Abstract
Loess is a highly porous and easily erosive aeolian sediment covering approximately 10% of the Earth’s surface. The weak vegetation cover and high wind speeds in many of these regions make loess sediment the main source of dust in the atmosphere. Dust particles deteriorate air quality and affect soils, crops, water systems, and animal and human health. The commonly used method for combating desertification is revegetation. However, planting various vascular plant species in loess landscapes did not show any long-lasting positive effects. This study aims to assess the potential of cyanobacterial strains for the restoration of exposed loess surfaces through the assisted development of biological loess crusts (BLCs). Isolated cyanobacterial loess strains were screened for the traits (toxicity, biomass and polysaccharide production) desirable for their use in restoration purposes. By simulating semi-arid environmental conditions in specially designed chambers, the potential of cyanobacterial loess strains for assisted development of BLCs and the mechanisms of loess stabilization have been evaluated by chlorophyll a accumulation and microscopic examination. It was confirmed that cyanobacteria have the ability to interact with loess particles resulting in BLC formation, which keeps the particles immobilized and the sediment below the particles stabilized.
1. Introduction
Loess is an aeolian sediment with a homogeneous and porous structure that is easily eroded by wind and water. It consists mainly of quartz particles (40–80%), but also includes feldspars, micas, calcite and dolomite in lesser amounts, and heavy minerals in varying amounts [1]. Loess sediments cover approximately 10% of Earth’s land surface [2] with a thickness from a few centimeters to hundreds of meters [2,3]. Most of the loess deposits were accumulated during the Pleistocene and it represents a valuable archive of climate and environmental changes from the past [4,5,6]. Despite the importance and wide distribution of loess sediments, their mode of formation is not yet fully understood [7], and no model of their origin is yet fully accepted. The definition of loess sediment based on aeolian origin neglects processes involved in the transformation of dust particles into the stable loess sediment [7]. A significant process in loess formation is loessification, which includes post-depositional processes responsible for the transformation of wind-blown silt to loess [8]. Recent hypothesis on the formation of loess (the BLOCDUST model: Biological loess crusts (BLCs) dust trapping model) emphasizes the role of BLCs and especially their cyanobacterial component in this process [9,10].
Loess sediments are mostly present in many semi-arid environments of inner Eurasia [3]. These dry environments are either threatened or already affected by desertification. According to the United Nations Convention to Combat Desertification (UNCCD), desertification represents “land degradation in drylands resulting from various factors, including climatic variations and human activities” [11]. Between 1982 and 2015, of 44.5 million km2 of the drylands, 6% experienced desertification due to unsustainable land use and anthropogenic climate change, while in 12.6% of drylands area anthropogenic climate change contributed to desertification [12]. According to Huang et al. [13] areas with desertification risk account for 29% of the global area and will increase in the future. Desertification leads to a reduction of vegetation cover [14], leading to reduced soil fertility, biodiversity, quality and quantity of freshwater supplies, crops, etc. According to the UNCCD, 24 billion tons of fertile soil is lost every year due to land degradation and desertification [15]. Minerals and organic material transported by dust affect terrestrial, as well as aquatic ecosystems [16]. As a result of inappropriate land use and military conflicts on the Loess Plateau, a high concentration of sediment ended up in the Yellow River and caused frequent floods [17]. The available literature points to desertification as a fairly common problem of loess landscapes and exposed loess surfaces. According to Sweeney and Mason [18], disturbed loess landscapes without vegetation cover are potentially major sources of dust. High wind speeds in such disturbed environments lead to an increase in wind-related erosive activities [19] (Figure 1A). The Loess Plateau in China, one of the most widespread and most eroded loess regions on Earth, provides an example of a degraded loess environment. From ~650,000 km2 of loess cover in Central China, ~470,000 km2 is erodible [20]. The Chinese Loess Plateau is characterized by a relatively scarce vegetation cover [21]. If higher biomass would be present, the vegetation could control erosion and water loss. Because of its current environmental state, 12.8% of the Plateau’s area experiences soil/sediment erosion greater than 10,000 t/km2/a [22]. It has been demonstrated that the high sediment concentration in the Yellow River (~16.4 billion tons of sediment annually) is a result of erosion from the sediments and soils on the Loess Plateau [21]. Dust particles can affect human and animal health either directly or by causing damage to the vegetation (Figure 1B), enhancing eutrophication of water systems (e.g., stimulating toxic algal blooms), or causing traffic accidents [23]. The dust causes mostly respiratory, ophthalmic, cardiac, and skin diseases [23]. Health consequences of desertification in loess regions have also been recognized. For more than a decade, a relationship between the distribution of loess/loess-like deposits and esophageal cancer has been adduced [24]. According to Jabbari et al. [25], long-term ingestion of silica particles leads to an increased risk of esophageal cancer.
