Abstract
For site-specific soil ecological risk assessments (SERAs), an integrated chemical, ecotoxicological, and ecological analysis needs to be performed. The SERA guidelines of international institutions and countries recommend that a SERA be initiated at the screening level to save time and social economic cost; however, they provide no unified test species for this screening level. This study performed SERAs for field soils and confirmed the importance of selecting bioassay test species that reflect the ecotoxicity of field soils at the screening level. To confirm test species that reflect the ecological risk of field soils, correlation analysis was performed on the results of each bioassay with the integrated ecotoxicological risk index (EtoxRI). Our results showed that soil algae, nematodes, and plants were the most representative species in soil assays, with high correlation coefficients with EtoxRI. The results imply the importance of selecting test species that represent ecological risk for the screening level of SERAs. Based on these findings, when using SERAs, species sensitivity, ecological relevance, and economic aspects should be considered when selecting the bioassay test species.
1. Introduction
Ecological risk in site-specific areas contaminated with heavy metals can be estimated using various factors, including the total heavy metal concentration. However, the concentration of heavy metals may not reflect the toxicity of the contaminated soil for the given bio-receptor owing to site-specific characteristics, such as soil properties, pH, organic matter content, clay content, and aging [1,2,3]. The guidelines of international organizations and several countries for soil ecological risk assessment (SERA) generally suggest performing chemical, ecotoxicological, and ecological assessments and comparing the results of these assessments for decision-making and environmental policy development [4,5,6,7,8].
The triad approach, an official guideline for “site-specific ecological risk assessment of soil contamination” published by ISO [8], requires lines of evidence (LoE) from chemistry, ecotoxicology, and ecology for site-specific SERA [1,9]. The data assessments are calculated separately and reported on scales of 0–1 for the chemical (ChemRI), ecotoxicological (EtoxRI), and ecological (EcoRI) risk indices. This results in a tiered system, where the site-specific risk of contaminated soil is estimated by integrating the three LoE. The results of lower tiers determine the need for higher-level tiers of ecological risk assessment (ERA), which require more analyses and reduce uncertainty. The lower tiers analyze the risk through a comparatively simple analysis that is faster and more economically feasible.
Each country and institution recommends lists of bioassays for the ecotoxicological analysis of SERAs by level; however, no unified test species for this screening level has been proposed. Uncertainties in actual ecological risk (including under- and overestimation) arise when bioassays for this screening level are performed by selecting species based only on cost-effectiveness [10,11]. Jensen et al. [1] suggest that all aspects, such as reproducibility, sensitivity, economics, applicability, and ecological relevance, should be considered when selecting species for bioassays. Although some previous studies have considered reproducibility, economic feasibility, and applicability, most have ranked sensitivity and ecological relevance as the least important features. In soil toxicology, earthworms and springtails are commonly used; however, various other test species have been proposed, and improved understanding of biodiversity and suitability of species is a research focus [3,12,13]. However, to the best of our knowledge, few studies have estimated the suitability of test species for ecotoxicity tests in site-specific SERAs. Niemeyer et al. [2] evaluated the suitability of bioassays by deriving the sensitivity of bioassays in ERA and considering cost-effectiveness; however, the test duration of the bioassays that they compared was too long to be used as a tool at the screening level (with a minimum of 14 days and a maximum of 56 days). Therefore, it is necessary to consider which species are more suitable as test species for the screening level of bioassays for triad methods.
Therefore, the aim of this study was to find test species for bioassays that are suitable at the screening level of SERAs. We conducted chemical and ecotoxicological analyses using field soil contaminated by heavy metals. The total concentrations of heavy metals in the soils were measured in the chemical assessment, and the ecotoxicological assessment was performed using six soil species. The test duration and endpoints of bioassays were minimized to ensure cost-effectiveness for the screening level of SERAs. In addition, we estimated the EtoxRI by integrating the results of all bioassays and analyzed the correlations with each bioassay to confirm the test species that best represent ecotoxicity at the screening level of SERAs. The appropriate reflection of ecotoxicity in soils will optimize screening in ecological risk assessments.
