Abstract
The study aimed to investigate exopolysaccharides (EPSs) produced by two Antarctic yeasts isolated from Livingston Island. The species were identified as Vishniacozyma victoriae (V) and Tremellomycetes sp. (T) based on a molecular genetic analysis of ITS1-5.8S-ITS4 regions of the 18S rRNA gene. The EPS production was investigated under stress conditions in culture flasks and a bioreactor. Different chromatographic (HPLC-RID, HPSEC-RID) and spectral (FT-IR) analyses were employed to characterize EPSs. Tremellomycetes sp. accumulated 7 g/L biomass and 4.5 g/L EPS after 120 h of cultivation. The total carbohydrate content of V-EPS and T-EPS was 75.4% and 79.0%, respectively. The EPSs mainly consisted of mannose (30–32%), which was followed by glucose, xylose, galactose, and small amounts of uronic acids (6.3–7.0%). EPSs had appreciable amounts of proteins (11–12%). The FT-IR spectra contained absorption bands typical for hetero-mannans and β-glucans (797–1033 cm−1). EPSs were heterogeneous with a broad molecular weight distribution range (47 × 104–68 × 104 g/mol). In conclusion, both yeasts synthesized high-molecular-weight heteromannans, and Tremellomycetes sp. stood out as being a better producer than V. victoriae. The current study also formed a basis for a better assessment of the potential for practical application of EPSs and yeasts in biochemical engineering and biotechnology.
1. Introduction
The Antarctic is among the most scarcely investigated regions of the world. What is more, Antarctica is a niche pressurized microorganisms into a synthesis of unusual molecules for adaptation to extreme environmental conditions such as freezing temperatures, numerous freeze–thaw cycles, strong winds, high sublimation, evaporation, and UV radiation. Several yeast genera such as Cryptococcus, Mrakia, Candida, Rhodotorula, Leucosporidium, Debaryomyces, and Vishniacozyma have already been described as part of the microbial communities living in Antarctica [1]. In general, different yeast species are applied for the industrial production of wines, spirit drinks, bakery products (Saccharomyces cerevisiae), single-cell protein (Hansenula sp.), biofuels (S. cerevisiae), pharmaceuticals (Pichia methanolica), enzymes (Candida antarctica), pigments (Xanthophyllomyces dendrorhous), and aromas (Zygosaccharomyces rouxii) [2]. A great biotechnological potential of psychrophilic yeasts is also drawing scientists’ attention. Producers of various cold enzymes, including α-amylases, glucoamylases, β-fructosidases, β-glucosidases, lipases, proteases, xylanases have been reported [3]. Unsaturated and saturated fatty acids, phospholipids, sterols, as well as tocopherols were determined by Antarctic strains of the genera Cryptococcus, Rhodotorula, and Sporobolomyces [4]. The psychrophilic strain S. salmonicolor AL1 is a producer of antioxidant compounds (ergosterol, β-carotene, coenzyme Q10, torulene, and torularhodin) [5]. On the one hand, there are some research findings on the yeast biosynthetic potential and role of EPSs that have been published. A recent study by Debnath et al. emphasized, for example, the leading role of EPSs in yeast physiology as protectors against phagocytosis, drying, freezing, and as a barrier to heavy metal ions, rather than as reserve compounds [6]. Other studies have shown that Antarctic yeasts most often synthesize hetero-PSs composed mainly of mannose, glucose, galactose, arabinose, xylose, etc. [7,8,9]. It should be noted that a very diverse chemical composition determines completely different physicochemical, functional, and biological properties of the EPSs. For example, according to previous research, a high molecular weight (gluco)mannan, synthesized by Rhodotorula sp. and Sporobolomyces sp., has demonstrated excellent emulsifying and stabilizing properties [10]. On the other hand, a great diversity of EPSs with promising functional properties is an important prerequisite to successful application in the field of food industry as emulsifiers, stabilizers, flocculants, film-forming, binding, and gelling agents [11,12]. Interestingly, scientists have attributed antioxidant, anti-inflammatory, anti-tumor, and immunomodulating properties to EPSs, which are prerequisites to application in cosmetic and pharmaceutical industries [13,14,15,16,17]. In general, that is the underlying reason for considering the yeast EPSs a good alternative to those isolated from plants and microalgae [12]. Therefore, EPSs from Antarctic yeasts can also find industrially relevant applications.
Unfortunately, however, a large number of yeast genera have not been studied for their polysaccharide constituents. Some chemical features are still insufficiently investigated. There is a lack of knowledge about yield, monosaccharide composition, physicochemical, as well as basic spectral characteristics of the EPSs, which are still not revealed. Moreover, even preliminary studies on the biosynthesis and accumulation of polysaccharides by a large number of Antarctic yeast genera have not been conducted yet. Such kind of interesting species could be found in genus Vishniacozyma and class Tremellomycetes based on available information on the biosynthetic potential for the accumulation of other bioactive secondary metabolites. For example, V. victoriae (homotypic synonym of Cryptococcus victoriae) grows the best in different Antarctic plant species (moss, lichens, and vascular plants), and molten ice biofilm. This species has already been isolated from samples collected in King George and Deceptions Islands, and it has been reported as a lipase producer [18,19,20]. Psychrophilic lipases are important hydrolases that find applications in organic synthesis, foods, different cosmetic and cleaning agents, pharmaceutical products, and the textile industry [21]. Compared to other Antarctic species, V. victoriae had relatively high levels of ergosterol and linoleic acid as part of polyunsaturated fatty acids [22]. Interestingly, it has also been found that V. victoriae is safe for humans and can be useful in the biocontrol of postharvest diseases of fruits in semi-commercial conditions [23]. Class Tremellomycetes is a large and heterogeneous group of yeasts; most often, its species are described in associations with plants. However, their biotechnological potential is scarcely studied [24]. It was hypothesized that Vishniacozyma and Tremellomycetes species are also able to synthesize structurally new EPSs, whose mass-scale production deserves more attention. In fact, the study of the yeast biosynthetic potential (biomass/product yield, conditions, etc.), together with a few basic properties of the formed polymer are enormously important from a biotechnological and practical point of view. Therefore, the current study aimed to identify two yeast species collected in Livingston Island (Antarctica), to investigate their potential for synthesizing polysaccharides, and to characterize the obtained EPSs. The identification of new Antarctic yeast EPS producers will fill the huge gaps in scientists’ knowledge, and it will keep scientists’ interest open for a search of new practical applications as well.
