Abstract
The aim of the study was to perform a mapping and umbrella review with meta-meta-analysis (MMA) to synthesise and critically evaluate the effectiveness of manual therapy (MT) and aerobic exercise (AE) in relation to pain intensity, frequency, disability and quality of life in patients with migraines, tension-type headaches (TTH) and cervicogenic headaches (CGH). A systematic search was conducted in PubMed, PEDro, Scielo and Google Scholar up to December 2020. A total of 18 articles met the inclusion criteria, and only 8 were included in the quantitative analysis. The MMA showed results in favour of the interventions in terms of pain intensity and quality of life in migraine, TTH and CCH. Data were also in favour of the intervention in terms of pain frequency in migraine and in terms of disability in TTH. However, there were no significant effects on pain frequency in TTH and CGH. The results showed moderate evidence to suggest that AE reduces pain intensity in patients with migraine. In addition, the evidence in favour of MT or a mixed intervention (including therapeutic exercise) was also moderate in terms of reducing pain intensity in patients with TTH.
1. Introduction
Headaches are categorised worldwide into 2 groups: primary and secondary [1]. Among the primary headaches, migraine and tension-type headaches (TTH) are the most prevalent [2]. Secondary headaches include cervicogenic headaches (CGHs), among others [1].
Headaches lead to important deteriorations in patients’ quality of life and involve significant economic repercussions, medical expenses, work incapacity and social and familiar impact. Headache disorders were the third-leading cause of disability in 2016 [2].
Regarding the therapeutic approach for headaches, there are both pharmacological and nonpharmacological interventions. Pharmacological treatment appears to be efficient for some acute cases and for prophylaxis [3]. However, this treatment is not effective in all cases, and they can become chronic disorders. Chronic headaches generate an increase in the number of medical consultations at the cost of the health system and can even generate medication-overuse headache [4,5,6].
The nonpharmacological approach provides therapeutic options to be assessed for the treatment of headaches, such as therapeutic exercise and manual therapy. Some proposed exercise modalities are aerobic exercise (AE) and exercise focused on retraining the cervical and shoulder muscles, with moderate evidence of reduced pain intensity, symptom frequency and disability and improved quality of life in patients in the short- to medium-term. It should also be noted that these exercise modalities do not generate adverse effects in these patients [7,8].
Manual cervical therapy has shown improvements in the symptomatology of headaches [9]. The most well-founded theory to justify these effects is that manual therapy produces neurophysiological effects on the central and peripheral nervous system, leading to changes in the symptomatology of these patients [10]. There is also evidence of the benefits of therapeutic exercise and manual therapy for individuals with headaches.
Therefore, the aim of the study was to perform a mapping and umbrella review with meta-meta-analysis (MMA) to synthesise and critically evaluate the current evidence on the effectiveness of manual therapy and exercise in relation to pain intensity, frequency, disability and quality of life in patients with migraines, TTHs and CGHs.
2. Materials and Methods
This umbrella and mapping review was performed according to the Preferred Reporting Items for Overviews of Systematic Reviews including the harms checklist (PRIO-harms). The PRIO-harms tool is composed of 27 items and 56 sub-items [11]. The protocol of this systematic review and meta-analysis was registered in an international register prior to starting the review (PROSPERO, CRD42020222573).
2.1. Review Inclusion Criteria
The inclusion criteria for this review were based on methodological and clinical factors, including population, intervention, control, outcomes and study type [12].
2.1.1. Population
The individuals selected for the articles were patients older than 18 years, diagnosed with migraine, TTH and CGH.
2.1.2. Intervention and Control
The interventions were any type of therapeutic exercise and/or manual therapy performed by a physical therapist or health professional. Studies in which the intervention was performed by chiropractors or osteopaths were excluded. The intervention could be provided as an independent treatment or combined with other types of intervention. The control group could include any type of intervention, when it was possible to isolate and evaluate the effectiveness of manual therapy and/or therapeutic exercise.
2.1.3. Outcomes
The measures employed to assess the results and effects were pain intensity, frequency of symptoms, disability and/or quality of life.
2.1.4. Study Design
We included systematic reviews (with or without meta-analysis) of randomised controlled trials (RCTs) or controlled clinical trials (CCTs). No language restrictions were applied, as recommended by international criteria [13].
2.2. Search Strategy
We conducted a search for articles on PubMed, PEDro, Scielo and Google Scholar. The last search was run on December 2020.
The following PubMed search strategy was employed and was adapted to the rest of the databases: (headache [MeSH Terms]) OR (“migraine disorders” [MeSH Terms]) OR (migraine) OR (“tension-type headache” [MeSH Terms]) OR (“tensional headache”) OR (“cervicogenic headache”) AND (pain [MeSH Terms]) OR (ache [MeSH Terms]) OR (frequency) OR (disability) OR (“quality of life” [MeSH Terms])) OR (“pain intensity”) AND (exercise [MeSH Terms]) OR (“exercise therapy” [MeSH Terms]) OR (“physical exercise” [Title/Abstract]) OR (“physical therapy” [Title/Abstract]) OR (“musculoskeletal manipulations” [MeSH Terms]) OR (“manual therapy” [MeSH Terms]) OR (“manual therapy”) OR (“manipulation spinal”). We also used the following search filters: “systematic review” and “meta-analysis”.
Two independent reviewers conducted the search using the same methodology, and differences during this phase were resolved by consensus. The reference sections of the original studies were screened manually, and the authors were contacted for further information if necessary.
2.3. Selection Criteria and Data Extraction
Initially, analyses were performed by two independent reviewers who assessed the relevance of the systematic reviews (with and without a meta-analysis) regarding the study questions and objectives. The first analysis was performed based on each study’s title information, abstract and keywords. If there was no consensus or the abstract did not contain enough information, the full text was reviewed.
In the second phase of the analysis, the full text was assessed if the studies met all of the inclusion criteria. Differences between the reviewers were resolved by a process of discussion, and consensus was moderated by a third reviewer [14]. Data described in the Results section were extracted by means of a structured protocol that ensured that the most relevant information was obtained from each study [15].
2.4. Methodology Quality Assessment
The two independent reviewers assessed the methodological quality of the selected systematic reviews based on the Modified Quality Assessment Scale for Systematic Reviews (AMSTAR) developed by Barton et al. This scale presents 13 items, each worth 2 points (with “yes” scoring 2; “in part” scoring 1; “no” scoring 0), and the maximum possible score is 26. A high-quality cutoff of 20 or more points was provided by the developers of the scale [16].
The two independent reviewers assessed the quality of the studies employing the same methods, and disagreements on the final quality assessment score were resolved by consensus with a third independent reviewer. The inter-rater reliability was estimated using the kappa coefficient (κ): κ > 0.7 indicated a high level of agreement between the reviewers; κ of 0.5–0.7 indicated a moderate level of agreement, and κ < 0.5 indicated a low level of agreement [17].
