Polysaccharides and Derivatives from Africa to Address and Advance Sustainable Development and Economic Growth in the Next Decade
Abstract
:1. Introduction
2. A Spoon of (African) Sugar?
2.1. Common Knowledge about Polysaccharides
| Type | Name | Composition | Type of Sources (Organism, Part of the Plant, …) | Ref. for Instance |
|---|---|---|---|---|
| Plants | Arabinogalactan | β-(1,4)-d-arabinogalactan (type I) | Cactus (cladodes) | [22] |
| Arabinoxylan | Branched β-(1,3)-d-arabinoxylan | Plant seeds | [23] | |
| Cellulose | β-(1,4)-d-glucan | Grains, fruits, vegetables, | [21] | |
| Galactomannan | β-(1,4)-d-mannan randomly substituted at O-6 position with α-Galp | Plant seeds | [24] | |
| Β-Glucans | β-(1,4)-d-glucan β-(1,3)-d-glucan | Barley grains, Fruits, seeds, Oats | [25] | |
| “Gums” | (Arabino)galactan, xylan, xyloglucan, glucuronic mannan type | Exudates of trees or isolated from seeds | [26] | |
| Hemicellulose | Xylan, mannan, β-glucan and xyloglucan | Vegetative and storage tissues | [27] | |
| Heteroxylan | Highly branched β-(1,3)-d-Xylp and β-(1,4)-d-Xylp backbone | Plant seeds | [28] | |
| Inulin | β-(1,2)-d-fructan | Onion, root, wheat | [29] | |
| Pectin | α-(1,4)-d-GalA and Rha backbone, Ara, Gal, Xyl side chains | Plant primary cell wall, leaves, soft tissues of fruit and vegetable | [30] | |
| Xyloglucan | β-(1,4)-d-glucan backbone with α-(1,6)-d-xylose branches | Tree fruits, seeds | [31] | |
| Macroalgae | Alginate | α-l-guluronate (G)/ β-d-mannuronate (M) block structure | Brown algae | [32] |
| Glucan | Cellulose, laminaran, starch | Brown/Green algae | [33] | |
| Porphyran | Alternating β-(1,3)-linked-d-Gal units and α-(1,4)-linked l-Gal, (1,6)-sulfate, or 3,6-anhydro-α-l-Gal units | Red algae | [34] | |
| Sulfated fucoidan | Branched α-(1,3), α-(1,4)-l-fucan, O-2, O-3, O-4 sulfation | Brown algae | [32] | |
| Sulfated galactan | Backbone of alternating β-(1,3)-linked d-Gal units and α-(1,4)-linked l-Gal, (1,6)-sulfate or 3,6-anhydro-α-l-Gal units. d-Gal units linked on C-3 and C-6, and sulfation mostly on O-4. | Red algae | [35] | |
| Sulfated polysaccharides | (1,3(6))-linked Gal, (1,3(4))-linked Ara, (1,4)-linked Glc and T-Glc, (1,4)-linked Xyl residues | Green algae | [36] | |
| Sulfated rhamnan | (1-2)-l-rhamnan substituted by sulfate groups at C-3, and/or C-4 | Green algae | [37] | |
| Ulvan | Repeating disaccharide ulvanobiouronic acid with Xyl, Glc, Rha, and sulfate groups | Green algae | [38] | |
| Xylan | β-(1,3)-xylan | Green algae | [15] |
2.2. Screening the Biological Potential of Polysaccharides: Randomly or Not?
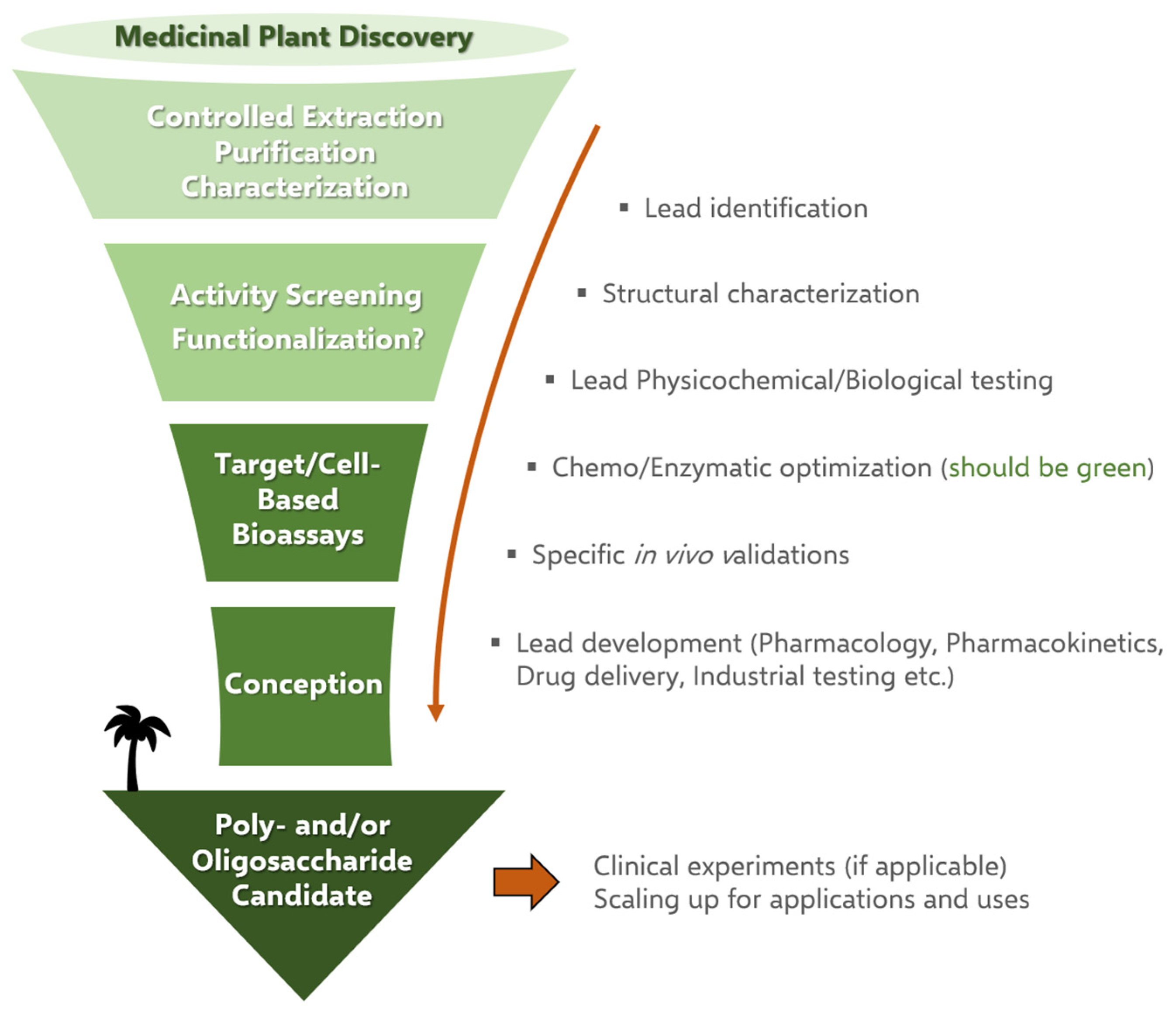
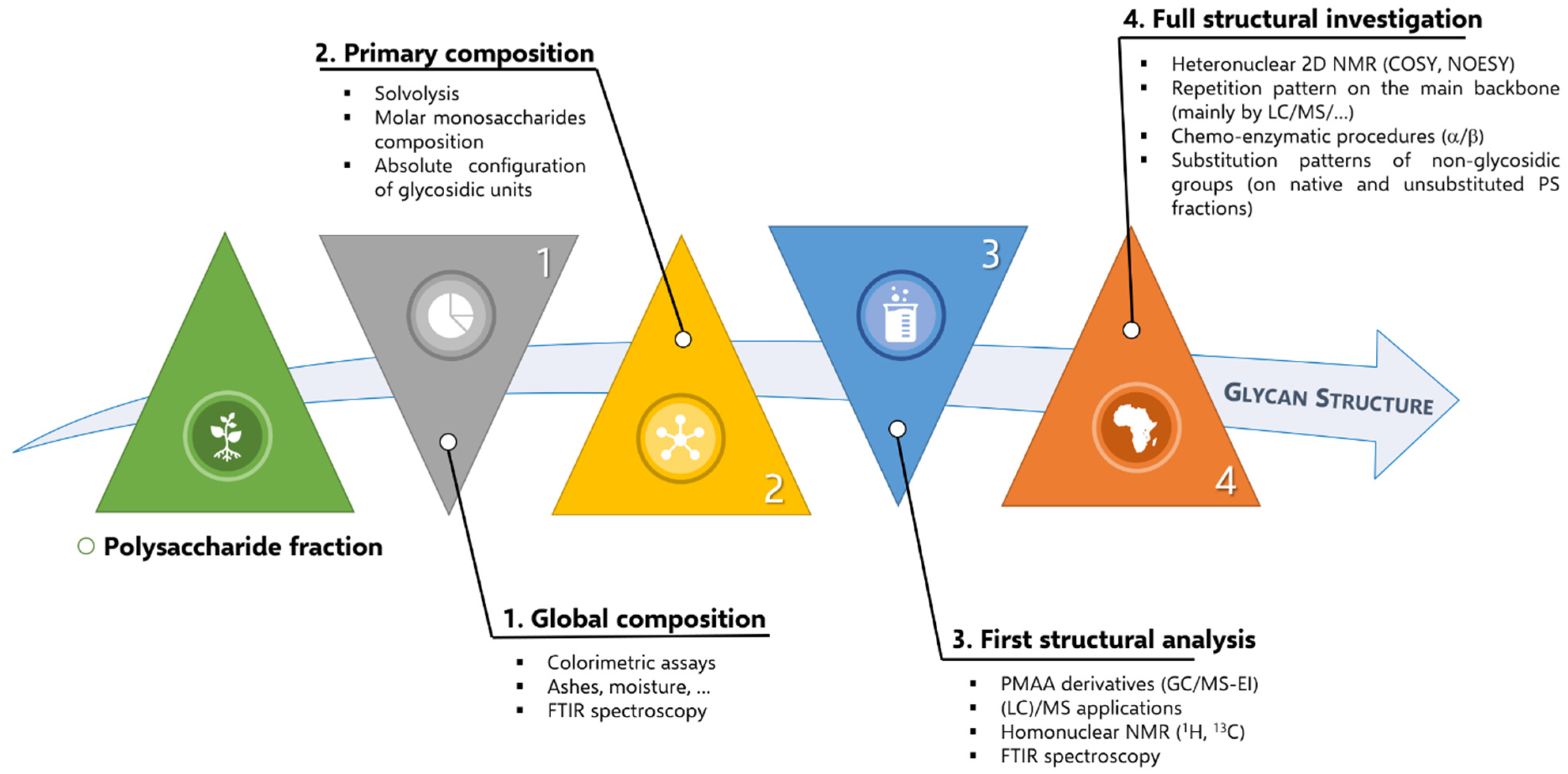
3. Discovering Polysaccharides in Africa
3.1. Research Methodology
3.2. The Concept of Ethnobotany for Giving Birth to Ethnopharmacology and Phytochemistry
- (i)
- Discovering new natural drugs or reusing existing ones for treating disorders,
- (ii)
- Developing new chemicals mimicking active structural features,
- (iii)
- Rising knowledge on:
- Characteristics and functions of medicinal plants;
- Toxicity level of plants,
- Biosynthetic pathways and metabolomics;
- Classification, chemical variability (inter and intraspecific);
- Biotechnology and genetic engineering for optimizing the synthesis of specific compounds;
- Phytoremediation, plant growing and elicitation.
