Abstract
Legumes are an essential food source worldwide. Their high-quality proteins, complex carbohydrates, dietary fiber, and relatively low-fat content make these an important functional food. Known to possess a multitude of health benefits, legume consumption is associated with the prevention and treatment of cardiovascular diseases (CVD). Legume crude protein isolates and purified peptides possess many cardiopreventive properties. Here, we review selected economically valued legumes, their taxonomy and distribution, biochemical composition, and their protein components and the mechanism(s) of action associated with cardiovascular health. Most of the legume protein studies had shown upregulation of low-density lipoprotein (LDL) receptor leading to increased binding and uptake, in effect significantly reducing total lipid levels in the blood serum and liver. This is followed by decreased biosynthesis of cholesterol and fatty acids. To understand the relationship of identified genes from legume studies, we performed gene network analysis, pathway, and gene ontology (GO) enrichment. Results showed that the genes were functionally interrelated while enrichment and pathway analysis revealed involvement in lipid transport, fatty acid and triglyceride metabolic processes, and regulatory processes. This review is the first attempt to collate all known mechanisms of action of legume proteins associated with cardiovascular health. This also provides a snapshot of possible targets leading to systems-level approaches to further investigate the cardiometabolic potentials of legumes.
1. Introduction
Food is considered as a good source of biologically active compounds that exhibit a great impact to improve the overall status of human health. Individuals worldwide gradually shift their diet to foods rich in vitamins, polyphenols, carotenoids, essential oils, proteins, and peptides [1]. Interestingly, bioactive peptides found in different food have been a subject of many studies to understand their potential effects on the prevention of CVD, hypertension, dyslipidemia, cancer, and microbial infections [2].
Cardiovascular diseases are the number one cause of death globally and accounts for 17.9 million lives lost each year [3]. An estimated 130 million people are predicted to contract this disease by 2035 in the United States alone [4,5]. In 2016, 17.6 million deaths were recorded globally. Highest mortality rates were found to be in Eastern Europe and Central Asia. Categories of CVD that affect human health are coronary artery disease, congenital heart disease, stroke, heart failure, and rheumatic heart disease [6]. Coronary artery disease and stroke account for 80% and 75% of CVD deaths in males and females, respectively [6]. Epidemiologic studies suggest that increased consumption of fruits and vegetables coupled with a healthy lifestyle may lower the risk of CVD. Because of this, the development of nutritionally enhanced and functional foods to address health concerns is rapidly increasing [7].
Functional foods are those that produce beneficial health effects to humans beyond nutrition. These foods include legumes, berries, and herbs that are rich in vitamins, minerals, antioxidants, and proteins. Their consumption appears to be associated with decreased occurrence of cardiovascular and gastrointestinal diseases [8]. As recorded in 2018, the global market of functional foods is growing very rapidly with a projected market estimates of 150 billion US dollars [9]. Although many foods exhibit therapeutic potentials, legume-based food is currently being valued worldwide due to their importance, functionality, and their availability as a meat alternative. As a functional food, legumes contain approximately 20–45% proteins, 60% complex carbohydrates, 5–37% dietary fiber and have a relatively low fat content [10]. Published reports showed that bioactive peptides isolated from various legumes demonstrate hypocholesterolemic, antihypertensive, antiatherogenic, and hypoglycemic properties among others. For instance, kidney beans (Phaseolus vulgaris L.) and peanuts (Arachis hypogaea L.) showed significant cholesterol lowering effects in vitro [11]. Peptides extracted from hyacinth bean (Lablab purpureus L.), mungbean (Vigna radiata) and cowpea seed (Vigna unguiculata) exhibit remarkable antihypertensive activity [12,13,14,15].
To date, pharmacological therapy is the first-in-line treatment for patients with CVD. Effective and new therapies such as aspirin, lipid-lowering treatments, and antihypertensive drugs are commonly used [16]. Over the years, non-pharmacologic interventions such as the Mediterranean diet and the Dietary Approaches to Stop Hypertension (DASH) also present important contributions to the management of CVD that significantly impact an individual’s stability and quality of life. These interventions include the incorporation of legumes, vegetables, fruits, and dairy foods in the diet to proffer cardiovascular protective effects [17]. However, the mechanisms on how these bioactive peptides and other functional components from legumes provide beneficial health effects are still uncertain though several clinical trials exhibited a positive correlation of such legume intake and CVD prevention. Some other bioactive peptides have the ability to inhibit the angiotensin-I converting enzyme (ACE1) which has a vital role in increasing blood pressure in the body by converting angiotensin-I to angiotensin-II [1,18]. Inhibiting ACE1 can effectively reduce the risk of hypertension and, thereby, prevent heart diseases.
Food legumes have been acknowledged as a good source of ACE-inhibitory peptides. Kinetic studies reveal that many of these peptides act as competitive inhibitors to the active site of ACE, indicating the possible peptide structural similarities to that of the endogenous substrate of the enzyme.
Legume proteins are also potential sources of hypocholesterolemic peptides. For instance, glycinin from soy is the parent protein of the LPYPR hypocholesterolemic peptide. This peptide is structurally similar to enterostatin, a hypocholesterolemic peptide intrinsically secreted in the gastric and intestinal mucosa [19,20]. Aside from being a structural homologue of enterostatin, LPYPR along with another glycinin derived peptide, IAVPGEVA, are also competitive inhibitors of 3-hydroxy-3-methylglutaryl CoA (HMG-CoA) reductase, the main enzyme targeted for the regulation of cholesterol biosynthesis. Structure-activity studies identified some key features in the effectivity of both peptides, namely, their hydrophobic region, having less than four amino acids in length, and the presence of a proline residue at any position except at the N-terminal [21,22].
Inhibition of 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (HMG-CoA reductase) and the ability to bind bile acids could also prevent hypercholesterolemia by inducing cholesterol conversion to additional bile salts reducing low density lipoprotein-cholesterol (LDL-C) levels. There are also other bioactive peptides that can reduce plasma triglycerides (TAGs) by lowering hepatic very low-density lipoprotein (VLDL) synthesis. Hence, the further integration of legume-based diets can provide beneficial effects against the severe effects of CVD compared to some conventional therapies supplemented with reduced health benefits and adverse side effects with long-term use.
This review explores the importance of legumes as a functional food and the effects of some of their proteins and isolated bioactive peptides to reduce and/or prevent CVDs and their associated risk factors.
2. General Characteristics of Legumes
2.1. Legumes Taxonomy and Distribution
Legumes are flowering plants under the family Leguminosae (or Fabaceae) which includes around 770 genera and over 19,500 species [23]. Legumes is a collective term for all plants under the family of Leguminosae including both agricultural crops and weeds. It is the third largest family of angiosperms next to the sunflower (Asteraceae, 23,000 species) and the orchid (Orchidaceae, 22,000 species) families [24]. It includes several horticultural varieties and many species are harvested as food, medicine, dye, oil, timber, and for soil enrichment [25].
The previous classification of the legume subfamilies was based on a few conspicuous floral characteristics; however, these characteristics are prone to evolutionary modifications. By 2017, the Legume Phylogeny Working Group published the new phylogenetic classification system for the Leguminosae based on morphological and molecular data. The family now consists of six sub-families: Cercidoideae, Detarioideae, Duparquetioideae, Caesalpinioideae, Faboideae (Papilionoideae), and Dialioideae [23]. Among the sub-families, Papilionoideae is the most widespread and includes more than 60 domesticated grain legumes that are grown and consumed all over the world [25,26]. Legumes are valued worldwide as an inexpensive meat alternative providing a rich source of protein and micronutrients that can benefit health and livelihood particularly in developing countries [24]. Table 1 lists some examples of cultivated grain legumes along with their distribution and uses that are described in this manuscript [27].

Table 1.
Some examples of members of the Leguminosae family, their distribution, and their uses described in this manuscript [27].
2.2. Biochemical Composition of Legumes
Generally, legume seeds contain relatively high amounts of proteins ranging as low as 16–26% (moisture free, dry mass basis) in moth bean, chickpea, common bean, and hyacinth bean [28,29] to as high as 34–47% (moisture free, dry mass basis) in soybean, lupin, jack bean, and peanut, which are comparable to meats (18–25%) but are higher than in other food types such as cereals (7–13%) (Table S1) [30,31].
Legume carbohydrate content varies widely across species, with some exceeding 60% (moisture free, dry mass basis) of the total seed components such as those in common bean, hyacinth bean, lentil, moth bean, and mungbean. The fat and oil content also varies considerably among species ranging between 1% to 49% (moisture free, dry mass basis) in lentils and peanuts, respectively. The lipid profiles of seed legumes also show substantial combinations of saturated and unsaturated fatty acids, including omega-3 and -6 fatty acids, that may ultimately affect the shelf life and health benefits of the oil products derived from these legumes. The moisture and ash contents are generally less than 15% and 10%, respectively, of the total seed components (Table S1) [28,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61].
3. Legumes as Functional Food for Cardiovascular Diseases
Over the last few years, consumption of plant proteins including those from various legumes such as pea, lupin, cowpea, jack bean, mungbean, chickpea, lentils, and soybean had increased due to their numerous associated health benefits [30,62,63,64,65]. Consumption of legumes was found to be beneficial in the prevention and treatment of various diseases including CVD [30,62]. Frequent legume ingestion was shown to reduce serum total cholesterol (TC), LDL-C, and TAG levels and increase high-density-lipoprotein-cholesterol (HDL-C) levels [62,66]. Bioactive peptides from storage proteins such as albumins and globulins are responsible for several properties relating to disease prevention including several cardiovascular related conditions [67]. For instance, protein hydrolysates from the common bean showed hypocholesterolemic activity by preventing oxidative stress and inflammation in vivo through the regulation of adipocytokines. In addition, these black bean protein hydrolysates showed hypoglycemic activity by inhibiting the GLUT2 and SGLT1 glucose transporters [68].
Other legume bioactive components that are beneficial in reducing the risk of CVD are polyphenols. These are naturally occurring substances that vary in structure and are commonly enriched in fruits, vegetables, tea, and wine. Flavonoids are made up most of different polyphenols such as flavones, flavanol, flavonols, and flavononones. Daidzein and genistein, two of the major isoflavones from soybeans, showed activity in regulating pancreatic beta cells in organisms with insulin-dependent diabetes. Moreover, ingestion of soy isoflavones resulted in increased adenosine monophosphate protein kinase (AMPK) activation which regulates lipid and glucose metabolism [69,70]. Soybean genistein was also reported to increase endothelial nitric oxide synthase (eNOS) gene transcription and protein synthesis in human vascular endothelial cells which produced protective effects and prevented the occurrence of hypertension [71]. In addition, other phenolic compounds from the common bean lowered lipid levels by inhibiting lipid accumulation in 3T3-L1 adipocytes cells [68]. Saponins from chickpeas were found to inhibit pancreatic lipase [68]. Rats fed with the common bean (P. vulgaris L.) manifested weight loss, improved plasma lipid profiles, and decreased plasma TC and LDL-C without affecting HDL-C and total TAG levels even just after several hours post-feeding [72]. Such rats also showed reduced visceral adiposity and adipocyte size while increasing hepatic carnitine palmitoyl-transferase I (CPT-1), a key enzyme regulating long chain fatty acid oxidation resulting in lowered levels of circulating TAGs [73].
A pulse-rich diet consisting of two daily servings of beans, chickpeas, lentils, or peas fed for two months to more than 100 people who are 50 years of age or above lowered their total cholesterol (TC) by 8.3% and low density lipoprotein-cholesterol (LDL-C) by 7.9% and was ascribed to decrease the risk against CVD [74]. In hypercholesterolemic adults, consumption of a single half-cup serving of Phaseolus vulgaris L. for eight weeks led to decreased serum LDL-C and TC by 5% and 6%, respectively, reducing coronary heart disease risk by 5–12% [75]. The risk of death is reduced by 8% for every 20 g intake of legumes [76]. Men and women consuming legumes four or more times per week have a lowered risk of coronary heart disease by 22% and CVD by 11% compared to people who consume legumes only once a week [77].
There is only a handful of literature describing how the recipient’s genotypic variation influences the cardiovascular benefits of these legumes. Jang et al., (2010) [78] found significant variation in TAG and HDL-C levels associated with the T->C allelic change at position 1131 of the Apolipoprotein A5 (ApoA5) gene (ApoA5-1131T->C). ApoA5-overexpressing mice showed 65% reduced plasma TAG concentrations while the ApoA5-knockout mice exhibited approximate four times higher TAG levels [78,79]. Based on human Apo5 polymorphism studies, the most frequently analyzed ApoA5 variant is 1131T->C with the C allele associated with higher TAG levels [80,81,82,83]. This SNP was observed to interact with various dietary factors especially dietary fat that affect TAG concentrations such as found in diabetic dyslipidemia [84] and is associated with increased coronary arterial disease (CAD) risk [80,81]. In the work of Jang et al., (2010) [78], modification of a third of the participants’ diets from refined rice to legumes as a carbohydrate source three times a day, supplementation of increased vegetable intake (30–70 g/unit/day) and a regular 30-min walk after dinner each day (Dietary Intervention and Regulatory Exercise, DIRE) for 12 weeks resulted in the reduced rate of decrease in TAG levels from 20% for the TT genotype to only 11.7% connected to the TC/CC genotype (p < 0.001) and for HDL-C from 9% for the TT genotype to 5% associated with the TC/CC genotype (p < 0.001) while ApoA5 plasma concentrations increased by 7.37% (p < 0.1) for the TT genotype, but was shown to be statistically insignificant to the 5.12% increase associated with the TC/CC genotype [85]. The same group later studied humans with impaired fasting glucose and those who were newly diagnosed with Type II diabetes whose diets were modified to replace refined rice intake with an equal proportion of legumes, barleys, and whole grains thrice a day and supplementation of increased vegetable intake (30–70 g/unit/day) for 12 weeks [86]. In this study, the reduction of TAG levels decreased from 11.6% for the TT genotype to only 7.2% for the TC/CC genotype (p < 0.05) while there is an increase, though not statistically significant, in HDL-C levels from 4.97% correlated with the TT genotype to only 5.35% when associated with the C allele. ApoA5 plasma concentrations were detected to be 10.5% for the TT genotype while it was found to be 13.5% when associated with the C allele [86].
Not only does consumption of legume seeds associated with cardiovascular health but also the ingestion of their specific seed proteins. Ferreira et al., (2015) [87] showed that rats fed with either the 7S globulin from cowpea or adzuki beans decreased serum TC and non-HDL-C while increasing HDL-C comparable to the simvastatin-fed group. They also found that these 7S cowpea proteins lowered hepatic cholesterol and TAG levels.
Over the last few years, consumption of plant proteins including those from various legumes such as pea, lupin, cowpea, jack bean, mungbean, chickpea, lentils, and soybean had increased due their numerous associated health benefits [30,62,63,64,65]. Legume proteins demonstrate antioxidant [63,64], blood pressure (BP) lowering [88,89,90,91,92], and anti-thrombotic properties. These proteins also reduce the risk of CVD by lowering serum TC due to decreased synthesis and secretion of hepatic TAG and of serum LDL-C, TAGs and VLDL while increasing serum HDL-C levels and hepatic uptake of VLDL [90,93,94,95,96,97,98,99]. We, herein, review various legumes that are found to be associated with cardiovascular health through the ingestion of the legumes themselves or by the introduction of certain protein groups and/or specific proteins into the diet as summarized in Table 2.

Table 2.
Functional protein components of selected legumes associated with cardiovascular-related conditions discussed in this manuscript.
3.1. Soybean (Glycine Max L.)
3.1.1. Benefits of Soybeans Relating to Cardiovascular Health
Soybean (Glycine max L.) is one of the most abundant plant sources constituting 36–56% protein [129]. Soybean proteins are categorized as complete proteins for human health and growth since they are highly digestible, supply all of the essential amino acids, and have a Protein Digestibility Corrected Amino Acid Score (PDCAAS) score of 1.0 which is comparable to casein and egg protein. These soybean proteins, though, are low in methionine:glycine and lysine:arginine ratios compared to casein [129]. Soybean proteins are ascribed to reduce cholesterol levels, control lipid metabolism and fatty acid oxidation, improve glucose homeostasis, are anti-hypertensive and anti-oxidative, and have the ability to control hunger and obesity [112,113,129,135,146,195,196,197,198,199,200]. Alcalase-digested soy proteins yield peptides (less than 3kDa) that were found to inhibit lipid peroxidation. These antioxidative soybean peptides were high in hydrophobic amino acids such as phenylalanine which are converted into tyrosine upon hydroxyl attack that are useful to scavenge hydroxyl radicals [121,122,123,124,125].
Cardiovascular Benefits of Soybean Proteins
Consumption of soybean proteins (47 g per day) was initially found to decrease TC, LDL levels, and TAGs as first evidenced in 1967 [107,108]. A meta-analysis of 38 studies in 1995 showed that soybean protein consumption was associated with decreased plasma TC, LDL-C, and TAG concentrations by 9.3%, 12.9%, and 10.5%, respectively, after ingestion of averagely 47 g soy proteins per day [109]. Several authors found that rats fed with soybean protein isolates had significantly lower serum TC levels than those fed with casein [112,113]. Following a meta-analysis of Reynolds et al., (2006) [96], soy proteins were associated with the significant reduction of TC, LDL-C, and TAGs while increasing HDL-C levels. Jenkins (2010) [111] showed that the consumption of 25 g of soy protein in humans reduced LDL-levels by 4%.
In 1998, Nagata et al. found that there was an inverse correlation between increasing soybean protein intake and serum TC levels in the Japanese population and that this same trend was found by Ho et al., (2000) [114,115] in a Chinese population. Moreover, in women, soybean protein consumption of 3–185 mg/day were ascribed to significantly decrease serum TC by 3.8%, LDL-C by 5.3%, and TAGs by 7.3% while significantly increasing serum HDL-C by 3% in a meta-analysis of 23 randomized studies between 1995 through 2002 [201]. Soy proteins were also seen to lower cholesterol in children and in renal patients [116,117].
Feeding studies of soybean proteins in various laboratory animals showed improvement of dyslipidemia. Mice (db/db) fed with black soybean (Phaseolus vulagris L.) peptides for five weeks exhibited downregulation of the hepatic fatty acid synthase (FASN), thereby, resulting in reduced TAG and HDL levels [106]. A significant anti-atheromatous effect (67%) was evident in female mice fed with soy protein containing diets [199] as well as the reduction in blood pressure [88,89,91,202,203]. A 20% alcohol washed-soy protein diet fed to Sprague Dawley rats markedly decreased plasma TAGs levels compared to the control casein diet [126].
Singh (2014) [129] reviewed that the hypocholesterolemic activity of the soybean peptides involved the stimulation of the bile acid secretion; changing the hepatic metabolism of cholesterol due to this increased fecal bile acid secretion and the enhanced the removal of LDL in young male cynomolgus macaques. In rabbits, soybean proteins stimulated less neutral steroids and bile acid production and enhanced the excretion of cholesterol leading to their decreased levels [128]. Further, Sugano et al., (1988 and 1990) [118,127] found that enzyme-digested soybean proteins yielded an undigested, insoluble, high-molecular-weight protein fraction capable of binding bile acids and enhanced the excretion of acidic and neutral steroids leading to the significant decrease in plasma and hepatic cholesterol levels in rats. In humans, this high molecular weight fraction also increased bile acid secretion, raised plasma HDL-C, and decreased LDL-C levels compared to individuals who consumed either casein- or a soy-protein diet [118,127,131]. In both rats and humans, soybean peptides stimulated fecal steroid excretion that led to lower serum TC levels [118,119].
Soybean proteins also decreased plasma and hepatic TAGs due to the reduced transcription and translation of the hepatic sterol regulatory element binding protein (SREBP)-1 either through an insulin-dependent or -independent pathway [100,148,204,205,206,207]. These SREBPs are basic helix-loop-helix leucine zipper (bHLH-leu) and membrane-bound transcription factors that activate about 30 genes involved in fatty acid and cholesterol biosynthesis [158,159,208,209,210]. There are three SREBP isoforms, SREBP-1 (1a and 1c), which activate fatty acid synthesis and result from the altered transcription splicing of the SREBF1 gene, and SREBP-2, which activate genes in the cholesterol pathway. These SREBPs are further regulated through autophagy and by the PI3K-mTORC1-AKT pathway through an unknown mechanism [158,208]. These SREBPs undergo a two-step proteolytic cleavage in the Golgi apparatus to release their N-terminal fragment that still contains the bHLH-leu motif. This N-terminal fragment enters the nucleus and binds to steroid response elements (SREs) found in a number of genes involved in cholesterol biosynthesis such as squalene synthase and HMG-CoA reductase [158,208]. Aside from the bHLH-leu motif, these SREBPs also contain an internal intronic region that produces miR-33 that reduces translation of other fatty acid oxidation genes including ATP binding cassette subfamily A member 1 (ABCA1) and ATP-binding cassette sub-family G member 1-like (ABCG). This miR-33 activity control fatty acid oxidation genes and is complementary to the activity of the SREBP-N-terminal domain found in SREBP-1a, -1c, and -2 described herein [208].
Via the insulin-dependent pathway, soybean proteins decrease insulin, thereby, reducing SREBP-1 expression in the liver and in rat primary hepatocytes [103], and increase the removal of hepatic insulin [204]. Through the insulin-independent pathway, soy proteins reduce SREBP-1 expression through the negative regulation of liver X receptor (LXR) mRNA expression [206] In either case, the reduction of SREBP-1 by the soybean proteins reduces the mRNA of various hepatic lipogenic enzymes such as glucose-6-phosphate dehydrogenase (G6PDH), malate enzyme, FASN, acetyl-CoA carboxylase alpha, and beta (ACCα and ACCβ) and ATPase/ATP synthase reducing the biosynthesis of endogenous fatty acids [100,126,149,205,206,211,212,213,214]. Soybean proteins were found to reduce malate enzyme and fatty acid synthetase gene expression in rats [100,205,213]. ACC regulation mediated by soybean proteins is through the thyroid-receptor (TR)-dependent pathway, whereby thyroid hormones regulate the binding to the thyroid hormone receptor response elements found on the ACC promoter. Through the SREBP-dependent pathway, soybean protein isolates inhibit the binding of SREBP-1 to one of the two promoters controlling ACC-alpha gene expression [103,215,216]. Soybean proteins also decrease dephosphorylation of the mitochondrial ATPase/ATP synthase β subunit, thus, increasing the enzyme’s activity [214].
The hypocholesterolemic benefit of soybean proteins was also associated with the control of other cholesterol synthesis genes and their translated proteins. Soybean isolates significantly reduced HMG-CoA reductase in rat livers reducing cholesterol biosynthesis, thereby, decreasing TC and VLDL-C [100,101]. Consumption of soybean proteins also significantly reduced the severity of retinoic-acid induced hypertriglyceridemia in rats through the interaction with the retinoid receptors [102,217]. Xiao et al., (2008) found that soybean isolate consumption in rats elevated both hepatic c (RARβ) translation and/or through its post-translational modification(s), thus, suppressing its ability to bind to the DNA of its target genes to alleviate this retinoid-induced hypertriglyceridemia [103,104,218]. Soybean proteins also stimulated LDL receptor gene expression in cultured human hepatic cells [116,145,150] and in various animals such as rats [101,219], in moderate hypercholesterolemics [220], and in those with familial hypercholesterolemia and hypercholesterolemic type 2 diabetic patients [221].