2. Materials and Methods
2.1. Antarctic Yeasts
Yeast strains were isolated from soil samples collected in Livingston Island, Antarctica by the Bulgarian Antarctic Expedition, which provided the logistics of the samples. The obtained isolates were included in the Antarctic yeast collection of the Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences. An 18S-rRNA gene sequence analysis was used for the identification of the yeast species. The isolation of the total yeast DNA was carried out by a PCR/DNA Clean-Up purification Kit (GeneMatrix Series, EURx, Gdańsk, Poland) following the protocol in the product manual. Furthermore, PCR amplification of the genomic DNA was carried out with the universal primers ITS1 and ITS4. The sequencing of the isolated DNA was performed by Macrogen Europe, B.V. (Amsterdam, the Netherlands). The resulting sequences were processed by Sequence Scanner v1.0. Then, they were compared with the available data of the National Center for Biotechnology Information (NCBI) GenBank (http://www.ncbi.nlm.nih.gov/; last accessed on 30 December 2021) to prove the species affiliation.
2.2. Growth Medium and Cultivation Conditions
The agar medium used for an identification of the isolates had the following ingredients (w/v): tryptone—1.0%; yeast extract—0.5%; glucose—1.0%; NaCl—1.0%; agar—2.0% (LB medium). The pH of the medium was adjusted to 7.5 before sterilization. The medium was autoclaved (20 min, 1 atm, 121 °C), and then, it was used for surface cultivation (4 °C, 14–20 days). The medium used for submerged cultivation had the following composition (w/v): 4.0% sucrose as a main carbon source, 0.1% yeast extract (Sigma-Aldrich Chemie, Taufkirchen, Germany), 0.25% (NH4)2SO4, 0.1% KH2PO4, 0.05% MgSO4 × 7 H2O, 0.01% NaCl, and 0.01% CaCl2 × 2H2O. It has already been optimized for the yeast growth and accumulation of EPSs as previously described [4]. The fermentation process was performed in 500 mL Erlenmeyer flasks under stirring on a rotary shaker at 220 rpm, 22 °C for 120 h. In order to get an inoculum, the seed culture (10% w/v) was incubated in 500 mL flasks on a rotary shaker at 220 rpm, 22 °C for 48 h.
According to our previous studies with C. laurentii AL100, S. salmonicolor AL1, and C. laurentii AL65, we determined the parameters for the transfer of the cultivation process in a bioreactor [25,26,27]. In brief, the fermentation process was performed in a Sartorius Aplus bioreactor (4 L) at 22 °C, with mechanical agitation—400 rpm and aeration rate—1.00 L/L/min, for 120 h. The bioreactor was equipped with a turbine stirrer, oxygen (Hamilton, Bonaduz AG, Switzerland), and pH (Hamilton, Bonaduz AG, Switzerland) electrodes that measure dissolved oxygen (DO2) and pH, correspondingly. The processes were monitored and controlled by a software program BioPAT® MFCS/DA 3.0 (Sartorius Stedim Biotech GmbH, Göttingen, Germany).
2.3. Determination of the Biomass and EPS in the Culture Medium
A gravimetric quantification of the biomass and EPS in the culture medium was done at the end of cultivation (120 h). In brief, biomass was separated through centrifugation (5000× g, 30 min) from the culture suspension. Part of the supernatant (5 mL) was used for the isolation of EPSs through precipitation with ethanol (24 h, 4 °C). The precipitated EPSs were dried at 105 °C to a constant weight. Parallel analysis of biomass was conducted after washing it twice with ultrapure water. Finally, the biomass was dried at 105 °C until there was no change in the mass of the sample.
2.4. Isolation of EPSs
Before isolating the EPS, the biomass was separated from the culture suspension through centrifugation at 5000× g for 25 min at 20 °C. Then, the volume of the supernatant was reduced by about one-third through a vacuum concentration at 50 °C (−0.1 MPa). To the concentrate, 3.0 volumes of 96% (v/v) cold ethanol were added in order to precipitate EPSs, and then, it was stood overnight at 4 °C, and the resulting precipitate was collected by centrifugation (20 min, 5000× g). In order to purify the EPSs, the precipitate was additionally dissolved in ultrapure water and extensively dialyzed (VISKING®, SERVA Electrophoresis, Germany, MWCO 12–14 kDa) against distilled water for 72 h at 4 °C, with a periodic water change. The dialyzed EPSs were centrifuged at room temperature, filtered through a Büchner funnel, and freeze-dried. The obtained EPSs were named V-EPS (V. victoriae) and T-EPS (Tremellomycetes sp.).