3.3. A Focus on Bioactive and/or Functional Polysaccharides from Arid and Semi-Arid Lands
3.3.1. Acacia
3.3.2. Argania
3.3.3. Opuntia
3.3.4. Plantago
3.3.5. Astragalus
3.3.6. Phoenix
3.3.7. Retama
3.3.8. Zizyphus
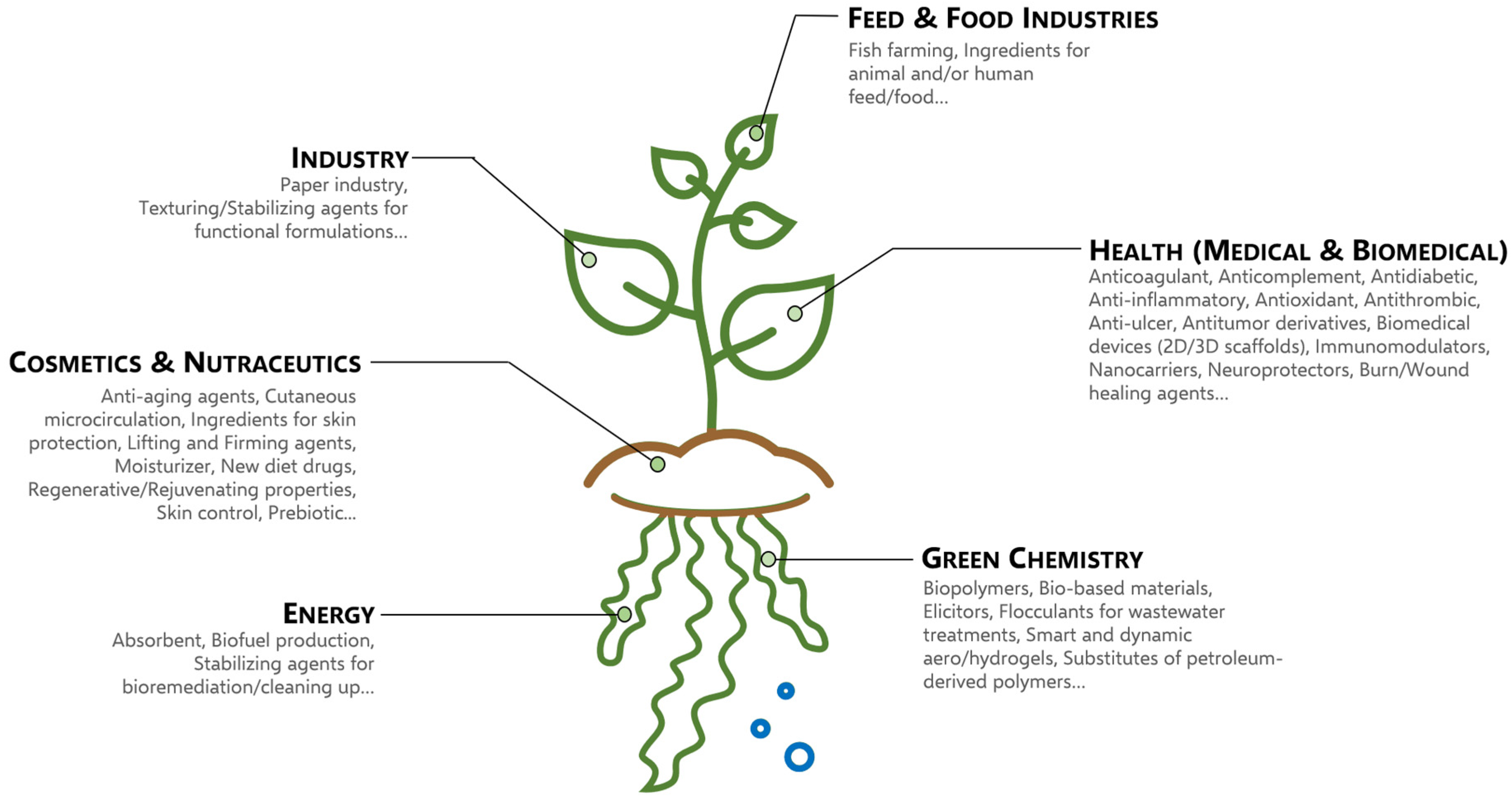
4. Economic Interests
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wijesinghe, W.A.J.P.; Jeon, Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: A review. Carbohydr. Polym. 2012, 88, 13–20. [Google Scholar]
- Cooke, C.L.; An, H.J.; Kim, J.; Solnick, J.V.; Lebrilla, C.B. Method for profiling mucin oligosaccharides from gastric biopsies of rhesus monkeys with and without Helicobacter pylori infection. Anal. Chem. 2007, 79, 8090–8097. [Google Scholar] [PubMed]
- Grønhaug, T.E.; Ghildyal, P.; Barsett, H.; Michaelsen, T.E.; Morris, G.; Diallo, D.; Inngjerdingen, M.; Paulsen, B.S. Bioactive arabinogalactans from the leaves of Opilia celtidifolia Endl. ex Walp. (Opiliaceae). Glycobiology 2010, 20, 1654–1664. [Google Scholar] [CrossRef] [Green Version]
- Bouissil, S.; El Alaoui-Talibi, Z.; Pierre, G.; Michaud, P.; El Modafar, C.; Delattre, C. Use of Alginate Extracted from Moroccan Brown Algae to Stimulate Natural Defense in Date Palm Roots. Molecules 2020, 25, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngwuluka, N.C. Responsive polysaccharides and polysaccharides-based nanoparticles for drug delivery. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Makhlouf, A.S.H., Abuthabit, N.Y., Eds.; Woodhead Publishing: Cambridge, UK, 2018; Volume 1, pp. 531–554. [Google Scholar]
- Anderson, J.M. Biocompatibility. In Polymer Science: A Comprehensive Reference; Matyjaszwski, K., Möeller, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2012; Volume 9, pp. 363–383. [Google Scholar]
- Gu, B.; Burgess, D.J. Chapter 20—Polymeric materials in drug delivery. In Natural and Synthetic Biomedical Polymers; Kumbar, S.G., Laurencin, C.T., Deng, M., Eds.; Elsevier Science: Boston, MA, USA, 2014; pp. 333–349. [Google Scholar]
- Ziani, B.E.C.; Rached, W.; Bachari, K.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Ferreira, I.C.F.R. Detailed chemical composition and functional properties of Ammodaucus leucotrichus Cross. & Dur. and Moringa oleifera Lamarck. J. Funct. Foods 2019, 53, 237–247. [Google Scholar]
- Idm’hand, E.; Msanda, F.; Cherifi, K. Medicinal uses, phytochemistry and pharmacology of Ammodaucus leucotrichus. Clin. Phytosci. 2020, 6, 6. [Google Scholar] [CrossRef]
- An, H.J.; Lebrilla, C.B. Structure elucidation of native N- and O-linked glycans by tandem mass spectrometry (tutorial). Mass Spectrom. Rev. 2011, 30, 560–578. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, L.-M. Chemical structural and chain conformational characterization of some bioactive polysaccharides isolated from natural sources. Carbohydr. Polym. 2009, 76, 349–361. [Google Scholar] [CrossRef]
- Matricardi, P.; Alhaique, F.; Coviello, T. Polysaccharide Hydrogels: Characterization and Biomedical Applications, 1st ed.; Jenny Stanford Publishing: Singapore, 2016; p. 540. [Google Scholar]
- Ji, X.; Hou, C.; Guo, X. Physicochemical properties, structures, bioactivities and future prospective for polysaccharides from Plantago L. (Plantaginaceae): A review. Int. J. Biol. Macromol. 2019, 135, 637–646. [Google Scholar] [CrossRef]
- Gorshkova, T.A.; Kozlova, L.V.; Mikshina, P.V. Spatial structure of plant cell wall polysaccharides and its functional importance. Biochemistry 2013, 78, 836–853. [Google Scholar]
- Liu, J.; Willför, S.; Xu, C. A review of bioactive plant polysaccharides: Biological activities, functionalization, and biomedical applications. Bioact. Carbohydr. Diet. Fibre 2015, 5, 31–61. [Google Scholar] [CrossRef]
- Onyechi, U.A.; Judd, P.A.; Ellis, P.R. African plant foods rich in non-starch polysaccharides reduce postprandial blood glucose and insulin concentrations in healthy human subjects. Br. J. Nutr. 1998, 80, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Angone, S.A.; Bardor, M.; Nguema-Ona, E.; Rihouey, C.; Ishii, T.; Lerouge, P.; Driouich, A. Structural characterization of cell wall polysaccharides from two plant species endemic to central Africa, Fleurya aestuans and Phragmenthera capitata. Carbohydr. Polym. 2009, 75, 104–109. [Google Scholar] [CrossRef]
- Ren, Y.; Bai, Y.; Zhang, Z.; Cai, W.; Del Rio Flores, A. The preparation and structure analysis methods of natural polysaccharides of plants and fungi: A review of recent development. Molecules 2019, 24, 3122. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Huang, G. Extraction, separation, modification, structural characterization, and antioxidant activity of plant polysaccharides. Chem. Biol. Drug. Des. 2020, 96, 1209–1222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, G. Extraction and derivatisation of active polysaccharides. J. Enzyme Inhib. Med. Chem. 2019, 34, 1690–1696. [Google Scholar] [CrossRef]
- Ullah, S.; Khalil, A.A.; Shaukat, F.; Song, Y. Sources, extraction and biomedical properties of polysaccharides. Foods 2019, 8, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petera, B.; Delattre, C.; Wadouachi, A.; Elboutachfaiti, R.; Engel, E.; Poughon, L.; Michaud, P.; Fenoradosoa, T.A. Characterization of arabinogalactan-rich mucilage from Cereus triangularis cladodes. Carbohydr. Polym. 2015, 127, 372–380. [Google Scholar] [CrossRef]
- Addoun, N.; Boual, Z.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Hentati, F.; Ould El-Hadj, M.D.; Michaud, P.; et al. Structural features and rheological behavior of a water-soluble polysaccharide extracted from the seeds of Plantago ciliata Desf. Int. J. Biol. Macromol. 2020, 155, 1333–1341. [Google Scholar] [CrossRef]
- Chouana, T.; Pierre, G.; Vial, C.; Gardarin, C.; Wadouachi, A.; Dailleu, D.; Le Cerf, D.; Boual, Z.; Ould El Hadj, M.D.; Michaud, P.; et al. Structural characterization and rheological properties of a galactomannan from Astragalus gombo Bunge seeds harvested in Algerian Sahara. Carbohydr. Polym. 2017, 175, 387–394. [Google Scholar] [CrossRef]
- Rjeibi, I.; Feriani, A.; Hentati, F.; Hfaiedh, N.; Michaud, P.; Pierre, G. Structural characterization of water-soluble polysaccharides from Nitraria retusa fruits and their antioxidant and hypolipidemic activities. Int. J. Biol. Macromol. 2019, 129, 422–432. [Google Scholar] [CrossRef]
- De, A.; Malpani, D.; Das, B.; Mitra, D.; Samanta, A. Characterization of an arabinogalactan isolated from gum exudate of Odina wodier Roxb.: Rheology, AFM, Raman and CD spectroscopy. Carbohydr. Polym. 2020, 250, 116950. [Google Scholar] [CrossRef] [PubMed]
- Wangensteen, H.; Diallo, D.; Paulsen, B.S. Medicinal plants from Mali: Chemistry and biology. J. Ethnopharmacol. 2015, 176, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Benaoun, F.; Delattre, C.; Boual, Z.; Ursu, A.V.; Vial, C.; Gardarin, C.; Wadouachi, A.; Le Cerf, D.; Varacavoudin, T.; Ould El-Hadj, M.D.; et al. Structural characterization and rheological behavior of a heteroxylan extracted from Plantago notata Lagasca (Plantaginaceae) seeds. Carbohydr. Polym. 2017, 175, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ledesma, F.R.; Sánchez-Moreno, V.E.; Vera, E.; Ciobotă, V.; Jentzsch, P.V.; Jaramillo, L.I. Extraction of Inulin from Andean Plants: An Approach to Non-Traditional Crops of Ecuador. Molecules 2020, 25, 5067. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, K.; Iboukhoulef, L.; Petit, E.; Benouatas, H.; Pierre, G.; Delattre, C. Anti-inflammatory and antioxidant effect of a d-galactose-rich polysaccharide extracted from Aloe vera leaves. Adv. Appl. Chem. Biochem. 2018, 1, 18–26. [Google Scholar]
- Ren, Y.; Picout, D.R.; Ellis, P.R.; Ross-Murphy, S.B.; Reid, J.S. A novel xyloglucan from seeds of Afzelia africana Se. Pers.—Extraction, characterization, and conformational properties. Carbohydr. Res. 2005, 340, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Le Cerf, D.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural characterization and antioxidant activity of water-soluble polysaccharides from the Tunisian brown seaweed Cystoseira compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef]