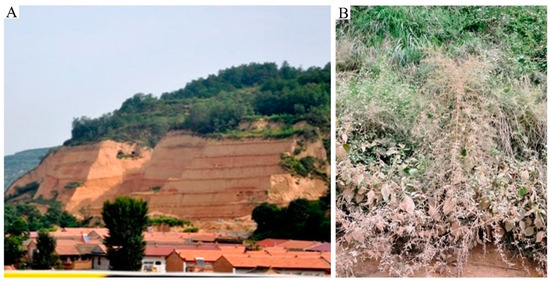
Figure 1.
Disturbed loess surfaces present a main source of dust. Disturbed vegetation cover exposes a large surface of loess landscape which acts as a source of dust (Luochuan Xiefeng, China) (A) and consequently causes damage to the vegetation cover (Luochuan Xiefeng, China) (B).
Considering the economic, social, environmental, and health problems caused by desertification, finding a natural and environment-friendly solution for the restoration of loess surfaces, as well as other desertified landscapes, is essential. So far, a commonly used method for combating desertification and dust storms and for the restoration of degraded land has been revegetation [26]. In 1999, the project ‘Grain for Green’ was established as the most substantial financial ecosystem investment, with a budget of 40 billion dollars, in order to prevent and control erosion on the Chinese Loess Plateau [27,28]. Although China had invested much effort and money in afforestation, there have been negative consequences to the environment as well, including a reduction in vegetation cover, loss of soil moisture [29], increase in soil erosion and above all, increased desertification rate [30] and continued sand storms [26]. According to Lamb et al. [31], one of the main reasons for failures in afforestation projects is the use of inappropriate plant species. Planting of trees or shrubs in areas where non-woody species present natural vegetation is not practical and leads to a decrease in water availability and loss of planted species or even native vegetation [26,29].
It is thus justified to find a long-term, environmentally friendly, and ecosystem-adapted solution, which recognizes native bioprocesses and uses native biota for the stabilization and restoration of loess landscapes. Considering the diversity of factors influencing desertification, there is no universal solution to combat desertification [32]. The appropriate solution depends on the local environmental (including water availability) and social conditions [33,34]. The crucial action in combating desertification is the restoration of appropriate vegetation and topsoil stability [35]. Even though the ‘Grain for Green’ project aimed to improve the state of the loess environment by planting trees and shrubs, an unexpected extensive development of biocrusts was observed, which later played a significant role in controlling soil erosion [28,36].
Biocrusts are bio-sedimentary complexes composed of organisms (bacteria, cyanobacteria, algae, fungi, lichens, and mosses) associated with the particles [37,38]. A special type of biocrust, BLCs, have been recognized to colonize loess sediment and can present significant biomass on the loess surfaces [3,9,39]. Biocrusts support many ecosystem functions by improving soil fertility and stability, reducing erosion by water and wind [40,41], increasing soil infiltration and delaying runoff [42] and increasing the abundance of soil fungi and bacteria [43]. Biocrusts influence primary ecosystem processes [44] and play a significant role in maintaining and improving the state of the environment [45]. Thus, they improve surface properties and provide an environment for numerous organisms [46]. Biocrusts are developing successively from cyanobacteria-dominated biocrusts to biocrusts dominated by lichens and mosses [47]. However, the process of natural succession could be long-lasting [48,49]. The study conducted by Hu and Liu [48] showed that the first community-building species in the biocrust were dominant even after 42 years. The full development of mature biocrusts can last even hundreds of years [50].
