Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes
Abstract
Featured Application
Abstract
1. Introduction
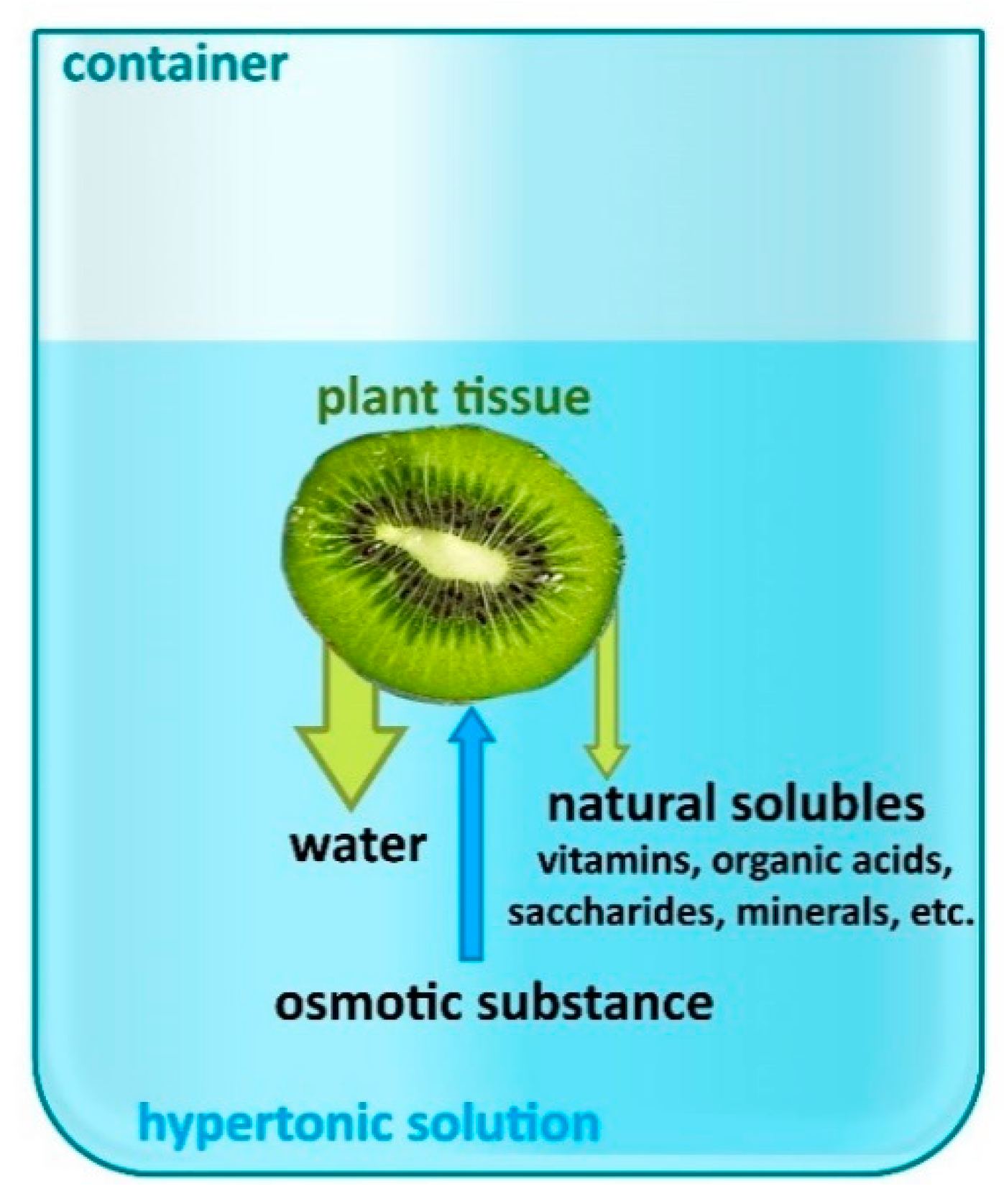
2. Osmotic Dehydration Process
3. Sonication
3.1. Ultrasound Treatment
3.2. Ultrasound Mechanism
- “sponge effect”;
- cavitation;
- absorption of acoustic energy; and
- effects accompanying cavitation (in particular at the solid/liquid border), such as microstreaming, microjetting or standing wave pattern.
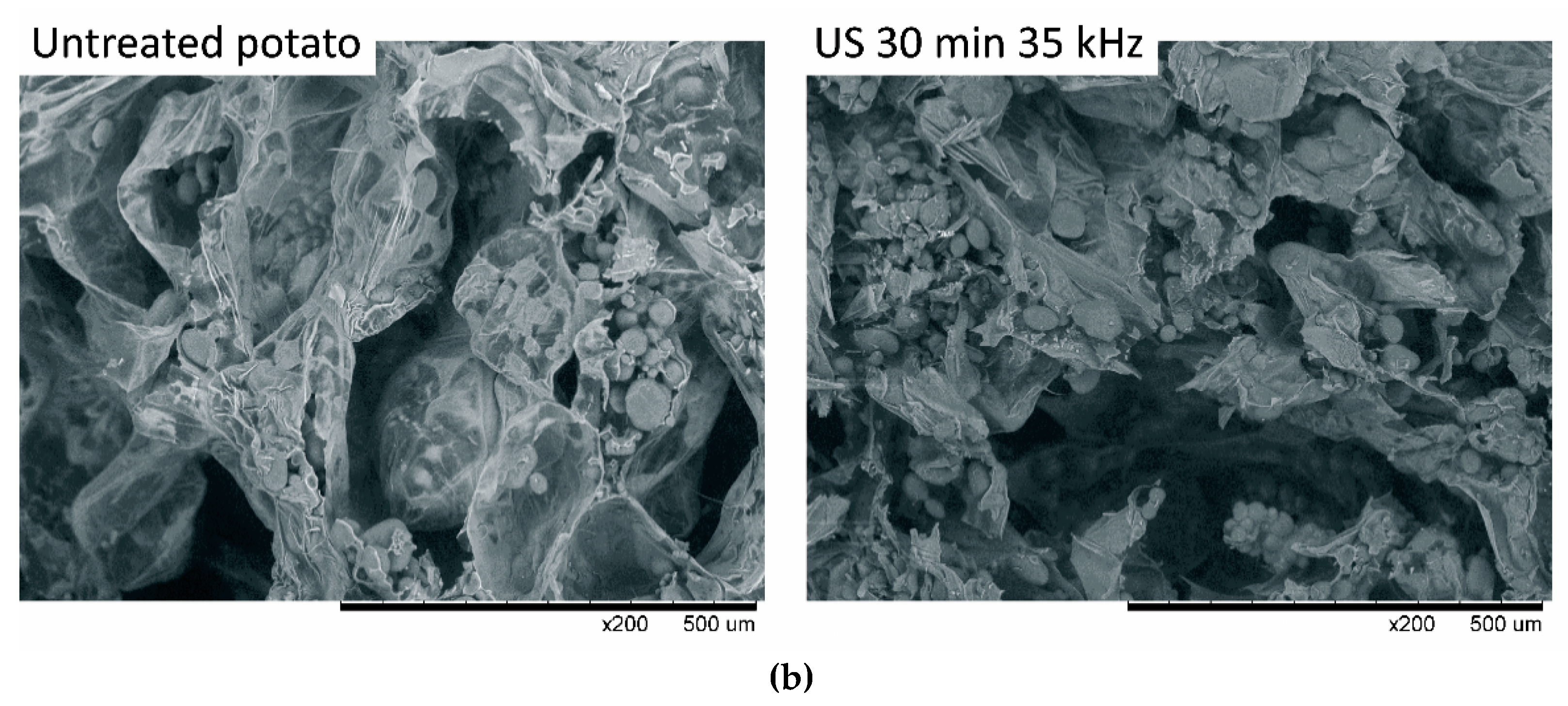
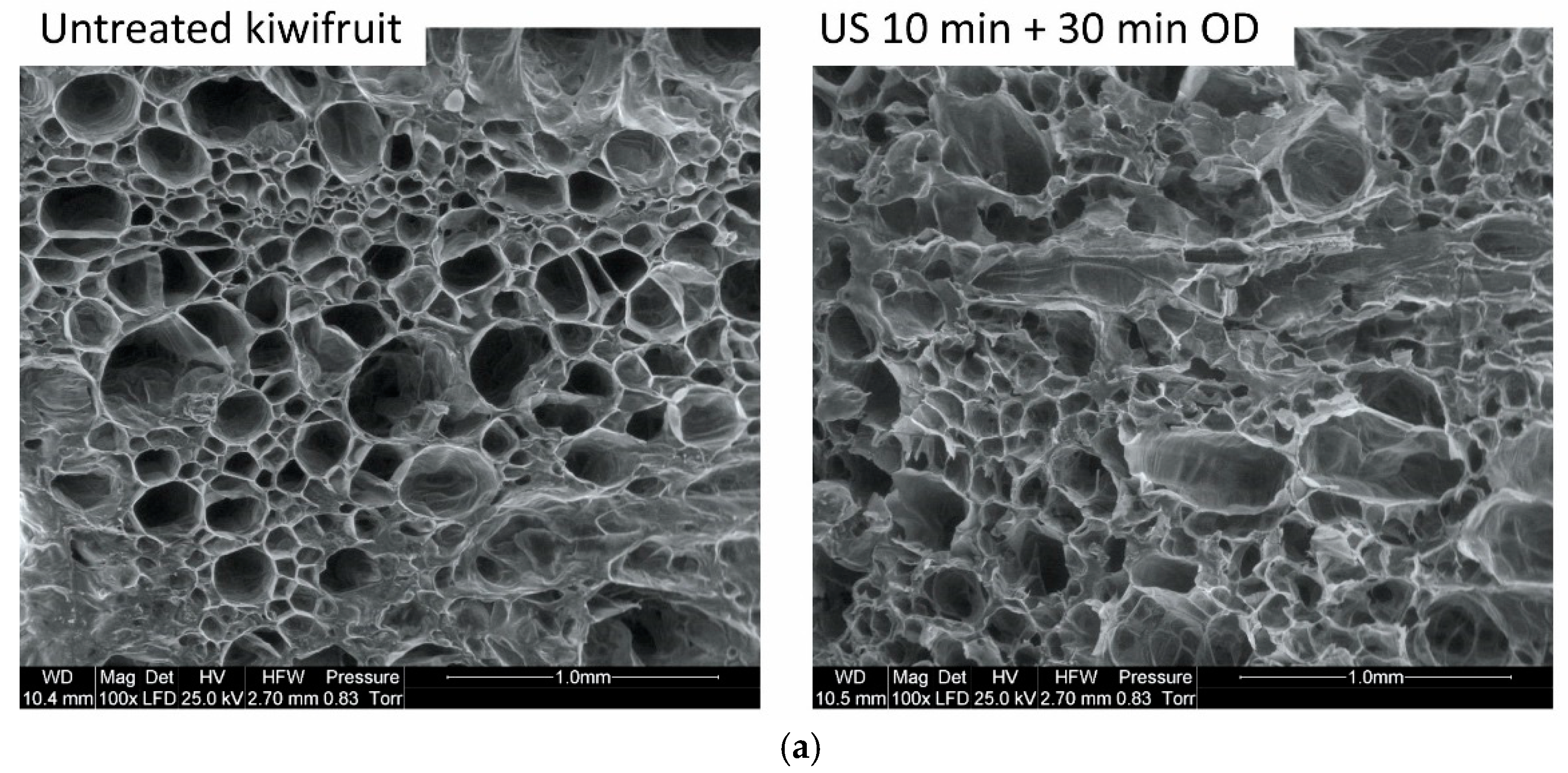
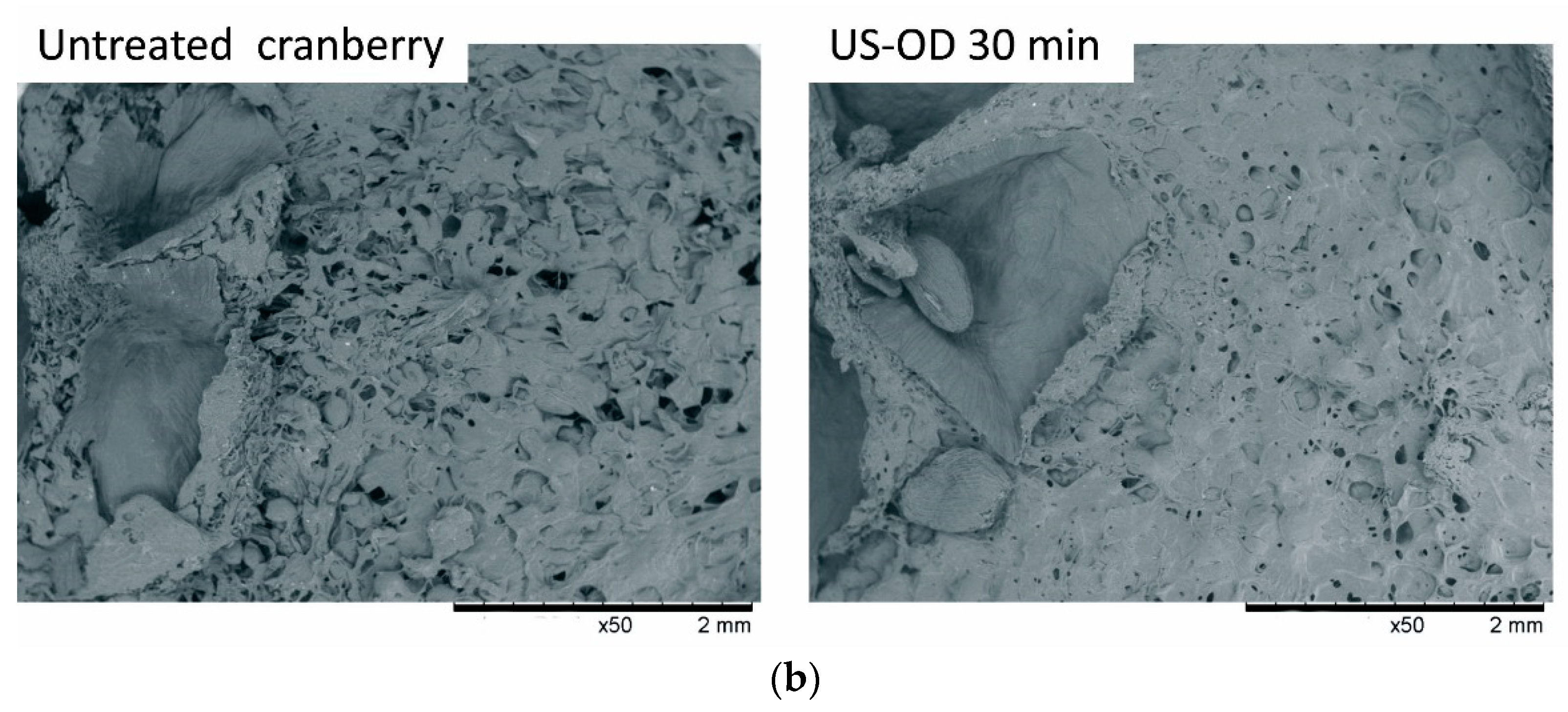
3.3. Structure Changes of Material Subjected to Sonication and Osmotic Process
3.4. Effect of Sonication and Osmotic Dehydration Process on Microbial Growth
3.5. Use of Sonication in Food Technology
4. Ultrasound-Assisted Osmotic Dehydration
4.1. Comparison of Ultrasound Assisted Osmotic Dehydration to Sonication Treatment—Kinetics Aspects
4.2. Synergic Effect of US and Other Innovative Technics/Treatments in Osmotic Dehydration
5. Properties of the Product Treated with US before or during OD
5.1. Physical Properties of the Product Treated with US before or during OD
5.2. Functional Properties of the Product Treated with US before or during OD
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lotito, S.B.; Frei, B. The increase in human plasma antioxidant capacity after apple consumption is due to the metabolic effect of fructose on urate, not apple-derived antioxidant flavonoids. Free Radic. Biol. Med. 2004, 37, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, J.B.; Camesano, T.A.; Cassidy, A.; Kris-etherton, P.; Howell, A.; Manach, C.; Ostertag, L.M.; Sies, H.; Skulas-ray, A.; Vita, J.A. Cranberries and Their Bioactive Constituents. Adv. Nutr. 2013, 4, 618–632. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: WHO. Diet, Nutrition and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; WHO Technical Report Series, No. 916; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Ojha, K.S.; Kerry, J.P.; Tiwari, B.K.; O’Donnell, C. Freezing for Food Preservation; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780081005965. [Google Scholar]
- Witrowa-Rajchert, D.; Rzaca, M. Effect of drying method on the microstructure and physical properties of dried apples. Dry. Technol. 2009, 27, 903–909. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. Low-cost drying methods for developing countries. Trends Food Sci. Technol. 2003, 14, 519–528. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. New hybrid drying technologies. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2005; pp. 535–551. ISBN 9780126767575. [Google Scholar]
- Aguilera, J.M.; Chiralt, A.; Fito, P. Food dehydration and product structure. Trends Food Sci. Technol. 2003, 14, 432–437. [Google Scholar] [CrossRef]
- Prinzivalli, C.; Brambilla, A.; Maffi, D.; Lo Scalzo, R.; Torreggiani, D. Effect of osmosis time on structure, texture and pectic composition of strawberry tissue. Eur. Food Res. Technol. 2006, 224, 119–127. [Google Scholar] [CrossRef]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Ciurzyńska, A.; Czajkowska, K.; Cichowska, J.; Rybak, K.; Lenart, A. Osmotic dehydration of Honeoye strawberries in solutions enriched with natural bioactive molecules. LWT Food Sci. Technol. 2017, 85, 500–505. [Google Scholar] [CrossRef]
- Rzaca, M.; Witrowa-Rajchert, D.; Tylewicz, U.; Rosa, M.D. Wymiana masy w procesie odwadniania osmotycznego owoców Kiwi. Zywn. Nauk. Technol. Jakosc 2009, 16, 140–149. [Google Scholar]
- Kowalska, J.; Kowalska, H.; Marzec, A.; Brzeziński, T.; Samborska, K.; Lenart, A. Dried strawberries as a high nutritional value fruit snack. Food Sci. Biotechnol. 2018, 27, 799–807. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, J.; Bórquez, R. Quality retention in strawberries dried by emerging dehydration methods. Food Res. Int. 2014, 63, 42–48. [Google Scholar] [CrossRef]
- Wiktor, A.; Nowacka, M.; Anuszewska, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Drying Kinetics and Quality of Dehydrated Cranberries Pretreated by Traditional and Innovative Techniques. J. Food Sci. 2019, 84, 1820–1828. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, H.; Marzec, A.; Kowalska, J.; Trych, U.; Masiarz, E.; Lenart, A. The Use of a Hybrid Drying Method with Pre-Osmotic Treatment in Strawberry Bio-Snack Technology. Int. J. Food Eng. 2020, 16, 1–14. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Wiktor, A.; Sledz, M.; Nowacka, M. Selected Emerging Technologies to Enhance the Drying Process: A Review. Dry. Technol. 2014, 32, 1386–1396. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Dadan, M.; Rybak, K.; Anuszewska, A.; Materek, L.; Witrowa-Rajchert, D. The application of combined pre-treatment with utilization of sonication and reduced pressure to accelerate the osmotic dehydration process and modify the selected properties of cranberries. Foods 2019, 8, 283. [Google Scholar] [CrossRef] [PubMed]
