Featured Application
The ethanol-ultrasound pre-treatment has the potential to be applied before drying of plant tissue—as it was proven in the case of apple, carrot, pumpkin, etc. The treatment may improve product quality and/or reduce drying time and energy consumption. Current work proved that the carotenoid content and the rehydration ability were improved while the colour remained unchanged which indicates the possibility to obtain a product with designed properties. However, it is worth emphasizing that the biggest limitation in the industrial use of the treatment is the price of ethanol which makes it necessary to develop solutions for recovery or re-use of ethanol and optimize its amount. Therefore, the utilization of ethanol-ultrasound pre-treatment in the industry is challenging and requires comprehensive studies concerning food quality, the optimization of ethanol dose and an evaluation of the ethanol residue in the product.
Abstract
The purpose of this study was to investigate the effect of pre-treatment in ethyl alcohol for 5, 15, 60 and 180 s with the application of ultrasound on the course of convective drying and properties of carrot tissue directly after the treatment and after the drying process. The treatment in ethanol resulted in loss of mass, increase of dry matter, ethanol conductivity, extractivity of carotenoids with a slight effect on the colour of carrot tissue after the treatment. The utilization of ultrasound during immersion in ethanol contributed to additional increase of conductivity of ethanol, and extractivity of carotenoids. The immersion in ethanol virtually did not affect the drying kinetics, which can be explained by the increase of shrinkage of the tissue in relation to the untreated dried tissue. Despite the lack of the influence on the drying course in the ethanol-immersed carrot, an increase of the carotenoid content (up to 135%) and the rehydration ability (up to 19%) was noted with the simultaneously unchanged colour of dried carrot in comparison to untreated dried material, which indicates the possibility to improve the quality of dried carrot after immersion in ethanol.
1. Introduction
Carrot is one of the most widely grown root vegetables around the world. Consumption of carrots and carrot products is constantly increasing due to their richness in natural nutrients, including carotenoids or dietary fibre [1,2]. Apart from being consumed in a fresh form, the root is usually dried to extend the shelf life. The most commonly used convective drying is a thermal process of simultaneous heat and mass exchange, the purpose of which is to evaporate some of the water from the material into the surrounding air, which is both the medium providing heat and carrying moisture away from the material. In general, the aim is to ensure the microbiological stability of the food, which results in a significant slowing down of many chemical reactions and even elimination of enzymatic changes in the dry material [3]. The drying of vegetables is predominantly carried out by the means of the convective method due to its simplicity, ease of process control and low costs of such dryers [4]. The disadvantages of this method, however, include high energy consumption and relatively low quality of the dried product, in particular low rehydration ability, high shrinkage and high losses of nutrients [5,6,7]. Considering the further use of dried carrot as a component of, e.g., instant soups, seasoning mixes, etc., it is especially important to select a processing method to provide a product characterizing by a good rehydration ratio, high content of carotenoids and stable colour. Therefore, novel pre-treatment methods are sought in order to reduce the negative aspects of convective drying.
One of such types of innovative processing is the use of ethyl alcohol as a dehydrating substance, using it either during pre-treatment or during the drying process (drying in an ethanol atmosphere). In principle, such enhancement of drying is to shorten the drying time and also to increase the rehydration capacity and reduce the degradation of chemical compounds. Literature indicates that the use of ethanol in the drying process shortened the drying time and allowed for obtaining a product of high quality with a simultaneous large loss of water from the tissue and thus a better structure [8,9,10]. Ethanol is an organic solvent that is able to extract some components of cell walls and membranes, e.g., polyphenols, lipids, or proteins and which maintain the cellulose, pectin, lignin, or hemicellulose in the cell wall. Thus, the thickness of the cell wall may be reduced and the permeability may increase [11,12]. Considering also that ethanol has both a lower boiling point and surface tension than water, and the fact that ethanol mixes with the aqueous solution on the surface of material, which increases the vapour pressure as well as the Marangoni effect, the heat and mass transfer processes are accelerated [13,14]. Corrêa et al. [15] reported that the most effective matter in reducing the drying time was to spread the ethanol over the material in a thin layer than to dry in an ethanol atmosphere. These preliminary studies have become a precursor to the use of ultrasound during immersion in ethanol as a pre-treatment [9,11,16,17,18].
Ultrasound (US) is pressure wave causing vibration of air in the frequency range inaudible for humans—from 18 kHz to 100 MHz. This mechanical wave may be propagated through liquid or solid media by the means of series of alternating compression and rarefaction [19,20]. The high intensity ultrasound (from 18 up to 100 kHz) is used for modification of tissue structure (by disruption of the cells and microscopic channels’ formation) and acceleration of mechanical or mass and/or heat transfer processes [21,22,23,24,25]. These effects can be obtained due to “sponge effect”, cavitation phenomenon and the effects accompanying cavitation, such as microstreaming or release of fountain of microbubbles [19].
Nowadays, the simultaneous pre-treatment in ethanol with the application of ultrasound has been studied, which may bring the benefits of both pre-treatments. As it was reported, the immersion in ethanol with ultrasound was more effective in drying time reduction than single ultrasound or ethanol treatments [9,16,17,26]. Costa Santos et al. [11] reported around 50% reduction of carrot drying time subjected to ethanol-ultrasound treatment for 30 min with enhanced rehydration ability of dried material and unchanged carotenoid content. The drying time of scallion stalk treated by means of ethanol (75%) with ultrasound (for 10 min) was reduced from 110 min (for control) to around 60 min [26]. Different studies reported a better rehydration ability, unchanged colour [26], decreased content of polyphenols [9,17] and worse antioxidant capacity [17] after EtOH+US treatment than the untreated dried material. The combined treatment was studied for different times (5 s–30 min) in different concentrations of ethanol (30–99.8%), but usually a longer treatment time (10–30 min) and higher concentrations (>95%) were investigated [9,11,16,17,18,26]. However, from the further utilization of ethanol-ultrasound pre-treatment in industry, it seems reasonable to reduce the treatment time. Zubernik et al. [9] reported that even 15 s of combined ethanol-ultrasound treatment caused a significant reduction of the drying time of apple. They also noted a higher reduction of drying time when a longer ultrasound-ethanol pre-treatment of apple was performed. The shortening of drying time was in the range of 9.8–18.3% when treatment was carried out for 5–180 s, in comparison to the untreated sample. However, prolongation of the treatment to 3 min caused significant degradation of polyphenols (from 16% for 1 min up to 40% for 3 min EtOH+US treatment in relation to the untreated dried apple) due to extraction of polyphenols by ethanol. Therefore, the time of treatment and the ethanol concentration should be adjusted to each material individually based not only on the energetic aspects but also on the quality of dried material and obtaining the product with designed properties. Furthermore, the impact of ultrasound depended on the applied frequency of ultrasound [24]. Therefore, optimization of the US frequency during immersion in ethanol is required.
The aim of the study was to analyse the impact of pre-treatment in ethanol with application of ultrasound on the drying kinetics and selected properties of carrot directly before the treatments and after convective drying. The immersive treatment was carried out in 96% of ethanol for various time (5–180 s) without and with ultrasound assistance of different frequency (21 and 40 kHz).
2. Materials and Methods
2.1. Material
Carrot was selected as a research material because air-dried carrot usually is characterized by poor rehydration ability, which limits its wider use. Carrot (var. Baltimore) conventionally cultivated in Poland was purchased at a local market from one producer (one batch delivered to the market). The carrot was delivered to the market the same day it was purchased. In order to assure homogeneity of material, uniformly coloured roots with a similar degree of maturity were selected and stored before the experiments in refrigerated conditions (2–4 °C) for up to seven days. Defect-free roots of a maintained texture (turgor) were taken out of the storage compartment, washed and left to reach ambient temperature. Afterwards, the material was cut into 5-millimetre-thick slices and then peel-free cylindrical discs with a diameter of 15 mm were cut out.
