Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material Description
2.2. Chemicals, Reagents and References
2.3. Plant Extracts Preparation
2.4. Laser-Assisted Extraction Installation Description
2.5. Analytical Qualitative and Quantitative Studies
2.6. Antioxidant Activity Studies
2.7. Pharmacological Studies In Vitro
3. Results
3.1. Analytical Results
3.1.1. Polyphenols Content in Extracts
3.1.2. Total Extractible Compounds and Total Phenolics in Extracts
3.1.3. Minerals and Micro-Elements Content in Extracts
3.2. Antioxidant Activity Results
3.3. Pharmacological Results
Effects on the Viability of Caco-2 Cells
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lighfoot, D.A. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Luna, S.L.; Ramirez-Garza, R.E.; Serna Saldivar, S.O. Environmentally Friendly Methods for Flavonoid Extraction from Plant Material: Impact of Their Operating Conditions on Yield and Antioxidant Properties. Sci. World J. 2020, 2020, 6792069. [Google Scholar] [CrossRef] [PubMed]
- Galanakis, C. Food Waste Recovery: Processing Technologies, Industrial Techniques, and Applications, 2nd ed.; Elsevier: Vienna, Austria, 2020. [Google Scholar]
- Panchev, I.; Kirtchev, N.A.; Dimitrov, D.D. Possibilities for application of laser ablation in food technologies. Innov. Food Sci. Emerg. Technol. 2011, 12, 369–374. [Google Scholar] [CrossRef]
- Batirtze, P.M.; Bock, P.; Schroffenegger, M.; Toca-Herrera, J.L.; Gierlinger, N. Following laser induced changes of plant phenylpropanoids by Raman microscopy. Sci. Rep. 2018, 8, 1804. [Google Scholar]
- Monties, B. Plant-Cell Walls as Fibrous Lignocellulosic Composites—Relations with Lignin Structure and Function. Anim. Feed Sci. Technol. 1991, 32, 159–175. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Holwerda, E.K.; Worthen, R.S.; Kothari, N.; Lasky, R.C.; Davison, B.H.; Fu, C.; Wang, Z.-Y.; Dixon, R.A.; Biswal, A.K.; Mohnen, D.; et al. Multiple levers for overcoming the recalcitrance of lignocellulosic biomass. Biotechnol. Biofuels 2019, 12, 15. [Google Scholar] [CrossRef]
- Zoghlami, A.; Paes, G. Lignocellulosic Biomass: Understanding Recalcitrance and Predicting Hydrolysis. Front. Chem. 2019, 7, 874. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Dixon, R.A. Lignin modification improves fermentable sugar yields for biofuel production. Nat. Biotechnol. 2007, 25, 759–761. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Ragauskas, A.J.; Beckham, G.T.; Biddy, M.J.; Chandra, R.; Chen, F.; Davis, M.F.; Davison, B.H.; Dixon, R.A.; Gilna, P.; Keller, M.; et al. Lignin Valorization: Improving Lignin Processing in the Biorefinery. Science 2014, 344, 1246843. [Google Scholar] [CrossRef] [PubMed]
- Rashid, G.M.M.; Bugg, T.D.H. Enhanced biocatalytic degradation of lignin using combinations of lignin-degrading enzymes and accessory enzymes. Catal. Sci. Technol. 2021, 11, 10. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Guo, X. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Nazario, F.E.A.; Sanches-Silva, A.; Ribeiro-Santos, R.; Ramos de Melo, N. Psyllium (Plantago ovata Forsk): From evidence of health benefits to its food application. Trends Food Sci. Technol. 2020, 96, 166–175. [Google Scholar]
- Fierascu, R.C.; Fierascu, I.; Ortan, A.; Paunescu, A. Plantago media L.-Explored and Potential Applications of an Underutilized Plant. Plants 2021, 10, 265. [Google Scholar] [CrossRef]
- Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-plantago-lanceolata-l-folium_en.pdf (accessed on 25 January 2022).
