1. Introduction
The hard tissue examination, including the native bone without decalcification, can be useful for special medical purposes (e.g., stem cell preparation, DNA-sampling, anthropological studies) and the implantology in dentistry. In the case of dental implants which must be tightly integrated into the recipient’s hard tissue to provide appropriate supporting/substituting functions, there is a need for rapid osseointegration at the interface to become a steady and stable osteo-metal unification. Therefore, hard tissue studies without decalcification may have great values both in the general medicine (e.g., orthopedic surgery) and in dentistry practice. The use of a specimen exhibiting a dental implant in conjunction with bone border surfaces, without artificial decalcination as an alternative slide preparation technique may have a major role in dental implants’ evaluations. With this method, the grade of osseointegration and the extent of newly formed extracellular hard (calcified) tissues in association with implants can be in situ examined. In the case of the traditional histological slide preparation, the paraffin-embedded samples were prepared decalcification prior cut with a traditional microtome for sectioning and staining. However, metal samples cannot be sliced with this traditional microtome. The solution of the processability in metal content cases was first examined by Gross and Strunz in 1977 [
1], and later the complete technique was established in 1985 by K. Donath [
2] in detail. He was the first who mentioned the hard tissue microtome, which can cut evenly the metal material, including the dental implants. This is the only way to create a correctly evaluable histological slide with metal content substance. Without the decalcification technique, the lime content of the samples is not removed/extracted, so the osseointegration can be examined much better. Accordingly, the embedded samples are cut with hard tissue microtome. These 1 mm thick slices have to be ground until reaching the optimal thickness (less than 10 microns) for a histological slide examination. However, due to mechanical forces, the grinding can remove the sample from the glass, or a part of the sample. Thus, the different adhesive materials have an important role to resist the mechanical forces during the preparation process but without influencing the quality of the specimen for the pathological examination. The dental universal adhesives have been used in clinical practice since 2011. These multicomponent systems contain incorporable monomers to produce chemical and micromechanical bonds to dental substrates. The most often applied incorporable monomers have carboxylate and/or phosphate functional groups that can bind to calcium ions of hydroxylapatite through ionic bonds thus can increase the bonding efficacy [
3]. Some universal adhesives may contain silane [
4]. Silane and a light-cure adhesive combination were presented in an undecalcified hard tissue histotechnology study by Caropreso et al. [
5]. Water cooling is necessary during the grinding process that can bring the main drawback of silane into focus, a tendency to hydrolysis [
6], raising the question in the preservation of section.
The other molecule that is often used in the universal adhesive is 10-methacryloyloxydecyl dihydrogen phosphate (10-MDP; shortly MDP). This is a non-hydrolysis sensitive, polar functional monomer that is found in new dental adhesives and able to bond to apatite, or dentin, collagen, metals, and zirconia. There is much research that investigates the chemical bond of the 10-MDP molecule to titanium, ceramics, and tooth structure. Researchers described that 10-MDP monomer (10-MDP) formed a strong and stable chemical bond with apatitic substrates like human hard tissues (dentin, enamel, and bone) [
7,
8]. The 10-MDP is a bifunctional monomer. One side contains hydrophilic phosphate groups until the other side is an unsaturated carbon double bond that connects to a hydrophobic hydrocarbon chain. The reactive phosphate group reacts with the calcium ions of hydroxyapatite and forms stable calcium phosphate salts [
9]. The other reactive site is a photopolymerizable reactive group that reacts with the monomers of resin-based cement or resin-based composite components during the light curing. Meerbek et al. revealed that the 10-MDP forms a self-assembled monolayer in 4 nm thickness on the surface of synthetic hydroxylapatite. In this layer the phosphate groups directed away from each other, the methacrylate groups directed toward each other [
9]. This molecule is developed by Kuraray co. Ltd. The numbers and intensity of the studies are increased further because the patents of the monomer have expired. The most important challenge is the purity of the 10-MDP in its synthesis [
10].
The components of a thin bone section are bone replacement material and/or dental implants, bone tissue, and any kind of embedding material. These components should be attached strongly to the microscopic slide and adhesion force should withstand the shear force that acts during the grinding of the section. The appropriate adhesion between the surfaces of the microscope slide and section is a determining factor to achieve an effective microscopic analysis of the histological section. The main chemical composition of a microscope glass slide and dental glass-ceramics is silica. More parallels are found in the composition of adherents (undecalcified thin bone, titanium, or bone replacement material) and glass substratum that led to 10-MDP application in histotechnology.
The micromechanical interlocking is commonly used next to the chemical adhesion in the adhesive bonding procedure that is usually achieved by a frosting of the surface. In the case of a well moisturizing bond, the surface irregularities are not going to disturb the microscopy evaluation. Goldman et al. made attempts to improve the quality of the final section for light and scanning electron microscopy examinations by hydrofluoric acid treatment of glass slide with silane and photopolymerizable adhesive treatments. The final thickness of the section was 100 µm ± 5 µm [
11]. In the histotechnology of undecalcified thin bone section, the expected, optimal section thickness is one cell thick (under 10 µm) for high-quality microscopy analysis.
The strong mineral acids, like hydrofluoric acid or nitric acid, are used to etch and clean the glass surface. The result of the acid etching is a rough surface glass. The other consequence of acid etching of glass is corrosion, which means alkali ions release from the crystalline structure of the glass. The places of the alkali ions will be replaced by H
+ ions to form Si-OH bonds resulted in increasing of Si-O bond percentage [
12]. Nitric acid is a stronger acid than hydrofluoric acid based on the pKa values, therefore, stronger glass protonation can be achieved by nitric acid than hydrofluoric acid. On the other hand, hydrofluoric acid can react with Si-O bonds which leads to the formation of hexafluorosilicic acid that decreases the numbers of Si-O bonds that are needed for protonation. Jang et al. investigated the effects of nitric acid chemical etching on different parameters (like chemical composition, surface roughness, and optical transmittance) of the glass [
13], those surface properties are important in adhesive bonding. They found that the optical transmittance of the glass was not affected by chemical etching, the surface roughness increased in the nanometer scale, and acid treatment caused dealkalization on glass similar to hydrofluoric acid.
In our research study, the combination of 10-MDP active molecule and nitric acid etched glass surface was used in the adhesive technique of histotechnology to improve bond strengths between the glass slide and the undecalcified bone section and the microscopic evaluation of the section. The primary aim of this study was to evaluate the protonation effect of the microscopic glass surface between the frosted glass slide and the histological section content (epoxy embedding material, bone, and titanium) using 10-MDP containing bond material with comparing of measured data of shear bond strength (SBS), contact angle, X-ray photoelectron spectra, zeta potential on the protonated and unprotonated glass. The secondary aim was to compare the efficacy of 10-MDP containing bond material to metallography used thermoplastic adhesive with SBS data comparison. The final aim was to improve the microscopic histological examination by safely thinning the undecalcified, titanium-containing thin bone section during the thinning processes of the section.
