Changes in Selected Quality Indices in Microbially Fermented Commercial Almond and Oat Drinks
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Reagents
2.2. Experimental Part
2.2.1. Fermentation
- Lactobacillus delbrueckii ssp. bulgaricus 27/23.
- Lactiplantibacillus plantarum ATCC 8014.
- Lactiplantibacillus plantarum PK 1.1.
- Candida antarctica CAN 0001.
- Torula casei TCS 0001.
- Yarrowia lipolytica YLP 0001.
- Kluyveromyces marxianus KF 0001.
- Candida lipolytica (Yarrowia lipolytica) CLP 0001.
2.2.2. Measurements of PB Beverage Acidification
2.2.3. Measurement of PB Beverage Viscosity
2.2.4. Measurement of the Fatty Acids Contents in the PB Beverages
2.2.5. E-Nose Analysis of the Volatile Compounds of the PB Beverages
2.2.6. Statistical Analysis
3. Results and Discussion
3.1. Oat and Almond Drinks as Mediums for the Growth of the LAB and Yeast Strains Used
3.2. Acidity Changes after the Fermentation of the Used PB Beverages
3.3. Viscosity of PB Beverages
3.4. Fatty Acid Composition Changes following the Fermentation of PB Beverages
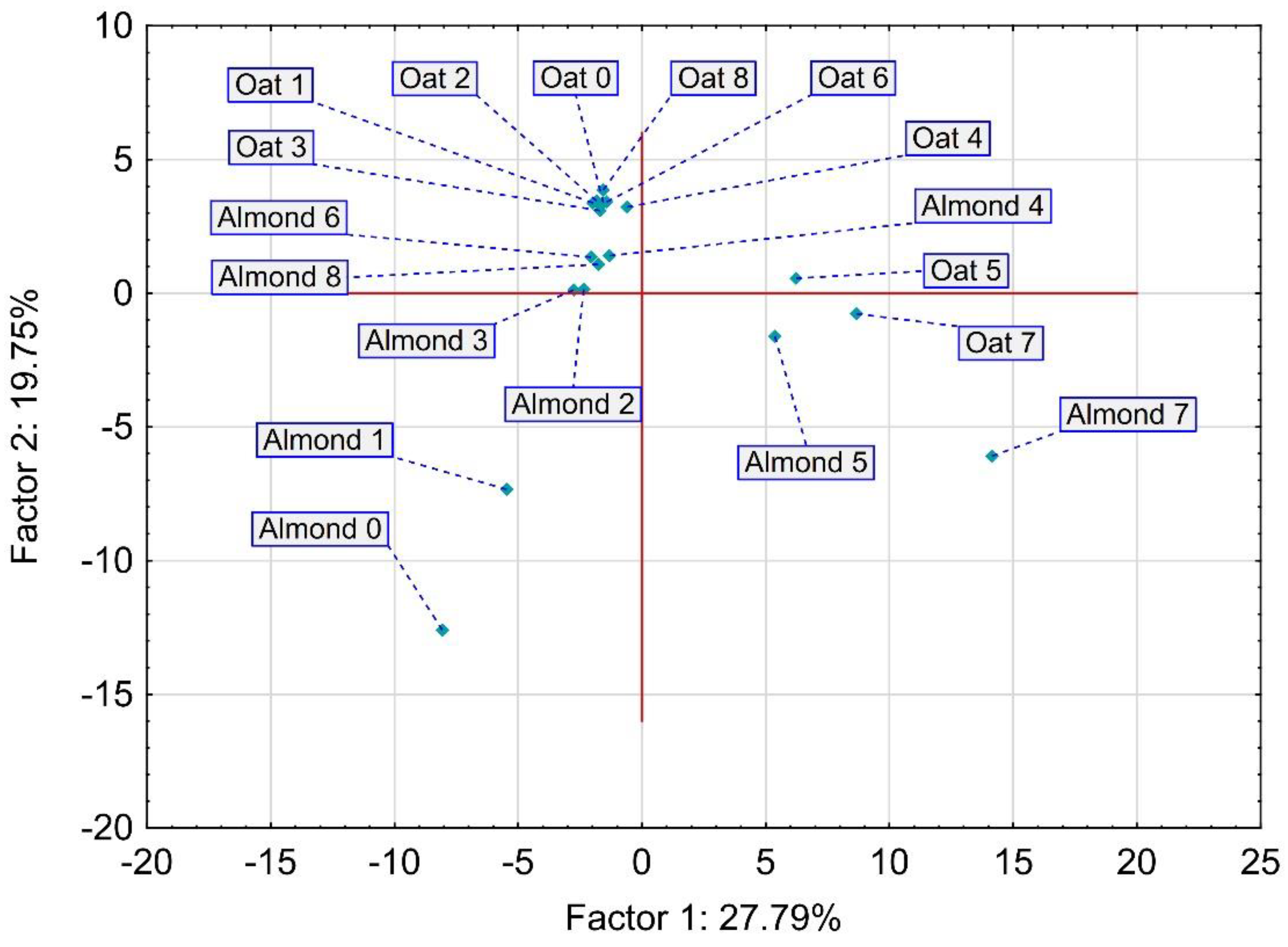
3.5. Volatile Compound Changes after the Fermentation of PB Beverages
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arenas-Jal, M.; Suñé-Negre, J.M.; Pérez-Lozano, P.; García-Montoya, E. Trends in the food and sports nutrition industry: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2405–2421. [Google Scholar] [CrossRef] [PubMed]
- Smart Protein Project. Smart Protein: From Farm to Fork. Available online: https://smartproteinproject.eu/plant-based-food-sector-report/ (accessed on 18 December 2021).
- Top Trends in Prepared Foods 2017: Exploring Trends in Meat, Fish and Seafood; Pasta, Noodles and Rice; Prepared Meals; Savory Deli Food; Soup; and Meat Substitutes; 2017. Available online: https://www.reportbuyer.com/product/4959853/top-trends-in-prepared-foods-exploring-trends-in-meat-fish-and-seafood-pasta-noodles-and-rice-prepared-meals-savory-deli-food-soup-and-meat-substitutes.html (accessed on 18 December 2021).