- Schmidgall, J.; Schnetz, E.; Hensel, A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Med. 2000, 66, 48–53. [Google Scholar] [CrossRef]
- McRorie, J.W. Evidence-based approach to fiber supplements and clinically meaningful health benefits, Part 1. Nutrition. Today 2015, 50, 82–89. [Google Scholar] [CrossRef] [Green Version]
- McRorie, J.W.; McKeown, N.M. Understanding the physics of functional fibers in the gastrointestinal tract: An evidence-based approach to resolving enduring misconceptions about insoluble and soluble fiber. J. Acad. Nutr. Diet. 2017, 117, 251–264. [Google Scholar] [CrossRef] [Green Version]
- Galisteo, M.; Sanchez, M.; Vera, R.; Gonzalez, M.; Anguera, A.; Duarte, J.; Zarzuelo, A. A diet supplemented with husks of Plantago ovata reduces the development of endothelial dysfunction, hypertension, and obesity by affecting adiponectin and TNF-alpha in obese Zucker rats. J. Nutr. 2005, 10, 2399–2404. [Google Scholar] [CrossRef] [Green Version]
- Ghiasian, M.; Niroomandi, Z.; Dastan, D.; Poorolajal, J.; Zare, F.; Ataei, S. Clinical and phytochemical studies of Plantago major in pressure ulcer treatment: A randomized controlled trial. Complement. Ther. Clin. Pract. 2021, 43, 101325. [Google Scholar] [CrossRef]
- Kartini, K.; Wati, N.; Gustav, R.; Wahyuni, R.; Angada, Y.F.; Hidayani, R.; Raharjo, A.; Islamie, R.; Dwi Putra, S.E. Wound healing effects of Plantago major extract and its chemical compounds in hyperglycemic rats. Food Biosci. 2021, 41, 100937. [Google Scholar] [CrossRef]
- Haddadian, K.; Haddadian, K.; Zahmatkash, M. A review of Plantago plant. Indian J. Tradit. Know 2014, 13, 681–685. [Google Scholar]
- Adom, M.B.; Taher, M.; Mutalabisin, M.F.; Amri, M.S.; Kudos, M.B.A.; Sulaiman, M.W.A.W.; Sengupta, P.; Susanti, D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017, 96, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Cicero, A.G.; Derosa, G.; Bove, M.; Imola, F.; Borghi, C.; Gaddi, A. Psyllium improves dyslipidaemia, hyperglycaemia and hypertension, while guar gum reduces body weight more rapidly in patients affected by metabolic syndrome following an AHA Step 2 diet. Mediterr. J. Nutr. Metab. 2010, 3, 47–54. [Google Scholar] [CrossRef]
- Anderson, J.W.; Davidson, M.H.; Blonde, L.; Brown, W.V.; Howard, W.J.; Ginsberg, H.; Allgood, L.D.; Weingand, K.W. Long-term cholesterol-lowering effects of psyllium as an adjunct to diet therapy in the treatment of hypercholesterolemia. Am. J. Clin. Nutr. 2000, 71, 1433–1438. [Google Scholar] [CrossRef]
- Story, J.A.; Donkin, S.S.; Furumoto, E.J.; Buhman, K.K. Dietary psyllium increases fecal bile acid excretion, total steroid excretion and bile acid biosynthesis in rats. J. Nutr. 1998, 128, 1199–1203. [Google Scholar] [CrossRef]
- Turley, S.D.; Daggy, B.P.; Dietschy, J.M. Psyllium augments the cholesterol-lowering action of cholestyramine in hamsters by enhancing sterol loss from the liver. Gastroenterology 1994, 107, 444–452. [Google Scholar] [CrossRef]
- Brum, J.M.; Gibb, R.D.; Peters, J.C.; Matte, R.D. Satiety effects of psyllium in healthy volunteers. Appetite 2016, 105, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.; Harris, P.J.; Ferguson, L.R. Potential Benefits of Dietary Fibre Intervention in Inflammatory Bowel Disease. Int. J. Mol. Sci. 2016, 17, 919. [Google Scholar] [CrossRef] [Green Version]
- Ogata, M.; Ogita, T.; Tari, H.; Arakawa, T.; Suzuki, T. Supplemental psyllium fibre regulates the intestinal barrier and inflammation in normal and colitic mice. Br. J. Nutr. 2017, 118, 661–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Available online: https://www.cambridge.org/core/journals/british-journal-of-nutrition/article/supplemental-psyllium-fibre-regulates-the-intestinal-barrier-and-inflammation-in-normal-and-colitic-mice/B03DA9C101028A6167873660B5555D3C (accessed on 25 January 2022).