Aside from the reduced hepatic TAG levels, ingestion of soybean proteins also decreased epididymal adipose tissue weight and increased the expression of the skeletal muscle carnithine-palmitoyl transferase 1 (CPT1), a key enzyme involved in the transfer of fatty acids into the mitochondria to promote hepatic β-oxidation [222] and of the peroxisome proliferator-activated receptor-alpha (PPARα) in male Sprague Dawley rats [223]. It is the activation PPARα gene expression that leads to the activation of CPT1 in the rat liver [206]. CPT-1 is part of the mitochondrial fatty acid β-oxidation system that permits acyl-CoA to be transferred through the mitochondrial matrix and cross into the inner mitochondrial membrane to enter this fatty acid pathway [224]. In dogs, CPT-1 inhibition regulates this β-oxidation system and slows cardiac left ventricle remodeling and the deterioration of the function in pacing-induced heart failure [225].
The peroxisome proliferator-activated receptors (PPARs) including alpha (α), beta (β), delta (δ), and gamma (γ) are transcription activators that regulate glucose and lipid metabolism [226], including those for the 3-hydroxyl-CoA-dehydrogenase (HAD) and CPT-1 [226,227,228]. Pellieux et al., (2009) found that PPARα, and β/δ inhibit the angiotensin-II mediated reduction of the fatty acid β-oxidation pathway by activating the TNF-α in adult rat cardiac (ARC) myocytes [229].
PPARα regulates lipid metabolism in various tissues such as the heart, liver and skeletal muscle to decrease circulating lipid levels and improve hyperlipidemia. Stimulation of this transcription activator suppresses post-prandial lipidemia by enhancing fatty acid β-oxidation in enterocytes, thus, reducing circulating lipid levels [230]. Much like the other PPARs, PPARα promotes the expression of key enzymes involved in fatty acid oxidative metabolism [231,232,233]. In the nucleus of various cells including the myocardium, PPARα heterodimerizes with the retinoid X-receptors (RXR), a common nuclear receptor binding partner of the PPARs. The PPARα-RXR heterodimer binds to the DNA of their specific targets such as acyl-CoA [231,234,235,236] and CPT-1 [231,237] to activate the enzymes involved in lipid metabolism including that for free fatty acids. In healthy hearts, these free fatty acids serve as the primary energy source while a failing heart reverts back to a fetal metabolic pathway with reduced enzyme activity for free fatty acid oxidation [231,238,239]. In canine failing hearts, there is a significant decrease of CPT-1 and medium chain acyl-coenzyme A dehydrogenase (MCAD) enzyme activity compared to normal hearts by 18% and 38%, respectively, as well as PPARα and RXR-alpha (RXRα) [231]. In heart tissues, PPARα also significantly increase cardiac CPT-1 levels in humans with dilated cardiomyopathy compared to the control group [240].
Soybean fed pregnant rats showed increased PPARα transcription although having the same levels of PPARα translated products while decreasing ACCα and β mRNA levels, body and liver weights, and levels of lipids and glycogen as compared to those fed with a casein-rich diet. It was opined that such reduction of the hepatic lipid concentrations in these soybean-fed rats was via the downregulation of the transcription and translation of these ACC genes rather than changes in their phosphorylation states leading to increased rates of β-oxidation and decreased lipogenesis [241].
Soy proteins also increased PPARγ, a key regulator of glucose homeostasis and adipogenesis that is required for normal adipocyte differentiation in the adipose tissue of Zucker diabetic fatty fa/fa rats [242]. PPARγ is involved in adipogenesis by regulating various genes including fatty acid-binding protein (FABP4) in adipocytes, lipoprotein lipase (LPL), and the fatty acid transporter and to maintain this adipocyte phenotype [243,244,245].
The great potential of soybean protein intake to reduce blood TC, LDL, and TAG levels [109] and coronary heart disease led the Food and Drug Administration of America (FDA) in 1999 to recommend the ingestion of 25 g per day [246] and was later adopted by other countries [218,247,248]. For example, in Asian populations, the consumption of 6g or more of soy proteins reduced TC and LDL levels as well as ischemic and cerebrovascular events [110].
Cardiovascular Benefits of Soybean 7S and 11S Globulin Proteins
Soy proteins are predominantly the 7S β-conglycinin and 11S glycinin which collectively constitute 65–85% of total soybean proteins [129,132] and were found to have hypocholesterolemic, hypolipidemic, antioxidant, and antimicrobial benefits.
These storage proteins were found to exert hypocholesterolemic effects comparable to the commercially-available fenofibrate in rats fed with a high-cholesterol diet [133] and have an atheroprotective role [249]. Ferreira et al., (2010) [133] found that diets supplemented with either 7S β-conglycinin or 11S glycinin (300 mg/kg/day) for 28 days were able to significantly reduce plasma cholesterol and TAG levels while increasing HDL-C concentrations compared to a hypercholesterolemic diet. However, these proteins vary in their contributions compared to the effect of a diet supplemented with fenofibrate (30 mg/kg/day). Both 7S and 11S globulins were able to reduce plasma cholesterol by 22.95% and 16% (p < 0.01), respectively, but not as more as when the drug was used which was able to reduce cholesterol levels by 35.8% (p < 0.001). The 7S β-conglycinin and 11S glycinin and the enriched diets were able to reduce plasma TAG levels by 34.98% (p < 0.01) and 16.3% (p < 0.05), respectively, but neither globulin was able to reduce TAG levels more than fenofibrate which could reduce plasma TAG levels by 45.7% (p < 0.001). All three treatments were able to increase plasma HDL-C levels by 55.5%, 20.0%, and 95.5% for the 7S β-conglycinin, 11S glycinin, and fenofibrate diets, respectively, compared to the rats fed with a high cholesterol diet but the effect of the 11S glycinin group was determined to be not significant (p < 0.05). The 7S β-conglycinin-fed rats showed decreased hepatic levels of TC and TAGs at 20.9% and 14.8%, respectively, while the 11S glycinin-fed rats showed decreased hepatic TAG levels (17.9%, p < 0.001) but no significant change in TC levels compared to the hypercholesterolemic fed rats. Rats fed with fenofibrate had a more significant hepatic TC reduction (32.1%, p < 0.001) but higher TAG levels (5.4%, p < 0.05) compared to the high-cholesterol fed rat group [133].
Cardiovascular Benefits of Soybean 7S β-Conglycinin
The soybean 7S β-conglycinin are ascribed in various preventive roles against oxidative stress, hypertension, hypercholesterolemia, dyslipidemia and obesity and cancer [64,116,133,134,135,136,137,138,139,140,218]. In vitro studies showed that the soy 7S protein stimulated the expression of the genes involved in fatty acid β-oxidation and LDL-receptors and the degradation of LDL, reduced cholesterol ester synthesis and suppressed apolipoprotein B-100 (APO B-100) secretion in cultured HepG2 hepatocytes [142,143,144]. These results were later confirmed where such cholesterol reduction was attributed to the stimulation of the LDL receptors and the degradation of LDL in cultured HepG2 hepatocytes [142,146].
This soybean β-conglycinin was also shown to control dyslipidemia in both animal models and humans comparable to the commonly used drugs such as statins [133,134,135,136,137]. Lovati et al., (1992) [142] showed the first in vivo evidence involving this soybean 7S globulin to reduce TAG and TC levels in rats by 35% that is comparable to clofibrate. In human clinical trials, this β-conglycinin consumption led to decreased levels in serum TAGs, fat, lipid accumulation and body:fat ratio [100,148,149].
Mochizuki et al., (2009) [144] also found that soy 7S β-conglycinin peptides also stimulated the nuclear transcription factor SREBP-2 which led to reduced levels of cholesterol. These SREBP transcription factors induce genes associated with cholesterol biosynthesis, the LDL receptor, and the regulators of the entry of cholesterol [144]. SREBP-2 is further controlled by the mTORC1 pathway through autophagy as the SREBP-2 transcription factor can bind to the promoter of several autophagy-related genes including LC3, Atg4B and Atg4d. Cells with SREBP-2 knockout led to the decreased formation of autophagosomes and the induction of the co-localization of lipid droplets with the autophagy LC3 marker [159].
Enzymatic digestion of soybean protein produced small (3–20 kDa) peptides and comparing to a small synthetic LRVPAGTTFYVVNPDNDENLRMIA peptide (MW = 2.3 kDa), corresponding to 127–150th position of the soybean 7S globulin, exhibited the same effect to modulate LDL receptor activity as that of the complete 7S globulin in cultured hepatic HepG2 cells [116]. Consumption of this 7S globulin decreased serum TAGs in both normal and genetic obese mice [108,147] and in humans [108,136]. Ferreira et al., (2010) [134] showed that supplementation of this major β-conglycinin soybean storage globulin (200 mg/day) to Wistar rats fed with a high-cholesterol diet for 28 days lowered plasma TC and TAG levels and LDL:HDL ratios and upregulated the β-VLDL receptor.
The 7S β-conglycinin is made up of three subunits (alpha, alpha’ and beta) that share a large degree of amino acid homology [103,108,129]. Of these three subunits, it is the alpha’ subunit that is directly involved in the hypocholesterolemic effect of soybean as evidenced by the upregulation of the LDL receptors in human HepG2 cultured cells [116,145,146,150]. Duranti et al., (2004) [135] showed that it is this alpha’-subunit of the soybean 7S globulin that was associated with the lowered plasma lipid and upregulation of β-VLDL receptors in rats fed with a high-cholesterol diet. In rat studies, this 7S globulin alpha’ subunit decreased plasma TC and TAGs. In this study, increasing concentrations of either the 7S globulin (100 or 200 mg/kg/day) or the 7S globulin alpha’-subunit (10 or 20 mg/kg/day) was observed to significantly decrease plasma TC and TAG levels. Using either the 7S globulin (100 or 200 mg/kg/day) or 7S globulin alpha’-subunit (20 mg/kg/day) exhibited statistically similar contributions (p < 0.001) in lowering plasma TC and TAG levels compared to clofibrate (200 mg/kg/day) [135]. The LLPHH pentapeptide from the soybean β-conglycinin derived from protease-hydrolysis was also found to be antioxidative as it inhibited linoleic acid auto-oxidation in an aqueous system since the imidazole ring of histidine contained therein conferred the ion chelating and lipid radical tapping abilities with tyrosine as the H-donor in these peptides [121,125].
Cardiovascular Benefits of Soybean 11S Globulin Peptides
To date, there is only a handful of reports relating the cardiovascular benefit of 11S globulin proteins and their peptide components. Pepsin hydrolysis of the soybean 11S globulin liberated the IAVPGEVA octapeptide that exerted its hypocholesterolemic effect by binding bile salts and reduced bile salt and cholesterol absorption [141]. The LPYP tetrapeptide from the soy 11S glycinin was found to have a hypocholesterolemic effect [250].
3.1.2. Soybean Peptides and Their Associated Health Benefits
Peptides are small protein molecules having between 2 to 21 amino acid residues (<10 kDa), that may exist naturally or be derived from cryptic sequences of native proteins [129,251,252] including plants. These peptides may be obtained from different plant sources including various legumes such as soybeans and peas [64,129]. Originally, these biopeptides are latent but are released through various methods to invoke their biological and/or physiological functions such as their hormone-like activity and influence over various major organ systems [129]. Such methods include hydrolysis via digestion or the proteolytic-enzyme activity of either microbial or plant-origin or from the digestive system [197,253], from microbial fermentation [105,254,255,256] or during food processing. These peptides offer different health benefits including reduction of blood pressure; anti-hypertensive, hypocholesterolemic, anti-oxidative, anti-thrombotic, anti-obesity, antiproliferation, anti-cancer, anti-diabetes, immunomodulatory, anxiolytic, and antimicrobial benefits; and the enhancement of mineral absorption and bioavailability [129,257,258].
Soybean oligopeptides derived after protease hydrolysis and lactobacilli-fermentation were found to inhibit ACE1, an important enzyme associated with hypertension and CVD [259,260,261]. Lactic acid bacteria released ACE inhibitory peptides derived from soybean, soymilk, and soy yoghurt [255]. Shimagake et al., (2012) [260], detected five novel ACE inhibitory proteins upon protease treatment of hikiwari natto and eight novel ACE inhibitory proteins from protease treated soymilk with the latter having 36-fold ACE-inhibition compared to when untreated soymilk was employed. Vallabha and Tiku (2014) [261] discovered that the LIVTQ pentapeptide that was released via the soybean fermentation with Lactobacillus casei spp. pseudoplantarum and exhibited ACE enzyme inhibition. Using different peptide analogues of this LIVTQ peptide, they found that the glutamine (Q) and threonine (T) residues have an important role towards this ACE enzyme inhibition.
ACE1 is a component of the renin-angiotensin-aldosterone system (RAAS) that regulates blood pressure, blood volume, and fluid balance and plays an important role in the physiology of CVD [129]. ACE1 is a non-specific dipeptidyl carboxypeptidase that catalyzes the catabolism of the vasodilator peptide bradykinin, a blood pressure lowering agent belonging to the kallikrenin–kinin system [262,263,264]. ACE1 is widely distributed in mammalian tissues as a membrane-bound ectoenzyme in the vascular endothelial cells among others [265]. In RAAS, cleavage of the C-terminus side of the inactive angiostensin I decapeptide results in the active angiotensin II (ACEII) octapeptide. ACEII is also involved in the release of aldosterone from the glomerulosa cells of the adrenal cortex which lowers blood pressure by increasing renal reabsorption of sodium ions and water [266]. Inhibition of ACE is a useful approach to treat hypertension and usually occurs by blocking the first step in RAAS and the negative feedback to angiotensin II [129], thereby, decreasing ACEII production with the corresponding increase of bradykinin levels and decrease in aldosterone secretion [267]. The presence of proline, threonine, glutamine, isoleucine, valine, and leucine in these ACE inhibitory peptides accounted for their ACE-inhibitory properties and most of these peptides were protease-resistant in the gastrointestinal tract [259,268].
Numerous studies have already claimed the hypocholesterolemic activity of the peptides released from the major storage proteins of soybean. Lactostatin (IIAEK) is a pentapeptide derived from bovine β-lactoglobulin. The sequence contains nearly equal amounts of hydrophobic and charged residues with no digestive cleavage sites for pepsin or trypsin [269]. There had been reports that the in vivo hypocholesterolemic activity of lactostatin was observed to be higher than that of β-sitosterol to reduce the level of serum cholesterol through a novel regulatory MAPK-dependent pathway involved in cholesterol degradation [270]. Lactostatin consumption could provide a convenient way to avoid high serum LDL cholesterol and its negative effects such as in cardiovascular diseases (CVD). This study also introduced tandem multimers of the nucleotide sequence encoding for the IIAEK peptide into the five DNA variable regions of the soybean glycinin A1aB1b subunit through protein engineering. Five mutants, each containing four IIAEK sequences in each of these five variable regions, were expressed in Escherichia coli. Mutants with four IIAEK sequences in the variable regions IV and V gave the highest expression level and were the most soluble compared to the other mutants. The introduction of the fifth IIAEK sequence to variable region IV did not change the expression level and solubility behavior but the introduction of 7 and 10 IIAEK sequences decreased its expression level and solubility to 40% and 1%, respectively. Furthermore, various combinations of such mutations were introduced into this soybean protein and the combined mutations in regions IV and V, and variable regions II and III had the best permutation for both expression and solubility. Purification and subsequent trypsin-digestion of these mutants showed that the desired IIAEK peptide yield was about 80% [269]. Recently, Medina et al., (2020) engineered this peptide into mungbean 8sα globulin using site-directed mutagenesis by substituting the IIAEK lactostatin peptide into the variable region II, expressed through a bacterial system, and validated through peptide mapping, Liquid Chromatography–Mass Spectrometry (LC-MS), and densitometric analysis. The group found that the HIC-purified, trypsin-chymotrypsin digests of this mutant 8S globulin were able to in vitro bind the taurocholate bile acid and reduce such levels significantly better (p < 0.05) than the wild-type globulin but not better than the rosuvastatin positive control [271].
3.1.3. Soybean Lunasin
Another important soybean peptide that has reported anti-inflammatory, antioxidant, anti-hypertensive, hypocholesterolemic, and anti-cancer benefits is lunasin. Lunasin is a 43-amino acid (SKWQHQQDSCRKQLQGVNLTPCEKHIMEKIQGRGDDDDDDDDD) (MW = 5.5 kDa) peptide derived from the methionine-rich Gm2S-1 gene encoding the 2S albumin found in the soybean cotyledon [259,272,273,274,275,276,277]. In soybeans, lunasin was found to constitute 4.4–70.5 mg/soybean protein [259]. Lunasin was found to be highly bioavailable and heat-resistant [275]. Though this protein was originally found in soybean, it has been later detected in cereals and pseudocereals including wheat, barley, rice, rye, triticale, and amaranth [278,279,280,281].
Lunasin’s hypocholesterolemic effect is associated with reduced HMG-CoA reductase gene levels, thus, reducing cholesterol biosynthesis. Furthermore, lunasin reduced HMG-CoA expression and induced LDLR production leading to increased LDL binding causing enhanced clearance of plasma LDL-C as shown in HepG2 liver cells. Lunasin was also found to reduce LDL-C levels in pigs [151].
Lunasin was also found to possess anti-oxidant properties by scavenging peroxyl and superoxide radicals in vitro [282] and to be able to chelate Fe2+ ions, thereby, preventing hydroxyl radical formation through a Fenton reaction [30,283]. Lunasin was also found to protect cell viability and antioxidant defenses in human Caco2 cells treated with hydrogen peroxide and tert-butylhydroperoxide after 24 h [282].
Lunasin was also found to have anti-inflammatory effects as shown by the inhibition of various pro-inflammatory biomarkers such as the IL6 and IL-1β interleukins, TNF-α, NF-κB, cyclooxygenase 2 (COX2), prostaglandin E2 (PGE2), and inducible nitric oxide synthase (iNOS) by activating various inflammatory pathways such as AKT/NF-κB in macrophages [284,285]. Aguzzi et al., (2010) found that the RGD tripeptide motif from lunasin binds to the αVβ3 integrin and is involved in macrophage aggregation during inflammation that might inhibit pro-inflammatory pathways [286].
3.2. Lupin (Lupinus spp.)
Lupin is a generic term for proteins derived from four domesticated Lupinus species-Lupinus albus (white lupin), L. luteus (yellow lupin), L. mutabilis (blue lupin) and L. angustifolius (narrow-leaf lupin) [153,287]. These are mostly produced in Australia, France, Poland, Russia, Hungary, and Italy [153]. These are excellent legumes since their protein content is comparable to soybean and is a good source of minerals, unsaturated fatty acids, vitamins, and tocopherols while having negligible amounts of phytoestrogens such as isoflavones and other antinutrients [153,288,289,290,291,292].
Lupin and its protein isolates were found to decrease cholesterol levels in various model animal systems such as rats, hamsters, pigs, and humans [152,153,157,160,290,293]. Lupin proteins are ascribed in lowering plasma TC and LDL-C in rats fed with a high-fat diet [153] and the significant improvement of LDL:HDL-C ratios in humans [160].
Male Sprague Dawley rats fed with 50 mg/rat of the lupin total protein extract for two weeks showed significant reduction in TAGs by 16% (p < 0.01), TC by 21%, and VLDL-LDL-C by 30% (p < 0.001) while increasing HDL-C by 20% (p < 0.10) compared to the vehicle-fed rats [152]. Pregnant female rats fed with 200 g/kg lupin proteins during pregnancy until the post-lactation stage showed decreased levels of plasma TAG (by 55%), plasma TC, VLDL, LDL, and hepatic cholesterol compared to dams fed with a casein-rich diet. Such lupin protein-fed rats also exhibited higher TAG and LDL receptors levels in their liver by more than twofold and in their milk by 81% and were found to have significantly (p < 0.05) upregulated lipogenic genes in their mammary glands [290].
In human trials, lupin protein isolates (25 g per day) significantly lowered blood pressure (p ≤ 0.044), LDL-C levels (p ≤ 0.036), and LDL:HDL-C ratios (p = 0.003) after four weeks compared to individuals who consumed a milk protein isolate or a casein diet [157,160]. According to the Weiße et al., (2010), the significant reduction in plasma TC due to the ingestion of the lupin proteins was mainly due to the decrease HDL-C levels, independent of LDL-C changes, leading to lower cardiovascular risk [160].
Sirtori et al., (2004) [153] demonstrated significant reduction of VLDL and LDL in rats fed with high-cholesterol and cholic acid that were supplemented with lupin protein isolates (50 mg/day). In a leporine model, Marchesi et al., (2008) [155] detected significantly lower cholesterol levels after 60 days by 40% and after 90 days by 33% (p < 0.05) and a significant reduction in focal lesion progression by 37% (p < 0.05) after feeding such animals with lupin proteins compared to the casein-fed group.
Lupin proteins were found to downregulate the SREBP-1 transcription factor in rat livers resulting in decreased fatty acid synthesis [153,154,294] and upregulated LDL receptors in human hepatoma cells [153,290,294]. Lupin proteins also significantly reduced the inflammation-associated C-reactive protein (CPT) biomarker that is linked to the risk of cardiovascular events [157,295,296].
Lupinus albus and L. angustifolius were shown to improve the lipoprotein profile and lower blood pressure in rat models [153,156,157,290,297,298]. In in vitro studies using HepG2 cell lines, Lammi et al., (2014) [299] discovered that pepsin and trypsin-digested L. albus proteins induced the LDL receptor protein resulting in the subsequent LDL uptake and stimulated the AKT-GSK3β signaling pathway suggesting that the AKT-GSKβ is involved in the upregulation of the LDL-receptor-SREBP2 pathway. Interestingly, Jang et al., (2012) determined that black soybean (Glycine max L.) peptides also stimulated the same AKT-GSK3β signaling pathway via the phosphorylation of GSK3β and FoxO1 in HepG2 cells [106]. Fontanari et al., (2012) [152] found that there was a significant reduction of plasma TC and non-HDL while increasing HDL-C levels in a hamster model for dyslipidemia fed with either whole L. albus seed or its protein isolate compared to the high-casein fed group. They also found low levels of steatosis upon histological examination of these hamster livers. L. albus proteins were also found to slow down atheromatous plaque progression induced by a hyperlipidemic diet in rabbits [155]. L. angustifolius (blue lupin) also showed a cholesterol-controlling effect in rats through the upregulation of SREBP-2 and other genes involved in lipid homeostasis as discussed above [156] and in humans [157,158,159,208,300]
Lupin proteins are predominantly albumins and globulins with an albumin:globumin ratio of 1:9. The globulin proteins are further classically classified as those belonging to the 7S and 11S globulins termed conglutin-beta and alpha, respectively. Lupins have also other minor distinct globulin types termed gamma and delta conglutin [153,301]. Lupin conglutin gamma is an oligomeric seed protein that constitutes about 5% of total lupin proteins [302]. The hypocholesterolemic potential of lupin conglutin-γ is widely believed to induce LDL-receptor activity. In 2004, Sirtori et al. found that the purified conglutin-γ increased LDL uptake by 53% by inducing LDL receptor activity in HepG2 cells [153]. In rat studies, addition of the purified lupin conglutin-γ (25, 50, and 100 mg/kg) into diets showed an inverse correlation to control plasma glucose levels with the 100 mg/kg levels performing as statistically significant (p < 0.001) compared to the benefits obtained from metformin (50 mg/kg) supplementation [135,137]. Radtke et al., (2015) found that conglutin-γ significantly decreased serum TC by 15% (p = 0.011) and increased serum levels of the pro-Vitamin D metabolite by 50% in ApoE knockout mice [162]. The LILPKHSDAD and LTFPGSAED lupin β-conglutin oligopeptides were found to inhibit HMG-CoA reductase, thereby improving LDL-protein levels in HepG2 cells [161].