- Bryant, C.J. We can’t keep meating like this: Attitudes towards vegetarian and vegan diets in the United Kingdom. Sustainability 2019, 11, 6844. [Google Scholar] [CrossRef]
- Neuman, N.; Mylan, J.; Paddock, J. Exploring (non-)meat eating and “translated cuisines” out of home: Evidence from three English cities. Int. J. Consum. Stud. 2020, 44, 25–32. [Google Scholar] [CrossRef]
- Grand View Research. Plant-Based Beverages Market Size, Share & Trends Analysis Report by Type (Soy-Based, Oats-Based), by Product (Plain, Flavored), by Region (Europe, Asia Pacific), and Segment Forecasts, 2021–2028. Available online: https://www.grandviewresearch.com/industry-analysis/plant-based-beverages-market (accessed on 18 December 2021).
- Wehrli, F.; Taneri, P.E.; Bano, A.; Bally, L.; Blekkenhorst, L.C.; Bussler, W.; Metzger, B.; Minder, B.; Glisic, M.; Muka, T.; et al. Oat intake and risk of type 2 diabetes, cardiovascular disease and all-cause mortality: A systematic review and meta-analysis. Nutrients 2021, 13, 2560. [Google Scholar] [CrossRef]
- Zurbau, A.; Noronha, J.; Khan, T.; Sievenpiper, J.; Wolever, T.M.S. Oat beta-glucan and postprandial blood glucose regulation: A systematic review and meta-analysis of acute, single-meal feeding, controlled trials. Curr. Dev. Nutr. 2020, 4, 677. [Google Scholar] [CrossRef]
- Dreher, M.L. A comprehensive review of almond clinical trials on weight measures, metabolic health biomarkers and outcomes, and the gut microbiota. Nutrients 2021, 13, 1968. [Google Scholar] [CrossRef]
- Høst, A.; Husby, S.; Østerballe, O. A prospective study of cow’s milk allergy in exclusively breast-fed infants. Acta Paediatr. 1988, 77, 663–670. [Google Scholar] [CrossRef]
- Edwards, C.W.; Younus, M.A. Cow Milk Allergy; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Manasa, R.; Harshita, M.; Prakruthi, M.; Shekahara Naik, R.; Mahesh, S. Non-dairy plant based beverages: A comprehensive review. Pharm. Innov. J. 2020, 9, 258–271. [Google Scholar]
- Salo, P.M.; Arbes, S.J.; Jaramillo, R.; Calatroni, A.; Weir, C.H.; Sever, M.L.; Hoppin, J.A.; Rose, K.M.; Liu, A.H.; Gergen, P.J.; et al. Prevalence of allergic sensitization in the united states: Results from the national health and nutrition examination survey (NHANES) 2005–2006. J. Allergy Clin. Immun. 2014, 134, 350–359. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regulation (EU) No 1169/2011 of 25 October 2011 on the Provision of Food Information to Consumers, Amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and Repealing Commission Directive 87/250/EEC, C 2011. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32011R1169 (accessed on 20 December 2021).
- Lu, Q.; Zuo, L.; Wu, Z.; Li, X.; Tong, P.; Wu, Y.; Fan, Q.; Chen, H.; Yang, A. Characterization of the protein structure of soymilk fermented by Lactobacillus and evaluation of its potential allergenicity based on the sensitized-cell model. Food Chem. 2022, 366, 130569. [Google Scholar] [CrossRef]
- Pi, X.; Sun, Y.; Fu, G.; Wu, Z.; Cheng, J. Effect of processing on soybean allergens and their allergenicity. Trends Food Sci. Technol. 2021, 118, 316–327. [Google Scholar] [CrossRef]
- Dąbrowski, G.; Konopka, I. Update on food sources and biological activity of odd-chain, branched and cyclic fatty acids—A review. Trends Food Sci. Technol. 2022, 119, 514–529. [Google Scholar] [CrossRef]
- Nkhata, S.G.; Ayua, E.; Kamau, E.H.; Shingiro, J.-B. Fermentation and germination improve nutritional value of cereals and legumes through activation of endogenous enzymes. Food Sci. Nutr. 2018, 6, 2446–2458. [Google Scholar] [CrossRef] [PubMed]
- Fontes, A.L.; Pimentel, L.; Rodríguez-Alcalá, L.M.; Gomes, A. Effect of PUFA substrates on fatty acid profile of Bifidobacterium breve Ncimb 702258 and CLA/CLNA production in commercial semi-skimmed milk. Sci. Rep. 2018, 8, 15591. [Google Scholar] [CrossRef]
- Czerwiec, Q.; Idrissitaghki, A.; Imatoukene, N.; Nonus, M.; Thomasset, B.; Nicaud, J.; Rossignol, T. Optimization of cyclopropane fatty acids production in Yarrowia lipolytica. Yeast 2019, 36, 143–151. [Google Scholar] [CrossRef]
- Grogan, D.W.; Cronan, J.E. Cyclopropane ring formation in membrane lipids of bacteria. Microbiol. Mol. Biol. R 1997, 61, 429–441. [Google Scholar]
- Velly, H.; Bouix, M.; Passot, S.; Penicaud, C.; Beinsteiner, H.; Ghorbal, S.; Lieben, P.; Fonseca, F. Cyclopropanation of unsaturated fatty acids and membrane rigidification improve the freeze-drying resistance of Lactococcus lactis subsp. lactis TOMSC161. Appl. Microbiol. Biotechnol. 2015, 99, 907–918. [Google Scholar] [CrossRef]