2.2. Immersion in Ethanol (EtOH) and Ultrasound-Assisted Immersion in Ethanol (EtOH+US)
The pre-treatment was carried out by immersion of carrot cylinders in 96% of ethanol for varied time: 5, 15, 60 and 180 s. Slices of a mass of 30 g obtained from at least two roots of carrot were flooded with approximately 60 mL of ethanol (the ratio of the material weight to the volume of ethanol was set at 1:2). Full immersion of the slices was assured. The treatment in alcohol was repeated twice for each treatment time.
The second variant of the pre-treatment in ethanol (96%) was performed with the assistance of ultrasound of the frequency of 21 kHz and power of 300 W and 40 kHz with power of 180 W in ultrasonic bath (MKD-3, MKD Ultrasonics, Warsaw, Poland). The same time (5, 15, 60 and 180 s) and the same ratio of the mass of carrot to the volume of ethanol (1:2) as for EtOH treatment were set. The experiment was carried out in two repetitions.
Carrot tissue not immersed in ethanol was the reference material for both EtOH and EtOH+US treatments.
Before and after the pre-treatment with ethyl alcohol, EtOH, EtOH+US21 and EtOH+US40 treatments, the changes of weight of the material, dry matter content, the conductivity of ethanol, colour, as well as carotenoid content were measured. On the basis of this preliminary research the parameters of 21 kHz and 300 W of US treatment were chosen for further research. The material after this treatment was characterized by similar properties in comparison to the intact sample. In dried carrot the following measurements were performed: dry matter, water activity, colour, carotenoid content, shrinkage (volume and density), rehydration ability and hygroscopic properties (kinetics of water vapour adsorption).
2.3. Analysis of Changes in Raw Material Caused by Pre-treatments
The weight of the carrot before and after the pre-treatments was checked with an accuracy of ± 0.001 g. Measurements were made for each repetition of the pre-treatment in duplicate. On their basis, the average weight loss in relation to the initial weight was calculated (%):
where Δm—relative mass change (%); mk—final weight of the treated slices (g); m0—initial weight of the slices before processing (g).
Δm = (mk − m0)/m0 × 100%,
The dry matter content of untreated, EtOH, EtOH+US-treated carrot was measured according to AOAC [27], whereas the water activity—by the means of hygrometer AquaLab CX-2 (Dekagon Devices Inc., Pullman, WA, USA) with accuracy of ±0.001 at a temperature of 20 °C. The measurements were conducted thrice for each repetition of treatment.
Moreover, the changes of the electrical conductivity of ethanol solution as a result of immersion of carrot during the treatment were measured by means of the Elmetron CX-505 conductometer (Zabrze, Poland) in four repetitions for each treatment.
2.4. Convective Drying
Air-drying of pre-treated and untreated carrot was carried out in a laboratory dryer (Warsaw, Poland) at 70 °C and air velocity of 2 m/s. The discs of the carrot were put in a single layer on sieves with a load of 2.9 kg/m2. The air flows parallel to the material layer. During the process the mass of the material was recorded digitally every 1 min with accuracy of ± 0.1 g (METTLER TOLEDO, AE 204S, Columbus, OH, USA). The drying was stopped when the constant mass was reached. The experiments were performed in two repetitions. The moisture ratio (MR) was calculated as follows [28]:
where Mt—moisture content during drying (kg H2O/kg d.m.) and M0—initial moisture content (kg H2O/kg d.m.).
MR = Mt/M0,
The differences in the drying kinetics were also presented in a table as a drying time until samples obtained MR equal 0.02. Then, the dry matter content and the water activity of dried carrot were measured in three repetitions.
2.5. Shrinkage
The volume and density of the carrot slices were measured by the ground sea sand method. For this purpose, a weighted carrot slice was placed in a measuring cylinder and covered with a known volume of sand. The volume of the slices was determined from the difference in volume. The density ρ was calculated from the following formula:
where Vpp—volume of carrot slices in sand (cm3); Vpm—the volume of sea sand (cm3).
ρ = m/(Vpp − Vpm),
Based on the volume of the material before and after drying, the shrinkage of the material was calculated [29]:
where S—drying shrinkage (%); Vk—volume of dried slice (cm3); V0—volume of slice before drying (cm3).
S = (1 − (Vk/V0)) × 100%,
2.6. Rehydration Ability and Hygroscopicity
The rehydration ability of dried material was analysed in accordance with the methodology presented by Fijalkowska et al. [30]. For this purpose, the weight of two dried carrot slices (with an initial weight of about 1 g) was measured before and after 1, 2 and 3 h of rehydration in distilled water at a temperature of 20 ± 1 °C. After each rehydration time, the material was filtered through a sieve and dried on filter paper before weighing. The test was performed in duplicate.
The water vapour adsorption (hygroscopicity) of the dried carrot tissue was measured by the desiccator method over saturated NaCl solution providing an environment with a water activity of 0.75. The material which was previously weighed on an analytical balance (METTLER TOLEDO, AE 204S, OH, USA) was placed in a desiccator and the weight changes over time at 0.5, 1, 3, 5, 24 and 72 h were measured. Measurements were made at the temperature of 20 ± 2 °C in three repetitions. The results were expressed as the relative water content (in relation to the initial value) during water vapour adsorption (U) [28].
2.7. Colour
Colour was measured with a Konica Minolta CM-5 colorimeter (Konica Minolta, Japan) using the CIE L*a*b* system with a standard observer setting of 2°. The device was equipped with a D65 light source. The measurement was performed in at least 10 replicates for fresh, treated and dried material. For this purpose, carrot slices were placed on the measuring area with a diameter of 3 mm, each time using different slices. Additionally, the hue angle (h°) and total colour change (ΔE) were calculated based on the relationship [31]:
where L*—lightness; a*—chromatic coordinate characterizing the colour on a scale from red (+a*) to green (−a*) (−); b*—chromatic coordinate characterizing the colour on a scale from yellow (+b*) to blue (−b*) (−); ∆L*, ∆a*, ∆b*—difference in lightness, a* and b* coordinate of the colour between the carrot tissue being determined and the fresh carrot tissue (−).
h° = tan−1 (b*/a*),
2.8. The Total Carotenoid Content (TCC)
The total carotenoid content was measured using spectrophotometric methods according to the Polish Standard PN-EN 12136:2000 [32]. In brief, 0.7 g of homogenized sample (m1), 20 mL of distilled water, 1 mL of Carrez I solution (VWR Chemicals BDH Prolabo, Leuven, Belgium) were added to the probe, mixed and after 1 min 1 mL of Carrez II solution (VWR Chemicals BDH Prolabo, Leuven, Belgium) was added and again mixed for 5 min. The colourless solution was then decanted, and the 20 mL of acetone was added and mixed in a vortex for 5 min. This step was repeated with 25 mL of petroleum ether and then again with 20 mL of acetone, and at the end, 15 mL of distilled water was used. Then, 1 g of anhydrous sodium sulphate was weighed into dry centrifuge tubes and the ether phase was added from the separator. The separator was rinsed with 10 mL of petroleum ether and the residue was poured into a centrifuge tube. It was thoroughly mixed and then centrifuged for 5 min. The solution (V) was poured from above the sediment into a 50 mL volumetric flask and made up to volume with petroleum ether solution. The absorbance was measured at the wavelength λ = 450 nm in the Heλios Thermo Electron v. 7.03 spectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The measurement was carried out thrice for each material on independently prepared extracts. The total carotenoid content was calculated from the formula:
where ρ (C40H56)—total carotenoids, in mg/kg of fresh matter (f.m.); A450—absorbance of the petroleum ether extract; 4.00—average in the conversion factor determined on the basis of the ring test, taking into account the average β-carotene absorption coefficient in petroleum ether and dilutions made during the analysis; V—the volume of the extract in petroleum ether (50 mL). The results were then calculated in mg/100 g d.m. (dry matter).
