Performance Study of Grass-Derived Nano-Cellulose and Polycaprolactone Composites for 3D Printing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Preparation of CNC/PCL Composite Materials
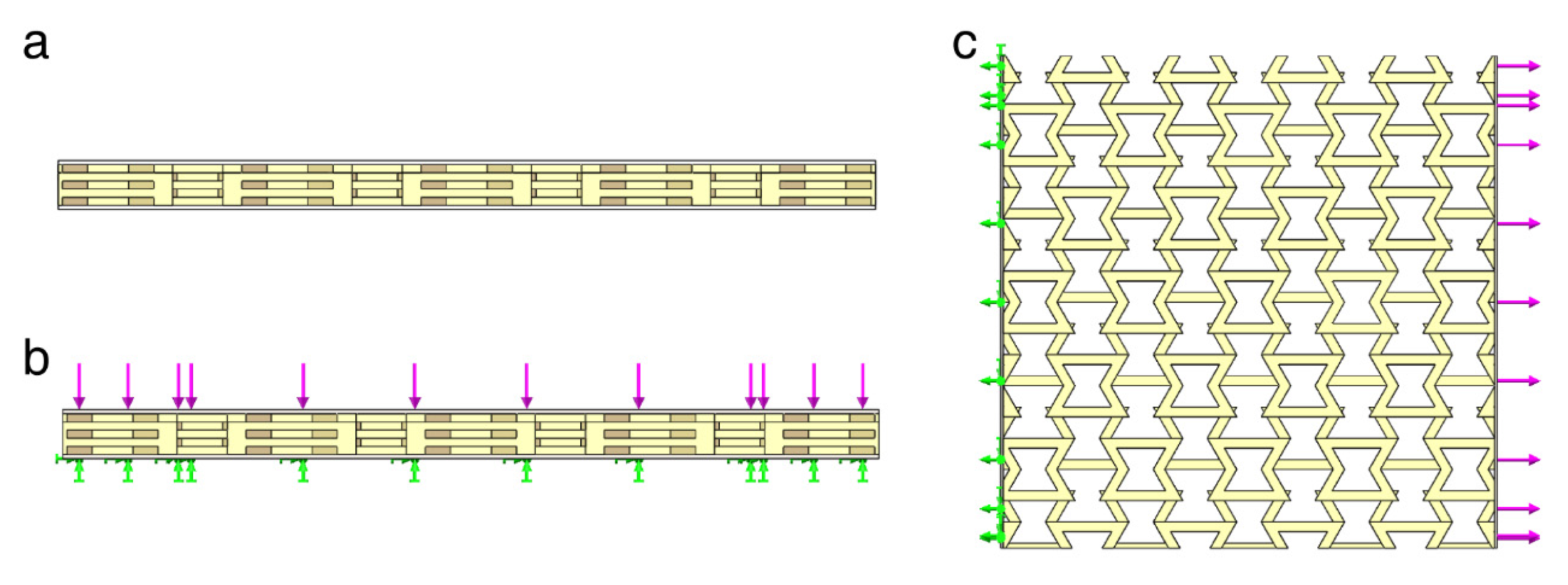
2.1.2. Mechanical Simulation with Solid Works Simulink
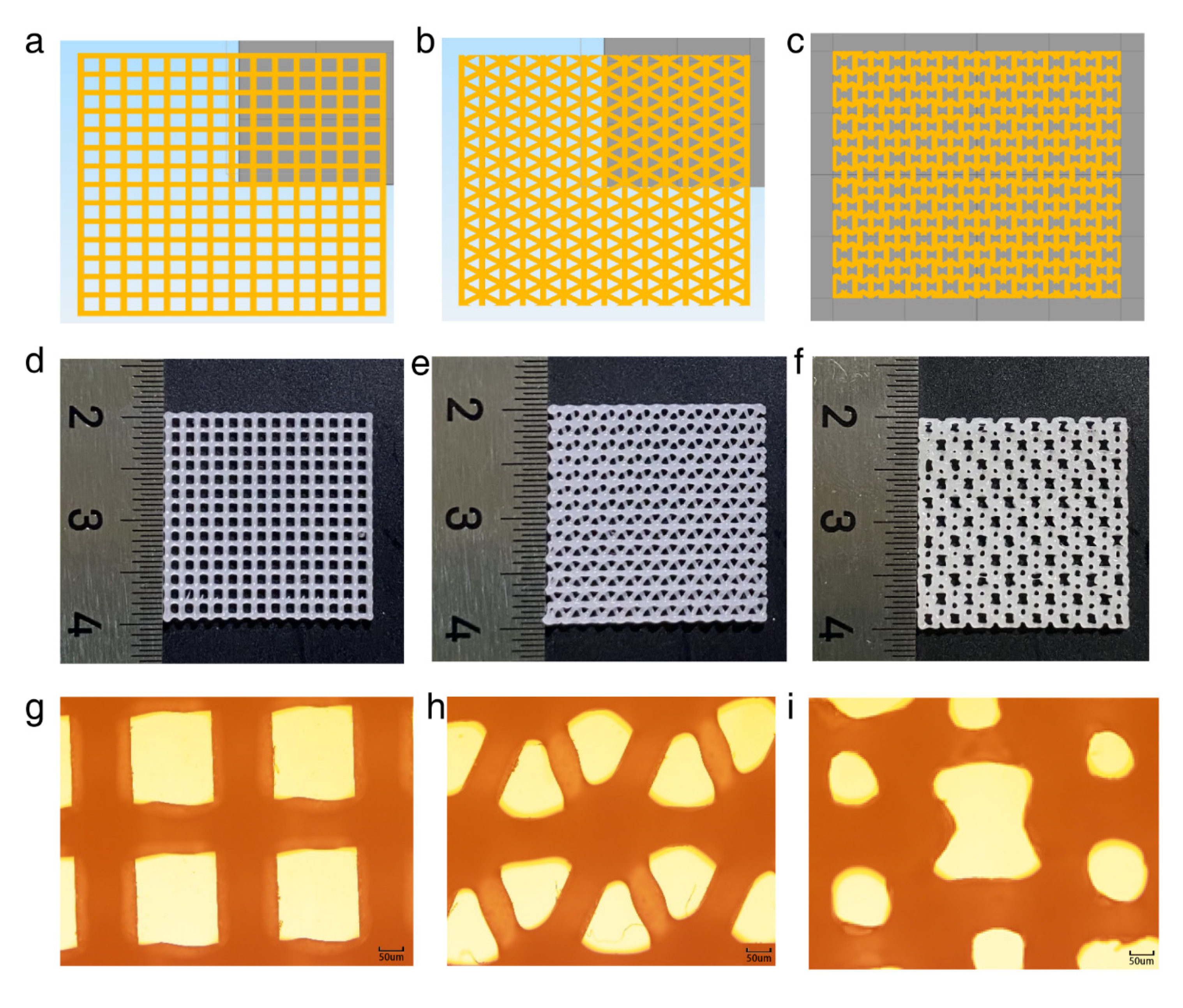
2.1.3. Printing of the Scaffold
2.1.4. Numerical Simulation of the Flow Field in the Scaffolds
2.2. Characterizations
2.2.1. Scanning Electron Microscopy (SEM)
2.2.2. Assessment of Rheological Property
2.2.3. Assessment of Mechanical Properties
2.2.4. Biocompatibility Assessment
3. Results and Discussion
3.1. Bioprinted Material Characterization Test Results
3.1.1. SEM Observation of Surface Morphology
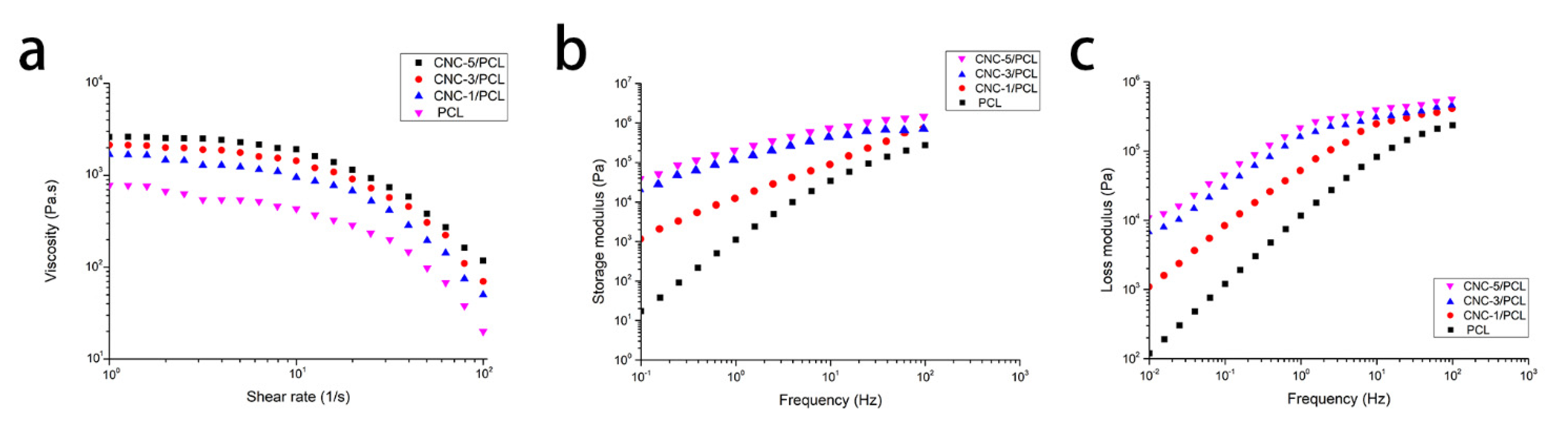
3.1.2. Analysis of Rheological Property
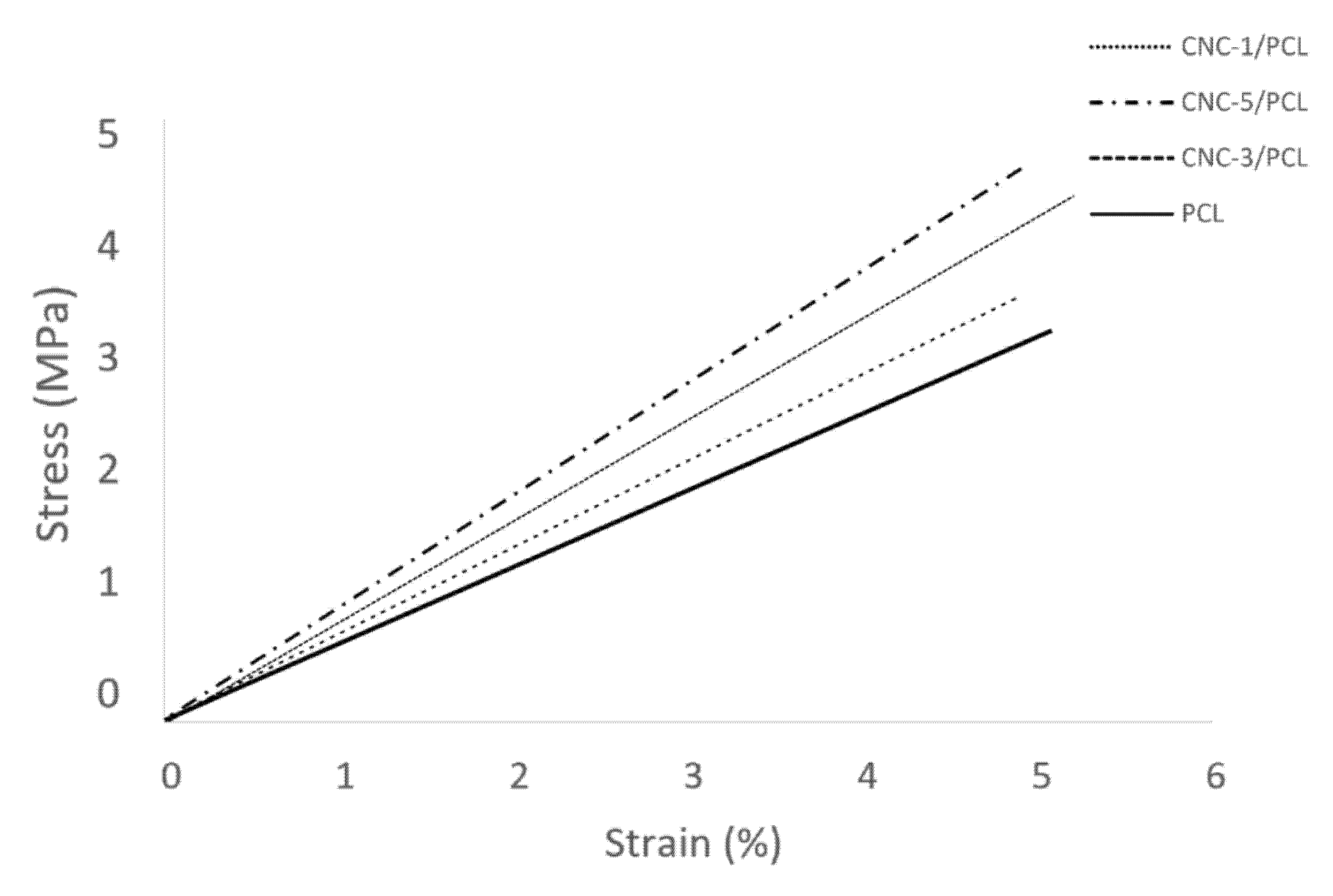
3.1.3. Analysis of the Mechanical Properties of the Materials
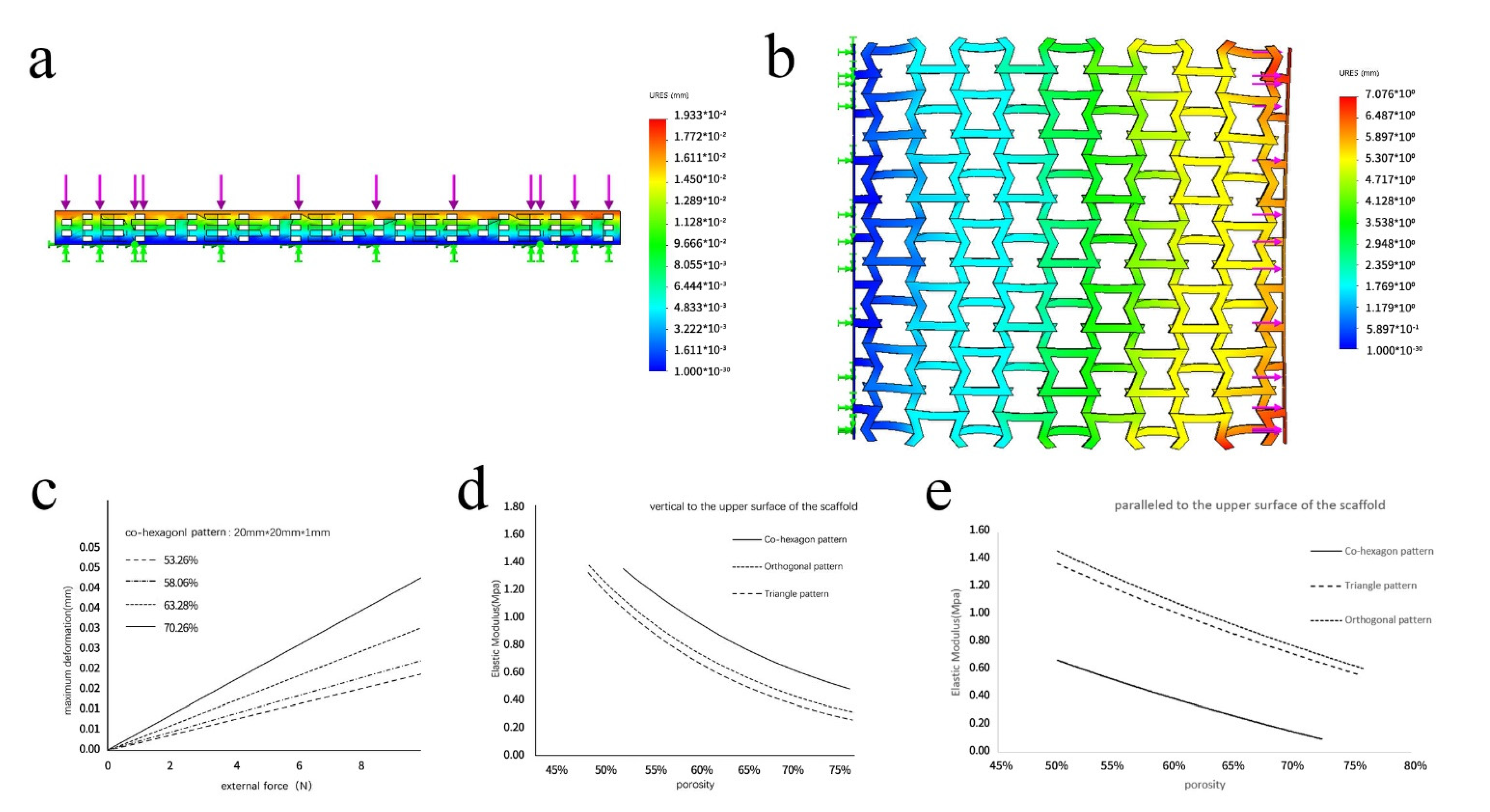
3.2. Analysis of the SolidWorks Simulation Results
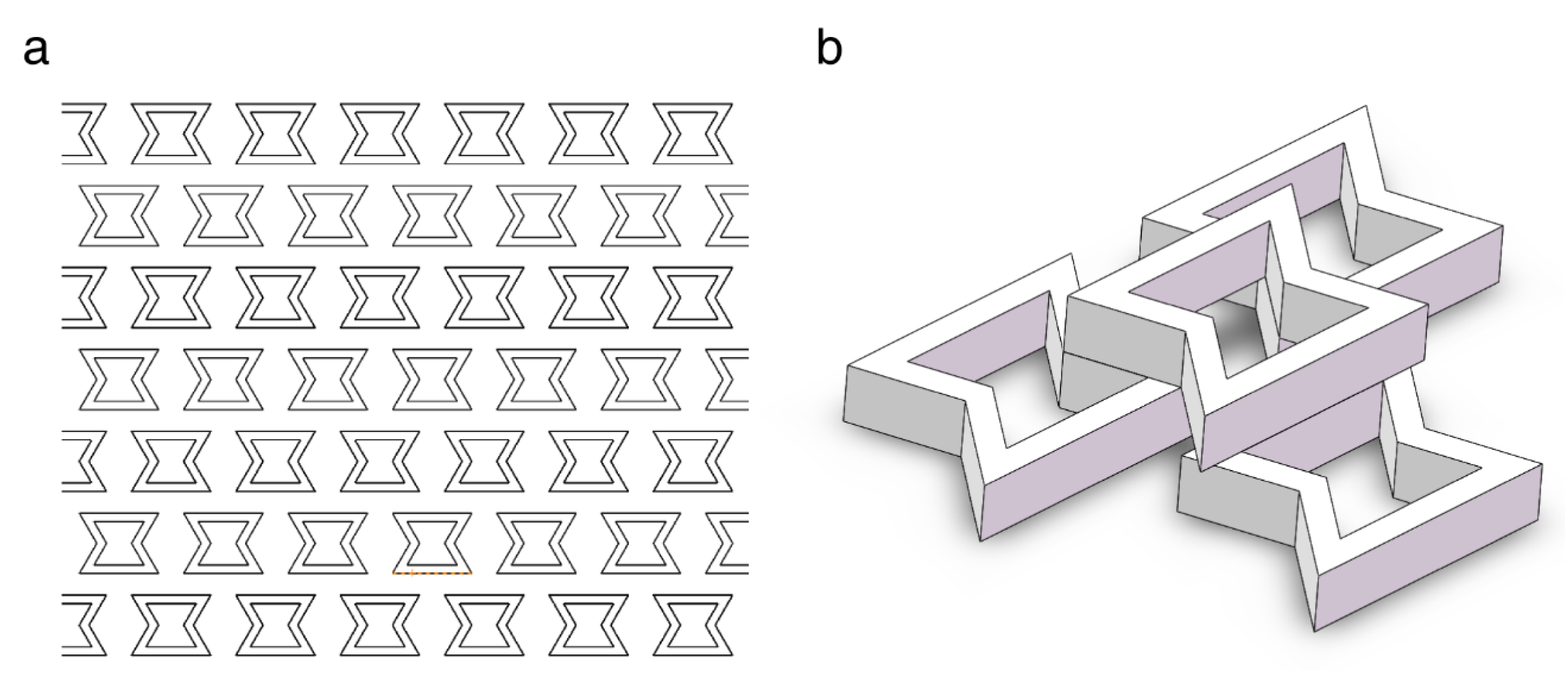
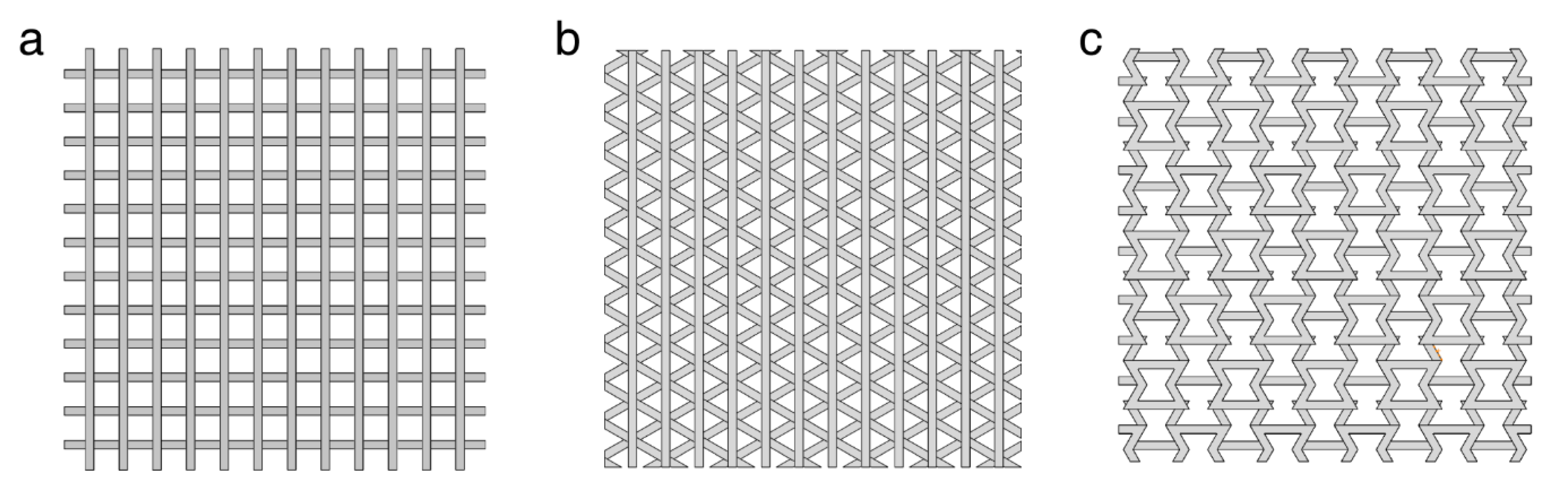
3.3. Analysis of the Simulation Results of the Different Scaffold Patterns
3.4. Structure Analysis of the Printed Cube Model
3.5. Analysis of the Mechanical Properties of the Scaffolds
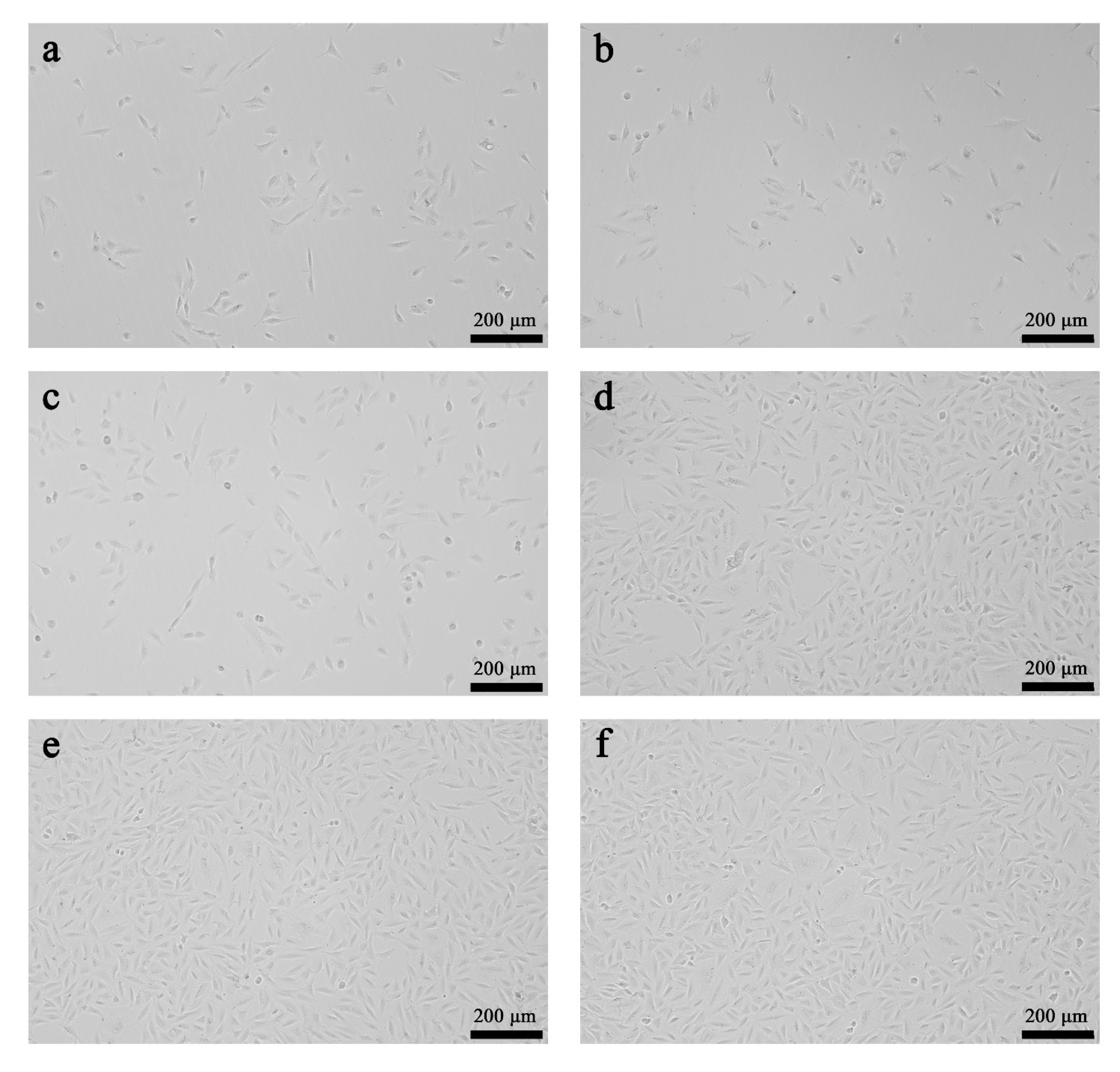