- Venn-Watson, S.; Lumpkin, R.; Dennis, E.A. Efficacy of dietary odd-chain saturated fatty acid pentadecanoic acid parallels broad associated health benefits in humans: Could it be essential? Sci. Rep. 2020, 10, 8161. [Google Scholar] [CrossRef]
- Su, X.; Magkos, F.; Zhou, D.; Eagon, J.C.; Fabbrini, E.; Okunade, A.L.; Klein, S. Adipose tissue monomethyl branched-chain fatty acids and insulin sensitivity: Effects of obesity and weight loss. Obesity 2015, 23, 329–334. [Google Scholar] [CrossRef]
- Vahmani, P.; Salazar, V.; Rolland, D.C.; Gzyl, K.E.; Dugan, M.E.R. Iso- but not anteiso-branched chain fatty acids exert growth-inhibiting and apoptosis-inducing effects in MCF-7 cells. J. Agric. Food Chem. 2019, 67, 10042–10047. [Google Scholar] [CrossRef]
- Yan, Y.; Wang, Z.; Wang, D.; Lawrence, P.; Wang, X.; Kothapalli, K.S.D.; Greenwald, J.; Liu, R.; Gyu, P.H.; Brenna, J.T. BCFA-enriched vernix-monoacylglycerol. reduces LPS-induced inflammatory markers in human enterocytes in vitro. Pediatr. Res. 2018, 83, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Meza, S.; Rodríguez-Landa, J.F.; Martínez, A.J.; Herrera-Meza, G.; Fernández-Demeneghi, R.; Reyes-Saldaña, K.; Oliart-Ros, R.M. Behavioral effect of Sterculia apetala seed oil consumption in male zucker rats. J. Med. Food 2017, 20, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Higuera, A.; Peña-Montes, C.; Herrera-Meza, S.; Mendoza-López, R.; Valerio-Alfaro, G.; Oliart-Ros, R.M. Preventive action of sterculic oil on metabolic syndrome development on a fructose-induced rat model. J. Med. Food 2020, 23, 305–311. [Google Scholar] [CrossRef]
- Matela, K.S.; Pillai, M.K.; Thamae, T. Evaluation of PH, Titratable acidity, syneresis and sensory profiles of some yoghurt samples from the Kingdom of Lesotho. Food Res. 2019, 3, 693–697. [Google Scholar] [CrossRef]
- Paulauskienė, A.; Tarasevičienė, Ž.; Šileikienė, D.; Česonienė, L. The quality of ecologically and conventionally grown white and brown Agaricus bisporus mushrooms. Sustainability 2020, 12, 6187. [Google Scholar] [CrossRef]
- Pestana, J.M.; Gennari, A.; Monteiro, B.W.; Lehn, D.N.; Souza, C.F.V. Effects of pasteurization and Ultra-High Temperature processes on proximate composition and fatty acid profile in bovine milk. Am. J. Food Technol. 2015, 10, 265–272. [Google Scholar] [CrossRef]
- Harper, A.R.; Dobson, R.C.J.; Morris, V.K.; Moggré, G. Fermentation of plant-based dairy alternatives by lactic acid bacteria. Microb. Biotechnol. 2022, 15, 1404–1421. [Google Scholar] [CrossRef]
- Battey, A.S.; Duffy, S.; Schaffner, D.W. Modeling yeast spoilage in cold-filled ready-to-drink beverages with Saccharomyces cerevisiae, Zygosaccharomyces bailii, and Candida lipolytica. Appl. Environ. Microbiol. 2002, 68, 1901–1906. [Google Scholar] [CrossRef]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Prado, M.R.; Blandón, L.M.; Vandenberghe, L.P.S.; Rodrigues, C.; Castro, G.R.; Thomaz-Soccol, V.; Soccol, C.R. Milk kefir: Composition, microbial cultures, biological activities, and related products. Front. Microbiol. 2015, 6, 1177. [Google Scholar] [CrossRef]
- Cardoso, V.M.; Borelli, B.M.; Lara, C.A.; Soares, M.A.; Pataro, C.; Bodevan, E.C.; Rosa, C.A. The influence of seasons and ripening time on yeast communities of a traditional Brazilian cheese. Food Res. Int. 2015, 69, 331–340. [Google Scholar] [CrossRef]
- Varela, J.A.; Puricelli, M.; Ortiz-Merino, R.A.; Giacomobono, R.; Braun-Galleani, S.; Wolfe, K.H.; Morrissey, J.P. Origin of lactose fermentation in Kluyveromyces lactis by interspecies transfer of a neo-functionalized gene cluster during domestication. Curr. Biol. 2019, 29, 4284–4290. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Ji, L.; Xu, Y.; Xu, S.; Lin, Y.; Iqbal, H.M.N.; Cheng, H. Bioprospecting Kluyveromyces marxianus as a robust host for industrial biotechnology. Front. Bioeng. Biotechnol. 2022, 10, 851768. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Corona, R.; Vázquez Marrufo, G.; Cortés Penagos, C.; Madrigal-Pérez, L.A.; González-Hernández, J.C. Bioinformatic characterization of the extracellular lipases from Kluyveromyces marxianus. Yeast 2020, 37, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; Pelaez, C.; et al. Safety of Yarrowia lipolytica yeast biomass as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05594. [Google Scholar] [CrossRef]