- Thygesen, A.; Ami, J.; Fernando, D.; Bentil, J.; Daniel, G.; Mensah, M.; Meyer, A.S. Microstructural and carbohydrate compositional changes induced by enzymatic saccharification of green seaweed from West Africa. Algal Res. 2020, 47, 101894. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, T.; Yang, Y.; Meng, F.; Zhan, F.; Jiang, Q.; Sun, X. Anti-Cancer Activity of Porphyran and Carrageenan from Red Seaweeds. Molecules 2019, 24, 4286. [Google Scholar] [CrossRef] [Green Version]
- Pierre, G.; Delattre, C.; Laroche, C.; Michaud, P. Galactans and its applications. In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer: Cham, Switzerland, 2014; pp. 1–37. [Google Scholar] [CrossRef]
- Ghosh, P.; Adhikari, U.; Ghosal, P.K.; Pujol, C.A.; Carlucci, M.J.; Damonte, E.B.; Ray, B. In vitro anti-herpetic activity of sulfated polysaccharide fractions from Caulerpa racemosa. Phytochemistry 2004, 65, 3151–3157. [Google Scholar] [CrossRef]
- Ciancia, M.; Fernández, P.V.; Leliaert, F. Diversity of sulfated polysaccharides from cell walls of coenocytic green algae and their structural relationships in view of green algal evolution. Front. Plant Sci. 2020, 11, 554585. [Google Scholar] [CrossRef]
- Kidgell, J.T.; Magnusson, M.; de Nys, R.; Glasson, C.R.K. Ulvan: A systematic review of extraction, composition and function. Algal Res. 2019, 39, 101422. [Google Scholar] [CrossRef]
- Hromádková, Z.; Ebringerová, A.; Valachovič, P. Comparison of classical and ultrasound-assisted extraction of polysaccharides from Salvia officinalis. Ultrason. Sonochem. 1999, 5, 163–168. [Google Scholar] [CrossRef]
- Riesz, P.; Kondo, T. Free radical formation induced by ultrasound and its biological implications. Free Radic. Biol. Med. 1992, 13, 247–270. [Google Scholar] [CrossRef]
- Srivastava, R.; Kulshreshtha, D.K. Bioactive polysaccharides from plants. Phytochemistry 1989, 28, 2877–2883. [Google Scholar] [CrossRef]
- Espinosa-Leal, C.A.; Garcia-Lara, S. Current methods for the discovery of new active ingredients from natural products for cosmeceutical applications. Planta Med. 2019, 85, 535–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delattre, C.; Pierre, C.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Pebers, P.A.; Smith, F. Colorimetric method for determination of sugar and relayed substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Monsigny, M.; Petit, C.; Roche, A.C. Colorimetric determination of neutral sugars by a resorcinol sulfuric acids micromethod. Anal. Biochem. 1988, 175, 525–530. [Google Scholar] [CrossRef]
- Montreuil, J.; Spick, G.; Chosson, A.; Segard, E.; Scheppler, N. Methods of study of the structure of glycoproteins. J. Pharm. Belg. 1963, 18, 529–546. [Google Scholar]
- Sloneker, J.H.; Orentas, D.G. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature 1962, 194, 478–479. [Google Scholar] [CrossRef]
- Voragen, A.G.J.; Schols, H.A.; Pilnik, W. Determination of the degree of methylation and acetylation of pectins by HPLC. Food Hydrocoll. 1986, 1, 65–70. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharide. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaques, L.B.; Ballieux, R.E.; Dietrich, C.P.; Kavanagh, L.W. A microelectrophoresis method for heparin. Can. J. Physiol. Pharmacol. 1968, 46, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Bradford, H.M. A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299C, 152–178. [Google Scholar]
- Kamerling, J.P.; Gerwig, G.J. Strategies for the structural analysis of carbohydrates. In Comprehensive Glycoscience—From Chemistry to Systems Biology; Boons, G.-J., Kamerling, J.P., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G.J., Eds.; Elsevier B.V.: Oxford, UK, 2007; Volume 2, pp. 1–68. [Google Scholar]
- Kitson, F.G.; Larsen, B.S.; McEwen, C.N. Gas Chromatography and Mass Spectrometry: A Practical Guide; Academic Press, Inc.: Berlin, Germany, 1996; p. 381. [Google Scholar]
- Pawliszyn, J. Comprehensive Sampling and Sample Preparation, 1st ed.; Academic Press, Inc.: Berlin, Germany, 2012; p. 3200. [Google Scholar]
- Kamerling, J.P.; Gerwig, G.J.; Vliegenthart, J.F.G.; Clamp, J.R. Characterization by gas liquid chromatography-mass spectrometry and proton-magnetic-resonance spectroscopy of pertrimethylsilylmethyl glycosides obtained in the methanolysis of glycoproteins and glycopeptides. Biochem. J. 1975, 151, 491–495. [Google Scholar] [CrossRef] [Green Version]
- Peña, M.J.; Tuomivaara, S.T.; Urbanowicz, B.R.; O’Neill, M.A.; York, W.S. Methods for structural characterization of the products of cellulose- and xyloglucan-hydrolyzing enzymes. Methods Enzymol. 2012, 510, 121–139. [Google Scholar]
- Duus, J.Ø.; Gotfredsen, C.H.; Bock, K. Carbohydrate structural determination by NMR spectroscopy: Modern methods and limitations. Chem. Rev. 2000, 100, 4589–4614. [Google Scholar] [CrossRef]
- Størseth, T.R.; Chauton, M.S.; Krane, J. HR MAS NMR spectroscopy of marine microalgae, part 2: 13C and 13C HR MAS NMR analysis used to study fatty acid composition and polysaccharide structure. In Modern Magnetic Resonance; Webb, G.A., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 953–957. [Google Scholar]
- Boual, Z.; Pierre, G.; Delattre, C.; Benaoun, F.; Petit, E.; Gardarin, C.; Michaud, P.; Ould El Hadj, M.D. Mediterranean semi-arid plant Astragalus armatus as a source of bioactive galactomannan. Bioact. Carbohydr. Diet. Fibre 2015, 5, 10–18. [Google Scholar] [CrossRef]
- Deng, Y.; Yi, Y.; Zhang, L.; Zhang, R.; Zhang, Y.; Wei, Z.; Tang, X.; Zhang, M. Immunomodulatory activity and partial characterisation of polysaccharides from Momordica charantia. Molecules 2014, 19, 13432–13447. [Google Scholar] [CrossRef] [Green Version]
- Yamada, H.; Kiyohara, H. Immunomodulating activity of plant polysaccharide structures. In Comprehensive Glycoscience—From Chemistry to Systems Biology; Boons, G.-J., Kamerling, J.P., Lee, Y.C., Suzuki, A., Taniguchi, N., Voragen, A.G.J., Eds.; Elsevier B.V.: Oxford, UK, 2007; Volume 4, pp. 663–694. [Google Scholar]
- Paulsen, B.S.; Barsett, H. Bioactive pectic polysaccharides. Adv. Polym. Sci. 2005, 186, 69–101. [Google Scholar]
- Inngjerdingen, K.T.; Meskini, S.; Austarheim, I.; Ballo, N.; Inngjerdingen, M.; Michaelsen, T.E.; Diallo, D.; Paulsen, B.S. Chemical and biological characterization of polysaccharides from wild and cultivated roots of Vernonia kotschyana. J. Ethnopharmacol. 2012, 139, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Grønhaug, T.E.; Kiyohara, H.; Sveaass, A.; Diallo, D.; Yamada, H.; Paulsen, B.S. β-d-(1→4)-galactan-containing side chains in RG-I regions of pectic polysaccharides from Biophytum petersianum Klotzsch. contribute to expression of immunomodulating activity against intestinal Peyer’s patch cells and macrophages. Phytochemistry 2011, 72, 2139–2147. [Google Scholar] [CrossRef] [PubMed]
- Kouakou, K.; Schepetkin, I.A.; Jun, S.; Kirpotina, L.N.; Yapi, A.; Khramova, D.S.; Pascual, D.W.; Ovodov, Y.S.; Jutila, M.A.; Quinn, M.T. Immunomodulatory activity of polysaccharides isolated from Clerodendrum splendens: Beneficial effects in experimental autoimmune encephalomyelitis. BMC Complement. Altern. Med. 2013, 13, 149. [Google Scholar] [CrossRef] [Green Version]
- Šutovská, M.; Fraňová, S.; Sadloňová, V.; Grønhaug, T.E.; Diallo, D.; Paulsen, B.S.; Capek, P. The relationship between dose-dependent antitussive and bronchodilatory effects of Opilia celtidifolia polysaccharide and nitric oxide in guinea pigs. Int. J. Biol. Macromol. 2010, 47, 508–513. [Google Scholar] [CrossRef]