Assisted development of biocrusts and acceleration of their succession is considered as a potential solution for loess restoration. Many studies confirmed the role of cyanobacteria in sand stabilization through the artificial formation of biocrusts e.g., [46,51,52,53,54,55,56,57,58,59]. Regarding loess surfaces and biocrust organisms it has been shown that using a natural moss-dominated biocrust inoculum obtained from the Loess Plateau in China, the bare loess surface was covered entirely after 10 months in the laboratory conditions [42]. In the study of Zhao et al. [60], the inoculation of native moss and moss-lichen crusts from the Loess Plateau resulted in the coverage of more than 65% of the bare loess surface after 10 weeks in laboratory conditions. However, the full development of moss-dominated biocrusts in field conditions was accomplished after four years [61]. On the other hand, it has been shown that the successful cultivation of cyanobacteria-dominated biocrusts under appropriate environmental conditions may require a few weeks only [46,52]. Cyanobacteria are primary colonizers, soil stabilizers, and nitrogen fixers within biocrusts [62]. The significance of cyanobacteria in the biological soil crusts (BSCs) is reflected in their ability to survive and grow in harsh environmental conditions which is made possible by their specific traits (migration capacity, ability to survive desiccation and extreme temperatures, production of the ultraviolet (UV)-protective pigments, and production of exopolysaccharides (EPSs)) [54,63,64,65].
EPSs produced by cyanobacteria form a protective layer between the cell and the environment [66]. They also bind soil particles [51] and make nutrients and water, two commonly limiting factors in drylands, available for the biocrust community [67,68]. Cyanobacteria produce three types of EPSs: sheaths, capsules, and slimes. Capsules and slimes release polysaccharides into the cultivation medium thus forming released exopolysaccharides (RPSs) [66]. Recent hypothesis on the partly biogenic origin of loess (BLOCDUST) emphasizes the role of cyanobacteria in loess formation. The model establishes the role of the EPSs in this context and discusses the necessary morphological and ecophysiological features of cyanobacteria [9,10].
The main aim of this manuscript was to examine the potential of cyanobacteria for the restoration of the loess surfaces by initiating biocrust development. The experiments studied the cyanobacterial traits necessary for the successful and sustainable establishment of the BLCs, i.e., non-toxicity and high production of biomass and polysaccharides. Furthermore, the experiments explored the interaction of the cyanobacteria/polysaccharides with airborne particles in the formation of loess. The study also identified processes and mechanisms important for the stabilization of loess particles in their interaction with cyanobacterial colonies.
2. Materials and Methods
2.1. Screening of Cyanobacterial Strains for Toxin, Biomass and Polysaccharide Production
2.1.1. Isolation and Cultivation of Cyanobacterial Strains
Five cyanobacterial strains were selected among those isolated from the BLCs collected at the Ruma loess section (N 45°00′43.8″, E 19°51′28.8″) in 2006. BLCs were collected together with approximately 10 cm of sediment below them using a spatula, packed in storage bags, and transported to the laboratory where they were further used to isolate cyanobacterial strains. 1 cm2 of cyanobacterial BLCs were inoculated in the BG11 N− (BG11 without NaNO3) and BG11 N+ (BG11 supplemented with 1.5 g/L of NaNO3) media. Upon the initial cultivation in the liquid media, strains were isolated through cultivation on a solid (supplemented with 1.5% agar) and liquid BG11 media [69]. Separation of strains was done manually under the stereomicroscope (Motic, SMZ-168) by separating and transferring singular colonies and/or filaments from the solid to the liquid medium. Isolation and separation processes were repeated until the monocultures of cyanobacteria were obtained. Strain determination to the genus level was performed on an Olympus BX 51 microscope with the support of recognized determination keys for cyanobacterial species [70,71,72].