- Bitencourt, T.B.; Souza, F.A.; Gomes da Silva, V.; Kleinert, E.J.; Martins, A. Nutrient biomass production from agro-industrial residues using Yarrowia lipolytica: Screening and optimization of growing conditions. Braz. J. Food Technol. 2022, 25, e2020287. [Google Scholar] [CrossRef]
- Montemurro, M.; Pontonio, E.; Coda, R.; Rizzello, C.G. Plant-Based Alternatives to Yogurt: State-of-the-Art and Perspectives of New Biotechnological Challenges. Foods 2021, 10, 316. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Applications of Biotechnology to Fermented Foods: Report of an ad hoc Panel of the Board on Science and Technology for International Development; National Academies Press (US): Washington, DC, USA, 1992; pp. 43–57. [Google Scholar]
- Batt, C.A.; Tortorello, M.L. Encyclopedia of Food Microbiology, 2nd ed.; Batt, C.A., Tortorello, M.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; ISBN 978-0-12-384733-1. [Google Scholar]
- Todorov, S.D.; Franco, B.D.G.D.M. Lactobacillus plantarum: Characterization of the species and application in food production. Food Rev. Int. 2010, 26, 205–229. [Google Scholar] [CrossRef]
- Landete, J.M.; Plaza-Vinuesa, L.; Montenegro, C.; Santamaría, L.; Reverón, I.; de las Rivas, B.; Muñoz, R. The Use of Lactobacillus plantarum esterase genes: A biotechnological strategy to increase the bioavailability of dietary phenolic compounds in lactic acid bacteria. Int. J. Food Sci. Nutr. 2021, 72, 1035–1045. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Liong, M.-T.; Tsai, Y.-C. New perspectives of Lactobacillus plantarum as a probiotic: The gut-heart-brain axis. J. Microbiol. 2018, 56, 601–613. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa; Singh, R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef] [PubMed]
- Szczepaniak, G.; Wojtatowicz, M. Dobór szczepów Yarrowia lipolytica i Debaryomyces hansenii do szczepionki wspomagającej proces dojrzewania sera. Żywność. Nauka. Technologia. Jakość 2011, 6, 192–203. [Google Scholar]
- Kurat, C.F.; Natter, K.; Petschnigg, J.; Wolinski, H.; Scheuringer, K.; Scholz, H.; Zimmermann, R.; Leber, R.; Zechner, R.; Kohlwein, S.D. Obese yeast: Triglyceride lipolysis is functionally conserved from mammals to yeast. J. Biol. Chem. 2006, 281, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Carsanba, E.; Papanikolaou, S.; Fickers, P.; Agirman, B.; Erten, H. Citric acid production by Yarrowia lipolytica. In Non-conventional Yeasts: From Basic Research to Application; Springer International Publishing: Cham, Switzerland, 2019; pp. 91–117. [Google Scholar]
- Cavallo, E.; Charreau, H.; Cerrutti, P.; Foresti, M.L. Yarrowia lipolytica: A model yeast for citric acid production. FEMS Yeast Res. 2017, 17, fox084. [Google Scholar] [CrossRef]
- Sabra, W.; Bommareddy, R.R.; Maheshwari, G.; Papanikolaou, S.; Zeng, A.-P. Substrates and oxygen dependent citric acid production by Yarrowia lipolytica: Insights through transcriptome and fluxome analyses. Microb Cell. Fact. 2017, 16, 78. [Google Scholar] [CrossRef]
- Gao, C.; Yang, X.; Wang, H.; Rivero, C.P.; Li, C.; Cui, Z.; Qi, Q.; Lin, C.S.K. Robust succinic acid production from crude glycerol. using engineered Yarrowia lipolytica. Biotechnol. Biofuels 2016, 9, 179. [Google Scholar] [CrossRef]
- Do Espirito-Santo, A.P.; Mouquet-Rivier, C.; Humblot, C.; Cazevieille, C.; Icard-Vernière, C.; Soccol, C.R.; Guyot, J.-P. Influence of cofermentation by amylolytic Lactobacillus strains and probiotic bacteria on the fermentation process, viscosity and microstructure of gruels made of rice, soy milk and passion fruit fiber. Food Res. Int. 2014, 57, 104–113. [Google Scholar] [CrossRef]
- House, J.D.; Hill, K.; Neufeld, J.; Franczyk, A.; Nosworthy, M.G. Determination of the protein quality of almonds (Prunus Dulcis L.) as assessed by in vitro and in vivo methodologies. Food Sci. Nutr. 2019, 7, 2932–2938. [Google Scholar] [CrossRef]
- UniProtKB. UniProtKB Database. Available online: https://www.uniprot.org (accessed on 10 June 2022).
- Qiao, B.; Jiménez-Ángeles, F.; Nguyen, T.D.; Olvera de la Cruz, M. Water follows polar and nonpolar protein surface domains. Proc. Natl. Acad. Sci. USA 2019, 116, 19274–19281. [Google Scholar] [CrossRef]
- Kouřimská, L.; Sabolová, M.; Horčička, P.; Rys, S.; Božik, M. Lipid Content, Fatty acid profile, and nutritional value of new oat cultivars. J. Cereal. Sci. 2018, 84, 44–48. [Google Scholar] [CrossRef]