- Ramya, V.; Jain, N.K. A Review on Osmotic Dehydration of Fruits and Vegetables: An Integrated Approach. J. Food Process Eng. 2017, 40, 1–22. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Braga, T.R.; Silva, E.O.; Rodrigues, S. Use of ultrasound for dehydration of mangoes (Mangifera indica L.): Kinetic modeling of ultrasound-assisted osmotic dehydration and convective air-drying. J. Food Sci. Technol. 2019, 56, 1793–1800. [Google Scholar] [CrossRef]
- Kumari, V.; Yadav, B.S.; Yadav, R.; Nema, P.K. Effect of osmotic agents and ultasonication on osmo-convective drying of sweet lime (Citrus limetta) peel. J. Food Process Eng. 2020, 43. [Google Scholar] [CrossRef]
- Soltani Firouz, M.; Farahmandi, A.; Hosseinpour, S. Recent advances in ultrasound application as a novel technique in analysis, processing and quality control of fruits, juices and dairy products industries: A review. Ultrason. Sonochem. 2019, 57, 73–88. [Google Scholar] [CrossRef]
- Allahdad, Z.; Nasiri, M.; Varidi, M.; Varidi, M.J. Effect of sonication on osmotic dehydration and subsequent air-drying of pomegranate arils. J. Food Eng. 2019, 244, 202–211. [Google Scholar] [CrossRef]
- Dellarosa, N.; Frontuto, D.; Laghi, L.; Dalla Rosa, M.; Lyng, J.G. The impact of pulsed electric fields and ultrasound on water distribution and loss in mushrooms stalks. Food Chem. 2017, 236, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Tylewicz, U.; Tappi, S.; Nowacka, M.; Wiktor, A. Safety, quality, and processing of fruits and vegetables. Foods 2019, 8, 569. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Dadan, M.; Rybak, K.; Wiktor, A.; Witrowa-Rajchert, D. Effect of ultrasound treatment during osmotic dehydration on bioactive compounds of cranberries. Ultrasonics 2018, 83, 18–25. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Dadan, M.; Tylewicz, U.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of power ultrasound on the main quality properties and cell viability of osmotic dehydrated cranberries. Ultrasonics 2018, 83, 33–41. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of osmosis and ultrasound on pineapple cell tissue structure during dehydration. J. Food Eng. 2009, 90, 186–190. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Laghi, L.; Dalla Rosa, M.; Witrowa-Rajchert, D. Effect of ultrasound treatment on the water state in kiwifruit during osmotic dehydration. Food Chem. 2014, 144, 18–25. [Google Scholar] [CrossRef]
- Rastogi, N.K.; Raghavarao, K.S.M.S.; Niranjan, K. Recent Developments in Osmotic Dehydration, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; ISBN 9780124114791. [Google Scholar]
- Tylewicz, U.; Panarese, V.; Laghi, L.; Rocculi, P.; Nowacka, M.; Placucci, G.; Rosa, M.D.M.D. NMR and DSC Water Study During Osmotic Dehydration of Actinidia deliciosa and Actinidia chinensis Kiwifruit. Food Biophys. 2011, 6, 327–333. [Google Scholar] [CrossRef]
- Tylewicz, U.; Tappi, S.; Mannozzi, C.; Romani, S.; Dellarosa, N.; Laghi, L.; Ragni, L.; Rocculi, P.; Dalla Rosa, M. Effect of pulsed electric field (PEF) pre-treatment coupled with osmotic dehydration on physico-chemical characteristics of organic strawberries. J. Food Eng. 2017, 213, 2–9. [Google Scholar] [CrossRef]
- Kulczyński, B.; Suliburska, J.; Rybarczyk, M.; Gramza-Michałowska, A. The effect of osmotic dehydration conditions on the calcium content in plant matrice. Food Chem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Rząca, M.; Witrowa-Rajchert, D.; Tylewicz, U.; Rosa, M.D. Mass exchange in osmotic dehydration process of Kiwi fruits (in Polish). Zywn. Nauk. Technol. Jakosc 2009, 6, 140–149. [Google Scholar]
- Ciurzyńska, A.; Kowalska, H.; Czajkowska, K.; Lenart, A. Osmotic dehydration in production of sustainable and healthy food. Trends Food Sci. Technol. 2016, 50, 186–192. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Tappi, S.; Siroli, L.; Lanciotti, R.; Romani, S.; Witrowa-Rajchert, D. Ultrasound assisted osmotic dehydration of organic cranberries (Vaccinium oxycoccus): Study on quality parameters evolution during storage. Food Control 2018, 93, 40–47. [Google Scholar] [CrossRef]
- Cichowska, J.; Witrowa-Rajchert, D.; Stasiak-Rózánska, L.; Figiel, A. Ultrasound-assisted osmotic dehydration of apples in polyols and dihydroxyacetone (DHA) solutions. Molecules 2019, 24, 3429. [Google Scholar] [CrossRef]
- Tylewicz, U.; Mannozzi, C.; Romani, S.; Castagnini, J.M.; Samborska, K.; Rocculi, P.; Dalla Rosa, M. Chemical and physicochemical properties of semi-dried organic strawberries enriched with bilberry juice-based solution. LWT Food Sci. Technol. 2019, 114, 108377. [Google Scholar] [CrossRef]