2. Materials and Methods
2.1. Study Site and Soil Sampling
The study site, an abandoned mine that produced Au, Cu, Pb, and Zn from 1961 to 1976, is located within North Chungcheong Province, Korea; there is a freshwater lake located ~1 km away from the site (Figure 1). Two muck fields (totaling ~7,500 m3; A1 and A3) were expected to have very high contamination levels. The areas in front of the mine head (A2) and at the bottom of the muck field (A4) were expected to have lower contamination levels than the muck fields. A reference site (R) was selected under the following conditions: (1) no contaminated material was detected, (2) it was close enough to the study site to have similar vegetation, ecology, and climate conditions, and (3) it had similar physical and chemical soil characteristics. Soil sampling was performed by collecting five sub-samples at depths of 0–20 cm at each site and combining them into one sample. Samples were collected in August 2020 and immediately moved to the laboratory for preparation and analysis. The concentrations of heavy metals (including As, Cd, Cu, Ni, Pb, and Zn) were found to exceed the thresholds of the Worrisome Level of Soil Contamination and Standards of Countermeasures against Soil Contamination of Korea [14,15].
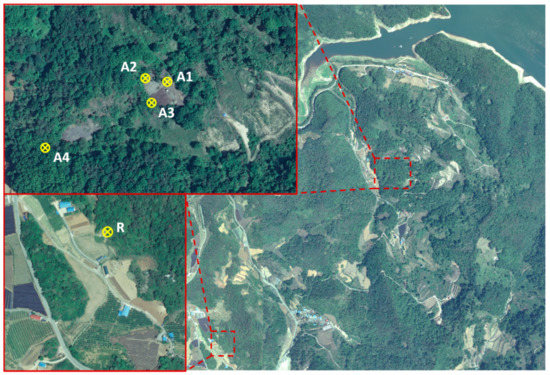
Figure 1.
Image of the study site. The yellow circles show each sampling area. The aerial photograph was provided by the National Geographic Information Institute of Korea (https://www.ngii.go.kr, accessed 10 May 2021).
2.2. Sample Preparation and Characterization
The soil samples were completely dried in a greenhouse under natural conditions and were sieved using 2 mm mesh. The soils were placed in a 2 L glass bottle and rolled overnight using a roller for homogenization. They were used for chemical and ecotoxicological analyses after stabilization at 25 ± 1 °C. To determine the physical characteristics, the contents of sand, silt, and clay were measured; to determine the chemical characteristics, the soil pH, electrical conductivity (EC), total organic carbon (TOC), and major and total cationic contents were measured. The total concentrations for seven metals and metalloids were measured by inductively coupled plasma optic emission spectrometry (ICP-OES, Vista Pro, Varian Inc., Mulgrave, Victoria, Australia).
2.3. Bioassays
For bioassays using soil samples, six test species were selected: Vigna radiata, Oryza sativa, Eisenia andrei, Folsomia candida, Caenorhabditis elegans, and Chlorococcum infusionum. For the plant assays, dicotyledoneae mung bean (V. radiata) and monocotyledoneae rice (O. sativa) were selected as test species. The plant assay methods followed those of Kim et al. [16] and the modified Organization for Economic Cooperation and Development (OECD) test guideline no. 208 [17]. For each pot, five seeds were exposed, and four replicates were run. For V. radiata, the experimental conditions were 25 ± 1 °C, 16 h:8 h of light-dark photoperiod, and 80% humidity, while those for O. sativa were 32 ± 1 °C, 16 h:8 h of light-dark photoperiod, and 100% humidity. The experimental conditions were adjusted to reflect the growing environment of each species. After 7 days and 14 days of exposure, the length of the shoots were measured.
In the earthworm assay, 310–600 mg of adult earthworm (E. andrei) was tested after removing gut contents on a moistened filter paper (3 h, 20 ± 1 °C, darkness). The earthworm soil acute assay followed a previous study [18]. Earthworms were maintained in the darkness at 20 ± 1 °C for 14 days. Ten replicates for each soil sample were run and the assay was repeated twice. After the 14-day assessment, worms were moved to trays and the survival rate was assessed.
The collembola assays were conducted according to the OECD test guideline no. 232 using F. candida [19]. F. candida exposed to soil samples were kept in 20 °C incubator with 16 h:8 h light-dark photoperiod for 14 days (acute), and 28 days (chronic). For chronic assays, ventilation was performed every week and food was supplied every 14 days. The survival rate (acute) and reproduction rate (chronic) of F. candida were measured. Every test was run with four replicates.