- Zamany, A.J.; Samadi, G.R.; Kim, D.H.; Keum, Y.-S.; Saini, R.K. Comparative study of tocopherol. contents and fatty acids composition in twenty almond cultivars of Afghanistan. J. Am. Oil Chem. Soc. 2017, 94, 805–817. [Google Scholar] [CrossRef]
- Duran, F.E.; Özdemir, N.; Güneşer, O.; Kök-Taş, T. Prominent strains of kefir grains in the formation of volatile compound profile in milk medium; the Role of Lactobacillus kefiranofaciens subsp. kefiranofaciens, Lentilactobacillus kefiri and Lentilactobacillus parakefiri. Eur. Food. Res. Technol. 2022, 248, 975–989. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, J.; Lv, M.; Shao, Z.; Hungwe, M.; Wang, J.; Bai, X.; Xie, J.; Wang, Y.; Geng, W. Metabolism characteristics of lactic acid bacteria and the expanding applications in food industry. Front. Bioeng. Biotechnol. 2021, 9, 378. [Google Scholar] [CrossRef] [PubMed]
- Popova, T.N.; Pinheiro de Carvalho, M.Â.A. Citrate and isocitrate in plant metabolism. Biochim. Biophys. Acta (BBA) Bioenerg. 1998, 1364, 307–325. [Google Scholar] [CrossRef]
- El Kafsi, H.; Binesse, J.; Loux, V.; Buratti, J.; Boudebbouze, S.; Dervyn, R.; Kennedy, S.; Galleron, N.; Quinquis, B.; Batto, J.-M.; et al. Lactobacillus delbrueckii ssp. lactis and ssp. bulgaricus: A chronicle of evolution in action. BMC Genom. 2014, 15, 407. [Google Scholar] [CrossRef]
- Kaseleht, K.; Paalme, T.; Mihhalevski, A.; Sarand, I. Analysis of volatile compounds produced by different species of Lactobacilli in rye sourdough using multiple headspace extraction. Int. J. Food Sci. Technol. 2011, 46, 1940–1946. [Google Scholar] [CrossRef]
- Liu, T.; Li, Y.; Yang, Y.; Yi, H.; Zhang, L.; He, G. the influence of different lactic acid bacteria on sourdough flavor and a deep insight into sourdough fermentation through RNA sequencing. Food Chem. 2020, 307, 125529. [Google Scholar] [CrossRef]
- Ogunremi, O.R.; Agrawal, R.; Sanni, A. Production and characterization of volatile compounds and phytase from potentially probiotic yeasts isolated from traditional fermented cereal foods in Nigeria. J. Genet. Eng. Biotechnol. 2020, 18, 16. [Google Scholar] [CrossRef]
| Sample | Oat Beverage | Almond Beverage | ||||||
|---|---|---|---|---|---|---|---|---|
| Inoculation Stage | After Fermentation (48 h) | Inoculation Stage | After Fermentation (48 h) | |||||
| LAB | Yeast | LAB | Yeast | LAB | Yeast | LAB | Yeast | |
| control | <10 | <10 | <10 | <10 | <10 | <10 | <10 | <10 |
| 1 | 1.2·104 | <10 | 1.0·109 | <10 | 1.0·104 | <10 | 3.1·109 | <10 |
| 2 | 1.0·104 | <10 | 2.4·109 | <10 | 2.2·104 | <10 | 2.6·109 | <10 |
| 3 | 1.6·104 | <10 | 2.5·109 | <10 | 1.8·104 | <10 | 4.8·109 | <10 |
| 4 | <10 | 2.0·103 | <10 | 4.7·107 | <10 | 3.4·103 | <10 | 4.5·107 |
| 5 | <10 | 6.5·103 | <10 | 7.6·107 | <10 | 4.0·103 | <10 | 8.4·107 |
| 6 | <10 | 2.0·103 | <10 | 7.0·106 | <10 | 2.5·103 | <10 | 1.4·107 |
| 7 | <10 | 9.8·102 | <10 | 1.2·108 | <10 | 1.2·103 | <10 | 7.4·107 |
| 8 | <10 | 3.9·103 | <10 | 1.4·106 | <10 | 2.6·103 | <10 | 8.9·105 |
| Sample | Oat Beverage | Almond Beverage | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Titratable Acidity (% of Lactic Acid) | pH | Apparent Viscosity (mPa × s) | Titratable Acidity (% of Lactic Acid) | pH | Apparent Viscosity (mPa × s) | |||||||||||||
| control | 0.08 | ± | 0.00 a | 7.05 | ± | 0.01 h | 20.53 | ± | 0.55 e | 0.07 | ± | 0.00 a | 7.99 | ± | 0.01 i | 29.60 | ± | 0.22 b |
| 1 | 0.76 | ± | 0.00 h | 2.80 | ± | 0.01 a | 19.10 | ± | 0.64 ab | 0.53 | ± | 0.02 e | 3.42 | ± | 0.01 d | 101.25 | ± | 0.10 h |
| 2 | 0.53 | ± | 0.00 g | 2.82 | ± | 0.01 a | 19.33 | ± | 0.55 abc | 0.72 | ± | 0.00 f | 3.13 | ± | 0.01 a | 97.80 | ± | 0.00 g |
| 3 | 0.47 | ± | 0.00 e | 2.90 | ± | 0.01 c | 18.44 | ± | 0.43 a | 0.73 | ± | 0.00 f | 3.23 | ± | 0.01 b | 90.63 | ± | 0.60 f |
| 4 | 0.11 | ± | 0.00 b | 4.96 | ± | 0.01 f | 19.52 | ± | 0.61 bcd | 0.07 | ± | 0.01 a | 6.51 | ± | 0.00 h | 23.40 | ± | 0.35 a |
| 5 | 0.17 | ± | 0.01 c | 4.51 | ± | 0.01 e | 19.72 | ± | 0.52 bcde | 0.19 | ± | 0.00 b | 5.39 | ± | 0.01 g | 60.51 | ± | 0.16 d |
| 6 | 0.50 | ± | 0.01 f | 2.85 | ± | 0.01 b | 19.44 | ± | 0.43 abcd | 0.79 | ± | 0.01 g | 3.28 | ± | 0.00 c | 108.84 | ± | 0.26 i |
| 7 | 0.22 | ± | 0.01 d | 4.43 | ± | 0.01 d | 19.94 | ± | 0.38 cde | 0.22 | ± | 0.00 c | 5.31 | ± | 0.01 f | 62.43 | ± | 0.21 e |