- Ahmed, I.; Qazi, I.M.; Jamal, S. Developments in osmotic dehydration technique for the preservation of fruits and vegetables. Innov. Food Sci. Emerg. Technol. 2016, 34, 29–43. [Google Scholar] [CrossRef]
- Nowacka, M.; Tappi, S.; Tylewicz, U.; Luo, W.; Rocculi, P.; Wesoły, M.; Ciosek-Skibińska, P.; Dalla Rosa, M.; Witrowa-Rajchert, D. Metabolic and sensory evaluation of ultrasound-assisted osmo-dehydrated kiwifruit. Innov. Food Sci. Emerg. Technol. 2018, 50, 26–33. [Google Scholar] [CrossRef]
- Torres, J.D.; Castelló, M.L.; Escriche, I.; Chiralt, A. Quality characteristics, respiration rates, and microbial stability of osmotically treated mango tissue (Mangifera indica L.) with or without calcium lactate. Food Sci. Technol. Int. 2008, 14, 355–365. [Google Scholar] [CrossRef]
- Qiu, L.; Zhang, M.; Tang, J.; Adhikari, B.; Cao, P. Innovative technologies for producing and preserving intermediate moisture foods: A review. Food Res. Int. 2019, 116, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.A.N.; Rodrigues, S.; Gaspareto, O.C.P.; Oliveira, E.L. Optimization of osmotic dehydration of papaya followed by air-drying. Food Res. Int. 2006, 39, 492–498. [Google Scholar] [CrossRef]
- Barman, N.; Badwaik, L.S. Effect of ultrasound and centrifugal force on carambola (Averrhoa carambola L.) slices during osmotic dehydration. Ultrason. Sonochem. 2017, 34, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Alolga, R.N.; Osae, R.; Essilfie, G.; Saalia, F.K.; Akaba, S.; Chikari, F. Sonication, osmosonication and vacuum-assisted osmosonication pretreatment of Ghanaian garlic slices: Effect on physicochemical properties and quality characteristics. Food Chem. 2021, 343, 128535. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The physical and chemical effect of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barosa-Canovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12. ISBN 9781441974716. [Google Scholar]
- Dadan, M.; Nowacka, M.; Wiktor, A.; Sobczynska, A.; Witrowa-Rajchert, D. Ultrasound to improve drying processes and prevent thermolabile nutrients degradation. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Barba, F.J., Cravotto, G., Chemat, F., Lorenzo Rodriguez, J.M., Munekata, P.E.S., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 55–110. ISBN 978-0-12-818275-8. [Google Scholar]
- Bromberger Soquetta, M.; Schmaltz, S.; Wesz Righes, F.; Salvalaggio, R.; de Marsillac Terra, L. Effects of pretreatment ultrasound bath and ultrasonic probe, in osmotic dehydration, in the kinetics of oven drying and the physicochemical properties of beet snacks. J. Food Process. Preserv. 2018, 42, 1–9. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- McClements, D.J. Advances in the Application of Ultrasound. Trends Food Sci. Technol. 1995, 6, 293–299. [Google Scholar] [CrossRef]
- Azoubel, P.M.; Baima, M.d.A.M.; Amorim, M.d.R.; Oliveira, S.S.B. Effect of ultrasound on banana cv Pacovan drying kinetics. J. Food Eng. 2010, 97, 194–198. [Google Scholar] [CrossRef]
- Zubernik, J.; Dadan, M.; Cichowska, J.; Witrowa-Rajchert, D. The Impact of the Pre-Treatment in Ethanol Solution on the Drying Kinetics and Selected Properties of Convective Dried Apples. Int. J. Food Eng. 2020, 1–11. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Rybak, K.; Nowacka, M.; Witrowa-Rajchert, D. The impact of ultrasound and steam blanching pre-treatments on the drying kinetics, energy consumption and selected properties of parsley leaves. Appl. Acoust. 2016, 103, 148–156. [Google Scholar] [CrossRef]
- Knorr, D.; Zenker, M.; Heinz, V.; Lee, D.U. Applications and potential of ultrasonics in food processing. Trends Food Sci. Technol. 2004, 15, 261–266. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Chemat, F.; Vian, M.A. Ultrasonic food processing. RSC Green Chem. 2011, 388–414. [Google Scholar]
- Kapturowska, A.; Stolarzewicz, I.; Chmielewska, I.; Białecka-Florjańczyk, E. Ultrasounds—A tool to inactivate yeast and to extract intracellular protein. Food Sci. Technol. Qual. 2011, 4, 160–171. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Al Khawli, F.; Zhou, J.; Wang, M.; Lorenzo, J.M.; Munekata, P.E.S.; Ferrer, E.; Barba, F.J. Mind the gap in the knowledge of the potential food applications of ultrasound based on its mechanism of action. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Barba, F.J., Cravotto, G., Chemat, F., Lorenzo Rodriguez, J.M., Munekata, P.E.S., Eds.; Elsevier Academic Press: London, UK, 2021; pp. 1–13. [Google Scholar]