For the soil nematode assay, C. elegans (wild type, Bristol strain N2) was used as the test species. The test was conducted according to a modified method of Kim et al. [20]. Five young, adult C. elegans (54–60 h after synchronization) were exposed to each soil sample and four replicates were run. Exposure conditions were 20 ± 1 °C in the dark for 24 h; after exposure, the offspring were counted.
In the soil algae assay, C. infusionum, obtained from SAG (Göttingen, Germany), was used as test species and precultured in Bold’s basal medium (BBM). The tests were conducted in a 12-well plate following the method of Nam et al. [21]. They were incubated at 24 ± 2 °C under 16 h:8 h of light-dark photoperiod for 6 days. After 6 days, for the extraction of algae in the soil, the BBM medium was added to each well and plates were cultured for 24 h under exposed conditions. After 24 h, chlorophyll in the supernatant was extracted for 3 h and measured using a microplate reader (Varioskan LUX Multimode Microplate Reader, Thermo Fisher Scientific, MA, United States) at an excitation of 420 nm and emission of 671 nm.
2.4. Estimation of the EtoxRI
EtoxRIs (based on the ecotoxicological risk index) were calculated by integrating data from all bioassays, following the method of Dagnino et al. [9]. A relative toxic response (RTR) was calculated using Equation (1), with the toxic response (TR) obtained by inserting data from the ecotoxicological test:
where represents the result for ith endpoints by exposure to the j soil sample and indicates the result for ith endpoints by exposure to the reference soil sample (i.e., a control group). In this study, was set to 100 and was calculated as a percentage of the reference soil sample.
For each endpoint, the RTR was scored as a risk index (RI) by comparing it with two thresholds (Th1 and Th2). If the RTR was less than Th1, the RI was calculated as 0; if the RTR was greater than Th2, the RI was 1. For Th1 < RTR < Th2, the RI was calculated using Equation (2), where the RI is from 0 to 1:
Dagnino et al. [9] specified Th1 and Th2 in the ecotoxicological assessment to be 0.2 and 0.8, respectively. The EtoxRI was obtained by integrating values from each RI using Equation (3):
2.5. Data Analysis
All datasets for bioassays were processed as a percentage of the reference soil. The statistical difference of data for contaminated soils were analyzed using SPSS Statistics version 24 (IBM) (p < 0.05). Moreover, correlation analysis of datasets of bioassays with EtoxRIs were performed, and Pearson’s product-moment correlation coefficient were calculated.
3. Results and Discussion
3.1. Soil Characterization
The physiochemical characteristics of the field soils are shown in Table 1. The pH of the soils was in the range of 7.1–7.5, excluding A3 (6.5), with an EC of 0.011–0.024 S/m. The TOC contents of R, A1, and A3 were lower than those of A2 and A4, in which there were many different types of vegetation. The range for the sum of cations was 112.6–254.8 cmol/kg soil. The field soils were sandy loam, except for the A4 soil, which was loamy sand.

Table 1.
Physicochemical characteristics of reference and test soils in North Chungcheong Province, Korea, used in soil ecological risk assessments.
The total concentrations of heavy metals in the soil are shown in Table 2. The contamination of the R soil was found to be non-hazardous, with low levels of heavy metals. The contamination levels were identified to be in the order of A1, A3, A2, and A4. The concentrations of As were the highest for all of the contaminated soils, followed by Pb, Zn, Cu, and Ni. The A1 and A3 soils were found to be highly contaminated and, although the A2 and A4 soils were found to have significantly lower concentrations of heavy metals than A1 and A3, they were also highly contaminated.

Table 2.
Total concentrations of heavy metals in test soil samples in North Chungcheong Province, Korea, used in soil ecological risk assessments, as determined with inductively coupled plasma optic emission spectrometry (ICP-OES).