| 8 | 0.12 | ± | 0.00 b | 5.45 | ± | 0.00 g | 20.36 | ± | 0.26 de | 0.37 | ± | 0.00 d | 4.32 | ± | 0.00 e | 48.13 | ± | 0.16 c |
| FA | Control | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Major fatty acids (g/100 g of lipids) | |||||||||
| C16:0 | 15.3 bcd | 13.9 abc | 13.8 abc | 16.0 cd | 16.6 d | 13.5 ab | 14.9 bcd | 17.0 d | 12.5 a |
| C18:0 | 1.51 a | 1.15 a | 1.09 a | 1.41 a | 1.17 a | 1.02 a | 1.17 a | 1.42 a | 1.35 a |
| C18:1 | 26.6 abc | 24.5 ab | 23.3 a | 31.2 c | 26.6 abc | 22.6 a | 27.0 abc | 29.6 bc | 30.2 c |
| C18:2 | 29.5 abc | 27.4 ab | 26.5 a | 32.7 c | 31.2 bc | 26.8 a | 29.9 abc | 33.1 c | 26.0 a |
| C18:3 | 1.273 a | 1.005 a | 0.901 a | 1.083 a | 0.915 a | 0.868 a | 0.991 a | 1.139 a | 0.846 a |
| total content of major FAs | 74.3 ab | 68.0 a | 65.6 a | 82.4 b | 76.4 ab | 64.7 a | 73.9 ab | 82.2 b | 70.9 ab |
| Minor fatty acids (mg/100 g of lipids) | |||||||||
| C14:0 | 384 c | 264 ab | 260 ab | 300 abc | 329 bc | 250 ab | 286 abc | 314 abc | 222 a |
| methyl 9-methyltetradecanoate | nd a | nd a | nd a | nd a | nd a | nd a | nd a | nd a | 10.2 b |
| C15:0 | 46.6 a | 27.6 a | 29.1 a | 36.8 a | 64.1 a | 28.8 a | 23.6 a | 29.1 a | 27.6 a |
| methyl (z)-5-dodecenoate | 21.2 bc | 19.0 bc | 15.5 bc | 25.3 c | nd a | 13.1 b | 17.6 bc | 21.0 bc | 15.6 bc |
| C16:1 | 314 b | 208 a | 206 a | 233 a | 256 ab | 207 a | 212 a | 277 ab | 266 ab |
| methyl 18-methylnonadecanoate | 101 b | 70 b | 70 b | 95 b | nd a | 59 ab | 71 b | 82 b | 72 b |
| C20:1 | 693 a | 548 a | 494 a | 681 a | 449 a | 418 a | 562 a | 621 a | 449 a |
| C20:2 | nd a | nd a | 20.5 ab | 15.4 ab | nd a | nd a | 24.4 b | 20.8 ab | 18.4 ab |
| 9-(3,3-dimethyloxiran-2-yl)-2,7-dimethylnona-2,6-dien-1-ol | 92 a | 112 a | 146 ab | 288 b | 170 ab | 81 a | 156 ab | 202 ab | 210 ab |
| C22:0 | 60.9 a | 42.9 a | 33.5 a | 37.8 a | 21.2 a | 40.0 a | 34.8 a | 50.3 a | 37.0 a |
| methyl (11r,12r,13s)-(z)-12,13-epoxy-11-methoxy-9-octadecenoate | 205 a | 227 a | 273 ab | 561 b | 308 ab | 162 a | 309 ab | 338 ab | 299 ab |
| methyl 8-(3-octyl-2-oxiranyl)octanoate | nd a | 60 b | 107 d | 75 bc | nd a | nd a | 98 cd | nd a | nd a |
| methyl 5,13-docosadienoate | 71.2 ab | 85.8 b | 41.9 ab | 66.3 ab | nd a | 72.6 ab | 73.5 ab | nd a | 44.7 ab |
| methyl 4-[2-[[2-[[2-[(2-pentylcyclopropyl)methyl]cyclopropyl]methyl]cyclopropyl]methyl]cyclopropyl]butanoate | nd a | nd a | 215 ab | 249 b | nd a | nd a | nd a | 296 b | 151 ab |
| methyl 7,11,14-eicosatrienoate | nd a | nd a | 202 b | 255 b | nd a | 213 b | 198 b | 328 b | 197 b |
| methyl octadec-17-ynoate | nd a | nd a | 114 ab | 148 ab | nd a | nd a | nd a | 249 b | 117 ab |
| methyl (z)-5,11,14,17-eicosatetraenoate | nd a | nd a | 72 a | 145 ab | nd a | 399 c | 125 a | 281 bc | 76 a |
| methyl 8-[2-((2-[(2-ethylcyclopropyl)methyl]cyclopropyl)methyl)cyclopropyl]octanoate | nd a | nd a | 68.3 ab | 138.9 bc | nd a | nd a | 80.0 ab | 234.3 c | 82.4 ab |
| methyl 10,13,16-docosatrienoate | 153 bc | 86 ab | 151 bc | 138 bc | nd a | 101 ab | 125 abc | 250 c | 142 bc |
| total content of minor FAs | 2143 a | 1751 a | 2519 abc | 3490 bc | 1598 a | 2044 a | 2395 ab | 3593 c | 2439 ab |
| Control | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| Major fatty acids (g/100 g of lipids) | |||||||||
| C16:0 | 6.93 c | 6.69 c | 6.99 c | 6.72 c | 6.94 c | 6.41 bc | 5.96 ab | 5.72 a | 6.88 c |
| C18:0 | 2.27 b | 2.23 b | 2.34 b | 2.26 b | 2.26 b | 2.05 ab | 1.96 ab | 1.74 a | 2.27 b |
| C18:1 | 57.3 d | 50.1 ab | 52.1 bc | 49.5 ab | 51.6 abc | 49.3 ab | 48.1 a | 50.4 ab | 55.2 cd |
| C18:2 | 19.0 b | 18.2 b | 19.1 b | 18.1 b | 19.0 b | 18.2 b | 16.5 a | 16.7 a | 19.1 b |
| total content of major FAs | 85.5 d | 77.2 ab | 80.5 bcd | 76.6 ab | 79.8 bc | 75.9 ab | 72.5 a | 74.6 a | 83.4 cd |
| Minor fatty acids (mg/100 g of lipids) | |||||||||
| C14:0 | 33.0 c | 27.0 abc | 29.1 abc | 26.9 abc | 28.0 abc | 24.4 a | 26.4 ab | 31.0 bc | 30.6 abc |
| C15:0 | 24.5 a | 22.8 a | 21.6 a | 22.3 a | 22.9 a | 21.6 a | 20.4 a | 22.8 a | 22.7 a |
| methyl (z)-5-dodecenoate | 11.8 a | 8.0 a | 9.6 a | 8.8 a | 7.2 a | 9.2 a | 8.7 a | 10.4 a | 12.1 a |
| C16:1 | 588 bc | 526 ab | 553 abc | 536 abc | 541 abc | 492 a | 518 ab | 498 a | 609 c |
| C17:1 | 128 c | 119 bc | 122 bc | 122 bc | 118 bc | 112 ab | 113 ab | 103 a | 127 c |
| methyl 8-(2-hexylcyclopropyl)octanoate | 62.8 b | 59.4 b | 48.2 ab | 49.1 ab | 41.6 ab | 29.0 a | 37.3 ab | 29.7 a | 51.4 ab |