- Cárcel, J.A.; García-Pérez, J.V.; Benedito, J.; Mulet, A. Food process innovation through new technologies: Use of ultrasound. J. Food Eng. 2012, 110, 200–207. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying Kinetics, Microstructure and Antioxidant Properties of Basil Treated by Ultrasound. J. Food Process Eng. 2017, 40, 1–13. [Google Scholar] [CrossRef]
- Jambrak, A.R.; Mason, T.J.; Lelas, V.; Krešić, G. Ultrasonic effect on physicochemical and functional properties of α-lactalbumin. LWT Food Sci. Technol. 2010, 43, 254–262. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Rodrigues, S. Ultrasound as pre-treatment for drying of fruits: Dehydration of banana. J. Food Eng. 2007, 82, 261–267. [Google Scholar] [CrossRef]
- Garcia-Noguera, J.; Oliveira, F.I.P.; Gallão, M.I.; Weller, C.L.; Rodrigues, S.; Fernandes, F.A.N. Drying Technology: An International Journal Ultrasound-Assisted Osmotic Dehydration of Strawberries: Effect of Pretreatment Time and Ultrasonic Frequency Ultrasound-Assisted Osmotic Dehydration of Strawberries: Effect of Pretreatment Time and Ultrasoni. Dry. Technol. 2010, 28, 294–303. [Google Scholar] [CrossRef]
- Hammami, C.; René, F. Determination of Freeze-drying Process Variables for Strawberries. J. Food Eng. 1997, 32, 133–154. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Gallão, M.I.; Rodrigues, S. Effect of osmotic dehydration and ultrasound pre-treatment on cell structure: Melon dehydration. LWT Food Sci. Technol. 2008, 41, 604–610. [Google Scholar] [CrossRef]
- Stojanovic, J.; Silva, J.L. Influence of osmotic concentration, continuous high frequency ultrasound and dehydration on antioxidants, colour and chemical properties of rabbiteye blueberries. Food Chem. 2006, 101, 898–906. [Google Scholar] [CrossRef]
- Goula, A.M.; Kokolaki, M.; Daftsiou, E. Use of ultrasound for osmotic dehydration. The case of potatoes. Food Bioprod. Process. 2017, 105, 157–170. [Google Scholar] [CrossRef]
- Jansrimanee, S.; Lertworasirikul, S. Synergetic effects of ultrasound and sodium alginate coating on mass transfer and qualities of osmotic dehydrated pumpkin. Ultrason. Sonochem. 2020, 69, 105256. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Zhang, M.; Wang, W.; Bhandari, B. A novel method of osmotic-dehydrofreezing with ultrasound enhancement to improve water status and physicochemical properties of kiwifruit. Int. J. Refrig. 2020, 113, 49–57. [Google Scholar] [CrossRef]
- Nowacka, M.; Laghi, L.; Rybak, K.; Dalla Rosa, M.; Witrowa-Rajchert, D.; Tylewicz, U. Water state and sugars in cranberry fruits subjected to combined treatments: Cutting, blanching and sonication. Food Chem. 2019, 299, 125122. [Google Scholar] [CrossRef]
- Bellary, A.N.; Sowbhagya, H.B.; Rastogi, N.K. Osmotic dehydration assisted impregnation of curcuminoids in coconut slices. J. Food Eng. 2011, 105, 453–459. [Google Scholar] [CrossRef]
- Siucińska, K.; Dyki, B.; Murgrabia, A.; Pieczywek, P.M.; Konopacka, D. Assessment of changes in structure of dried tissue of sour cherry pretreated using ultrasound-assisted osmotic dehydration. FOOD. Sci. Technol. Qual. 2015, 3, 123–137. [Google Scholar] [CrossRef]
- Shamaei, S.; Emam-Djomeh, Z.; Moini, S. Ultrasound-assisted osmotic dehydration of cranberries: Effect of finish drying methods and ultrasonic frequency on textural properties. J. Texture Stud. 2012, 43, 133–141. [Google Scholar] [CrossRef]
- Karizaki, V.M.; Sahin, S.; Sumnu, G.; Mosavian, M.T.H.; Luca, A. Effect of Ultrasound-Assisted Osmotic Dehydration as a Pretreatment on Deep Fat Frying of Potatoes. Food Bioprocess Technol. 2013, 6, 3554–3563. [Google Scholar] [CrossRef]
- Sharma, M.; Dash, K.K. Effect of ultrasonic vacuum pretreatment on mass transfer kinetics during osmotic dehydration of black jamun fruit. Ultrason. Sonochem. 2019, 58, 104693. [Google Scholar] [CrossRef] [PubMed]
- de São José, J.F.B.; de Andrade, N.J.; Ramos, A.M.; Vanetti, M.C.D.; Stringheta, P.C.; Chaves, J.B.P. Decontamination by ultrasound application in fresh fruits and vegetables. Food Control 2014, 45, 36–50. [Google Scholar] [CrossRef]
- Duckhouse, H.; Mason, T.J.; Phull, S.S.; Lorimer, J.P. The effect of sonication on microbial disinfection using hypochlorite. Ultrason. Sonochem. 2004, 11, 173–176. [Google Scholar] [CrossRef]
- Zenker, M.; Heinz, V.; Knorr, D. Application of Ultrasound-Assisted Thermal Processing for Preservation and Quality Retention of Liquid Foods. J. Food Prot. 2003, 66, 1642–1649. [Google Scholar] [CrossRef]
- Ugarte-Romero, E.; Feng, H.; Martin, S.E.; Cadwallader, K.R.; Robinson, S.J. Inactivation of Escherichia coli with power ultrasound in apple cider. J. Food Sci. 2006, 71. [Google Scholar] [CrossRef]
- Ananta, E.; Voigt, D.; Zenker, M.; Heinz, V.; Knorr, D. Cellular injuries upon exposure of Escherichia coli and Lactobacillus rhamnosus to high-intensity ultrasound. J. Appl. Microbiol. 2005, 99, 271–278. [Google Scholar] [CrossRef]
