Presence of Cyanotoxins in a Mexican Subtropical Monomictic Crater Lake
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling
Mat Biomass and Phytoplankton
2.3. Chemicals and Standards
2.4. Extraction and Analysis
2.4.1. Microcystin Extraction
UHPLC-MS/MS and Microcystin Quantitation
2.4.2. PST Extraction
PST Analysis
2.5. Cyanobacteria Bloom Forming Species Identification and Quantification
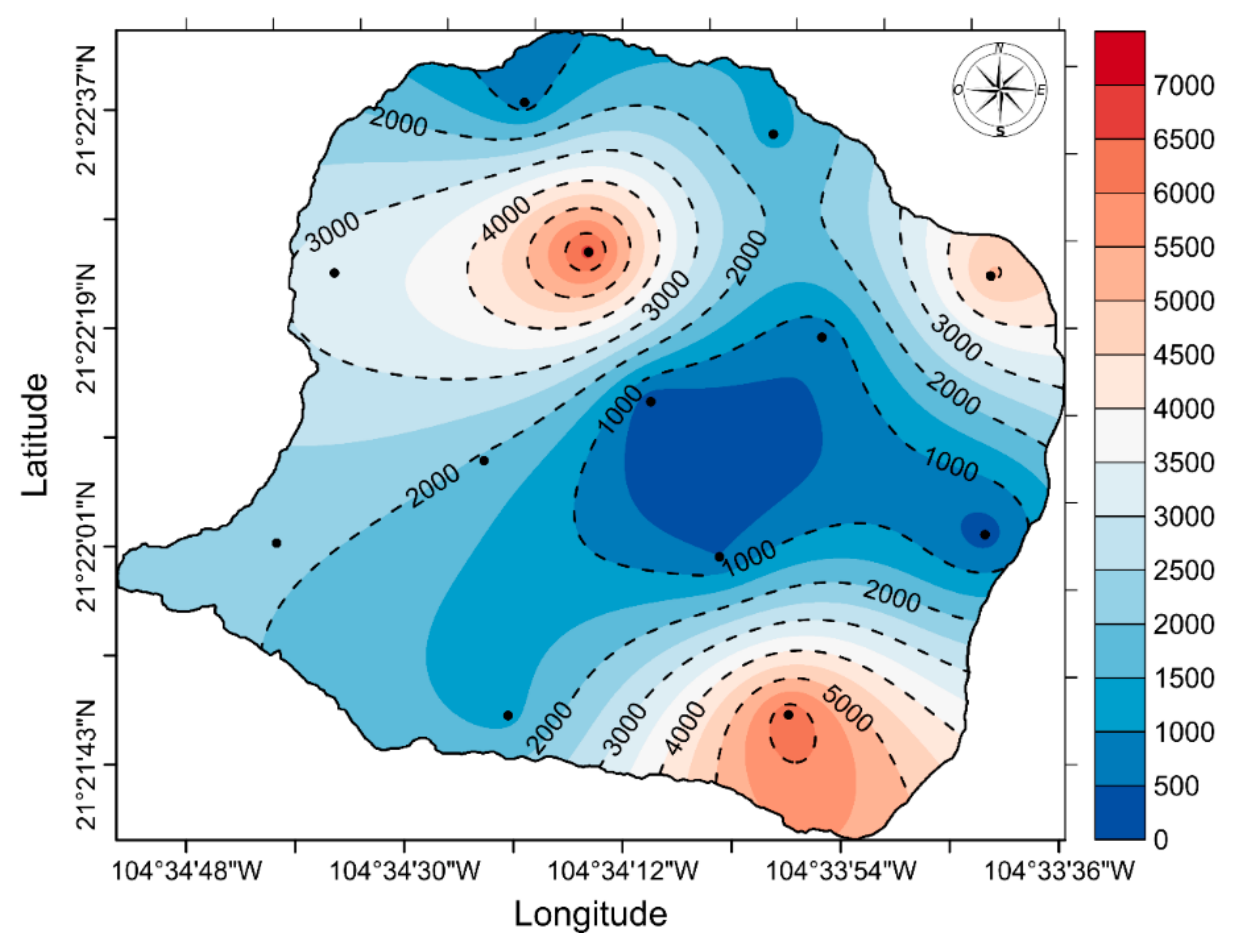
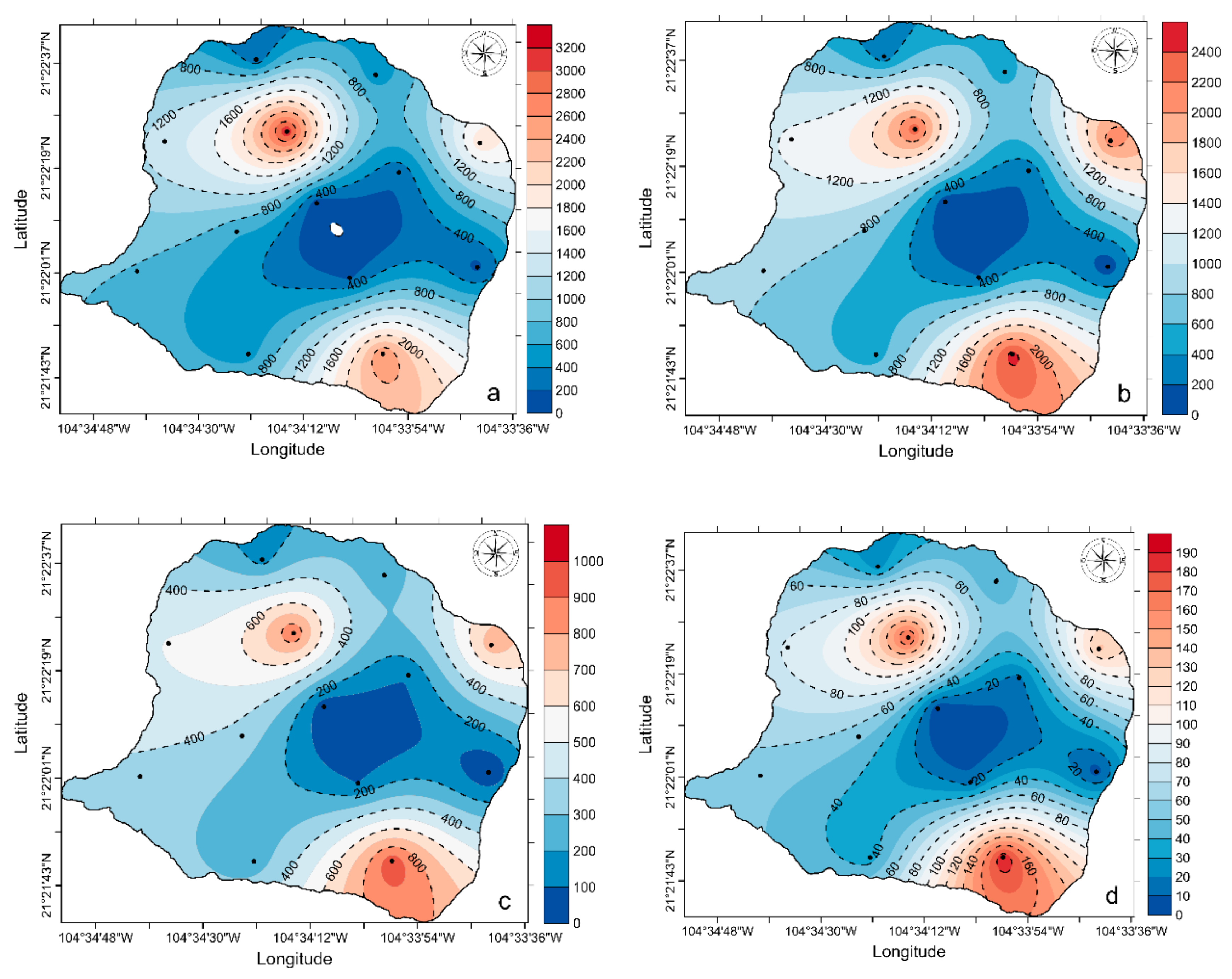
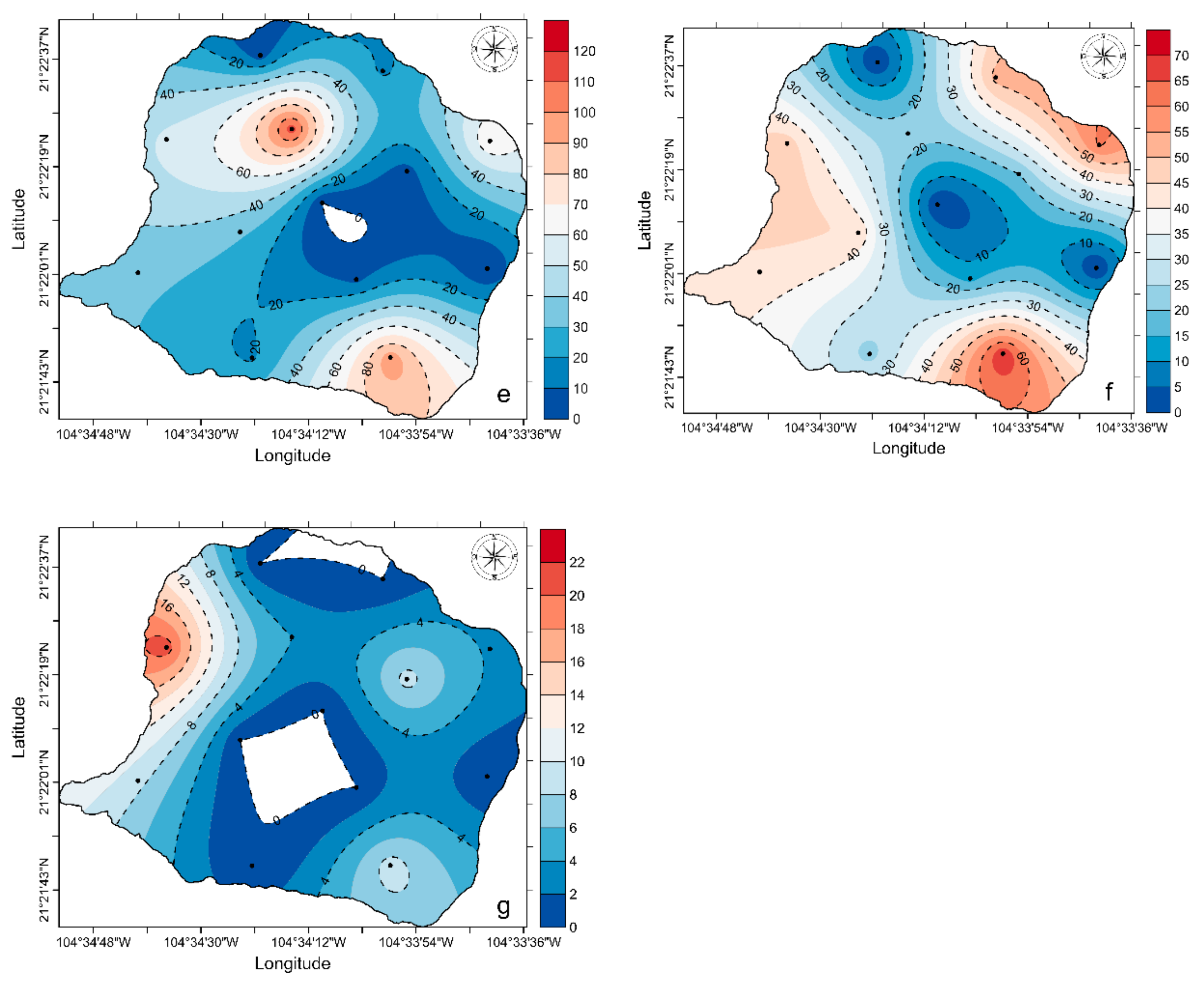
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Anderson, D. HABs in a changing world: A perspective on harmful algal blooms, their impacts, and research and management in a dynamic era of climactic and environmental change. Harmful Algae 2012, 3–17. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4667985/ (accessed on 10 April 2020).
- Bouaïcha, N.; Miles, C.O.; Beach, D.G.; Labidi, Z.; Djabri, A.; Benayache, N.Y.; Nguyen-Quang, T. Structural diversity, characterization and toxicology of Microcystins. Toxins 2019, 11, 714. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, P. Mechanisms of Microcystin-induced cytotoxicity and apoptosis. Mini Rev. Med. Chem. 2016, 16, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Kotak, B.G.; Zurawell, R.W. Cyanobacterial toxins in Canadian freshwaters: A review. Lake Reservoir Manag. 2007, 23, 109–122. [Google Scholar] [CrossRef]
- Funari, E.; Testai, E. Human health risk assessment related to cyanotoxins exposure. Crit. Rev. Toxicol. 2008, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Pearson, L.; Mihali, L.; Moffitt, M.; Kellmann, R.; Neilan, B. On the chemistry, toxicology and genetics of the cyanobacterial toxins, microcystin, nodularin, saxitoxin and cylindrospermopsin. Mar. Drugs. 