Lammi et al., (2016) [303] found that lupin peptides derived from pepsin and trypsin hydrolysis had a positive influence on the cholesterol metabolism in Caco2 cells by reducing the secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9). In another publication from the same group, the lupin LILPKHSDAD oligopeptide further inhibited PCSK9-LDLR binding in a dose-dependent manner allowing for the increased extracellular entry of LDL as observed through both in silico and in vitro approaches [304] since PCSK9 regulates the degradation of the hepatic LDL receptor [156,297,305].
3.3. Pea (Pisum Sativum L.)
Peas (Pisum sativum L.) contain an average of 22% protein (ranging between 13–33% protein) and such proteins were found to improve against CVD and its risks [306,307,308,309]. Rimm et al., (1996) found that a daily serving of peas contributed to the reduced risk of heart attack in human male professionals [307]. Using rat models, Lasekan et al., (1995) found that 20% of dietary pea proteins significantly reduced plasma TC by 61%, TAGs by 47% and hepatic cholesterol levels by 94% (p < 0.05) and increased HDL-C concentrations versus the casein-fed group [164]. Rigamonti et al., (2010) [165] found that rats fed with pea proteins for 14 and 28 days had significantly lower plasma TC and TAGs, lower expression of genes involved in fatty acid synthesis including FASN and stearoyl-CoA desaturase, and increased hepatic LDL-receptor mRNA than those fed with casein (p < 0.05). Rats fed with 200 g pea protein per kg body weight for 16 days showed significantly (p < 0.05) reduced hepatic TC and VLDL proteins and increased levels of cholesterol-7-alpha-hydroxylase levels, hepatic SREBP-2 and its target genes such as HMG-CoA and LDL-receptor, and fecal bile acid excretion [166]. Employing obese Dutch people with untreated elevated BP, Teunissen-Beekman et al., (2014) found that a pea protein meal significantly sustained blood pressure levels (p ≤ 0.01) and induced the production of nitrite, nitro, and NOx compounds [310].
Hydrolysis of pea proteins may yield peptides with various bioactivities such as those for ACE1 inhibition and improvement against CVD [258,306]. For example, Alcalase-digestion of pea proteins liberated three dipeptides (IR, KF, and EF) that exerted ACE-inhibitory effects in vitro [163]. Recent publications reported that the LRW tripeptide and its similar isomer IWR exhibited their antihypertensive activity by activating angiotensin II [167,311,312,313]. Incubation of vascular smooth muscle cells (VSMCs) with this LRW pea tripeptide reduced angiotensin-triggered superoxide production, inflammation and proliferation by inducing the ACE2-Ang-(1–7)-MasR axis and modulating the nuclear factor-kappa B (NF-κB) pathway [167].
3.4. Cowpea (Vigna Unguiculata (L.) Walp.)
Cowpea (Vigna unguiculata (L.) Walp.) is a widely consumed legume in the world. It is rich in protein and carbohydrates while having a low fat content [31,314,315]. The protein content of cowpea is 2-4-fold higher than cereals and tuber crops [316,317] although the cowpea’s amino acid profile is comparable to that of cereal grains. Cowpea proteins are rich in lysine [318] but deficient in sulfur-containing amino acids such as methionine and cysteine [319]. Cowpea proteins have a low lysine:arginine ratio comparable to soybean suggesting that cowpea proteins have the potential to decrease cholesterol [320].
Some cowpea isolates and peptides were found to be hypocholesterolemic. In hamsters, Frota et al., (2008) [170] found that cowpea whole seed and protein-fed animals have significantly reduced plasma TC and non-HDL-C and promoted reductions in liver steatosis. This hypocholesteromic effect was attributed to the ability of the proteins to bind bile acids disrupting cholesterol micelles in the gastrointestinal tract as well as altering adipocytic enzyme activity and increased the transcription of lipogenic and cholesterol biosynthetic proteins which influence lipid profiles [170,321,322,323,324]. Spielmann et al., (2008) [166] further stated that these cowpea proteins interfere with hepatic cholesterol absorption through the inhibition of the intestinal cholesterol transporter Niemann–Pick C1 Like-1 (NPC1L1) gene expression, thereby, reducing cholesterol synthesis by lowering SREBP-2 gene expression and, consequently, HMG-CoA reductase and LDL receptors. NPC1L1 is involved in the cholesterol absorption at the apical membrane of enterocytes through an unknown mechanism. The cowpea KD dipeptide and three black bean pea peptides (YAAAT, ERAF, and FATGT) were found in silico to have binding affinities to the N-terminal domain of NPC1L1 and were opined to have great potential to disrupt the interaction between NPC1L1 and membrane proteins facilitating cholesterol absorption better than Ezetimibe [325].
Cowpea proteins are predominantly globulins (50–70%) followed by glutelins, albumins and prolamins [326,327,328]. The 7S globulin from cowpea (300 mg per body weight per day) was shown to significantly decrease average body weight gain, liver TC by 14%, serum non-HDL-C by 54%, hepatic TAG levels by 17% and the atherogenic index, a marker for predisposition of heart disease, while increasing serum HDL-C by 57%, lipoprotein lipase activity by 92%, and excreted total fat by 12% and total cholesterol 7% in male Wistar rats compared to the casein-fed group after feeding for 28 days. Ferreira et al., (2015) found that rats fed with cowpea 7S globulin (300 mg/kg/day) was able to decrease TC by 32.5% compared to just 20.3% by simvastatin (50 mg/kg/day) (p < 0.001) after their 28-day feeding experiment. In the same study, they found that the 7S cowpea globulin-fed mice had significantly reduced serum non-HDL-C and atherogenic index by 46% and 70.6%, respectively, while simvastatin was able to significantly decrease serum non-HDL-C and the atherogenic index by 30.7% and 0.31%, respectively, compared to the hypercholesterolemic-fed mice (p < 0.001). Supplementation of the cowpea 7S globulin also led to an significant increase of serum HDL-C by 157% when compared against a high cholesterol diet (p < 0.001) outperforming simvastatin, which only contributed a non-significant increase of 18.5% As compared to the rats fed with a high cholesterol diet, hepatic TC and TAG levels were significantly reduced by 14% (p < 0.01) and 17% (p < 0.001), respectively, compared to the effect of simvastatin which significantly reduced TC levels by 11% (p < 0.05) but not significantly reducing TAG (6%) [87].
Bioactive peptides in cowpea are ascribed to their antihypertensive property due to ACE1 inhibition [13,168,169,329]. Hydrolysis of cowpea proteins yielded peptide fractions that inhibit ACE1-activity compared to the unhydrolyzed protein [13,168,169].
3.5. Jack Bean (Canavalia Ensiformis (L.) DC.)
Jack bean (Canavalia ensiformis (L.) DC.) is a tropical legume originally from South America but is widely planted in South Asia, Africa, and Latin America. Macarulla et al., (2001) determined that jack bean seed is rich in protein (23–28%). They further discovered that even at a 10% jack bean protein diet, albino rats had decreased weight gain by 79% and PER (weight gain per amount of protein fed) by 64%; TC levels in the plasma by 10%, liver by 16%, kidney 13%, and in the heart by 42%; and total lipids in the plasma by 33%, liver by 37%, and heart (by 25%) (p < 0.05) [171].
One of jack bean’s proteins, concanavalin A, was shown to significantly decrease serum TC by 81%, liver cholesterol and TAG levels but increased HDL-C by 87% compared to the control group. Kayashima et al., (2005) further noted that the cholesterol lowering ability of concanavalin A (conA) was directly related to its lectin activity. ConA was found to be fairly resistant to degradation in the gut and confers various changes in the gastrointestinal environment. These changes promote fecal excretion of neutral sterols and triglycerides leading to inhibition of intestinal absorption of cholesterol and lowering of serum triglyceride concentration [172].
3.6. Mungbean (Vigna Radiata L.)
Mungbean contains 21–31% protein per dry weight basis (Table 3) [54]. In high-fat diet induced male rats, mungbean protein isolate supplementation for four weeks increased secondary:primary bile acid ratio; lowered TC, TAGs, non-HDL-C, non-HDL-C:HDL-C and TC:HDL-C ratios; and increased members of the Bacteroidetes and Firmicutes phyla [176]. Further, humans with hyperlipidemia had significantly decreased TAG levels and obese subjects were seen to decrease the visceral fat accumulation [177].

Table 3.
Pathway/GO term enrichment analysis of the different genes using Reactome as discussed in this manuscript.
Mungbean protein supplementation also decreased several lipogenic genes and some glycolytic enzymes. These mungbean proteins also decreased serum alanine amino transferase (ALT) and aspartate transferase (AST) levels. Kohno et al., (2017) further found that these mungbean protein isolates stimulated de novo lipogenesis-related genes such as SREBP-1, FASN, SCD1 in primary hepatocyte cells; alleviated hepatic TAG accumulation; and suppressed inflammation and fibrosis in the liver in a non-alcohol steatohepatitis model, thereby, reducing TAG accumulation [177]. Watanabe et al., (2017) found that mungbean protein isolates fed to male, hypercholesterolemic mice resulted in decreased FASN activity and hepatic TAG levels [330]. Consumption of mungbean proteins also reduced plasma TC, TAGs, and non-HDL-C levels in high cholesterol diet-fed hamsters. The same study showed that HMG-CoA reductase and CYP7A1 were both upregulated transcriptionally and translationally [173,174,175].
Mungbean protein hydrolyzed with Alcalase were found to have a high ACE inhibitory activity in spontaneously hypertensive rat models. These protein hydrolysates were shown to exhibit antihypertensive activity by reducing systolic blood pressure in such rats that received a single administration of this mungbean protein hydrolysate [173]. In another study, such Alcalase-hydrolyzed proteins from mungbean (Vigna radiata L.) protein hydrolysates released peptides that show ACE1 inhibition and exhibited antioxidant (including DPPH and hydroxyl radical and metal-chelating scavenging activities), hypolipidemic, and antihypertensive properties [330,331,332,333]. The KDYRL, VTPALR, and KLPAGTLF peptides from this Alcalase-hydrolyzed mungbean protein isolate were found to have novel ACE inhibitory activity [334].
3.7. Chickpea (Cicer Aretinum L.)
Chickpea (Cicer aretinum L.) is one of the oldest and widely consumed legumes [185]. The seeds are an excellent source of dietary protein, has a well-balanced amino acid composition, and has a substantial bioavailable protein [335]. Chickpea consumption is attributed to several cardiovascular benefits such as mitigation of CVD as well as the prevention of hyperlipidemia [336,337]. Boualga et al., (2009) found that male Wistar rats fed with 200 g/kg of either chickpea or lentil diets for four weeks showed significant (p < 0.05) reduction in body weight, food efficiency ratio, weight gain (g/day/rat), epididymal fat relative weight and fat lipoprotein lipase activity as well as decreased plasma TAG and VLDL; liver cholesterol and TAG; plasma VLDL mass; and APOB-100, esterified and unesterified cholesterol, and VLDL phospholipid levels. Further, ingestion of such chickpea or lentil diets led to increased hepatic phospholipid content and lipase activity in these rats [181]. The decreased plasma VLDL mass and APOB-100 levels in the chickpea-fed rats indicated a lower number of VLDL particles as evidenced by diminished VLDL-TAG and ApoB concentrations as seen in other soybean protein studies [94,338]. Chickpea consumption also decreased serum TC levels in humans [90,183,184], and chickpea proteins were shown to normalize TAG levels in hypercholesterolemic rats [180] further suggesting the beneficial effects of chickpea protein consumption towards the prevention of coronary heart disease and metabolic syndrome [66].
Shi et al., (2019) [179] found that HepG2 cells treated with chickpea proteins resulted in reduced cellular TC while increasing uptake of NBD-cholesterol. They further found that rats fed with chickpea proteins had decreased body and adipose tissue weight; serum TC, TAG, and LDL-C and atherogenic index; and hepatic TC and TAG levels while increasing the levels of serum HDL-C and hepatic and fecal bile acids. They also found that these chickpea proteins also inhibited FASN, HMG-CoA reductase, PPARγ, and SREBP-1c while activating liver X receptor (LXR) and the estrogen receptors α and β (ER-α and ER-β). They also determined that the chickpea VFVRN pentapeptide could inhibit HMG-CoA reductase expression, thus, inhibiting TC biosynthesis [179].
Mice fed with a hydrolysate rich in chickpea albumin decreased serum LDL-C in a dose-dependent manner and serum TC and TAG by 44% and 24%, respectively, while increasing HDL-C compared to the high-fat diet fed group.
3.8. Lentil (Lens Culinaris Medik.)
Bioactive peptides derived from lentils (Lens culinaris Medik.) were shown to be anti-hypertensive, ACE inhibitory and anti-oxidative [187,188]. In the study by Hanson et al., (2016), Wistar-Kyoto and spontaneously hypertensive rats showed significantly reduced levels of TC and LDL-C (p < 0.05) after whole lentils feeding for eight weeks [186].
The lentil convicillin storage protein is ascribed to inhibit ACE and dipeptidyl aminopeptidase III and IV peptidases, key enzymes involved in the complex interactions between cyclic nucleotides and the calcium-dependent secondary messenger systems [188] and stimulate vasoactive substances [187]. Three oligopeptides (LLSGTQNQPSFLSGF, NSLTLPILRYL, TLEPNSVFLPVLLH) derived from the Savinase-digestion of cultivated lentil proteins were identified to have the highest potentials against ACE activity in vitro. The peptides’ antihypertensive benefit was linked to the primary structure of the hexapeptides at the C-terminal end as these residues affect ACE inhibition [189]. Enzyme-hydrolyzed protein isolates also yielded oligopeptides of less than six amino acids that strongly inhibited ACE [190,191,192,193].
3.9. Other Legumes
A number of other legumes and their proteins have been briefly reported to improve cardiovascular health. Protein concentrates (12%) from Phaseolus angularis, Phaseolus calcaratus, and Dolichos lablab significantly decreased levels of TAGs, TC, and LDL-C and increased hepatic total lipids in male Golden Syrian hamsters after 30 days [14,339]. Trinidad et al., (2010) [11] found that kidney beans (Phaseolus vulgaris L.) showed significant reduction in TC (by 6%) and LDL-C (by 9%) while peanuts (Arachis hypogaea L.) significantly decreased TC levels by 7% in humans (p < 0.05). More recently, Distor et al., (2015) [14] found ACE inhibitory activities in vitro from the enzyme-digested 7S globulin of the hyacinth bean (Lablab purpureus L.) that is comparable to a drug widely used to treat hypertension, congestive heart failure, and increases the survival following a heart attack. In vitro ACE1 inhibition assays revealed that either pepsin- or α-chymotrypsin-digested purified hyacinth bean 7S globulins (1 mg/mL) performed statistically similarly compared to an equivalent concentration of captopril [14].
Moth bean (Phaseolus aconitifiolius Jacq.) seed proteins were found to significantly reduce plasma TAG, TC, LDL-C and total lipids as well as hepatic total TC levels while increasing HDL-C in Wistar rats fed with 10% of the bean’s protein for 45 days [194].
The faba bean (Vicia faba L.) is commonly consumed in some European countries particularly those found along the Mediterranean Sea. In male Wistar, hypercholesterolemic rat models, Macarulla et al., (2001) [171] demonstrated significant decreased body weights and plasma TAG when fed with whole faba bean seed diet. Rats fed with either the whole seed or protein isolate increased serum LDL + VLDL-C [171]. They further opined that this hypercholesterolemic effect was not due to the reduced cholesterol synthesis due to the HMG-CoA reductase activity but rather to the increased excretion of fecal steroids. Faba bean proteins demonstrated the reduction of blood cholesterol levels that is comparable to soybean protein through the use of hypercholesterolemic rats [171,340]. The amino acid profile of faba bean seeds and its protein isolates are ascribed to their hypocholesterolemic potential as the seeds have a lysine:arginine ratio and a methionine:glycine ratio 0.33 and 0.17, respectively, while its protein isolates have a lysine:arginine ratio and a methionine:glycine ratio of 0.26 and 0.29, respectively, which are lower than the corresponding levels in casein [171,341,342].
Ferreira et al., (2015) [87] showed that male Winstar rats fed with 300 mg of the purified adzuki bean or red mungbean (Vicia angularis (Willd.) Ohwi and Ohashi) 7S globulins (protein per kg body weight) for 28 days exhibited significant decrease in mean body weight gain (23%), which is comparable to the casein-simvastatin fed group and decreased hepatic tissue weight (10%); serum cholesterol, non-HDL-C, and TAGs; lipoprotein lipase activity (62%); and atherogenic index while increased serum HDL-C (52%) and excreted total fat (17%) and cholesterol (13%) levels compared to the casein-fed group. Supplementation of the adzuki 7S globulin (300 mg/kg/day) was able to significantly reduce serum TC and TAG levels by 33% (p < 0.001) and 17.8% (p < 0.05) compared to the rats fed with simvastatin (50 mg/kg/day). Simvastatin was still able to significantly reduce such TC levels by 20.3% (p < 0.001) but could not significantly decrease serum TAG levels. With reference to the rats fed with a high cholesterol diet for 28 days, addition of the adzuki 7S globulin also decreased serum non-HDL-C concentrations and the atherogenic index by 46% and 67%, respectively, when compared to only a reduction of 30.7% and 0.31%, respectively, due to simvastatin supplementation (p < 0.001). Serum HDL-C levels significantly increased by 153% (p < 0.001) but the simvastatin fed group did not statistically increase (18.5%) when compared to the rats fed with a hypercholesterolemic diet. Contrary, hepatic TC levels were not significantly decreased (9%) with the addition of the adzuki 7S globulin while simvastatin was able significantly decrease such hepatic TC levels by 11% (p < 0.05) [87].
4. Peptide Structure, Length, and Hydrophobicity Analysis of Legume Peptides with Associated Cardiovascular Benefits
This manuscript discussed the reported information on how legume diets, legume proteins, and/or specific legume peptides play a positive role towards cardiovascular health. The chemical structures of these 22 legume peptides [19,20,21,22,116,121,125,161,163,167,179,189,250,259,261,269,272,273,274,275,276,277,286,304,334] developed using ChemDraw Ultra 12.0 (PerkinElmer, Cambridge, MA, USA) are depicted in Figure 1.
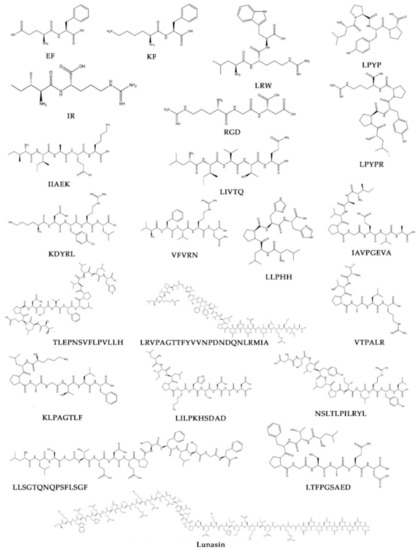
Figure 1.
Chemical structures of the 22 peptides associated with cardiovascular health discussed in this manuscript. These chemical structures were developed using ChemDraw Ultra 12.0 (PerkinElmer, Cambridge, MA, USA).
Several physico-chemical properties including the length of the peptide and their hydrophobicity properties influence their associated functional roles and biological activities [343]. Peptides with relatively smaller molecular weights are less prone to proteolytic digestion and could easily be absorbed through the intestine as such peptides can readily transverse the intestinal barrier [344]. These 22 peptides are relatively short (Figure 2 and Table S2) (mean of 8.8 residues; range = 2 to 43 amino acids) with 21 (91%) of the discussed peptides having a peptide length of 15 amino acids or shorter. These relatively short sizes enable these peptides to transverse readily through the digestive system. Wang et al., (2019) [345] stated that di- and tri-peptides are recognized and actively transported intactly through the PepT1 transporter via the intestinal epithelium. Relating to cardiovascular health, several short-chained peptides (2–12 amino acids) are ACE-inhibitory [266,346,347,348,349,350]. Even longer peptides (10–27 residues) can readily transverse through the cell in a receptor-independent mechanism [351], allowing these peptides to be readily bioavailable intracellularly, and may also be used deliver various important cargoes such as clinically-important drugs and proteins.
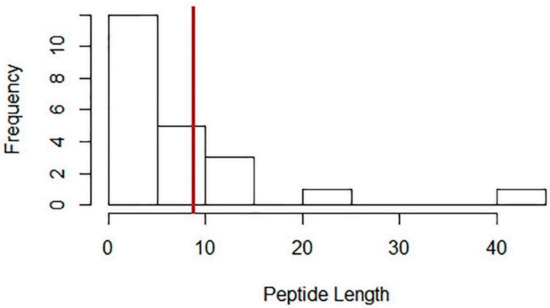
Figure 2.
Histogram of the frequency of the lengths of various peptides (n = 22) discussed in this manuscript. The mean number of amino acid residues is indicated as a brown-colored line on the x-axis.
The hydrophobic nature of peptides can influence cardiovascular health. Guha and Mahumder et al., (2019) [352] reviewed that it is this hydrophobic property of peptides, even in very small di-, tri-, or oligopeptides, which is one of the major factors associated with their anti-inflammatory benefit. Nan et al., (2009) [353] revealed that the high hydrophobicity promote the binding of peptides to membranes leading to the latter’s depolarization as such as exemplified in microbial cells. The hydrophobicity values of the 22 peptides discussed in this manuscript were obtained by calculating their individual Grand average of hydropathicity index (GRAVY) values [354] through the Protein GRAVY website (https://www.bioinformatics.org/sms2/protein_gravy.html accessed on 11 February 2021) [355] (Table S2). A peptide’s GRAVY score represents its hydrophobicity with positive GRAVY values indicating hydrophobic peptides while negative values are for hydrophilic peptides [354,356]. The GRAVY scores of these 22 peptides were then visualized through RStudio version 1.1.456 (Rstudio, Boston, MA, USA) (Figure 3a). The GRAVY scores of these peptides reveal that they are slightly hydrophilic (mean GRAVY score = −0.21, ranging from −2.8 for RGD through 1.66 for LIVTQ).
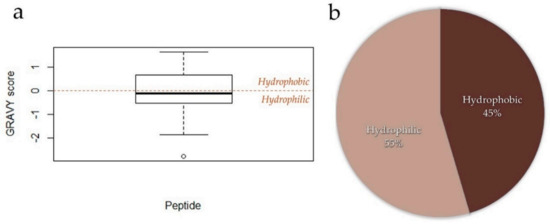
Figure 3.