- Castelló, M.L.; Igual, M.; Fito, P.J.; Chiralt, A. Influence of osmotic dehydration on texture, respiration and microbial stability of apple slices (Var. Granny Smith). J. Food Eng. 2009, 91, 1–9. [Google Scholar] [CrossRef]
- Mauro, M.A.; Dellarosa, N.; Tylewicz, U.; Tappi, S.; Laghi, L.; Rocculi, P.; Rosa, M.D. Calcium and ascorbic acid affect cellular structure and water mobility in apple tissue during osmotic dehydration in sucrose solutions. Food Chem. 2016, 195, 19–28. [Google Scholar] [CrossRef]
- Gianotti, A.; Sacchetti, G.; Guerzoni, M.E.; Dalla Rosa, M. Microbial aspects on short-time osmotic treatment of kiwifruit. J. Food Eng. 2001, 49, 265–270. [Google Scholar] [CrossRef]
- Dermesonlouoglou, E.; Paraskevopoulou, E.; Andreou, V.; Taoukis, P. Osmotic dehydration for the production of novel pumpkin cut products of enhanced nutritional value and sustainability. Appl. Sci. 2020, 10, 6225. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Soltanzadeh, M.; Peressini, D. Properties of dried apricots pretreated by ultrasound-assisted osmotic dehydration and application of active coatings. Food Technol. Biotechnol. 2020, 58, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Bhargava, N.; Mor, R.S.; Kumar, K.; Sharanagat, V.S. Advances in application of ultrasound in food processing: A review. Ultrason. Sonochem. 2021, 70, 105293. [Google Scholar] [CrossRef] [PubMed]
- Patist, A.; Bates, D. Industrial applications of high power ultrasonics. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barosa-Canovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 599–616. [Google Scholar]
- Dadan, M.; Rybak, K.; Wiktor, A.; Nowacka, M.; Zubernik, J.; Witrowa-Rajchert, D. Selected chemical composition changes in microwave-convective dried parsley leaves affected by ultrasound and steaming pre-treatments–An optimization approach. Food Chem. 2018, 239, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Dadan, M.; Matys, A.; Kamińska-Dworznicka, A.; Lammerskitten, A.; Toepfl, S.; Parniakov, O. Improvement of freezing processes assisted by ultrasound. In Design and Optimization of Innovative Food Processing Techniques Assisted by Ultrasound; Barba, F.J., Cravotto, G., Chemat, F., Lorenzo Rodriguez, J.M., Munekata, P.E.S., Eds.; Elsevier Academic Press: London, UK, 2020; pp. 217–273. [Google Scholar]
- Chen, F.; Zhang, M.; Yang, C. hui Application of ultrasound technology in processing of ready-to-eat fresh food: A review. Ultrason. Sonochem. 2020, 63, 104953. [Google Scholar] [CrossRef]
- Alizehi, M.H.; Niakousari, M.; Fazaeli, M.; Iraji, M. Modeling of vacuum- and ultrasound-assisted osmodehydration of carrot cubes followed by combined infrared and spouted bed drying using artificial neural network and regression models. J. Food Process Eng. 2020, 1–16. [Google Scholar] [CrossRef]
- Khin, M.M.; Zhou, W.; Perera, C.O. Impact of process conditions and coatings on the dehydration efficiency and cellular structure of apple tissue during osmotic dehydration. J. Food Eng. 2007, 79, 817–827. [Google Scholar] [CrossRef]
- Fernandes, F.A.N.; Oliveira, F.I.P.; Rodrigues, S. Use of ultrasound for dehydration of papayas. Food Bioprocess Technol. 2008, 1, 339–345. [Google Scholar] [CrossRef]
- Prithani, R.; Dash, K.K. Mass transfer modelling in ultrasound assisted osmotic dehydration of kiwi fruit. Innov. Food Sci. Emerg. Technol. 2020, 64, 102407. [Google Scholar] [CrossRef]
- Bchir, B.; Bouaziz, M.A.; Ettaib, R.; Sebii, H.; Danthine, S.; Blecker, C.; Besbes, S.; Attia, H. Optimization of ultrasound-assisted osmotic dehydration of pomegranate seeds (Punica granatum L.) using response surface methodology. J. Food Process. Preserv. 2020, 44, 1–17. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Rasia, M.C.; Mulet, A.; Cárcel, J.A. Influence of ultrasound application on both the osmotic pretreatment and subsequent convective drying of pineapple (Ananas comosus). Innov. Food Sci. Emerg. Technol. 2017, 41, 284–291. [Google Scholar] [CrossRef]
- Kowalska, K.; Olejnik, A. Beneficial effects of cranberry in the prevention of obesity and related complications: Metabolic syndrome and diabetes–A review. J. Funct. Foods 2016, 20, 171–181. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, X.; Yagoub, A.E.G.A.; Xu, B.; Wu, B.; Zhang, L.; Zhou, C. Vacuum pretreatment coupled to ultrasound assisted osmotic dehydration as a novel method for garlic slices dehydration. Ultrason. Sonochem. 2019, 50, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Prosapio, V.; Norton, I. Simultaneous application of ultrasounds and firming agents to improve the quality properties of osmotic + freeze-dried foods. LWT 2018, 96, 402–410. [Google Scholar] [CrossRef]