- Schepetkin, I.A.; Kouakou, K.; Yapi, A.; Kirpotina, L.N.; Jutila, M.A.; Quinn, M.T. Immunomodulatory and Hemagglutinating Activities of Acidic Polysaccharides Isolated from Combretum racemosum. Int. Immunopharmacol. 2013, 15, 628–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yusuf, A.J.; Abdullahi, M.I. The phytochemical and pharmacological actions of Entada africana Guill. & Perr. Heliyon 2019, 5, 23–32. [Google Scholar]
- Zhang, Y.; Wang, C.; Liu, C.; Wang, X.; Chen, B.; Yao, L.; Qiao, Y.; Zheng, H. Recent developments in stigma maydis polysaccharides: Isolation, structural characteristics, biological activities and industrial application. Int. J. Biol. Macromol. 2020, 150, 246–252. [Google Scholar] [CrossRef]
- Lebrun, J.-P.; Stork, A.L. Enumération des Plantes à Fleurs d’Afrique Tropicale. Volume II. Chrysobalanaceae à Apiaceae; Conservatoire et Jardin Botaniques de la Ville de Genève: Genève, Switzerland, 1992; p. 257. ISBN 2-8277-0109-X. [Google Scholar]
- Magadula, J.J.; Erasto, P. Bioactive natural products derived from the East African flora. Nat. Prod. Rep. 2009, 26, 1535–1554. [Google Scholar] [CrossRef] [PubMed]
- Orthen, B. A survey of the polysaccharide reserves in geophytes native to the winter-rainfall region of South Africa. S. Afr. J. Bot. 2001, 67, 371–375. [Google Scholar] [CrossRef] [Green Version]
- Cowling, R.M.; Procheş, Ş.; Vlok, J.H.J.; van Staden, J. On the origin of southern African subtropical thicket vegetation. S. Afr. J. Bot. 2005, 71, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Young, K.J.; Hopkins, W.G. Ethnobotany, 1st ed.; Chelsea House Publishers: New York, NY, USA, 2006; p. 112. ISBN 0791089630. [Google Scholar]
- Kpodar, M.S.; Karou, S.D.; Katawa, G.; Anani, K.; Gbekley, H.E.; Adjrah, Y.; Tchacondo, T.; Batawila, K.; Simpore, J. An ethnobotanical study of plants used to treat liver diseases in the Maritime region of Togo. J. Ethnopharmacol. 2016, 181, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Pardo de Santayana, M.; Pieroni, A.; Puri, R.K. Ethnobotany in the New Europe. People, Health and Wild Plant Resources, 1st ed.; Berghahn Books: New York, NY, USA, 2010; p. 408. ISBN 978-1-84545-456-2. [Google Scholar]
- Egbuna, C.; Ifemeje, J.C.; Udedi, S.C.; Kumar, S. Phytochemistry. Fundamentals, Modern Techniques, and Applications, 1st ed.; Apple Academic Press: Florida, FL, USA, 2019; p. 684. ISBN 1774634325. [Google Scholar]
- Addoun, N.; Delattre, C.; Boual, Z.; Ould El Hadj, M.D.; Michaud, P.; Pierre, G. Ethnic medicine and ethnobotany concept to identify and characterize new polysaccharide-based drug from arid and semi-arid lands. Adv. Appl. Chem. Biochem. 2018, 1, 37–39. [Google Scholar]
- Kiyohara, H.; Yamada, H. Structure of an anti-complementary arabinogalactan from the root of Angelica acutiloba Kitagawa. Carbohydr. Polym. 1986, 193, 173–195. [Google Scholar] [CrossRef]
- Diallo, D.; Sogn, C.; Samaké, F.B.; Paulsen, B.S.; Michaelsen, T.E.; Keita, A. Wound healing plants in Mali, the Bamako region. An ethnobotanical survey and complement fixation of water extracts from selected plants. Pharm. Biol. 2002, 40, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Tsouh Fokou, P.V.; Nyarko, A.K.; Appiah-Opong, R.; Tchokouaha Yamthe, L.R.; Addo, P.; Asante, I.K.; Boyom, F.F. Ethnopharmacological reports on anti-Buruli ulcer medicinal plants in three West African countries. J. Ethnopharmacol. 2015, 172, 297–311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salihu Shinkafi, T.; Bello, L.; Wara Hassan, S.; Ali, S. An ethnobotanical survey of antidiabetic plants used by Hausa-Fulani tribes in Sokoto, Northwest Nigeria. J. Ethnopharmacol. 2015, 172, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Hostettmann, K.; Marston, A.; Ndjoko, K.; Wolfender, J.L. The Potential of African Plants as a Source of Drugs. Curr. Org. Chem. 2000, 4, 973–1010. [Google Scholar] [CrossRef]
- Bedi, M.K.; Shenefelt, P.D. Herbal therapy in dermatology. Arch. Dermatol. 2002, 138, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Petera, B.; Delattre, C.; Pierre, G.; Vial, C.; Michaud, P.; Fenoradosoa, T.A. Rheological study and prebiotic potential of Cereus triangularis cladodes extract. Int. J. Adv. Res. Pub. 2020, 4, 1–11. [Google Scholar]
- Nehdi, I.A.; Sbihi, H.; Tan, C.P.; Al-Resayes, S.I. Evaluation and characterisation of Citrullus colocynthis (L.) Schrad seed oil: Comparison with Helianthus annuus (sunflower) seed oil. Food Chem. 2013, 136, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-F.; Zhang, Y.-Y.; Fu, Y.-P.; Inngjerdingen, K.T.; Paulsen, B.S.; Feng, B.; Zhu, Z.-K.; Li, L.-X.; Jia, R.-Y.; Huang, C.; et al. A Polysaccharide Isolated from Codonopsis pilosula with Immunomodulation Effects Both In Vitro and In Vivo. Molecules 2019, 24, 3632. [Google Scholar] [CrossRef] [Green Version]
- Boual, Z.; Pierre, G.; Kemassi, A.; Mosbah, S.; Benaoun, F.; Delattre, C.; Michaud, P.; Ould El Hadj, M.D. Chemical composition and bioloigcal activities of water-solbule polysaccharides from Commiphora myrrha (Nees) Engl. Gum. Analele Univ. din Oradea Fasc. Biol. 2020, 27, 50–55. [Google Scholar]
- Freiesleben, S.H.; Soelberg, J.; Jäger, A.K. Medicinal plants used as excipients in the history in Ghanaian herbal medicine. J. Ethnopharmacol. 2015, 174, 561–568. [Google Scholar] [CrossRef]
- Djomdi, D.; Hamadou, B.; Gibert, O.; Tran, T.; Delattre, C.; Pierre, G.; Michaud, P.; Ejoh, R.; Ndjouenkeu, R. Innovation in Tigernut (Cyperus Esculentus L.) Milk Production: In Situ Hydrolysis of Starch. Polymers 2020, 12, 1404. [Google Scholar] [CrossRef]
- Diallo, D.; Paulsen, B.S.; Liljebäck, T.H.; Michaelsen, T.E. Polysaccharides from the roots of Entada africana Guill. et Perr., Mimosaceae, with complement fixing activity. J. Ethnopharmacol. 2001, 74, 159–171. [Google Scholar] [CrossRef]
- Wani, S.A.; Kumar, P. Fenugreek: A review on its nutraceutical properties and utilization in various food products. J. Saudi Soc. Agric. Sci. 2018, 17, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Sangare, M.M.; Zina, H.; Dougnon, J.; Bayala, B.; Ategbo, J.-M.; Dramane, K.L. Etude ethnobotanique des plantes hépatotropes et de l’usage traditionnel de Gomphrena celosioides Mart. (Amaranthaceae) au Bénin. Int. J. Biol. Chem. Sci. 2012, 6, 5008–5021. [Google Scholar] [CrossRef] [Green Version]
- Magassouba, F.B.; Diallo, A.; Kouyate, M.; Mara, F.; Mara, O.; Bangoura, O.; Camara, A.; Traore, S.; Diallo, A.K.; Zaoro, M.; et al. Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. J. Ethnopharmacol. 2007, 114, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Adeleye, O.O.; Ayeni, O.J.; Ajamu, M.A. Traditional and medicinal uses of Morinda lucida. J. Med. Plants Stud. 2018, 6, 249–254. [Google Scholar]
- Rjeibi, I.; Hentati, F.; Feriani, A.; Hfaiedh, N.; Delattre, C.; Michaud, P.; Pierre, G. Novel Antioxidant, Anti-α-Amylase, Anti-Inflammatory and Antinociceptive Water-Soluble Polysaccharides from the Aerial Part of Nitraria retusa. Foods 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anjaneyalu, Y.V.; Tharanathan, R.N. Composition and preliminary fractionation of the seed mucilage of Ocimum canum. Austr. J. Chem. 1971, 24, 1501–1507. [Google Scholar] [CrossRef]
- Nadour, M.; Laroche, C.; Pierre, G.; Delattre, C.; Moulti-Mati, F.; Michaud, P. Structural characterization and biological activities of polysacharides from olive mill wastewater. Appl. Biochem. Biotechnol. 2015, 177, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Lefsih, K.; Delattre, C.; Pierre, G.; Michaud, P.; Aminabhavi, T.M.; Dahmoune, F.; Madani, K. Extraction, characterization and gelling behavior enhancement of pectins from the cladodes of Opuntia ficus indica. Int. J. Biol. Macromol. 2016, 82, 645–652. [Google Scholar] [CrossRef]
- Lawson-Evi, P.; Eklu-Gadegbeku, K.; Agbonon, A.; Aklikokou, K.; Creppy, E.E.; Gbeassor, M. Antihyperglycemic activity of Phyllanthus amarus (Schum & Thonn) in rats. J. Rech. Sci. Univ. Lomé 2011, 13, 131–138. [Google Scholar]