3.6. The Results of the Biocompatibility Assessment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jing, X.; Peng, X.F. Research progress in preparation of biodegradable polymer porous scaffolds. China Plast. 2012, 26, 1–6. [Google Scholar] [CrossRef]
- Johnson, C.; Sheshadri, P.; Ketchum, J.M.; Narayanan, L.K.; Weinberger, P.M.; Shirwaiker, R.A. In vitro characterization of design and compressive properties of 3D-biofabricated/decell ularized hybridgrafts for tracheal tissue engineering. Mech. Behav. Biomed. Mater. 2016, 59, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Jung, J.W.; Kang, H.W.; Joo, Y.H.; Lee, J.S.; Cho, D.W. Development of a 3D bellows tracheal graft: Mechanical behavior analysis, fabrication and an in vivofeasibility study. Biofabrication 2012, 4, 035004. [Google Scholar] [CrossRef]
- Kuehn, B.M. Clinicians Embrace 3D Printers to Solve Unique Clinical Challenges. JAMA 2016, 315, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D. New paradigms in hierarchical porous scaffold design for tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1759–1772. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271–275. [Google Scholar] [CrossRef]
- Goetzendorfer, B.; Mohr, T.; Hellmann, R. Hybrid Approaches for Selective Laser Sintering by Building on Dissimilar Materials. Materials 2020, 13, 5285. [Google Scholar] [CrossRef]