2.5. Risk of Bias Assessment
We assessed the risk of bias using the Risk of Bias in Systematic Reviews tool (ROBIS), which evaluates the quality across 3 phases: (1) relevance assessment; (2) identification of concerns with the review process through 4 domains related to study eligibility, study identification and selection, data collection and study appraisal, and synthesis and findings; and (3) judgment on the risk of bias. The ROBIS tool includes signalling questions to evaluate specific domains by answering “yes”, “probably yes”, “probably no”, “no” or “no information”. The risk of bias is therefore judged as “low”, “high” or “unclear” [18].
The two independent reviewers evaluated the risk of bias in the selected studies using the same methodology; disagreements were resolved through consensus and mediation by a third reviewer. The interrater reliability was estimated using the same κ cutoffs described in the section Methodology quality assessment.
2.6. Evidence Map
We presented the scientific evidence of each systematic review with meta-analysis through a visual map. We created 2 different maps, one descriptive and the other using effect size data, and the information from each review was provided using the following criteria:
- Number of studies (figure size): The size of each figure is directly proportional to the number of original studies included in each of the meta-analyses.
- Study Population (bubble colour) and type of intervention (symbol): The type of population evaluated in each study is represented by a colour (green: migraine; blue: TTH; yellow: CGH). The type of therapeutic intervention determined each symbol inside the bubble (x: manual therapy; −: therapeutic exercise; +: mixed intervention). In addition, in the second mapping, the plot of the figure represents the study variable.
- x-Axis: In the descriptive mapping, each of the study variables is represented on the x-axis. In the second mapping, each of the reviews was classified according to the size effect as described by Hopkins (Hopkins et al., 2009). The categorisation of the effect size is described in the section Data synthesis and analysis.
- y-Axis: The descriptive mapping represents the quality of each of the reviews on the y-axis according to the AMSTAR scale. In the second mapping, the reviews were sorted into the following 4 categories according to the Physical Activity Guidelines Advisory Committee (PAGAC): strong, moderate, limited or not assignable.
2.7. Qualitative Analysis
As described earlier, we relied on the assessments of each systematic review with meta-analysis for the methodological quality of the primary studies, using AMSTAR for the included reviews and PAGAC for assessing the evidence across reviews.
For the PAGAC analysis, the findings were evaluated according to 5 criteria: (1) the applicability of the study sample, exposures and outcomes to the research question, (2) generalisability to the population of interest, (3) the risk of bias or study limitations, (4) the quantity and consistency of findings across studies and (5) the magnitude and precision of the effect. The strength of the evidence was classified as strong, moderate, limited or not assignable [19].
2.8. Data Synthesis and Analysis
The statistical analysis was performed using meta-analyses with MetaXL software [20,21].
The same inclusion criteria for the systematic review were employed, but 2 criteria were added: (1) The Results section contained detailed information on the comparative statistical data (mean, standard deviation and/or 95% confidence interval [CI]) of the main variables and (2) data for the analysed variables were represented in at least 2 studies. The summary statistics are presented in the form of forest plots [22], which consist of a weighted compilation of all standardised mean differences (SMDs) and corresponding 95% CIs reported by each study. They provide an indication of heterogeneity among the studies. The statistical significance of the pooled SMDs were examined using Hedges’ g to account for a possible overestimation of the true population effect size in small studies [23]. We interpreted the statistical significance of the pooled SMDs as described by Hopkins [24]; that is, we considered an SMD of 4.0 an extremely large clinical effect, 2.0–4.0 a very large effect, 1.2–2.0 a large effect, 0.6–1.2 a moderate effect, 0.2–0.6 a small effect and 0.0–0.2 a trivial effect. When the statistical significance of the pooled data was presented as mean difference, the meta-analysis was replicated using Meta-Essentials (ERIM, Erasmus University Rotterdam, Netherlands) with Microsoft Excel to obtain the SMD values [25]. The degree of heterogeneity among the studies was estimated by employing Cochran’s Q statistic test (p < 0.1 was considered significant) and the inconsistency index (I2) [26]. An I2 > 25% is considered to represent low heterogeneity, while an I2 > 50% is considered medium and an I2 > 75% is considered to represent large heterogeneity [27]. The I2 index is complementary to the Q test, although it has a similar problem with power as does the Q test with a small number of studies [27]. A study was therefore considered heterogeneous when it fulfilled one or both of the following conditions: (1) the Q-test was significant (p < 0.1) and (2) the result of I2 was >75%. To obtain a pooled estimate of the effect in the meta-analysis of the heterogeneous studies, we performed a random-effects model, as described by DerSimonian and Laird (1986) [28]. Publication bias through the funnel plot and the sensitivity exclusion analysis was not evaluated due to the impossibility of performing it when the MMA includes fewer than 2 studies.
3. Results
The study screening strategy is shown in the flow chart (Figure 1). Eighteen articles met the inclusion criteria and were selected, 9 of which were systematic reviews [7,29,30,31,32,33,34,35,36], while the remaining 9 were systematic reviews with a meta-analysis [8,37,38,39,40,41,42,43,44]. The characteristics of the included studies (study design, original studies included, demographic characteristics, interventions, main outcomes and conclusions) are presented in Table 1 and Table 2.
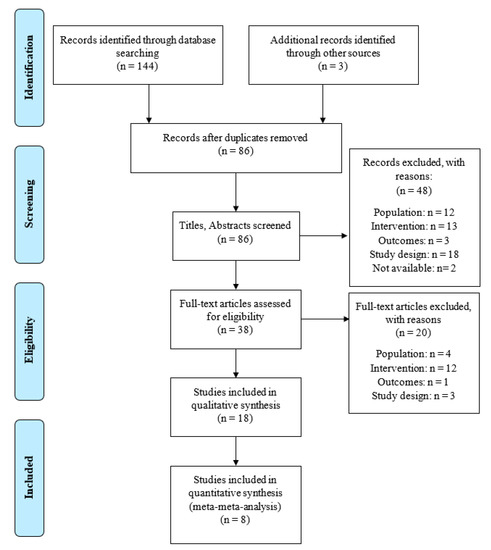
Figure 1.
Flow chart according to PRISMA.

Table 1.
Characteristics of the included studies.

Table 2.
Interventions included in each of the studies.
Eight of the studies were included in eight independent MMAs. The MMAs assessed pain intensity and the frequency of symptoms in patients with migraine, TTH and CGH independently, as well as disability in patients with TTH.
3.1. Characteristics of the Included Systematic Reviews
Our study included 18 systematic reviews (with or without a meta-analysis), comprising 95 original studies, 90 RCTs, 4 CCTs and 1 non-controlled clinical trial (included by [29]), with a total of 9188 participants. Several original studies appeared in different systematic reviews, but none of the reviews included the same studies (Table S1).