- Zou, Y.-F.; Zhang, B.-Z.; Inngjerdingen, K.T.; Barsett, H.; Diallo, D.; Miachelsen, T.E.; El-Soubair, E.; Paulsen, B.S. Polysaccharides with immunomodulating properties from the bark of Parkia biglobosa. Carbohydr. Polym. 2014, 101, 457–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukova, P.; Nikolova, M.; Petit, E.; Elboutachfaiti, R.; Vasileva, T.; Katsarov, P.; Manev, H.; Gardarin, C.; Pierre, G.; Michaud, P.; et al. Prebiotic activity of poly- and oligosaccharides obtained from Plantago major L. leaves. Appl. Sci. 2020, 10, 2648. [Google Scholar] [CrossRef] [Green Version]
- Akindele, A.J.; Wani, Z.A.; Sharma, S.; Mahajan, G.; Satti, N.K.; Adeyemi, O.O.; Mondhe, D.M.; Saxena, A.K. In Vitro and In Vivo Anticancer Activity of Root Extracts of Sansevieria liberica Gerome and Labroy (Agavaceae). Evid. Based Compl. Altern. Med. 2015, 2015, 560404. [Google Scholar] [CrossRef] [Green Version]
- Oladeji, O.S.; Adelowo, F.E.; Oluyori, A.P.; Bankole, D.T. Ethnobotanical Description and Biological Activities of Senna alata. Evid. Based Compl. Alt. Med. 2020, 2020, 2580259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchenna, E.O.; Ezugwu, C.O.; Ugwuoke, C.E.C.; Ezea, S.C.; Onu, S. Evaluation of the anti-diabetic and antihyperlipidemic effect of methanol extract of Strophanthus hispidus roots D.C. (apocynaceae). Planta Med. 2015, 81, PX59. [Google Scholar] [CrossRef]
- Shao, H.; Zhang, H.; Tian, Y.; Song, Z.; Lai, P.F.H.; Ai, L. Composition and Rheological Properties of Polysaccharide Extracted from Tamarind (Tamarindus indica L.) Seed. Molecules 2019, 24, 1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, P.; Mukherjee, S.; Bera, K.; Ghosh, K.; Ali, I.; Khawas, S.; Ray, B.; Ray, S. Polysaccharides from Thymus vulgaris leaf: Structural features, antioxidant activity and interaction with bovine serum albumin. Int. J. Biol. Macromol. 2019, 125, 580–587. [Google Scholar] [CrossRef]
- Yin, X.; Chávez León, M.A.S.C.; Osae, R.; Linus, L.O.; Qi, L.-W.; Alolga, R.N. Xylopia aethiopica Seeds from Two Countries in West Africa Exhibit Differences in Their Proteomes, Mineral Content and Bioactive Phytochemical Composition. Molecules 2019, 24, 1979. [Google Scholar] [CrossRef] [Green Version]
- Clifford, S.C.; Arndt, S.M.; Popp, M.; Jones, H.G. Mucilages and polysaccharides in Ziziphus species (Rhamnaceae): Localization, composition and physiological roles during drought-stress. J. Exp. Bot. 2002, 53, 131–138. [Google Scholar] [CrossRef]
- Tigrine-Kordjani, N.; Meklati, B.Y.; Chemat, F. Analysis by gas chromatography–mass spectrometry of the essential oil of Zygophyllum album L., an aromatic and medicinal plant growing in Algeria. Int. J. Aromather. 2006, 16, 187–191. [Google Scholar] [CrossRef]
- Lslam, A.M.; Phillips, G.O.; Sljivo, A.; Snowden, M.J.; Williams, P.A. A review of recent developments on the regulatory, structural and functional aspects of gum Arabic. Food Hydrocoll. 1997, 11, 493–505. [Google Scholar]
- De Pinto, G.L.; Martinez, M.; Sanabria, L. Structural features of the polysaccharide gum from Acacia glomerosa. Food Hydrocoll. 2001, 15, 461–467. [Google Scholar] [CrossRef]
- Martinez, M.C.; De Pinto, L.G.; Rivas, C.; Ocando, E. Chemical and spectroscopic studies of the gum polysaccharide from Acacia macracantha. Carbohydr. Polym. 1996, 29, 241–252. [Google Scholar] [CrossRef]
- Verbeken, D.; Dierckx, S.; Dewettinck, K. Exudate gums: Occurrence, production, and applications. Appl. Microbiol. Biotechnol. 2003, 63, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.W.; Dea, I.C.M.; Hirst, S.E. Studies on uronic acid materials part XXXII*. Some structural features of the gum exudate from Acacia seyal DEL. Carbohydr. Res. 1968, 8, 460–476. [Google Scholar] [CrossRef]
- Kumar Lakhera, A.; Kumar, V. Monosaccharide composition of acidic gum exudates from Indian Acacia tortilis ssp. raddiana (Savi) Brenan. Int. J. Biol. Macromol. 2017, 94, 45–50. [Google Scholar] [CrossRef]
- Beltrán, O.; Leon de Pinto, G.; Martínez, M.; Rincón, F. Comparación de los datos analíticos de las gomas de Acacia macracantha, Acacia tortuosa y otras Gummiferae. Afinidad 2005, 62, 237–241. [Google Scholar]
- Martinez, M.C.; De Pinto, L.G.; Rivas, C. Composition of Acacia macracantha Gum exudates. Phytochemistry 1992, 31, 535–536. [Google Scholar] [CrossRef]
- Beltrán, O.; De Pinto, G.; Rincon, F.; Picton, L.; Cozic, C.; Le Cerf, D.; Muller, G. Acacia macracantha gum as a possible source of arabinogalactan-protein. Carbohydr. Polym. 2008, 72, 88–94. [Google Scholar] [CrossRef]
- Sanchez, C.; Nigen, M.; Mejia Tamayo, V.; Doco, T.; Williams, P.; Amine, C.; Renard, D. Acacia gum: History of the Future. Food Hydrocoll. 2018, 78, 140–160. [Google Scholar] [CrossRef]
- Binwei, B.; Yang, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Characterization and emulsifying properties of b-lactoglobulin-gum Acacia seyal conjugates prepared via the Maillard reaction. Food Chem. 2017, 214, 614–621. [Google Scholar]
- Nie, S.P.; Wang, C.; Cui, S.W.; Wang, Q.; Xie, M.Y.; Phillips, G.O. A further amendment to the classical core structure of gum arabic (Acacia senegal). Food Hydrocoll. 2013, 31, 42–48. [Google Scholar] [CrossRef]
- Siddig, N.; Osman, M.E.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on acacia exudate gums, part IV. Distribution of molecular components in Acacia seyal in relation to Acacia senegal. Food Hydrocoll. 2005, 19, 679–686. [Google Scholar] [CrossRef]
- Anderson, D.M.W.; Weiping, W. The characterization of gum arabic (Acacia senegal) samples from Uganda. Food Hydrocoll. 1991, 5, 297–306. [Google Scholar] [CrossRef]
- Street, C.A.; Anderson, D.M.W. Refinement of structures previously proposed for gum arabic and other acacia gum exudates. Talanta 1983, 30, 887–893. [Google Scholar] [CrossRef]
- Biswas, B.; Biswas, S.; Phillips, G.O. The relationship of specific optical rotation to structural composition for Acacia and related gums. Food Hydrocoll. 2000, 14, 601–608. [Google Scholar] [CrossRef]
- Gashua, I.B.; Williams, P.A.; Baldwin, T.C. Molecular characteristics, association and interfacial properties of gum Arabic harvested from both Acacia senegal and Acacia seyal. Food Hydrocoll. 2016, 61, 514–522. [Google Scholar] [CrossRef]
- Hassan, E.A.; Al-Assaf, S.; Phillips, G.O.; Williams, P.A. Studies on Acacia gums: Part III molecular weight characteristics of Acacia seyal var. seyal and Acacia seyal var fistula. Food Hydrocoll. 2005, 19, 669–677. [Google Scholar] [CrossRef]
- Lopez-Torrez, L.; Nigen, M.; Williams, P.; Doco, T.; Sanchez, C. Acacia senegal vs. Acacia seyal gums—Part 1: Composition and structure of hyper branched plant exudates. Food Hydrocoll. 2015, 51, 41–53. [Google Scholar] [CrossRef]
- Embaby, H.E.; Rayan, A.M. Chemical composition and nutritional evaluation of the seeds of Acacia tortilis (Forssk.) Hayne ssp. raddiana. Food Chem. 2016, 200, 62–68. [Google Scholar] [CrossRef]
- Sebaa, H.S.; Kaid Harche, M. Anatomical structure and ultrastructure of the endocarp cell walls of Argania spinosa (L.) Skeels (Sapotaceae). Micron 2014, 67, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Zunzunegui, M.; Boutaleb, S.; Díaz Barradas, M.C.; Esquivias, M.P.; Valera, J.; Jáuregui, J.; Tagma, T.; Ain-Lhout, F. Reliance on deep soil water in the tree species Argania spinosa. Tree Physiol. 2018, 38, 678–689. [Google Scholar] [CrossRef]
- Aboughe-Angone, S.; Nguema-Ona, E.; Ghosh, P.; Lerouge, P.; Ishii, T.; Ray, B.; Driouich, A. Cell wall carbohydrates from fruit pulp of Argania spinosa: Structural analysis of pectin and xyloglucan polysaccharides. Carbohydr. Res. 2008, 343, 67–72. [Google Scholar] [CrossRef]
- Ray, B.; Loutelier-Bourhis, C.; Lange, L.; Condamine, E.; Driouich, A.; Lerouge, P. Structural investigation of hemicellulosic polysaccharides from Argania spinosa: Characterisation of a novel xyloglucan motif. Carbohydr. Res. 2004, 339, 201–208. [Google Scholar] [CrossRef]
