 | [97] |
| Food By-Product | Solvent | Electric Field Strengths (kV/cm) | Pulse Duration (μs) | Number of Pulses | TPC 1 in Final Extract (mg GAE 2/g) | Reference |
|---|---|---|---|---|---|---|
| Lemon residues | Water | 7 | 3 | 30 | 1.61 | [105] |
| Pomegranate peels | Water | 10 | NR 3 | n | 39 ± 2 | [106] |
| Mango peels | NR | 13.3 | 8.3 | 300 | 2.169 | [107] |
| Borage leaves | Acidic water | 5 | 3 | 20 | Lower than 1.2 | [108] |
| Orange peels | Water | 10 | 70 | n | 22 | [109] |
| Sesame cake | Water | 13.3 | 10 | up to 700 | Lower than 4 | [110] |
| Spearmint leaves | Mannitol | 4.5 | 10 | 99 | Lower than 10 | [111] |
| Food By-Product | Solvent | Voltage (V) | Electrical Field Strength (V/cm) | Temperature (°C) | Time (min) | TPC 1 in Final Extract (mg GAE 2/g) | Reference |
|---|---|---|---|---|---|---|---|
| Yacon leaves (red, fresh) | NaCl solution (0.3%) | 150 | NR 3 | NR | 10 | 76.67 ± 21.67 | [116] |
| Grape skins | Water | NR | 16 | 100 | 1 s | 3.2 | [118] |
| Tomato peels | Ethanol (70%) | NR | NR | 70 | 15 | 2.550 ± 0.072 | [119] |
| Vine pruning residue | Ethanol (45%) | NR | 840 | 80 | 60 | 3.1 ± 0.2 | [120] |
| Stevia rebaudiana leaves | Water | NR | 150 | NR | 1 | 84.36 ± 4.61 | [121] |
| Technique | Treated Food/Compound | Treatment Condition | Result | Reference |
|---|---|---|---|---|
| Flat sweep frequency and pulsed ultrasound | Mushroom PPO | The ultrasound was applied with a frequency that moved up and down within a predetermined range. | Treatment with dual-frequency of 22/40 kHz mode decreased PPO activity significantly. | [140] |
| Ultrasound | Potato | Power: 540 W, time: 15 min, temperature: 20 °C. | The optimal condition had the highest PPO inhibitory effect. | [141] |
| Ohmic heating | Water chestnut juice | Voltage: 220 V; electric field strength: 22, 27.5, and 36.7 V/cm; with a titanium electrode. | PPO activity decreased rapidly with ohmic heating treatment at the critical deactivation temperature (35 °C). | [142] |
| Ohmic heating | Coconut water | Electric field strength: 10 and 20 V/cm, time: 3–15 min. | At 90 °C, PPO activity decreased to about 10% of its initial activity at only 3 min. | [143] |
| Ultrasound | Spinach juice | Power: 180 W, frequency: 40 kHz, time: 21 min, temperature: 30 °C. | PPO activity decreased by 36%. | [144] |
| Cold plasma | Cut apple and potato | Time: 10 min, frequency: 2.45 GHz, power: 1.2 kW, gas flow: 20 L/min. | PPO activity was reduced by about 62% and 77% in fresh cut apple and potato tissue, respectively. | [145] |
| Pulsed electric field | Spinach juice | Electric field strength:9 kV/cm, frequency: 1 kHz, treatment time: 335 µs. | PPO activity decreased by 44%. | [144] |
| Ultrasound and pulsed electric field | Spinach juice | Ultrasound was performed before pulsed electric field. | PPO activity decreased by 56%. | [144] |
| Microwave | Peach puree | Different power densities (4.4, 7.7, and 11.0 W/g) were applied, and cooking value was observed. | The PPO significantly decreased from around 50% to around 5% with increasing the cook value level, regardless of power density applied. | [146] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, P.; Lante, A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Appl. Sci. 2022, 12, 1923. https://doi.org/10.3390/app12041923
Ebrahimi P, Lante A. Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Applied Sciences. 2022; 12(4):1923. https://doi.org/10.3390/app12041923
Chicago/Turabian StyleEbrahimi, Peyman, and Anna Lante. 2022. "Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review" Applied Sciences 12, no. 4: 1923. https://doi.org/10.3390/app12041923
APA StyleEbrahimi, P., & Lante, A. (2022). Environmentally Friendly Techniques for the Recovery of Polyphenols from Food By-Products and Their Impact on Polyphenol Oxidase: A Critical Review. Applied Sciences, 12(4), 1923. https://doi.org/10.3390/app12041923