In terms of the populations in the systematic reviews, 9 studies (n = 3693) included patients diagnosed with migraine [7,8,29,33,35,37,39,40,41], 13 systematic reviews (n = 4435) included patients diagnosed with TTH [7,29,30,32,33,34,35,37,38,40,41,42,43] and 8 studies included patients diagnosed with CGH (n = 1592) [31,33,35,36,37,41,43,44]. One of the RCTs included patients with post-traumatic headache (n = 23) (included by Bronfort et al., 2001 [33]), and four of them (n = 173) did not specify which type of headache was studied (included by Chaibi and Russell 2014; Mesa-Jiménez et al., 2015; Luedtke et al., 2016 [30,41,42]).
In three of the systematic reviews, the Intervention group used AE with other forms of exercise therapy in patients with migraine [8,39] or TTH [29]. Ten systematic reviews had an Intervention group that used various types of manual therapy in patients with TTH [30,32,34,38], CGH [44] or populations with various types of headaches included in this study [33,35,37,40,43]. In the remaining five studies, the intervention used a combination of both treatments in patients with TTH [42], CGH [31,36] or several types of headache [7,41].
3.2. Results of the Methodology Quality Analysis
The scores ranged from 7 to 24 out of a possible 26 points, with a mean score of 17.28 ± 4.75 points. Only 6 (33.33%) of the 18 studies were considered high quality, with a score above 20 points [8,37,40,41,42,44] (Table 3).

Table 3.
Quality assessment scores.
The items with the highest scores were those related to the assessment of selection bias and the adequate description of the quality assessment. The items with the lowest scores were those related to language restrictions and the reporting of confidence intervals and effect sizes. The inter-rater reliability of the methodological quality assessment was high (κ = 0.790).
3.3. Results of the Risk of Bias Analysis
Of the 18 studies, 6 (33.33%) had a low risk of bias [8,35,37,40,41,44]. The remaining 12 (66.66%) had a high risk of bias [7,29,30,31,32,33,34,36,38,39,42,43] (Table 4 and Figure 2).

Table 4.
Risk of bias assessment in systematic reviews through the ROBIS scale.
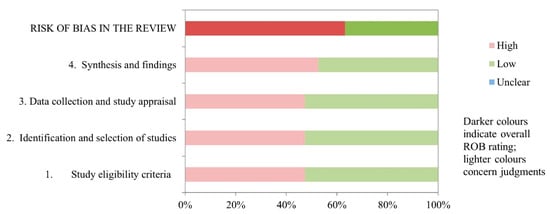
Figure 2.
Graphical representation of the ROBIS results.
The domain for “synthesis and findings” had the highest risk of bias, with 10 (55.55%) studies scoring a high risk of bias. In domains for “study eligibility criteria”, “identification and selection of studies” and “data collection and study appraisal”, 9 (50%) of the 18 studies had a low risk of bias. The inter-rater reliability for the risk of bias assessment was high (κ = 0.849).
3.4. Evidence Map
Figure 3 and Figure 4 present the results of the evidence map for the 18 studies. In addition, Table 5 shows the levels of evidence of the meta-analyses included in the study according to the PAGAC.

Figure 3.
Descriptive evidence map of meta-analyses. TE, therapeutic exercise; MT, mid-term; LT, long-term. On the x-axis the studies are classified according to the study variable, while on the y-axis they are categorized according to their score on the Modified Quality Assessment Scale for Systematic Reviews (AMSTAR), with a score above 20 being considered high-quality.
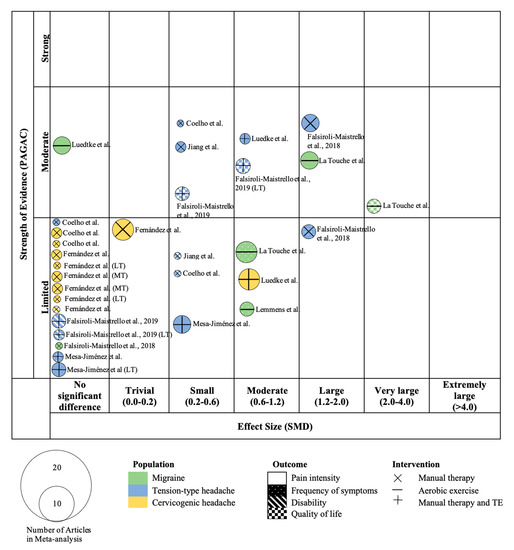
Figure 4.
Mapping based on effect size of meta-analyses. TE, therapeutic exercise; MT, mid-term; LT, long-term. On the x-axis the studies are classified according to the effect size of the meta-analysis with standard mean difference (SMD). On the y-axis they are categorized according to the strength of evidence based on the Physical Activity Guidelines Advisory Committee (PAGAC).

Table 5.
Committee assigned grades for the effects of manual therapy or therapeutic exercise on headache-related outcomes.
3.5. Pain Intensity
Seventeen studies assessed pain intensity in patients with headache; 8 of the studies included meta-analyses [8,38,39,40,41,42,43,44], and the remaining 9 performed a qualitative synthesis [7,29,30,31,32,33,34,35,36].
Interventions using AE with or without resistance exercise were an effective approach in patients with migraine or TTH in the short term [8,29,39]. Interventions based on manual therapy showed differences in favour of the Intervention group in patients with TTH [30,32,34,38], and in populations that included patients with migraine, TTH and CGH in the short-term [33,35,40,43] and at follow-up [35]. In patients with CGH, manual therapy showed positive results in the short-term [36,44], but not in the medium- or long-term [44]. The combination of manual therapy and therapeutic exercise showed differences favouring the Intervention group in patients with migraine, TTH or CGH in the short-term [41] and medium-term [7,31]. In one study, when comparing manual therapy with or without exercise and medication, they found significant differences in the short-term, but not in the long-term in patients with TTH [42].
With regard to the quantitative analysis, the MMA of pain intensity in patients with migraine revealed significant differences using aerobic exercise in two meta-analyses [8,41] (SMD = −1.16; 95% CI −1.90 to −0.41; p < 0.05) without evidence of heterogeneity (Q = 0.61, p = 0.44, (I2 = 0%) (Figure 5).
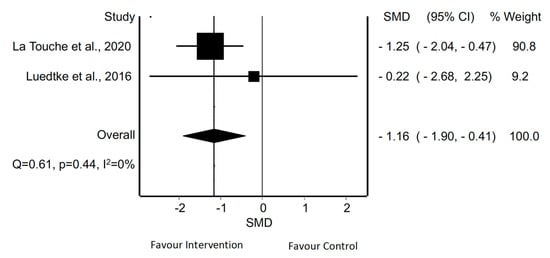
Figure 5.