- Hachem, K.; Faugeron, C.; Kaid-Harche, M.; Gloaguen, V. Structural investigation of cell wall xylan polysaccharides from the leaves of Algerian Argania spinosa. J. Mol. 2016, 21, 1587. [Google Scholar] [CrossRef] [Green Version]
- Habibi, Y.; Vignon, M.R. Isolation and characterization of xylans from seed pericarp of Argania spinosa fruit. Carbohydr. Res. 2005, 340, 1431–1436. [Google Scholar] [CrossRef]
- Hachem, H.; Benabdesslem, Y.; Ghomari, S.; Hasnaoui, O.; Kaid-Harche, M. Partial structural characterization of pectin cell wall from Argania spinosa leaves. Heliyon 2016, 2, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in bioremediation of wastewaters. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Cheikh Rouhou, M.; Abdelmoumen, S.; Thomas, S.; Attia, H.; Ghorbel, D. Use of green chemistry methods in the extraction of dietary fibers from cactus rackets (Opuntia ficus indica): Structural and microstructural studies. Int. J. Biol. Macromol. 2018, 116, 901–910. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. d-Xylans from seed endosperm of Opuntia ficus-indica prickly pear fruits. C. R. Chimie 2005, 8, 1123–1128. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Isolation and structure of d-xylans from pericarp seeds of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2002, 337, 1593–1598. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Maraisa, M.F.; Vignon, M.R. An arabinogalactan from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Res. 2004, 339, 1201–1205. [Google Scholar] [CrossRef]
- Habibi, Y.; Mahrouz, M.; Vignon, M.R. Isolation and structural characterization of protopectin from the skin of Opuntia ficus-indica prickly pear fruits. Carbohydr. Polym. 2005, 60, 205–213. [Google Scholar] [CrossRef]
- Matsuhiro, B.; Lillo, L.E.; Saenz, C.; Urzua, C.C.; Zarate, O. Chemical characterization of the mucilage from fruits of Opuntia ficus indica. Carbohydr. Polym. 2006, 63, 263–267. [Google Scholar] [CrossRef]
- Ishurd, O.; Zgheel, F.; Elghazoun, M.; Elmabruk, M.; Kermagi, A.; Kennedy, J.F.; Knill, C.J. A novel (1→4)-α-d-glucan isolated from the fruits of Opuntia ficus indica (L.) Miller. Carbohydr. Polym. 2010, 82, 848–853. [Google Scholar] [CrossRef]
- Fons, F.; Gargadennec, A.; Rapior, S. Culture of Plantago species as bioactive components resources: A 20-year review and recent applications. Acta Bot. Gall. 2008, 155, 277–300. [Google Scholar] [CrossRef]
- Gazer, M.H.; Shalabi, L.F. The role of pollen morphology in the identification and classification of Plantago (Plantaginaceae). Egypt J. Exp. Biol. 2016, 12, 125–132. [Google Scholar] [CrossRef]
- Thakur, V.K.; Thakur, M.K. Recent Trends in Hydrogels based on Psyllium Polysaccharide: A Review. J. Clean Prod. 2014, 81, 1–15. [Google Scholar] [CrossRef]
- Boual, Z.; Chouana, T.; Kemassi, A.; Hamid Oudjana, A.; Daddi Bouhoun, M.; Michaud, P.; Ould El Hadj, M.D. Chemical composition and bioactivity of water-soluble polysaccharides from leaves of Plantago notata Lagasca (Plantaginaceae). Phytothérapie 2015, 13, 396–402. [Google Scholar] [CrossRef]
- Mishra, B.K.; Singh, B.; Dubey, P.N.; Joshi, A.; Kant, K.; Maloo, S.R. Biochemical characteristics and microbial association of Isabgol (Plantago ovata Forks.) growing soils in Western Arid region of India. Afr. J. Microbiol. Res. 2015, 9, 695–700. [Google Scholar]
- Sharma, P.K.; Koul, A.K. Mucilage in seeds of Plantago ovata and its wild allies. J. Ethnopharmacol. 1986, 17, 289–295. [Google Scholar] [CrossRef]
- Pawar, H.; Varkhade, C. Isolation, characterization and investigation of Plantago ovata husk polysaccharide as superdisintegrant. Int. J. Biol. Macromol. 2014, 69, 52–58. [Google Scholar] [CrossRef]
- Labed, A.; Ferhat, M.; Labed-Zouad, I.; Kaplaner, E.; Zerizer, S.; Voutquenne-Nazabadioko, L.; Alabdul Magid, A.; Semra, Z.; Kabouche, A.; Kabouche, Z.; et al. Compounds from the pods of Astragalus armatus with antioxidant, anticholinesterase, antibacterial and phagocytic activities. Pharm. Biol. 2016, 54, 3026–3032. [Google Scholar] [CrossRef] [Green Version]
- Medjekal, S.; Ghadbane, M.; Bodas, R.; Bousseboua, H.; López, S. Volatile fatty acids and methane production from browse species of Algerian arid and semi-arid Areas. J. Appl. Anim. Res. 2018, 46, 44–49. [Google Scholar] [CrossRef]
- Mahdhi, M.; Houidheg, N.; Mahmoudi, N.; Msaadek, A.; Rejili, M.; Mars, M. Characterization of Rhizobial Bacteria Nodulating Astragalus corrugatus and Hippocrepis areolata in Tunisian Arid Soils. Pol. J. Microbiol. 2016, 65, 331–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Snafi, A. Chemical constituents and pharmacological effects of Astragalus hamosus and Astragalus tribuloides grown in Iraq. Asi. J. Pharm. Sci. Technol. 2015, 5, 321–328. [Google Scholar]
- Gros-Balthazard, M. Hybridization in the genus Phoenix: A review. Emir. J. Food Agric. 2013, 25, 831–842. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.A.; Hegazi, S.M. Chemical Composition of the Egyptian Dry Dates. J. Sci. Fd. Agric. 1971, 22, 632–633. [Google Scholar] [CrossRef]
- Ishrud, O.; Zahid, M.; Zhou, H.; Pan, Y. A water-soluble galactomannan from the seeds of Phoenix dactylifera L. Carbohydr. Res. 2001, 335, 297–301. [Google Scholar] [CrossRef]
- Ishurd, O.; Ali, Y.; Wei, W.; Bashir, F.; Ali, A.; Ashour, A.; Pan, Y. An alkali-soluble heteroxylan from seeds of Phoenix dactylifera L. Carbohydr. Res. 2003, 338, 1609–1612. [Google Scholar] [CrossRef]
- Bendahou, A.; Dufresne, A.; Kaddami, H.; Habibi, Y. Isolation and structural characterization of hemicelluloses from palm of Phoenix dactylifera L. Carbohydr. Polym. 2007, 68, 601–608. [Google Scholar] [CrossRef]
- Mahdhi, M.; Nzoue, A.; De Lajudie, P.; Mars, M. Characterization of root-modulating bacteria on Retama raetam in arid Tunisian soils. Progr. Nat. Sci. 2008, 18, 43–49. [Google Scholar] [CrossRef]
- Ishurd, O.; Kermagi, A.; Zgheel, F.; Flefla, M.; Elmabruk, M.; Yalin, W.; Kennedy, J.F.; Yuanjiang, P. Structural aspects of water-soluble galactomannans isolated from the seeds of Retama raetam. Carbohydr. Polym. 2004, 58, 41–44. [Google Scholar] [CrossRef]
- Wu, Y.; Pan, Y.; Sun, C.; Hu, N.; Ishurd, O. Structural Analysis of an Alkali-Extractable Polysaccharide from the Seeds of Retama raetam ssp. gussonei. J. Nat. Prod. 2006, 69, 1109–1112. [Google Scholar] [CrossRef]
- Elaloui, M.; Laamouri, A.; Fabre, J.; Mathieu, C.; Vilarem, G.; Hasnaoui, B. Distribution of free amino acids, polyphenols and sugars of Ziziphus jujuba pulps harvested from plants grown in Tunisia. Nat. Prod. Res. 2015, 29, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdeddaim, M.; Lombarkia, O.; Bacha, A.; Fahloul, D.; Abdeddaim, D.; Farhat, R.; Saadoudi, M.; Noui, Y.; Lekbir, A. Biochemical characterization and nutritional properties of Zizyphus lotus L. fruits in aures region, northeastern of Algeria. Food Sci. Technol. 2014, 15, 75–81. [Google Scholar]
- Chouaibi, M.; Rezig, L.; Ben Daoued, K.; Mahfoudhi, N.; Bouhafa, H.; Hamdi, S. Extraction of polysaccharide from Zizyphus lotus fruits. Int. J. Food Eng. 2012, 8, 1–24. [Google Scholar] [CrossRef]
- Mkadmini Hammi, K.; Hammami, M.; Rihouey, C.; Le Cerf, D.; Ksouri, R.; Majdoub, H. Optimization extraction of polysaccharide from Tunisian Zizyphus lotus fruit by response surface methodology: Composition and antioxidant activity. Food Chem. 2016, 212, 476–484. [Google Scholar] [CrossRef] [PubMed]
- Boual, Z.; Kemassi, A.; Chouana, T.; Michaud, P.; Ould El Hadj, M.D. Chemical Characterization and Prebiotic Effect of Water-Soluble Polysaccharides from Zizyphus lotus Leaves. Int. J. Biol. Biomol. Agric. Food Biotechnol. Engin. 2015, 9, 1148–1158. [Google Scholar]
- Chen, L.; Ge, M.; Zhu, Y.; Song, Y.; Cheung, P.C.K.; Zhang, B.; Liu, L. Structure, bioactivity and applications of natural hyperbranched polysaccharides. Carbohydr. Polym. 2019, 223, 115076. [Google Scholar] [CrossRef]
- Into The Minds. Available online: https://intotheminds.com (accessed on 7 July 2020).