3.2. Ecotoxicological Analysis
The bioassay results are shown in Figure 2. In the plant assay, the inhibition of shoot growth for V. radiata compared with that in the reference soil was observed in the order of A1, A3, and A2; that for O. sativa was observed in the order of A1 and A3 (p < 0.05). The growth of both plants was higher in the A4 soil, and the growth of rice was significantly higher in the A2 soil (p < 0.05) than in the reference soil. In the earthworm soil acute assay, the mortality of E. andrei after 14 days of exposure was not significantly higher in the contaminated soil (A1–A4) than in the reference soil (p < 0.05). For the collembola assay, none of the contaminated soil samples showed a significant decrease in the survival of adults. However, the reproduction rate significantly decreased in A3 (p < 0.05). The reproduction rate of nematodes was significantly decreased in the order of A3, A1, and A4 (p < 0.05). The biomass of C. infusionum was significantly lower in A1 and A3 than in the reference soil (p < 0.05); however, there was no significant difference between A2 and A4 (p > 0.05). For soil bioassays, species sensitivity was high for C. infusionum, V. radiata, C. elegans, F. candida, O. sativa, and E. andrei (Figure 3). Except for the E. andrei and acute F. candida assays, almost all species used in this study were found to be sensitive to A1 and A3. This appears to reflect the high total concentrations of heavy metals in the A1 and A3 soils.
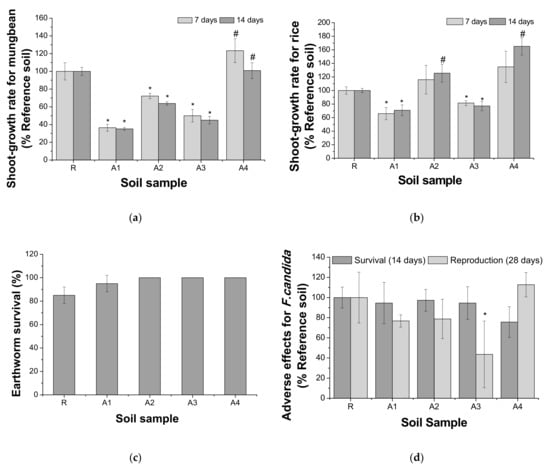
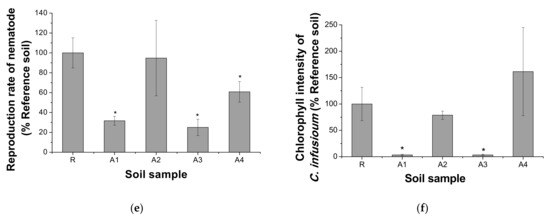
Figure 2.
Soil bioassays using soil organisms, where (a) and (b) show the growth rates in plant assays on mung bean (V. radiata), and rice (O. sativa) after a 7-day (left) and 14-day exposure (right), respectively, (c) shows the survival rate of earthworms (E. andrei) after a 14-day exposure, (d) represents the adverse effects on collembola (F. candida) after 14 days (left; the survival of adults) and 28 days (right; the reproduction of juvenile), and (e) and (f) show the reproduction rate of the soil nematode (C. elegans) after a 24 h exposure and the chlorophyll intensity of soil algae (C. infusionum) after a 6-day exposure, respectively. Line bars indicate standard deviation, and the asterisk (*) indicates a significant adverse effect compared with the control (R). The hash (#) indicates a significant positive effect compared with the R.
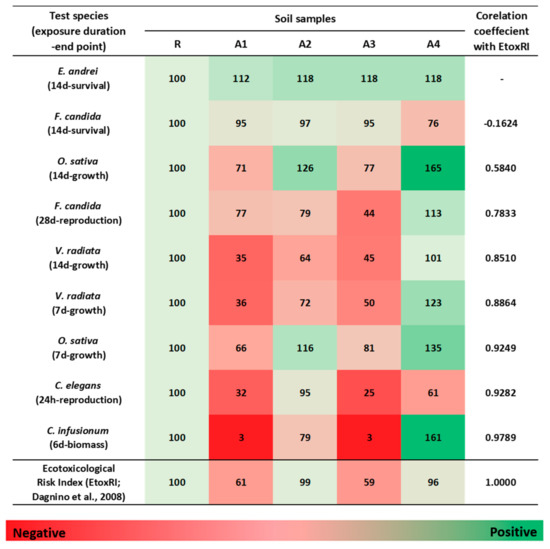
Figure 3.
Comparison of each bioassay and the ecotoxicological risk index (EtoxRI). To compare with bioassay results, the EtoxRI (calculated as 0–1 in this study) was converted to a percentage of the R soil (control). Red and green colors denote negative and positive effects, respectively. The test species are placed in the order of the correlation coefficients calculated from the correlation with the EtoxRI. The higher the correlation with the EtoxRI, the lower it is placed. The right-most column presents the correlation coefficients of the EtoxRI and the bioassay results.