2. Materials and Methods
2.1. Materials and Sample Preparation
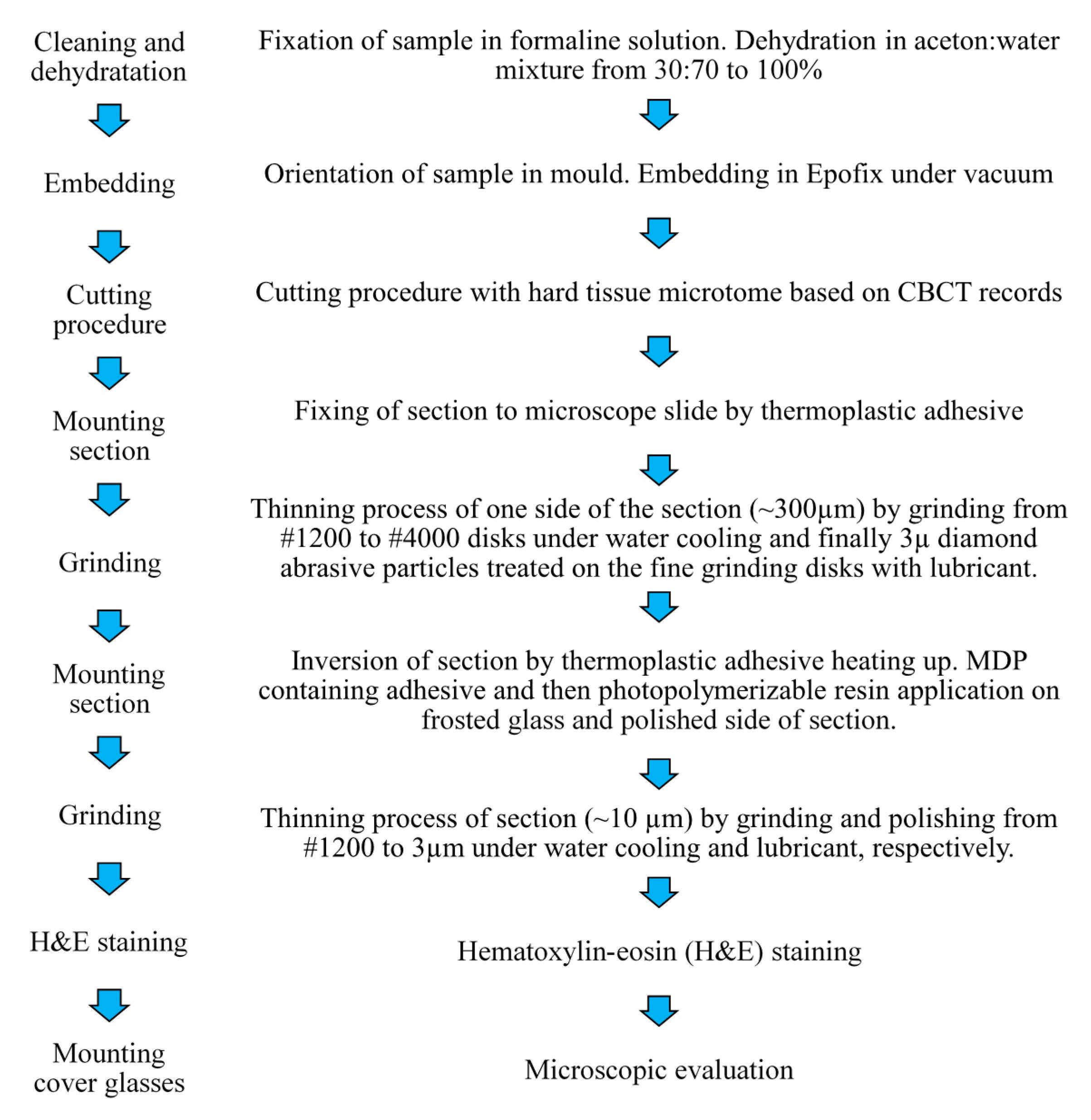
Epoxy is a routinely used material at embedding of the undecalcified bone. In this study, epoxy samples were prepared with the mixing of EpoFix resin and Epofix hardener (EpoFix Kit, Struers, Ballerup, Denmark) and were hardened under vacuum in CitoVac (Struers, Denmark) device. Bone samples were prepared from a beef bone that was embedded into the epofix system under vacuum. Grade 5 titanium samples (d = 5.3 mm, h = 2 mm) were cast under an argon atmosphere (Allergo Dent Bt., Hu). The casted titanium samples were ground and polished on Struers LaboPol 35 (Denmark) grinding machine on 500, 1000, 1200, 2400, and 4000 grit silicon carbide grinding paper under water cooling and then polished by a 3 µm diamond particles containing textile surface. The samples were placed in the ultrasound bath to eliminate the abrasive particles.
Epoxy and bone samples were prepared from solidified epoxy and embedded bone with the help of a trepan drill and diamond-covered saw equipped hard tissue microtome (Leitz 1600, Nussloch, Germany) under water cooling. The drill provides cylinders with 5.3 mm internal diameters and the microtome cut 2 mm slices from these.
In this study, microscope glass slides (Thermo Scientific Menzel Gläser LOT#8501777) were used and cut into 13 × 25 mm pieces. To chemical–micromechanical adhesion, the glass slides were gently sandblasted by 50 μm Al2O3 particles at 7 bar pressure from 1 cm distance for 30 s to achieve a frosted glass slide. After the frosting procedure glass slides were sonicated in water and acetone for 10 min three times in an ultrasound bath then were dried with oil-free air.
2.2. Applied Adhesives and Resin Matrix
Two types of adhesives were used to epoxy, bone and titanium samples adhesion. In metallography routinely used thermoplastic adhesive (Crystalbond 509 Mounting Adhesive, SPI Supplies, USA LOT#1180228) melts around 160 °C and it becomes solid again at room temperature cooling to bond mechanically the samples to a microscope glass slide.
The other adhesive was an MDP containing bond material in which the chemically active molecule is 10-MDP (Biosynth AG, Switzerland LOT#0000018236). The concertation of 10-MDP was 5 wt% in absolute ethanol. The final resin matrix concertation was 5 wt%. The resin matrix consists of bisphenol A-glycidyl methacrylate (BisGMA, Sigma-Aldrich, St. Louis, MO, USA):diurethane dimethacrylate (UDMA, Sigma-Aldrich, Darmstadt, Germany):triethylene glycol dimethacrylate (TEGDMA, Sigma-Aldrich, USA) in 21.4:25.4:53.3 wt% ratio.
A photopolymerizable resin matrix is needed for the MDP containing bond material to achieve a chemical bonding between the frosted, bonded surface and epoxy, bone, titanium samples. This resin matrix contains Bis-GMA:UDMA:TEGDMA in 21.4:25.4:53.3 wt% ratio, camphorquinone (CQ, Sigma-Aldrich, Germany) as a photoinitiator in 0.2 wt% and ethyl 4-(dimethylamino)benzoate (EDMAB, Sigma-Aldrich, Shanghai, China) in 2 mol% referred to monomers [
14].