2.5. General Analytical Methods
The crude protein content of the EPSs was estimated by the micro-Kjeldahl method [28]. The determination of nitrogen expressed as ammonia content of the digested sample was performed by the acetylacetone–formaldehyde colorimetric method, using ammonium sulfate as a standard [29]. The results were calculated using 5.78 as a conversion factor. The total carbohydrate content of the EPSs was analyzed by the phenol-sulfuric acid method using mannose for the calibration curve construction [30]. EPSs were solubilized in 72% (w/w) H2SO4 (1 h, at 30 °C), and after dilution with water to 1 M H2SO4, hydrolysis was completed in 3 h at 100 °C in a block heater (SBH200D, Stuart®, Staffordshire, UK). The obtained hydrolyzates were used as samples for analysis. The absorbance was measured at 490 nm. A part of the hydrolysate was taken for the analysis of total uronic acid content. An automated 3-phenylphenol analysis was conducted by a continuous flow analyzer Skalar San++ system (Skalar Analytical BV, Breda, The Netherlands), according to the instructions of the manufacturer. Absorption was measured at 530 nm, and glucuronic acid (12.5–100.0 μg/mL) was used for a calibration curve construction. The qualitative estimation of polysaccharide-linked rare sugars was performed by the periodate thiobarbituric acid colorimetric assay of Karkhanis et al., as described by Ognyanov et al. [31,32]. The acetyl content of EPSs was estimated photometrically by the hydroxamic acid reaction method of McComb & McCready, using β-D-glucose pentaacetate (24–120 μg/mL) as a standard [33].
2.6. Monosaccharide Composition Analysis
V-EPS and T-EPS (20 mg) were hydrolyzed with 2 M trifluoroacetic acid (10 mL) for 1.5 h at 121 °C for the release of neutral monosaccharide constituents. Hydrolyzates were dried in vacuo at 40 °C and re-dissolved in distilled water. This step was repeated twice in order to evaporate completely trifluoroacetic acid. Furthermore, the hydrolyzates (10 mg/mL) were filtered (0.45 μm) and analyzed on a Nexera-i LC2040C Plus UHPLC system (Shimadzu Corporation, Kyoto, Japan), coupled with a Zorbax Carbohydrate column (4.6 × 150 mm, 5 μm) and Zorbax Reliance Cartridge guard-column operating at 35 °C. The samples were auto-injected (10 μL) and eluted with a mobile phase composed of a mixture of acetonitrile/H2O (80/20 v/v) at a flow rate of 1.0 mL/min. The eluate was monitored using a refractive index detector RID-20A (cell temperature 40 °C). The concentration of sugars in the sample was deduced using a calibration curve constructed by plotting the peak area (x-axis) against five different concentrations (y-axis) for each sugar. The peak corresponding to different sugars in the sample was confirmed by a comparison of retention time with that of the standards.
2.7. Molecular Weight Distribution Analysis
The molecular weight distribution of EPSs (2 mg/mL) was carried out on a Nexera-i LC2040C Plus UHPLC system (Shimadzu Corporation, Kyoto, Japan), coupled with a RID-20A detector, using a Bio SEC-3 column (4.6 × 300 mm, 300 Å, 3 μm, Agilent). Ten microliters of the sample were auto-injected and eluted at 30 °C with a mobile phase of 0.15 M NaH2PO4 (pH 7.0), applying a flow rate of 0.5 mL/min. Pullulan standards (Shodex® standard P-82 kit, Showa Denko-K.K., Kawasaki, Japan) with molecular weights in the range of 0.59 × 104 to 78.8 × 104 g/mol were used for the construction of a standard curve. Initially, the samples were dissolved in distilled water for 24 h and then filtered (0.45 μm) before analysis.
2.8. Fourier Transform Infrared (FT-IR) Spectroscopy
The FT-IR spectra of the EPS samples (2 mg) were recorded in the region 4000–500 cm−1 using the attenuated total reflection technique on Tenzor 27 (Bruker, Germany), controlled by OPUS 8.7. software. The two spectra were analyzed in Spectragryph software (Friedrich Menges, Oberstdorf, Germany).
2.9. Statistics
All cultivations were performed in duplicates. All cultivations were performed in duplicates. The HPLC determination of monosaccharide composition and molecular weight distribution were performed at least in duplicates, whereas the other spectrophotometric analyses were run at least in triplicates. The quantitative results were expressed as mean values ± standard deviations. SigmaPlot 2001 (Systat Software Inc., Palo Alto, CA, USA) and Microsoft Excel, 2016 (Microsoft Corporation, Redmond, DC, USA) were used for statistical data processing and graphical layout.
3. Results
3.1. Identification and Cultivation of Yeast Strains
Based on the molecular genetic analysis of ITS1-5.8S-ITS4 regions of the 18S rRNA gene, the first strain was found to belong to Vishniacozyma victoriae, while the second one was identified only at the genus level as Tremellomycetes sp. The most likely explanation could be the lack of available data in NCBI GenBank, which is used for comparison during the identification process. It is highly probable that this strain is a new one. Its taxonomy will be further revealed in full detail.
Furthermore, the yeast growth, accumulation of EPSs, and the change of pH during the fermentation process of both strains in flasks and bioreactor were monitored and assessed. The results are illustrated in Figure 1 and Figure 2, respectively. The relatively high temperature of cultivation (22 °C) was chosen to induce heat stress in the psychrophilic yeast cultures, aiming for an extensive accumulation of EPSs as a protective response mechanism. The kinetic of the fermentation process of V. victoriae showed preservation of the standard course of the exponential curve and entering the stationary phase after 72 h (Figure 1A). The amount of accumulated biomass at the 120th h of cultivation was about 6 g/L. At this stage, a carbon source was utilized, and EPS was formed. The quantity of accumulated EPS was over 4 g/L. Considering Tremellomycetes sp., a similar trend was observed, as the synthesized biopolymer at the end of the fermentation was 4.5 g/L. The accumulated biomass was about 7 g/L at the 96th h; after that, it entered the stationary phase of growth (Figure 1B). Interestingly, we found a considerable decrease in pH from the initial 5.3 to 2 for both producers during the first 24 h of cultivation.
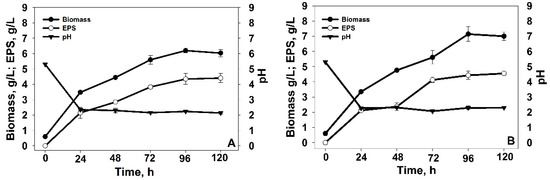
Figure 1.