3.3. EtoxRI
The EtoxRI based on soil bioassays was confirmed to be 0.39 (A1), 0.01 (A2), 0.41 (A3), and 0.04 (A4). No interval classification for the EtoxRI is presented because it is expressed as an environmental RI through integration with ChemRI and EcoRI [9]. However, EtoxRI is expressed as a range from 0.00 to 1.00 in relation to the reference soil; that is, the smaller the number, the lower the ecotoxicological risk. Therefore, we quantified the EtoxRI for the soil bioassays, and confirmed that the R, A2, and A4 soils had very low ecotoxicological risk, whereas the A1 and A3 soils had a moderate risk (Figure 3). Chemical analysis showed that the contamination levels of all contaminated soils were very high, whereas the ecotoxicological analysis showed that the risk degree varied depending on the soil. It was assumed that the discrepancies in the degrees of risk for the soils were due to changes in the bioavailability of heavy metals.
3.4. Correlation of EtoxRI and Ecotoxicological Data
Our comparison of EtoxRI with ecotoxicological data showed that the test species that best reflected the overall ecotoxicity of field soils were in the order of soil algae (C. infusionum), soil nematodes (C. elegans), and plants (especially acute assays for O. sativa and V. radiata); their correlation coefficients were 0.979 (p < 0.01), 0.928, 0.925, and 0.886 (p < 0.05), respectively. Other species had lower correlation coefficients. Therefore, in this study, we determined that soil algae, soil nematodes, and plants are ideal for well-represented assays that reflect site-specific SERA.
According to Jensen et al. [1] (the progenitors of the triad approach), commercial test kits for luminescent bacteria (ostracod and cladoceran) are recommended as toxicology tools for tier 1. For tier 2, the acute toxicity test using earthworms and avoidance tests for soil invertebrates are suggested. Finally, for tier 3 toxicology tools to evaluate the potential risk of soils for entire ecosystems, plant assays, reproduction tests using earthworms and collembola, and enchytraeid tests are recommended for soil bioassays, whereas algae tests, aquatic plant tests, and bioassays using Daphnia magna are suggested for soil extract bioassays. The findings of this study raise uncertainties that the list of test species may not adequately reflect the ecotoxicity of field soils for site-specific SERA. Therefore, it is assumed that discussion on the recommended bioassays for site-specific SERA will be necessary.
We compiled a list of the test species used in triad approach studies (Table 3). The ecotoxicological LoE in the tier 1 assessment was mainly estimated from bioassays using only one soil type. Only two or three studies performed bioassays using two or three soil species. Two other studies used no soil species at all [22,23]. Most studies performed bioassays using plants or earthworms. Bioassays using aquatic organisms were evaluated using soil eluates, extract, and leachate, among others, for the tier 1 triad approach. Among them, D. magna and Aliivibrio fischeri were usually used as test species [23,24,25,26,27,28] as they are reliable, rapid, and cheap [1,29]. Five studies among nine researched used algae or plant assays in solution tests [27,28,30,31,32]. Few studies selected test species based on the ecological relevance of the site (Table 3). The main justifications for the selection of a test species are common use in soil ecotoxicity tests, simplicity, and low cost of the assay. The author van Gestel [33] suggested the following criteria to select test species for ERA: practicability, including feasibility and cost-effectiveness; acceptability, including standardization, reproducibility, and validity; and ecological meaning, such as sensitivity and ecological realism. In addition, as identified in this study, performing bioassays by selecting test species based on economic reasons does not achieve the goal of screening for ecological risk. Using a single species is also likely to underestimate or overestimate the ecotoxicological risk when screening for a SERA. Therefore, even at the screening level, at least two test species for soil ecosystems should be used. In addition, the ecological relevance or species sensitivity should be considered in the selection of test species.

Table 3.
Summary of previous studies on the criteria for selecting test species for the screening of site-specific soil ecological risk assessments (SERAs). The asterisk (*) indicates research that performed bioassays using only one test media.