2.3. Specimen Preparation for Determination of Optimal Etching Time on Nitric Acid Etched Glass Surface
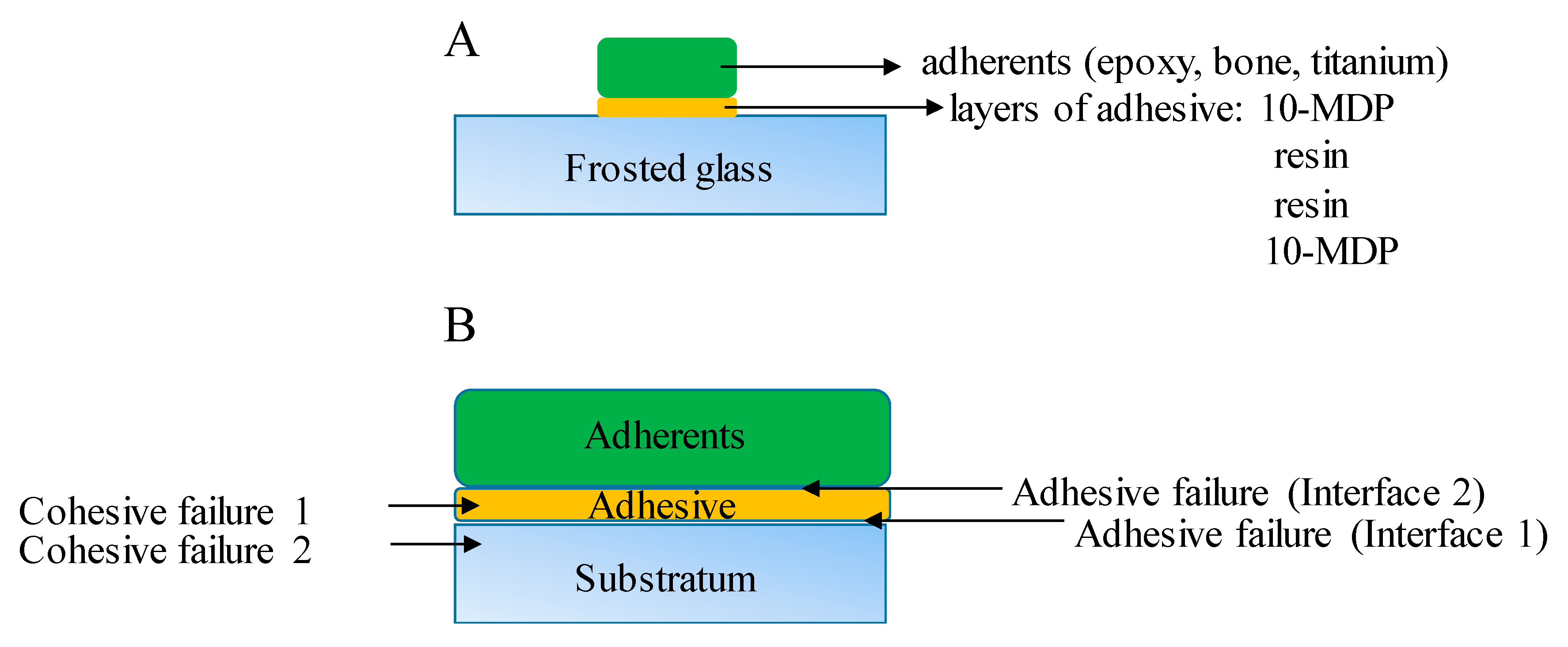
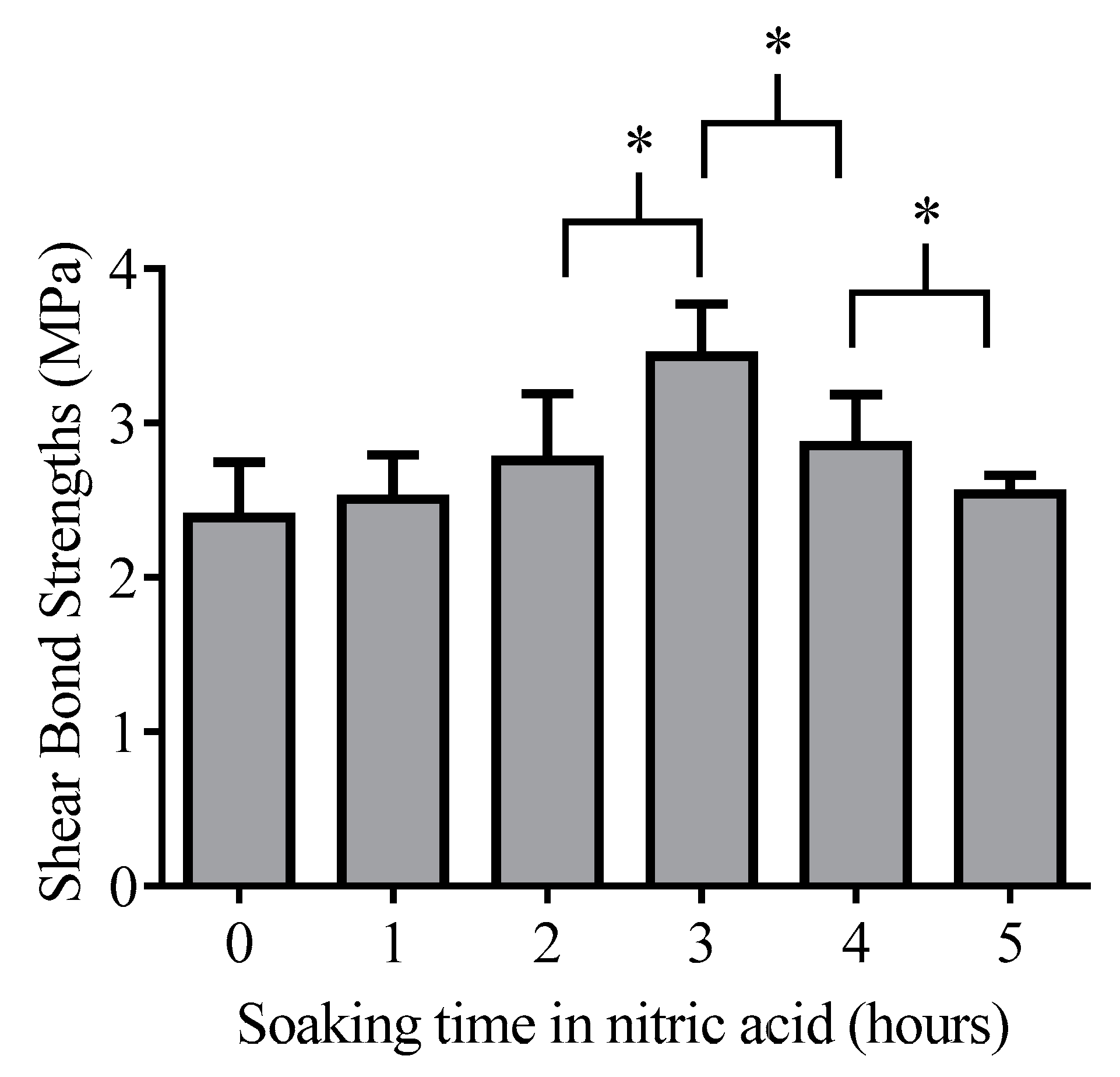
The optimal acid etching time was determined in a preliminary experiment. The inert epoxy embedding materials were used to determine the optimal etching time because it has the lowest affinity to the glass surface thus pure protonation effect can analyze from the side of the glass. The 13 × 25 mm glass pieces (n = 42, 7 pieces per hour) were soaked 68% nitric acid (VWR, Debrecen, Hungary) containing Teflon dish from 0 to 5 h in dark during gentle agitation to make the diffusion layer smaller. After soaking the glass pieces were briefly rinsed with distilled water and were gently dried with cleaned oil-free air. Epoxy samples (n = 42) adhered to the frosted glass surface by creating of layered structure (Figure 2). MDP containing bond material was brushed and air-dried on the frosted glass surface and epoxy sample surface, then the photopolymerizable resin matrix was applied both on epoxy and glass surface also. The specimens were placed into a clamping device to reach a uniform adhesive layer and the samples were fixed in the device by photopolymerization with the help of dental hand lamp (Bluephase 20i, Ivoclar Vivadent, Liechtenstein, Austria) at high intensity for 20 s onto the glass surface. Then the specimens were placed into a light chamber unit (Scheu LC-6 Light Oven, Iserlohn, Germany) for 5 min to reach complete photocuring. The prepared samples were tested immediately in a mechanical analyzer for shear bond strength measurements.
2.4. Determination of Glass Surface Charge by Zeta Potential Measurements before and after Nitric Acid Etching
For the zeta potential measurements, the glass slab pieces were ground with Analysette 3 Vibratory Sieve Shaker (Fritsch, Weimar, Germany) at 6 min 1.5 mm amplitude. Zirconium balls were used combined with zirconia lined milling bowl. The ground glass powder was washed three times by suspending and centrifuging in distilled water. The particles were dried at 90 °C. Silica powder (0.1 g) was placed into nitric acid for 3 h (soaking time is determined by preliminary experiment). After the decanting of nitric acid supernatant, the powder was rinsed ultra-pure water (3 × 8 mL). The suspension was made in 0.001 M NaCl containing ultra-pure water from protonated silica powder. Unprotonated bare glass powder suspension was prepared by 0.1 g glass powder dispersed in 0.001 M NaCl background electrolyte. The pH of suspensions and background electrolyte was measured by Orion 2 Star (Thermo Scientific, Singapore). The zeta potential measurements of suspensions and electrolytes were performed with the help of Zetasizer Nano ZS (Malvern Instruments Ltd., Grovewood, Worcestershire, UK) based on electrophoretic mobility in folded capillary cells next to continuous 0.001 M NaCl as a background in ultrapure water. Each sample was measured five times (n = 5). Zeta potential was calculated using the Smoluchowski approximation.