Time course of cell growth, EPS accumulation, and pH during the cultivation of (A) Vishniacozyma victoriae and (B) Tremellomycetes sp. in flasks.
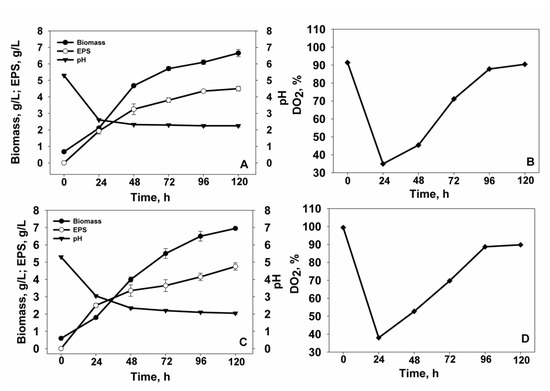
Figure 2.
Time course of cell growth, EPS accumulation, pH, and dissolved oxygen (DO2) during the cultivation of (A,B) Vishniacozyma victoriae and (C,D) Tremellomycetes sp. in a bioreactor.
Figure 2 shows the growth kinetics of the yeast strains in a 4 L bioreactor. Trends in cell growth and EPS accumulation were similar to that of flask cultivation. Interestingly, the Tremellomycetes sp. (Figure 2C) cell growth had a longer exponential phase by comparison with that of V. victoriae strain (Figure 2A). It seemed that both species were similar in regard to EPS production, although Tremellomycetes sp. produced a slightly higher amount of EPS (4.8 g/L), while the amount of EPS in the culture medium of V. victoriae reached not more than 4.5 g/L at the 120th h. The biomass productivity of V. victoriae was 0.055 g/L/h, and that of Tremellomycetes sp. was 0.058 g/L/h. The accumulation of EPSs was 0.038 g/L/h and 0.040 g/L/h, respectively. Interestingly, a large part of EPSs (>50%) was synthesized during the first 24 h of the cultivation, which was also accompanied by high oxygen consumption and effective microbial growth. The DO2 level was plotted in Figure 2B,D. It is shown that after the initial decrease in DO2, the level of DO2 gradually increased, which was closely associated with a smooth transition between the exponential and stationary phase. It is worth noting that the DO2 is a key parameter for the support and activation of cell growth and other anabolic processes of the aerobic metabolism, such as accumulation of the secondary metabolites (EPSs). It may be that a better stirring and aeration of the growth medium contributed to a slightly higher EPS synthesis by Tremellomycetes sp. during the cultivation in the bioreactor.
3.2. Chemical Characterization of EPSs
The yield and chemical characteristics of V-EPS and T-EPS are summarized in Table 1. From the table, it was evident that carbohydrates were the major constituent occupying 75% and 80% of the EPSs obtained, respectively. V-EPS and T-EPS were characterized as neutral polysaccharides because neutral sugars considerably predominated by comparison to smaller amounts of uronic acids (6–7%). The monosaccharide composition analysis showed that EPSs consisted mainly of mannose and glucose, suggesting that V-EPSs and T-EPS were of mannan-, glucan-, or hetero-mannan type. Mannose and glucose represented more than 60% of the total carbohydrates. Interestingly, V-EPS bore close similarity to T-EPS in regard to the qualitative and quantitative composition, suggesting the similarity in the structure. This statement should be additionally investigated. Furthermore, a thiobarbituric acid assay was employed to obtain information about the presence of rare sugars. It can be noticed that EPS contained small amounts of thiobarbiturate-positive substances, since a very typical pink color was formed (Table 1). EPSs had appreciable amounts of proteins (11–12%) that could be incorporated into the yeast cell wall or directly linked to the EPSs. It is evident that yeast EPSs were not O-acetylated (Table 1).

Table 1.
Yield, monosaccharide composition, and protein content of EPSs.
3.3. Molecular Weight Distribution Analysis of EPSs
The molecular weight distribution of the V-EPS and T-EPS was determined by HPSEC, as illustrated in Figure 3A,B, respectively. It can be seen that V-EPS consisted of three high molecular weight populations (Figure 3A). The main peak was eluted between 5.9 and <7.5 min covering the mass range between 47 × 104 g/mol and 68 × 104 g/mol, while the other peaks covered the range between 10 × 104 g/mol and 0.9 × 104 g/mol. The first three peaks represented 34%, 25%, and 29% of the total peak area. The low molecular weight compounds (RT 8.182 and 11.081 min) occupied a very small percentage of the total peak area (11%) and therefore a small percentage of EPS.
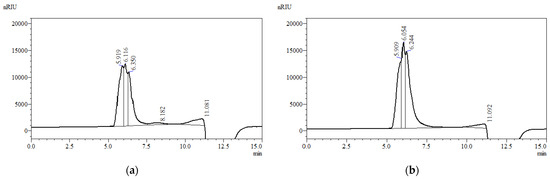
Figure 3.
HPSEC elution pattern of EPS produced by: (a) Vishniacozyma victoriae; (b) Tremellomycetes sp.
Considering the T-EPS elution profile, there was no significant difference in the range of high molecular weight of V-EPS and T-EPS, which was illustrated as a similarity in elution pattern. This suggested the similarity in the structure and molecular weight. Three high molecular weight populations were recognized and eluted early before 7.0 min at 5.9, 6.0, and 6.2 min. Different peaks represented 27%, 26%, and 43% of the total peak area and cover the range between 68 × 104 g/mol and 51 × 104 g/mol. An additional small peak (<10,000 g/mol) was eluted later after the main peaks at RT 11.0 min, and it occupied only 4% of the total peak area.
3.4. FT-IR Spectral Characterization of EPSs
The FT-IR spectrum of V-EPS and T-EPS are presented in Figure 4. Both spectra were typical for polysaccharides with the characteristic signals at 3290.51 (3313.80) and 2927.72 (2926.56) cm−1, arising from ν(OH) and ν(CH and CH2) vibrations, respectively [34].