| 2-cis,cis-9,12-octadecadienyloxyethanol | 41.3 a | 49.6 a | 46.8 a | 46.3 a | 48.8 a | 41.0 a | 41.0 a | 36.9 a | 46.3 a |
| C18:3 | 18.0 a | 17.5 a | 20.7 a | 23.4 a | 22.8 a | 22.8 a | 14.5 a | 17.0 a | 18.8 a |
| methyl 18-methylnonadecanoate | 51.5 a | 57.1 a | 71.0 a | 68.0 a | 64.1 a | 59.4 a | 52.6 a | 48.3 a | 57.2 a |
| C20:1 | 36.5 abc | 34.2 abc | 43.9 c | 40.8 bc | 37.7 abc | 31.3 abc | 29.9 ab | 25.8 a | 37.8 abc |
| 9-(3,3-dimethyloxiran-2-yl)-2,7-dimethylnona-2,6-dien-1-ol | 376 abc | 514 bc | 490 bc | 463 abc | 380 abc | 198 a | 311 abc | 252 ab | 531 c |
| methyl (11r,12r,13s)-(z)-12,13-epoxy-11-methoxy-9-octadecenoate | 126 ab | 201 b | 201 b | 197 b | 158 ab | 85 a | 124 ab | 93 ab | 191 ab |
| 9-octadecene, 1,1-dimethoxy-, (z)- | 16.8 a | 33.6 ab | 41.6 ab | 40.1 ab | 39.3 ab | 26.2 a | 46.4 ab | 35.9 ab | 63.1 b |
| methyl 8-(3-octyl-2-oxiranyl)octanoate | nd a | nd a | nd a | nd a | nd a | nd a | nd a | 76.8 a | nd a |
| methyl 5,13-docosadienoate | 34.3 a | 33.7 a | 34.9 a | 33.4 a | 33.8 a | 28.9 a | 26.7 a | 91.6 a | 96.3 a |
| methyl 9-oxooctadecanoate | 68 a | 97 a | 87 a | 90 a | 116 a | 84 a | 126 a | 90 a | 119 a |
| methyl 4-[2-[[2-[[2-[(2-pentylcyclopropyl)methyl]cyclopropyl]methyl]cyclopropyl]methyl]cyclopropyl]butanoate | 163 a | 239 a | 203 a | 210 a | 219 a | 108 a | 193 a | 168 a | 276 a |
| methyl 5-[(1s,2s)-2-undecylcyclopropyl]pentanoate | 430 a | 451 a | 364 a | 372 a | 329 a | 146 a | 253 a | 199 a | 454 a |
| methyl (z)-5,11,14,17-eicosatetraenoate | 35.1 bc | 42.8 c | 36.8 bc | 33.9 bc | 29.1 bc | 12.1 ab | 26.8 bc | nd a | 34.5 bc |
| methyl 8-[2-((2-[(2-ethylcyclopropyl)methyl]cyclopropyl)methyl)cyclopropyl]octanoate | 40.7 a | 46.3 a | 37.2 a | 38.4 a | 34.7 a | 16.9 a | 24.5 a | 51.6 a | 48.8 a |
| methyl 10,13,16-docosatrienoate | 23.5 a | 48.4 ab | 46.6 ab | 43.3 ab | 62.1 ab | 43.6 ab | 48.4 ab | 41.7 ab | 76.0 b |
| total content of minor FAs | 2309 abc | 2628 bc | 2507 abc | 2464 abc | 2334 abc | 1590 a | 2041 abc | 1923 ab | 2903 c |
| Class of Compounds | Identified Compound | Kovats Retention Indices | Almond Beverages | Oat Beverages | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| KI MXT-5 | KI MXT-17 | ||||||||||||||||||||
| C | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | C | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||
| alcohols | n-butanol | 654 | 781 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| 1-hexanol | 859 | 986 | + | + | |||||||||||||||||
| 2-phenylethanol | 1099 | 1273 | + | + | + | + | + | + | + | ||||||||||||
| 1-propanol, 2-methyl- | 619 | 728 | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| propylenglycol | 741 | 941 | + | + | + | + | + | + | + | + | + | ||||||||||
| terpinen-4-ol | 1175 | 1274 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| 2-propanol | 489 | 597 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| (z)-3-hexen-1-ol | 854 | - | + | ||||||||||||||||||
| aldehydes | acetaldehyde | 446 | 505 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| propanal | 466 | 590 | + | + | + | + | |||||||||||||||
| 2-methylpropanal | 519 | 621 | + | + | + | + | + | + | + | + | + | + | + | + | |||||||
| p-anisaldehyde | 1251 | 1447 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| 2,4-decadienal, (e,e)- | 1304 | 1456 | + | + | |||||||||||||||||
| trans-2-undecenal | 1369 | 1482 | + | + | |||||||||||||||||
| alpha-hexyl cinnamaldehyde | 1754 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| 3-methylbutanal | 646 | 730 | + | + | + | ||||||||||||||||
| hexanal | 790 | 885 | + | + | + | + | + | + | + | + | + | + | + | ||||||||
| octanal | 983 | 1096 | + | + | + | ||||||||||||||||
| benzaldehyde | 958 | 1085 | + | + | + | + | + | + | + | ||||||||||||
| pentanal | 696 | 775 | + | + | + | + | + | ||||||||||||||
| furfural | 827 | 954 | + | + | |||||||||||||||||
| decanal | 1193 | 1294 | + | ||||||||||||||||||
| amines | pyrrole | 777 | 928 | + | + | + | + | + | + | + | + | ||||||||||
| cyclic compounds | myristicin | 1509 | 1677 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| esters | isopropyl acetate | 646 | 720 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| ethyl 3-(methylthio)propanoate | 1107 | 1220 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||
| benzyl phenyl acetate | 1810 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| benzyl salicylate | 1864 | - | + | + | + | + | |||||||||||||||
| 2-phenylethyl phenyl acetate | 1906 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||