- Korpela, J.; Kokkari, A.; Korhonen, H.; Malin, M.; Närhi, T.; Seppälä, J. Biodegradable and bioactive porousscaffold structures prepared using fused deposition modeling. J. Biomed. Mater. Res. Part B Appl. Biomater. 2013, 101, 610–619. [Google Scholar] [CrossRef]
- Bourell, D.; Espalin, D.; Arcaute, K.; Rodriguez, D.; Medina, F.; Posner, M.; Wicker, R. Fused deposition modeling of patient-specific polymethylmethacrylate implants. Rapid Prototyp. J. 2010, 16, 164–173. [Google Scholar] [CrossRef]
- Luong, D.X.; Subramanian, A.K.; Lopez Silva, G.A.; Yoon, J.; Cofer, S.; Yang, K.; Owuor, P.S.; Wang, T.; Wang, Z.; Lou, J.; et al. Laminated Object Manufacturing of 3D-Printed Laser-Induced Graphene Foams. Adv. Mater. 2018, 30, 1707416. [Google Scholar] [CrossRef]
- Ahn, D.; Kweon, J.H.; Choi, J.; Seokhee, L. Quantification of surface roughness of partsprocessed by laminated object manufacturing. J. Mater. Process. Technol. 2012, 212, 339–346. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and itsapplications in biomedical enginccring. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [PubMed]
- Arcaute, K.; Mann, B.K.; Wicker, R.B. Practical use of hydrogels in stereolithography for tissue engineering applications. In Stereolithography; Springer: Boston, MA, USA, 2011; pp. 299–331. [Google Scholar] [CrossRef]
- Anne, G.; David, B.V.; Carlos, B.P.J.; Marcos, L.; Ana, A.P.; Abad, J.L.M. Experimental and Numerical Investigation of the Extrusion and Deposition Process of a Poly(lactic Acid) Strand with Fused Deposition Modeling. Polymers 2020, 12, 2885. [Google Scholar] [CrossRef]