- Amiri, M.S.; Mohammadzadeh, V. Plant-Based Gums and Mucilages Applications in Pharmacology and Nanomedicine: A Review. Molecules 2021, 26, 1770. [Google Scholar] [CrossRef] [PubMed]
- Bashir, S.; Erum, A.; Saghir, S.; Tulain, U.R.; Rashid, A. Physicochemical characterization and evaluation of suspending properties of arabinoxylan from Ispaghula (Plantago ovata) husk. Pak. J. Pharm. Sci. 2014, 27, 1761–1766. [Google Scholar] [PubMed]
- Abbas, S.; Sherazi, M.; Khan, A.; Alyami, H.S.; Latif, M.; Qureshi, Z.U.R.; Majeedullah; Hassan Bin Asad, M.H. Investigation of Plantago ovata Husk as Pharmaceutical Excipient for Solid Dosage Form (Orodispersible Tablets). Biomed. Res. Int. 2021, 14, 5538075. [Google Scholar] [CrossRef]
- Zubair, M.; Nybom, H.; Lindholm, C.; Rumpunen, K. Major polyphenols in aerial organs of greater plantain (Plantago major L.), and effects of drying temperature on polyphenol contents in the leaves. Sci. Hortic. 2011, 128, 523–529. [Google Scholar] [CrossRef]
- Beara, I.N.; Lesjak, M.M.; Jovin, E.D.; Balog, K.J.; Anackov, G.T.; Orcic, D.Z.; Mimica-Dukic, N.M. Plantain (Plantago L.) species as novel sources of flavonoid antioxidants. J. Agric. Food Chem. 2009, 57, 9268–9273. [Google Scholar] [CrossRef]
- Samuelsen, A.B. The traditional uses, chemical constituents and biological activities of Plantago major. J. Ethnopharmacol. 2000, 71, 1–21. [Google Scholar] [CrossRef]
- Nazaizadeh, A.; Mikaili, P.; Moloudizargari, M.; Aghajanshakeri, S.; Javaherypour, S. Therapeutic uses and pharmacological properties of Plantago major L. and its active constituents. J. Basic Appl. Sci. Res. 2013, 3, 212–221. [Google Scholar]
- Carrillo-Ocampo, D.; Bazaldúa-Gómez, S.; Bonilla-Barbo, J.R.; Aburto-Amar, R.; Rodríguez-López, V. Anti-Inflammatory Activity of Iridoids and Verbascoside Isolated from Castilleja tenuiflora. Molecules 2013, 18, 12109–12118. [Google Scholar] [CrossRef]
- González, L.; Pereira-Crespo, S.; Dagnac, T.; Resch-Zafra, C.; Fernández-Lorenzo, B.; Botana, A.; Veiga, M.; Aguión, A.; Flores-Calvete, G. Fatty acid, carotenoid and vitamin-E contents of Plantago lanceolata at different maturity stages. In Conference Paper: The Multiple Roles of Grassland in the European Bioeconomy. Proceedings of the 26th General Meeting of the European Grassland Federation, Trondheim, Norway, 4–8 September 2016; NIBIO: Ås, Norway, 2016; pp. 302–304. [Google Scholar]
- Dzomeku, B.M.; Wald, J.P.; Wünsche, J.N.; Nohr, D.; Biesalski, H.K. Climate Change Enhanced Carotenoid Pro-Vitamin A Levels of Selected Plantain Cultivars. Plants 2020, 9, 541. [Google Scholar] [CrossRef]
- Hussan, F.; Mansor, A.S.; Hassan, S.N.; Effendy Kamaruddin, N.T.T.; Budin, S.B.; Othman, F. Anti-Inflammatory Property of Plantago major Leaf Extract Reduces the Inflammatory Reaction in Experimental Acetaminophen-Induced Liver Injury. Evid. Based Complement. Alternat Med. 2015, 347861. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.; Imran, M.Z.; Hasan, M. Choleretic and cholagogic effects of anti- cholelithiatic plants. J. Pharmacogn. Phytochem. 2021, 10, 1–7. [Google Scholar] [CrossRef]
- Jazayeri, S.F.; Ghods, R.; Dabaghian, F.H.; Shojaii, A.; Al-Hadi Moravej, S.A.; Khadem, E.; Seyedian, S. The Efficacy of Plantago major Seed on Liver Enzymes in Nonalcoholic Fatty Liver Disease: A Randomized Double-Blind Clinical Trial. Evid. Based Complement Alternat Med. 2021, 2021, 6693887. [Google Scholar] [CrossRef] [PubMed]
- Israel Cardoso, F.C.; Peruzzo Apolinário, P.; Cunha Breder, J.S.; Paranhos, T.; Oliveira, H.C.; Dini Polidoro, A.; Souza Oliveira Kumakura, A.R.; Melo Lima, M.H. A protocol for systematic review of Plantago major L. effectiveness in accelerating wound-healing in animal models. Syst. Rev. 2019, 8, 337. [Google Scholar] [CrossRef] [Green Version]
- Najafian, Y.; Hamedi, S.S.; Farshchi, M.K.; Feyzabadi, Z. Plantago major in Traditional Persian Medicine and modern phytotherapy: A narrative review. Electron. Physician 2018, 10, 6390–6399. [Google Scholar] [CrossRef] [Green Version]
- Fleer, H.; Verspohl, E.J. Antispasmodic activity of an extract from Plantago lanceolata L. and some isolated compounds. Phytomedicine 2007, 14, 409–415. [Google Scholar] [CrossRef]
- Sadraei, H.; Ghanadian, M.; Asghari, G.; Sekhavati, N. Antispasmodic activity of apigenin and luteolin, two components of Dracocephalum kotschyi extract, on rat ileum contractions. J. Herbmed Pharmacol. 2018, 7, 100–105. [Google Scholar] [CrossRef] [Green Version]
- Fabrizio Ferrazzano, G.; Cantile, T.; Roberto, L.; Ingenito, A.; Catania, M.R.; Roscetto, E.; Palumbo, G.; Zarrelli, A.; Pollio, A. Determination of the In Vitro and In Vivo Antimicrobial Activity on Salivary Streptococci and Lactobacilli and Chemical Characterisation of the Phenolic Content of a Plantago lanceolata Infusion. Biomed. Res. Int. 2015, 2015, 286817. [Google Scholar] [CrossRef] [Green Version]