ρ (C40H56) = A450 × 4.00 × (V/m1),
2.9. Statistical Analysis
The results were analysed using one-way analysis of variance ANOVA (Tukey’s test). The level of significance was set at α = 0.05. The analysis was performed using the Statistica 13.1 software (TIBCO Software, Palo Alto, CA, USA). In addition, the significance of the type of treatment or time on the studied variables was determined using a two-way analysis of variance without repetitions by the means of F-test at the significance level of α = 0.05. The analysis was performed in MS Excel with Analysis ToolPak (Microsoft, the USA). The results of the one-way ANOVA are presented in tables and figures by different letters, whereas the results of two-way ANOVA are presented by indicating the significance (*) of the influence of time or type of treatment (based on the p-values).
3. Results and Discussion
3.1. Changes in Raw Material Caused by Pre-Treatment in Ethanol Solution with and without Ultrasound
The changes in raw material caused by pre-treatment in ethanol solution without (EtOH) and with ultrasound (EtOH + US) are presented in Table 1. Both in the case of the pre-treatment in ethanol and the treatment in ethanol with the use of ultrasound, there was a weight loss in the carrot slices in the range of 2.22–8.97% when using alcohol alone, 5.38–14.05% for the EtOH+US21 treatment, and 6.95–14.84% for the EtOH+US40 treatment (Table 1). The weight loss was mainly related to the reduction of the water content after the treatments. In almost all cases with a longer time of treatment, the weight loss was greater. Additionally, the application of ultrasound caused a further increase in weight loss. The weight loss was greater when a higher frequency was used, which, with the greater conductivity of the solution (Figure 1), may explain the greater leakage of water-soluble components into the ethanol solution. The significance of the influence of both treatment type and time on the relative weight loss was confirmed (p < 0.05; Table 1). This means that the weight loss was caused by the treatment in ethanol, while sonication additionally intensified these changes, probably as a result of partial damage to the structure. It is well known that the sonication results in structural changes due to the influence of sound waves on the material [2,24,30,33,34]. Similarly, Zhou et al. [26] noticed the increase of the water loss in scallion slices immersed in ethanol, and sonication intensified the changes, which means that the ultrasound cavitation and other mechanical effects of sonication can intensify the migration between the tissue and medium. Furthermore, after carrot treatment in ethanol and ethanol with sonication assistance for 30 min, Costa Santos et al. [11] observed shrinkage of the cell wall in comparison to the fresh sample. As they stated, the shrinkage has an impact on the permeability of the cell and it has an influence on water loss with other compounds.

Table 1.
Relative mass change (Δm) of carrot slices (%) in relation to the initial weight, dry matter content and water activity changes of carrot tissue pre-treated without and with ultrasound; error bars indicate ± SD (standard deviation calculated from four repetitions in the case of relative mass change and six repetitions in the case of dry matter content and water activity).
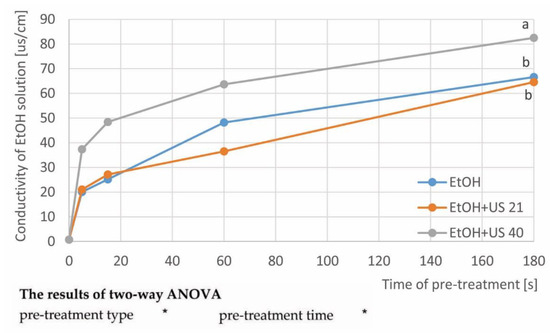
Figure 1.
Conductivity of the ethanol solution after carrot pre-treatment without and with ultrasound (21 and 40 kHz): a, b, etc.—different letters indicate different homogeneous groups (one-way ANOVA, α = 0.05).
In fresh carrot (untreated), the dry matter content was 9.70 ± 0.79% (Table 1). Both in the case of EtOH and EtOH+US treatments, the dry matter content of carrot slices in most cases increased after treatment. The increase was of up to 27 and 19% for samples treated in ethanol alone and in ethanol with the application of ultrasound, respectively. The two-way analysis of variance did not reveal a significant influence of type nor time of treatment (Table 1). Additionally, Zhou et al. [26] observed an increase in dry matter content in scallion slices, when the immersion in ethanol was applied, and with the assistance of sonication the value even increased. Changes in the dry mass content are related to two opposite phenomena: the penetration of solvent into the material and dry matter components into the environment [35,36]. In the case of the current research, the ultrasound treatment was carried out in ethyl alcohol, which allowed to obtain the desired effect, i.e., increasing the dry substance content, and thus water loss as a result of its partial, surface replacement by alcohol. In the case of water activity, all samples did not differ significantly, however, the two-factor analysis of variance showed the existence of a statistically significant effect of the type of pre-treatment on the value of water activity in the material after treatment (p < 0.05; Table 1). Higher values were obtained in the case of EtOH+US in comparison to the EtOH sample.
The conductivity of the ethanol solution after carrot pre-treatment with ultrasound (EtOH + US), depending on the ultrasound frequency (21 and 40 kHz), in comparison with the conductivity of the EtOH samples, is presented in Figure 1. This method of measurement is mainly used to assess the properties of food products, their origin, quality after pre-treatment, e.g., by evaluating tissue leakage after the blanching process [20]. Ethyl alcohol, thanks to its properties, helps to extract substances from plant material. In carrot tissue ethanol positively influenced extraction, especially of the substances with hydroxyl groups in their structure [37]. Thus, the measurement of the electrical conductivity of ethanol used during the pre-treatments allows the assessment of the degree of leaking out of dissociating compounds from the carrot tissue.
The conductivity of pure ethanol was 0.75 ± 0.10 µS/cm. A significant (p < 0.05) effect of both the treatment type and time on the conductivity of the ethanol solution was proven (Figure 1). During all pre-treatments, the electrolyte losses from the plant material to the surrounding medium was most intense in the first 5 s. With longer pre-treatment, increasing conductivity was observed, especially when ultrasound frequency of 40 kHz was used. In the case of EtOH+US 21 and EtOH samples, the conductivity values were similar in almost the entire process. It means that the use of a higher frequency and longer time caused the higher compounds to leak from the carrot tissue. The different influence of the two types of US frequencies proves the need to select processing parameters for a given material. Ultrasound causes the phenomenon of cavitation, as a result of which there is a local increase in pressure and temperature in the material, which destroys the structure of plant tissue cells, making it easier for substances to leak from the tissue [38,39]. The highest conductivity value (82.52 ± 2.30 µS/cm) was obtained after the longest treatment time (180 s) for EtOH+US40. Thus, the increase in the conductivity value was over 19.3% with respect to the conductivity value for the alcohol solution after the same treatment time without US assistance (EtOH—66.60 ± 1.99 µS/cm).
Colour is one of the most important parameters for assessing the quality of raw materials and food products. From the perspective of a potential consumer, colour is one of the basic factors that influence the acceptability of a product [31,33]. Therefore, it is very important to control colour changes after pre-treatments. This allows to check whether the application of the treatment or process is useful for industrial purposes and allows to maintain the colour of the raw material. Table 2 shows the changes in colour parameters of carrot slices after pre-treatment in ethyl alcohol with or without ultrasound support. For the fresh carrot, the L*, a * and b* parameters were equal to 40.6 ± 2.1, 12.3 ± 3.0, and 23.4 ± 3.8, respectively. The fresh material has an orange colour with the hue angle (h°) equal to 62.6 ± 2.2. The treatment in the ethyl alcohol caused alteration of colour parameters. There was a lack of any trend in the L* value changes and this parameter decreased at the beginning of the treatment and after the longest time of treatment increased in comparison to fresh sample. The sonication assistance resulted in a higher L* value. In the case of a* and b* values, the treatment in alcohol resulted in most cases lowering of the value. Therefore, the total colour difference, describing the colour changes in comparison to fresh material, was characterized by value in the range of 5.4–12.3. According to Tiwari et al. [40] the value higher than 2 indicates a visible change, which can be noted by an inexperienced observer as a consumer. It means that all treatments with ethanol resulted in significant colour modifications. Despite the changes in colour parameters, the majority of the parameters of the preliminary treatments did not significantly affect the hue angle (h°) of the researched carrot slices. The h° values were in ranges from 61.1 to 67.2 (EtOH), 62.9–68.4 (EtOH+US21) and 61.8–74.0 (EtOH+US40). Therefore, all values did not exceed 90°, which means that both fresh carrots and those subjected to preliminary treatments were characterized by a red-orange colour.