2.5. X-ray Photoelectron Spectroscopy (XPS) Measurements
X-ray photoelectron spectra were obtained using an Al/Mg twin anode non-monochromatized radiation source and a Phoibos100 MCD-5 series hemispherical energy analyzer produced by SPECS (Berlin). The measurements were conducted with Al K-α (E = 1486 eV) rays on both acid-etched and untreated samples. Roughly 1 × 1 cm pieces were cut from a slide, their surfaces cleaned with a nitrogen jet, and were either mounted directly onto the XPS sample holder (untreated sample) or left to soak in the nitric acid for 3 h at room temperature, in the dark (etched sample). After the acid treatment, the samples were thoroughly washed with distilled water, dried by nitrogen jet, mounted onto the sample holder, and left to further dry and outgas in a vacuum (10-7 mbar) for 20 h before measurement.
Core level spectra were recorded for Ca, Na, O, C, and Si and processed with CasaXPS (
http://www.casaxps.com). Peak positions were normalized to that of carbon (284.5 eV), fitting mixed Gaussian/Lorentzian curves after Shirley baseline correction.
2.6. Contact Angle (CA) Measurements
The sessile drop method was used to measure the water and adhesive contact angle (CA) on bare, protonated glass, epoxy, bone, and titanium surfaces by using DSA 30 Drop Shape Analyzer (Krüss GmbH, Hamburg, Germany) at room temperature (25 °C). Drops of water (3 µL) were deposited on the top of the surface with an automatic dosing system (0.5 mm diameter needle). Drops of adhesives (3 µL) were deposited on the surface with a manual dosing system holding a 1 mL of syringe (0.3 mm diameter needle). The contact angles were automatically calculated by fitting the captured drop shape to the degree calculated from the Young–Laplace equation. The average contact angle was determined from 10 drops measurements (n = 10) on the bare and protonated glass slab and section ingredients surface.
2.7. Specimen Preparation for Shear Bond Strength (SBS) Measurements
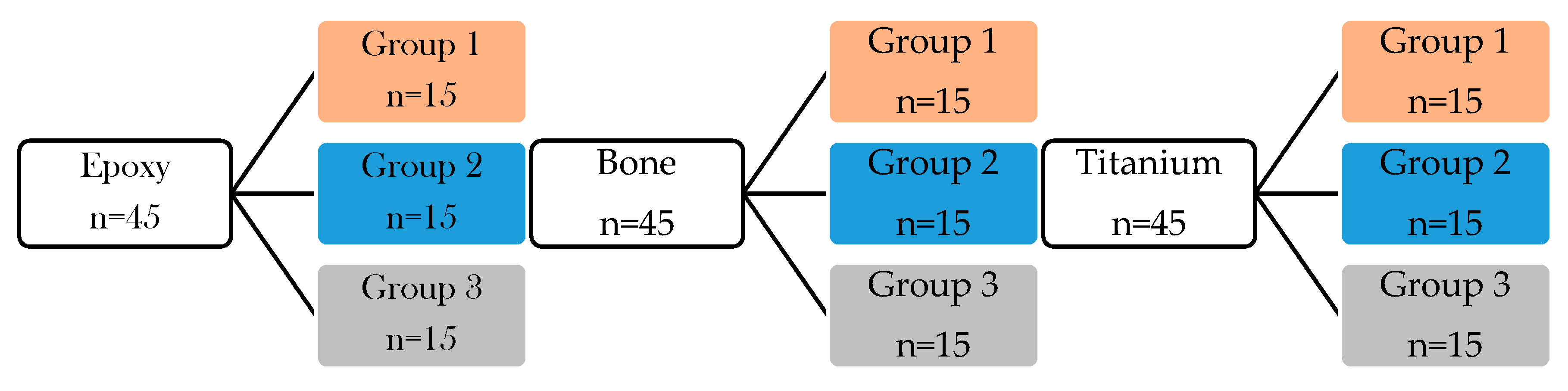
Table 1 summarizes the created groups for shear bond strength measurements and surface treatment steps for each group. The classification was performed based on the applied adhesive (thermoplastic, 10-MDP containing bond material) and glass surface modification (bare, frosted, protonated). The SBS were measured immediately after the specimen preparations.
The detailed description of specimen preparations are the following:
GROUP 1: The samples (bone (n = 15), epoxy (n = 15), titanium (n = 15)) were stuck to a glass slide (n = 45) with a small amount of thermoplastic adhesive at 120 °C and then the specimens were placed into a clamping device to ensure the uniform thickness of the adhesive layer between the sample and glass slide until the thermoplastic adhesive cooled down. (
Figure 1 orange color-coded boxes).
GROUP 2: In this adhesive technique, the surface of the frosted glass (n = 45) and epoxy (n = 15), bone (n = 15), and titanium samples (n = 15) were moistened with MDP containing bond material by brushing of adhesive. The adhesive was gently dried with air blowing. Thereafter, resin matrix was applied on glass and sample surfaces to form a layered structure (
Figure 2A). The specimens were placed into a clamping device and were photopolymerized by a dental hand lamp (Bluephase 20i, Ivoclar Vivadent, Liechtenstein, Austria) at high intensity for 20 s onto the glass surface. Then the specimens were placed into a light chamber unit (Scheu LC-6 Light Oven, Iserlohn, Germany) for 5 min (
Figure 1 blue color-coded boxes).
GROUP 3: The frosted glass (n = 45) was previously chemically etched by nitric acid for 3 h (determined in the preliminary experiment) after briefly distilled water rinsing and gently air drying, the epoxy, bone, and titanium samples were attached to protonated, frosted glass surface as it is described in previously presented at Group 2 specimen preparation steps (
Figure 1 grey color-coded boxes).
Figure 1 shows the chart flow of the experiments with different surface treatments and applied adhesive on the glass surface.
Figure 2 shows a schematic picture from the specimen preparation to shear bond strength measurements and cohesive and adhesive failure modes detection.
2.8. Shear Bond Strength (SBS) Measurements
The shear bond strength measurements were performed on a mechanical testing device (INSTRON 5544, Norwood, USA) in compression mode with 2 kN load cell and 2 mm/min crosshead speed. The specimens were placed into a custom-designed apparatus. During the measurements, the applied compression force (N) was recorded. The SBS was calculated with the following formula:
where F is the applied compression force, A is the attached surface area of the samples that is calculated with the following formula:
2.9. Detection of Failure Mode
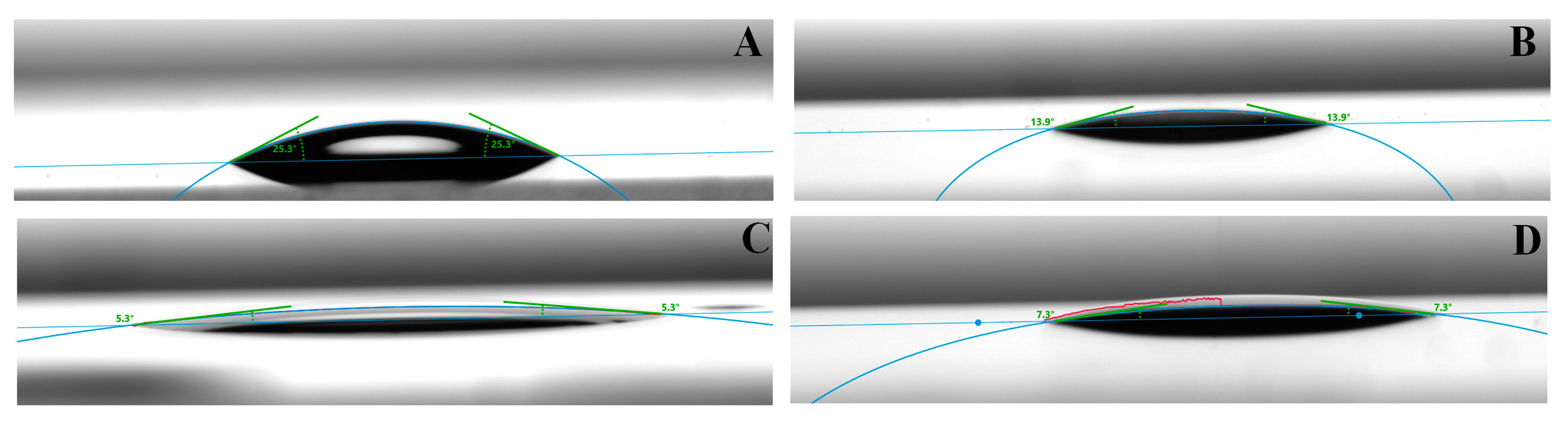
Adhesive and cohesive failure mode was determined under an optical microscope (Olympus SZ61) at 45× magnification after SBS measurements. The fracture modes were identified as adhesive failure at interface 1 where the failure was between the glass (substratum) and adhesive material (thermoplastic material or 10-MDP containing adhesive layer) (
Figure 2B). Failure mode was coded as an adhesive failure at interface 2 when the failure was between the adherents (epoxy, bond, titanium) and adhesive (10-MDP containing adhesive interface) (
Figure 2B). The fractures were identified as a cohesive failure when the failure was within the adhesive layer (cohesive failure 1,
Figure 2B) or within the glass (cohesive failure 2,
Figure 2B).