Isolated cyanobacterial strains were cultivated in triplicate in batch cultures using the BG11 N+ and the BG11 N− medium and 1 mL inoculum per 100 mL medium. The strains were cultivated under room temperature, without mechanical shaking but shaken manually on a daily basis and illuminated for 14 h a day. White cool fluorescent tubes provided a light intensity of 720 lux.
2.1.2. Toxicity Assessment
Total toxicity (cell-bound and extracellular toxicity) of isolated cyanobacterial strains was assessed using the Artemia salina toxicity test. Toxicity was evaluated in two cultivation periods, after seven and 16 weeks of cultivation. The release of the cell contents was ensured through freezing and thawing of 10 mL of each culture five times and by sonication (Bransonic 12). Thereafter, extracts containing extracellular and released intracellular content were centrifuged (Tehtnica, Železniki, Slovenia) at 1150× g for 10 min and supernatants were used for toxicity assessment. Toxicity of the five cyanobacterial strains, each cultivated in the BG11 N+ and BG11 N− media, was assessed using the A. salina larvae according to Kiviranta et al. [73]. The toxicities of the strains were expressed as the percentage of mortality, by subtracting the mortality in control samples from mortality caused by the cyanobacterial sample. Strains causing the mortality of <50% larvae were regarded as non-toxic or low-toxic, while medium or high toxicity was assigned to the strains that caused mortality of ≥50% e.g., [74]).
2.1.3. Quantification of Biomass Production
Biomass production of the strains was expressed through the concentration of chlorophyll a (Chl a), measured spectrophotometrically (SPECTROstar Nano, BMG LABTECH GmbH, Ortenberg, Germany) at seven-day intervals for seven weeks. The Chl a concentrations were measured according to the Mackinney [75], while the corresponding biomasses were calculated indirectly according to the APHA method [76]. Samples for determination of Chl a concentration were prepared in the following way: 10 mL of each sample was filtered through the filter paper (Filtratech QL05, Saran, France), extracted in dark at 4 °C for 24 h in glass tubes containing 5 mL of 100% methanol. The samples were further sonicated for 10 min in an ultrasonic bath (Bransonic 12) and centrifuged (Tehtnica, Železniki, Slovenia) at 1150× g for 10 min. The supernatant was separated and analyzed spectrophotometrically at 663 nm. 100% methanol was used as a blank.
2.1.4. Polysaccharide Production
The production of polysaccharides was examined spectrophotometrically (SPECTROstar Nano) by quantifying the released polysaccharide (RPS) and total polysaccharide (intracellular, RPS, and cell-bound (sheath, capsule and slime, CPS)) content in seven-week-old cultures. Polysaccharide production was analyzed by the phenol-sulfuric method for quantification of carbohydrates [77], adapted for measurement in microtiter plates [78] and using glucose as a standard. RPSs were separated from the capsular exopolysaccharides and intact cells by centrifugation (Eppendorf 5810 R) of cultures at 8150× g for 15 min at 19 °C. The supernatant was separated and the RPSs were precipitated by the gradual addition of cold 96% ethanol in a 1:3 (v/v) ratio. After 18 h of precipitation, RPSs were sedimented by centrifugation at 8150× g for 10 min. The residual ethanol was removed by evaporation at 37 °C and the precipitates were dissolved in sterile distilled water. Total polysaccharides were quantified after freezing and thawing the strain cultures five times. The cultures were further centrifuged, precipitated, and processed in the same manner as RPSs. The samples were kept in a freezer until quantification.