Table 2.
Colour parameters (L*, a*, b*), hue angle (h°) and total colour difference (ΔE, in comparison to raw material) of carrot tissue pre-treated without and with ultrasound; error bars indicate ± SD (standard deviation calculated from at least 10 repetitions).
Carotenoids are an important group of pigments naturally found in plants. The best-known carotenoid is carotene, which has an orange colour. Carrots are a vegetable rich in carotenoids, in particular, α- and β-carotene, which are precursors to vitamin A [11,41]. The scientific literature reported a wide range of the carotenoid content in carrot roots, depending on the variety of the carrot, climatic aspects, soil conditions [42,43], year of growth [43], extraction methods [44], and methods of processing [45]. The total content of carotenoids in the edible part of carrot roots ranges from around 5.8 to 311 mg/100 g f.w. (fresh weight) [2,43,46]. Thus, the content of pigments in the product affects its colour and it has an impact on product acceptability. Figure 2 presents changes in the carotenoid content in raw tissue (untreated) and after 60 and 180 s of treatment in ethanol with and without sonication assistance. The samples subjected to a shorter time of immersion in ethanol (5 and 15 s) were not analysed due to the negligible effect on the total carotenoid content in carrots. The content of carotenoids in raw carrot was 53.60 ± 4.78 mg/100 g d.m. (dry matter), which referred to the content of 5.20 ± 0.46 mg/100 g f.w. It was thus practically in the range reported in the literature. The application of the pre-treatment, consisting of the immersion of raw carrot slices in EtOH for 60 and 180 s, resulted in an increase of the carotenoid content by 15 and 52%, respectively. The use of ultrasonic waves with a frequency of 21 (EtOH+US21) and 40 kHz (EtOH+US40) further increased the extractivity of carotenoids. During the 60 s of treatment at 21 kHz, the carotenoid content was higher by 32%, and for the frequency of 40 kHz, by 44% compared to the raw material. In the case of a treatment lasting 180 s, the carotenoid content was higher, in comparison to fresh carrot, by 104% (EtOH+US21) and 144% (EtOH+US40). However, the two-factor ANOVA did not confirm the significance of both factors (p > 0.05, Figure 2). A similar increase in the content of active compounds as a result of sonication was obtained by Wiktor et al. [47] in the case of apple tissue. The polyphenol content after sonication at the frequency of 21 and 40 kHz was from 27 to 145% higher in comparison to the raw tissue. The increase of polyphenol content was observed with increasing the processing time and the frequency from 21 to 40 kHz.
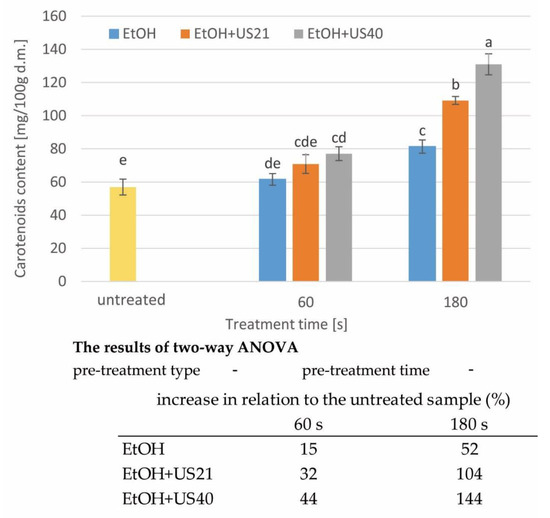
Figure 2.
The carotenoid content in raw carrot tissue pre-treated with and without ultrasound; a, b, etc.—different letters indicate different homogeneous groups (α = 0.05), the results of two-way ANOVA indicate the significance (*) or insignificance (-) of the influence of the type and time of treatment (α = 0.05). In table—the increase in carotenoid content in relation to the untreated sample.
On the basis of the obtained results, it can be concluded that the use of immersion in ethyl alcohol changed the structure [11,18] and increased the extractivity of carotenoid from carrot tissue, especially with ultrasound application and the prolongation of the processing time.
3.2. Effect of the Pre-Treatment on the Drying Process of Carrot Tissue
The pre-treatments in ethanol with and without ultrasound assistance, regardless of the type of processing, virtually did not affect the kinetics of the convective drying of carrot tissue, as shown in Figure 3. The drying of raw carrot slices, not subjected to any pre-treatment, to a relative water content (MR) of 0.02, took around 101 min (Table 3). In the case of EtOH treatment, the shortest processing time (5 s) caused reduction of the drying time only by 1.5%. On the other hand, the extension of the processing time (15–180 s) resulted in slightly longer drying (by 3.5–6.0%). Furthermore, the EtOH+US processing led to both a slight reduction (by 2.5% in the case of 15 s) and a slight prolongation (by 3.5–6.5% for 5, 60 and 180 s) of the drying time in relation to the tissue not subjected to the treatment. It can be summed up that despite the statistical differences, the immersion in ethanol practically did not affect the drying time (changes around 6 min are marginal from the industrial point of view), due to the increase of shrinkage of the tissue in comparison to untreated dried sample (Table 4). However, other researchers observed a reduction in drying time, when ethanol treatment was used. A shorter drying time about 48–50% [18] and about 34–53% [17] was reported during convective drying of pumpkin and apple, respectively, but for longer pre-treatment time (15–30 min), with or without ultrasound. A similar effect was noticed by Costa Santos et al. [11] for carrot subjected to 30 min ethanol pre-treatment, with and without US assistance, where the drying time was shortened (in comparison to control) of around 51 and 50%, respectively. While ethanol treatment lasted from 5 to 180 s for apples, the reduction of drying time from 4.9 to 18.3% was noted [9]. This phenomenon can be explained by ethanol entering the surface of the tissue, which caused the reduction of the water content at the beginning of drying. Furthermore, ultrasound facilitates the process [11]. Moreover, Rojas et at. [16], who subjected potato to ethanol pre-treatment with and without US followed by infrared drying, mentioned that the creation of the microchannels might positively influence the drying time. Nonetheless, in the present study, both the treatment in ethanol alone and with ultrasound assistance resulted in an increase in the density of dried material (Table 4) and reduction of the volume, which is discussed below. This means that the treatment in ethanol causes such changes in the structure of carrots, which, contrary to the hypothesis concerning the acceleration of drying with ethanol, limits the possibility of faster evaporation of water during drying. It is also possible that US intensified the changes in the plant structure, therefore, the drying time was even longer, with some exceptions.