- Reddy, P.R.T.; Vandana, K.V.; Prakash, S. Antibacterial and anti-inflammatory properties of Plantago ovata Forssk. leaves and seeds against periodontal pathogens: An in vitro study. Ayu 2018, 39, 226–229. [Google Scholar] [CrossRef]
- Sharifa, A.A.; Neoh, Y.L.; Iswadi, M.I.; Khairul, O.; Abdul Halim, M.; Jamaludin, M.; MohamedAzman, A.B.; Hing, H.L. Effects of methanol, ethanol and aqueous extract of Plantago major on gram positive bacteria, gram negative bacteria and yeast. Ann. Microsc. 2008, 8, 42–44. [Google Scholar]
- Tsai, T.H.; Chien, Y.C.; Lee, C.W.; Tsai, P.J. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.A.; See, T.L.; Khuay, L.Y.; Osman, K.; Bakar, M.A.A. In vitro effects of Plantago major on urolithiasis. Malays. J. Med. Sci. 2005, 12, 22–26. [Google Scholar] [PubMed]
- Committee on Herbal Medicinal Products (HMPC) Community Herbal Monograph on Plantago lanceolata L., Folium. 2011. 28 January 2014 EMA/HMPC/437858/2010, Corrigendum 28 January 2014. Available online: https://www.ema.europa.eu/en/documents/herbal-monograph/final-community-herbal-monograph-plantago-lanceolata-l-folium_en.pdf (accessed on 25 January 2022).
- Wagner, H.; Bladt, S. Plant. Drug Analysis. A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 1996. [Google Scholar]
- Reikh, E.; Schibli, A. HPTLC for the Analysis of Medicinal Plants; Thieme: New York, NY, USA; Stuttgart, Germany, 2008. [Google Scholar]
- Collective Work. Romanian Pharmacopoeia, Xth ed.; Medicala: Bucharest, Romania, 1993. [Google Scholar]
- Rusu, N.; Meghea, A. Bioacumulation of toxic metals in fish oils capsules. Ecosystems 2015, 77, 131–140. [Google Scholar]
- Iftimie, N.; Giurginca, M.; Meghea, A. The estimation of the antioxidant effect of plants extracts by chemiluminescence. Rev. Chem. 2003, 55, 1025–1028. [Google Scholar]
- Protocols & Applications Guide. Available online: www.promega.com (accessed on 12 August 2021).
- Ronsted, N.; Franzyk, H.; Molgaard, P.; Jaroszevski, W.; Jensen, S.R. Chemotaxonomy and evolution of Plantago L. Plant. Syst. Evol. 2003, 242, 63–82. [Google Scholar] [CrossRef]
- Li, Y.; Gan, L.; Li, G.Q.; Deng, L.; Zhang, X. Pharmacokinetics of plantamajoside and acteoside from Plantago asiatica in rats by liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2014, 89, 251–256. [Google Scholar] [CrossRef]
- Pol, M.; Schmidtke, K.; Lewandowska, S. Plantago lanceolata—An overview of its agronomically and healing valuable features. Open Agric. 2021, 6, 479–488. [Google Scholar] [CrossRef]
- Petrova, S.; Velcheva, I.; Yurukova, L.D.; Berova, M. Plantago lanceolata L. as a Biomonitor of Trace Elements in an Urban Area. Bulg. J. Agric. Sci. 2014, 20, 325–329. [Google Scholar]
- Drava, G.; Cornara, L.; Giordani, P.; Minganti, V. Trace elements in Plantago lanceolata L., a plant used for herbal and food preparations: New data and literature review. Environ. Sci. Pollut. Res. Int. 2019, 26, 2305–2313. [Google Scholar] [CrossRef]
- Skrynetska, I.; Karcz, J.; Barczyk, G.; Kandziora-Ciupa, M.; Ciepa, R.; Socha, N. Using Plantago major and Plantago lanceolata in environmental pollution research in an urban area of Southern Poland. Environ. Sci. Pollut. Res. Int. 2019, 26, 23359–23371. [Google Scholar] [CrossRef] [Green Version]
- Sanna, F.; Piluzza, G.; Campesi, G.; Molinu, M.G.; Re, G.A.; Sulas, L. Antioxidant Contents in a Mediterranean Population of Plantago lanceolata L. Exploited for Quarry Reclamation Interventions. Plants 2022, 11, 791. [Google Scholar] [CrossRef]
- Jirasek, A.; Georg Schulze, H.; Hughesman, C.H.; Creagh, A.L.; Haynes, C.A.; Blades, M.W.; Turner, R.F.B. Discrimination between UV radiation-induced and thermally induced spectral changes in AT-paired DNA oligomers using UV resonance Raman spectroscopy. J. Raman Spectrosc. 2006, 37, 1368–1380. [Google Scholar] [CrossRef]
- La Plant, F. Lasers, spectrographs, and detectors. In Emerging Raman Applications and Techniques in Biomedical and Pharmaceutical Fields; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Atalla, R.H. In-situ Raman microprobe studies of plant cell walls: Macromolecular organization and compositional variability in the secondary wall of Picea mariana (Mill.) B.S.P. Planta 1986, 169, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Verrier, S.; Romanska, H.; Bishop, A.E.; Polak, J.M.; Hench, L.L. In situ characterisation of living cells by Raman spectroscopy. Spectroscopy 2002, 16, 43–5143. [Google Scholar] [CrossRef] [Green Version]
- Segers-Nolten, G.M.J.; Otto, C.; de Mul, F.F.M.; Greve, J. Laser irradiation and Raman spectroscopy of single living cells and chromosomes: Sample degradation occurs with 514.5 nm but not with 660 nm laser light. Exp. Cell Res. 1991, 192, 361–367. [Google Scholar]
- Britannica. The Editors of Encyclopaedia. “Light Summary”. Encyclopedia Britannica. Available online: https://www.britannica.com/science/light (accessed on 18 May 2022).