- AP News. Available online: https://apnews.com/press-release/pr-businesswire/89c039188e1f48a9990a99ac2a59e260 (accessed on 14 April 2021).
- Angone, S.A.; Nguema-Ona, E.; Driouich, A. La thérapie par les plantes en Afrique: Activités immunostimulantes des polysaccharides de la paroi végétale. Phytothérapie 2010, 8, 223–230. [Google Scholar] [CrossRef]
- Song, E.-C.; Shang, J.; Ratner, D.M. Polysaccharides. In Polymer Science: A Comprehensive Reference, 1st ed.; Matyjaszewski, K., Möller, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2012; Volume 9, pp. 137–155. [Google Scholar]
- Li, Y.; Xu, F.; Zheng, M.; Xi, X.; Cui, X.; Han, C. Maca polysaccharides: A review of compositions, isolation, therapeutics and prospects. Int. J. Biol. Macromol. 2018, 111, 894–902. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Othman, S.I.; Allam, A.A.; Morsy, O.M. Synthesis, drying process and medical application of polysaccharide-based aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Mordor Intelligence. Available online: https://www.mordorintelligence.com/ (accessed on 26 June 2020).
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216–224. [Google Scholar] [CrossRef] [PubMed]
- RES, Réseau Environnement Santé. Available online: http://www.reseau-environnement-sante.fr/ (accessed on 22 June 2020).
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Mohan, K.; Ravichandran, S.; Muralisankar, T.; Uthayakumar, V.; Chandirasekar, R.; Seedevi, P.; Abirami, R.G.; Rajan, D.K. Application of marine-derived polysaccharides as immunostimulants in aquaculture: A review of current knowledge and further perspectives. Fish Shellfish. Immunol. 2019, 86, 1177–1193. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui-Essoussi, L.; Zahaf, M. The Organic Food Market: Opportunities and Challenges. In Organic Food and Agriculture: New Trends and Developments in the Social Sciences, 1st ed.; Reed, M., Ed.; IntechOpen: London, UK, 2012; pp. 1–28. [Google Scholar] [CrossRef]
- GlobeNewswire. Environmental Remediation Market Size to Reach USD 122.80 Billion by 2022—Zion Market Research. Available online: https://www.globenewswire.com/en/news-release/2018/01/17/1295780/0/en/Environmental-Remediation-Market-Size-to-Reach-USD-122-80-Billion-by-2022-Zion-Market-Research.html (accessed on 14 April 2021).
- Musarurwa, H.; Tavengwa, N.T. Application of carboxymethyl polysaccharides as bio-sorbents for the sequestration of heavy metals in aquatic environments. Carbohydr. Polym. 2020, 237, 116142. [Google Scholar] [CrossRef]
- Dun&Bradstreet. Remediation & Environmental Cleanup Services Industry, Insights from D&B Hoovers. Available online: https://www.dnb.com/business-directory/industry-analysis.remediation-environmental-cleanup-services.html (accessed on 14 April 2021).
- Statista. Market value of Biofuels Worldwide in 2019 and 2024. Available online: https://www.statista.com/statistics/217179/global-biofuels-market-size/ (accessed on 14 April 2021).
- Prado, H.J.; Matulewicz, M.C. Cationization of polysaccharides: A path to greener derivatives with many industrial applications. Eur. Polym. J. 2014, 52, 53–75. [Google Scholar] [CrossRef]
- GlobeNewswire. Global Biofuels Market Report 2020: Provides a Look at the Regulatory Framework Regarding the Use of Biofuels, Incentives for Fuel Production, and the Number & Capacity of Manufacturing Plants. Available online: https://www.globenewswire.com/news-release/2020/04/02/2010529/0/en/Global-Biofuels-Market-Report-2020-Provides-a-Look-at-the-Regulatory-Framework-Regarding-the-Use-of-Biofuels-Incentives-for-Fuel-Production-and-the-Number-Capacity-of-Manufacturing.html (accessed on 14 April 2021).


| Name | Region | Type | Part(s) | Properties and Uses | Active Molecules | Ref. |
|---|---|---|---|---|---|---|
| Aloe vera Barbadensis Miller | Northern Algeria | Plant | Leaves | Anti-inflammatory, antioxidant | Pectin-like structure | [30] |
| Angelica acutiloba | Sahara | Perennial herb | Roots | Anti-complement activity | Arabinogalactan | [83] |
| Annona senegalensis Pers. | Western Mali | Plant | Bark, roots | Anti-complement, antiparasitic, insecticide, antiulcer, antispasmodic, wound healing | Glucan, pectin-like structure | [84] |
| Astragalus armatus | Septentrional Algerian Sahara | Perennial plant | Seeds | Anti-complement activity, antioxidant | Galactomannan | [63] |
| Astragalus gombo | Septentrional Algerian Sahara | Perennial plant | Seeds | Antioxidant, prebiotic, texturing agent | Galactomannan | [24] |
| Bauhinia thonningii Schumach. | Western Mali | Savanna tree | Leaves | Anti-complement, antitussive, hemostatic activity, wound healing | Arabinan | [84] |
| Biophytum petersianum Klotzsch | Western Mali | Flowering plant | Aerial parts | Anti-complement, wound healing | Pectic arabinogalactan | [84] |
| Burkea Africana Hook. | Western Mali | Savanna tree | Bark | Anti-complement, immunomodulator, hemostatic activities, wound healing | Arabinan, glucan, pectic-like structure | [84] |
| Carica papaya L. | Western Africa | Flowering plants | Leaves | Buruli ulcer, liver damage, dysentery, diabetes, constipation, and chronic indigestion | Extracts | [85] |
| Cassia sieberiana | Western Africa | Leguminous plant | Bark, roots, stem | Diabetes, malaria | - | [86] |
| Catharanthus roseus | Madagascar | Flowering plant | Areal parts | Anti-leukemic agents | Glycosides | [87] |
| Ceratonia siliqua | Middle East | Tree | Seeds | Diarrhea, eye infection, visual disturbances, intestinal parasite infestation | Glycosides | [88] |
| Cereus triangularis | Madagascar | Cactus | Cladodes | Anti-inflammatory, anti-complementary, gastro-protectors, immuno-modulators, prebiotic | Arabinogalactan (Type I) (poly- and oligosaccharides) | [22,89] |
| Chamaecrista nigricans (Vahl) Green | Western Mali | Woody plant | Leaves | Anti-complement, antiulcerogenic property, wound healing | Arabinan, pectin-like structure | [84] |
| Citrullus colocynthis | Sahara | Desert viny plant | Fruits | Diabetes, asthma, gastrointestinal disorders, different microbial infections | Glycosides, oils | [90] |
| Cochlospermum tinctorium | Western Africa | Flowering plants | Bark, roots | Anti-complement, anti-Malaria, anti-viral, hepatoprotective | (Rhamno)galactan, glucan | [84] |
| Codonopsis pilosula | Sahara | Flowering plant | Roots | Anti-complement activity | RG-I containing AG-I and AG-II sidechains | [91] |
| Cola cordifolia (Cav.) R. Br. | Western Africa | Tree | Bark, leaves, stems | Abdominal pain, anti-complement, fever, anti-ulcer, headache, wound healing | Pectic arabinogalactan, glucan | [84] |
| Commiphora myrrha Engl. | Septentrional Algerian Sahara | Small tree or large shrub | Gum-resin | Antihyperglycemic and phagocytic activities | Arabinogalactan-like structure | [92] |
| Crossopteryx febrifuga (Afzel. ex G. Don) Benth. | Western Mali | Tree | Bark, fruits | Anti-complement, antimicrobial property, respiratory disorder, wound healing | Glucan, pectic-like structure | [84] |
| Cymbopogon citratus | Madagascar | Tropical plant | Leaves | Fever | Extracts | [93] |
| Cyperus esculentus | Cameroon | Edible plant | Tubers | Prebiotic, texturing agent | Starch | [94] |
| Entada africana | Tropical and subtropical Africa | Tree | Bark, leaves, roots | Hepatic diseases | Pectin-like structure, RG-I, AG-II | [95] |
| Fenugreek | Northern Africa | Leguminous plant | Leaves, seeds | Promoting digestion and reducing blood sugar levels in diabetics | Galactomannan, glycosides | [96] |
| Gomphrena celosioides | Western Africa | Herbaceous perennial | Areal parts and roots | Viral hepatitis A and C, liver damage, urinary tract, kidney stones | Extracts | [97] |
| Harrisonia abyssinica | Tropical Africa | Shrub | Bark, roots | Infectious diseases | Extracts | [98] |
| Lannea velutina A. Rich. | Western Africa | Tree | Bark (stem) | Anticomplement, anti-inflammatory effect, wound healing | Arabinogalactan | [94] |
| Morinda lucida | Central Africa | Flowering plant | Leaves, roots | anti-allergic, anti-carcinogenic, anti- inflammatory, antioxidant, anti-proliferative, anti-viral | Crude extract including polysaccharides | [99] |