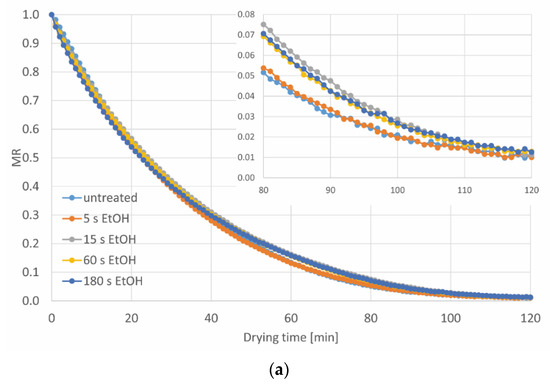
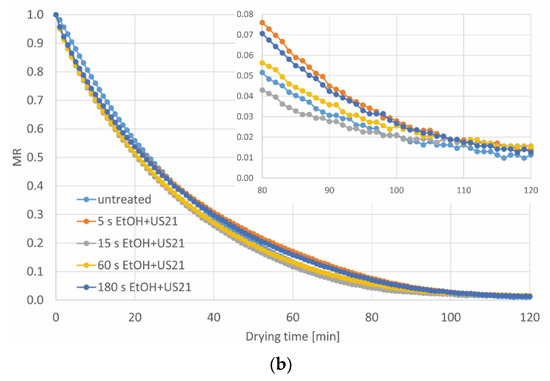
Figure 3.
The drying kinetics of carrot tissue pre-treated (a) without and (b) with ultrasound, a, b, etc.—different letters indicate different homogeneous groups (α = 0.05).

Table 3.
Drying time to MR = 0.02, dry matter content, water activity of dried carrot tissue pre-treated without and with ultrasound; error bars indicate ± SD (standard deviation calculated from three repetitions).

Table 4.
Shrinkage, density, rehydration rate (after 3 h of rehydration), hygroscopic properties (after 72 h above NaCl solution) of dried carrot tissue pre-treated without and with ultrasound; error bars indicate ± SD (standard deviation calculated from three repetitions).
The shelf life of dried products depends on the obtained water activity level and the storage conditions (ambient humidity, type of packaging). Water activity affects the course of biological processes, in particular the development and ability to divide microorganisms. It is assumed that microbiologically safe dried products have a water activity not exceeding 0.6 [48]. In all cases, the water activity of dried samples was below the limit (0.250–0.274; Table 3). The dried untreated carrot contained 94.05 ± 0.71% of dry matter, whereas for the ethanol-treated samples, it was in the range of 92.19–95.46%. The type and time of treatment did not affect both dry matter content and water activity in dried carrots (p > 0.05, Table 3). Moreover, there was no correlation between the final water content after drying and the water activity of dried materials (p = 0.255; r = −0.424).
3.3. Effect of the Pre-Treatment on the Properties of Dried Material
During pre-treatment and drying, the physical properties, i.e., volume and density of the plant tissue, change. The volume changes depend mainly on the quality of the raw material subjected to the process, as well as the method of processing [29]. Shrinkage and density of dried carrot slices immersed in ethanol are summarized in Table 4. The shrinkage of the untreated dried carrot was 65.3 ± 2.0%, while the density was equal to 0.79 ± 0.10 g/cm3. Both in the case of EtOH and EtOH+US treatments, there were no significant differences in these parameters of dried materials. Nevertheless, there was a noticeable tendency regarding the shrinkage and density, which increased in most cases in comparison to the untreated dried carrot. The higher shrinkage was observed for scallion stalk and apple treated with ethanol (with and without sonication) [9,26]. Furthermore, the density increased with the increasing time for both treatment variants in ethanol, and the two-factor analysis of variance showed the significant influence of the type of treatment on the material density (p = 0.022). The increased density was a result of the decreased volume of the slices and increased shrinkage, along with the prolongation of the processing time and the application of US. The increase in shrinkage, as a result of ultrasound treatment prior to drying, was also noted by Nowacka et al. [25]. Similarly, Żubernik et al. [13] noticed increase in shrinkage for apples immersed in ethanol before drying. On the other hand, Funebo et al. [49] reported a reduction in shrinkage after treatment with ethyl alcohol in the case of apples. However, it should be mentioned that the treatment was carried out under different conditions, hence probably different trends.
Rehydration ability is an important physical property of dried products, as the amount of water absorbed by dried material may affect the textural and visual properties [11,18,49]. A good rehydration capacity is especially important in the case of dried carrot tissue, due to its further use in, e.g., soups. In conducted research, the dried untreated material was characterized by a rehydration rate equal to 5.35 ± 0.07 (Table 4). EtOH treatment, especially when it was combined with US, resulted in increased rehydration capacity, which was in the range of 5.27–6.01 for EtOH (increase of up to 12%) and from 5.99 to 6.37 for EtOH+US (increase of up to 19%). It was proven that type of treatment significantly influenced (p < 0.05, Table 4) the rehydration ability, which was probably related to the increased porosity of the material as a result of damage to the internal structure during sonication [30]. This means that the use of treatment in ethanol, especially when this treatment is supported by the ultrasound, increases the possibility of re-absorption of water of dried carrots. Funebo et al. [49] came to similar conclusions that the pre-treatment in ethanol improved the rehydration properties and porosity of dehydrated apples. The authors explained the higher rehydration capacity of apples treated with ethanol by changes in the microstructure, including thinned cell walls, which swelled upon rehydration. The same results were obtained for rehydrated pumpkin [18], carrot [11] and scallion stalk [26] when the material was treated by ethanol with and without sonication. These changes were explained by the better access of the ethanol molecules into the tissue, which improved the resistance of plant cells to shrinkage during the drying.
Hygroscopic properties depend on chemical composition, structure, enzymatic and chemical reactions. These properties in dried products can help in assessing changes in the product during the drying process [30]. In general, the greater damage of the structure, the worse is the water vapour adsorption capacity [28]. Changes in the water vapour adsorption of dried carrots pre-treated in EtOH and EtOH+US are shown in Figure 4 and in Table 4. On the basis of Figure 4, it can be noted that the highest intensity of water vapour adsorption for all tested samples took place within the first 24 h. The high value of hygroscopic properties in the case of dried carrot subjected before drying to 5 s EtOH and 15 s EtOH+US may indicate slight changes in the internal structure and preserved adsorption centres. It is worth mentioning that in the case of these materials, the drying time was the shortest (less than for untreated carrot). There was no observed trend in changes of hygroscopic properties depending on the time of treatment, both with and without sonication (p > 0.05, Table 4). Żubernik et al. [13] also observed different water vapour adsorption properties for dried apples immersed in ethanol. They recorded the lowest relative water content after the longest treatment time (3 min), which was explained by the increased shrinkage of apples dried after this treatment. However, it can probably also be caused both by changes in the internal structure of dried carrots caused by ethanol and different behaviour of the material during drying (e.g., structure collapse or shrinkage). It could be noticed that in most cases the material subjected to ethanol with US assistance showed worse hygroscopic properties, which could be due to increased damage of the structure. On the basis of the presented results, it can be concluded that with the selected parameters of the pre-treatment, it is possible to influence the hygroscopic properties of dried products. Reduced water vapour adsorption may increase the storage stability of dried material.
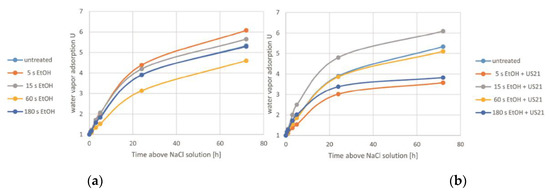
Figure 4.
Kinetics of the water vapour adsorption (hygroscopic properties) of dried carrot tissue pre-treated in ethanol without (a) and with ultrasound (b).