2.1.5. Viscosity of the Medium after Cultivation
The viscosity of the cultivation medium of seven weeks old cultures, after cell removal by centrifugation (Eppendorf 5810 R) at 8150× g for 15 min, was determined using a rotational viscometer Rheotest 2 RV-2 (RHEOTEST Medingen GmbH, Otendorf-Okrila, Germany) with a double gap coaxial cylinder sensor system, spindle N. Based on the deflection of the measuring instrument (α, Skt), shear stress (τ, Pa) was calculated under defined values of shear rates (D, 1/s) using the Equation (1):
where z is the constant with a value of 3.08 dyn/cm2·Skt. The data obtained for each sample was fitted using the Ostwald-de-Waelle (power law) model in Microsoft® Excel 2010 software (MS Office, Microsoft Corporation, Philadelphia, PA, USA) in order to determine the consistency factor (K, Pa·sn) and flow behavior index (n). The goodness of fit was evaluated by the coefficient of determination which had a value higher than 0.940. Obtained values of rheological parameters were used for the calculation of apparent viscosity (ηa, mPa·s) from Equation (2):
where D is the shear rate with a value of 10 1/s.
τ = 0.1·z·α,
ηa = K·Dn−1,
2.1.6. Statistical Analysis
All measurements were carried out in triplicate and the results were averaged. The experimental data of biomass and polysaccharide production and cell-free medium apparent viscosity were processed by two-way analysis of variance (two-way ANOVA) at the significance level of α = 0.05 using Statistica 13.5 software (TIBCO Software Inc., Palo Alto, CA, USA).
2.2. Ex Situ Particle Trapping and Stabilization Study
The potential of isolated cyanobacterial strains to trap and stabilize airborne dust particles was examined in specially designed rectangular glass chambers with a surface of 3.5 m2 each. The chambers comprised two sections. The front section for the “dust storm” generation was equipped with a fan that simulates a wind speed of 12 m/s and had an opening for the insertion of loess particles on the top. The fan directed the particles towards the back section, which was used to study the biocrust development, particle trapping and the stabilization mechanism of cyanobacterial biocrusts. The back section was equipped with fluorescent tubes to provide irradiation ranging from 560 to 700 lux at different points.
A mixed cyanobacterial culture comprising isolates from the BLCs collected at the Ruma loess section (N 45°00′43.8″, E 19°51′28.8″) was inoculated over the glass surface of the back section in one chamber (hereinafter referred to as experimental chamber). Another chamber was used as a control, where cyanobacteria were not inoculated (hereinafter referred to as the control chamber). Upon inoculation, the cyanobacterial biofilm was developing in the following manner: it was watered with approximately 250 mL of distilled water five days a week and with the same volume of BG11 medium (0.9 g/L NaNO3) once a month, shifts of wet and dry seasons were performed by desiccation once a week. After the cyanobacterial biofilm had developed in such a way that it completely covered the back section of the experimental chamber, dust storms were simulated in both chambers in order to examine the model of particle trapping and stabilization ex situ. The experiment lasted 10 months and during that time 37 dust storms were simulated. Dust storms were simulated every third week, using 400 g of fine loess particles, one hour after watering. Loess sediment used in this study was collected at the Titel Loess Plateau, and stored in a dark and dry place until use. Prior to application in the chambers, the sediment was disaggregated in a grinder to provide a dust-like application material. During the ex situ study, the back sections of both chambers were watered and desiccated in the same manner as the biofilm during its development. This amount of water proved to be optimal for the growth of cyanobacteria and did not contribute to an excess of water on the test surface in the control chamber. By subtracting the amount of sediment remaining in the front section of each chamber from the amount of the loess sediment used for dust storm generation, the sediment quantities that were blown into the back section of the experimental chamber (3225 g) and the control chamber (2850 g) were calculated. Sampling was performed after 10 months of biocrust growth under the described conditions and the biocrusts development, dust particle trapping, and stabilization were examined.
2.2.1. Microscopic Examination of Developed Biocrusts
The development of biocrusts, and the dust particle trapping and stabilization mechanism were visually examined under a binocular stereo microscope (Leica M205 C) and scanning electron microscope (SEM, Jeol JSM-6610LV). The stereo microscope examinations were conducted on biocrusts in the dry and wet states. The stereo microscope images were taken using a digital camera (Leica DFC290 HD) and software LAS, version V4.11. To examine the biocrust structure under SEM, the samples were coated with gold to a thickness of 15 nm and a density of 19.32 g/cm3, optimization was undertaken using copper. The additional conditions were the following: the source of electrons: W wire; acceleration voltage of electrons: 20 kV; recording under ultra-high vacuum conditions: 10−9 Pa.