| Nitraria retusa | Northern Africa | Shrub plant | Aerial parts | Antioxidant, anti-α-amylase, anti-inflammatory, antinociceptive activities, anti-edematous effects | Pectin-like structure | [100] |
| Northern Africa | Shrub plant | Fruits | Antioxidant, hypolipidemic activity | β-(1→3)-glucan, traces of pectin | [25] | |
| Ocimum canum | Sahara | Perennial herbs | Mucilage, roots, seeds | Antiparasitic, antioxidant, antiulcer | Acidic “bacterial-like” polysaccharide, acidic xylan, (galacto-) glucomannan | [101] |
| Olive tree | Northern Africa | Tree | Wastewater | Antioxidant, biobased polymer films, prebiotic | Glucan, xyloglucans, pectin fractions | [102] |
| Opilia celtidifolia Endl. Ex Walp. | Western Africa | Tree | Leaves | Complement fixing, immunomodulator, macrophage stimulator, regulating inflammatory | Type II arabinogalactan, Rhamnogalacturonan I regions | [3] |
| Opuntia ficus indica | Northern Africa | Cactus | Cladodes | Antioxidant, bioassay applications, texturing agent | Pectin fractions | [103] |
| Phyllanthus amarus | Western Africa | Flowering plant | Roots | Anti-hyperglycemic, antiviral, anti-ulcer | Crude extract including polysaccharides | [104] |
| Parkia biglobosa | Western Africa | Perennial tree | Bark, seeds | Antiviral, complement fixation, immunomodulator, | Type II arabinogalactan | [105] |
| Podaxon aegyptiacus Mont. | Western Mali | Mushroom | Spores | Anticomplement, burn/wound healing | (Galacto)mannan | [84] |
| Plantago ciliata Desf. | Septentrional Algerian Sahara | Spontaneous flowering plant | Seeds | Anti-inflammatory, medicinal cream, prebiotic, wound healing | Arabinoxylan | [23] |
| Plantago major | Sahara | Flowering plant | Leaves | Anti-complement activity, prebiotic | Pectin-like structure (poly- and oligosaccharides) | [106] |
| Plantago notata | Septentrional Algerian Sahara | Semi-annual flowering plant | Seeds | Antioxidant, prebiotic | Heteroxylan | [28] |
| Pterocarpus erinaceus Poir. | Western Mali | Tree | Bark | Anticomplement, wound healing | Glucan, pectin-like structure | [84] |
| Sansevieria liberica | Western Africa | Flowering plants | Leaves, roots | Anti-inflammatory, antioxidant | Crude extract including glycosides | [107] |
| Senna Alata L. (Cassia alata) | Western Africa | Flowering plant/herb | Bark, flowers, leaves, roots, seeds | antibacterial, antidiabetic, antifungal, anti-inflammatory, antihelmintic, antimicrobial, antioxidant, antitumor, wound healing activities | Crude extract including reducing sugars | [108] |
| Stereospermum kunthianum Cham. | Western Mali | Tree | Bark, leaves | Anticomplement, burn/wound healing | (Rhamno)glucan, pectic-like structure | [84] |
| Strophanthus hispidus | Africa | Liana | Roots, seeds | Antidiabetic, antihyperlipidemic, cardiac insufficiency | Crude extract including polysaccharides | [109] |
| Tamarindus indica | Eastern Africa | Leguminous tree | Fruits, seeds | Antidiabetic, anti-inflammatory, anti-hepatotoxic, Antioxidant, antimutagen, antimitotic, blood tonic, digestive, carminative, expectorant, immunomodulator, laxative, texturing agent | Heteropolysaccharide (Gal, Man, Glc), xyloglucan | [110] |
| Trichilia emetica Vahl. | Western Mali | Tree | Leaves | Anticomplement, anti-inflammatory, immunomodulatory, phagocytic activities, wound healing | Arabinogalactan, traces of pectin | [84] |
| Thymus vulgaris | Sahara | Flowering plant | Leaves | Anti-complement, antioxidant, complement activator | Type II arabinogalactan, type I rhamnogalacturonan | [111] |
| Vernonia kotschyana (Baccharoides adoensis var. kotschyana) | Sahara | Annual plant | Roots | Anti-ulcer properties arthritis, complement fixing activity, immunomodulator, | Glucan, inulin, pectic arabinogalactan, type II arabinogalactan | [67] |
| Xeroderris stuhlmannii (Taub.) Mendoça and E.C. Sousa | Tropical Africa | Tree | Leaves | Anticomplement, wound healing | Pectic arabinogalactan | [84] |
| Ximenia americana L. | Western Africa | Tree | Bark, roots, leaves | Anticomplement, carcinostatic, antibacterial activity, wound healing | Arabinogalactan | [84] |
| Xylopia aethiopica | Western Africa | Aromatic tree | Bark, fruits, seeds | Antioxidant, Buruli ulcer, excipient, post-partum care | Mainly phenols and flavonoids | [112] |
| Ziziphus mauritiana | Eastern and Western Africa | Tree | Bark, leaves, mucilage, roots | Anti-diabetic, epithelium wounds and mucous membrane irritation, skin treatment, | Galactan, glucan, rhamnan, pectic-like structure | [113] |
| Zygophyllum album | Mediterranean Africa | Halophytic plant | Areal parts | Asthma, diabetes, diuretic agent, dermatosis, indigestion, local anesthetic, rheumatism | Essential oil | [114] |
| Acacia Species | Main Monosaccharides (% Molar Ratio) | Reference | |||
|---|---|---|---|---|---|
| Gal | Ara | Rha | GlcA | ||
| A. glomerosa | 46 | 27 | 15 | - | [116] |
| A. macracantha | 43 | 30 | 5 | 22 | [117] |
| A. senegal | 39–42 | 24–27 | 12–16 | 15–16 | [118] |
| A. seyal | 38 | 45 | 4 | 7 | [119] |
| A. tortilis var. raddiana | 19 | 78 | 2 | 4.4 | [120] |
| Characteristics | General Information | A. senegal Gum | A. seyal Gum |
|---|---|---|---|
| Specific rotations | Differences were described is due to the variation of monosaccharide. A. seyal gum contained more Ara than Rha residues [127] | Negative specific rotations: | Positive specific rotations: |
| −26 to −34° [128] | +60° [129] | ||
| −30° [127] | +54° [127] | ||
| Rheological behavior | Gums are more described for their emulsifying properties | Viscous [130] | Low viscosity [130] |
| Molecular weight | Molecular weights are often higher than 1M Da | 0.485 × 106 g.mol−1 [131] | 1.14 × 106 g.mol−1 for A. seyal [131] |
| 2.1 × 106 for A. seyal var. fistula [132] | |||
| 1.7 × 106 for A.seyal var. seyal [132] | |||
| Monosaccharide composition | Both are rich in d-Gal and l-Ara in addition to some minor carbohydrates, including l-Rha, d-GlcA and 4-O-Me-GlcA [18] | Ara/Gal ratio ˂ 1 [126,130] | Ara/Gal > 1 [126,130] |
| Higher proportion of rhamnose [130] | Low rhamnose content [130] | ||
| Structural features | Main chain structures of β-(1,3)-d-Gal with numerous branching points in O-6 positions of d-Gal residues. Lateral chains have units of α-l-Araf, α-l-Rhap, β-d Glcp and 4-O-Me-β-d Glcp, the last two mainly as end-units [8,17,21,26]. | Hyperbranched structure with degree of branching up to 78% with more branched Galp, shorter Araf ramifications, and more Rhap in terminal positions [125,133]. | Less degree of branching (around 59%) [125,133] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarraf, A.; Verton, E.; Addoun, N.; Boual, Z.; Ould El Hadj, M.D.; El Alaoui-Talibi, Z.; El Modafar, C.; Abdelkafi, S.; Fendri, I.; Delattre, C.; et al. Polysaccharides and Derivatives from Africa to Address and Advance Sustainable Development and Economic Growth in the Next Decade. Appl. Sci. 2021, 11, 5243. https://doi.org/10.3390/app11115243
Sarraf A, Verton E, Addoun N, Boual Z, Ould El Hadj MD, El Alaoui-Talibi Z, El Modafar C, Abdelkafi S, Fendri I, Delattre C, et al. Polysaccharides and Derivatives from Africa to Address and Advance Sustainable Development and Economic Growth in the Next Decade. Applied Sciences. 2021; 11(11):5243. https://doi.org/10.3390/app11115243
Chicago/Turabian StyleSarraf, Antony, Emeline Verton, Noura Addoun, Zakaria Boual, Mohamed Didi Ould El Hadj, Zainab El Alaoui-Talibi, Cherkaoui El Modafar, Slim Abdelkafi, Imen Fendri, Cédric Delattre, and et al. 2021. "Polysaccharides and Derivatives from Africa to Address and Advance Sustainable Development and Economic Growth in the Next Decade" Applied Sciences 11, no. 11: 5243. https://doi.org/10.3390/app11115243
APA StyleSarraf, A., Verton, E., Addoun, N., Boual, Z., Ould El Hadj, M. D., El Alaoui-Talibi, Z., El Modafar, C., Abdelkafi, S., Fendri, I., Delattre, C., Dubessay, P., Michaud, P., & Pierre, G. (2021). Polysaccharides and Derivatives from Africa to Address and Advance Sustainable Development and Economic Growth in the Next Decade. Applied Sciences, 11(11), 5243. https://doi.org/10.3390/app11115243