Table 5 shows the colour parameters of dried carrots, which were subjected to pre-treatment in ethanol (EtOH) and ethanol-ultrasound (EtOH+US21). Dried carrot slices, untreated before drying, had an L* value equal to 51.5 ± 4 and after the immersion treatment in ethyl alcohol (EtOH), darkening of the dried carrots was observed. Whereas, the sonication assistance did not significantly change the lightness of dried material in comparison to the untreated dried carrot. The different effect of both treatments, with and without ultrasound assistance, was confirmed by the two-way analysis of variance (p < 0.05, Table 5). However, the treatment time did not have a significant effect (p > 0.05, Table 5). Nowacka and Wedzik [2] noted that the sonication parameters influenced both the darkening of dried sonicated carrots, as well as causing no changes in carrot colour, compared to those not pre-treated. The lack of changes in the lightness of dried carrots treated with ethanol and ultrasound means that there is a positive effect of this type of treatment on the colour of dried carrots. The a* value of the untreated dried material was 17.6 ± 2.9 and for all dried materials subjected to pre-treatments in ethanol, with and without sonication, no statistical changes in red colour were noted. Similarly, Nowacka and Wedzik [2] did not find a significant difference in the a* and b* value between the dried untreated carrot and the dried one subjected to US for 10–30 min. On the other hand, in our research, in the case of b* value and hue angle (h°), a significant decrease was observed for almost all dried samples subjected to both EtOH and EtOH+US pre-treatments, in comparison to control (dried, untreated). It is worth noting that for both treatments the obtained values for hue angle did not exceed 90° (ranged from 51.9 to 56.2), which means that the dried carrot slices were still characterized by a red-orange colour. The pre-treatment, regardless of the variant and its duration, significantly decreased the hue angle, which was probably related to immersion of the material in ethanol.

Table 5.
Colour parameters (L*, a*, b*), hue angle (h°) and total colour difference (ΔE, in comparison to raw material) of carrot tissue pre-treated in ethanol without (EtOH) and with ultrasound (EtOH+US21); error bars indicate ± SD (standard deviation calculated from at least six repetitions).
The above changes in all colour parameters of dried carrots were calculated in comparison to the raw carrots and presented as the total colour difference (ΔE). In the case of processing in ethyl alcohol (EtOH), a significant colour change was demonstrated in relation to the untreated dried material, except for the longest processing time. The total colour difference ranged from 9.7 to 13.8. It is worth noting that in the case of US-assisted treatment, ΔE ranged from 16.1 to 18.1 and was statistically identical to the control dried sample. These results indicate a colour change (ΔE > 5) noticeable by the eye of an inexperienced observer, which can be due to different reflection of light source from fresh and dried material. Similar results were obtained by Zhou et al. [26] for infrared-convective dried scallion slices when the water and ethanol, with and without sonication assistance, were used before drying. Treatment in ethanol of scallion slices without US resulted in lower ΔE value in comparison to control (soak in distilled water) and sonicated sample in ethanol.
Convective drying is usually conducted at high temperatures [29,50], which can lead to the degradation of many biologically active ingredients in food [11,51,52]. Carotenoids are such ingredients in carrots [53]. The carotenoid content in dried carrot tissue pre-treated in ethanol without (EtOH) and with the ultrasound of 21 kHz (EtOH+US 21) are presented in Figure 5. The carotenoid content decreased after drying in comparison to fresh samples (Figure 2). Dried carrots not subjected to pre-treatments were characterized by the content of carotenoids of 21.00 ± 0.28 mg/100 g d.m. Compared to raw carrots, the retention of these compounds after drying of untreated sample was 39%. Rawson et al. [54] reported 28% retention of carotenoids after convective drying of carrots. The degradation of carotenoid content during the processing of food products depends on the composition of the product and the parameters of the drying process itself. Carotenoids are sensitive to the action of oxygen, light, heating and enzymes, hence their losses during processing can be significant [53,54]. However, in properly processed products, changes in carotenoid content can be limited [55]. For example, when a carrot was dried at 40 °C the retention of carotenoid content was 92% [56]. However, when the higher temperatures of 60, 70, 80 and 90 °C were used the retention was 17, 27, 30 and 36%, respectively [52].
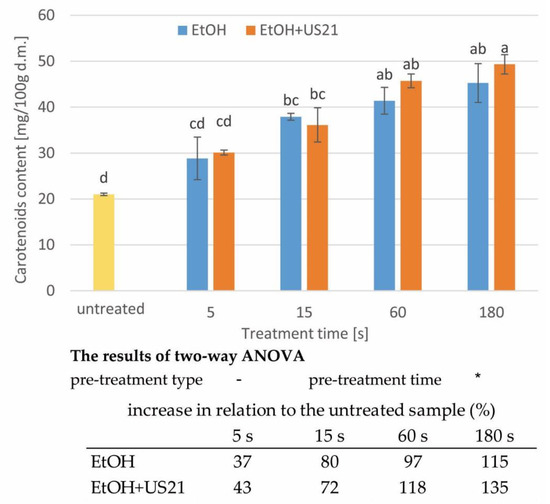
Figure 5.
The carotenoid content in dried carrot tissue pre-treated in ethanol without (EtOH) and with ultrasound (EtOH+US21); a, b, etc.—different letters indicate different homogeneous groups (α = 0.05), the results of two-way ANOVA indicate the significance (*) or insignificance (-) of the influence of the type and time of treatment (α = 0.05). In table—the increase in carotenoid content in relation to the untreated dried sample.
As in the case of the material directly after processing in ethanol (Figure 2), as well as in the case of dried carrots, increased content of carotenoids after treatment in ethanol was observed (up to 135% increase in comparison to untreated dried carrot). This was linked to the use of immersion in ethanol, and higher carotenoid content was obtained with longer processing time (p < 0.05, Figure 5) and with sonication assistance. However, the second factor did not affect significantly the obtained results (p > 0.05, Figure 5). The increased carotenoid contents in relation to untreated dried material was in the range of 37–115% and 43–135% in the case of EtOH and EtOH+US, respectively. Ultrasound caused thus only a slight increase in carotenoid content in dried carrots in relation to the dried material immersed in ethanol. These results are confirmed by the reports of Rawson et al. [54] and Nowacka and Wedzik [2]. However, pumpkin subjected to 30 min of ethanol with US and convective drying at 50 °C retained 100% of carotenoids, while control samples showed 23% degradation of carotenoid content [18]. Furthermore, Costa Santos [11] observed unchanged carotenoid contents in dried carrots treated with ethanol for 30 min compared to the untreated dried material. It should be, however, mentioned that in our study the increased content of carotenoids after a shorter amount of time indicates the possibility of obtaining product of designed properties when the pre-treatment parameters are adjusted. Furthermore, it is worth mentioning that due to enhanced extractivity of carotenoids, the immersion in ethyl alcohol with US assistance for 180 s resulted in limited degradation of carotenoids (8%) in dried material when compared to fresh tissue. It can be therefore stated that dried carrot subjected to EtOH+US treatment contained a similar amount of carotenoids as raw root.
4. Conclusions
The pre-treatment of carrots in ethanol contributed to the reduction of water content, mass loss and an increase of carotenoid content in tissue as well as an increase of ethyl alcohol conductivity due to partial, surface replacement of water by ethanol, leakage of dissociating compounds from the tissue to alcohol and simultaneously increased extractivity of carotenoids from pre-processed carrots. The changes were more pronounced with increasing pre-treatment time and with ultrasound application, especially when the frequency of 40 kHz was used. The colour of ethanol-treated carrot was generally unchanged in comparison to the fresh sample. This indicates a greater disruption of the structure after sonication and thus a greater leakage of cellular contents into the ethanol solution and an increased extractability of carotenoids caused by a higher ultrasound frequency. Therefore, the US frequency of 21 kHz was selected for further investigation during the drying process.