Hydrophobicity analysis of the 22 peptides discussed in this manuscript. (a). Boxplot of the Grand average of hydropathicity index (GRAVY) values of these peptides calculated through the Protein GRAVY website (https://www.bioinformatics.org/sms2/protein_gravy.html accessed on 11 February 2021) [355] and visualized using RStudio version 1.1.456 (Rstudio, Boston, MA, USA). GRAVY scores greater than 0 are considered hydrophobic while scores less than 0 are considered hydrophilic [354,356] as demarcated by the brown, dashed, horizontal line. (b) Proportion of the 22 peptides classified based on their hydrophobicity GRAVY scores.
Twelve of these 22 peptides (57%) were found to be hydrophilic (Figure 3b). There is a dearth in the available literature associating the hydrophobicity of plant proteins on their cardiovascular benefit. Yea et al., (2014) found that some acidic and basic peptides with partially higher hydrophobicity values from winged bean (Psophocarpus tetragonolobus (L.) DC) seed proteins were ascribed to convey high ACE-inhibition and anti-oxidative properties [357]. Further identification of other peptides and the association of their hydrophobicity value will further associate peptide hydrophobicity with their ascribed cardiovascular potentials.
5. Gene Network Analyses
Based on this review, we performed a gene-to-gene network analysis using the GeneMANIA plugin of the Cytoscape software platform [358,359] and showed that 18 of the genes from all of these legume studies are functionally interrelated (Figure 4). Meanwhile, pathway and gene ontology (GO) terms enrichment using the Reactome functional interaction program [360] revealed that most of these genes are involved in lipid transport, fatty acid, and TAG metabolic processes and in the regulation of these processes (Table 3), suggesting that these pathways may be key and/or target processes to understand the mechanism of action of these legume proteins. Notably, genes such as CPT1A, SREBF2, PPARG, and LDLR are shown to be commonly involved in pathways related to lipid and fatty acid metabolism. These may serve as target genes and proteins for further studies to elucidate how legume and their proteins affect these pathways as well as how these genes regulate other genes and proteins such as those involved in cardiovascular health.
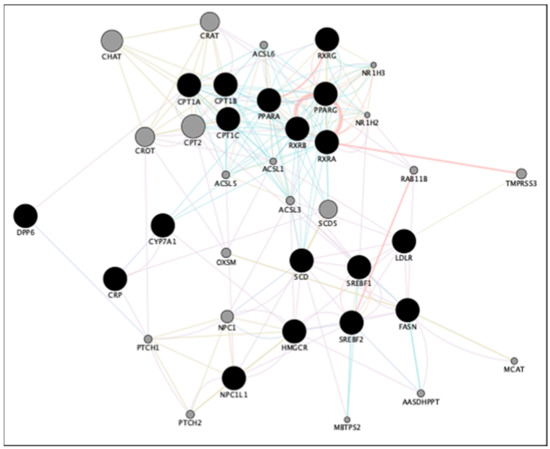
Figure 4.
Functional network of genes associated with cardiovascular disease and legumes proteins. Black nodes denote query genes; gray nodes denote attribute genes.
6. The Mechanism of Action (MOA) of Legumes Proteins and Peptides Associated with Cardiovascular Health
Based on the comprehensive literature search [361,362,363], the known genes, proteins, enzymes and corresponding cellular localization have been integrated to depict the highly possible mechanisms of action of isolated legume proteins and/or peptides in relation to their cardiovascular benefits (Figure 5). This figure illustrates how the induction of these protein and peptide isolates affects the cellular physiology and metabolism in the hepatic and blood serum systems that can lead to improved cardiovascular health. Two major pathways are emphasized in these mechanisms of action: (a) cholesterol and fatty acid degradation and (b) the inhibition of angiotensin converting enzyme (ACE).
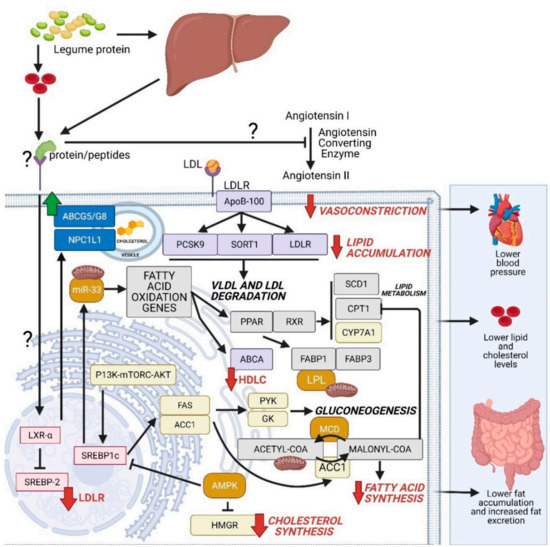
Figure 5.
Proposed mechanism of action of legume proteins and/or peptides towards cardiovascular health. This figure was created using BioRender.com
(accessed on 25 February 2021).
The first pathway showed a series of mechanisms with the ultimate goal of lowering lipid accumulation in the system. In order to decrease the lipid levels, legume protein isolates target cholesterol and fatty acid metabolism. LDL receptor genes are observed to be upregulated in order to produce more LDR receptor proteins that can receive and engulf LDL and VLDL for subsequent lysosomal degradation. Essential nuclear transcription regulators like SREBP1c and SREBP-2 play critical roles in lowering cholesterol and fatty acid synthesis. Aside from lowering lipid and cholesterol levels, such processes subsequently lower fat accumulation and an increased fat excretion.
The second major effect of legume isolates is their ability to regulate hypertension. From the available literature, results suggest that Angiotensin I and II are regulated by blocking the functions of ACE through an unknown mechanism. This observation leads to more relaxed blood vessels, lesser vasoconstriction, lowered blood pressure, and ultimately contributing to improved cardiovascular health.
7. Conclusions and Perspectives
Cardiovascular diseases are the leading cause of mortality in the world. Incorporation of functional foods to improve the human diet in order to lower CVD risk is a growing trend. Among these functional foods are the various legumes described herein which have been a popular part of the normal diet. However, other legumes that show promising bioactivities are yet to be explored or are still underutilized. More in-depth research should be performed to determine the latent benefits, up-to-date information on their effective concentrations, salient structures, and accompanying modes of action of legume bioactive peptides, which could enhance their potential towards improved cardiovascular health and for future alternative drug development. In addition, omics-based studies on these legumes could also be done to map out salient genomic sites corresponding to high bioactive peptide activities such as for cardiovascular health. These associated mapped genomic sites can be the bases for breeding platforms for targeted improvement of crops relating to cardiovascular health. Allergenicity studies of these endemic legume varieties should also be done to ensure their safety since some legumes such as peanuts have known allergens. Genome-wide association studies on diverse cardiovascular responses due to the recipient’s genotypic variation may also be embarked.
Several factors such as palatability, smell, and texture that affect the consumers’ preference in eating these legumes despite the fact that they have health benefits could be explored. Formulation of new food products such as milk and flour from these legumes could show great potential to increase its economic value. Additional studies should also be done to assess if the processing of these legumes will affect their innate and latent bioactive peptide activities.
Since bioactive peptides show great potential in preventing or lowering CVD risks, these can also be synthesized or produced in large scale quantities to be used as supplements and possibly, as alternative medicine. This poses an advantage since there are no reported side effects of bioactive peptides compared to the synthetic drugs when used long-term.
Considering the public health sector, health professionals such as nutritionists and doctors can help elevate the value of these crops by promoting the incorporation of these legumes into the diet such as for improved cardiovascular health. Various government agencies and the private sector could be tapped to promote these products through enhanced information dissemination and increased research funding.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/app11125475/s1, Table S1: Proximate biochemical composition (moisture-free basis) described in this manuscript. Table S2: Peptide length and GRAVY hydrophobicity scores of the 22 peptides with ascribed cardiovascular benefits discussed in this manuscript.
Author Contributions
Conceptualization, J.G.C.A., M.A.O.T., M.A.C.E., J.C.V., and L.Y.C.U.; introduction (Section 1), J.C.V., L.Y.C.U., and M.A.C.E.; legume taxonomy and distribution (Section 2.1), S.M.Q.M.; biochemistry composition of legumes (Section 2.2) and Table S1, M.R.N.A.; legumes as functional food for CVD (Section 3), J.G.C.A., J.C.V., L.Y.C.U., S.M.Q.M., M.C.L.T., J.P.L., M.R.N.A., M.C.M.B.-C., M.A.C.E. and M.A.O.T.; peptide structure, length, and hydrophobicity analysis of legume peptides with associated cardiovascular benefits (Section 4), J.G.C.A., J.C.V., and M.C.M.B.-C.; gene network analysis (Section 5), M.C.L.T.; the mechanism of action (MOA) of legumes proteins and peptides associated with cardiovascular health (Section 6), L.Y.C.U., J.P.L., M.C.L.T., and J.G.C.A.; conclusions and perspectives (Section 7), M.A.O.T., L.Y.C.U., M.A.C.E., and J.G.C.A.; writing—original draft preparation, J.G.C.A., J.C.V., L.Y.C.U., S.M.Q.M., M.C.L.T., J.P.L., M.R.N.A., M.C.M.B.-C. and M.A.O.T.; writing—review and editing, J.G.C.A., J.C.V., L.Y.C.U., S.M.Q.M., M.C.L.T., J.P.L., M.A.C.E. and M.A.O.T.; visualization, L.Y.C.U., M.C.L.T., J.C.V., J.P.L., M.C.M.B.-C. and J.G.C.A.; supervision, J.G.C.A., M.A.C.E. and M.A.O.T.; funding acquisition, M.A.O.T. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Emerging Interdisciplinary Research Program, Office of the Vice President for Academic Affairs, University of the Philippines, EIDR C05-002.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We would like to thank Cerrone S. Cabanos (Kyoto University) for his invaluable technical guidance on the analyses of the peptide sequences we described in this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jakubczyk, A.; Karaś, M.; Rybczyńska-Tkaczyk, K.; Zielińska, E.; Zieliński, D. Current Trends of Bioactive Peptides—New Sources and Therapeutic Effect. Foods 2020, 9, 846. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Fogacci, F.; Colletti, A. Potential Role of Bioactive Peptides in Prevention and Treatment of Chronic Diseases: A Narrative Review. Br. J. Pharmacol. 2017, 174, 1378–1394. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cardiovascular Diseases (CVD). 2017. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 6 September 2020).
- Kypreos, K.E.; Bitzur, R.; Karavia, E.A.; Xepapadaki, E.; Panayiotakopoulos, G.; Constantinou, C. Pharmacological Management of Dyslipidemia in Atherosclerosis: Limitations, Challenges, and New Therapeutic Opportunities. Angiology 2018, 70, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Stavropoulos, K.; Imprialos, K.; Athyros, V.; Doumas, M.; Karagiannis, A. Pharmacological Management of Cardiac Disease in Patients with Type 2 Diabetes: Insights into Clinical Practice. Curr. Vasc. Pharmacol. 2020, 18, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, K.; Ajmal, I.; Naz, T.; Fazili, A.; Bai, X.; Song, Y. Bioactive Functional Foods for Cardiovascular Diseases. Am. J. Biochem. Biotechnol. 2020, 16, 354–369. [Google Scholar] [CrossRef]
- Erdmann, K.; Cheung, B.W.Y.; Schröder, H. The Possible Roles of Food-Derived Bioactive Peptides in Reducing the Risk of Cardiovascular Disease. J. Nutr. Biochem. 2008, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Elmaliklis, I.-N.; Liveri, A.; Ntelis, B.; Paraskeva, K.; Goulis, I.; Koutelidakis, A. Increased Functional Foods’ Consumption and Mediterranean Diet Adherence May Have a Protective Effect in the Appearance of Gastrointestinal Diseases: A Case–Control Study. Medicines 2019, 6, 50. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Functional Food: What Are They? And Why Are They So Popular? Acta Sci. Nutr. Health 2018, 2, 26–27. [Google Scholar]
- Maphosa, Y.; Jideani, V. The Role of Legumes in Human Nutrition. In Functional Food—Improve Health through Adequate Food Maria Chavarri Hueda; Department of Food Science and Technology, Cape Peninsula University of Technology: Bellville, South Africa, 2017; pp. 103–122. ISBN 978-953-51-3440-4. [Google Scholar]
- Trinidad, T.P.; Mallillin, A.C.; Loyola, A.S.; Sagum, R.S.; Encabo, R.R. The Potential Health Benefits of Legumes as a Good Source of Dietary Fibre. Br. J. Nutr. 2010, 103, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Castillo, I.; Angelia, M.; Torio, M.; Belina-Aldemita, M. Antihypertensive Property of the Peptic and Chymotryptic Hydrolysates Derived from the Crude Protein Extract of Okra [Abelmoschus esculentus (L.) Moench} Seeds. Int. Food. Res. J. 2017, 24, 2586–2592. [Google Scholar]
- De Leon, R.; Torio, M.; Manalo, M.; Aguda, R. Isolation, Purification and Characterization of the Major Storage Protein in Cowpea (Vigna unguiculata) Seed with Bioactive Peptides Exhibiting Antiangiotensin I-Converting Enzyme Activity. In Proceedings of the 42nd Annual Convention of the Kapisanang Kimika ng Pilipinas—Southern Tagalog Chapter, Southeast Asian Regional Center for Graduate Study and Research in Agriculture (SEARCA), University of the Philippines Los Baños, Laguna, Philippines, 29–30 October 2013; p. 26. [Google Scholar]
- Distor, N.; Angelia, M.; San Pascual, J.; Torio, M.; Recuenco, M. Potential Antihypertensive Activities from the Peptic and A-Chymotryptic Hydrolysates of Purified 7S Globulins of Hyacinth Bean (Lablab purpureus L.). In Proceedings of the 44th Annual Convention of the Kapisanang Kimika ng Pilipinas—Southern Tagalog Chapter, B.P. International—Makiling, Jamboree Site, University of the Philippines Los Baños, College, Laguna, Philippines, 5–6 November 2015; p. 12. [Google Scholar]
- Viernes, L.; Garcia, R.; Torio, M.; Garcia, R. Antihypertensive Peptides from Vicilin, the Major Storage Protein of Mung Bean (Vigna radiata (L.) R. Wilczek). J. Biol. Sci. 2012, 12, 393–399. [Google Scholar] [CrossRef]
- Gomes, M.J.; Pagan, L.U.; Okoshi, M.P. Non-Pharmacological Treatment of Cardiovascular Disease | Importance of Physical Exercise. Arq. Bras. Cardiol. 2019, 113, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Alissa, E.M.; Ferns, G.A. Functional Foods and Nutraceuticals in the Primary Prevention of Cardiovascular Diseases. J. Nutr. Metab. 2012, 2012, 569486. [Google Scholar] [CrossRef] [PubMed]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric Assay and Properties of the Angiotensin-Converting Enzyme of Rabbit Lung. Biochem. Pharmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Karami, Z.; Akbari-Adergani, B. Bioactive Food Derived Peptides: A Review on Correlation between Structure of Bioactive Peptides and Their Functional Properties. J. Food Sci. Technol. 2019, 56, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Doyama, N.; Maruyama, N.; Utsumi, S.; Yoshikawa, M. Introduction of DPR, an Enterostatin Fragment Peptide, into Soybean β-Conglycinin α′ Subunit by Site-Directed Mutagenesis. Biosci. Biotechnol. Biochem. 2004, 68, 253–256. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Lee, N.; Kim, M.S.; Kwon, D.Y. Structure-Activity Relationships of the Peptide Ile-Ala-Val-Pro and Its Derivatives Revealed Using the Semi-Empirical AM1 Method. Chem. Nat. Compd. 2005, 41, 454–460. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.; Kwon, D.Y.; Yun, L. Design of a Highly Potent Inhibitory Peptide Acting as a Competitive Inhibitor of HMG-CoA Reductase. Amino Acids 2012, 43, 2015–2025. [Google Scholar] [CrossRef] [PubMed]
- Azani, N.; Babineau, M.; Bailey, C.D.; Banks, H.; Barbosa, A.R.; Pinto, R.B.; Boatwright, J.S.; Borges, L.M.; Brown, G.K.; Bruneau, A.; et al. (Legume Phylogeny Working Group) A New Subfamily Classification of the Leguminosae Based on a Taxonomically Comprehensive Phylogeny: The Legume Phylogeny Working Group (LPWG). Taxon 2017, 66, 44–77. [Google Scholar] [CrossRef]
- Yahara, T.; Javadi, F.; Onoda, Y.; de Queiroz, L.P.; Faith, D.P.; Prado, D.E.; Akasaka, M.; Kadoya, T.; Ishihama, F.; Davies, S.; et al. Global Legume Diversity Assessment: Concepts, Key Indicators, and Strategies. Taxon 2013, 62, 249–266. [Google Scholar] [CrossRef]
- Cardoso, D.; de Queiroz, L.P.; Pennington, R.T.; de Lima, H.C.; Fonty, É.; Wojciechowski, M.F.; Lavin, M. Revisiting the Phylogeny of Papilionoid Legumes: New Insights from Comprehensively Sampled Early-Branching Lineages. Am. J. Bot. 2012, 99, 1991–2013. [Google Scholar] [CrossRef]
- Sprent, J. Global distribution of legumes. In Legume. Nodulation: A Global Perspective; Sprent, J.I., Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2009; pp. 35–50. ISBN 978-1-4051-8175-4. [Google Scholar]
- Shuster-Gajzágó, I. Nutritional aspects of legumes. In Cultivated. Plants, Primarily as Food Sources; Fuleky, G., Ed.; Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO, Eolss Publishers: Paris, France, 2009; Volume 1, ISBN 1-84826-100-4. [Google Scholar]
- Bastianelli, D.; Grosjean, F.; Peyronnet, C.; Duparque, M.; Régnier, J.M. Feeding Value of Pea (Pisum sativum L.) 1. Chemical Composition of Different Categories of Pea. Anim. Sci. 1998, 67, 609–619. [Google Scholar] [CrossRef]
- Hove, E.L. Composition and Protein Quality of Sweet Lupin Seed. J. Sci. Food Agric. 1974, 25, 851–859. [Google Scholar] [CrossRef]
- Bouchenak, M.; Lamri-Senhadji, M. Nutritional Quality of Legumes, and Their Role in Cardiometabolic Risk Prevention: A Review. J. Med. Food 2013, 16, 185–198. [Google Scholar] [CrossRef]
- Cruz, F.; de Almeida, H.; dos Santos, D. Growth, Nutritional Status and Nitrogen Metabolism in Vigna unguiculata (L.) Walp. Is Affected by Aluminum. Aust. J. Crop. Sci. 2014, 8, 1132–1139. [Google Scholar]
- Adamu, A.; Ajayi, M.; Oyetunde, J. Inorganic and Proximate Nutritional Composition of Common Beans in Nigeria. EJPAC 2016, 3, 25–28. [Google Scholar]
- Caprioli, G.; Giusti, F.; Ballini, R.; Sagratini, G.; Vila-Donat, P.; Vittori, S.; Fiorini, D. Lipid Nutritional Value of Legumes: Evaluation of Different Extraction Methods and Determination of Fatty Acid Composition. Food Chem. 2016, 192, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Rincón, F.; Martínez, B.; Ibáñez, M.V. Proximate Composition and Antinutritive Substances in Chickpea (Cicer arietinum L.) as Affected by the Biotype Factor. J. Sci. Food Agric. 1998, 78, 382–388. [Google Scholar] [CrossRef]
- Marioli Nobile, C.; Carreras, J.; Grosso, R.; Inga, M.; Silva, M.; Aguilar, R.; Allende, M.; Badini, R.; Martinez, M. Proximate Composition and Seed Lipid Components of “Kabuli”-Type Chickpea (Cicer arietinum L.) from Argentina. Agric. Sci. 2013, 4, 729–737. [Google Scholar] [CrossRef]
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical Characteristics and Amino Acid Profile of Chickpea Cultivars Grown in Pakistan. J. Foodserv. 2006, 17, 94–101. [Google Scholar] [CrossRef]
- Nwadike, C.; Okere, A.; Nwosu, D.; Okoye, C.; Vange, T.; Apuyor, B. Proximate and Nutrient Composition of Some Common Bean (Phaseolus vulgaris L.) and Cowpea (Vigna unguiculata L. Walp.) Accessions of Jos-Plateau, Nigeria. J. Agric. Ecol. 2018, 15, 1–9. [Google Scholar] [CrossRef]
- Antova, G.A.; Stoilova, T.D.; Ivanova, M.M. Proximate and Lipid Composition of Cowpea (Vigna unguiculata L.) Cultivated in Bulgaria. J. Food Compos. Anal. 2014, 33, 146–152. [Google Scholar] [CrossRef]
- Punia, K.P. Darshan Proximate Composition, Phytic Acid, Polyphenols and Digestibility (In Vitro) of Four Brown Cowpea Varieties. Int. J. Food Sci. Nutr. 2000, 51, 189–193. [Google Scholar] [CrossRef]
- Henshaw, F. Varietal Differences in Physical Characteristics and Proximate Composition of Cowpea (Vigna unguiculata). WJAS 2008, 4, 302–306. [Google Scholar]