2.2.2. Chl a Analysis
The analyses of Chl a in the developed biocrusts were performed on 12 random samples from each chamber during their wet and dry state. The final concentration represents a mean value of 12 samples in each state. Using the methodology by Lan et al. [54], approximately 1 cm2 of biocrusts were ground in 10 mL of 100% acetone with mortar and pestle in triplicates. After extraction for 18 h at 4 °C, the samples were centrifuged (Tehtnica, Železniki, Slovenia) for 10 min at 1150× g. The supernatant was separated, and the absorbances at 663 nm, 384 nm, and 490 nm were measured spectrophotometrically (SPECTROstar Nano). The contents of the Chl a were quantified according to Garcia-Pichel and Castenholz [79] and Garcia-Pichel et al. [80], using Equation (3).
Chl a = (1.02 A663 − 0.027 A384 − 0.01 A490)/CChl a * V/S,
Chl a represents the content of Chl a expressed in mg/m2; CChl a is the extinction coefficient of Chl a (L g−1 cm−1), and its value is 92.5; A663, A490 and A384 are the absorbances of extracts at 663 nm, 490 nm and 384 nm; V is the extract volume; S is the area of the crust sample.
3. Results
3.1. Screening of Cyanobacterial Strains for Toxin, Biomass and Polysaccharide Production
3.1.1. Toxicity Assessment
None of the tested strains caused high mortality (>50%) of brine shrimp larvae after 24 and 48 h (Table 1). The highest mortality was expressed by a 16-week old culture (Tolypothrix sp. L4) after 48 h of exposure. By causing the death of only 10.2% and 16.1% of larvae in BG11 N+ and BG11 N-, respectively, this strain together with the other strains could be regarded as low-toxic or non-toxic. High standard deviations were observed in some samples (Table 1) but the overall toxicity related to strains L1-L6 was low and the high standard deviations were mainly caused by single outliers.

Table 1.
Toxicity of isolated cyanobacterial loess strains. Artemia salina bioassay results on total toxicity of seven and 16 weeks old cultures grown in BG11 N+ and BG11 N− media. Microcystis PCC7806 from the Pasteur Culture Collection of Cyanobacteria was used as a reference strain for the toxicity assessment as it is a known producer of microcystin. The strain was grown in a modified BG11 N+/− medium (0.9 g/L NaNO3).
3.1.2. Quantification of Biomass Production
The biomass production and the effects of nitrate presence in the culture medium are summarized in Table 2. The highest biomass (501.4 ± 25.6 mg/L) in the medium BG11 N+ was produced by the strain Nostoc sp. L5. The strain Tolypothrix sp. L4 produced the highest biomass in medium BG11 N− (89.2 ± 6.0 mg/L). Regarding the effects of nitrate on biomass production, four tested strains (Chroococcidiopsis sp. L1, Nostoc sp. L2, Tolypothrix sp. L4, Nostoc sp. L5) were observed to have a higher biomass production when cultivated in the medium supplemented with nitrate, while one strain (Nostoc sp. L6) showed higher biomass production under diazotrophic cultivation conditions. The continuous growth of all strains throughout the cultivation period of seven weeks was observed in the strains cultivated in the medium BG11 N+. However, three cyanobacterial strains (Chroococcidiopsis sp. L1, Nostoc sp. L5, Nostoc sp. L6, each in the 4th, 5th and 6th weeks of cultivation, respectively) reached the highest cell density quicker when cultivated under diazotrophic conditions. A gradual decrease in biomass production followed afterward.

Table 2.
Biomass production (mg/L) by isolated cyanobacterial strains. The biomass production by the strains cultivated in BG11 N+ and BG11 N− media was measured at seven-day intervals. The highest biomass produced by each strain in the appropriate medium is marked with boldface.