2010, 8, 1650–1680. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.; Vasconcelos, V. Use of qPCR for the study of hepatotoxic cyanobacteria population dynamics. Arch. Microbiol. 2011, 193, 615–627. [Google Scholar] [CrossRef]
- Williams, C.D.; Aubel, M.T.; Chapman, A.D.; D’Aiuto, P.E. Identification of cyanobacterial toxins in Florida’s freshwater systems. Lake Reser. Manag. 2007, 23, 144–152. [Google Scholar] [CrossRef]
- Winter, J.G.; DeSellas, A.M.; Flecher, R.; Heintsch, L.; Morley, A.; Nakamoto, L.; Utsumi, K. Algal blooms in Ontario, Canada: Increases in reports since 1994. Lake Reser. Manag. 2011, 27, 107–114. [Google Scholar] [CrossRef]
- Arzate-Cárdenas, M.A.; Olvera-Ramírez, R.; Martínez-Jerónimo, F. Microcystis toxigenic strains in urban lakes: A case of study in Mexico City. Ecotoxicology 2010, 19, 1157–1165. [Google Scholar] [CrossRef]
- Hu, Y.; Chen, J.; Fan, H.; Xiel, P.; He, J. A review of neurotoxicity of microcystins. Environ. Sci. Pollut. Res. 2016, 23, 7211–7219. [Google Scholar] [CrossRef]
- Wiese, M.; D’Agostino, P.M.; Mihali, T.K.; Moffitt, M.C.; Neilan, B.A. Neurotoxic alkaloids: Saxitoxin and its analogs. Mar. Drugs 2010, 8, 2185–2211. [Google Scholar] [CrossRef] [PubMed]
- Durán-Riveroll, L.M.; Cembella, A.D.; Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; Correa-Basurto, J. Docking simulation of the binding interactions of saxitoxin analogs produced by the marine dinoflagellate Gymnodinium catenatum to the voltage-gated sodium channel Nav1.4. Toxins 2017, 8, 129. [Google Scholar] [CrossRef] [PubMed]