Synthesis forest plot for pain intensity in patients with migraine treated with aerobic exercise. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
The MMA of pain intensity in patients with TTH treated by manual therapy or mixed treatment revealed significant differences in three [38,40,43] and two meta-analyses [41,42], respectively (SMD = −0.83; 95% CI −1.47 to −0.19; p < 0.05 and SMD = −0.59; 95% CI −0.85 to −0.33; p < 0.05, respectively). There was evidence of heterogeneity in the analysis of manual therapy (Q = 6.39, p = 0.04, I2 = 69%) but not in the case of mixed treatment (Q = 0.01, p = 0.91; I2 = 0%) (Figure 6 and Figure 7).

Figure 6.
Synthesis forest plot for pain intensity in patients with tension-type headache treated with manual therapy. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).

Figure 7.
Synthesis forest plot for pain intensity in patients with tension-type headache and mixed intervention (manual therapy and/or therapeutic exercise). The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
In the case of patients with CGH, the MMA of pain intensity also revealed significant differences in three meta-analyses including manual therapy or therapeutic exercise [41,43,44] (SMD = −0.49; 95% CI −0.86 to −0.12.33; p < 0.05) without evidence of heterogeneity according to Cochran’s Q statistical test (Q = 3.60, p = 0.16), but with heterogeneity according to I2 (I2 = 45%) (Figure 8).

Figure 8.
Synthesis forest plot for pain intensity in patients with cervicogenic headache and a mixed intervention. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
3.6. Frequency of Symptoms
Sixteen studies evaluated the frequency of symptoms in patients with headache; 9 performed a qualitative synthesis [7,29,30,31,32,33,34,35,36], while the remaining 7 included a meta-analysis [8,39,40,41,42,43,44].
Interventions using aerobic exercise have shown significant differences in headache frequency reduction in patients with migraine [8,39] or migraine and TTH [29]. Treatment with manual therapy showed positive results in studies involving patients with TTH in the short-term [30,32,34]. There was also significant differences in patients with CGH in the short-term [36,44] and medium-term [44]. Studies that included populations with migraine, TTH or CGH found positive results favouring the manual therapy group in the short-term [35,40,43] and medium-term [35,43]. Studies using a combination of therapeutic exercise and manual therapy showed a decrease in headache in the days immediately following treatment in patients with migraine, TTH or CGH [33,42] and in both the short- and medium-term [7,31]. Luedtke et al. found significant differences in patients with migraine and TTH but not in the case of CGH [41].
With regard to the quantitative analysis, the MMA of the frequency of symptoms in patients with migraine treated with AE revealed a statistically significant difference in two meta-analyses [8,39] (SMD = −0.75; 95% CI −1.08 to −0.43; p < 0.05) and without evidence of significant heterogeneity (Q = 0.00, p = 0.95, I2 = 0%) (Figure 9). In patients with TTH, the MMA of the frequency of symptoms revealed no significant difference in two meta-analyses that employed manual therapy [40,43] (SMD = −0.91; 95% CI −2.18 to 0.36; p > 0.05) with evidence of significant heterogeneity (Q = 9.40, p = 0.00; I2 = 89%) (Figure 10). The MMA of patients with CGH revealed no significant difference in 2 meta-analyses in which treatment included manual therapy or exercise in terms of the frequency of symptoms [43,44] (SMD = −0.30; 95% CI −0.84 to 0.23; p > 0.05) without evidence of heterogeneity (Q = 0.06, p = 0.80, I2 = 0%) (Figure 11).

Figure 9.
Synthesis forest plot for the frequency of symptoms in patients with migraine treated with aerobic exercise. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs, and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
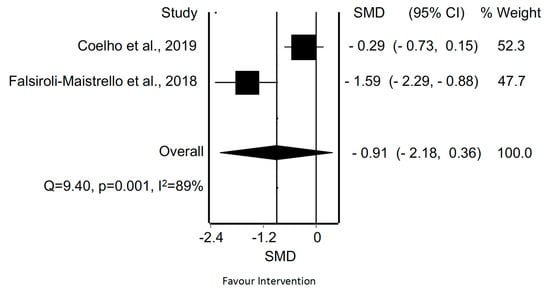
Figure 10.
Synthesis forest plot for the frequency of symptoms in patients with tension-type headache treated with manual therapy. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
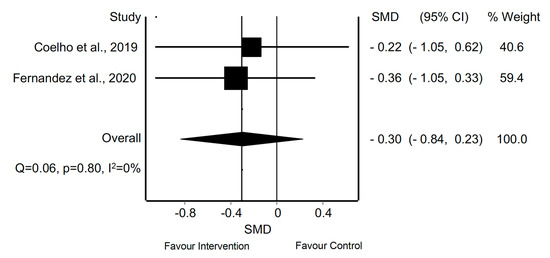
Figure 11.
Synthesis forest plot for the frequency of symptoms in patients with cervicogenic headache and mixed intervention. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
3.7. Quality of Life
Quality of life was evaluated in five studies: two included a meta-analysis [8,37], while three performed a qualitative synthesis [32,34,40]. Two studies showed significant differences in the increase in quality of life when using aerobic exercise in patients with migraine [8] or manual therapy in patients with migraine, TTH or CGH [37]. However, the remaining three studies showed no significant differences between groups or showed inconclusive results when the intervention included different manual therapy techniques in patients with TTH [32,34] or migraine and TTH [40].
3.8. Disbility
Ten studies assessed disability in patients with headache; seven were systematic reviews [7,31,32,33,34,35,40], and the remaining three systematic reviews included meta-analysis [37,38,43].
Most studies that evaluated disability associated with headache employed manual therapy, finding positive results in favour of the Intervention group in the short-term in patients with TTH [32,34,38] or GCH [44]. Studies that included patients with migraine, TTH or CGH also showed favourable results after employing manual therapy in the short-term [33,35,37,40,43] and at follow-up [37]. Manual therapy with or without therapeutic exercise produced medium-term improvements in patients with migraine or TTH [7], with conflicting evidence because only one study evaluated disability [31].
With regard to the quantitative analysis, the MMA of disability in patients with TTH revealed a statistically significant difference in two meta-analyses through manual therapy [38,43] (SMD = −0.27; 95% CI −0.47 to −0.06; p < 0.05). Based on Cochran’s Q statistical test, the heterogeneity among the reviews was not considered significant (Q = 1.37, p = 0.24). However, we found low evidence of heterogeneity according to the I2 (I2 = 27%) (Figure 12).
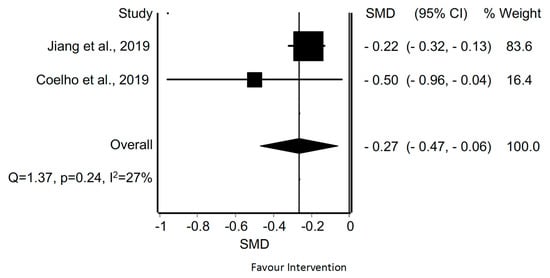
Figure 12.