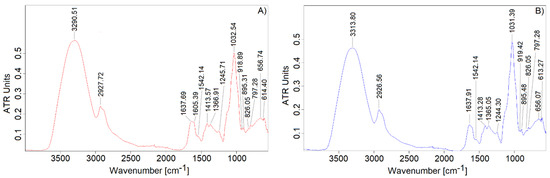
Figure 4.
FT-IR spectra of EPS produced by: (A) Vishniacozyma victoriae; (B) Tremellomycetes sp.
The presence of proteins in the samples was confirmed by the signal at 1542.14 cm−1 assigned for δ(N-H) and ν(C-N) of amide II structure. Interestingly, the band at 1637.69 (1637.91) cm−1 can be related to the absorbed water in the EPSs; however, it should be also linked to the ν(C-O) of amide I structure vibrations in detected proteins [35]. Furthermore, the absorption bands at 1605.39 and 1413.57 (1413.28) cm−1 could be attributed to νas(COO-) and νs(COO-) vibrations of the ionized form of the carboxylic group of uronic acids present in EPSs [19]. However, the signal at 1413–1414 cm−1 might be a result of δs(-CH2) and δs(-CH3) of proteins because of the low uronic acid content in the samples [36]. The signal at 1366.91 (1365.05) cm−1, which generally is associated with the presence of C-H bond oscillation in methyl groups and CH2 groups, can originate from C-H vibrations in β-glucose units [36]. It was suggested that the signal at 1245.71 (1244.30) cm−1 was a result of amide III (δ(N-H) and ν(C-N)) in proteins in EPSs [34,35]. The peak at 1032.54 (1031.39) cm−1 arose from ν(CC)(CO) interactions in the monosaccharide rings [19]. The FT-IR spectra revealed the existence of both α- (826.05 cm−1) and β-anomer configurations (895.31(48) cm−1) in the monosaccharide units in the EPSs [37]. Two EPSs were rich in mannose and glucose (Table 1), and therefore, the absorption bands at 1031–1033 and 895 cm−1 should be linked to the presence of β-glucan [38]. On the other hand, bands at 919 and 797 cm−1 might be associated with mannans in EPS studied [39,40]. It can be hypothesized that V-EPS and T-EPS contained (non-O-acetylated) hetero-mannans, β-glucans, or a mixture of them.
4. Discussion
The current study aimed mainly at investigating two yeast strains collected in Livingston Island (Antarctica). The producers belonged to Vishniacozyma victoriae and Tremellomycetes sp., respectively. However, there is not any information, to the best of our knowledge, in the available literature on examining the EPSs of these species, and therefore, our study makes a meaningful contribution to scientific knowledge.
As the submerged cultivation was the most often used for the production of EPSs, we performed submerged fermentation in flasks and in a bioreactor. It provides not only higher productivity than solid-phase cultivation but also ensures precise control of physical factors during cultivation. In general, a uniform transformation of nutrients into biomass and EPSs when using a stirred-tank bioreactor—the most common type of bioreactor—could be obtained [6]. For example, the Antarctic yeast strain C. laurentii AL65 synthesized about 3 g/L EPS under similar bioreactor conditions with 0.067 g/L/h productivity of accumulated biomass at the 96th h by comparison with that of the current research [26]. The V. victoriae strain differed from C. laurentii in that it produced a higher amount of EPS, whereas the latter strain had higher biomass productivity. The differences, logically, could be explained mainly by the differences in the metabolism of yeasts. The submerged cultivation of Antarctic yeast strains had a distinct advantage of a lower pH value. The lower pH of the medium after 24 h would provide adequate protection against microbial contaminants, which has already been observed [26]. In comparison between the flask and bioreactor cultivation, the differences in the amounts of biomass and accumulated EPSs were small, but it should much prefer the bioreactor to flask fermentation due to a higher available volume and better control of the technological process. Furthermore, the use of a temperature of 22 °C aimed at the development of a relatively cost-effective process.
In a similar way to the industrially applied yeast species, some of the promising biotechnological applications of psychrophilic yeasts have already been revealed for the synthesis of cryoprotective molecules, cold-active enzymes for use in the food industry, wastewater treatment, bioremediation, and biofuels, as well as bioactive EPSs [2]. Particularly, the biosynthesis of extracellular carbohydrate polymers is of specific interest, because our previous studies have demonstrated that Antarctic yeasts are very good producers of EPSs [9,26]. In the current research, V. victoriae and Tremellomycetes sp. produced appreciable amounts of EPSs, which were much higher, by comparison to other yeast species, such as Candida famata and Candida guilliermondii. They were also cultivated on 5% sucrose but for 96 h in flasks [41]. Under the preferred carbon source of 5% maltose, both Candida species did not produce more than 2.10–2.98 g/L EPS within 120 h of cultivation. Similarly, the probiotic yeast Lipomyces starkeyi produced 4.86 g/L EPS on 2% sucrose for 30 days in flasks [42]. The carbon source is extremely important for the proper accumulation of EPSs. From a practical point of view, the production of EPSs on different by-products from the food industry containing sugars as cheap carbon sources could be examined also. In general, beet or cane molasses are industrially used to replace sucrose; however, this could lead to the production of EPSs with lower purity because of the possible physical and chemical interaction with the complex waste matrices.