- Ghosal, K.; Manakhov, A.; Zajíčková, L.; Thomas, S. Structural and surface compatibility study of modified electrospun poly(ε-caprolactone) (PCL) composites for skin tissue engineering. AAPS PharmSciTech 2017, 18, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.J.; Jung, S.M.; Kang, M.; Shin, H.S.; Youk, J.H. Preparation of hydrophilic PCL nanofiber scaffolds via electrospinning of PCL/PVP-b-PCL block copolymers for enhanced cell biocompatibility. Polymer 2015, 69, 95–102. [Google Scholar] [CrossRef]
- Li, Z.; Tan, B.H. Towards the development of polycaprolactone based amphiphilic block copolymers: Molecular design, self-assembly and biomedical applications. Mater. Sci. Eng. C 2014, 45, 620–634. [Google Scholar] [CrossRef]
- Cobos, M.; Ramos, J.R.; Guzmán, D.J.; Fernández, M.D.; Fernández, M.J. PCL/POSS Nanocomposites: Effect of POSS Derivative and Preparation Method on Morphology and Properties. Polymers 2018, 11, 33. [Google Scholar] [CrossRef]
- Propst, E.J.; Prager, J.D.; Meinzen-Derr, J.; Clark, S.L.; Cotton, R.T.; Rutter, M.J. Pediatric tracheal reconstruction using cadaveric homograft. Arch. Otolaryngol. Head Neck Surg. 2011, 137, 583–590. [Google Scholar] [CrossRef][Green Version]