- Hacıseferoǧulları, H.; Gezer, İ.; Bahtiyarca, Y.; Mengeş, H.O. Determination of Some Chemical and Physical Properties of Sakız Faba Bean (Vicia faba L. var. Major). J. Food Eng. 2003, 60, 475–479. [Google Scholar] [CrossRef]
- Grela, E.R.; Günter, K.D. Fatty Acid Composition and Tocopherol Content of Some Legume Seeds. Anim. Feed. Sci. Technol. 1995, 52, 325–331. [Google Scholar] [CrossRef]
- Millar, K.A.; Gallagher, E.; Burke, R.; McCarthy, S.; Barry-Ryan, C. Proximate Composition and Anti-Nutritional Factors of Fava-Bean (Vicia faba), Green-Pea and Yellow-Pea (Pisum sativum) Flour. J. Food Compost. Anal. 2019, 82, 103233. [Google Scholar] [CrossRef]
- Hossain, S.; Ahmed, R.; Bhowmick, S.; Mamun, A.A.; Hashimoto, M. Proximate Composition and Fatty Acid Analysis of Lablab purpureus (L.) Legume Seed: Implicates to Both Protein and Essential Fatty Acid Supplementation. Springerplus 2016, 5, 1899. [Google Scholar] [CrossRef]
- Habib, H.M.; Theuri, S.W.; Kheadr, E.E.; Mohamed, F.E. Functional, Bioactive, Biochemical, and Physicochemical Properties of the Dolichos lablab Bean. Food Funct. 2017, 8, 872–880. [Google Scholar] [CrossRef]
- Vadivel, V.; Janardhanan, K. Diversity in Nutritional Composition of Wild Jack Bean (Canavalia ensiformis L. DC) Seeds Collected from South India. Food Chem. 2001, 74, 507–511. [Google Scholar] [CrossRef]
- Abitogun, A.; Olasehinde, E. Nutritional Evaluation of Seed and Characterization of Crude Jack Bean (Canavalia ensiformis) Oil. IOSR-JAC 2012, 1, 36–40. [Google Scholar] [CrossRef]
- Ramdath, D.D.; Padhi, E.M.T.; Sarfaraz, S.; Renwick, S.; Duncan, A.M. Beyond the Cholesterol-Lowering Effect of Soy Protein: A Review of the Effects of Dietary Soy and Its Constituents on Risk Factors for Cardiovascular Disease. Nutrients 2017, 9, 324. [Google Scholar] [CrossRef]
- De Carvalho, I.M.M.S. Effects of Water Stress on the Proximate Composition and Mineral Contents of Seeds of Two Lupins (Lupinus albus and Lupinus mutabilis). J. Food Qual. 2005, 28, 325–332. [Google Scholar] [CrossRef]
- Garcıa-López, P.M.; Muzquiz, M.; Ruiz-Lopez, M.A.; Zamora-Natera, J.F.; Burbano, C.; Pedrosa, M.M.; Cuadrado, C.; Garzón-De la Mora, P. Chemical Composition and Fatty Acid Profile of Several Mexican Wild Lupins. J. Food Compost. Anal. 2001, 14, 645–651. [Google Scholar] [CrossRef]
- Musco, N.; Cutrignelli, M.I.; Calabrò, S.; Tudisco, R.; Infascelli, F.; Grazioli, R.; Lo Presti, V.; Gresta, F.; Chiofalo, B. Comparison of Nutritional and Antinutritional Traits among Different Species (Lupinus albus L., Lupinus luteus L., Lupinus angustifolius L.) and Varieties of Lupin Seeds. J. Anim. Physiol. Anim. Nutr. 2017, 101, 1227–1241. [Google Scholar] [CrossRef] [PubMed]
- Opara, C.; Egbuonu, A.; Obike, C. Assessment of Proximate, Vitamins, Minerals and Anti-Nutrients Compositions of Unprocessed Vigna aconitifolia (Moth Bean) Seeds. ACRI 2017, 11, 1–7. [Google Scholar] [CrossRef]
- Shet, M.S.; Murugiswamy, B.; Madaiah, M. Lipid Profile and Fatty Acid Composition of Moth Bean (Vigna aconitefolia) Seeds. Fette Seifen Anstrichm. 1986, 88, 264–266. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Przybylski, R.; Sultana, B.; Ashraf, M. Chemical Composition and Antioxidant Activity of Seeds of Different Cultivars of Mungbean. J. Food Sci. 2007, 72, S503–S510. [Google Scholar] [CrossRef]
- El-Adawy, T.A.; Rahma, E.H.; El-Bedawey, A.A.; El-Beltagy, A.E. Nutritional Potential and Functional Properties of Germinated Mung Bean, Pea and Lentil Seeds. Plant Foods Hum. Nutr. 2003, 58, 1–13. [Google Scholar] [CrossRef]
- Abdel-Rahman, E.-S.A.; El-Fishawy, F.A.; El-Geddawy, M.A.; Kurz, T.; El-Rify, M.N. The Changes in the Lipid Composition of Mung Bean Seeds as Affected by Processing Methods. Int. J. Food Eng. 2007, 3. [Google Scholar] [CrossRef]
- Hove, E.L.; King, S.; Hill, G.D. Composition, Protein Quality, and Toxins of Seeds of the Grain Legumes Glycine max, Lupinus spp., Phaseolus spp., Pisum sativum, and Vicia faba. N. Z. J. Agric. Res. 1978, 21, 457–462. [Google Scholar] [CrossRef]
- Atasie, V.; Akinhanmi, T.; Ojiodu, C. Proximate Analysis and Physico-Chemical Properties of Groundnut (Arachis hypogaea L.). Pak. J. Nut. 2009, 8, 194–197. [Google Scholar] [CrossRef]
- Etiosa, O.; Chika, N.; Benedicta, A. Mineral and Proximate Composition of Soya Bean. Asian J. Phys. Chem. Sci. 2017, 4, 1–6. [Google Scholar] [CrossRef]
- Padgette, S.R.; Taylor, N.B.; Nida, D.L.; Bailey, M.R.; MacDonald, J.; Holden, L.R.; Fuchs, R.L. The Composition of Glyphosate-Tolerant Soybean Seeds Is Equivalent to That of Conventional Soybeans. J. Nutr. 1996, 126, 702–716. [Google Scholar] [CrossRef]
- Fehr, W.R. Breeding for Modified Fatty Acid Composition in Soybean. Crop. Sci. 2007, 47, S-72. [Google Scholar] [CrossRef]
- Bazzano, L.; Tees, M.T.; Nguyen, C.H. Effect of Non-Soy Legume Consumption on Cholesterol Levels: A Meta-Analysis of Randomized Controlled Trial. Circulation 2008, 118, S1122. [Google Scholar]
- Wang, H.X.; Ng, T.B. An Antifungal Peptide from Red Lentil Seeds. Peptides 2007, 28, 547–552. [Google Scholar] [CrossRef]
- Malaguti, M.; Dinelli, G.; Leoncini, E.; Bregola, V.; Bosi, S.; Cicero, A.; Hrelia, S. Bioactive Peptides in Cereals and Legumes: Agronomical, Biochemical and Clinical Aspects. Int. J. Mol. Sci. 2014, 15, 21120–21135. [Google Scholar] [CrossRef] [PubMed]
- Jayathilake, C.; Visvanathan, R.; Deen, A.; Bangamuwage, R.; Jayawardana, B.C.; Nammi, S.; Liyanage, R. Cowpea: An Overview on Its Nutritional Facts and Health Benefits. J. Sci. Food Agric. 2018, 98, 4793–4806. [Google Scholar] [CrossRef] [PubMed]
- Rizkalla, S.W.; Bellisle, F.; Slama, G. Health Benefits of Low Glycaemic Index Foods, Such as Pulses, in Diabetic Patients and Healthy Individuals. Br. J. Nutr. 2002, 88, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Serventi, L.; Dsouza, L. Bioactives in legumes. In Upcycling Legume Water: From Wastewater to Food Ingredients; Springer: Cham, Switzerland, 2020; pp. 139–153. ISBN 978-3-030-42468-8. [Google Scholar]
- Moreno-Valdespino, C.A.; Luna-Vital, D.; Camacho-Ruiz, R.M.; Mojica, L. Bioactive Proteins and Phytochemicals from Legumes: Mechanisms of Action Preventing Obesity and Type-2 Diabetes. Food Res. Int. 2019, 108905. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Entezari, M.H.; Iraj, B.; Askari, G.R. The Effects of Consumption of Bread Fortified With Soy Bean Flour on Metabolic Profile in Type 2 Diabetic Women: A Cross-over Randomized Controlled Clinical Trial. Int. J. Prev. Med. 2014, 5, 1529–1536. [Google Scholar] [PubMed]
- Garg, S.; Lule, V.K.; Malik, R.K. Soy Bioactive Components in Functional Perspective: A Review. Int. J. Food Prop. 2016, 19, 2550–2574. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Genistein, a Soy Phytoestrogen, Up-Regulates the Expression of Human Endothelial Nitric Oxide Synthase and Lowers Blood Pressure in Spontaneously Hypertensive Rats. J. Nutr. 2008, 138, 297–304. [Google Scholar] [CrossRef]
- Zhu, Z.; Jiang, W.; Thompson, H.J. Edible Dry Bean Consumption (Phaseolus vulgaris L.) Modulates Cardiovascular Risk Factors and Diet-Induced Obesity in Rats and Mice. Br. J. Nutr. 2012, 108, S66–S73. [Google Scholar] [CrossRef]
- Thompson, H.J.; McGinley, J.N.; Neil, E.S.; Brick, M.A. Beneficial Effects of Common Bean on Adiposity and Lipid Metabolism. Nutrients 2017, 9, 998. [Google Scholar] [CrossRef] [PubMed]
- Abeysekara, S.; Chilibeck, P.D.; Vatanparast, H.; Zello, G.A. A Pulse-Based Diet Is Effective for Reducing Total and LDL-Cholesterol in Older Adults. Br. J. Nutr. 2012, 108, S103–S110. [Google Scholar] [CrossRef] [PubMed]
- Winham, D.M.; Hutchins, A.M. Baked Bean Consumption Reduces Serum Cholesterol in Hypercholesterolemic Adults. Nutr. Res. 2007, 27, 380–386. [Google Scholar] [CrossRef]
- Kabagambe, E.K.; Baylin, A.; Ruiz-Narvarez, E.; Siles, X.; Campos, H. Decreased Consumption of Dried Mature Beans Is Positively Associated with Urbanization and Nonfatal Acute Myocardial Infarction. J. Nutr. 2005, 135, 1770–1775. [Google Scholar] [CrossRef] [PubMed]
- Flight, I.; Clifton, P. Cereal Grains and Legumes in the Prevention of Coronary Heart Disease and Stroke: A Review of the Literature. Eur. J. Clin. Nutr. 2006, 60, 1145–1159. [Google Scholar] [CrossRef]
- Pennacchio, L.A.; Olivier, M.; Hubacek, J.A.; Cohen, J.-C.; Cox, D.R.; Fruchart, J.C.; Krauss, R.M.; Rubin, E.M. An Apolipoprotein Influencing Triglycerides in Humans and Mice Revealed by Comparative Sequencing. Science 2001, 294, 169. [Google Scholar] [CrossRef] [PubMed]
- Plump, A.S.; Smith, J.D.; Hayek, T.; Aalto-Setälä, K.; Walsh, A.; Verstuyft, J.G.; Rubin, E.M.; Breslow, J.L. Severe Hypercholesterolemia and Atherosclerosis in Apolipoprotein E-Deficient Mice Created by Homologous Recombination in ES Cells. Cell 1992, 71, 343–353. [Google Scholar] [CrossRef]
- Jang, Y.; Kim, J.Y.; Kim, O.Y.; Lee, J.E.; Cho, H.; Ordovas, J.M.; Lee, J.H. The −1131T→C Polymorphism in the Apolipoprotein A5 Gene Is Associated with Postprandial Hypertriacylglycerolemia; Elevated Small, Dense LDL Concentrations; and Oxidative Stress in Non-Obese Korean Men. Am. J. Clin. Nutr. 2004, 80, 832–840. [Google Scholar] [CrossRef][Green Version]
- Jang, Y.; Paik, J.K.; Hyun, Y.J.; Chae, J.S.; Kim, J.Y.; Choi, J.R.; Lee, S.H.; Shin, D.-J.; Ordovas, J.M.; Lee, J.H. The Apolipoprotein A5 -1131T>C Promoter Polymorphism in Koreans: Association with Plasma APOA5 and Serum Triglyceride Concentrations, LDL Particle Size and Coronary Artery Disease. Clin. Chim. Acta 2009, 402, 83–87. [Google Scholar] [CrossRef]
- Talmud, P.J.; Martin, S.; Taskinen, M.-R.; Frick, M.H.; Nieminen, M.S.; Kesäniemi, Y.A.; Pasternarck, A.; Humphries, S.E.; Syvänne, M. APOA5 Gene Variants, Lipoprotein Particle Distribution, and Progression of Coronary Heart Disease: Results from the LOCAT Study. J. Lipid. Res. 2004, 45, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Nabika, T.; Nasreen, S.; Kobayashi, S.; Masuda, J. The Genetic Effect of the Apoprotein AV Gene on the Serum Triglyceride Level in Japanese. Atherosclerosis 2002, 165, 201–204. [Google Scholar] [CrossRef]
- Charriere, S.; Bernard, S.; Aqallal, M.; Merlin, M.; Billon, S.; Perrot, L.; Le Coquil, E.; Sassolas, A.; Moulin, P.; Marcais, C. Association of APOA5 −1131T>C and S19W Gene Polymorphisms with Both Mild Hypertriglyceridemia and Hyperchylomicronemia in Type 2 Diabetic Patients. Clin. Chim. Acta 2008, 394, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.; Chae, J.S.; Kim, O.Y.; Park, H.J.; Kim, J.Y.; Paik, J.K.; Lee, S.-H.; Lee, J.H. APOA5-1131T>C Genotype Effects on Apolipoprotein A5 and Triglyceride Levels in Response to Dietary Intervention and Regular Exercise (DIRE) in Hypertriglyceridemic Subjects. Atherosclerosis 2010, 211, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kim, M.; Chae, J.S.; Lee, S.-H.; Lee, J.H. Consumption of Whole Grains and Legumes Modulates the Genetic Effect of the APOA5 -1131C Variant on Changes in Triglyceride and Apolipoprotein A-V Concentrations in Patients with Impaired Fasting Glucose or Newly Diagnosed Type 2 Diabetes. Trials 2014, 15, 100. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Amaral, A.L.S.; Demonte, A.; Zanelli, C.F.; Capraro, J.; Duranti, M.; Neves, V.A. Hypocholesterolaemic Effect of Rat-Administered Oral Doses of the Isolated 7S Globulins from Cowpeas and Adzuki Beans. J. Nutr. Sci. 2015, 4, e7. [Google Scholar] [CrossRef]
- Washburn, S.; Burke, G.; Morgan, T.; Anthony, M. Effect of Soy Protein Supplementation on Serum Lipoproteins, Blood Pressure, and Menopausal Symptoms in Perimenopausal Women. Menopause 1999, 6, 7–13. [Google Scholar] [CrossRef]
- Burke, V.; Hodgson, J.; Beilin, L.; Giangiulioi, N.; Rogers, P.; Puddey, I. Dietary Protein and Soluble Fiber Reduce Ambulatory Blood Pressure in Treated Hypertensives. Hypertension 2001, 38, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Darmadi-Blackberry, I.; Wahlqvist, M.L.; Kouris-Blazos, A.; Steen, B.; Lukito, W.; Horie, Y.; Horie, K. Legumes: The Most Important Dietary Predictor of Survival in Older People of Different Ethnicities. Asia Pac. J. Clin. Nutr. 2004, 13, 217–220. [Google Scholar] [PubMed]
- He, J.; Gu, D.; Wu, X.; Chen, J.; Duan, X.; Chen, J.; Whelton, P.K. Effect of Soybean Protein on Blood Pressure: A Randomized, Controlled Trial. Ann. Intern. Med. 2005, 143, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boye, J.; Maltais, A. Pulses a Novel Protein Source. Agro Food Ind. Hi Tech 2011, 22, 24–26. [Google Scholar]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Monetti, M.; Pazzucconi, F.; Gatti, E. Soy and Cholesterol Reduction: Clinical Experience. J. Nutr. 1995, 125, 598S–605S. [Google Scholar] [CrossRef]
- Madani, S.; Prost, J.; Narce, M.; Belleville, J. VLDL Metabolism in Rats Is Affected by the Concentration and Source of Dietary Protein. J. Nutr. 2003, 133, 4102–4106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Anderson, J.W.; Major, A.W. Pulses and Lipaemia, Short- and Long-Term Effect: Potential in the Prevention of Cardiovascular Disease. Br. J. Nutr. 2002, 88, 263–271. [Google Scholar] [CrossRef]
- Reynolds, K.; Chin, A.; Lees, K.A.; Nguyen, A.; Bujnowski, D.; He, J. A Meta-Analysis of the Effect of Soy Protein Supplementation on Serum Lipids. Am. J. Cardiol. 2006, 98, 633–640. [Google Scholar] [CrossRef]
- Bazzano, L.A.; Thompson, A.M.; Tees, M.T.; Nguyen, C.H.; Winham, D.M. Non-Soy Legume Consumption Lowers Cholesterol Levels: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 94–103. [Google Scholar] [CrossRef]
- Belski, R. Chapter 4—Fiber, protein, and lupin-enriched foods: Role for improving cardiovascular health. In Advances in Food and Nutrition Research; Henry, J., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 66, pp. 147–215. ISBN 1043-4526. [Google Scholar]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Murguía, F.; Cruz, C.; Hernández-Pando, R.; Aguilar-Salinas, C.A.; Pedraza-Chaverri, J.; Correa-Rotter, R.; Torres, N. A Soy Protein Diet Alters Hepatic Lipid Metabolism Gene Expression and Reduces Serum Lipids and Renal Fibrogenic Cytokines in Rats with Chronic Nephrotic Syndrome. J. Nutr. 2002, 132, 2562–2569. [Google Scholar] [CrossRef]
- Sirtori, C.; Galli, G.; Lovati, M.; Carrara, P.; Bosisio, E.; Kienle, M. Effects of Dietary Proteins on the Regulation of Liver Lipoprotein Receptors in Rats. J. Nutr. 1984, 114, 1493–1500. [Google Scholar] [CrossRef]
- Vu-Dac, N.; Gervois, P.; Torra, I.P.; Fruchart, J.C.; Kosykh, V.; Kooistra, T.; Princen, H.M.; Dallongeville, J.; Staels, B. Retinoids Increase Human Apo C-III Expression at the Transcriptional Level via the Retinoid X Receptor. Contribution to the Hypertriglyceridemic Action of Retinoids. J. Clin. Investig. 1998, 102, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Mei, J.; Wood, C. Effect of Soy Proteins and Isoflavones on Lipid Metabolism and Involved Gene Expression. Front. Biosci. 2008, 13, 2660–2673. [Google Scholar] [CrossRef]
- Xiao, C.W.; Mei, J.; Huang, W.; Wood, C.; L’Abbé, M.R.; Gilani, G.S.; Cooke, G.M.; Curran, I.H. Dietary Soy Protein Isolate Modifies Hepatic Retinoic Acid Receptor-β Proteins and Inhibits Their DNA Binding Activity in Rats. J. Nutr. 2007, 137, 1–6. [Google Scholar] [CrossRef]
- Seppo, L.; Jauhiainen, T.; Poussa, T.; Korpela, R. A Fermented Milk High in Bioactive Peptides Has a Blood Pressure–Lowering Effect in Hypertensive Subjects. Am. J. Clin. Nutr. 2003, 77, 326–330. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Ko, J.H.; Ahn, C.W.; Lee, H.H.; Shin, J.K.; Chang, S.J.; Park, C.S.; Kang, J.H. In Vivo and in Vitro Application of Black Soybean Peptides in the Amelioration of Endoplasmic Reticulum Stress and Improvement of Insulin Resistance. Life Sci. 2010, 86, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.E.; Krehl, W.A.; Stone, D.B.; Lopez, A. Dietary Carbohydrates and Low Cholesterol Diets: Effects on Serum Lipids of Man. Am. J. Clin. Nutr. 1967, 20, 198–208. [Google Scholar] [CrossRef]
- Lyn-Cook, B.D.; Blann, E.; Payne, P.W.; Bo, J.; Sheehan, D.; Medlock, K. Methylation Profile and Amplification of Proto-Oncogenes in Rat Pancreas Induced with Phytoestrogens. Proc. Soc. Exp. Biol. Med. 1995, 208, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Johnstone, B.M.; Cook-Newell, M.E. Meta-Analysis of the Effects of Soy Protein Intake on Serum Lipids. N. Engl. J. Med. 1995, 333, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Sirtori, C.; Galli, C.; Anderson, J.; Sirtori, E.; Arnoldi, A. Functional Foods for Dyslipidaemia and Cardiovascular Risk Prevention. Nutr. Res. Rev. 2009, 22, 244–261. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, D.J.A.; Mirrahimi, A.; Srichaikul, K.; Berryman, C.E.; Wang, L.; Carleton, A.; Abdulnour, S.; Sievenpiper, J.L.; Kendall, C.W.C.; Kris-Etherton, P.M. Soy Protein Reduces Serum Cholesterol by Both Intrinsic and Food Displacement Mechanisms. J. Nutr. 2010, 140, 2302S–2311S. [Google Scholar] [CrossRef] [PubMed]
- Forsythe, W.A., III. Comparison of Dietary Casein or Soy Protein Effects on Plasma Lipids and Hormone Concentrations in the Gerbil (Meriones unguiculatus). J. Nutr. 1986, 116, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Sugano, M. Effects of Addition of Sulfur-Containing Amino Acids and Glycine to Soybean Protein and Casein on Serum Cholesterol Levels of Rats. J. Nutr. Sci. Vitaminol. 1989, 35, 323–332. [Google Scholar] [CrossRef]
- Nagata, C.; Takatsuka, N.; Kurisu, Y.; Shimizu, H. Decreased Serum Total Cholesterol Concentration Is Associated with High Intake of Soy Products in Japanese Men and Women. J. Nutr. 1998, 128, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Ho, S.C.; Woo, J.L.F.; Leung, S.S.F.; Sham, A.L.K.; Lam, T.H.; Janus, E.D. Intake of Soy Products Is Associated with Better Plasma Lipid Profiles in the Hong Kong Chinese Population. J. Nutr. 2000, 130, 2590–2593. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Arnoldi, A.; Kurowska, E.; Carroll, K.K.; Sirtori, C.R. Soy Protein Peptides Regulate Cholesterol Homeostasis in Hep G2 Cells. J. Nutr. 2000, 130, 2543–2549. [Google Scholar] [CrossRef]
- Imai, S. Soybean and Processed Soy Foods Ingredients, and Their Role in Cardiometabolic Risk Prevention. Recent Pat. Food Nutr. Agric. 2015, 7, 75–82. [Google Scholar] [CrossRef]
- Sugano, M.; Goto, S.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. Cholesterol-Lowering Activity of Various Undigested Fractions of Soybean Protein in Rats. J. Nutr. 1990, 120, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-F.; Yamamoto, S.; Chung, H.-M.; Chung, S.-Y.; Miyatani, S.; Mori, M.; Okita, T.; Sugano, M. Antihypercholesterolemic Effect of Undigested Fraction of Soybean Protein in Young Female Volunteers. J. Nutr. Sci. Vitaminol. 1995, 41, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Mackay, S.; Ball, M. Do Beans and Oat Bran Add to the Effectiveness of a Low-Fat Diet? Eur. J. Clin. Nutr. 1992, 46, 641–648. [Google Scholar] [PubMed]
- Chen, H.; Muramoto, K.; Yamauchi, F. Structural Analysis of Antioxidative Peptides from Soybean β-Conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar] [CrossRef]