3.1.3. Polysaccharide Production
The production of RPSs and total polysaccharides by isolated strains is summarized in Table 3. The production of RPSs and total polysaccharides was proportional in both growth media. The highest observed amounts per liter of RPSs and total polysaccharides were produced by Tolypothrix sp. L4 strain cultivated in the BG11 N+ medium (2.7 ± 0.1 mg/L and 7.1 ± 0.6 mg/L, respectively). The highest amounts of RPSs and total polysaccharides among strains cultivated in the BG11 N− medium was observed in the Nostoc L6 strain (1.6 ± 0.1 mg/L and 3.7 ± 0.2 mg/L, respectively).

Table 3.
Production of exopolysaccharides (RPSs) and total polysaccharides by isolated cyanobacterial strains. The polysaccharide content of strains in the 7th week of cultivation in BG11 N+ and BG11 N− media is presented.
The presence of nitrate in the cultivation media had an effect on polysaccharide production. The potential of isolated strains to release polysaccharides into their surrounding was estimated by the share of RPSs in the total amount of polysaccharides (Table 3, RPS share). Four of five strains (Chroococcidiopsis sp. L1, Nostoc sp. L2, Tolypothrix sp. L4, Nostoc sp. L5) showed the ability to excrete a higher portion of polysaccharides when grown in the BG11 N+ medium. The potential of these strains for polysaccharide production was assessed by calculating the specific polysaccharide production in each strain. The production of RPSs and total polysaccharides were correlated with their biomass (Table 3, total polysaccharide and RPS yield). Strain Chroococcidiopsis sp. L1 showed the highest total polysaccharide and RPS yield when cultivated in diazotrophic conditions by producing 196.6 mg/g biomass and 77.4 mg/g biomass, respectively. However, such yields are ‘falsely’ high since at the time of polysaccharide analyses (7th week of cultivation) a drastic decrease of biomass production by the strain Chroococcidiopsis sp. L1 was observed. If the observations of the yields of strain L1 in the medium BG11 N− are excluded, the two highest RPS and total polysaccharide yields were obtained by the same strain, Nostoc sp. L6, in the medium with N source (61.0 mg/g biomass and 145.6 mg/g biomass, RPS and total polysaccharide yield, respectively) and without N supply (33.7 mg/g biomass and 75.9 mg/g biomass, respectively). The results of the total polysaccharide yield indicated that the presence of N source stimulated the production of polysaccharides in Tolypothrix sp. L4 and Nostoc sp. L6, while in Nostoc sp. L2 and Nostoc sp. L5 cultivation conditions without added N source stimulated the production of polysaccharides. Nitrate presence positively influenced the RPS production of strains Nostoc sp. L2, Tolypothrix sp. L4, Nostoc sp. L6, contrary to strain Nostoc sp. L5 where N deficiency stimulated the RPS production.
3.1.4. Viscosity of the Cell-Free Medium
The higher viscosity of the cell-free cultivation medium was observed in three out of five strains when cultivated in the medium BG11 N− (Figure 2). In contrast, higher viscosity of the cell-free BG11 N+ medium of the Chroococcidiopsis sp. L1 strain was observed (12.01 mPa·s). This value also represents the highest viscosity measured in this study. The viscosity of Tolypothrix sp. L4 cultures was similar in both cultivation media.
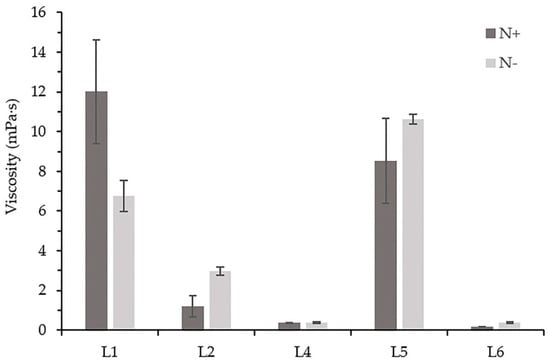
Figure 2.