Not only the yield but also the structural features and molecular weight distribution of Antarctic yeast EPSs are important for the manifestation of biological activity and explanation of their interesting functional properties. V-EPS and T-EPS are rich in mannose, followed by glucose, xylose, and galactose, and based on the FT-IR analysis, it was suggested that both samples contained heteromannans and β-glucans. It was found that mannose in heteromannans from other yeast species can be in α-anomeric configuration as 1,2-, 1,3-, and 1,6-linked or in β-anomeric configuration as 1,4,6-linked [43,44]. The uronic acid content in V-EPS and T-EPS was low, which meant that uronic acids were not the main structural components in the EPSs. It was assumed that both samples contained glucuronic acid, as it has already been found in other yeast EPSs, where it was terminally linked [43,45]. It is worth mentioning that both EPSs did not contain O-acetyl groups, which was in contrast to a previous study that reported the presence of acidic glucuronoxylomannan carrying O-acetyl groups (3–10%) [46]. The molecular weight distribution analysis revealed that both EPSs consisted mainly of high molecular weight populations (Figure 3). However, the molecular weight of yeast EPSs can reach several million g/mol, which considerably affects their hydrodynamic properties [45,46]. From a purely technological point of view, it could be speculated that EPSs with higher molecular weights can express better film-forming, stabilizing, and gelling properties, which are absolutely necessary for the development of food, nutraceutical, cosmetic, or pharmaceutical products.
A previously done study on the yeast Rhodotorula acheniorum MC and S. salmonicolor AL(1) revealed the mannan and glucomannan nature of their EPSs. Moreover, EPSs have shown good emulsifying properties [10]. Furthermore, EPSs from psychrophilic strains of Cryptococcus laurentii and Sporobolomyces salmonicolor have been used to develop cosmetic creams with UV protection [47]. Interestingly, a 1,2,6-linked α-mannan with in vitro anti-inflammatory activity has been isolated from Antarctic bacteria Sphingobacterium sp., and an anti-tumor 1,3,6-β-glucan has been obtained from the Antarctic fungus Thelebolus sp. [48,49,50]. Therefore, it is worth further studying the functional properties and biological activity of V-EPS and T-EPS, which will be our next task.
5. Conclusions
The study provided useful insights into the EPS-synthetic potential of Antarctic yeasts V. victoriae and Tremellomycetes sp. To the best of our knowledge, there are no published reports on the production and characterization of EPSs from these yeast strains. Chemical, chromatographical, and spectral investigations indicated that yeast strains produced high molecular weight hetero-mannan and β-glucan type EPSs. In addition, yeast strains may be cultivated relatively easily in a bioreactor, which can guarantee the accumulation of EPS in a higher amount, taking a short time of cultivation. In conclusion, the key findings from this study may form a basis for a better assessment of the potential for the practical application of EPS in the food, cosmetic, and pharmaceutical industries and yeasts in biochemical engineering and industrial biotechnology.
Author Contributions
Conceptualization, S.R.-V., M.O. and Y.G.; methodology, S.R.-V., M.O. and Y.G.; formal analysis, M.O., Y.G. and M.K.; investigation, S.R.-V., M.O., M.K., A.A. and V.K.; resources, S.R.-V., M.O. and M.K; data curation, S.R.-V., M.O., Y.G. and M.K.; writing—original draft preparation, M.O., Y.G. and S.R.-V.; writing—review and editing, M.O., S.R.-V., Y.G. and M.K.; visualization, S.R.-V., M.O. and Y.G.; supervision, S.R.-V. and M.O.; project administration, S.R.-V.; funding acquisition, S.R.-V. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Bulgarian Ministry of Education and Science through the National Centre for Polar Studies, and Sofia University “St. Kliment Ohridski” in the frames of the National Program for Polar Studies 2017–2021, grant number 70.25-173/22.11.2019.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Acknowledgments
We thank the staff of the Bulgarian Antarctic Expedition for their logistic support. M.O. would like to express his gratitude to Ani Petrova, M.Sc. for her irreplaceable technical assistance.
Conflicts of Interest
The authors declare no conflict of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Buzzini, P.; Margesin, R. Cold-Adapted Yeasts: A Lesson from the Cold and a Challenge for the XXI Century. In Cold-Adapted Yeasts: Biodiversity, Adaptation Strategies and Biotechnological Significance; Buzzini, P., Margesin, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–22. ISBN 978-3-642-39681-6. [Google Scholar]
- Żymańczyk-Duda, E.; Brzezińska-Rodak, M.; Klimek-Ochab, M.; Duda, M.; Zerka, A. Yeast as a versatile tool in biotechnology. In Yeast: Industrial Applications; Morata, A., Loira, I., Eds.; IntechOpen: London, UK, 2017; pp. 3–40. [Google Scholar]
- Buzzini, P.; Branda, E.; Goretti, M.; Turchetti, B. Psychrophilic yeasts from worldwide glacial habitats: Diversity, adaptation strategies and biotechnological potential. FEMS Microbiol. Ecol. 2012, 82, 217–241. [Google Scholar] [CrossRef] [PubMed]
- Zlatanov, M.; Pavlova, K.; Antova, G.; Angelova-Romova, M.; Georgieva, K.; Rusinova-Videva, S. Biomass production by Antarctic yeast strains: An investigation on lipid composition. Biotechnol. Biotechnol. Equip. 2010, 24, 2096–2101. [Google Scholar] [CrossRef]