- Ligh Science Technologies. The Visible Wavelength Range and Its Impact on Plant Growth. Available online: https://lightsciencetech.com/visible-wavelength-range-plant-growth/ (accessed on 18 May 2022).
- Zhang, S.; Zhang, L.; Zou, H.; Qui, L.; Zheng, Y.; Yang, D.; Wang, Y. Effects of Light on Secondary Metabolite Biosynthesis in Medicinal Plants. Front. Plant. Sci. 2021, 11, 497. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B 2016, 159, 1–7. [Google Scholar] [CrossRef]
- Xie, D.J.; Tarin, M.W.K.; Chen, L.Y.; Ren, K.; Yang, D.M.; Zhou, C.C.; Wan, J.; He, T.; Rong, J.; Zheng, Y. Consequences of LED lights on root morphological traits and compounds accumulation in Sarcandra glabra seedlings. Int. J. Mol. Sci. 2021, 22, 7179. [Google Scholar] [CrossRef]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Haider Abbasi, B. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE 2020, 15, e0233963. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Pintilie, O.; Stoleru, T.; Burducea, M.; Oroian, M.; Zamfirache, M.M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [Green Version]
- Silva, T.D.; Batista, D.S.; Fortini, E.A.; de Castro, K.M.; Sousa Felipe, S.H.; Fernandes, A.M.; JesusSousa, K.C.; da Silva, J.V.S.; Freitas Correia, L.N.; Monteiro Farias, L.; et al. Blue and red light affects morphogenesis and 20-hydroxyecdisone content of in vitro Pfaffia glomerata accessions. J. Photochem. Photobiol. B 2020, 203, 111761. [Google Scholar] [CrossRef] [PubMed]
- Kubica, P.; Szopa, A.; Prokopiuk, B.; Komsta, L.; Pawlowska, B.; Ekiert, H. The influence of light quality on the production of bioactive metabolites-verbascoside, isoverbascoside and phenolic acids and the content of photosynthetic pigments in biomass of Verbena officinalis L. cultured in vitro. J. Photochem. Photobiol. B 2020, 203, 111768. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.Y.; Li, L.T.; Hu, Y.R.; Zhou, C.; Wang, X.C.; Wang, L.; Zeng, J.; Yang, Y. Transcriptomic analysis of the effects of three different light treatments on the biosynthesis of characteristic compounds in the tea plant by RNA-Seq. Tree Genet. Genomes 2016, 12, 118. [Google Scholar] [CrossRef]