In this study, we identified test species that best represent the ecological risk of field soils; however, the results are limited because they reflect site specificity of the field soil. Moreover, various factors (e.g., diversity of texture of soils and pollutants, and low-contaminated soil samples) were not considered. However, in ecotoxicity assessment, the results may vary depending on soil characteristics and the degree of contamination [34,35,36]. Despite these limitations, this study is meaningful in that it identified test species that can represent the ecological risk of field soils at the screening level of SERAs by comparing the ecotoxicological index with ecotoxicity data. Future studies should identify test species suitable for use at the screening level of SERAs more carefully; the accumulation of such data could lead to test species that have site-specific characteristics and ecological relevance.
4. Conclusions
In this study, we attempted to identify test species to represent ecological risk at the screening level of a site-specific SERA using nine bioassays of soil species. We found that soil algae, nematodes, and plants have the highest statistical correlation with the EtoxRI of soil. These results also indicate that previous studies conducted using earthworm or marine bacteria bioassays did not reflect site-specific ecological risk. Furthermore, the previous studies using only one or two bioassays, likely had high uncertainty in terms of the ecological risk while screening. Therefore, future studies for site-specific SERAs should select test species that reflect site characteristics, including sensitivity and ecological relevance.
Author Contributions
Conceptualization, D.K. and Y.-J.A.; methodology, D.K., T.-Y.L., L.K., R.C., J.I.K., H.K., W.H., and J.-I.K.; investigation, D.K., T.-Y.L., L.K., R.C., J.I.K., H.K., S.-H.N., M.K., W.H., and S.H.; writing—original draft preparation Y.-J.A.; writing—review and editing, Y.-J.A.; supervision, Y.-J.A.; funding acquisition, Y.-J.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Institute of Environment Research (NIER), funded by the Ministry of Environment (MOE) of the Republic of Korea (NIER-SP2020-223). This research was funded by Basic Science Research Program through the National Research Foundation (NRF) of Korea funded by the Ministry of Science, ICT and future planning (2020R1A2B5B02001734). This paper was also supported by the Konkuk University Research Fund in 2020, and the MOE through the graduate school of specialization for safe management of chemicals.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank the Korea Basic Science Institute (KBSI) for the ICP-AES analysis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jensen, J.; Mesman, M.; Loibner, A.P.; Erlacher, E.; Rutgers, M.; Archibald, G.; Ehlers, C.; Dirven-van Breemen, L.; Bogolte, B.T.; Sorokin, N.; et al. Ecological Risk Assessment of Contaminated Land: Decision Support for Site Specific Investigations; RIVM: Bilthoven, The Nederland, 2006. [Google Scholar]
- Niemeyer, J.C.; da Silva, E.M.; Sousa, J.P. Soil ecotoxicology in environmental risk assessment: A case study in a metal contaminated site in Brazil. In Ecotoxicology in Latin America; Araújo, C.V.M., Shinn, C.H., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2017; pp. 1–19. [Google Scholar]
- Römbke, J.; Martin-Laurent, F. Microbial, Plant, and Invertebrate Test Methods in Regulatory Soil Ecotoxicology. In Bioavailability of Organic Chemicals in Soil and Sediment; Ortega-Calvo, J.J., Parsons, J.R., Eds.; Springer International Publishing: Berlin, Germany, 2020; pp. 369–388. [Google Scholar]
- USEPA (United States Environmental Protection Agency). Guidelines for Ecological Risk Assessment; USEPA: Washington, DC, USA, 1998.
- EA (Environment Agency). An Ecological Risk Assessment Framework for Contaminants in Soil; EA: Bristol, UK, 2008.
- NEPC (National Environment Protection Council). Guideline on Ecological Risk Assessment, Schedule B5a; NEPC: Canberra, Australia, 2010. [Google Scholar]
- CCME (Canadian Council of Ministers of the Environment). Federal Contaminated Sites Action Plant (FCSAP)-Ecological Risk Assessment Guidance; Environment Canada: Gatineau, QC, Canada, 2012.