Synthesis forest plot for disability in patients with tension-type headache treated with manual therapy. The forest plot summarizes the results of included studies (review size, standardized mean differences [SMDs] and weight). The small boxes with the squares represent the point estimate of the effect size and sample size. The lines on either side of the box represent a 95% confidence interval (CI).
4. Discussion
The present research aimed to synthesise and critically evaluate the evidence on the effectiveness of manual therapy and therapeutic exercise in patients with migraine, TTH or CGH. Results showed, in general, that the interventions with MT and exercise were beneficial for patients with TTH and CCH, and aerobic exercise was beneficial for patients with migraine. Most of the quantitative results favoured the interventions, with statistically significant results supporting the use of MT and exercise to reduce pain intensity in CGH and TTH but not to reduce the frequency of headaches. In addition, the MMA suggested a positive effect of AE for reducing the pain and frequency of migraine. The methodological quality of the eight meta-analyses included in our MMA is low for three of them [38,39,43] and high for the rest; and the risk of bias in general is high, except for three studies that had good methodological quality and also a low risk of bias [8,40,41].
4.1. Migraine
Currently, the management of patients with migraine is complex, given there is no specific treatment that fits all patients, and it sometimes leads to medication overuse and other adverse effects [45]. A lot of effort has been made to find a conservative non-pharmacological approach [46,47]. Therapeutic exercise prescriptions have added some hope for the treatment of this population [48,49]. This non-pharmacological intervention, which lacks adverse effects, helps minimise the need for drugs or other invasive interventions. If the headaches can be improved by reducing the frequency or intensity of the symptoms, medication overuse and adverse effects would be prevented.
Most of the included reviews on migraine have compared AE with other interventions, and the results of moderate, continuous AE appear to be positive. Along these lines, the results of the present MMA showed that AE can reduce pain intensity and frequency of headache in migraine patients, with moderate and limited evidence, respectively, and large effect sizes and low heterogeneity. The sensitivity analyses could not be performed since only two studies were included in each MMA (this also occurred in six out of the eight analyses performed).
In recent decades, the exercise intervention studied for migraine in most of the RCTs comprised moderate, continuous AE performed 2–5 days per week and for 40–60 min at 60–75% VO2max. The new approaches to therapeutic exercise for migraine use high-intensity exercise or interval exercise (of moderate or high intensity) [50,51]. There is still a lack of evidence regarding the difference between AE intensities and modalities, because the new approaches have yet to be evaluated in depth. In this regard, contradictory results have been obtained when assessing the effects of continuous moderate versus high-intensity interval training, although in general both interventions might reduce headache frequency and pain [50,51].
It is crucial to determine whether the patient with migraines also presents kinesiophobia, because it could impede successful completion of the exercise program. The first step in implementing exercise treatment for these patients should be to detect any irrational beliefs about movement, so that we can establish adequate management with a biobehavioural approach (therapeutic patient education) [52].
This section is not mandatory but can be added to the manuscript if the discussion is unusually long or complex.
4.2. Tension-Type Headache
Among the treatment options for patients with TTH, the conservative interventions applied by a physical therapist have been given a lot of weight. This type of intervention is justified based on the characteristics of patients with TTH and the involvement of the neck in TTH, which has been described and accepted worldwide [1]. Interventions such as cervical manual therapy and local exercises directed to the neck muscles are the most popular and studied approaches. These interventions are based on findings that suggest the presence of referred pain to the head coming from the cervical structures after manual provocation tests [53,54,55].
The results of the MMA showed that interventions based on MT might reduce the pain intensity (limited evidence) and disability of patients with TTH (limited evidence). The effect size is large for pain intensity with large heterogeneity. A small effect size and low heterogeneity was found for disability. No effect on frequency was found for manual therapy (limited evidence). The analyses of combined interventions (MT and/or local exercises) favoured the intervention, with moderate evidence; however the effect size was small, although with low heterogeneity.
According to the results of the present review, the physical therapy techniques included in conservative treatment for TTH were articular and soft-tissue techniques (in most cases) combined with therapeutic exercise. The articular approach included cervical and thoracic mobilisations or manipulations, mobilisations of several neck segments and tractions and soft tissue interventions applied to cervical muscle trigger points. In addition to these techniques, neck exercises were employed focused on the strength and endurance of the target muscles. In this regard, manual therapy has been largely studied; however there is a need to study other conservative exercise treatments that could also have positive effects on patients with TTH. This approach might be particularly indicated for those with chronic pain, for whom a generalised exercise intervention based on AE could have positive effects, as has been demonstrated for patients with migraine. It has been reported that patients with chronic TTH have an impairment of pain inhibition in a similar manner as has been observed in other chronic pain conditions [56].
Another interesting point is that patients with migraine as well as TTH typically have low levels of physical activity, and inactivity among pain-free individuals is a risk factor for developing a non-migraine type of headache [57]. These findings justify the implementation of interventions to increase physical activity/exercise among patients with TTH and also CGH. The combination of therapeutic patient education with exercise seems likely to lead to better adherence to exercise [58].
4.3. Cervicogenic Headache
CGH has been included in the International Classification of Headache Disorders as a secondary headache arising from musculoskeletal disorders in the cervical spine, but not necessarily accompanied by neck pain [1,59]. Structures such as the upper cervical synovial joints, upper cervical muscles and C2–C3 intervertebral disc have been raised as possible origins of CGH [60,61]. Some findings suggest the involvement of the neck structures in CGH; for example a reduction in upper cervical rotation [62] reduced cervical flexion/extension or painful upper cervical joints as assessed by manual palpation [63]. Given the problem of relapses at the neck, conservative treatment has focused on the neck structures. Manual therapy techniques are usually employed for patients with CGH [64,65]. In the present MMA, the data suggest that MT can influence pain intensity, but not pain frequency, both with limited evidence. The effect sizes for both findings were small, with low heterogeneity.
The interventions used in the RCT included in the analysed reviews were MT and/or therapeutic local exercise or MT alone. There is evidence suggesting positive effects for therapeutic exercise on patients with neck pain [66]; thus, it would be interesting to assess the effect of therapeutic exercise alone in CGH, given that there is scarce but favourable evidence for it [67].
4.4. Clinical Implications
The present review attempts to provide clear data and conclusions to be applied in practical terms to patients with headaches. More high-quality research is needed to be able to offer more specific recommendations, but with the results of this MMA, some suggestions can be made. Patients with headache might benefit from manual therapy and exercise. Physical therapists should be able to establish a specific and appropriate treatment for patients with headache according to their type of headache. Based on the data, for example, it would be preferable to employ a general exercise intervention for patients with migraine instead of a specific MT intervention.
From a clinical point of view, even a small intervention of 2 min per day of resistance exercise can reduce headache frequency by up to 50% [68]. This result highlights the importance of frequent therapeutic exercise for patients with headaches.