- Dimitrova, S.; Pavlova, K.; Lukanov, L.; Korotkov, E.; Petrova, E.; Zagorchev, P.; Kuncheva, M. Production of metabolites with antioxidant and emulsifying properties by Antarctic strain Sporobolomyces salmonicolor AL1. Appl. Biochem. Biotechnol. 2013, 169, 301–311. [Google Scholar] [CrossRef]
- Debnath, A.; Das, B.; Devi, M.; Ram, R. Fungal exopolysaccharides: Types, production and application. In Microbial Polymers, Applications and Ecological Perspectives; Vaishnav, A., Choudhary, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 45–68. [Google Scholar]
- Elinov, N.; Ananieva, E.; Vitovskaya, G. Features of the biosynthesis and characteristics of the exoglycanns in yeasts of the genus Sporobolomyces. Microbiologiya 1992, 60, 466–470. [Google Scholar]
- Pavlova, K.; Rusinova-Videva, S.; Kuncheva, M.; Kratchanova, M.; Gocheva, M.; Dimitrova, S. Synthesis and characterization of an exopolysaccharide from Antarctic yeast strain Cryptococcus laurentii AL100. Appl. Biochem. Biotechnol. 2011, 163, 1038–1052. [Google Scholar] [CrossRef] [PubMed]
- Rusinova-Videva, S.; Pavlova, K.; Georgieva, K. Effect of different carbon sources on biosynthesis of exopolysaccharide from Antarctic strain Cryptococcus laurentii AL62. Biotechnol. Biotechnol. Equip. 2011, 25, 80–84. [Google Scholar] [CrossRef][Green Version]
- Kuncheva, M.; Pavlova, K.; Panchev, I.; Dobreva, S. Emulsifying power of mannan and glucomannan produced by yeasts. Int. J. Cosmetic. Sci. 2007, 29, 377–384. [Google Scholar] [CrossRef]
- Williams, P.; Hickey, M. Fluid gels based on natural polymers for cosmetic applications. Cosmet. Toilet. 2003, 118, 51–59. [Google Scholar]
- Freitas, F.; Alves, V.; Reis, M. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Nishinari, K. Polysaccharide rheology and in-mouth perception. In Food Polysaccharides and Their Applications, 2nd ed.; Alistair, M., Glyn, O., Peter, A., Eds.; CRC Press: Boca Raton, FL, USA, 2006; pp. 541–588. [Google Scholar]
- Nicolaus, B.; Kambourova, M.; Oner, E. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Chen, J.; Mao, D.; Yong, Y.; Li, J.; Wei, H.; Lu, L. Hepatoprotective and hypolipidemic effects of water-soluble polysaccharidic extract of Pleurotus eryngii. Food Chem. 2012, 130, 687–694. [Google Scholar] [CrossRef]
- Hu, S.; Liang, Z.; Chia, Y.; Lien, J.; Chen, K.; Lee, M.; Wang, J. Antihyperlipidemic effect of polysaccharide from fermented broth of Pleurotus citrinopileatus. Appl. Microbiol. Biotechnol. 2006, 70, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Hristova, D.; Rusinova-Videva, S.; Konstantinov, S. Biologically active substances and extracts of fungal original. Pharmacong. Rev. 2021, 15, 12–19. [Google Scholar]
- Vaz, A.B.; Rosa, L.H.; Vieira, M.L.; Garcia, V.D.; Brandao, L.R.; Teixeira, L.C.; Rosa, C.A. The diversity, extracellular enzymatic activities and photoprotectivecompounds of yeasts isolated in Antarctica. Braz. J. Microbiol. 2011, 42, 937–947. [Google Scholar] [CrossRef]
- Ogaki, M.B.; Teixeira, D.R.; Vieira, R.; Lírio, J.M.; Felizardo, J.P.; Abuchacra, R.C.; Cardoso, R.P.; Zani, C.L.; Alves, T.M.; Junior, P.A.; et al. Diversity and bioprospecting of cultivable fungal assemblages in sediments of likes in the Antarctic of lakes in the Antarctic Peninsula. Fungal Biol. 2020, 124, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Baeza, M.; Alcaíno, J.; Cifuentes, V.; Turchetti, B.; Buzzini, P. Cold active enzymes from cold-adapted yeasts. In Biotechnology of Yeasts and Filamentous Fungi; Sibirny, A.A., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 297–324. [Google Scholar] [CrossRef]
- Joseph, B.; Ramteke, P.W.; Thomas, G. Cold active microbial lipases: Some hot issues and recent developments. Biotechnol. Adv. 2008, 26, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, P.; Carrasco, M.; Barahona, S.; Alcaíno, J.; Cifuentes, V.; Baeza, M. Antarctic yeasts: Analysis of their freeze-thaw tolerance and production of antifreeze proteins, fatty acids and ergosterol. BMC Microbiol. 2018, 18, 66. [Google Scholar] [CrossRef]
- Lutz, M.C.; Lopes, C.A.; Sosa, M.C.; Sangorrín, M.P. Semi-commercial testing of regional yeasts selected from North Patagonia Argentina for the biocontrol of pear postharvest decays. Biol. Control 2020, 150, 104246. [Google Scholar] [CrossRef]
- Andrade, P.; de Sousa, J.; Lira, P.; Assis, A.; Lerlinck, S.; Andreote, D. The bacterial and fungal communities associated with Anthurium ssp. leaves: Insight into plant endemism and microbe association. Microbiol. Res. 2021, 244, 126667. [Google Scholar] [CrossRef]
- Rusinova-Videva, S.; Radchenkova, N.; Dobrev, G.; Pavlova, K. Purification of arabinomannan synthesized by Cryptococcus laurentii AL100. Acta Microbiol. Bulg. 2015, 31, 141–144. [Google Scholar]
- Rusinova-Videva, S.; Nachkova, S.; Adamov, A.; Dimitrova-Dyulgerova, I. Antarctic yeast Cryptococcus laurentii (AL65): Biomass and exopolysaccharide production and biosorption of metals. J. Chem. Technol. Biotechnol. 2020, 95, 1372–1379. [Google Scholar] [CrossRef]
- Vlaev, S.; Pavlova, K.; Rusinova-Videva, S.; Georgieva, K.; Georgiev, D. Agitation effects and kinetic constants of exoglucomannan production by Antarctic yeast strain in a stirred tank bioreactor. Chem. Biochem. Eng. Q. 2016, 30, 393–400. [Google Scholar] [CrossRef]
- Bradstreet, R.B. The Kjeldahl Method for Organic Nitrogen; Academic Press Inc.: New York, NY, USA, 1965; pp. 9–145. [Google Scholar]
- GB 5009.5-2016; Determination of Protein in Foods. National Food Safety Standard (NFSS) of the People’s Republic of China. China National Center for Food Safety Risk Assessment: Beijing, China, 2016.