- Codd, G.A.; Bell, S.G.; Kaya, K.; Ward, C.; Beattie, K.A.; Metcalf, J. Cyanobacterial toxins, exposure routes and human health. Eur. J. Phycol. 1999, 34, 405–415. [Google Scholar] [CrossRef]
- Aráoz, R.; Molgó, J.; Tandeau de Marsac, N. Neurotoxic cyanobacterial toxins. Toxicon 2010, 56, 813–828. [Google Scholar] [CrossRef]
- Zamyadi, A. Emerging Toxic Cyanobacterial Issues in Freshwater Sources: Influence of Climate Change. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: New York, NY, USA, 2014; pp. 105–120. [Google Scholar]
- Vale, P. Saxitoxin and Analogs: Ecobiology, Origin, Chemistry, and Detection. In Seafood and Freshwater Toxins: Pharmacology, Physiology, and Detection, 3rd ed.; Botana, L.M., Ed.; CRC Press: New York, NY, USA, 2014; pp. 991–1011. [Google Scholar]
- Band-Schmidt, C.J.; Bustillos-Guzmán, J.J.; López-Cortés, D.J.; Gárate-Lizárraga, I.; Núñez-Vázquez, E.J.; Hernández-Sandoval, F.E. Ecological and physiological studies of Gymnodinium catenatum in the Mexican Pacific: A review. Mar. Drugs. 2010, 8, 1935–1961. [Google Scholar] [CrossRef]
- Berry, J.P.; Lind, O. First evidence of ‘‘paralytic shellfish toxins’’ and cylindrospermopsin in a Mexican freshwater system, Lago Catemaco, and apparent bioaccumulation of the toxins in ‘‘tegogolo’’ snails (Pomacea patula catemacensis). Toxicon 2010, 55, 930–938. [Google Scholar] [CrossRef]
- Caballero, M.; Rodríguez, A.; Vilaclara, G.; Ortega, B.; Roy, P.; Lozano, S. Hydrochemistry, ostracods and diatoms in a deep, tropical, crater lake in Western Mexico. J. Limnol. 2013, 72, 512–523. [Google Scholar] [CrossRef]