4.5. Research Implications
In general terms, further research with a combination of interventions, such as exercise, education and various MT techniques, is needed for the several types of headaches. This approach could establish new models of combined treatments for patients with headache. Regarding the exercise interventions, there is a great variety of exercise options to be compared, such as high-intensity general exercise for migraine and TTH or general AE for TTH. Another pending subject to assess is the dosage of the interventions, which needs to be established through further investigation.
It would also be interesting to assess whether a high-intensity interval training intervention would be more effective than a moderate AE intervention. Finally, general strength training should be assessed in patients with migraine.
4.6. Limitations
The present review has some limitations. First, many of the included studies had low methodological quality and a high risk of bias. The results should be analysed with caution. Second, there was considerable variability between the systematic reviews in terms of the interventions used. Third, six of the MMAs performed included only two meta-analyses, and the other two MMAs included three meta-analyses. Fourth, with the small number of studies included in each of the MMAs, the sensitivity was impossible to calculate. Sixth, due to the lack of data, it was not possible to conduct an MMA for quality of life and disability. Finally, there were significant inconsistencies among the RCTs included in the meta-analyses regarding the diagnostic criteria used to classify the patients. Likewise, it could have been interesting to evaluate the effectiveness of exercise and manual therapy according to the existing subdivisions within each of the headache types, for example, in patients with migraine with or without aura; however, that information was not present in the included studies.
5. Conclusions
The present umbrella and mapping review with MMA provide an overview of the effects of physical therapy interventions on pain intensity, pain frequency, disability and quality of life of patients with primary headaches. The MMAs showed results in favour of the interventions in terms of pain intensity and quality of life in patients with migraine, TTH and CCH. The data are also in favour of the intervention in terms of frequency of migraine and disability in TTH. However, there were no significant effects on pain frequency in patients with TTH and CGH.
There is moderate evidence to suggest that AE reduces pain intensity in patients with migraine. In addition, the evidence in favour of MT or a mixed intervention (including therapeutic exercise) is also moderate in terms of reducing pain intensity in patients with TTH.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11156856/s1, Table S1: Citation matrix for the assessment of study overlap within systematic reviews.
Author Contributions
Conceptualization, R.L.T.; methodology, A.H.-G., I.G.-P., P.M.-I. and R.L.T.; formal analysis, A.H.-G. and R.L.T.; writing—review and editing, A.H.-G., I.G.-P., P.M.-I., R.L.T. and A.P.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- International Headache Society (IHS). Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd ed. Cephalalgia 2018, 38, 285. [Google Scholar] [CrossRef]
- Stovner, L.J.; Nichols, E.; Steiner, T.J.; Abd-Allah, F.; Abdelalim, A.; Al-Raddadi, R.M.; Ansha, M.G.; Barac, A.; Bensenor, I.M.; Doan, L.P.; et al. Global, Regional, and National Burden of Migraine and Tension-Type Headache, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef] [Green Version]
- Bendtsen, L.; Evers, S.; Linde, M.; Mitsikostas, D.D.; Sandrini, G.; Schoenen, J. EFNS Guideline on the Treatment of Tension-Type Headache—Report of an EFNS Task Force. Eur. J. Neurol. 2010, 17, 1318–1325. [Google Scholar] [CrossRef] [PubMed]
- Kristoffersen, E.S.; Grande, R.B.; Aaseth, K.; Lundqvist, C.; Russell, M.B. Management of Primary Chronic Headache in the General Population: The Akershus Study of Chronic Headache. J. Headache Pain 2012, 13, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Russell, M.B. Epidemiology and Management of Medication-Overuse Headache in the General Population. Neurol. Sci. 2019, 40, 23–26. [Google Scholar] [CrossRef]
- Volcy-Gómez, M. The Impact of Migraine and Other Primary Headaches on the Health System and in Social and Economic Terms. Rev. Neurol. 2006, 43, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Gil-Martínez, A.; Kindelan-Calvo, P.; Agudo-Carmona, D.; Muñoz-Plata, R.; López-de-Uralde-Villanueva, I.; La Touche, R. Therapeutic Exercise as Treatment for Migraine and Tension-Type Headaches: A Systematic Review of Randomised Clinical Trials. Rev. Neurol. 2013, 57, 433–443. [Google Scholar] [CrossRef] [Green Version]
- La Touche, R.; Fernández Pérez, J.J.; Proy Acosta, A.; González Campodónico, L.; Martínez García, S.; Adraos Juárez, D.; Serrano García, B.; Angulo-Díaz-Parreño, S.; Cuenca-Martínez, F.; Suso-Martí, L.; et al. Is Aerobic Exercise Helpful in Patients with Migraine? A Systematic Review and Meta-analysis. Scand. J. Med. Sci. Sports 2020, 30, 965–982. [Google Scholar] [CrossRef]
- Biondi, D.M. Physical Treatments for Headeache: A Structured Review. Headache 2005, 45, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Bialosky, J.E.; Beneciuk, J.M.; Bishop, M.D.; Coronado, R.A.; Penza, C.W.; Simon, C.B.; George, S.Z. Unraveling the Mechanisms of Manual Therapy: Modeling an Approach. J. Orthop. Sports Phys. Ther. 2018, 48, 8–18. [Google Scholar] [CrossRef]
- Bougioukas, K.I.; Liakos, A.; Tsapas, A.; Ntzani, E.; Haidich, A.B. Preferred Reporting Items for Overviews of Systematic Reviews Including Harms Checklist: A Pilot Tool to Be Used for Balanced Reporting of Benefits and Harms. J. Clin. Epidemiol. 2018, 93, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Stone, P. Popping the (PICO) Question in Research and Evidence-Based Practice. Appl. Nurs. Res. 2002, 15, 197–198. [Google Scholar] [CrossRef]
- Moher, D.; Pham, B.; Jones, A.; Cook, D.J.; Jadad, A.R.; Moher, M.; Tugwell, P.; Klassen, T.P. Does Quality of Reports of Randomised Trials Affect Estimates of Intervention Efficacy Reported in Meta-Analyses? Lancet 1998, 352, 609–613. [Google Scholar] [CrossRef]