Despite a decrease of water content after treatment in ethanol (EtOH) and ethanol with ultrasound (EtOH+US), both pre-treatments virtually did not change the drying time in comparison to the untreated sample, which was probably due to increased tissue shrinkage and partial breakdown of the structure during drying. The dried pre-treated in ethanol carrot exhibited an increased rehydration rate (up to 19%) and total carotenoid contents (up to 135%) with statistically identical total colour change as in the case of untreated dried material. Longer pre-treatment time, in particular when ultrasound was combined with ethanol treatment, caused additional increase of rehydration ability and carotenoid contents. It can be therefore stated that ethanol-ultrasound (EtOH+US) treatment for a short time (up to 3 min) creates the possibility of obtaining dried carrot with designed quality, corresponding to both the specific requirements of the consumer and the industry.
Author Contributions
Conceptualization, M.D.; methodology, M.D.; software, M.D. and M.N.; validation, M.D.; formal analysis, M.D. and M.N.; investigation, M.D.; resources, M.D.; data curation, M.D.; writing—original draft preparation, M.D. and M.N.; writing—review and editing, M.D. and M.N.; visualization, M.D. and M.N.; supervision, M.D. and M.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Food Sciences of Warsaw University of Life Sciences.
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors would like to thank Marta Szymanska for her help.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sharma, K.D.; Karki, S.; Thakur, N.S.; Attri, S. Chemical composition, functional properties and processing of carrot-A review. J. Food Sci. Technol. 2012, 49, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Wedzik, M. Effect of ultrasound treatment on microstructure, colour and carotenoid content in fresh and dried carrot tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Chua, K.J.; Chou, S.K. New hybrid drying technologies. In Emerging Technologies for Food Processing; Sun, D.-W., Ed.; Elsevier Academic Press: London, UK, 2005; pp. 535–551. ISBN 9780126767575. [Google Scholar]
- Wang, J.; Sheng, K. Far-infrared and microwave drying of peach. LWT Food Sci. Technol. 2006, 39, 247–255. [Google Scholar] [CrossRef]
- Rahman, M.S.; Perera, C.O. Drying and food preservation. In Handbook of Food Preservation, 2nd ed.; Rahman, M.S., Ed.; CRC Press LLC: London, UK, 2007; pp. 403–432. [Google Scholar]
- Moses, J.A.; Norton, T.; Alagusundaram, K.; Tiwari, B.K. Novel Drying Techniques for the Food Industry. Food Eng. Rev. 2014, 6, 43–55. [Google Scholar] [CrossRef]
- Motevali, A.; Minaei, S.; Khoshtagaza, M.H. Evaluation of energy consumption in different drying methods. Energy Convers. Manag. 2011, 52, 1192–1199. [Google Scholar] [CrossRef]
- Braga, A.M.P.; Pedroso, M.P.; Augusto, F.; Silva, M.A. Volatiles identification in pineapple submitted to drying in an ethanolic atmosphere. Dry. Technol. 2009, 27, 248–257. [Google Scholar] [CrossRef]
- Zubernik, J.; Dadan, M.; Cichowska, J.; Witrowa-Rajchert, D. The Impact of the Pre-Treatment in Ethanol Solution on the Drying Kinetics and Selected Properties of Convective Dried Apples. Int. J. Food Eng. 2020, 1–11. [Google Scholar] [CrossRef]
- Silva, M.G.; Celeghini, R.M.S.; Silva, M.A. Effect of ethanol on the drying characteristics and on the coumarin yield of dried guaco leaves (Mikania laevigata schultz bip. ex baker). Braz. J. Chem. Eng. 2018, 35, 1095–1104. [Google Scholar] [CrossRef]
- Costa Santos, K.; Guedes, J.S.; Rojas, M.L.; Carvalho, G.R.; Augusto, P.E.D. Enhancing carrot convective drying by combining ethanol and ultrasound as pre-treatments: Effect on product structure, quality, energy consumption, drying and rehydration kinetics. Ultrason. Sonochem. 2021, 70, 105304. [Google Scholar] [CrossRef]
- Canteri, M.H.G.; Renard, C.M.G.C.; Le Bourvellec, C.; Bureau, S. Corrigendum to “ATR-FTIR spectroscopy to determine cell wall composition: Application on a large diversity of fruits and vegetables” (Carbohydrate Polymers (2019) 212 (186–196), (S0144861719301638), (10.1016/j.carbpol.2019.02.021)). Carbohydr. Polym. 2020, 235, 115960. [Google Scholar] [CrossRef]
- Żubernik, J.; Dadan, M.; Czyżewski, J.; Witrowa-Rajchert, D. The impact of ethanol on drying process and selected properties of apple tissue. Zesz. Probl. Postępów Nauk Rol. 2017, 145–153. [Google Scholar] [CrossRef]
- Santos, P.H.S.; Silva, M.A. Retention of vitamin C in drying processes of fruits and vegetables - A review. Dry. Technol. 2008, 26, 1421–1437. [Google Scholar] [CrossRef]
- Corrêa, J.L.G.; Braga, A.M.P.; Hochheim, M.; Silva, M.A. The Influence of Ethanol on the Convective Drying of Unripe, Ripe, and Overripe Bananas. Dry. Technol. 2012, 30, 817–826. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D. Ethanol and ultrasound pre-treatments to improve infrared drying of potato slices. Innov. Food Sci. Emerg. Technol. 2018, 49, 65–75. [Google Scholar] [CrossRef]
- Rojas, M.L.; Augusto, P.E.D.; Cárcel, J.A. Ethanol pre-treatment to ultrasound-assisted convective drying of apple. Innov. Food Sci. Emerg. Technol. 2020, 61, 102328. [Google Scholar] [CrossRef]
- Rojas, M.L.; Silveira, I.; Augusto, P.E.D. Ultrasound and ethanol pre-treatments to improve convective drying: Drying, rehydration and carotenoid content of pumpkin. Food Bioprod. Process. 2020, 119, 20–30. [Google Scholar] [CrossRef]
- Kentish, S.; Ashokkumar, M. The physical and chemical effect of ultrasound. In Ultrasound Technologies for Food and Bioprocessing; Feng, H., Barosa-Canovas, G.V., Weiss, J., Eds.; Springer: New York, NY, USA, 2011; pp. 1–12. ISBN 9781441974716. [Google Scholar]
- Wiktor, A.; Gondek, E.; Jakubczyk, E.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. Acoustic and mechanical properties of carrot tissue treated by pulsed electric field, ultrasound and combination of both. J. Food Eng. 2018, 238, 12–21. [Google Scholar] [CrossRef]
- Leonelli, C.; Mason, T.J. Microwave and ultrasonic processing: Now a realistic option for industry. Chem. Eng. Process. Process Intensif. 2010, 49, 885–900. [Google Scholar] [CrossRef]
- Awad, T.S.; Moharram, H.A.; Shaltout, O.E.; Asker, D.; Youssef, M.M. Applications of ultrasound in analysis, processing and quality control of food: A review. Food Res. Int. 2012, 48, 410–427. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Wiktor, A.; Sledz, M.; Nowacka, M. Selected Emerging Technologies to Enhance the Drying Process: A Review. Dry. Technol. 2014, 32, 1386–1396. [Google Scholar] [CrossRef]
- Sledz, M.; Wiktor, A.; Nowacka, M.; Witrowa-Rajchert, D. Drying Kinetics, Microstructure and Antioxidant Properties of Basil Treated by Ultrasound. J. Food Process Eng. 2017, 40, 1–13. [Google Scholar] [CrossRef]
- Nowacka, M.; Wiktor, A.; Śledź, M.; Jurek, N.; Witrowa-Rajchert, D. Drying of ultrasound pretreated apple and its selected physical properties. J. Food Eng. 2012, 113, 427–433. [Google Scholar] [CrossRef]
- Zhou, C.; Wang, Z.; Wang, X.; Yagoub, A.E.A.; Ma, H.; Sun, Y.; Yu, X. Effects of tri-frequency ultrasound-ethanol pretreatment combined with infrared convection drying on the quality properties and drying characteristics of scallion stalk. J. Sci. Food Agric. 2020. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; 2002; Volume 920.15, Available online: https://www.aoac.org/aoac_prod_imis/AOAC/Publications/O_cial_Methods_of_Analysis/AOAC_Member/Pubs/OMA/AOAC_O_cial_Methods_of_Analysis.aspx (accessed on 11 January 2021).