2.10. Statistical Analysis
Statistical analysis for SBS and contact angle data were performed using Student’s t-test with SPSS 17.0 software (IBM, Armonk, NY, USA). All of the tests’ accuracies were set at a significance level of 0.05.
4. Discussion
Ideal bone implants need to be a scaffold that provides mechanical support at the place of the defect. Besides, the implants are simultaneously allowing for bone in-growth when osteogenic cells migrate to the bone defects and form a new bone matrix. The visualizing of the bone/implant interface with a light microscope technique is a commonly used method for evaluating the effectiveness of implants. The type of implants determines the method that helps the visualization of bone in-growth. This method is a multistep procedure in which many artifacts appear to limit the high-quality histological result evaluation. Troiano et al. reviewed the processing, embedding, and cutting techniques used for bone implants and their associated histopathology [
18]. The authors described that the preferred method for processing metal implants is undecalcified section preparation. After dehydration, embedding, and ground of titanium-containing bone specimen, generally, the section thickness is from 30 to 100 microns. This thickness is far from the one-cell-thick monolayer. However, the thinner thickness in the section can obtain a higher resolution of evaluation in visualizing of bone/implant interface. Pazzaglia et al. described that many fewer intersections with direct bone–implant contact are present in the thinner section (15 µm) compared with the 300 µm slide [
19] due to higher resolution. Many factors influence the successful histological evaluation of the titanium-containing bone section. Among these factors, the appropriate thickness of the section can take part. By grinding of both sides of the embedded thin bone section, the optimal thickness can be reached inasmuch the adhesion between the section and pathological grade glass slide is strong enough to withstand the shearing force during the grinding and polishing. The section should adhere to an inert glass surface otherwise the section loss may occur. There are several microscopic glass slides that are surface modified by different layers to improve the tissue retention to glass. The positively charged surface is reached by a basic polymer or by a chemical reaction that leaves chemical groups linked by covalent bonds to the silicon atoms of the glass. Above-mentioned glass slides related to those sections are in an aqueous medium. In our case, the dehydrated, epoxy polymer infiltrated section is almost hydrophobic so the above-mention glass slide is not relevant. In our study, the positively charged glass surface was achieved by nitric acid etching.
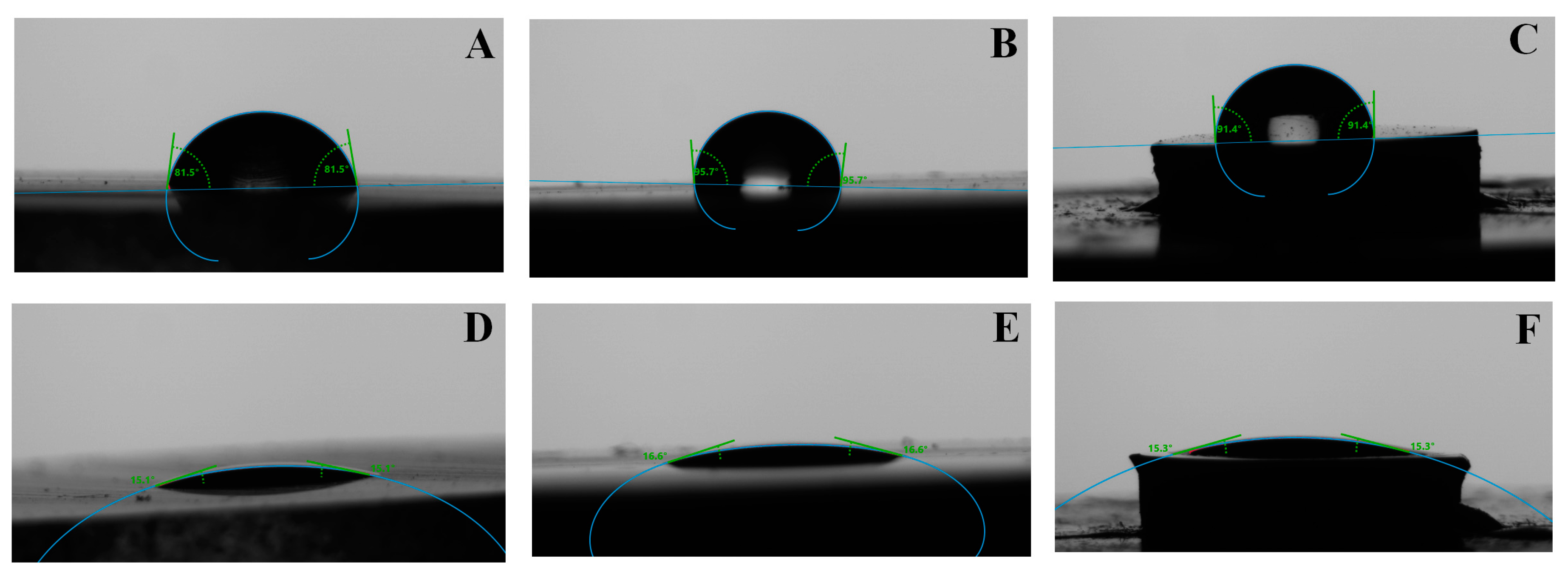
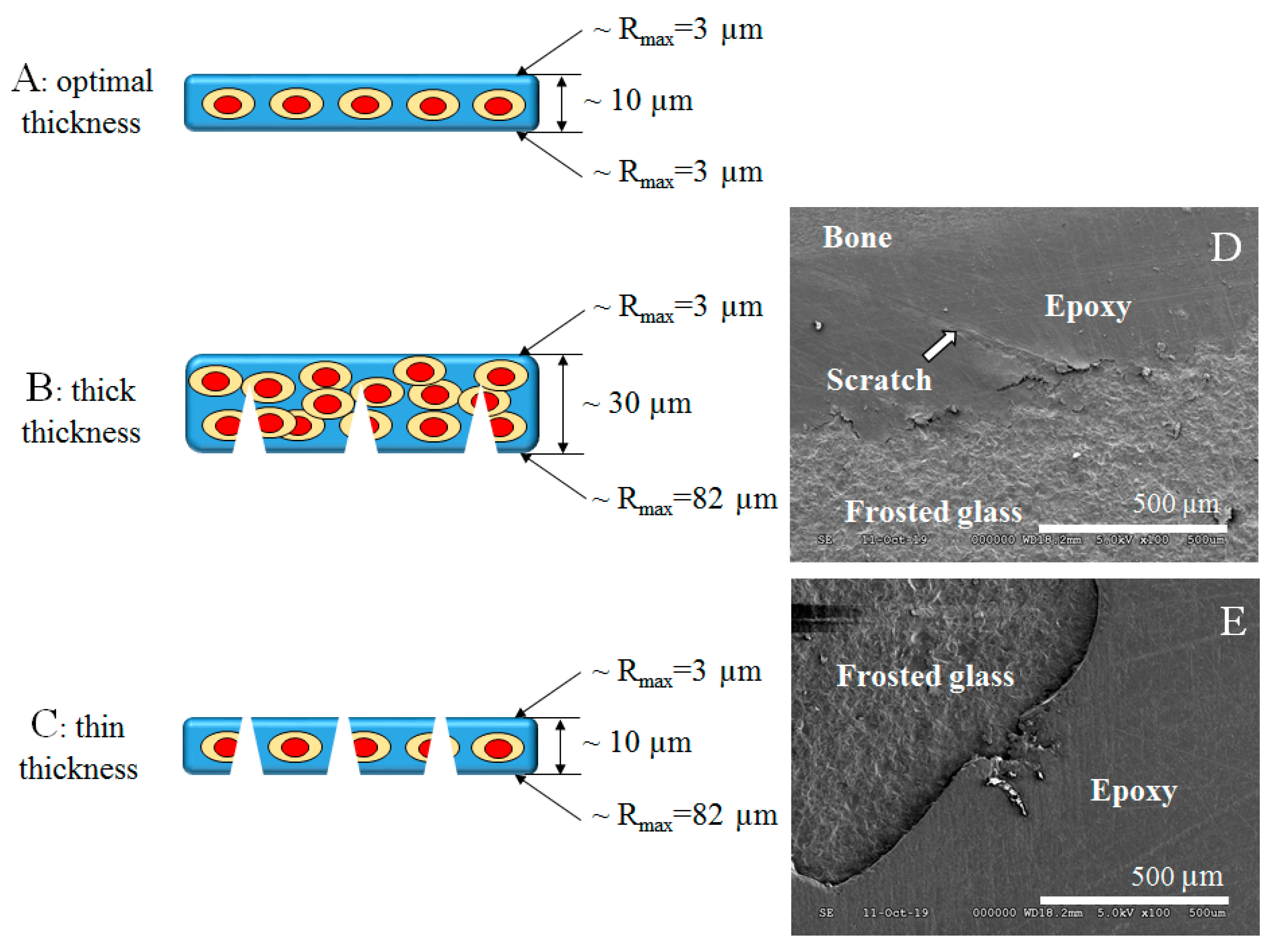
The other option is to increase the adhesion by the micromechanical interlocking that can be achieved by sandblasting or acid etching of the glass slide. Thereby fully frosted histological glass slides are available that are ideal for examining body fluid specimens. The frosting procedure in histotechnology is used to elevate the bond strength between the sample and the glass slide. The frosting creates microgrooves on the bonding side of the glass surface that means bigger adhesion surface area and higher bond strength. Nevertheless, the section surface also contains microgrooves, micro scratches that can contribute to better adhesion. At these dimensions, we have to compare the thickness of the section and depth of scratches left by abrasive particles. A well-chosen and well moisturizing bond can help with this comparison. This good wetting ability adhesive can penetrate easily the surface irregularities and fill them. In that case, if the surface of the section was unpolished by fine particles and the section is thick enough, the perforation of the section can occur (