- Furlan, A.D.; Pennick, V.; Bombardier, C.; Van Tulder, M. Updated Method Guidelines for Systematic Reviews in the Cochrane Back Review Group. Spine 2009, 34, 1929–1941. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V.; Collaboration, C. Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0; Wiley-Blackwell: Chichester, UK, 2008. [Google Scholar]
- Barton, C.J.; Webster, K.E.; Menz, H.B. Evaluation of the Scope and Quality of Systematic Reviews on Nonpharmacological Conservative Treatment for Patellofemoral Pain Syndrome. J. Orthop. Sports Phys. Ther. 2008, 38, 529–541. [Google Scholar] [CrossRef] [PubMed]
- McHugh, M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012, 22, 276–282. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R. ROBIS: A New Tool to Assess Risk of Bias in Systematic Reviews Was Developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, A.; Tennant, B.; Ribeiro-Lucas, I.; Vaux-Bjerke, A.; Piercy, K.; Bloodgood, B. Umbrella and Systematic Review Methodology to Support the 2018 Physical Activity Guidelines Advisory Committee. J. Phys. Act. Health 2018, 15, 805–810. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A.; Lee, Y.Y.; Norman, R.E.; Vos, T. Meta-Analysis of Prevalence. J. Epidemiol Community Health 2013, 67, 974–978. [Google Scholar] [CrossRef]
- Barendregt, J.J.; Doi, S.A. MetaXL User Guide; Version 5.3; EpiGear International Pty Ltd.: Queensland, Australia, 2016. [Google Scholar]
- Lewis, S.; Clarke, M. Forest Plots: Trying to See the Wood and the Trees. Br. Med. J. 2001, 322, 1479–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedges, L.V. Estimation of Effect Size from a Series of Independent Experiments. Psychol. Bull. 1982, 92, 490–499. [Google Scholar] [CrossRef]
- Hopkins, W.; Marshall, S.; Batterham, A.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suurmond, R.; van Rhee, H.; Hak, T. Introduction, Comparison, and Validation of Meta-Essentials: A Free and Simple Tool for Meta-Analysis. Res. Synth. Methods 2017, 8, 537–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring Inconsistency in Meta-Analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing Heterogeneity in Meta-Analysis: Q Statistic or I2 Index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DerSimonian, R.; Laird, N. Meta-Analysis in Clinical Trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Machado-Oliveira, L.; da Silva Gauto, Y.O.; de Santana Neto, F.J.; da Silva, M.G.; Germano-Soares, A.H.; Diniz, P.R.B. Effects of Different Exercise Intensities on Headache: A Systematic Review. Am. J. Phys. Med. Rehabil. 2020, 99, 390–396. [Google Scholar] [CrossRef]
- Chaibi, A.; Russell, M.B. Manual Therapies for Primary Chronic Headaches: A Systematic Review of Randomized Controlled Trials. J. Headache Pain 2014, 15, 67. [Google Scholar] [CrossRef] [Green Version]
- Racicki, S.; Gerwin, S.; DiClaudio, S.; Reinmann, S.; Donaldson, M. Conservative Physical Therapy Management for the Treatment of Cervicogenic Headache: A Systematic Review. J. Man. Manip. Ther. 2013, 21, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Fernández-De-Las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Miangolarra, J.C.; Barriga, F.J.; Pareja, J.A. Are Manual Therapies Effective in Reducing Pain from Tension-Type Headache?A Systematic Review. Clin. J. Pain 2006, 22, 278–285. [Google Scholar] [CrossRef]
- Bronfort, G.; Assendelft, W.J.; Evans, R.; Haas, M.; Bouter, L. Efficacy of Spinal Manipulation for Chronic Headache: A Systematic Review. J. Manip. Physiol. Ther. 2001, 24, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Cumplido-Trasmonte, C.; Fernández-González, P.; Alguacil-Diego, I.M.; Molina-Rueda, F. Terapia Manual En Adultos Con Cefalea Tensional: Revisión Sistemática. Neurologia 2018. [Google Scholar] [CrossRef] [PubMed]
- Brønfort, G.; Haas, M.; Evans, R.L.; Goldsmith, C.H.; Assendelft, W.J.; Bouter, L.M. Non-Invasive Physical Treatments for Chronic/Recurrent Headache. Cochrane Database Syst. Rev. 2009, 2017. [Google Scholar] [CrossRef] [Green Version]
- Fernández-de-las-Peñas, C.; Alonso-Blanco, C.; Cuadrado, M.L.; Pareja, J.A. Spinal Manipulative Therapy in the Management of Cervicogenic Headache. Headache J. Head Face Pain 2005, 45, 1260–1263. [Google Scholar] [CrossRef] [PubMed]
- Maistrello, L.F.; Rafanelli, M.; Turolla, A. Manual Therapy and Quality of Life in People with Headache: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Curr. Pain Headache Rep. 2019, 23, 1–14. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Z.; Wei, N.; Chang, W.; Chen, W.; Sui, H.J. Effectiveness of Physical Therapy on the Suboccipital Area of Patients with Tension-Type Headache. Medicine 2019, 98, e15487. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, J.; De Pauw, J.; Van Soom, T.; Michiels, S.; Versijpt, J.; Van Breda, E.; Castien, R.; De Hertogh, W. The Effect of Aerobic Exercise on the Number of Migraine Days, Duration and Pain Intensity in Migraine: A Systematic Literature Review and Meta-Analysis. J. Headache Pain 2019, 20, 16. [Google Scholar] [CrossRef] [PubMed]
- Falsiroli Maistrello, L.; Geri, T.; Gianola, S.; Zaninetti, M.; Testa, M. Effectiveness of Trigger Point Manual Treatment on the Frequency, Intensity, and Duration of Attacks in Primary Headaches: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Neurol. 2018, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Luedtke, K.; Allers, A.; Schulte, L.H.; May, A. Efficacy of Interventions Used by Physiotherapists for Patients with Headache and Migraine—Systematic Review and Meta-Analysis. Cephalalgia 2016, 36, 474–492. [Google Scholar] [CrossRef]
- Mesa-Jiménez, J.A.; Lozano-López, C.; Angulo-Díaz-Parreño, S.; Rodríguez-Fernández, Á.L.; De-La-Hoz-Aizpurua, J.L.; Fernández-De-Las-Peñas, C. Multimodal Manual Therapy vs. Pharmacological Care for Management of Tension Type Headache: A Meta-Analysis of Randomized Trials. Cephalalgia 2015, 35, 1323–1332. [Google Scholar] [CrossRef] [PubMed]
- Coelho, M.; Ela, N.; Garvin, A.; Cox, C.; Sloan, W.; Palaima, M.; Cleland, J.A. The Effectiveness of Manipulation and Mobilization on Pain and Disability in Individuals with Cervicogenic and Tension-Type Headaches: A Systematic Review and Meta-Analysis. Phys. Ther. Rev. 2019, 24, 29–43. [Google Scholar] [CrossRef]