- Witrowa-rajchert, D.; Lewicki, P.P. Original article Rehydration properties of dried plant tissues. Int. J. Food Sci. Technol. 2006, 41, 1040–1046. [Google Scholar] [CrossRef]
- Spinei, M.; Oroian, M. The influence of osmotic treatment assisted by ultrasound on the physico-chemical characteristics of blueberries (Vaccinium myrtillus L.). Ultrasonics 2021, 110, 106298. [Google Scholar] [CrossRef]
- Farhaninejad, Z.; Fathi, M.; Shahedi, M.; Sadeghi, M. Osmotic Dehydration of Banana Slices Using Direct and Indirect Sonication: Optimization and Microstructure Analysis. J. Food Process Eng. 2017, 40. [Google Scholar] [CrossRef]
- Nowacka, M.; Tylewicz, U.; Romani, S.; Dalla Rosa, M.; Witrowa-Rajchert, D. Influence of ultrasound-assisted osmotic dehydration on the main quality parameters of kiwifruit. Innov. Food Sci. Emerg. Technol. 2017, 41, 71–78. [Google Scholar] [CrossRef]
- Kroehnke, J.; Szadzińska, J.; Radziejewska-Kubzdela, E.; Biegańska-Marecik, R.; Musielak, G.; Mierzwa, D. Osmotic dehydration and convective drying of kiwifruit (Actinidia deliciosa)–The influence of ultrasound on process kinetics and product quality. Ultrason. Sonochem. 2021, 71. [Google Scholar] [CrossRef]
- Rahaman, A.; Zeng, X.A.; Kumari, A.; Rafiq, M.; Siddeeg, A.; Manzoor, M.F.; Baloch, Z.; Ahmed, Z. Influence of ultrasound-assisted osmotic dehydration on texture, bioactive compounds and metabolites analysis of plum. Ultrason. Sonochem. 2019, 58. [Google Scholar] [CrossRef]
- Çağlayan, D.; Barutçu Mazı, I. Effects of ultrasound-assisted osmotic dehydration as a pretreatment and finish drying methods on the quality of pumpkin slices. J. Food Process. Preserv. 2018, 42. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Changes of Mechanical and Thermal Properties of Cranberries Subjected to Ultrasound Treatment. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Nieto, A.B.; Vicente, S.; Hodara, K.; Castro, M.A.; Alzamora, S.M. Osmotic dehydration of apple: Influence of sugar and water activity on tissue structure, rheological properties and water mobility. J. Food Eng. 2013, 119, 104–114. [Google Scholar] [CrossRef]
- Amami, E.; Khezami, W.; Mezrigui, S.; Badwaik, L.S.; Bejar, A.K.; Perez, C.T.; Kechaou, N. Effect of ultrasound-assisted osmotic dehydration pretreatment on the convective drying of strawberry. Ultrason. Sonochem. 2017, 36, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Amanor-Atiemoh, R.; Zhou, C.; Wahia, H.; Mustapha, A.T.; Rashid, M.T.; Sampson, G.; Amoa-Owusu, A.; Ma, H.; Zhou, R. Acoustically-aided osmo-dehydration pretreatments under pulsed vacuum dryer for apple slices: Drying kinetics, thermodynamics, and quality attributes. J. Food Sci. 2020, 85, 3909–3919. [Google Scholar] [CrossRef]
- Yalcin, H.; Dursun Çapar, T. Bioactive Compounds of Fruits and Vegetables. In Minimally Processed Refrigerated Fruits and Vegetables; Yildiz, F., Wiley, R.C., Eds.; Springer: New York, NY, USA, 2017; pp. 723–746. ISBN 9781493970162. [Google Scholar]
- Oladejo, A.O.; Ma, H.; Qu, W.; Zhou, C.; Wu, B. Effects of Ultrasound on Mass Transfer Kinetics, Structure, Carotenoid and Vitamin C Content of Osmodehydrated Sweet Potato (Ipomea Batatas). Food Bioprocess Technol. 2017, 10, 1162–1172. [Google Scholar] [CrossRef]
- Tayyab Rashid, M.; Ahmed Jatoi, M.; Safdar, B.; Wali, A.; Muhammad Aadil, R.; Sarpong, F.; Ma, H. Modeling the drying of ultrasound and glucose pretreated sweet potatoes: The impact on phytochemical and functional groups. Ultrason. Sonochem. 2020, 68, 105226. [Google Scholar] [CrossRef]
- Sakooei-Vayghan, R.; Peighambardoust, S.H.; Hesari, J.; Peressini, D. Effects of osmotic dehydration (with and without sonication) and pectin-based coating pretreatments on functional properties and color of hot-air dried apricot cubes. Food Chem. 2020, 311, 125978. [Google Scholar] [CrossRef]
- Siucińska, K.; Mieszczakowska-Frąc, M.; Połubok, A.; Konopacka, D. Effects of Ultrasound Assistance on Dehydration Processes and Bioactive Component Retention of Osmo-Dried Sour Cherries. J. Food Sci. 2016, 81, C1654–C1661. [Google Scholar] [CrossRef]
- Luchese, C.L.; Gurak, P.D.; Marczak, L.D.F. Short Communication: Osmotic Dehydration of Physalis—Influence of Ultrasound Pretreatment. Food Eng. Rev. 2015, 7, 193–197. [Google Scholar] [CrossRef]
- Oladejo, A.O.; Ma, H. Optimisation of ultrasound-assisted osmotic dehydration of sweet potato (Ipomea batatas) using response surface methodology. J. Sci. Food Agric. 2016, 96, 3688–3693. [Google Scholar] [CrossRef] [PubMed]

| Product [References] | Treatment Applied | Physical Properties after OD | Physical Properties after Drying |
|---|---|---|---|
| Blueberries [106] | UOD (25 kHz, 400 W, 30–50 °C, 20–60 min and amplitude of 20, 60 and 100%) | US results in a higher lightness (L*) and decreased water activity. | - |