- Wade, A.M.; Tucker, H.N. Antioxidant Characteristics of L-Histidine. J. Nutr. Biochem. 1998, 9, 308–315. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-Oxidative Capacity of Enzymatically Released Peptides from Soybean Protein Isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Park, S.; Lee, J.; Baek, H.; Lee, H. Purification and Characterization of Antioxidant Peptides from Soy Protein Hydrolysate. J. Food Biochem. 2010, 34, 120–132. [Google Scholar] [CrossRef]
- Chen, H.-M.; Muramoto, K.; Yamauchi, F.; Nokihara, K. Antioxidant Activity of Designed Peptides Based on the Antioxidative Peptide Isolated from Digests of a Soybean Protein. J. Agric. Food Chem. 1996, 44, 2619–2623. [Google Scholar] [CrossRef]
- Xiao, C.; Wood, C.; Huang, W.; Abbé, M.; Gilani, G.; Gerard, M.; Curran, I. Tissue-Specific Regulation of Acetyl-CoA Carboxylase Gene Expression by Dietary Soya Protein Isolate in Rats. Br. J. Nutr. 2006, 95, 1048–1054. [Google Scholar] [CrossRef]
- Sugano, M.; Yamada, Y.; Yoshida, K.; Hashimoto, Y.; Matsuo, T.; Kimoto, M. The Hypocholesterolemic Action of the Undigested Fraction of Soybean Protein in Rats. Atherosclerosis 1988, 72, 115. [Google Scholar] [CrossRef]
- Huff, M.; Carroll, K. Effects of Dietary Protein on Turnover, Oxidation, and Absorption of Cholesterol, and on Steroid Excretion in Rabbits. J. Lipid Res. 1980, 21, 546–548. [Google Scholar] [CrossRef]
- Singh, B.P.; Vij, S.; Hati, S. Functional Significance of Bioactive Peptides Derived from Soybean. Peptides 2014, 54, 171–179. [Google Scholar] [CrossRef]
- Anthony, M.; Clarkson, T.; Bullock, B.; Wagner, J. Soy Protein versus Soy Phytoestrogens in the Prevention of Diet-Induced Coronary Artery Atherosclerosis of Male Cynomolgus Monkeys. Arterioscler. Thromb. Vasc. Biol. 1997, 17, 2524–2531. [Google Scholar] [CrossRef] [PubMed]
- Hall, W.L.; Vafeiadou, K.; Hallund, J.; Bugel, S.; Reimann, M.; Koebnick, C.; Zunft, H.J.F.; Ferrari, M.; Branca, F.; Dadd, T.; et al. Soy-Isoflavone-Enriched Foods and Markers of Lipid and Glucose Metabolism in Postmenopausal Women: Interactions with Genotype and Equol Production. Am. J. Clin. Nutr. 2006, 83, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, B.F.; Zougman, A.; Masse, R.; Mulligan, C. Production and Characterization of Bioactive Peptides from Soy Hydrolysate and Soy-Fermented Food. Food Res. Int. 2004, 37, 123–131. [Google Scholar] [CrossRef]
- Ferreira, E.D.S.; Silva, M.A.; Demonte, A.; Neves, V.A. β-Conglycinin (7S) and Glycinin (11S) Exert a Hypocholesterolemic Effect Comparable to That of Fenofibrate in Rats Fed a High-Cholesterol Diet. J. Funct. Foods 2010, 2, 275–283. [Google Scholar] [CrossRef]
- Ferreira, E.D.S.; Silva, M.A.; Demonte, A.; Neves, V.A. Soy β-Conglycinin (7S Globulin) Reduces Plasma and Liver Cholesterol in Rats Fed Hypercholesterolemic Diet. J. Med. Food 2010, 14, 94–100. [Google Scholar] [CrossRef]
- Duranti, M.; Lovati, M.R.; Dani, V.; Barbiroli, A.; Scarafoni, A.; Castiglioni, S.; Ponzone, C.; Morazzoni, P. The α′ Subunit from Soybean 7S Globulin Lowers Plasma Lipids and Upregulates Liver β-VLDL Receptors in Rats Fed a Hypercholesterolemic Diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar] [CrossRef] [PubMed]
- Kohno, M.; Hirotsuka, M.; Kito, M.; Matsuzawa, Y. Decreases in Serum Triacylglycerol and Visceral Fat Mediated by Dietary Soybean β-Conglycinin. J. Atheroscler. Thromb. 2006, 13, 247–255. [Google Scholar] [CrossRef]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular Nutraceutics as a Mean to Investigate the Positive Effects of Legume Seed Proteins on Human Health. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar] [CrossRef]
- Martinez-Villaluenga, C.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-Conglycinin Embeds Active Peptides That Inhibit Lipid Accumulation in 3T3-L1 Adipocytes in Vitro. J. Agric. Food Chem. 2008, 56, 10533–10543. [Google Scholar] [CrossRef]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; Gonzalez de Mejia, E. β-Conglycinins among Sources of Bioactives in Hydrolysates of Different Soybean Varieties That Inhibit Leukemia Cells in Vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Villaluenga, C.; Dia, V.P.; Berhow, M.; Bringe, N.A.; Gonzalez de Mejia, E. Protein Hydrolysates from β-Conglycinin Enriched Soybean Genotypes Inhibit Lipid Accumulation and Inflammation in Vitro. Mol. Nutr. Food Res. 2009, 53, 1007–1018. [Google Scholar] [CrossRef]
- Pak, V.V.; Koo, M.S.; Kasymova, T.D.; Kwon, D.Y. Isolation and Identification of Peptides from Soy 11S-Globulin with Hypocholesterolemic Activity. Chem. Nat. Compd. 2005, 41, 710–714. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Corsini, A.; Granata, A.; Frattini, R.; Fumagalli, R.; Sirtori, C.R. Low Density Lipoprotein Receptor Activity Is Modulated by Soybean Globulins in Cell Culture. J. Nutr. 1992, 122, 1971. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Corsini, A.; Granata, A.; Fumagalli, R.; Sirtori, C.R. 7S Globulin from Soybean Is Metabolized in Human Cell Cultures by a Specific Uptake and Degradation System. J. Nutr. 1996, 126, 2831. [Google Scholar]
- Mochizuki, Y.; Maebuchi, M.; Kohno, M.; Hirotsuka, M.; Wadahama, H.; Moriyama, T.; Kawada, T.; Urade, R. Changes in Lipid Metabolism by Soy β-Conglycinin-Derived Peptides in HepG2 Cells. J. Agric. Food Chem. 2009, 57, 1473–1480. [Google Scholar] [CrossRef]
- Manzoni, C.; Lovati, M.R.; Gianazza, E.; Morita, Y.; Sirtori, C.R. Soybean Protein Products as Regulators of Liver Low-Density Lipoprotein Receptors. II. A−α‘ Rich Commercial Soy Concentrate and α‘ Deficient Mutant Differently Affect Low-Density Lipoprotein Receptor Activation. J. Agric. Food Chem. 1998, 46, 2481–2484. [Google Scholar] [CrossRef]
- Manzoni, C.; Duranti, M.; Eberini, I.; Scharnag, H.; März, W.; Castiglioni, S.; Lovati, M. Subcellular Localization of Soybean 7S Globulin in HepG2 Cells and LDL Receptor Up-Regulation by Its α′ Constituent Subunit. J. Nutr. 2003, 133, 2149–2155. [Google Scholar] [CrossRef]
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean Beta-Conglycinin Diet Suppresses Serum Triglyceride Levels in Normal and Genetically Obese Mice by Induction of Beta-Oxidation, Downregulation of Fatty Acid Synthase, and Inhibition of Triglyceride Absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef]
- Shimomura, I.; Bashmakov, Y.; Horton, J.D. Increased Levels of Nuclear SREBP-1c Associated with Fatty Livers in Two Mouse Models of Diabetes Mellitus. J. Biol. Chem. 1999, 274, 30028–30032. [Google Scholar] [CrossRef] [PubMed]
- Shukla, A.; Brandsch, C.; Bettzieche, A.; Hirche, F.; Stangl, G.I.; Eder, K. Isoflavone-Poor Soy Protein Alters the Lipid Metabolism of Rats by SREBP-Mediated Down-Regulation of Hepatic Genes. J. Nutr. Biochem. 2007, 18, 313–321. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Gianazza, E.; Sirtori, C.R. Soybean Protein Products as Regulators of Liver Low-Density Lipoprotein Receptors. I. Identification of Active β-Conglycinin Subunits. J. Agric. Food Chem. 1998, 46, 2474–2480. [Google Scholar] [CrossRef]
- Galvez, A. Identification of Lunasin as the Active Component in Soy Protein Responsible for Reducing LDL Cholesterol and Risk of Cardiovascular Disease. Circulation 2012, 126, A10693. [Google Scholar] [CrossRef]
- Fontanari, G.G.; Batistuti, J.P.; Da Cruz, R.J.; Saldiva, P.H.N.; Arêas, J.A.G. Cholesterol-Lowering Effect of Whole Lupin (Lupinus albus) Seed and Its Protein Isolate. Food Chem. 2012, 132, 1521–1526. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Lovati, M.R.; Manzoni, C.; Castiglioni, S.; Duranti, M.; Magni, C.; Morandi, S.; D’Agostina, A.; Arnoldi, A. Proteins of White Lupin Seed, a Naturally Isoflavone-Poor Legume, Reduce Cholesterolemia in Rats and Increase LDL Receptor Activity in HepG2 Cells. J. Nutr. 2004, 134, 18–23. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Eder, K.; Stangl, G. Lupin Protein Acts Hypocholesterolemic and Increases Milk Fat Content in Lactating Rats by Influencing the Expression of Genes Involved in Cholesterol Homeostasis and Triglyceride Synthesis. Mol. Nutr. Food Res. 2009, 53, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, M.; Parolini, C.; Diani, E.; Rigamonti, E.; Cornelli, L.; Arnoldi, A.; Sirtori, C.R.; Chiesa, G. Hypolipidaemic and Anti-Atherosclerotic Effects of Lupin Proteins in a Rabbit Model. Br. J. Nutr. 2008, 100, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Parolini, C.; Rigamonti, E.; Marchesi, M.; Busnelli, M.; Cinquanta, P.; Manzini, S.; Sirtori, C.R.; Chiesa, G. Cholesterol-Lowering Effect of Dietary Lupinus angustifolius Proteins in Adult Rats through Regulation of Genes Involved in Cholesterol Homeostasis. Food Chem. 2012, 132, 1475–1479. [Google Scholar] [CrossRef] [PubMed]
- Bähr, M.; Fechner, A.; Krämer, J.; Kiehntopf, M.; Jahreis, G. Lupin Protein Positively Affects Plasma LDL Cholesterol and LDL:HDL Cholesterol Ratio in Hypercholesterolemic Adults after Four Weeks of Supplementation: A Randomized, Controlled Crossover Study. Nutr. J. 2013, 12, 107. [Google Scholar] [CrossRef]
- Vairappan, B. Chapter 15—Cholesterol Regulation by Leptin in Alcoholic Liver Disease. In Molecular Aspects of Alcohol and Nutrition; Patel, V.B., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 187–200. ISBN 978-0-12-800773-0. [Google Scholar]
- Robinet, P.; Smith, J.D. Chapter 13—Role of Autophagy in Atherogenesis. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Hayat, M.A., Ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 203–211. ISBN 978-0-12-801033-4. [Google Scholar]
- Weiße, K.; Brandsch, C.; Zernsdorf, B.; Nkengfack Nembongwe, G.S.; Hofmann, K.; Eder, K.; Stangl, G.I. Lupin Protein Compared to Casein Lowers the LDL Cholesterol:HDL Cholesterol-Ratio of Hypercholesterolemic Adults. Eur. J. Nutr. 2010, 49, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, C.; Aiello, G.; Arnoldi, A.; Lammi, C. Investigations on the Hypocholesterolaemic Activity of LILPKHSDAD and LTFPGSAED, Two Peptides from Lupin β-Conglutin: Focus on LDLR and PCSK9 Pathways. J. Funct. Foods 2017, 32, 1–8. [Google Scholar] [CrossRef]
- Radtke, J.; Schutkowski, A.; Brandsch, C.; Hirche, F.; Hasenkopf, K.; Stangl, G.I. Isolated Conglutin γ from Lupin, but Not Phytate, Lowers Serum Cholesterol without Influencing Vascular Lesion Development in the ApoE-Deficient Mouse Model. Plant Foods Hum. Nutr. 2015, 70, 113–118. [Google Scholar] [CrossRef]
- Li, H.; Aluko, R.E. Identification and Inhibitory Properties of Multifunctional Peptides from Pea Protein Hydrolysate. J. Agric. Food Chem. 2010, 58, 11471–11476. [Google Scholar] [CrossRef]
- Lasekan, J.B.; Gueth, L.; Khan, S. Influence of Dietary Golden Pea Proteins versus Casein on Plasma and Hepatic Lipids in Rats. Nutr. Res. 1995, 15, 71–84. [Google Scholar] [CrossRef]
- Rigamonti, E.; Parolini, C.; Marchesi, M.; Diani, E.; Brambilla, S.; Sirtori, C.R.; Chiesa, G. Hypolipidemic Effect of Dietary Pea Proteins: Impact on Genes Regulating Hepatic Lipid Metabolism. Mol. Nutr. Food Res. 2010, 54, S24–S30. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, J.; Stangl, G.I.; Eder, K. Dietary Pea Protein Stimulates Bile Acid Excretion and Lowers Hepatic Cholesterol Concentration in Rats. J. Anim. Physiol. Anim. Nutr. 2008, 92, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Bhullar, K.S.; Fan, H.; Liao, W.; Qiao, Y.; Su, D.; Wu, J. Regulatory Effects of a Pea-Derived Peptide Leu-Arg-Trp (LRW) on Dysfunction of Rat Aortic Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2020, 68, 3947–3953. [Google Scholar] [CrossRef] [PubMed]
- Guang, C.; Phillips, R. Angiotensin I-Converting Enzyme Inhibitory Peptides from Hydrolyzed Cowpea Flour. J. Food Agric. Environ. 2005, 10, 55–59. [Google Scholar]
- Segura-Campos, M.R.; Chel Guerrero, L.A.; Betancur Ancona, D.A. Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activities of Peptide Fractions Extracted by Ultrafiltration of Cowpea Vigna unguiculata Hydrolysates. J. Sci. Food Agric. 2010, 90, 2512–2518. [Google Scholar] [CrossRef]
- Frota, K.M.G.; Mendonça, S.; Saldiva, P.H.N.; Cruz, R.J.; Arêas, J.A.G. Cholesterol-Lowering Properties of Whole Cowpea Seed and Its Protein Isolate in Hamsters. J. Food Sci. 2008, 73, H235–H240. [Google Scholar] [CrossRef]
- Macarulla, M.T.; Medina, C.; Diego, M.A.D.; Chávarri, M.; Zulet, M.Á.; Martínez, J.A.; Nöel-Suberville, C.; Higueret, P.; Portillo, M.P. Effects of the Whole Seed and a Protein Isolate of Faba Bean (Vicia faba) on the Cholesterol Metabolism of Hypercholesterolaemic Rats. Br. J. Nutr. 2001, 85, 607–614. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, T.; Okazaki, Y.; Katayama, T.; Hori, K. Dietery Lectin Lowers Serum Cholesterol and Raises Fecal Neutral Sterols in Cholesterol-Fed Rats. J. Nutri. Sci. Vitaminol. 2005, 51, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shi, Y.; Liu, H.; Le, G. Antihypertensive Effect of Alcalase Generated Mung Bean Protein Hydrolysates in Spontaneously Hypertensive Rats. Eur. Food Res. Technol. 2006, 222, 733–736. [Google Scholar] [CrossRef]
- Yao, Y.; Hao, L.; Shi, Z.; Wang, L.; Cheng, X.; Wang, S.; Ren, G. Mung Bean Decreases Plasma Cholesterol by Up-Regulation of CYP7A1. Plant Foods Hum. Nutr. 2014, 69, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Zhu, Y.; Ren, G. Mung Bean Protein Increases Plasma Cholesterol by Up-Regulation of Hepatic HMG-CoA Reductase, and CYP7A1 in mRNA Levels. J. Food Nutr. Res. 2014, 2, 770–775. [Google Scholar] [CrossRef][Green Version]
- Nakatani, A.; Li, X.; Miyamoto, J.; Igarashi, M.; Watanabe, H.; Sutou, A.; Watanabe, K.; Motoyama, T.; Tachibana, N.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces High-Fat Diet-Induced Weight Gain by Modulating Host Bile Acid Metabolism in a Gut Microbiota-Dependent Manner. Biochem. Biophys. Res. Commun. 2018, 501, 955–961. [Google Scholar] [CrossRef]
- Kohno, M.; Motoyama, T.; Shigihara, Y.; Sakamoto, M.; Sugano, H. Improvement of Glucose Metabolism via Mung Bean Protein Consumption: A Clinical Trial of GLUCODIATM Isolated Mung Bean Protein in Japan. Funct. Foods Health. Dis. 2017, 7, 115–134. [Google Scholar] [CrossRef]
- Kohno, M.; Sugano, H.; Shigihara, Y.; Shiraishi, Y.; Motoyama, T. Improvement of Glucose and Lipid Metabolism via Mung Bean Protein Consumption: Clinical Trials of GLUCODIATM Isolated Mung Bean Protein in the USA and Canada. J. Nutr. Sci. 2018, 7, e2. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Hou, T.; Liu, W.; Guo, D.; He, H. The Hypolipidemic Effects of Peptides Prepared from Cicer arietinum in Ovariectomized Rats and HepG2 Cells. J. Sci. Food Agric. 2019, 99, 576–586. [Google Scholar] [CrossRef]
- Zulet, M.A.; Macarulla, M.T.; Portillo, M.P.; Noel-Suberville, C.; Higueret, P.; Martínez, J.A. Lipid and Glucose Utilization in Hypercholesterolemic Rats Fed a Diet Containing Heated Chickpea (Cicer aretinum L.): A Potential Functional Food. Int. J. Vitam. Nutr. Res. 1999, 69, 403–411. [Google Scholar] [CrossRef] [PubMed]
- Boualga, A.; Prost, J.; Taleb-Senouci, D.; Krouf, D.; Kharoubi, O.; Lamri-Senhadji, M.; Belleville, J.; Bouchenak, M. Purified Chickpea or Lentil Proteins Impair VLDL Metabolism and Lipoprotein Lipase Activity in Epididymal Fat, but Not in Muscle, Compared to Casein, in Growing Rats. Eur. J. Nutr. 2009, 48, 162–169. [Google Scholar] [CrossRef]
- Jansen, H. Hepatic Lipase: Friend or Foe and under What Circumstances? Curr. Atheroscler. Rep. 2004, 6, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Foster-Powell, K.; Holt, S.H.; Brand-Miller, J.C. International Table of Glycemic Index and Glycemic Load Values: 2002. Am. J. Clin. Nutr. 2002, 76, 5–56. [Google Scholar] [CrossRef] [PubMed]
- Martínez-González, M.Á.; De La Fuente-Arrillaga, C.; Nunez-Cordoba, J.M.; Basterra-Gortari, F.J.; Beunza, J.J.; Vazquez, Z.; Benito, S.; Tortosa, A.; Bes-Rastrollo, M. Adherence to Mediterranean Diet and Risk of Developing Diabetes: Prospective Cohort Study. BMJ 2008, 336, 1348. [Google Scholar] [CrossRef]
- Xue, Z.; Gao, J.; Zhang, Z.; Yu, W.; Wang, H.; Kou, X. Antihyperlipidemic and Antitumor Effects of Chickpea Albumin Hydrolysate. Plant Foods Hum. Nutr. 2012, 67, 393–400. [Google Scholar] [CrossRef]
- Hanson, M.G.; Taylor, C.G.; Wu, Y.; Anderson, H.D.; Zahradka, P. Lentil Consumption Reduces Resistance Artery Remodeling and Restores Arterial Compliance in the Spontaneously Hypertensive Rats. J. Nutr. Biochem. 2016, 37, 30–38. [Google Scholar] [CrossRef]
- Khazaei, H.; Subedi, M.; Nickerson, M.; Martínez-Villaluenga, C.; Frias, J.; Vandenberg, A. Seed Protein of Lentils: Current Status, Progress, and Food Applications. Foods 2019, 8, 391. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, R.; Raju, R.; Sharma, R. Calmodulin-Dependent Cyclic Nucleotide Phosphodiesterase (PDE1). Cell. Mol. Life Sci. 1999, 55, 1164–1186. [Google Scholar] [CrossRef]
- García-Mora, P.; Martín-Martínez, M.; Angeles Bonache, M.; González-Múniz, R.; Peñas, E.; Frias, J.; Martinez-Villaluenga, C. Identification, Functional Gastrointestinal Stability and Molecular Docking Studies of Lentil Peptides with Dual Antioxidant and Angiotensin I Converting Enzyme Inhibitory Activities. Food Chem. 2017, 221, 464–472. [Google Scholar] [CrossRef]
- Akıllıoğlu, H.G.; Karakaya, S. Effects of Heat Treatment and In Vitro Digestion on the Angiotensin Converting Enzyme Inhibitory Activity of Some Legume Species. Eur. Food Res. Techol. 2009, 229, 915–921. [Google Scholar] [CrossRef]
- Barbana, C.; Boye, J.I. Angiotensin I-Converting Enzyme Inhibitory Properties of Lentil Protein Hydrolysates: Determination of the Kinetics of Inhibition. Food Chem. 2011, 127, 94–101. [Google Scholar] [CrossRef]
- Jakubczyk, A.; Baraniak, B. Activities and Sequences of the Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Obtained from the Digested Lentil (Lens culinaris) Globulins. Int. J. Food Sci. Technol. 2013, 48, 2363–2369. [Google Scholar] [CrossRef]
- Boschin, G.; Scigliuolo, G.M.; Resta, D.; Arnoldi, A. ACE-Inhibitory Activity of Enzymatic Protein Hydrolysates from Lupin and Other Legumes. Food Chem. 2014, 145, 34–40. [Google Scholar] [CrossRef]
- Mayilvaganan, M.; Singh, S.; Johari, R. Hypocholesterolemic Effect of Protein Prepared from Phaseolus aconitifolius (Jacq.). Indian J. Exp. Bot. 2004, 42, 904–908. [Google Scholar]
- Potter, S.M. Overview of Proposed Mechanisms for the Hypocholesterolemic Effect of Soy. J. Nutr. 1995, 125, 606S–611S. [Google Scholar] [CrossRef] [PubMed]
- Shin, Z.I.; Yu, R.; Park, S.-A.; Chung, D.K.; Ahn, C.W.; Nam, H.S.; Kim, K.S.; Lee, H.J. His-His-Leu, an Angiotensin I Converting Enzyme Inhibitory Peptide Derived from Korean Soybean Paste, Exerts Antihypertensive Activity in Vivo. J. Agric. Food Chem. 2001, 49, 3004–3009. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Hypotensive and Physiological Effect of Angiotensin Converting Enzyme Inhibitory Peptides Derived from Soy Protein on Spontaneously Hypertensive Rats. J. Agric. Food Chem. 2001, 49, 501–506. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K.; Tachibana, N.; Wanezaki, S.; Tsuzaki, S.; Takamatsu, K.; Yamamoto, T.; Hashimoto, Y.; Shimoda, T. Isoflavone-Free Soy Protein Prepared by Column Chromatography Reduces Plasma Cholesterol in Rats. J. Agric. Food Chem. 2002, 50, 5717. [Google Scholar] [CrossRef]