- Delaere, P.; Vranckx, J.; Verleden, G.; Leyn, P.D.; Raemdonck, D.V. Leuven Tracheal Transplant Group Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N. Engl. J. Med. 2010, 362, 138–145. [Google Scholar] [CrossRef]
- Wurtz, A.; Hysi, I.; Kipnis, E.; Copin, M.C. Recent Advances in Circumferential Tracheal Replacement and Transplantation. Am. J. Transplant. 2016, 16, 1334–1335. [Google Scholar] [CrossRef][Green Version]
- Ma, T.; Lv, L.; Ouyang, C.; Hu, X.; Liao, X.; Song, Y.; Hu, X. Rheological behavior and particle alignment of cellulose nanocrystal and its composite hydrogels during 3D printing. Carbohydr. Polym. 2021, 253, 117217. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Shi, Q.; Guo, W.; Terrell, L.; Qureshi, A.T.; Hayes, D.J.; Wu, Q. Electrospun bio-nanocomposite scaffolds for bone tissue engineering by cellulose nanocrystals reinforcing maleic anhydride grafted PLA. ACS Appl. Mater. Interfaces 2013, 5, 3847–3854. [Google Scholar] [CrossRef]
- Huan, W.L.; Cheng, L.; Bai, G.; Liu, G. Fabrication and characterization of electrospun polystyrene/cellulose nanocrystals nanofibrous films. Polym. Mater. Sci. Eng. 2016, 32, 141–146. [Google Scholar] [CrossRef]
- Baguneid, M.S.; Seifalian, A.M.; Salacinski, H.J.; Murray, D.; Hamilton, G.; Walker, M.G. Tissue engineering of bloodvessels. Br. J. Surg. 2006, 93, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Vale, A.C.; Cunha, E.P.F.; Paiva, M.C.; Reis, R.L.; Vaquette, C.; Alves, N.M. 3D-printed cryomilled poly(ε-caprolactone)/graphene composite scaffolds for bone tissue regeneration. J. Biomed. Mater. Res. B Appl. Biomater. 2020. [CrossRef]