Figure 9C) that can lead to loss of the section next to inappropriate adhesion and can make it impossible to evaluate. In this situation, the chemical role of an adhesive molecule is very important. In the case of the thicker section the perforation may not occur, but the thickness of the section can disturb the microscopy evaluation. (
Figure 9B). In a case of ideal section thickness with fine polishing and strong (mechanical and chemical) adhesion, the microscopy evaluation of the section can be successful (
Figure 9A).
Figure 9D shows a scanning electron microscopy (SEM) picture that represents the separation of the section from the frosted glass slide in the continuity of the scratch.
Figure 9E SEM picture shows a perforated thin bone section on a glass slide.
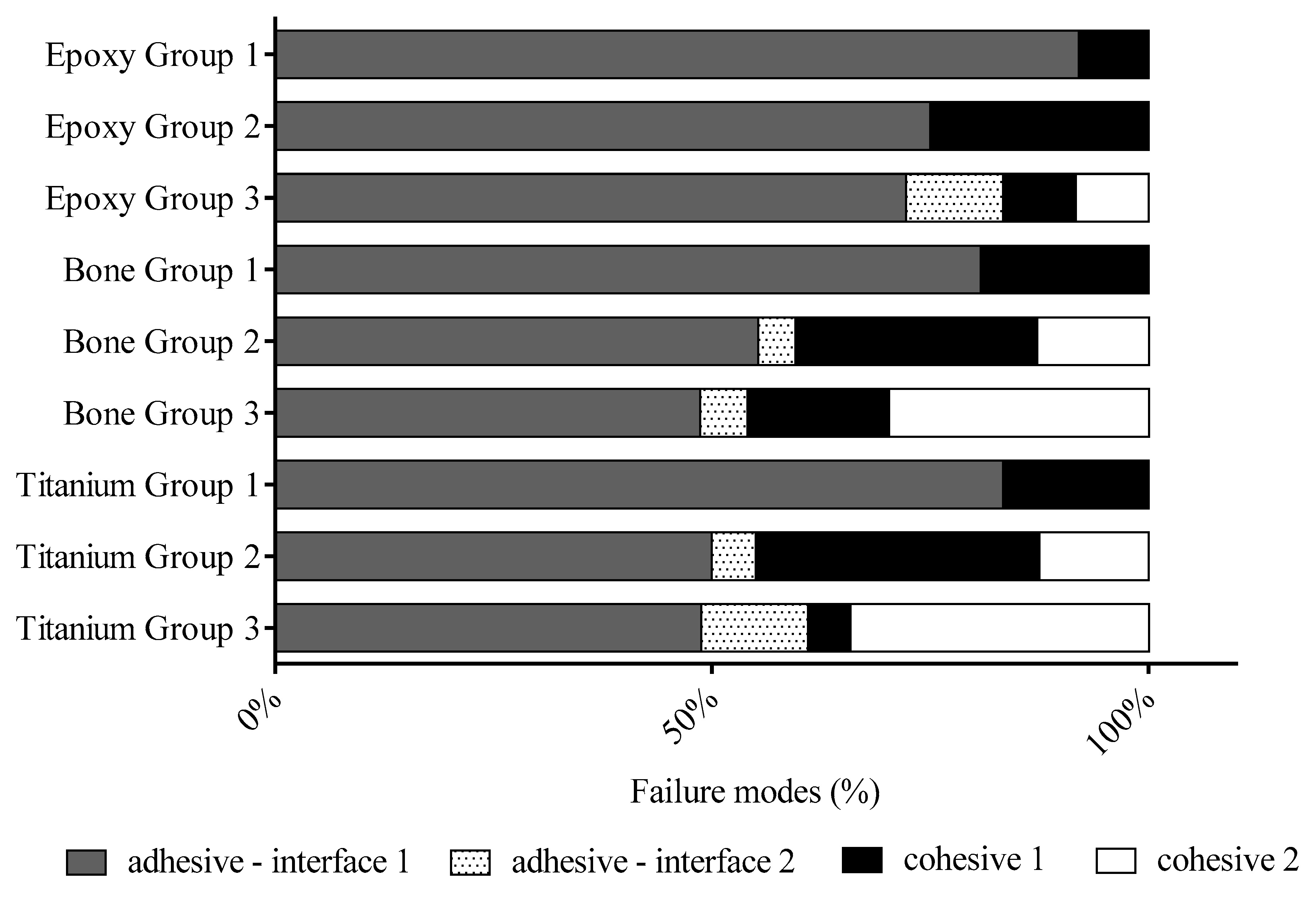
The preparation of a section to microscopy evaluation is a multistep process. The main drawback of the undecalcified thin bone section is insufficient thinness. In our study, we made an experiment to enhance the adhesion chemically and micromechanically between the pathological grade glass and components of the titanium-containing thin bone section to reduce the thickness of the section during the thinning process. Our primary goal was to investigate and increase the adhesion between the 10-MDP containing adhesive material and glass slide by frosting and protonation of glass. The chemical enhancements were achieved by 10-MDP containing bond material application and protonation of the glass slide, the micromechanical enhancements were frosting of the glass slide. A shear force acts between the surface of the section and abrasive paper during the grinding, therefore, the shear bond strength was a suitable measurement for the investigation of experimental groups. The SBS measurements revealed that the thermoplastic adhesive had the lowest bond strength (
Figure 7—Group 1). The thermoplastic adhesive is a high viscosity (6000 cps at 121 °C) thermo-responsive material. The penetration ability of adhesive is limited to the surface irregularities and not able to bond to a smooth glass surface that resulted above 80% adhesive failure modes at all samples (
Figure 8). In most of the cases, the thermoplastic adhesive is separated from the smooth glass surface. In a small percentage, the failure modes showed cohesive fracture inside the thermoplastic layer. Due to the high viscosity of thermoplastic adhesive small air bubbles entrapped after cooling down, that can be starting points of defects in the adhesive layer. Based on our measurements it was found that frosted slide combined with 10-MDP containing adhesive (
Figure 7—Group 2) gave significantly higher SBS data at bone and titanium compared to thermoplastic adhesive (
Figure 7—Group 1). At epoxy adherent, the difference was not significant, but the numbers of cohesive fractures increased compared to a thermoplastic that indicated the micromechanical retention (lower viscosity of 10-MDP containing bond), which is also supported by good wetting (
Table 4). A rougher surface created by the frosting procedure (gently sandblasting) provided an increased surface area for enhanced adhesion. It is well-known that micromechanical retention is a frequently used procedure to enhance the adhesion of prosthodontic work to tooth structure [
20]. The cured epoxy with an MDP containing bond material on frosted and protonated-frosted glass surface showed the same magnitude bond strength as a thermoplastic adhesive (
Figure 7). EpoFix was a mixture and cured polymer of diglycidyl ether of bisphenol A (DGEBPA) prepolymer resin and triethylenetetramine (TETA) hardener components. Hydroxyl groups are formed on the polymer chain at the end of crosslinking polymerization [
21]. The epoxy surface has hydrophobic nature based on contact angle measurements (
Table 4), but the MDP containing bond material can wet well this surface due to ethanol content (