- Ochoa-Zamora, G.G. Variación Espacio-Temporal de las Poblaciones de Cianobacterias Formadoras de Florecimientos en el Lago Cráter de Santa María del Oro, Nayarit. Bachelor’s Thesis, Universidad Autónoma de Nayarit, Tepic, Mexico, 2018. (In Spanish). [Google Scholar]
- Salazar-Alcaraz, I. Identificación y Aislamiento de Cianobacterias de un Lago Cráter Tropical. Master Thesis, Universidad Autónoma de Nayarit, Tepic, Mexico, 2018. (In Spanish). [Google Scholar]
- Vázquez-Castro, G.; Ortega-Guerrero, B.; Rodríguez, A.; Caballero, M.; Lozano-García, S. Mineralogía magnética como indicador de sequía en los sedimentos lacustres de los últimos ca. 2600 años de Santa María del Oro, occidente de México. Rev. Mex. Cienc. Geol. 2008, 25, 21–38. [Google Scholar]
- Serrano-Hernández, D.E. Procesos Termodinámicos en el Lago Volcánico de Santa María del Oro, Nayarit: México. Ph.D. Thesis, Universidad Nacional Autónoma de México, Tepic, Mexico, 2004. (In Spanish). [Google Scholar]
- Cardoso-Mohedano, A.; Sánchez-Cabeza, J.A.; Ruiz-Fernández, A.C.; Pérez-Bernal, L.H.; Lima-Rego, J.; Giralt, S. Fast deep water warming of a subtropical crater lake. Sci. Total Environ. 2019, 691, 1353–1361. [Google Scholar] [CrossRef]
- Berbery, E.H. Mesoscale moisture analysis of the North American monsoon. J. Clim. 2001, 14, 121–137. [Google Scholar] [CrossRef]
- Liebmann, B.; Bladé, I.; Bond, N.A.; Gochis, D.; Allured, D.; Bates, G.T. Characteristics of North American summertime rainfall with emphasis on the monsoon. J. Clim. 2008, 21, 1277–1294. [Google Scholar] [CrossRef]
- Castro, S.C.D. Variabilidad de los ciclones tropicales que afectan a México. Interciencia 2010, 35, 306–310. Available online: https://www.redalyc.org/pdf/339/33913156011.pdf (accessed on 20 June 2020).