- Aliabouzar, M.; Lee, S.J.; Zhou, X.; Zhang, G.L.; Sarkar, K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol. Bioeng. 2018, 115, 495–506. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, S.; Sun, R.; Scarpa, F.; Wang, J. Research and application of mechanical properties of negative Poisson’s ratio materials and structures. Prog. Mech. 2011, 41, 335–350. [Google Scholar] [CrossRef]
- Ingrole, A.; Hao, A.; Liang, R. Design and modeling of auxetic and hybrid honeycomb structures for in-plane property enhancement. Mater. Des. 2017, 117, 72–83. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, J.; Zhang, Q.G.; Zhao, L.; Heng, D.; Chen, D.; Liu, D. Preparation of cellulose nanocrystals from Humulus japonicus stem and the influence of high temperature pretreatment. Carbohydr. Polym. 2017, 164, 284–293. [Google Scholar] [CrossRef]
- Ferrua, M.J.; Xue, Z.; Singh, R.P. On the kinematics and efficiency of advective mixing during gastric digestion—A numerical analysis. J. Biomech. 2014, 47, 3664–3673. [Google Scholar] [CrossRef]
- Zhou, J.; Feng, C. Nanocomposite Gel Material Hardness and Stretch Automatic Detector. CN209690061U, 26 November 2019. [Google Scholar]