- Śledź, M.; Nowacka, M.; Wiktor, A.; Witrowa-Rajchert, D. Selected chemical and physico-chemical properties of microwave-convective dried herbs. Food Bioprod. Process. 2013, 91, 421–428. [Google Scholar] [CrossRef]
- Witrowa-Rajchert, D.; Rzaca, M. Effect of drying method on the microstructure and physical properties of dried apples. Dry. Technol. 2009, 27, 903–909. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The physical, optical and reconstitution properties of apples subjected to ultrasound before drying. Ital. J. Food Sci. 2017, 29, 343–356. [Google Scholar]
- Nowacka, M.; Wiktor, A.; Anuszewska, A.; Dadan, M.; Rybak, K.; Witrowa-Rajchert, D. The application of unconventional technologies as pulsed electric field, ultrasound and microwave-vacuum drying in the production of dried cranberry snacks. Ultrason. Sonochem. 2019, 56, 1–13. [Google Scholar] [CrossRef]
- Polish Standard. Fruit and Vegetable Juice—Total Carotenoids and Carotenoids Fraction Determination (in Polish) (PN-EN 12136); Poland, 2000. [Google Scholar]
- Pieczywek, P.M.; Kozioł, A.; Konopacka, D.; Cybulska, J.; Zdunek, A. Changes in cell wall stiffness and microstructure in ultrasonically treated apple. J. Food Eng. 2017, 197, 1–8. [Google Scholar] [CrossRef]
- Rajewska, K.; Mierzwa, D. Influence of ultrasound on the microstructure of plant tissue. Innov. Food Sci. Emerg. Technol. 2017, 43, 117–129. [Google Scholar] [CrossRef]
- Nowacka, M.; Fijalkowska, A.; Wiktor, A.; Rybak, K.; Dadan, M.; Witrowa-Rajchert, D. Changes of Mechanical and Thermal Properties of Cranberries Subjected to Ultrasound Treatment. Int. J. Food Eng. 2017, 13. [Google Scholar] [CrossRef]
- Fijalkowska, A.; Nowacka, M.; Wiktor, A.; Sledz, M.; Witrowa-Rajchert, D. Ultrasound as a Pretreatment Method to Improve Drying Kinetics and Sensory Properties of Dried Apple. J. Food Process Eng. 2016, 39, 256–265. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.G.; Lightfoot, D.A. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Ashokkumar, M. Applications of ultrasound in food and bioprocessing. Ultrason. Sonochem. 2015, 25, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Miano, A.C.; Ibarz, A.; Augusto, P.E.D. Mechanisms for improving mass transfer in food with ultrasound technology: Describing the phenomena in two model cases. Ultrason. Sonochem. 2016, 29, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, B.K.; Patras, A.; Brunton, N.; Cullen, P.J.; O’Donnell, C.P. Effect of ultrasound processing on anthocyanins and color of red grape juice. Ultrason. Sonochem. 2010, 17, 598–604. [Google Scholar] [CrossRef]
- Wiktor, A.; Dadan, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The impact of combination of pulsed electric field and ultrasound treatment on air drying kinetics and quality of carrot tissue. LWT 2019, 110, 71–79. [Google Scholar] [CrossRef]
- Fikselová, M.; Mareček, J.; Mellen, M. Carotenes content in carrot roots (Daucus carota L.) as affected by cultivation and storage. Veg. Crop. Res. Bull. 2010, 73, 47–54. [Google Scholar] [CrossRef]
- Koca Bozalan, N.; Karadeniz, F. Carotenoid profile, total phenolic content, and antioxidant activity of carrots. Int. J. Food Prop. 2011, 14, 1060–1068. [Google Scholar] [CrossRef]
- Saini, R.K.; Keum, Y.S. Carotenoid extraction methods: A review of recent developments. Food Chem. 2018, 240, 90–103. [Google Scholar] [CrossRef]
- Thane, C.; Reddy, S. Processing of fruit and vegetables: Effect on carotenoids. Nutr. Food Sci. 1997, 97, 58–65. [Google Scholar] [CrossRef]
- Arscott, S.A.; Tanumihardjo, S.A. Carrots of many colors provide basic nutrition and bioavailable phytochemicals acting as a functional food. Compr. Rev. Food Sci. Food Saf. 2010, 9, 223–239. [Google Scholar] [CrossRef]
- Wiktor, A.; Sledz, M.; Nowacka, M.; Rybak, K.; Witrowa-Rajchert, D. The influence of immersion and contact ultrasound treatment on selected properties of the apple tissue. Appl. Acoust. 2016, 103, 136–142. [Google Scholar] [CrossRef]
- Şen, F.; Karaçali, İ.; Eroğul, D. Effects of Storage Conditions and Packaging on Moisture Content, Water Activity and Tissue Hardness of Dried Apricots Depo Koşulları ve Ambalajların Kuru Kayısıların Su Miktarı, Su. Meyve Bilim. Sci. 2015, 2, 45–49. [Google Scholar]
- Funebo, T.; Ahrné, L.; Prothon, F.; Kidman, S.; Langton, M.; Skjöldebrand, C. Microwave and convective dehydration of ethanol treated and frozen apple—Physical properties and drying kinetics. Int. J. Food Sci. Technol. 2002, 37, 603–614. [Google Scholar] [CrossRef]
- Tylewicz, U.; Nowacka, M.; Rybak, K.; Drozdzal, K.; Dalla Rosa, M.; Mozzon, M. Design of Healthy Snack Based on Kiwifruit. Molecules 2020, 25, 3309. [Google Scholar] [CrossRef] [PubMed]
- Rzaca, M.; Witrowa-Rajchert, D. Changes in radical scavenging activity and in content of polyphenols in dried apples produced using infrared radiation | Zmiany aktywności przeciwrodnikowej i zawartości polifenoli w suszu jablkowym uzyskanym przy wykorzystaniu promieniowania podczerwoneg. Zywn. Nauk. Technol. Jakosc/Food. Sci. Technol. Qual. 2009, 16, 108. [Google Scholar]
- Zielinska, M.; Markowski, M. Color characteristics of carrots: Effect of drying and rehydration. Int. J. Food Prop. 2012, 15, 450–466. [Google Scholar] [CrossRef]
- Gheonea (Dima), I.; Aprodu, I.; Enachi, E.; Horincar, G.; Bolea, C.A.; Bahrim, G.E.; Râpeanu, G.; Stănciuc, N. Investigations on thermostability of carotenoids from tomato peels in oils using a kinetic approach. J. Food Process. Preserv. 2019, 44, e14303. [Google Scholar] [CrossRef]
- Rawson, A.; Tiwari, B.K.; Tuohy, M.G.; O’Donnell, C.P.; Brunton, N. Effect of ultrasound and blanching pretreatments on polyacetylene and carotenoid content of hot air and freeze dried carrot discs. Ultrason. Sonochem. 2011, 18, 1172–1179. [Google Scholar] [CrossRef]
- Tryzno, E.; Śledź, M.; Hankus, M.; Królikowski, K.; Witrowa-Rajchert, D. The application of accelerated shelf-life tests to assessment of storage life of dried beetroot, carrot and basil. Zesz. Probl. Postępów Nauk Rol. 2013, 573, 51–59. [Google Scholar]
- Frias, J.; Penas, E.; UIllate, M.; Vidal-Valverde, C. Influence of Drying by Convective Air Dryer or Power Ultrasound on the Vitamin C and β-Carotene Content of Carrots. J. Agric. Food Chem. 2010, 58, 10539–10544. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).