- Alrifai, O.; Hao, X.M.; Marcone, M.F.; Tsao, R. Current review of the modulatory effects of LED lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. [Google Scholar] [CrossRef] [PubMed]
- Orlando, M.; Trivellini, A.; Incrocci, L.; Ferrante, A.; Mensuali, A. The Inclusion of Green Light in a Red and Blue Light Background Impact the Growth and Functional Quality of Vegetable and Flower Microgreen Species. Horticulturae 2022, 8, 217. [Google Scholar] [CrossRef]

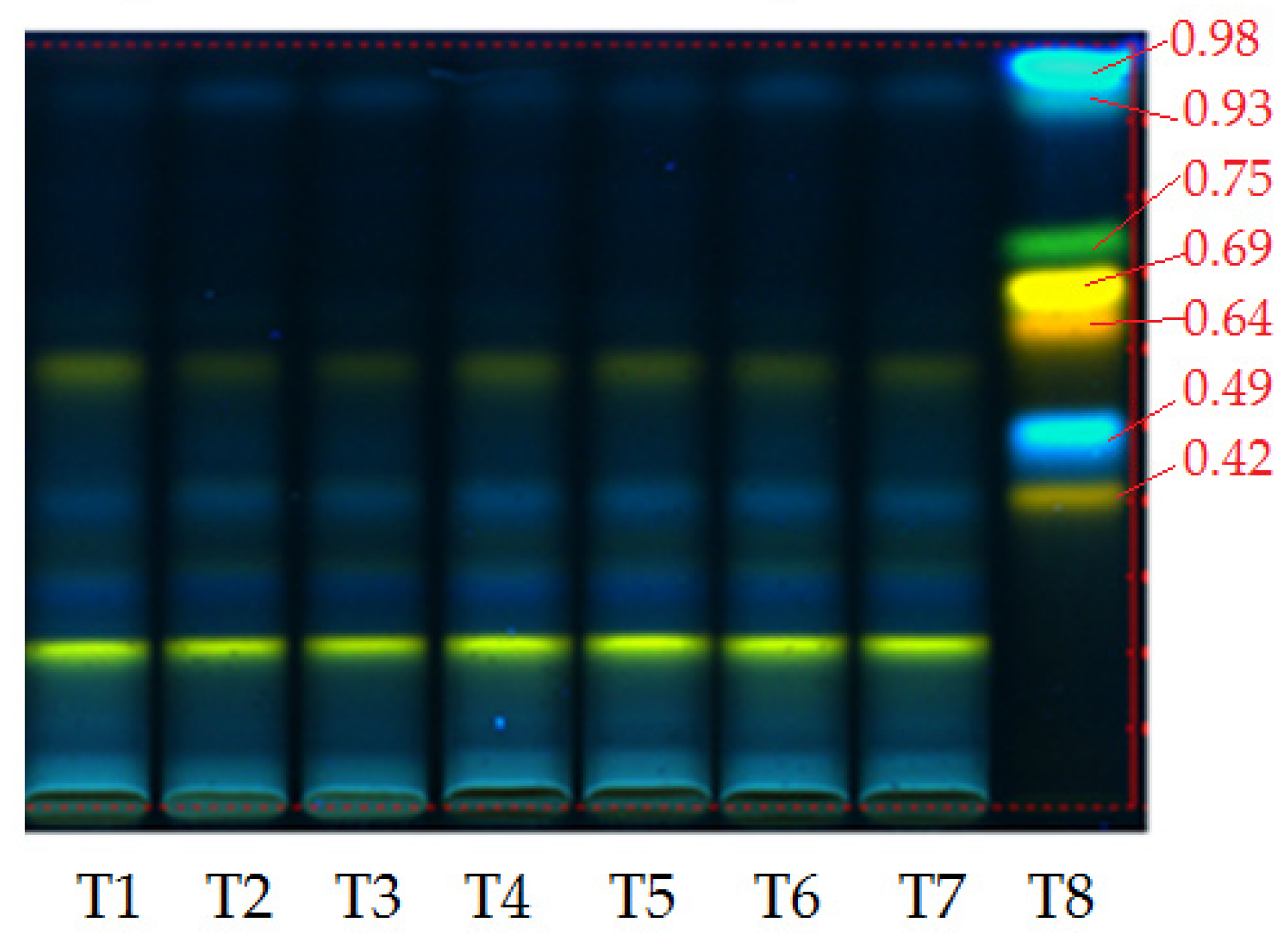



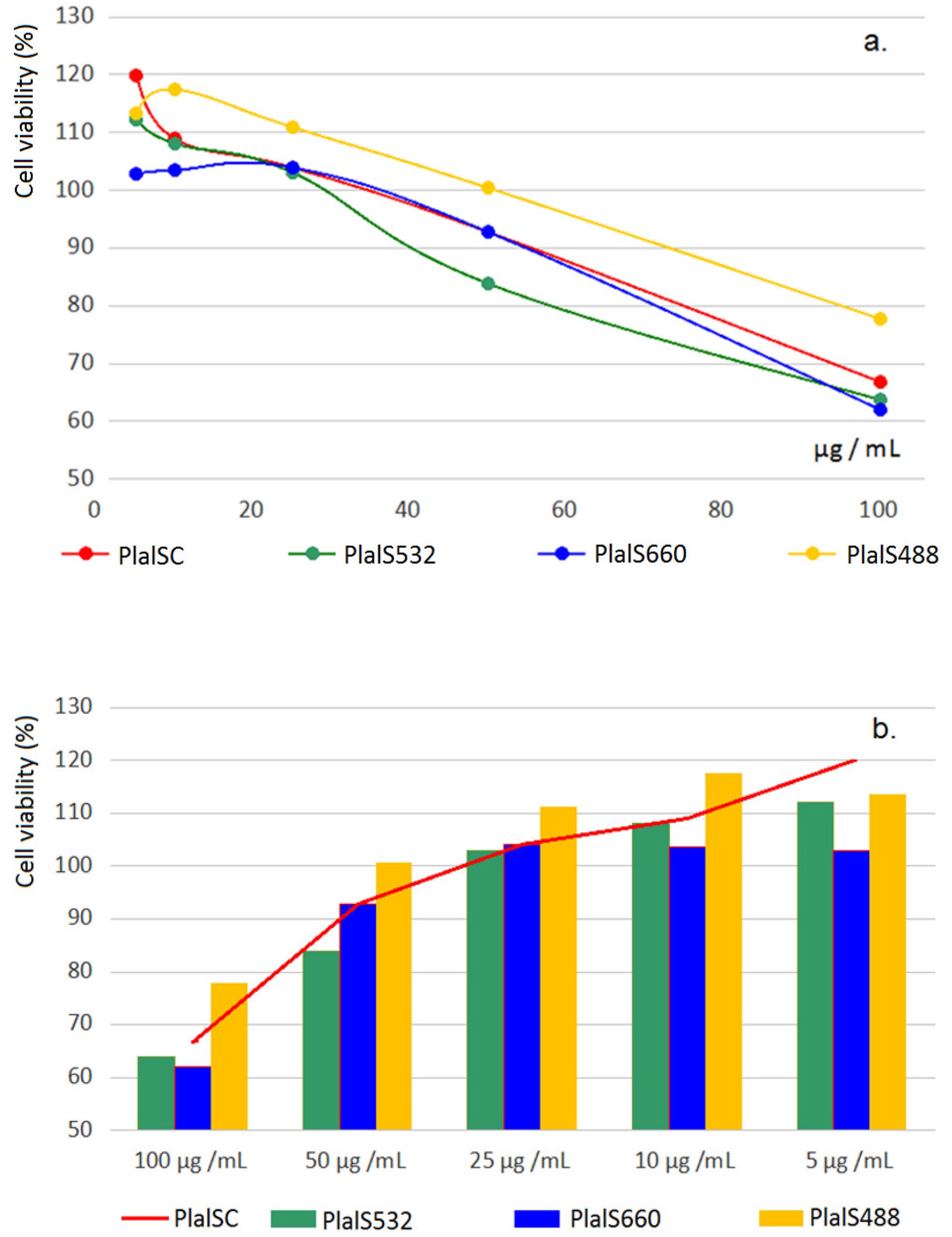
| Test Extract/(μg/g Plant) | Control Extract (PlalC) | Laser Extract Plal488 | PlalC/Plal488 (%) | Laser Extract Plal514 | PlalC/Plal514 (%) | Laser Extract Plal532 | PlalC/Plal532 (%) |
| Total extractible/dry matter | 331,204 | 334,564 | +1.01 | 352,802 | +6.52 | 333,516 | +0.69 |
| Total phenolics/[GAE] | 6201 | 5986 | −3.47 | 5240 | −15.50 | 6220 | +0.31 |
| Test Extract/(μg/g Plant) | Control Extract (PlalC) | Laser Extract Plal552 | PlalC/Plal552 (%) | Laser Extract Plal660 | PlalC/Plal660 (%) | Laser Extract Plal785 | PlalC/Plal785 (%) |
| Total extractible/dry matter | 331,204 | 352,793 | +6.52 | 331,195 | −0.01 | 324,110 | −2.14 |
| Total phenolics/[GAE] | 6201 | 6948 | +12.05 | 7162 | +15.50 | 7162 | +15.50 |
| Ref./(µg/g) | Control Extract (PlalC) | Laser Extract (Plal488) | PlalC/Plal488 (%) | Laser Extract (Plal514) | PlalC/Plal514 (%) | Laser Extract (Plal532) | PlalC/Plal532 (%) |
| K | 111,305 | 117,027 | +5.14 | 76,032 | −31.69 | 117,912 | +5.93 |
| Ca | 31,980 | 33,200 | +3.81 | 21,537 | −32.65 | 33,104 | +3.51 |
| Mg | 6733 | 7122 | +5.77 | 5760 | −14.45 | 7412 | +9.15 |
| Na | 1887 | 2192 | +16.16 | 1998 | +5.88 | 2032 | +7.68 |
| Total minerals | 151,905 | 159,541 | +5.03 | 105,327 | −30.66 | 160,460 | +5.63 |