- Xu, X.; Zhou, J.; Jiang, Y. 3D printing process of oxidized nanocellulose and gelatin scaffold. J. Biomater. Sci. Polym. Ed. 2018, 29, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.Y.; Jing, X.; Peng, J.; Salick, M.R.; Peng, X.F.; Turng, L.S. Poly(ε-caprolactone) (PCL)/cellulose nano-crystal (CNC) nanocomposites and foams. Cellulose 2014, 21, 2727–2741. [Google Scholar] [CrossRef]
- Feng, Q.; Yamasaki, N.; Yanagisawa, K. Hydrothermal syn-thesis and metal ion adsorptive property of birnessite-type po-tassium manganese oxide. J. Mater. Sci. Lett. 1996, 15, 963–965. [Google Scholar] [CrossRef]
- Alvesda, S.M.; Crawford, A.; Mundy, J.M. Chitosan/polyester-based scaffolds for cartilage tissue engineering: Assessment of extracelluar matrix formation. Acta Biomater. 2010, 6, 1149–1157. [Google Scholar] [CrossRef]
- Domingues, R.M.; Chiera, S.; Gershovich, P. Enhancing the biomechanical performance of anisotropic nanofibrous scaffolds intendon tissue engineering: Reinforcement with cellulose nanocrystals. Adv. Healthc. Mater. 2016, 5, 1364–1375. [Google Scholar] [CrossRef]
- Xu, S.L.; Li, T.C.; Lu, B.H. Numerical study of two-phase flow of cells and cell suspension in microchannels during artificial bone perfusion in vitro. J. Mech. Eng. 2006, 3, 40–45. [Google Scholar] [CrossRef]
- Guo, Y.J.; Yuan, M.Q.; Qian, X.M. Mechanical behavior and response characteristics of concave honeycomb structures under impact loading. J. Beijing Inst. Technol. 2019, 15, 5–10. [Google Scholar]