- Fernandez, M.; Moore, C.; Tan, J.; Lian, D.; Nguyen, J.; Bacon, A.; Christie, B.; Shen, I.; Waldie, T.; Simonet, D.; et al. Spinal Manipulation for the Management of Cervicogenic Headache: A Systematic Review and Meta-Analysis. Eur. J. Pain (UK) 2020, 24, 1687–1702. [Google Scholar] [CrossRef] [PubMed]
- González-Oria, C.; Belvís, R.; Cuadrado, M.L.; Díaz-Insa, S.; Guerrero-Peral, A.L.; Huerta, M.; Irimia, P.; Láinez, J.M.; Latorre, G.; Leira, R.; et al. Document of Revision and Updating of Medication Overuse Headache (MOH). Neurologia 2021, 36, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Grazzi, L.; Toppo, C.; D’Amico, D.; Leonardi, M.; Martelletti, P.; Raggi, A.; Guastafierro, E. Non-Pharmacological Approaches to Headaches: Non-Invasive Neuromodulation, Nutraceuticals, and Behavioral Approaches. Int. J. Environ. Res. Public Health 2021, 18, 1503. [Google Scholar] [CrossRef]
- Ashina, M.; Buse, D.C.; Ashina, H.; Pozo-Rosich, P.; Peres, M.F.; Lee, M.J.; Terwindt, G.M.; Halker Singh, R.; Tassorelli, C.; Do, T.P.; et al. Migraine: Integrated Approaches to Clinical Management and Emerging Treatments. Lancet 2021. [Google Scholar] [CrossRef]
- Krøll, L.S.; Hammarlund, C.S.; Linde, M.; Gard, G.; Jensen, R.H. The Effects of Aerobic Exercise for Persons with Migraine and Co-Existing Tension-Type Headache and Neck Pain. A Randomized, Controlled, Clinical Trial. Cephalalgia 2018, 38, 1805–1816. [Google Scholar] [CrossRef]
- Song, T.J.; Chu, M.K. Exercise in Treatment of Migraine Including Chronic Migraine. Curr. Pain Headache Rep. 2021, 25, 14. [Google Scholar] [CrossRef]
- Hanssen, H.; Minghetti, A.; Magon, S.; Rossmeissl, A.; Rasenack, M.; Papadopoulou, A.; Klenk, C.; Faude, O.; Zahner, L.; Sprenger, T.; et al. Effects of Different Endurance Exercise Modalities on Migraine Days and Cerebrovascular Health in Episodic Migraineurs: A Randomized Controlled Trial. Scand. J. Med. Sci. Sports 2018, 28, 1103–1112. [Google Scholar] [CrossRef]
- Eslami, R.; Parnow, A.; Pairo, Z.; Nikolaidis, P.; Knechtle, B. The Effects of Two Different Intensities of Aerobic Training Protocols on Pain and Serum Neuro-Biomarkers in Women Migraineurs: A Randomized Controlled Trail. Eur. J. Appl. Physiol. 2021, 121, 609–620. [Google Scholar] [CrossRef] [PubMed]
- Benatto, M.T.; Bevilaqua-Grossi, D.; Carvalho, G.F.; Bragatto, M.M.; Pinheiro, C.F.; Lodovichi, S.S.; Dach, F.; Fernandez-de-Las-Penas, C.; Florencio, L.L. Kinesiophobia Is Associated with Migraine. Pain Med. 2019, 20, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Amiri, M.; Jull, G.; Bullock-Saxton, J.; Darnell, R.; Lander, C. Cervical Musculoskeletal Impairment in Frequent Intermittent Headache. Part 2: Subjects with Concurrent Headache Types. Cephalalgia 2007, 27, 891–898. [Google Scholar] [CrossRef]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial Trigger Points in Migraine and Tension-Type Headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef]
- Watson, D.H.; Drummond, P.D. Head Pain Referral during Examination of the Neck in Migraine and Tension-Type Headache. Headache 2012, 52, 1226–1235. [Google Scholar] [CrossRef] [PubMed]
- Pielsticker, A.; Haag, G.; Zaudig, M.; Lautenbacher, S. Impairment of Pain Inhibition in Chronic Tension-Type Headache. Pain 2005, 118, 215–223. [Google Scholar] [CrossRef]
- Varkey, E.; Hagen, K.; Zwart, J.A.; Linde, M. Physical Activity and Headache: Results from the Nord-Trøndelag Health Study (HUNT). Cephalalgia 2008, 28, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Davenport, S.; Dickinson, A.; Minns Lowe, C. Therapy-Based Exercise from the Perspective of Adult Patients: A Qualitative Systematic Review Conducted Using an Ethnographic Approach. Clin. Rehabil. 2019, 33, 1963–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olesen, J.; Bes, A.; Kunkel, R.; Lance, J.W.; Nappi, G.; Pfaffenrath, V.; Rose, F.C.; Schoenberg, B.S.; Soyka, D.; Tfelt-Hansen, P.; et al. The International Classification of Headache Disorders, 3rd ed (Beta Version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [Green Version]
- Bogduk, N. Cervicogenic Headache: Anatomic Basis and Pathophysiologic Mechanisms. Curr. Pain Headache Rep. 2001, 5, 382–386. [Google Scholar] [CrossRef]
- Becker, W.J. Cervicogenic Headache: Evidence That the Neck Is a Pain Generator. Headache 2010, 50, 699–705. [Google Scholar] [CrossRef]
- Hall, T.M.; Robinson, K.W.; Fujinawa, O.; Akasaka, K.; Pyne, E.A. Intertester Reliability and Diagnostic Validity of the Cervical Flexion-Rotation Test. J. Manip. Physiol. Ther. 2008, 31, 293–300. [Google Scholar] [CrossRef]
- Zito, G.; Jull, G.; Story, I. Clinical Tests of Musculoskeletal Dysfunction in the Diagnosis of Cervicogenic Headache. Man. Ther. 2006, 11, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Togha, M.; Bahrpeyma, F.; Jafari, M.; Nasiri, A. A Sonographic Comparison of the Effect of Dry Needling and Ischemic Compression on the Active Trigger Point of the Sternocleidomastoid Muscle Associated with Cervicogenic Headache: A Randomized Trial. J. Back Musculoskelet. Rehabil. 2020, 33, 749–759. [Google Scholar] [CrossRef]
- Haas, M.; Bronfort, G.; Evans, R.; Schulz, C.; Vavrek, D.; Takaki, L.; Hanson, L.; Leininger, B.; Neradilek, M.B. Dose-Response and Efficacy of Spinal Manipulation for Care of Cervicogenic Headache: A Dual-Center Randomized Controlled Trial. Spine J. 2018, 18, 1741–1754. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, M.P.; Donaldson, M.; Griswold, D.; Learman, K.E.; Garcia, A.N.; Learman, S.M.; Cleland, J.A. The Effects of Exercise Dosage on Neck-Related Pain and Disability: A Systematic Review with Meta-Analysis. J. Orthop. Sports Phys. Ther. 2020, 50, 607–621. [Google Scholar] [CrossRef] [PubMed]
- Ylinen, J.; Nikander, R.; Nykänen, M.; Kautiainen, H.; Häkkinen, A. Effect of Neck Exercises on Cervicogenic Headache: A Randomized Controlled Trial. J. Rehabil. Med. 2010, 42, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, L.L.; Mortensen, O.S.; Zebis, M.K.; Jensen, R.H.; Poulsen, O.M. Effect of Brief Daily Exercise on Headache among Adults--Secondary Analysis of a Randomized Controlled Trial. Scand. J. Work Environ. Health 2011, 37, 547–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).