| Cranberries [112] | CUT or BL (90 °C for 5 min) + UOD (21 kHz, 30 or 60 min in two osmotic solution SA and SAG) | US reduced the maximum force necessary for conduction 90% of material deformation, whereas the work for cut and blanched fruits after UOD increased. | - |
| [30] | UOD (21 kHz, 180 W, 30 and 60 min) BL (90 °C for 5 min) + UOD (21 kHz, 180 W, 30 and 60 min) | After UOD of whole fruits water activity and volume did not change, while cut and blanched was unchanged or lower. Combined treatment (CUT/BL+UOD) led to increase the lightness and decrease a*. | - |
| Carrots [96] | OD (40 °C, 2 h) UOD (35 kHz, 130 W, 15 min) VOD (200 mbar, 15 min) UVOD (35 kHz, 130 W, 200 mbar, 15 min) | - | Better RR, lower shrinkage and hardness and higher lightness of UOD samples were observed. UVOD caused the smallest changes in color. |
| Garlic [48] | US (40 kHz, 600 W, 40 min, water) UOD (40 kHz, 600 W, 40 min, 30% CaCl2) UVOD (100 mbar, 40 kHz, 600 W, 40 min, 30% CaCl2) | - | All pretreatments increased the rehydration ability in the following trend: UVOD > UOD > US. |
| Apples [115] | OD (30 °C, 30 min); US (20 kHz, 300 W/L) UOD (20 kHz, 300 W/L, 30 °C, 30 min) | - | L*, b* and chroma values of color of UOD apple were higher compared to US, OD and control sample. |
| Banana [107] | OD (40, 45, 50, 55 and 60% sucrose, 15, 30, 60, 90, 120 and 180 min) Indirect UOD (40 kHz, 130 kW/m2, 5, 10, 15 and 20 min) Direct UOD (20 kHz, 200 W, intermittent mode 5 s on/5 s off, total time: 1, 3, 5, 7, 10 and 15 min) | UOD resulted in a lower total color change than in the case of mechanical agitation (OD). Indirect UOD caused lower changes than direct UOD. | - |
| Pomegranate arils [24] | OD (10-80 min, 50% sucrose) UOD (25 or 40 kHz, 10–80 min, 100 W, 50% sucrose) | UOD especially at 40 kHz greater decreased the water activity than OD. UOD did not change significantly the hardness and color compared with OD. | - |
| Pomegranate seeds [100] | OD (sucrose 0, 30, 60%) UOD (20 kHz, 30, 40, 50 °C for 20, 130, 240 min, respectively) | Hardness of OD and UOD seeds were significantly higher than for fresh seeds, while toughness values were lower. Between OD and UOD the texture factors were almost the same. | The application of UOD reduces the rehydration ratio. UOD and OD did not have a significant influence on the color of dried pomegranate seeds. |
| Strawberry [114] | UOD (40 kHz, 21 min, 20–31 °C; 47.5 °Brix) | - | A higher shrinkage and RR of UOD strawberries than dried without pretreatment was noted. The UOD caused only minor changes in color. |
| Pumpkin [111] | UOD (53 kHz, 40 and 60% sucrose, 40, 80 and 120 min | Dry matter increased with increasing both treatment time and sugar concentration. Increased treatment time caused increase of a* and b* color parameters but hue angle was not altered. | RR of dried pumpkin was dependent on drying method, treatment time and sugar concentration. For air and vacuum dried samples there was no apparent effect but for freeze-dried samples with increasing time the RR decreased. In comparison to untreated sample, UOD caused a decrease of L* and increase of a* color parameters. |
| Kiwifruits [108] | US (35 kHz, 10–30 min) and/or OD (61.5 °Brix, 120 min, 25 °C) | US pretreatment significantly enhanced the firmness after 120 min of OD, resulting in similar value for 20 and 30 min of US+OD than for fresh kiwifruit. US contributed to less changed color after OD than in the case of OD alone. | - |
| [109] | OD (50 °Brix, erythritol, sorbitol and sucrose, 120 min) UOD (25 kHz, 50 °Brix, erythritol, sorbitol and sucrose, 120 min) | - | The total color change of OD and UOD kiwifruit was dependent on the type of solution and method of drying. US caused both decrease and increase of color changes values. There were no significant changes in water activity due to a different treatment. |
| Plum [110] | UOD (25 kHz, 30 and 60 min, 50 °Brix glucose or sucrose, 30 °C) | - | In comparison to dried untreated plum, the UOD caused a higher and a lower hardness when sucrose and glucose were used, respectively. Color was significantly changed due to UOD—a lower lightness, a*, b* and chroma parameters were noted. |
| Product [References] | Treatment Applied | Functional Properties after OD | Functional Properties after Drying |
|---|---|---|---|
| Cranberries [29,30] | UOD (21 kHz, 180 W, 30 and 60 min) BL (90 °C for 5 min) + UOD (21 kHz, 180 W, 30 and 60 min) | Both pretreatments promoted a decrease in vitamin C, which was higher in BL+UOD samples. Anthocyanins, TPC and antioxidant activity were strongly influenced by the applied treatment type and duration. | - |