- Adams, M.R.; Golden, D.L.; Franke, A.A.; Potter, S.M.; Smith, H.S.; Anthony, M.S. Dietary Soy β-Conglycinin (7S Globulin) Inhibits Atherosclerosis in Mice. J. Nutr. 2004, 134, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Desroches, S.; Lamarche, B. Diet and Low-Density Lipoprotein Particle Size. Curr. Atheroscler. Rep. 2004, 6, 453–460. [Google Scholar] [CrossRef]
- Zhan, S.; Ho, S.C. Meta-Analysis of the Effects of Soy Protein Containing Isoflavones on the Lipid Profile. Am. J. Clin. Nutr. 2005, 81, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.-Y.; Tong, X.; Wu, Z.-W.; Xun, P.-C.; He, K.; Qin, L.-Q. Effect of Soya Protein on Blood Pressure: A Meta-Analysis of Randomised Controlled Trials. Br. J. Nutr. 2011, 106, 317–326. [Google Scholar] [CrossRef]
- Liu, X.X.; Li, S.H.; Chen, J.Z.; Sun, K.; Wang, X.J.; Wang, X.G.; Hui, R.T. Effect of Soy Isoflavones on Blood Pressure: A Meta-Analysis of Randomized Controlled Trials. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 463–470. [Google Scholar] [CrossRef]
- Lavigne, C.; Marette, A.; Jacques, H. Cod and Soy Proteins Compared with Casein Improve Glucose Tolerance and Insulin Sensitivity in Rats. Am. J. Physiol. Endocrinol. 2000, 278, E491–E500. [Google Scholar] [CrossRef] [PubMed]
- Ascencio, C.; Torres, N.; Isoard-Acosta, F.; Gómez-Pérez, F.J.; Hernández-Pando, R.; Tovar, A.R. Soy Protein Affects Serum Insulin and Hepatic SREBP-1 mRNA and Reduces Fatty Liver in Rats. J. Nutr. 2004, 134, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Tovar, A.R.; Torre-Villalvazo, I.; Ochoa, M.; Elías, A.L.; Ortíz, V.; Aguilar-Salinas, C.A.; Torres, N. Soy Protein Reduces Hepatic Lipotoxicity in Hyperinsulinemic Obese Zucker Fa/Fa Rats. J. Lipid Res. 2005, 46, 1823–1832. [Google Scholar] [CrossRef] [PubMed]
- Shin, E.S.; Lee, H.H.; Cho, S.Y.; Park, H.W.; Lee, S.J.; Lee, T.R. Genistein Downregulates SREBP-1 Regulated Gene Expression by Inhibiting Site-1 Protease Expression in HepG2 Cells. J. Nutr. 2007, 137, 1127–1131. [Google Scholar] [CrossRef]
- Bayly, G.R. CHAPTER 37—Lipids and disorders of lipoprotein metabolism. In Clinical. Biochemistry: Metabolic and Clinical Aspects, 3rd ed.; Marshall, W.J., Lapsley, M., Day, A.P., Ayling, R.M., Eds.; Churchill Livingstone: London, UK, 2014; pp. 702–736. ISBN 978-0-7020-5140-1. [Google Scholar]
- Robinet, P.; Védie, B.; Chironi, G.; Gariépy, J.; Simon, A.; Moatti, N.; Paul, J.-L. Characterization of Polymorphic Structure of SREBP-2 Gene: Role in Atherosclerosis. Atherosclerosis 2003, 168, 381–387. [Google Scholar] [CrossRef]
- Robinet, P.; Fradagrada, A.; Monier, M.-N.; Marchetti, M.; Cogny, A.; Moatti, N.; Paul, J.-L.; Vedie, B.; Lamaze, C. Dynamin Is Involved in Endolysosomal Cholesterol Delivery to the Endoplasmic Reticulum: Role in Cholesterol Homeostasis. Traffic 2006, 7, 811–823. [Google Scholar] [CrossRef]
- Iritani, N.; Nagashima, K.; Fukuda, H.; Katsurada, A.; Tanaka, T. Effects of Dietary Proteins on Lipogenic Enzymes in Rat Liver. J. Nutr. 1986, 116, 190–197. [Google Scholar] [CrossRef]
- Iritani, N.; Hosomi, H.; Fukuda, H.; Tada, K.; Ikeda, H. Soybean Protein Suppresses Hepatic Lipogenic Enzyme Gene Expression in Wistar Fatty Rats. J. Nutr. 1996, 126, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, A.; Fukui, K.; Kojima, M.; Kishida, K.; Maeda, N.; Nagaretani, H.; Hibuse, T.; Nishizawa, H.; Kihara, S.; Waki, M.; et al. Divergent Effects of Soy Protein Diet on the Expression of Adipocytokines. Biochem. Biophys. Res. Commun. 2003, 311, 909–914. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Wood, C.; L’Abbé, M.R.; Gilani, G.S.; Cooke, G.M.; Curran, I.H.; Xiao, C.W. Consumption of Soy Protein Isolate Modulates the Phosphorylation Status of Hepatic ATPase/ATP Synthase β Protein and Increases ATPase Activity in Rats. J. Nutr. 2007, 137, 2029–2035. [Google Scholar] [CrossRef]
- Luo, X.C.; Park, K.; Lopez-Casillas, F.; Kim, K.H. Structural Features of the Acetyl-CoA Carboxylase Gene: Mechanisms for the Generation of mRNAs with 5′ End Heterogeneity. Proc. Natl. Acad. Sci. USA 1989, 86, 4042–4046. [Google Scholar] [CrossRef]
- Aoki, H.; Kimura, K.; Igarashi, K.; Takenaka, A. Soy Protein Suppresses Gene Expression of Acetyl-CoA Carboxylase Alpha from Promoter PI in Rat Liver. Biosci. Biotechnol. Biochem. 2006, 70, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Standeven, A.; Beard, R.; Johnson, A.; Boehm, M.; Escobar, M.; Heyman, R.; Chandraratna, R. Retinoid-Induced Hypertriglyceridemia in Rats Is Mediated by Retinoic Acid Receptors. Fundam. Appl. Toxicol. 1996, 33, 264–271. [Google Scholar] [CrossRef]
- Xiao, C.W. Health Effects of Soy Protein and Isoflavones in Humans. J. Nutr. 2008, 138, 1244S–1249S. [Google Scholar] [CrossRef]
- Lovati, M.; Manzoni, C.; Agostinelli, P.; Ciapellano, S.; Sirtori, C.R. Studies on the Mechanism of the Cholesterol Lowering Activity of Soy Proteins. Nutr. Metab. Cardiovasc. Dis. 1991, 1, 18–24. [Google Scholar]
- Baum, J.A.; Teng, H.; Erdman, J.W., Jr.; Weigel, R.M.; Klein, B.P.; Persky, V.W.; Freels, S.; Surya, P.; Bakhit, R.M.; Ramos, E.; et al. Long-Term Intake of Soy Protein Improves Blood Lipid Profiles and Increases Mononuclear Cell Low-Density-Lipoprotein Receptor Messenger RNA in Hypercholesterolemic, Postmenopausal Women. Am. J. Clin. Nutr. 1998, 68, 545–551. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Canavesi, A.; Sirtori, M.; Vaccarino, V.; Marchi, M.; Gaddi, A.; Sirtori, C.R. Soybean Protein Diet Increases Low Density Lipoprotein Receptor Activity in Mononuclear Cells from Hypercholesterolemic Patients. J. Clin. Investig. 1987, 80, 125. [Google Scholar] [CrossRef]
- Ip, E.; Farrell, G.C.; Robertson, G.; Hall, P.; Kirsch, R.; Leclercq, I. Central Role of PPARα-Dependent Hepatic Lipid Turnover in Dietary Steatohepatitis in Mice. Hepatology 2003, 38, 123–132. [Google Scholar] [CrossRef]
- Morifuji, M.; Sanbongi, C.; Sugiura, K. Dietary Soya Protein Intake and Exercise Training Have an Additive Effect on Skeletal Muscle Fatty Acid Oxidation Enzyme Activities and mRNA Levels in Rats. Br. J. Nutr. 2006, 96, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Conti, R.; Mannucci, E.; Pessotto, P.; Tassoni, E.; Carminati, P.; Giannessi, F.; Arduini, A. Selective Reversible Inhibition of Liver Carnitine Palmitoyl-Transferase 1 by Teglicar Reduces Gluconeogenesis and Improves Glucose Homeostasis. Diabetes 2011, 60, 644. [Google Scholar] [CrossRef]
- Lionetti, V.; Linke, A.; Chandler, M.P.; Young, M.E.; Penn, M.S.; Gupte, S.; d’Agostino, C.; Hintze, T.H.; Stanley, W.C.; Recchia, F.A. Carnitine Palmitoyl Transferase-I Inhibition Prevents Ventricular Remodeling and Delays Decompensation in Pacing-Induced Heart Failure. Cardiovasc. Res. 2005, 66, 454–461. [Google Scholar] [CrossRef] [PubMed]
- Fraulob, J.C.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Beneficial Effects of Rosuvastatin on Insulin Resistance, Adiposity, Inflammatory Markers and Non-Alcoholic Fatty Liver Disease in Mice Fed on a High-Fat Diet. Clin. Sci. 2012, 123, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Tenenbaum, A.; Motro, M.; Fisman, E.Z. Dual and Pan-Peroxisome Proliferator-Activated Receptors (PPAR) Co-Agonism: The Bezafibrate Lessons. Cardiovasc. Diabetol. 2005, 4, 14. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Da-Silva, S.; Souza-Mello, V.; Magliano, D.C.; Marinho, T.D.S.; Aguila, M.B.; Mandarim-De-Lacerda, C.A. Singular Effects of PPAR Agonists on Nonalcoholic Fatty Liver Disease of Diet-Induced Obese Mice. Life Sci. 2015, 127, 73–81. [Google Scholar] [CrossRef]
- Pellieux, C.; Montessuit, C.; Papageorgiou, I.; Lerch, R. Angiotensin II Downregulates the Fatty Acid Oxidation Pathway in Adult Rat Cardiomyocytes via Release of Tumour Necrosis Factor-α. Cardiovasc. Res. 2009, 82, 341–350. [Google Scholar] [CrossRef]
- Kimura, R.; Takahashi, N.; Murota, K.; Yamada, Y.; Niiya, S.; Kanzaki, N.; Murakami, Y.; Moriyama, T.; Goto, T.; Kawada, T. Activation of Peroxisome Proliferator-Activated Receptor-α (PPARα) Suppresses Postprandial Lipidemia through Fatty Acid Oxidation in Enterocytes. Biochem. Biophys. Res. Commun. 2011, 410, 1–6. [Google Scholar] [CrossRef]
- Osorio, J.; Stanley, W.; Linke, A.; Castellari, M.; Quy, D.; Panchal, A.; Hintze, T.; Lopaschuk, G.; Recchia, F. Impaired Myocardial Fatty Acid Oxidation and Reduced Protein Expression of Retinoid X Receptor-α in Pacing-Induced Heart Failure. Circulation 2002, 106, 606–612. [Google Scholar] [CrossRef]
- Bishop-Bailey, D. Peroxisome Proliferator-Activated Receptors in the Cardiovascular System. Br. J. Pharmacol. 2000, 129, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Fujii, H.; Takahashi, T.; Kodama, M.; Aizawa, Y.; Ohta, Y.; Ono, T.; Hasegawa, G.; Naito, M.; Nakajima, T.; et al. Constitutive Regulation of Cardiac Fatty Acid Metabolism through Peroxisome Proliferator-Activated Receptor α Associated with Age-Dependent Cardiac Toxicity. J. Biol. Chem. 2000, 275, 22293–22299. [Google Scholar] [CrossRef] [PubMed]
- Keller, H.; Dreyer, C.; Medin, J.; Mahfoudi, A.; Ozato, K.; Wahli, W. Fatty Acids and Retinoids Control Lipid Metabolism through Activation of Peroxisome Proliferator-Activated Receptor-Retinoid X Receptor Heterodimers. Proc. Natl. Acad. Sci. USA 1993, 90, 2160–2164. [Google Scholar] [CrossRef]
- Tugwood, J.D.; Issemann, I.; Anderson, R.G.; Bundell, K.R.; McPheat, W.L.; Green, S. The Mouse Peroxisome Proliferator Activated Receptor Recognizes a Response Element in the 5′ Flanking Sequence of the Rat Acyl CoA Oxidase Gene. EMBO J. 1992, 11, 433–439. [Google Scholar] [CrossRef]
- Dreyer, C.; Krey, G.; Keller, H.; Givel, F.; Helftenbein, G.; Wahli, W. Control of the Peroxisomal β-Oxidation Pathway by a Novel Family of Nuclear Hormone Receptors. Cell 1992, 68, 879–887. [Google Scholar] [CrossRef]
- Huss, J.M.; Levy, F.H.; Kelly, D.P. Hypoxia Inhibits the Peroxisome Proliferator-Activated Receptor α/Retinoid X Receptor Gene Regulatory Pathway in Cardiac Myocytes: A Mechanism for O2-Dependent Modulation of Mitochondrial Fatty Acid Oxidation. J. Biol. Chem. 2001, 276, 27605–27612. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.; Rader, T.; Park, S.; Bastin, J.; McCune, S.; Kelly, D. Fatty Acid Oxidation Enzyme Gene Expression Is Downregulated in the Failing Heart. Circulation 1996, 94, 2837–2842. [Google Scholar] [CrossRef]
- Razeghi, P.; Young, M.E.; Alcorn, J.L.; Moravec, C.; Frazier, O.; Taegtmeyer, H. Metabolic Gene Expression in Fetal and Failing Human Heart. Circulation 2001, 104, 2923–2931. [Google Scholar] [CrossRef]
- Schupp, M.; Kintscher, U.; Fielitz, J.; Thomas, J.; Pregla, R.; Hetzer, R.; Unger, T.; Regitz-Zagrosek, V. Cardiac PPARα Expression in Patients with Dilated Cardiomyopathy. Eur. J. Heart Fail. 2006, 8, 290–294. [Google Scholar] [CrossRef]
- Milanski, M.; Souza, K.L.A.; Reis, S.R.L.; Feres, N.H.; de Souza, L.M.I.; Arantes, V.C.; Carneiro, E.M.; Boschero, A.C.; Reis, M.A.B.; Latorraca, M.Q. Soybean Diet Modulates Acetyl-Coenzyme A Carboxylase Expression in Livers of Rats Recovering from Early-Life Malnutrition. Nutrition 2009, 25, 774–781. [Google Scholar] [CrossRef]
- Katsurada, A.; Iritani, N.; Fukuda, H.; Matsumura, Y.; Nishimoto, N.; Noguchi, T.; Tanaka, T. Effects of Nutrients and Hormones on Transcriptional and Post-Transcriptional Regulation of Fatty Acid Synthase in Rat Liver. Eur. J. Biochem. 1990, 190, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Seo, C.-S.; Hwang, I.H.; Lee, M.W.; Song, K.H. Anti-Obesity Activities of Chikusetsusaponin IVa and Dolichos lablab L. Seeds. Nutrients 2018, 10, 1221. [Google Scholar] [CrossRef]
- Barak, Y.; Nelson, M.C.; Ong, E.S.; Jones, Y.Z.; Ruiz-Lozano, P.; Chien, K.R.; Koder, A.; Evans, R.M. PPARγ Is Required for Placental, Cardiac, and Adipose Tissue Development. Mol. Cell 1999, 4, 585–595. [Google Scholar] [CrossRef]
- Schoonjans, K.; Peinado-Onsurbe, J.; Lefebvre, A.M.; Heyman, R.A.; Briggs, M.; Deeb, S.; Staels, B.; Auwerx, J. PPARalpha and PPARgamma Activators Direct a Distinct Tissue-Specific Transcriptional Response via a PPRE in the Lipoprotein Lipase Gene. EMBO J. 1996, 15, 5336–5348. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Association. Food labeling: Health claims; soybean protein and coronary heart disease. In Federal. Register: Rules and Regulations; Food and Drug Administration: Silver Spring, MD, USA, 1999; Volume 64, pp. 57700–57733. [Google Scholar]
- Harland, J.I.; Haffner, T.A. Systematic Review, Meta-Analysis and Regression of Randomised Controlled Trials Reporting an Association Between an Intake of Circa 25g Soya Protein per Day and Blood Cholesterol. Atherosclerosis 2008, 200, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Benkhedda, K.; Boudrault, C.; Sinclair, S.; Marles, R.; Xiao, C.; Underhill, L. Food Risk Analysis Communication. Issued by Health Canada’s Food Directorate. Health Canada’s Proposal to Accept a Health Claim about Soy Products and Cholesterol Lowering. Int. Food Risk Anal. J. 2014, 4, 1–12. [Google Scholar]
- Nagarajan, S.; Burris, R.L.; Stewart, B.W.; Wilkerson, J.E.; Badger, T.M. Dietary Soy Protein Isolate Ameliorates Atherosclerotic Lesions in Apolipoprotein E-Deficient Mice Potentially by Inhibiting Monocyte Chemoattractant Protein-1 Expression. J. Nutr. 2008, 138, 332–337. [Google Scholar] [CrossRef]
- Kwon, D.; Oh, S.; Lee, J.; Yang, H.; Lee, S.; Lee, J.; Lee, Y.; Sohn, H. Amino Acid Substitution of Hypocholesterolemic Peptide Originated from Glycinin Hydrolyzate. Food Sci. Biotechnol. 2002, 11, 55–61. [Google Scholar]
- Farrokhi, N.; Whitelegge, J.P.; Brusslan, J.A. Plant Peptides and Peptidomics. Plant Biotechnol. J. 2008, 6, 105–134. [Google Scholar] [CrossRef]
- Nasri, R.; Nasri, M. Marine-Derived Bioactive Peptides as New Anticoagulant Agents: A Review. Curr. Protein Pept. Sci. 2013, 14, 199–204. [Google Scholar] [CrossRef]
- Coda, R.; Rizzello, C.G.; Pinto, D.; Gobbetti, M. Selected Lactic Acid Bacteria Synthesize Antioxidant Peptides during Sourdough Fermentation of Cereal Flours. Appl. Environ. Microbiol. 2012, 78, 1087. [Google Scholar] [CrossRef]
- Korhonen, H.; Pihlanto, A. Food-Derived Bioactive Peptides—Opportunities for Designing Future Foods. Curr. Pharm. Des. 2003, 9, 1297–1308. [Google Scholar] [CrossRef]
- Tsai, J.S.; Lin, Y.S.; Pan, B.S.; Chen, T.J. Antihypertensive Peptides and γ-Aminobutyric Acid from Prozyme 6 Facilitated Lactic Acid Bacteria Fermentation of Soymilk. Process Biochem. 2006, 41, 1282–1288. [Google Scholar] [CrossRef]
- Tsai, J.-S.; Chen, T.-J.; Pan, B.S.; Gong, S.-D.; Chung, M.-Y. Antihypertensive Effect of Bioactive Peptides Produced by Protease-Facilitated Lactic Acid Fermentation of Milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Zambrowicz, A.; Timmer, M.; Polanowski, A.; Lubec, G.; Trziszka, T. Manufacturing of Peptides Exhibiting Biological Activity. Amino Acids 2013, 44, 315–320. [Google Scholar] [CrossRef]
- Agyei, D. Bioactive Proteins and Peptides from Soybeans. Recent. Pat. Food Nutr. Agric. 2015, 7, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Hernandez-Ledesma, B.; Hsieh, C.-C.; De Lumen, B.O. Chemopreventive Properties of Peptide Lunasin: A Review. Protein Pept. Lett. 2013, 20, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Shimakage, A.; Shinbo, M.; Yamada, S. ACE Inhibitory Substances Derived from Soy Foods. J. Biol. Macromol. 2012, 12, 72–80. [Google Scholar] [CrossRef][Green Version]
- S Vallabha, V.; Tiku, P.K. Antihypertensive Peptides Derived from Soy Protein by Fermentation. Int. J. Pept. Res. Ther. 2014, 20, 161–168. [Google Scholar] [CrossRef]
- Johnston, J.; Franz, V. Renin-Angiotensin System: A Dual Tissue and Hormonal System for Cardiovascular Control. J. Hypertens. 1992, 10, 13–26. [Google Scholar] [CrossRef]
- Nguyen Dinh Cat, A.; Touyz, R.M. A New Look at the Renin–Angiotensin System—Focusing on the Vascular System. Peptides 2011, 32, 2141–2150. [Google Scholar] [CrossRef]
- Jao, C.L.; Huang, S.L.; Hsu, K.C. Angiotensin I-Converting Enzyme Inhibitory Peptides: Inhibition Mode, Bioavailability, and Antihypertensive Effects. BioMedicine 2012, 2, 130–136. [Google Scholar] [CrossRef]
- Sibony, M.; Gasc, J.M.; Soubrier, F.; Alhenc-Gelas, F.; Corvol, P. Gene Expression and Tissue Localization of the Two Isoforms of Angiotensin I Converting Enzyme. Hypertension 1993, 21, 827–835. [Google Scholar] [CrossRef]
- Natesh, R.; Schwager, S.L.U.; Sturrock, E.D.; Acharya, K.R. Crystal Structure of the Human Angiotensin-Converting Enzyme–Lisinopril Complex. Nature 2003, 421, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Donkor, O.N.; Henriksson, A.; Singh, T.K.; Vasiljevic, T.; Shah, N.P. ACE-Inhibitory Activity of Probiotic Yoghurt. Int. Dairy J. 2007, 17, 1321–1331. [Google Scholar] [CrossRef]
- Mallikarjun Gouda, K.G.; Gowda, L.R.; Rao, A.G.A.; Prakash, V. Angiotensin I-Converting Enzyme Inhibitory Peptide Derived from Glycinin, the 11S Globulin of Soybean (Glycine max). J. Agric. Food Chem. 2006, 54, 4568–4573. [Google Scholar] [CrossRef]
- Prak, K.; Maruyama, Y.; Maruyama, N.; Utsumi, S. Design of Genetically Modified Soybean Proglycinin A1aB1b with Multiple Copies of Bioactive Peptide Sequences. Peptides 2006, 27, 1179–1186. [Google Scholar] [CrossRef]
- Nagaoka, S. Structure-Function Properties of Hypolipidemic Peptides. J. Food Biochem. 2019, 43, e12539. [Google Scholar] [CrossRef] [PubMed]
- Medina, M.; Uy, L.; Manuel, M.; Recuenco, M.; Torio, M. Hypocholesterolemic Activity of Mungbean 8Sα Globulin Engineered with Lactostatin. PJCS 2020, 45, 13–26. [Google Scholar]
- Odani, S.; Koide, T.; Ono, T. Amino Acid Sequence of a Soybean (Glycine max) Seed Polypeptide Having a Poly(L-Aspartic Acid) Structure. J. Biol. Chem. 1987, 262, 10502–10505. [Google Scholar] [CrossRef]
- Galvez, A.F.; de Lumen, B.O. A Soybean cDNA Encoding a Chromatin-Binding Peptide Inhibits Mitosis of Mammalian Cells. Nat. Biotechnol. 1999, 17, 495–500. [Google Scholar] [CrossRef] [PubMed]
- De Lumen, B.O. Lunasin: A Cancer-Preventive Soy Peptide. Nutr. Rev. 2005, 63, 16–21. [Google Scholar] [CrossRef]
- Lule, V.K.; Garg, S.; Pophaly, S.D.; Hitesh; Tomar, S.K. Potential Health Benefits of Lunasin: A Multifaceted Soy-Derived Bioactive Peptide. J. Food Sci. 2015, 80, R485–R494. [Google Scholar] [CrossRef]
- Seber, L.E.; Barnett, B.W.; McConnell, E.J.; Hume, S.D.; Cai, J.; Boles, K.; Davis, K.R. Scalable Purification and Characterization of the Anticancer Lunasin Peptide from Soybean. PLoS ONE 2012, 7, e35409. [Google Scholar] [CrossRef] [PubMed]
- Rayaprolu, S.J.; Hettiarachchy, N.S.; Chen, P.; Kannan, A.; Mauromostakos, A. Peptides Derived from High Oleic Acid Soybean Meals Inhibit Colon, Liver and Lung Cancer Cell Growth. Food Res. Int. 2013, 50, 282–288. [Google Scholar] [CrossRef]