| P | 13,882 | 15,358 | +10.63 | 10,062 | −27.52 | 15,498 | +11.64 |
| Fe | 520.0 | 719.0 | +38.27 | 567.5 | +9.13 | 592.4 | +13.92 |
| Mn | 240.5 | 257.1 | +6.90 | 170.0 | −29.31 | 267.9 | +11.39 |
| Zn | 118.3 | 131.9 | +11.49 | 55.56 | −53.03 | 669.3 | +465.7 |
| Cu | 22.26 | 25.08 | +12.67 | 17.63 | −20.79 | 24.02 | +7.90 |
| Cr | 7.54 | 9.93 | +31.70 | 6.10 | −19.10 | 8.46 | +12.20 |
| Pb | 3.36 | 3.36 | 0.00 | 3.03 | −9.82 | 3.45 | +2.68 |
| As | 0.306 | 0.291 | −4.90 | 0.282 | −7.84 | 0.327 | +6.86 |
| Cd | 0.294 | 0.267 | −9.18 | 0.297 | +1.02 | 0.309 | +5.1 |
| Total micro-elements | 14,795 | 16,505 | +11.56 | 10,882 | −26.45 | 17,064 | +15.34 |
| Total elements | 166,700 | 176,046 | +5.60 | 116,209 | −30.29 | 177,524 | +6.49 |
| Ref./(µg/g) | Control Extract (PlalC) | Laser Extract Plal552 | PlalC/Plal552 (%) | Laser Extract Plal660 | PlalC/Plal660 (%) | Laser Extract Plal785 | PlalC/Plal785 (%) |
| K | 111,305 | 98,692 | −11.33 | 96,871 | −12.97 | 51,319 | −53.89 |
| Ca | 31,980 | 25,697 | −19.65 | 27,806 | −13.05 | 15,556 | −51.36 |
| Mg | 6733 | 5915 | −12.15 | 5875 | −12.74 | 3672 | −45.46 |
| Na | 1087 | 1923 | +76.91 | 1891 | +73.96 | 1174 | +8.00 |
| Total minerals | 151,905 | 132,227 | −12.95 | 132,444 | −12.81 | 71,720 | −52.79 |
| P | 13,882 | 12,452 | −10.30 | 13129 | −5.42 | 7001 | −49.57 |
| Fe | 520.0 | 598.2 | +15.04 | 615.2 | +18.31 | 381.6 | −26.61 |
| Mn | 240.5 | 182.3 | −24.20 | 190.1 | −20.95 | 121.7 | −49.40 |
| Zn | 118.3 | 64.77 | −45.24 | 73.70 | −37.70 | 111.9 | −5.41 |
| Cu | 22.26 | 17.68 | −20.57 | 23.80 | +6.92 | 12.96 | −41.78 |
| Cr | 7.54 | 7.58 | +0.53 | 30.41 | +307.3 | 7.83 | +3.84 |
| Pb | 3.36 | 3.38 | +0.59 | 3.37 | +0.29 | 3.25 | −3.27 |
| As | 0.306 | 0.348 | +13.72 | 0.372 | +21.57 | 0.303 | −0.98 |
| Cd | 0.294 | 0.360 | +22.454 | 0.327 | +11.22 | 0.300 | +2.04 |
| Total micro-elements | 14,795 | 13,327 | −9.92 | 14,066 | −4.93 | 7641 | −48.35 |
| Total elements | 166,700 | 145,554 | −12.68 | 146,510 | −12.11 | 79,361 | −52.39 |