- Rodríguez-Ramírez, A.; Caballero, M.; Roy, P.; Ortega, B.; Vázquez-Castro, G.; Lozano-García, S. Climatic variability and human impact during the last 2000 years in western Mesoamerica: Evidence of late Classic (AD 600 900) and Little Ice Age drought events. Clim. Past. 2015, 11, 1239–1248. [Google Scholar] [CrossRef]
- Órgano del Gobierno del Estado de Nayarit. Plan de desarrollo municipal de Santa María del Oro, Nayarit, 2017–2021; Periódico Oficial: Tepic, Mexico, 2017; Volume 130, 124p. (In Spanish)
- Valencia-Plascencia, M.A.; Jacome-Pérez, B.G. Cultivo Experimental de Tilapia (Oerochromis aureus) en Jaulas Flortantes de p.v.c. en el Lago de Santa María del Oro, Nay. Bachelor’s Thesis, Universidad Autónoma de Nayarit, Tepic, Mexico, 1993; 47p. (In Spanish). [Google Scholar]
- Órgano del Gobierno del Estado de Nayarit. Plan de desarrollo urbano del centro de población de Santa María del Oro, Nayarit; Periódico Oficial: Tepic, Mexico, 2005; Volume 63, 108p. (In Spanish)
- Turner, A.D.; Waack, J.; Lewis, A.; Edwards, C.; Lawton, L. Development and single laboratory validation of a UHPLC-MS/MS method for quantitation of microcystins and nodularin in natural water, cyanobacteria, shellfish and algal supplement tablet powders. J. Chromatogr. B. 2018, 1074–1075, 111–123. [Google Scholar] [CrossRef]
- Turner, A.D.; Dhanji-Rapkova, M.; O’Neill, A.; Coates, L.; Lewis, A.; Lewis, K. Analysis of microcystins in cyanobacterial blooms from freshwater bodies in England. Toxins 2018, 10, 39. [Google Scholar] [CrossRef]
- Yu, R.C.; Hummert, C.; Luckas, B.; Qian, P.Y.; Zhou, M.J. Modified HPLC method for analysis of PSP toxins in algae and shellfish from China. Chromatographia 1998, 48, 671–676. Available online: https://link.springer.com/article/10.1007/BF02467597 (accessed on 20 June 2020). [CrossRef]
- Fastner, J.; Wirsing, B.; Wiedner, C.; Heinze, R.; Neumann, U.; Chorus, I. Cyanotoxin occurrence in Germany: Microcystins and hepatocyte toxicity. In Cyanotoxins: Occurrence, Causes, Consequences; Chorus, I., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 22–37. [Google Scholar]
- IARC Working Group. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins. In Monographs on the Evaluation of Carcinogenic Risks to Humans; WHO, IARC: Geneva, Switzerland, 2010; Volume 94, p. 450. ISBN 978-92-832-1294-2. [Google Scholar]
- Serrano, D.; Filonov, A.; Tereshchenko, I. Dynamic response to valley breeze circulation in Santa María del Oro, a volcanic lake in Mexico. Geophys. Res. Lett. 2002, 29, 27–31. [Google Scholar] [CrossRef]
- Harke, M.J.; Steffen, M.M.; Glober, C.J.; Otten, T.G.; Wilhelm, S.W.; Wood, S.A.; Paerl, H.M. A review of the global ecology, genomics and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 2016, 54, 4–20. [Google Scholar] [CrossRef]
- Mowe, M.A.D.; Mitrovic, S.M.; Lim, R.P.; Furey, A.; Yeo, D.C.J. Tropical cyanobacterial blooms: A review of prevalence, problem taxa, toxins and influencing environmental factors. J. Limnol. 2015, 7, 205–224. [Google Scholar] [CrossRef]
- Nandini, S.; Sánchez-Zamora, C.; Sarma, S.S.S. Toxicity of cyanobacterial blooms from the reservoir Valle de Bravo (Mexico): A case study on the rotifer Brachionus calyciflorus. Sci. Total Environ. 2019, 688, 1348–1358. [Google Scholar] [CrossRef]