Figure 6). The molecular structure of ethanol can solvate polar and nonpolar parts of specimens and it has a high hydrogen-bonding capacity. The cured epoxy polymer surface can be considered as an inert surface due to the lack of chemically polymerizable or reactive molecular sites. Therefore, the epoxy primarily connected to the frosted glass surface through 10-MDP containing adhesive by micromechanical retention. In this aspect, the adhesive has a meaningful wetting role on the epoxy surface that contributes to the adhesion of the section to the glass surface. By the frosted glass protonation, adhesive fracture in interface 2 (
Figure 2 and
Figure 8) appeared in a small percentage (11%) until this fracture mode is missing at the unprotonated glass. This suggests that a stronger bond may be formed between the protonated glass surface and the MDP than without protonation. However, epoxy does not have a free valence to bond with MDP so that a fracture at interface 2 occurs between the substrate and the MDP. This fracture in interface 2 contributes to that there was no significant change between the protonated and non-protonated versions. In this study, a commercially available 10-MDP was applied to increase the adhesion between the glass slide and components of the undecalcified thin bone section. The base of usage of 10-MDP in histotechnology is that much research in the adhesive technique describes the positive effects of 10-MDP on bone and titanium surface. There are numerous studies in which the chemical bonding of 10-MDP to titanium or bone are justified [
7,
8]. The 10-MDP is a bifunctional molecule, one site can bind to the calcium ions of hydroxyl-apatite, the other site can polymerize the monomers of the applied resin matrix during the light-curing process. Meerbek et al. described that the phosphate group of 10-MDP creates a chemical bond with calcium ions of apatite and forms a self-assembled monolayer. In this layer, methacrylate groups are directed towards each other and their phosphate groups are directed away from each other [
8]. Tsuchimoto et al. investigated the effect of 10-MDP containing primer on titanium and resin-based cement bond strength measurements. They found that the highest bond strength was achieved at 10 wt% 10-MDP primer treated titanium surface [
22]. The X-ray photoelectron spectroscopic investigation revealed phosphor (P) traces on 10-MDP treated titanium surface despite the rising of distilled water. The P was under detectable limit at untreated titanium samples. The ratio of P and Ti depended on the concentration of polyphosphoric acid. These chemical connections contributed to the strong adhesion between bone, titanium, and glass surface. Based on our study it was found that the chemical bonding between the glass surface and samples (bone, titanium) by 10-MDP active molecule combined with the frosting of glass contributed to the elevated SBS values (
Figure 7). In the comparison of Groups 1 and 2 at bone adherent, there was a significant difference in SBS. The numbers of adhesive fractures in interface 1 decreased (
Figure 8) that indicated that the low viscose 10-MDP containing adhesive flow easily into the surface irregularities to ensure a micromechanical interlocking. The numbers of cohesive failure inside the adhesive layer (cohesive failure 1) elevated compared to epoxy Group 2. This indicated that a strong connection is formed between the Ca ions of apatite and 10-MDP active phosphate groups that are previously proved by several research studies.
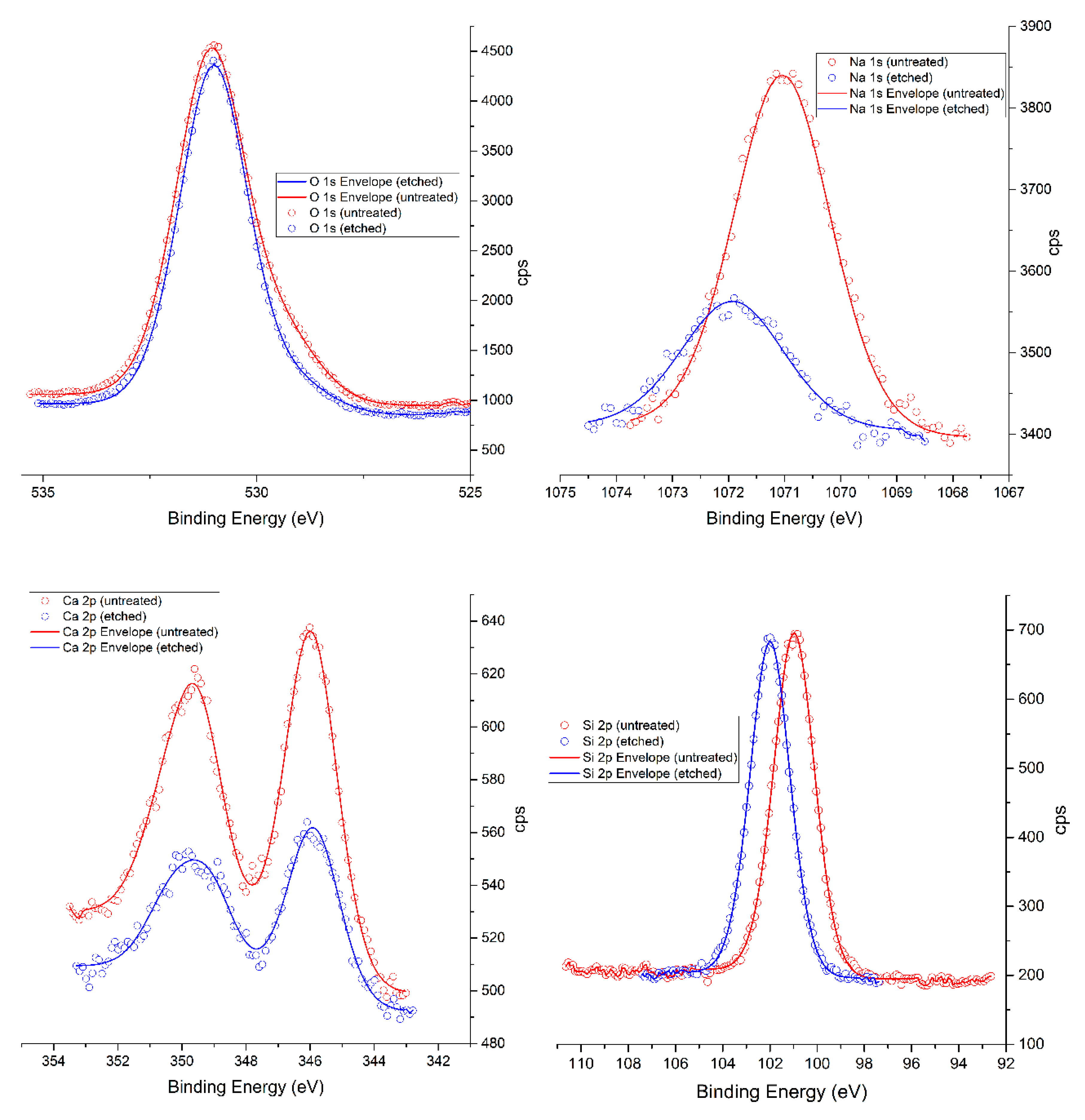
The glass protonation has generally used the process to clean or activate of the glass surface. Based on the XPS measurements it was found that sodium and calcium ions were released from the glass surface by the effect of acid etching (
Figure 4,
Table 3). These findings are related to the results of Jang et al. [
13]. In our study, the protonation by nitric acid of pathological grade glass is a new aspect in increasing of bond strength of the undecalcified thin bone section to glass. This study revealed that there is an optimum at 3 h for acid soaking of pathological grade borosilicate surface regarding shear bond strengths measurement (