| Antioxidant Activity (AA %)/ Laser Radiation (nm)/Laser Power (mW) | 488 nm (40 mW) | 514 nm (15 mW) | 532 nm (20 mW) | 552 nm (15 mW) | 660 nm (75 mW) | 785 nm (70 mW) | SD (n = 3) |
|---|---|---|---|---|---|---|---|
| Control positive sample at 1 h (* PlalCx) in comparison with Control negative sample (PlalC) | +14% | +13% | +13% | +13% | +11% | +11% | ±2% |
| Test irradiated sample (** Plalx) in comparison with Control negative sample (PlalC) | +25% | +20% | 0.0% | +18% | −37% | +27% | ±1% |
| Test irradiated sample (** Plalx) in comparison with Control positive sample at 1 h (*PlalCx) | +13% | +8% | −14% | +6% | −54% | +17% | ±2% |
| Test Sample | IC50 (µg GAE/mL Sample) |
|---|---|
| PlalC | 132.09 ± 1.94 |
| Plal532 | 122.59 ± 1.76 |
| Plal660 | 134.76 ± 1.61 |
| Plal488 | 170.94 ± 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, L.C.; Nita, S.; Rusu, N.; Bazdoaca, C.; Neagu, G.; Bubueanu, C.; Udrea, M.; Udrea, R.; Enache, A. Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability. Appl. Sci. 2022, 12, 5517. https://doi.org/10.3390/app12115517
Pirvu LC, Nita S, Rusu N, Bazdoaca C, Neagu G, Bubueanu C, Udrea M, Udrea R, Enache A. Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability. Applied Sciences. 2022; 12(11):5517. https://doi.org/10.3390/app12115517
Chicago/Turabian StylePirvu, Lucia Camelia, Sultana Nita, Nicoleta Rusu, Cristina Bazdoaca, Georgeta Neagu, Corina Bubueanu, Mircea Udrea, Radu Udrea, and Alin Enache. 2022. "Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability" Applied Sciences 12, no. 11: 5517. https://doi.org/10.3390/app12115517
APA StylePirvu, L. C., Nita, S., Rusu, N., Bazdoaca, C., Neagu, G., Bubueanu, C., Udrea, M., Udrea, R., & Enache, A. (2022). Effects of Laser Irradiation at 488, 514, 532, 552, 660, and 785 nm on the Aqueous Extracts of Plantago lanceolata L.: A Comparison on Chemical Content, Antioxidant Activity and Caco-2 Viability. Applied Sciences, 12(11), 5517. https://doi.org/10.3390/app12115517