- Zamora-Barrios, C.A.; Nandini, S.; Sarma, S.S.S. Effect of crude extracts from cyanobacterial blooms in Lake Texcoco (Mexico) on the population growth of Brachionus calyciflorus (Rotifera). Toxicon 2017, 13, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Zamora-Barrios, C.A.; Nandini, S.; Sarma, S.S.S. Bioaccumulation of microcystins in seston, zooplankton and fish: A case study in Lake Zumpango, Mexico. Environ. Pollut. 2019, 249, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, V.; Martins, A.; Vale, M.; Antunes, A.; Azevedo, J.; Welker, M.; López, O.; Montejano, G. First report on the occurrence of microcystins in planktonic cyanobacteria from Central Mexico. Toxicon 2010, 56, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Rodríguez, R.; Núñez-Vázquez, E.J.; Cordero-Tapia, A.; Gárate-Lizárraga, I.; Hiller, S.A.; Ochoa, J.L. Proliferación de Microcystis aeruginosa y presencia de microcistinas en un lago artificial en Culiacán, Sin., México. In Book of Abstracts of the XIV Reunión Nacional de la Sociedad Mexicana de Planctología, A.C. y VII Reunión Internacional de Planctología; Sociedad Mexicana de Planctología: Mexico City, Mexico, 2006. [Google Scholar]
- Ueno, Y.; Nagata, S.; Tsutsumi, T.; Hasegawa, A.; Watanabe, M.F.; Park, H.D.; Chen, G.C.; Chen, G.; Yu, S.Z. Detection of microcystins, a blue-green algal hepatotoxin, in drinking water sampled in Haimen and Fusui, endemic areas of primary liver cancer in China, by highly sensitive immunoassay. Carcinogenesis 1996, 17, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Komárek, J.; Zapomělová, E.; Šmarda, J.; Kopecký, J.; Rejmánková, E.; Woodhouse, J.; Neilan, B.; Komárková, J. Polyphasic evaluation of Limnoraphis robusta, a water–bloom forming cyanobacterium from Lake Atitlán, Guatemala, with a description of Limnoraphis gen. nov. Fottea Olomouc. 2013, 13, 39–52. [Google Scholar] [CrossRef]
- Li, J.; Li, R.; Li, J. Current research scenario for microcystins biodegradation–A review on fundamental knowledge, application prospects and challenges. Sci. Total Environ. 2017, 595, 615–632. [Google Scholar] [CrossRef]
- Romero-Oliva, C.S.; Contardo-Jara, V.; Block, T.; Pflugmacher, S. Accumulation of microcystin congeners in different aquatic plants and crops—A case study from lake Amatitlán, Guatemala. Ecotox. Environ. Safe. 2014, 102, 121–128. [Google Scholar] [CrossRef]
- Pekar, H.; Westerberg, E.; Bruno, O.; Lääne, A.; Persson, K.M.; Sundstrom, L.F.; Thim, A.M. Fast, rugged and sensitive ultra high pressure liquid chromatography tandem mass spectrometry method for analysis of cyanotoxins in raw water and drinking water—First findings of anatoxins, cylindrospermopsins and microcystin variants in Swedish source waters and infiltration ponds. J. Chromatogr. 2016, 1429, 265–276. [Google Scholar] [CrossRef]
- Zervou, S.K.; Christophoridis, C.; Kaloudis, T.; Triantis, T.M.; Hiskia, A. New SPE-LC-MS/MS method for simultaneous determination of multi-class cyanobacterial and algal toxins. J. Hazar. Mater. 2017, 321, 56–66. [Google Scholar] [CrossRef]
- Visser, P.; Ibelings, B.; Mur, L.R.; Walsby, A. 2005. The ecophysiology of the harmful cyanobacterium Microcystis: Features explaining its success and measures for its control. In Harmful Cyanobacteria; Huisman, J., Matthijs, H.C.P., Visser, P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 109–142. Available online: https://www.academia.edu/28066256/ (accessed on 5 May 2020).