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Karkhanis, Y.D.; Zeltner, J.Y.; Jackson, J.J.; Carlo, D.J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of gramnegative bacteria. Anal. Biochem. 1978, 85, 595–601. [Google Scholar] [CrossRef]
- Ognyanov, M.; Georgiev, Y.; Petkova, N.; Ivanov, I.; Vasileva, I.; Kratchanova, M. Isolation and characterization of pectic polysaccharide fraction from in vitro suspension culture of Fumaria officinalis L. Int. J. Polym. Sci. 2018, 2018, 5705036. [Google Scholar] [CrossRef]
- McComb, E.A.; McCready, R.M. Determination of acetyl in pectin and in acetylated carbohydrate polymers. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, J.; Matĕjka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Singh, B.R.; DeOliveira, D.B.; Fu, F.-N.; Fuller, M.P. Fourier transform infrared analysis of amide III bands of proteins for the secondary structure estimation. In Proceedings of the SPIE, Biomolecular Spectroscopy III, Los Angeles, CA, USA, 1 May 1993; Volume 1890, pp. 47–55. [Google Scholar]
- Farinha, I.; Duarte, P.; Pimentel, A.; Plotnikova, E.; Chagas, B.; Mafra, L.; Grandfils, C.; Freitas, F.; Fortunato, E.; Reis, M.A.M. Chitin–glucan complex production by Komagataella pastoris: Downstream optimization and product characterization. Carbohydr. Polym. 2015, 130, 455–464. [Google Scholar] [CrossRef]
- Kačuráková, M.; Capek, P.; Sasinková, V.; Wellner, N.; Ebringerová, A. FT-IR study of plant cell wall model compounds: Pectic polysaccharides and hemicelluloses. Carbohydr. Polym. 2000, 43, 195–203. [Google Scholar] [CrossRef]
- Šandula, J.; Kogan, G.; Kačuráková, M.; Machová, E. Microbial (1→3)-β-d-glucans, their preparation, physico-chemical characterization and immunomodulatory activity. Carbohydr. Polym. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Galichet, A.; Sockalingum, G.D.; Belarbi, A.; Manfait, M. FTIR spectroscopic analysis of Saccharomyces cerevisiae cell walls: Study of an anomalous strain exhibiting a pink-colored cell phenotype. FEMS Microbiol. Lett. 2001, 197, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Nitta, M.; Mizuno, T. Infrared spectroscopy of some mannans. Agric. Biol. Chem. 1973, 37, 433–435. [Google Scholar] [CrossRef]
- Gientka, I.; Bzducha-Wróbel, A.; Stasiak-Różańska, L.; Bednarska, A.A.; Błażejak, S. The exopolysaccharides biosynthesis by Candida yeast depends on carbon sources. Electron. J. Biotechnol. 2016, 22, 31–37. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Das, N. Optimization of exopolysaccharide production by probiotic yeast Lipomyces starkeyi VIT-MN03 using response surface methodology and its applications. Ann. Microbiol. 2019, 69, 515–530. [Google Scholar] [CrossRef]
- Breierová, E.; Hromádková, Z.; Stratilová, E.; Sasinková, V.; Ebringerová, A. Effect of salt stress on the production and properties of extracellular polysaccharides produced by Cryptococcus laurentii. Z. Nat. C 2005, 60, 444–450. [Google Scholar] [CrossRef]
- Hamidi, M.; Gholipour, A.R.; Delattre, C.; Sesdighi, F.; Seveiri, R.M.; Pasdaran, A.; Kheirandish, S.; Pierre, G.; Kozani, P.S.; Kozani, P.S.; et al. Production, characterization and biological activities of exopolysaccharides from a new cold-adapted yeast: Rhodotorula mucilaginosa sp. GUMS16. Int. J. Biol. Macromol. 2020, 151, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Oluwa, S.W. Structure and foaming properties of viscous exopolysaccharides from a wild grape-associated basidiomycetous yeast Papiliotrema flavescens formerly known as Cryptococcus flavescens. J. Microbiol. Biotechnol. 2020, 30, 1739–1749. [Google Scholar] [CrossRef]
- Van Bogaert, I.N.A.; De Maeseneire, S.L.; Vandamme, E.J. Extracellular polysaccharides produced by yeasts and yeast-like fungi. In Yeast Biotechnology: Diversity and Applications; Satyanarayana, T., Kunze, G., Eds.; Springer Science + Business Media, B.V.: Berlin/Heidelberg, Germany, 2009; pp. 651–671. [Google Scholar]
- Sajna, K.V.; Sukumaran, R.K.; Gottumukkala, L.D.; Jayamurthy, H.; Dhar, K.S.; Pandey, A. Studies on structural and physical characteristics of a novel exopolysaccharide from Pseudozyma sp. NII 08165. Int. J. Biol. Macromol. 2013, 59, 84–89. [Google Scholar] [CrossRef]
- Panchev, I.N.; Dobreva, S.; Karashanova, D.; Pavlova, K.; Kuncheva, M.; Georgieva, K. Physical properties of cosmetic creams containing exopolysaccharides synthesized from Antarctic yeast. In Proceedings of the Food Science, Engineering and Technologies, Plovdiv, Bulgaria, 19–20 October 2012. [Google Scholar]
- Chatterjee, S.; Mukhopadhyay, S.K.; Gauri, S.S.; Dey, S. Sphingobactan, a new α-mannan exopolysaccharide from Arctic Sphingobacterium sp. IITKGP-BTPF3 capable of biological response modification. Int. Immunopharmacol. 2018, 60, 84–95. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.K.; Chatterjee, S.; Gauri, S.S.; Das, S.S.; Mishra, A.; Patra, M.; Ghosh, A.K.; Das, A.K.; Singh, S.M.; Dey, S. Isolation and characterization of extracellular polysaccharide Thelebolan produced by a newly isolated psychrophilic Antarctic fungus Thelebolus. Carbohydr. Polym. 2014, 104, 204–212. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).