- ISO (International Organization for Standardization). Soil Quality—Procedure for Site-Specific Ecological Risk Assessment of Soil Contamination (Soil Quality TRIAD Approach); ISO 19204; ISO: Geneva, Switzerland, 2017. [Google Scholar]
- Dagnino, A.; Sforzini, S.; Dondero, F.; Fenoglio, S.; Bona, E.; Jensen, J.; Viarengo, A. A weight-of-evidence approach for the integration of environmental “triad” data to assess ecological risk and biological vulnerability. Integr. Environ. Assess. Manag. 2008, 4, 314–326. [Google Scholar] [CrossRef] [PubMed]
- Duke, L.D.; Taggart, M. Uncertainty factors in screening ecological risk assessments. Environ. Toxicol. Chem. 2000, 19, 1668–1680. [Google Scholar] [CrossRef]
- Semenzin, E.; Critto, A.; Rutgers, M.; Marcomini, A. Integration of bioavailability, ecology, and ecotoxicology by three lines of evidence into ecological risk indexes for contaminated soil assessment. Sci. Total Environ. 2008, 389, 71–86. [Google Scholar] [CrossRef] [PubMed]
- ISO (International Organization for Standardization). Soil Quality—Determination of Dry Matter and Water Content on a Mass Basis—Gravimetric Method; ISO 11465; ISO: Geneva, Switzerland, 1993. [Google Scholar]
- EFSA PPR Panel (Panel on Plant Protection Products and their Residues (PPR)). Scientific opinion addressing the state of the science on risk assessment of plant protection products for in-soil organisms. EFSA J. 2017, 15, 1–225. [Google Scholar]
- KECO (Korea Environment Corporation). A Survey on the Status of Soil Pollution in Waste Metal Mine; KECO: Incheon, Korea, 2007. [Google Scholar]
- MOE (Korean Ministry of Environment). Soil Environment Conservation Act; MOE: Seoul, Korea, 2020.
- Kim, D.; Cui, R.; Moon, J.; Kwak, J.I.; Kim, S.W.; Kim, D.; An, Y.-J. Estimation of the soil hazardous concentration of methylparaben using a species sensitivity approach. Environ. Pollut. 2018, 242, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- OECD (Organisation for Economic Co-operation and Development). OECD Guideline for Testing of Chemicals; Test No.208; “Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test”; OECD: Paris, France, 2006. [Google Scholar]
- Kwak, J.I.; Nam, S.-H.; Kim, S.W.; Bajagain, R.; Jeong, S.W.; An, Y.-J. Changes in soil properties after remediation influence the performance and survival of soil algae and earthworm. Ecotox. Environ. Safe. 2019, 174, 189–196. [Google Scholar] [CrossRef] [PubMed]
- OECD (Organisation for Economic Co-operation and Development). OECD Guidelines for Testing of Chemicals; Test No.232; “Collembolan Reproduction Test in Soil”; OECD: Paris, France, 2009. [Google Scholar]
- Kim, S.W.; Moon, J.; Jeong, S.W.; An, Y.-J. Development of a nematode offspring counting assay for rapid and simple soil toxicity assessment. Environ. Pollut. 2018, 236, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Nam, S.-H.; An, Y.-J. A rapid screening method to assess soil algal toxicity: Non-destructive sampling of algal cells using culture medium extraction. Appl. Soil Ecol. 2017, 120, 143–152. [Google Scholar] [CrossRef]
- Frick, H.; Tardif, S.; Kandeler, E.; Holm, P.E.; Brandt, K.K. Assessment of biochar and zero-valent iron for in-situ remediation of chromated copper arsenate contaminated soil. Sci. Total Environ. 2019, 655, 414–422. [Google Scholar] [CrossRef]
- Klimkowicz-Pawlas, A.; Maliszewska-Kordybach, B.; Smreczak, B. Triad-based screening risk assessment of the agricultural area exposed to the long-term PAHs contamination. Environ. Geochem. Health 2019, 41, 1369–1385. [Google Scholar] [CrossRef]
- Antunes, S.C.; Castro, B.B.; Pereira, R.; Gonçalves, F. Contribution for tier 1 of the ecological risk assessment of Cunha Baixa uranium mine (Central Portugal): II. Soil ecotoxicological screening. Sci. Total Environ. 2008, 390, 387–395. [Google Scholar] [CrossRef]
- Niemeyer, J.C.; Moreira-Santos, M.; Nogueira, M.A.; Carvalho, G.M.; Ribeiro, R.; Da Silva, E.M.; Sousa, J.P. Environmental risk assessment of a metal-contaminated area in the Tropics. Tier I: Screening phase. J. Soils Sediments 2010, 10, 1557–1571. [Google Scholar] [CrossRef]
- Ribé, V.; Aulenius, E.; Nehrenheim, E.; Martell, U.; Odlare, M. Applying the Triad method in a risk assessment of a former surface treatment and metal industry site. J. Hazard. Mater. 2012, 207, 15–20. [Google Scholar] [CrossRef]
- Chapman, E. Ecological Risk Screening of Metal (Pb and Zn) Contaminated Acidic Soil Using a Triad Approach. Ph.D. Thesis, University of Gothenburg, Gothenburg, Sweden, 4 June 2013. [Google Scholar]
- Pereira, R.; Bouguerra, S.; Lopes, I.; Santos, B.; Marques, C.R.; Silva, C.; Mestiri, A.; Frankenbach, S.; Hentati, O.; Khadraoui, M.; et al. Application of a standard risk assessment scheme to a North Africa contaminated site (Sfax, Tunisia)-Tier 1. Chemosphere 2021, 263, 128326. [Google Scholar] [CrossRef]
- EA (Environment Agency). Guidance on the Use of Bioassays in Ecological Risk Assessment; EA: Bristol, UK, 2008.