- Kim, S.G.; Rhee, S.K.; Ahn, C.Y.; Ko, S.R.; Choi, G.G.; Bae, J.W.; Park, Y.H.; Oh, H.M. Determination of cyanobacterial diversity during algal blooms in Daechung Reservoir, Korea, on the basis of cpcBA intergenic spacer region analysis. Appl. Environ. Microbiol. 2006, 72, 3252–3258. [Google Scholar] [CrossRef]
- Banack, S.A.; Caller, T.A.; Stommel, E.W. The cyanobacteria derived toxin beta-N-Methylamino-L-Alanine and amyotrophic lateral sclerosis. Toxins 2010, 2, 2837–2850. [Google Scholar] [CrossRef] [PubMed]
- Esterhuizen-Londt, M.; Baik, S.; Kwon, K.-S.; Ha, M.-H.; Oh, H.-M.; Pflugmacher, S. Toxicity and toxin composition of Microcystis aeruginosa from Wangsong reservoir. Toxicol. Environ. Health Sci. 2018, 10, 179–185. [Google Scholar] [CrossRef]
- Dörr, F.A.; Pinto, E.; Soares, R.M.; Feliciano de Oliveira e Azevedo, S.M. Microcystins in South American aquatic ecosystems: Occurrence, toxicity and toxicological assays. Toxicon 2010, 56, 1247–1256. [Google Scholar] [CrossRef] [PubMed]


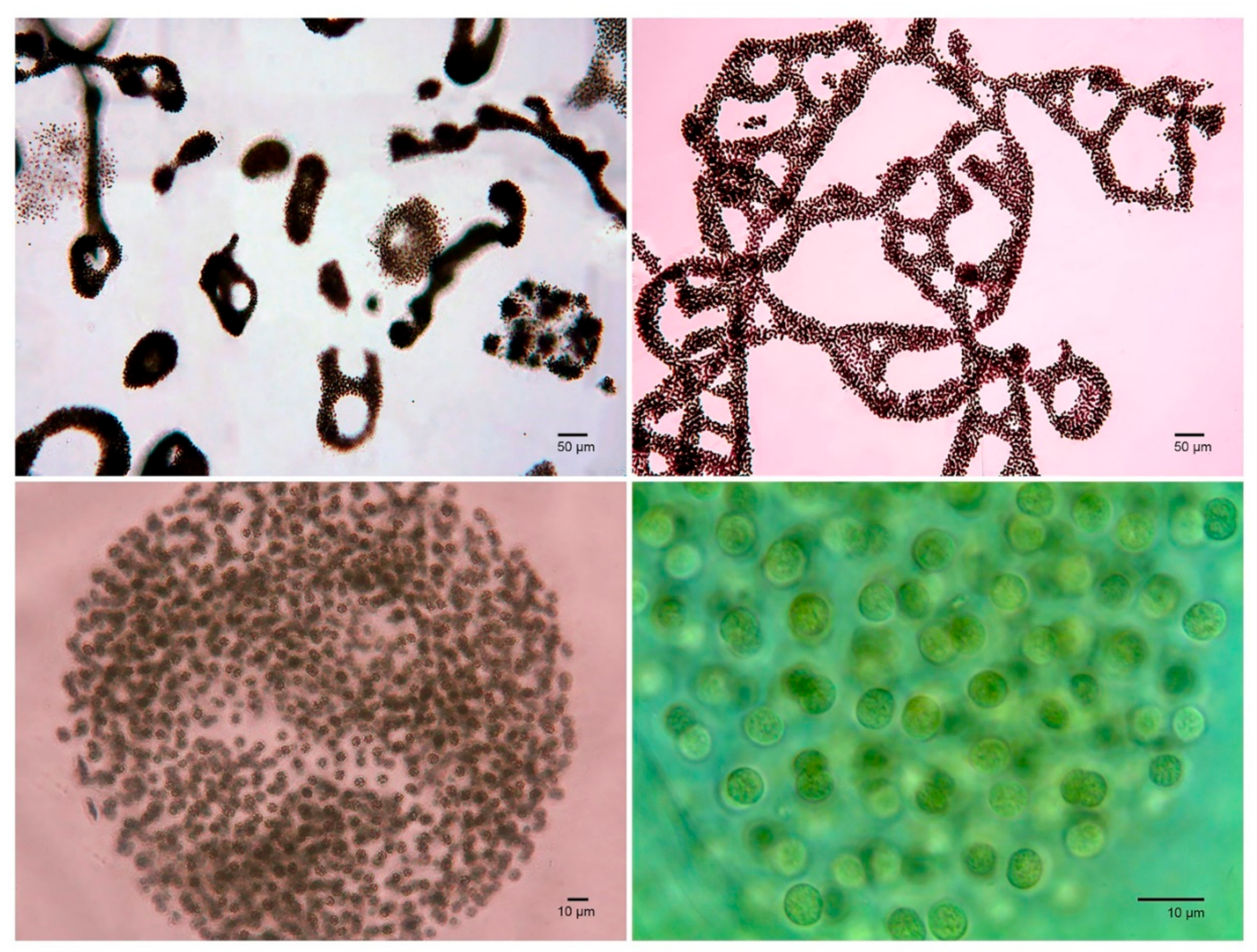


| Analyte | SRM Transitions | Cone, V | CE, eV |
|---|---|---|---|
| MC-RR | 519.9 > 134.9; 126.9; 102.8 | 30 | 30; 50; 70 |
| Nod | 825.5 > 135.1; 103.1 | 55 | 60; 100 |
| MC-LA | 910.1 > 135.1; 106.9 | 35 | 70; 80 |
| [Dha7]-MC-LR | 981.5 > 135.0; 106.8 | 75 | 75; 80 |
| Asp3-MC-LR | 981.5 > 134.9; 106.9 | 75 | 70; 80 |
| MC-LF | 986.5 > 213.0; 135.0 | 35 | 60; 65 |
| MC-LR | 995.6 > 135.0; 127.0 | 60 | 70; 90 |
| MC-LY | 1002.5 > 135.0; 106.9 | 40 | 70; 90 |
| MC-HilR | 1009.7 > 134.9; 126.9; 106.9 | 75 | 75; 90; 80 |
| MC-LW | 1025.5 > 134.9; 126.8 | 35 | 65; 90 |
| MC-YR | 1045.6 > 135.0; 126.9 | 75 | 75; 90 |
| MC-HtyR | 1059.6 > 134.9; 106.9 | 75 | 70; 90 |
| MC-WR | 1068.6 > 134.9; 106.9 | 80 | 75; 100 |
| Toxin | Mean (%) | SD |
|---|---|---|
| MC-WR | 39.9 | 7.65 |
| MC-LR | 38.2 | 4.25 |
| MC-LA | 16.5 | 4.25 |