| [19] | BL (90 °C for 5 min) + US (21 kHz, 10 or 20 min) + V (40 kPa, 10 or 20 min) + OD (72 h, 40 °C) | Combined treatment resulted in a better preservation of anthocyanins and similar retention of TPC, TF and vitamin C if compared to untreated samples. | - |
| [16] | BL (90 °C for 5 min) + US (21 kHz, 30 min) + PEF (5.5 kV/cm, 2.0 kJ/kg) + OD (72 h, 40 °C) | - | Combined treatment caused a decrease in total polyphenols, flavonoids and anthocyanins content if compared to untreated samples. |
| Carrots [96] | OD (40 °C, 2 h) UOD (35 kHz, 130 W, 15 min) VOD (200 mbar, 15 min) UVOD (35 kHz, 130 W, 200 mbar, 15 min) | - | Better preservation of total carotenoids was observed in UOD treated samples and dried at low temperature (55 °C). |
| Garlic [48] | US (40 kHz, 600 W, 40 min, water) UOD (40 kHz, 600 W, 40 min, 30% CaCl2) UVOD (100 mbar, 40 kHz, 600 W, 40 min, 30% CaCl2) | - | All pretreatments increased the TPC, TFC, antioxidant activity and allicin content, following the trend: UVOD > UOD > US |
| Apricot [119] | OD (55 °C, 30 and 45 min) + edible coating (P, P+CA, P+AA) UOD (25 and 35 kHz, 30 and 45 min) + edible coating (P, P+CA, P+AA) | Decrease in TPC and vitamin C content was observed in both OD and UOD samples if compared to untreated ones; higher decrease in UOD samples with 35 kHz and longer duration. | The highest retention of TPC and vitamin C was noticed in OD samples coated by P+AA followed by UOD and P+AA samples. Both treatments led to a higher antioxidant activity. UOD and P+CA coating preserved better the β-carotene content. |
| Blueberries [106] | UOD (25 kHz, amplitude of—20, 60 and 100%; 61.5% sucrose solution at 30, 40 and 50 ◦C for 20, 40 and 60 min) | The optimal conditions for the highest antioxidant activity and total content of anthocyanins, flavonoids and polyphenols were obtained by using 30 °C for 40 min at a 100% amplitude. | - |
| Sour cherries [120] | UOD (25 kHz, 60% sucrose solution for 0–120 min, shaking 30 rpm for 0–120 min, 40 °C) | Total anthocyanins and TPC decreased in all samples, however retention of these compounds was observed when US and shaking were applied for 60 min. | After drying, a further decrease of each compound was observed, confirming the trend observed in OD samples. |
| Sweet potatoes [118] | OD (10–20% glucose, 10–45 min US (20 kHz, 10–45 min) UOD (20 kHz, 10–20% glucose, 10–45 min) | - | Better preservation of TPC and TF was observed in OD samples, while antioxidant activities were improved by US alone or in combination with OD. The vitamin C was retained better in UOD samples. |
| [117] | UOD (28 kHz, 300 W, 20–60 min) | >70% of vitamin C retention, however higher loss of carotenoids was noticed in UOD samples in comparison to OD ones. | - |
| Apples [115] | OD (30 °C, 30 min); US (20 kHz, 300 W/L) UOD (20 kHz, 300 W/L, 30 °C, 30 min) | - | Higher retention in vitamin C content was reported in UOD samples (46.05%), than in US (31.28%) and OD ones (25.95%). |
| Strawberry [114] | UOD (40 kHz, 21 min, 20–31 °C; 47.5 °Brix) | - | TPC content decreased in the dried samples in the similar range for samples with UOD pretreatment and those just dried. |
| Kiwifruits [108] | US (35 kHz, 10–30 min) and/or OD (61.5 °Brix, 120 min, 25 °C) | US pretreatment for 20 and 30 min significantly enhanced the chlorophyll content in kiwifruit if compared to OD samples. | - |
| [109] | OD (50 °Brix, erythritol, sorbitol and sucrose, 120 min) UOD (25 kHz, 50 °Brix, erythritol, sorbitol and sucrose, 120 min) | - | UOD promoted a decrease in polyphenols and carotenoids if compared to OD samples; better retention of these compounds was achieved when hybrid conventional +US drying was applied. |
| Physalis [121] | US (20 kHz, 30 min) + OD (55 °C, 55 °Brix, 10 h) | No influence on carotenoids content was reported. | - |
| Plum [110] | UOD (25 kHz, 30 and 60 min, 50 °Brix glucose or sucrose, 30 °C) | - | Higher TPC and antioxidant activity when UOD was applied for 30 min in glucose solution. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, M.; Dadan, M.; Tylewicz, U. Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes. Appl. Sci. 2021, 11, 1269. https://doi.org/10.3390/app11031269
Nowacka M, Dadan M, Tylewicz U. Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes. Applied Sciences. 2021; 11(3):1269. https://doi.org/10.3390/app11031269
Chicago/Turabian StyleNowacka, Małgorzata, Magdalena Dadan, and Urszula Tylewicz. 2021. "Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes" Applied Sciences 11, no. 3: 1269. https://doi.org/10.3390/app11031269
APA StyleNowacka, M., Dadan, M., & Tylewicz, U. (2021). Current Applications of Ultrasound in Fruit and Vegetables Osmotic Dehydration Processes. Applied Sciences, 11(3), 1269. https://doi.org/10.3390/app11031269