- Karjalainen, A.M.; Kilpi-Koski, J.; Väisänen, A.O.; Penttinen, S.; van Gestel, C.A.; Penttinen, O.P. Ecological risks of an old wood impregnation mill: Application of the triad approach. Integr. Environ. Assess. Manag. 2009, 5, 379–389. [Google Scholar] [CrossRef]
- Sorvari, J.; Schultz, E.; Haimi, J. Assessment of ecological risks at former landfill site using TRIAD procedure and multicriteria analysis. Risk Anal. 2013, 33, 203–219. [Google Scholar] [CrossRef]
- Hong, Y.K.; Yoon, D.H.; Kim, J.W.; Chae, M.J.; Ko, B.K.; Kim, S.C. Ecological risk assessment of heavy metal-contaminated soil using the triad approach. J. Soils Sediments 2020, 1–12. [Google Scholar] [CrossRef]
- Van Gestel, C.A.M. Scientific basis for extrapolating results from soil ecotoxicity tests to field conditions and the use of bioassays. In Ecological Risk Assessment of Contaminants in Soil; van Straalen, N.M., Løkke, H., Eds.; Springer: Boston, MA, USA, 1997; pp. 25–50. [Google Scholar] [CrossRef]
- Płaza, G.A.; Nałęcz-Jawecki, G.; Pinyakong, O.; Illmer, P.; Margesin, R. Ecotoxicological and microbiological characterization of soils from heavy-metal-and hydrocarbon-contaminated sites. Environ. Monit. Assess. 2010, 163, 477–488. [Google Scholar] [CrossRef]
- Agnieszka, B.; Tomasz, C.; Jerzy, W. Chemical properties and toxicity of soils contaminated by mining activity. Ecotoxicology 2014, 23, 1234–1244. [Google Scholar] [CrossRef]
- Liu, K.; Li, C.; Tang, S.; Shang, G.; Yu, F.; Li, Y. Heavy metal concentration, potential ecological risk assessment and enzyme activity in soils affected by a lead-zinc tailing spill in Guangxi, China. Chemosphere 2020, 251, 126415. [Google Scholar] [CrossRef]
- Gworek, B.; Baczewska-Dąbrowska, A.H.; Kalinowski, R.; Górska, E.B.; Rekosz-Burlaga, H.; Gozdowski, D.; Olejniczak, I.; Graniewska, M.; Dmuchowski, W. Ecological risk assessment for land contaminated by petrochemical industry. PLoS ONE 2018, 13, e0204852. [Google Scholar] [CrossRef] [PubMed]
- Son, J.; Kim, J.G.; Hyun, S.; Cho, K. Screening level ecological risk assessment of abandoned metal mines using chemical and ecotoxicological lines of evidence. Environ. Pollut. 2019, 249, 1081–1090. [Google Scholar] [CrossRef] [PubMed]
- Buch, A.C.; Niemeyer, J.C.; Marques, E.D.; Silva-Filho, E.V. Ecological risk assessment of trace metals in soils affected by mine tailings. J. Hazard. Mater. 2021, 403, 123852. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).