Figure 3). In aqueous suspension, the hydroxylation of the glass surface can result in the formation of silanol groups (Si-OH). The surface silanol (Si-OH) groups can pick up a proton from the nitric acid solution through the following reaction [
23]:
That is why the glass particles are positively charged under acidic pH after nitric acid etching (
Table 3). Based on the contact angle measurements, it was revealed that the adhesive has a better wetting ability on the acid-etched, positively charged surface than unprotonated glass which can contribute to the higher bond strength as a part of chemical adhesion (
Table 4). The zeta potential measurements, which were calculated by Smoluchowski coagulation equation, revealed that the glass surface becomes positively charged after nitric acid etching (
Table 2). Pazinatto et al. described that insoluble silica salts formed on the surface after acid exposure of glass. These precipitates are not removable not even in an ultrasonic bath [
17]. Based on our XPS measurements, it was revealed that nitric acid treatment causes the dealkalization process in that alkaline ions are leached out from the glass surface (
Figure 4,
Table 3) and may form insoluble salts. These salts have a positive effect on the chemical bonding of PO
4 groups of 10-MDP to precipitated salts of the glass surface. Lee at al. reported mismatched interaction between zirconia primer (MDP) and the glazed surface [
24]. Therefore, it was assumed that insoluble salts on the glass surface by Pazinatto may have a determining factor in the chemical affinity of MDP containing bond to the etched glass surface.
At bone and titanium, the MDP containing bond material on the protonated glass surface resulted in a higher bond strength value compared to the unprotonated version (
Figure 7). Based on the research studies it can be concluded that positively charged surface—in the case of apatite is Ca
2+—favorably influences the connection of phosphate group of 10-MDP. By glass protonation SiOH
+2 positively charged ions are formed on the surface that can explain the larger number of cohesive fractures in interface 2 (from 13% to 30%) at bone substratum (
Figure 8). In the case of titanium, the 10-MDP adhesive has better adhesive strength than thermoplastic adhesive. By glass protonation, the SBS value increased significantly and the frequency of cohesive failure 2 was the highest at titanium. The explanation is a strong ionic bond between the titanium and 10-MDP that was justified by research studies. In that case, the SBS data are only analyzed from the side of the protonated glass slide and there would not be a difference in bond strengths measurements because the bond strength of 10-MDP and glass is measured at every measurement but there was a difference in SBS data at bone and titanium on protonated and unprotonated glass. Therefore, it can be concluded that the other side—adherents, 10-MDP—connection parallelly has an important role in the formation of failure modes and SBS values.
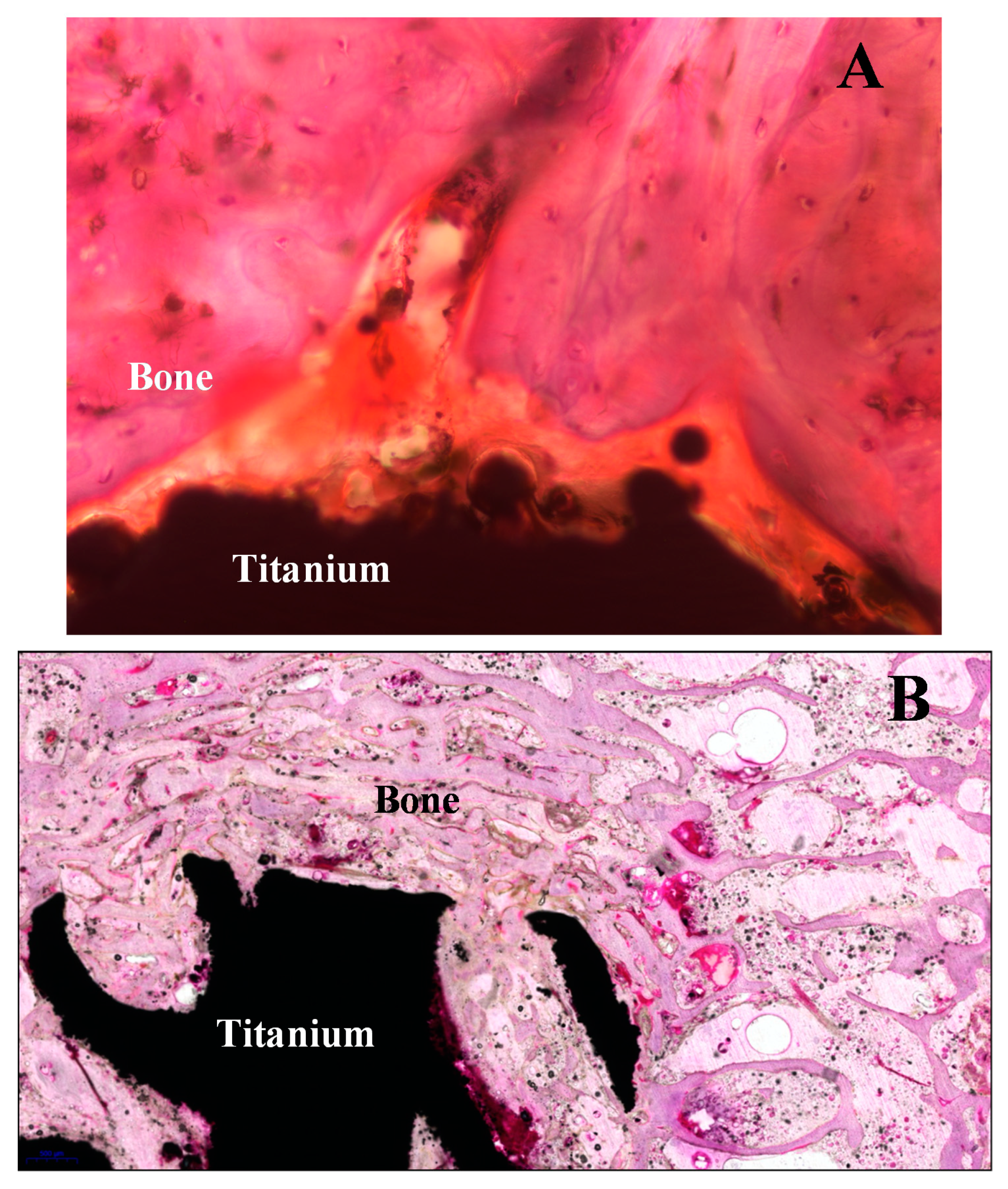
Figure 10 shows two sections with the thick thickness (A part) and optimum thickness (B part) after hematoxylin-eosin (H&E) staining. The procedure step by step for section preparation from sintered titanium implant in the animal bone sample is shown in
Figure 11.
Our findings suggest that the components of titanium implant containing undecalcified bone section showed higher SBS data with 10-MDP containing adhesive than in metallography routinely used thermoplastic adhesive. The MDP containing bond material provided chemical-micromechanical retention that resulted in higher cohesive failure mode frequencies and SBS values. The chemical etching with nitric acid provided a positively charged motif on the glass surface. This surface modification positively influences the shear bong strengths and wetting ability of MDP containing bond material.