- Saeedi, R.; Omrani, L.R.; Abbasi, M.; Chiniforush, N.; Kargar, M. Effect of Three Wavelengths of Diode Laser on the Efficacy of Bleaching of Stained Teeth. Front. Dent. 2019, 16, 458–464. [Google Scholar] [CrossRef]





| Sample Name | Specific Gravity of Dry CNC (%) | Specific Gravity of Dry PCL (%) |
|---|---|---|
| PCL | 0 | 100 |
| CNC-1/PCL | 1 | 99 |
| CNC-3/PCL | 3 | 97 |
| CNC-5/PCL | 5 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, C.; Zhou, J.; Xu, X.; Jiang, Y.; Shi, H.; Zhao, G. Performance Study of Grass-Derived Nano-Cellulose and Polycaprolactone Composites for 3D Printing. Appl. Sci. 2021, 11, 1273. https://doi.org/10.3390/app11031273
Feng C, Zhou J, Xu X, Jiang Y, Shi H, Zhao G. Performance Study of Grass-Derived Nano-Cellulose and Polycaprolactone Composites for 3D Printing. Applied Sciences. 2021; 11(3):1273. https://doi.org/10.3390/app11031273
Chicago/Turabian StyleFeng, Chen, Jiping Zhou, Xiaodong Xu, Yani Jiang, Hongcan Shi, and Guoqi Zhao. 2021. "Performance Study of Grass-Derived Nano-Cellulose and Polycaprolactone Composites for 3D Printing" Applied Sciences 11, no. 3: 1273. https://doi.org/10.3390/app11031273
APA StyleFeng, C., Zhou, J., Xu, X., Jiang, Y., Shi, H., & Zhao, G. (2021). Performance Study of Grass-Derived Nano-Cellulose and Polycaprolactone Composites for 3D Printing. Applied Sciences, 11(3), 1273. https://doi.org/10.3390/app11031273