| MC-HilR | 2.7 | 0.44 |
| MC-LF | 1.5 | 1.29 |
| MC-YR | 1.1 | 0.61 |
| MC-LY | 0.19 | 0.41 |
| Stations | L. robusta | M. aeruginosa |
|---|---|---|
| 1–2 | 4,504,067 | 550 |
| 2–3 | 4,987,405 | 133 |
| 3–4 | 299,032 | 18 |
| 4–5 | 3,962,304 | 20 |
| 5–6 | 5,428,251 | 214 |
| 6–7 | 43,129 | 3 |
| 7–8 | 4,206,629 | 356 |
| 8–9 | 50,936,326 | 5300 |
| 9–10 | 373,391 | 25 |
| 10–11 | 3,691,423 | 180 |
| 11–12 | 992,170 | 26 |
| 12–13 | 54,601,192 | 1700 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bustillos-Guzmán, J.J.; Turner, A.; Hernández-Almeida, O.U.; Band-Schmidt, C.J.; Romero-Bañuelos, C.A.; Hernández-Sandoval, F.E.; Núñez-Vázquez, E.J.; Palomino-Hermosillo, Y.A. Presence of Cyanotoxins in a Mexican Subtropical Monomictic Crater Lake. Appl. Sci. 2020, 10, 6719. https://doi.org/10.3390/app10196719
Bustillos-Guzmán JJ, Turner A, Hernández-Almeida OU, Band-Schmidt CJ, Romero-Bañuelos CA, Hernández-Sandoval FE, Núñez-Vázquez EJ, Palomino-Hermosillo YA. Presence of Cyanotoxins in a Mexican Subtropical Monomictic Crater Lake. Applied Sciences. 2020; 10(19):6719. https://doi.org/10.3390/app10196719
Chicago/Turabian StyleBustillos-Guzmán, José Jesús, Andrew Turner, Oscar Ubisha Hernández-Almeida, Christine Johanna Band-Schmidt, Carlos Alberto Romero-Bañuelos, Francisco Eduardo Hernández-Sandoval, Erick Julián Núñez-Vázquez, and Yolotzin Apatzingan Palomino-Hermosillo. 2020. "Presence of Cyanotoxins in a Mexican Subtropical Monomictic Crater Lake" Applied Sciences 10, no. 19: 6719. https://doi.org/10.3390/app10196719
APA StyleBustillos-Guzmán, J. J., Turner, A., Hernández-Almeida, O. U., Band-Schmidt, C. J., Romero-Bañuelos, C. A., Hernández-Sandoval, F. E., Núñez-Vázquez, E. J., & Palomino-Hermosillo, Y. A. (2020). Presence of Cyanotoxins in a Mexican Subtropical Monomictic Crater Lake. Applied Sciences, 10(19), 6719. https://doi.org/10.3390/app10196719