- Park, J.H.; Jeong, H.J.; de Lumen, B.O. Contents and Bioactivities of Lunasin, Bowman−Birk Inhibitor, and Isoflavones in Soybean Seed. J. Agric. Food Chem. 2005, 53, 7686–7690. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.J.; Jeong, J.B.; Kim, D.S.; Park, J.H.; Lee, J.B.; Kweon, D.-H.; Chung, G.Y.; Seo, E.W.; de Lumen, B.O. The Cancer Preventive Peptide Lunasin from Wheat Inhibits Core Histone Acetylation. Cancer Lett. 2007, 255, 42–48. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Jeong, J.; Park, J.; Cheong, Y.; de Lumen, B. The Preventive Seed Peptide Lunasin from Rye Is Bioavailable and Bioactive. Nutr. Cancer 2009, 61, 680–686. [Google Scholar] [CrossRef]
- Jeong, H.J.; Jeong, J.B.; Hsieh, C.C.; Hernández-Ledesma, B.; de Lumen, B.O. Lunasin Is Prevalent in Barley and Is Bioavailable and Bioactive in in Vivo and in Vitro Studies. Nutr. Cancer 2010, 62, 1113–1119. [Google Scholar] [CrossRef] [PubMed]
- García-Nebot, M.; Recio, I.; Hernández-Ledesma, B. Antioxidant Activity and Protective Effects of Peptide Lunasin against Oxidative Stress in Intestinal Caco-2 Cells. Food Chem. Toxicol. 2014, 65, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.B.; De Lumen, B.O.; Jeong, H.J. Lunasin Peptide Purified from Solanum nigrum L. Protects DNA from Oxidative Damage by Suppressing the Generation of Hydroxyl Radical via Blocking Fenton Reaction. Cancer Lett. 2010, 293, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ledesma, B.; Hsieh, C.-C.; de Lumen, B.O. Antioxidant and Anti-Inflammatory Properties of Cancer Preventive Peptide Lunasin in RAW 264.7 Macrophages. Biochem. Biophys. Res. Commun. 2009, 390, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Dia, V.P.; Wang, W.; Oh, V.L.; de Lumen, B.O.; de Mejia, E.G. Isolation, Purification and Characterisation of Lunasin from Defatted Soybean Flour and in Vitro Evaluation of Its Anti-Inflammatory Activity. Food Chem. 2009, 114, 108–115. [Google Scholar] [CrossRef]
- Aguzzi, M.S.; Fortugno, P.; Giampietri, C.; Ragone, G.; Capogrossi, M.C.; Facchiano, A. Intracellular Targets of RGDS Peptide in Melanoma Cells. Mol. Cancer 2010, 9, 84. [Google Scholar] [CrossRef]
- Gueguen, J.; Cerletti, P. Proteins of some legume seeds: Soybean bean, pea, faba bean and lupin. In New and Developing Sources of Food Proteins; Chapman and Hall: London, UK, 1994; pp. 145–193. ISBN 978-1-4615-2652-0. [Google Scholar]
- Katagiri, Y.; Ibrahim, R.; Tahara, S. HPLC Analysis of White Lupin Isoflavonoids. Biosci. Biotechnol. Biochem. 2000, 64, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A.; Kotlarz, A.; Strobel, W. Compositional and Nutritional Evaluation of Several Lupin Seeds. Food Chem. 2006, 98, 711–719. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Weiße, K.; Hirche, F.; Eder, K.; Stangl, G. Lupin Protein Influences the Expression of Hepatic Genes Involved in Fatty Acid Synthesis and Triacylglycerol Hydrolysis of Adult Rats. Br. J. Nutr. 2008, 99, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Boschin, G.; D’Agostina, A.; Annicchiarico, P.; Arnoldi, A. Effect of Genotype and Environment on Fatty Acid Composition of Lupinus Albus L. Seed. Food Chem. 2008, 108, 600–606. [Google Scholar] [CrossRef]
- Boschin, G.; Arnoldi, A. Legumes Are Valuable Sources of Tocopherols. Food Chem. 2011, 127, 1199–1203. [Google Scholar] [CrossRef]
- Brandsch, C.; Kappis, D.; Weiße, K.; Stangl, G.I. Effects of Untreated and Thermally Treated Lupin Protein on Plasma and Liver Lipids of Rats Fed a Hypercholesterolemic High Fat or High Carbohydrate Diet. Plant Foods Hum. Nutr. 2010, 65, 410–416. [Google Scholar] [CrossRef]
- Spielmann, J.; Shukla, A.; Brandsch, C.; Hirche, F.; Stangl, G.I.; Eder, K. Dietary Lupin Protein Lowers Triglyceride Concentrations in Liver and Plasma in Rats by Reducing Hepatic Gene Expression of Sterol Regulatory Element-Binding Protein-1c. Ann. Nutr. Metab. 2007, 51, 387–392. [Google Scholar] [CrossRef]
- Ridker, P.M.; Hennekens, C.H.; Buring, J.E.; Rifai, N. C-Reactive Protein and Other Markers of Inflammation in the Prediction of Cardiovascular Disease in Women. N. Engl. J. Med. 2000, 342, 836–843. [Google Scholar] [CrossRef]
- Naruszewicz, M.; Nowicka, G.; Klosiewicz-Latoszek, L.; Arnoldi, A.; Sirtori, C. Abstract 4055: Effect of Lupin Protein (Lupinus albus) on Cardiovascular Risk Factors in Smokers with Mild Hypercholesterolemia. Circulation 2006, 114, II_874. [Google Scholar] [CrossRef]
- Bettzieche, A.; Brandsch, C.; Schmidt, M.; Weiße, K.; Eder, K.; Stangl, G. Differing Effect of Protein Isolates from Different Cultivars of Blue Lupin on Plasma Lipoproteins of Hypercholesterolemic Rats. Biosci. Biotechnol. Biochem. 2008, 72, 3114–3121. [Google Scholar] [CrossRef]
- Sirtori, C.; Galli, C.; Anderson, J.W.; Arnoldi, A. Nutritional and Nutraceutical Approaches to Dyslipidemia and Atherosclerosis Prevention: Focus on Dietary Proteins. Atherosclerosis 2009, 203, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Lammi, C.; Zanoni, C.; Scigliuolo, G.M.; D’Amato, A.; Arnoldi, A. Lupin Peptides Lower Low-Density Lipoprotein (LDL) Cholesterol Through an Up-Regulation of the LDL Receptor/Sterol Regulatory Element Binding Protein 2 (SREBP2) Pathway at HepG2 Cell Line. J. Agric. Food Chem. 2014, 62, 7151–7159. [Google Scholar] [CrossRef]
- Sirtori, C.R.; Triolo, M.; Bosisio, R.; Bondioli, A.; Calabresi, L.; De Vergori, V.; Gomaraschi, M.; Mombelli, G.; Pazzucconi, F.; Zacherl, C.; et al. Hypocholesterolaemic Effects of Lupin Protein and Pea Protein/Fibre Combinations in Moderately Hypercholesterolaemic Individuals. Br. J. Nutr. 2012, 107, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Blagrove, R.; Gillespie, J. Isolation, Purification and Characterization of the Seed Globulins of Lupinus albus. Aust. J. Plant Physiol. 1981, 2, 13–27. [Google Scholar]
- Duranti, M.; Restani, P.; Poniatowska, M.; Cerletti, P. The Seed Globulins of Lupinus albus. Phytochemistry 1981, 20, 2071–2075. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Ferruzza, S.; Ranaldi, G.; Sambuy, Y.; Arnoldi, A. Hypocholesterolaemic Activity of Lupin Peptides: Investigation on the Crosstalk between Human Enterocytes and Hepatocytes Using a Co-Culture System Including Caco-2 and HepG2 Cells. Nutrients 2016, 8, 437. [Google Scholar] [CrossRef]
- Lammi, C.; Zanoni, C.; Aiello, G.; Arnoldi, A.; Grazioso, G. Lupin Peptides Modulate the Protein-Protein Interaction of PCSK9 with the Low Density Lipoprotein Receptor in HepG2 Cells. Sci. Rep. 2016, 6, 29931. [Google Scholar] [CrossRef]
- Bähr, M.; Fechner, A.; Kiehntopf, M.; Jahreis, G. Consuming a Mixed Diet Enriched with Lupin Protein Beneficially Affects Plasma Lipids in Hypercholesterolemic Subjects: A Randomized Controlled Trial. Clin. Nutr. 2015, 34, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Dahl, W.J.; Foster, L.M.; Tyler, R.T. Review of the Health Benefits of Peas (Pisum sativum L.). Br. J. Nutr. 2012, 108, S3–S10. [Google Scholar] [CrossRef] [PubMed]
- Rimm, E.B.; Ascherio, A.; Giovannucci, E.; Spiegelman, D.; Stampfer, M.J.; Willett, W.C. Vegetable, Fruit, and Cereal Fiber Intake and Risk of Coronary Heart Disease among Men. JAMA 1996, 275, 447–451. [Google Scholar] [CrossRef] [PubMed]
- Tzitzikas, E.N.; Vincken, J.-P.; de Groot, J.; Gruppen, H.; Visser, R.G.F. Genetic Variation in Pea Seed Globulin Composition. J. Agric. Food Chem. 2006, 54, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Hood-Niefer, S.D.; Warkentin, T.D.; Chibbar, R.N.; Vandenberg, A.; Tyler, R.T. Effect of Genotype and Environment on the Concentrations of Starch and Protein in, and the Physicochemical Properties of Starch from, Field Pea and Fababean. J. Sci. Food Agric. 2012, 92, 141–150. [Google Scholar] [CrossRef]
- Teunissen-Beekman, K.F.M.; Dopheide, J.; Geleijnse, J.M.; Bakker, S.J.L.; Brink, E.J.; de Leeuw, P.W.; Serroyen, J.; van Baak, M.A. Differential Effects of Proteins and Carbohydrates on Postprandial Blood Pressure-Related Responses. Br. J. Nutr. 2014, 112, 600–608. [Google Scholar] [CrossRef]
- Liao, W.; Bhullar, K.S.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Egg White-Derived Tripeptide IRW (Ile-Arg-Trp) Is an Activator of Angiotensin Converting Enzyme 2. J. Agric. Food Chem. 2018, 66, 11330–11336. [Google Scholar] [CrossRef]
- Majumder, K.; Liang, G.; Chen, Y.; Guan, L.; Davidge, S.T.; Wu, J. Egg Ovotransferrin-Derived ACE Inhibitory Peptide IRW Increases ACE2 but Decreases Proinflammatory Genes Expression in Mesenteric Artery of Spontaneously Hypertensive Rats. Mol. Nutr. Food Res. 2015, 59, 1735–1744. [Google Scholar] [CrossRef]
- Liao, W.; Chakrabarti, S.; Davidge, S.T.; Wu, J. Modulatory Effects of Egg White Ovotransferrin-Derived Tripeptide IRW (Ile-Arg-Trp) on Vascular Smooth Muscle Cells against Angiotensin II Stimulation. J. Agric. Food Chem. 2016, 64, 7342–7347. [Google Scholar] [CrossRef]
- Khalid, I.; Elharadallou, S. Functional Properties of Cowpea (Vigna ungiculata L. Walp), and Lupin (Lupinus termis) Flour and Protein Isolates. J. Nutr. Food Sci. 2013, 3, 1–6. [Google Scholar]
- Kirse, A.; Karklina, D. Integrated Evaluation of Cowpea (Vigna unguiculata (L.) Walp.) and Maple Pea (Pisum sativum var. Arvense L.) Spreads. Agron. Res. 2015, 13, 956–968. [Google Scholar]
- Sebetha, E.; Modi, A.; Owoeye, L. Cowpea Crude Protein as Affected by Cropping System, Site and Nitrogen Fertilization. J. Agric. Sci. 2014, 7, 224–234. [Google Scholar] [CrossRef]
- Trehan, I.; Benzoni, N.S.; Wang, A.Z.; Bollinger, L.B.; Ngoma, T.N.; Chimimba, U.K.; Stephenson, K.B.; Agapova, S.E.; Maleta, K.M.; Manary, M.J. Common Beans and Cowpeas as Complementary Foods to Reduce Environmental Enteric Dysfunction and Stunting in Malawian Children: Study Protocol for Two Randomized Controlled Trials. Trials 2015, 16, 520. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, A.; Goufo, P.; Barros, A.; Domínguez-Perles, R.; Trindade, H.; Rosa, E.A.S.; Ferreira, L.; Rodrigues, M. Cowpea (Vigna unguiculata L. Walp), a Renewed Multipurpose Crop for a More Sustainable Agri-Food System: Nutritional Advantages and Constraints. J. Sci. Food Agric. 2016, 96, 2941–2951. [Google Scholar] [CrossRef]
- Petchiammal, C.; Hopper, W. Antioxidant Activity of Proteins from Fifteen Varieties of Legume Seeds Commonly Consumed in India. Int. J. Pharm. 2014, 6, 476–479. [Google Scholar]
- Kanetro, B. Hypocholesterolemic Properties of Protein Isolate from Cowpeas (Vigna unguiculata) Sprout in Normal and Diabetic Rats. Procedia Food Sci. 2015, 3, 112–118. [Google Scholar] [CrossRef]
- Frota, K.; Matias, A.; Areas, J. Influence of Food Components on Lipid Metabolism: Scenarios and Perspective on the Control and Prevention of Dyslipidemias. Food Sci. Technol. 2010, 30, 7–14. [Google Scholar] [CrossRef]
- Frota, K.; dos Santos, F.; Ribeiro, V.; Areas, J. Cowpea Protein Reduces LDL-Cholesterol and Apolipoprotein B Concentrations, but Does Not Improve Biomarkers of Inflammation or Endothelial Dysfunction in Adults with Moderate Hypercholesterolemia. Nutr. Hosp. 2015, 31, 1611–1619. [Google Scholar]
- Marques, M.R.; Cerda, A.; Fontanari, G.G.; Pimenta, D.C.; Soares-Freitas, R.M.; Hirata, M.H.; Hirata, R.D.C.; Arêas, J.A.G. Transport of Cowpea Bean Derived Peptides and Their Modulator Effects on mRNA Expression of Cholesterol-Related Genes in Caco-2 and HepG2 Cells. Food Res. Int. 2018, 107, 165–171. [Google Scholar] [CrossRef]
- Marques, M.R.; Soares Freitas, R.A.M.; Corrêa Carlos, A.C.; Siguemoto, É.S.; Fontanari, G.G.; Arêas, J.A.G. Peptides from Cowpea Present Antioxidant Activity, Inhibit Cholesterol Synthesis and Its Solubilisation into Micelles. Food Chem. 2015, 168, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Real-Hernandez, L.M.; Gonzalez de Mejia, E. Bean Peptides Have Higher In Silico Binding Affinities than Ezetimibe for the N-Terminal Domain of Cholesterol Receptor Niemann-Pick C1 Like-1. Peptides 2017, 90, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Singh, R.; Malhotra, S.; Boora, K.S.; Singal, H.R. Characterization of Seed Storage Proteins in High Protein Genotypes of Cowpea [Vigna unguiculata (L.) Walp]. Physiol. Mol. Biol. Plants 2010, 16, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Park, S.J.; Kim, T.W.; Baik, B.-K. Relationship between Proportion and Composition of Albumins, and In Vitro Protein Digestibility of Raw and Cooked Pea Seeds (Pisum sativum L.). J. Sci. Food Agric. 2010, 90, 1719–1725. [Google Scholar] [CrossRef]
- Tchiagam, J.; Bell, J.; Antoine, N.; Njintang, N.; Youmbi, E. Genetic Analysis of Seed Proteins Contents in Cowpea (Vigna unguiculata L Walp). Afr. J. Biotechnol. 2011, 10, 3077–3086. [Google Scholar]
- Hachibamba, T.; Dykes, L.; Awika, J.; Minnaar, A.; Duodu, K.G. Effect of Simulated Gastrointestinal Digestion on Phenolic Composition and Antioxidant Capacity of Cooked Cowpea (Vigna unguiculata) Varieties. Int. J. Food Sci. Technol. 2013, 48, 2638–2649. [Google Scholar] [CrossRef]
- Watanabe, H.; Inaba, Y.; Kimura, K.; Asahara, S.; Kido, Y.; Matsumoto, M.; Motoyama, T.; Tachibana, N.; Kaneko, S.; Kohno, M.; et al. Dietary Mung Bean Protein Reduces Hepatic Steatosis, Fibrosis, and Inflammation in Male Mice with Diet-Induced, Nonalcoholic Fatty Liver Disease. J. Nutr. 2016, 147, 52–60. [Google Scholar] [CrossRef]
- Li, G.; Le, G.; Liu, H.; Shi, Y. Mung-Bean Protein Hydrolysates Obtained with Alcalase Exhibit Angiotensin I-Converting Enzyme Inhibitory Activity. Food Sci. Technol. Int. 2005, 11, 281–287. [Google Scholar] [CrossRef]
- Wongekalak, L.; Sakulsom, P.; Jirasripongpun, K.; Hongsprabhas, P. Potential Use of Antioxidative Mungbean Protein Hydrolysate as an Anticancer Asiatic Acid Carrier. Food Res. Int. 2011, 44, 812–817. [Google Scholar] [CrossRef]
- Xie, J.; Du, M.; Shen, M.; Wu, T.; Lin, L. Physico-Chemical Properties, Antioxidant Activities and Angiotensin-I Converting Enzyme Inhibitory of Protein Hydrolysates from Mung Bean (Vigna radiata). Food Chem. 2019, 270, 243–250. [Google Scholar] [CrossRef]
- Li, G.; Wan, J.; Le, G.; Shi, Y. Novel Angiotensin I-Converting Enzyme Inhibitory Peptides Isolated from Alcalase Hydrolysate of Mung Bean Protein. J. Pept. Sci. 2006, 12, 509–514. [Google Scholar] [CrossRef]
- Clemente, A.; Vioque, J.; Sánchez-Vioque, R.; Pedroche, J.; Bautista, J.; Millán, F. Protein Quality of Chickpea (Cicer arietinum L.) Protein Hydrolysates. Food Chem. 1999, 67, 269–274. [Google Scholar] [CrossRef]
- Pittaway, J.K.; Robertson, I.K.; Ball, M.J. Chickpeas May Influence Fatty Acid and Fiber Intake in an Ad Libitum Diet, Leading to Small Improvements in Serum Lipid Profile and Glycemic Control. J. Am. Diet. Assoc. 2008, 108, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor Components of Pulses and Their Potential Impact on Human Health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- Madani, S.; Prost, J.; Belleville, J. Dietary Protein Level and Origin (Casein and Highly Purified Soybean Protein) Affect Hepatic Storage, Plasma Lipid Transport, and Antioxidative Defense Status in the Rat. Nutrition 2000, 16, 368–375. [Google Scholar] [CrossRef]
- Chau, C.; Cheung, P.; Wong, Y. Hypocholesterolemic Effects of Protein Concentrates from Three Chinese Indigenous Legume Seeds. J. Agric. Food Chem. 1998, 46, 3698–3701. [Google Scholar] [CrossRef]
- Duranti, M. Grain Legume Proteins and Nutraceutical Properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Macarulla, M.T.; del Barrio, A.S.; Martínez, J.A. Composition and Functional Properties of Protein Isolates Obtained from Commercial Legumes Grown in Northern Spain. Plant Foods Hum. Nutr. 1997, 51, 331–341. [Google Scholar] [CrossRef]
- Fernández-Quintela, A.; Barrio, A.S.D.; Macarulla, M.T.; Martínez, J.A. Nutritional Evaluation and Metabolic Effects in Rats of Protein Isolates Obtained from Seeds of Three Legume Species. J. Sci. Food Agric. 1998, 78, 251–260. [Google Scholar] [CrossRef]
- Li, Y.W.; Li, B.; He, J.; Qian, P. Quantitative Structure–Activity Relationship Study of Antioxidative Peptide by Using Different Sets of Amino Acids Descriptors. J. Mol. Struct. 2011, 998, 53–61. [Google Scholar] [CrossRef]
- Roberts, P.R.; Burney, J.D.; Black, K.W.; Zaloga, G.P. Effect of Chain Length on Absorption of Biologically Active Peptides from the Gastrointestinal Tract. Digestion 1999, 60, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Xie, N.; Li, B. Influence of Peptide Characteristics on Their Stability, Intestinal Transport, and In Vitro Bioavailability: A Review. J. Food Biochem. 2019, 43, e12571. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure−Activity Relationship Study of Di- and Tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-Converting Enzyme (ACE)-Inhibitory Peptides from Plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Jang, J.-H.; Jeong, S.-C.; Kim, J.-H.; Lee, Y.-H.; Ju, Y.-C.; Lee, J.-S. Characterisation of a New Antihypertensive Angiotensin I-Converting Enzyme Inhibitory Peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural Requirements of Angiotensin I-Converting Enzyme Inhibitory Peptides: Quantitative Structure-Activity Relationship Modeling of Peptides Containing 4-10 Amino Acid Residues. QSAR Comb. Sci. 2006, 25, 873–880. [Google Scholar] [CrossRef]
- Iwaniak, A.; Minkiewicz, P.; Darewicz, M. Food-Originating ACE Inhibitors, Including Antihypertensive Peptides, as Preventive Food Components in Blood Pressure Reduction. Compr. Rev. Food Sci. Food Saf. 2014, 13, 114–134. [Google Scholar] [CrossRef]
- Temsamani, J.; Vidal, P. The Use of Cell-Penetrating Peptides for Drug Delivery. Drug. Discov. Today 2004, 9, 1012–1019. [Google Scholar] [CrossRef]
- Guha, S.; Majumder, K. Structural-Features of Food-Derived Bioactive Peptides with Anti-Inflammatory Activity: A Brief Review. J. Food Chem. 2019, 43, e12531. [Google Scholar] [CrossRef]
- Nan, Y.; Park, K.; Park, Y.; Jeon, Y.J.; Kim, Y.; Park, I.; Hahm, K.; Shin, S. Investigating the Effects of Positive Charge and Hydrophobicity on the Cell Selectivity, Mechanism of Action and Anti-Inflammatory Activity of a Trp-Rich Antimicrobial Peptide Indolicidin. FEMS. Microbiol. Lett. 2009, 292, 134–140. [Google Scholar] [CrossRef]
- Kyte, J.; Doolittle, R.F. A Simple Method for Displaying the Hydropathic Character of a Protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef]
- Stothard, P. The Sequence Manipulation Suite: JavaScript Programs for Analyzing and Formatting Protein and DNA Sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Yang, J. Analysis and Prediction of Highly Effective Antiviral Peptides Based on Random Forests. PLoS ONE 2013, 8, e70166. [Google Scholar] [CrossRef] [PubMed]
- Yea, C.S.; Ebrahimpour, A.; Hamid, A.A.; Bakar, J.; Muhammad, K.; Saari, N. Winged Bean [Psophorcarpus tetragonolobus (L.) DC] Seeds as an Underutilised Plant Source of Bifunctional Proteolysate and Biopeptides. Food Funct. 2014, 5, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Montojo, J.; Zuberi, K.; Rodriguez, H.; Kazi, F.; Wright, G.; Donaldson, S.L.; Morris, Q.; Bader, G.D. GeneMANIA Cytoscape Plugin: Fast Gene Function Predictions on the Desktop. Bioinformatics 2010, 26, 2927–2928. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sidiropoulos, K.; Viteri, G.; Sevilla, C.; Jupe, S.; Webber, M.; Orlic-Milacic, M.; Jassal, B.; May, B.; Shamovsky, V.; Duenas, C.; et al. Reactome Enhanced Pathway Visualization. Bioinformatics 2017, 33, 3461–3467. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).