| isoamyl acetate | 871 | 931 | + | + | + | + | + | + | + | + | + | + | |||||||||
| ethyl propanoate | 725 | 775 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| methyl 2-methylbutanoate | 786 | 835 | + | + | + | + | + | + | |||||||||||||
| ethyl formate | 479 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| ethyl acetate | 621 | 674 | + | + | + | ||||||||||||||||
| ethyl isobutyrate | 739 | 820 | + | + | + | + | + | + | |||||||||||||
| ethyl butyrate | 803 | 856 | + | + | |||||||||||||||||
| butyl acetate | 820 | 886 | + | + | + | ||||||||||||||||
| ethyl tetradecanoate | 1805 | - | + | ||||||||||||||||||
| benzyl acetate | 1174 | 1293 | + | + | + | + | + | + | + | + | + | + | |||||||||
| benzyl benzoate | 1738 | - | + | + | |||||||||||||||||
| pentyl octanoate | 1460 | - | + | ||||||||||||||||||
| ethyl hexanoate | 989 | 1061 | + | ||||||||||||||||||
| heterocyclic compounds | maltol | 1110 | 1294 | + | |||||||||||||||||
| ketones | 2-heptanone | 881 | 987 | + | |||||||||||||||||
| butane-2,3-dione | 559 | 697 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| butan-2-one | 587 | 679 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||||
| 2,3-pentanedione | 702 | 794 | + | + | + | + | + | + | + | ||||||||||||
| undecan-2-one | 1300 | 1407 | + | ||||||||||||||||||
| lactones | delta-undecalactone | 1505 | 1853 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| delta-decalactone | 1504 | 1731 | + | + | |||||||||||||||||
| 4-undecanolide | 1612 | 1836 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| dodecan-4-olide | 1689 | 1904 | + | + | + | + | + | + | + | + | + | ||||||||||
| delta-dodecalactone | 1722 | 1947 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| 4-octanolide | 1251 | 1498 | + | + | + | + | + | + | + | ||||||||||||
| organic acids | propanoic acid | 740 | 884 | + | + | + | + | + | + | + | |||||||||||
| 3-methylbutanoic acid | 875 | 1016 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |||
| (e)-9-octadecenoic acid | 1934 | - | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| acetic acid | 614 | 776 | + | + | + | + | + | + | + | ||||||||||||
| valerenic acid | 1864 | - | + | + | + | + | + | + | + | ||||||||||||
| butanoic acid | 804 | 955 | + | ||||||||||||||||||
| pentanoic acid | 887 | 1095 | + | + | + | + | + | + | + | + | |||||||||||
| phenolic compounds | 4-ethylguaiacol | 1288 | 1428 | + | + | + | + | + | + | + | + | ||||||||||
| anisyl alcohol | 1287 | 1506 | + | + | + | + | + | + | + | ||||||||||||
| vanillin | 1446 | 1646 | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | + | |
| anethole | 1286 | 1405 | + | + | |||||||||||||||||
| terpenes | citronellal | 1169 | 1252 | + | + | ||||||||||||||||
| thymol | 1299 | 1501 | + | + | + | + | + | + | + | + | + | + | + | + | + | ||||||
| gamma-terpinene | 1084 | 1108 | + | + | + | + | + | ||||||||||||||
| citronellol | - | 1332 | + | ||||||||||||||||||
| 1r-(+)-alpha-pinene | 952 | 943 | + | ||||||||||||||||||
| 1s-()-a-pinene | - | 955 | + | ||||||||||||||||||
| ()-.-beta-.-pinene | 983 | 1005 | + | + | |||||||||||||||||
| Number of identified volatile compounds in PB-beverages | 25 | 30 | 34 | 34 | 37 | 29 | 39 | 36 | 31 | 30 | 29 | 30 | 32 | 36 | 31 | 39 | 27 | 24 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dąbrowski, G.; Paulauskienė, A.; Baltušnikienė, A.; Kłębukowska, L.; Czaplicki, S.; Konopka, I. Changes in Selected Quality Indices in Microbially Fermented Commercial Almond and Oat Drinks. Appl. Sci. 2022, 12, 9983. https://doi.org/10.3390/app12199983
Dąbrowski G, Paulauskienė A, Baltušnikienė A, Kłębukowska L, Czaplicki S, Konopka I. Changes in Selected Quality Indices in Microbially Fermented Commercial Almond and Oat Drinks. Applied Sciences. 2022; 12(19):9983. https://doi.org/10.3390/app12199983
Chicago/Turabian StyleDąbrowski, Grzegorz, Aurelija Paulauskienė, Aldona Baltušnikienė, Lucyna Kłębukowska, Sylwester Czaplicki, and Iwona Konopka. 2022. "Changes in Selected Quality Indices in Microbially Fermented Commercial Almond and Oat Drinks" Applied Sciences 12, no. 19: 9983. https://doi.org/10.3390/app12199983
APA StyleDąbrowski, G., Paulauskienė, A., Baltušnikienė, A., Kłębukowska, L., Czaplicki, S., & Konopka, I. (2022). Changes in Selected Quality Indices in Microbially Fermented Commercial Almond and Oat Drinks. Applied Sciences, 12(19), 9983. https://doi.org/10.3390/app12199983