The viscosity of cell-free media of isolated strains in the 7th week of cultivation. The error bars indicate standard deviation.
3.1.5. Statistical Analyses
Using two-way ANOVA at the significance level of α = 0.05, the results related to biomass, carbohydrate production, and apparent viscosity of the cell-free media were analyzed. The results are presented in Table 4. A statistically significant effect (p < 0.05) of both variables (strain and medium composition) and their interaction on the production of biomass and polysaccharides was observed. The mean square (MS) results suggest that the medium composition had the highest effect on biomass production and the production of both RPSs and total polysaccharides. The medium composition did not express a statistically significant effect (p > 0.05) on the viscosity of the cell-free medium of the analyzed strains. Interaction of applied variables, medium composition and strains, as well as strain as single variable expressed a statistically significant effect (p < 0.05) on viscosity. Considering the MS values, cyanobacterial strains expressed the highest effect on viscosity of cell-free cultivation medium.

Table 4.
The results of statistical analyses (SS—sum of squares; DF—degrees of freedom; MS—mean square; F-Value—Fisher value; p-Value—probability value). Two-way analysis of variance (ANOVA) results showing the effect of isolated cyanobacterial strains and medium composition on analyzed parameters.
3.2. Ex Situ Particle Trapping and Stabilization Study
3.2.1. Development and Morphology of Biocrusts
During the simulated dust storms, airborne particles were inserted among the cyanobacterial colonies. This community, composed of organisms (inoculated cyanobacteria (Figure 3A)) and particles, formed hardened and crisp biocrust structures during the dry stage. The biocrusts formed were morphologically changing during the cultivation. Patchy biocrusts with flaky and curled features (Figure 3B, arrow 1) covered most of the surface in the experimental chamber. The non-colonized parts were later occupied by smooth biocrusts (Figure 3B, arrow 2).
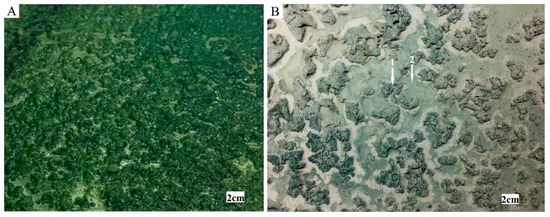
Figure 3.
Formation of biocrusts. The interaction between the cyanobacterial biofilm (A) and particles resulted in biocrust development (B). A patchy structure was formed by pinnacled and curled biocrusts (B, arrow 1). Smooth biocrusts appeared between the patches later (B, arrow 2).
As explained in the preceding paragraph and Figure 3, different growth patterns of cyanobacterial colonies were observed. The results of visual observations were the following growth patterns (Figure 4): panel A shows areas of smooth biocrusts, panel B shows an example of intercalated morphotypes consisting of curled, pinnacled and smooth structures, and panel C shows tentacle-like protrusions made of cyanobacterial filaments.
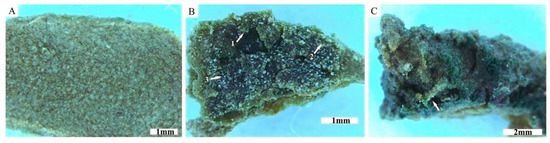
Figure 4.
Morphology of formed biocrusts. Morphologically different colonies covered the surface of the experimental chamber: smooth biocrusts (A); biocrusts consisting of pinnacled (B, arrow 1), curled (B, arrow 2) and smooth structures (B, arrow 3); pinnacled biocrusts (C, arrow).
Biocrusts changed their appearance upon water availability. The volume and the area of the biocrust/biofilm increased after wetting (Figure 5). During dry periods the volume of biocrusts shrank, the colour became more bleached and the surface became discontinuous over the sediment (Figure 5A). Upon wetting an increase in the biovolume and a transformation of the structure from discontinuous (Figure 5A) to continuous (Figure 5B) were observed. The discontinuous appearance (Figure 5A) became continuous, thicker, and dark-green coloured upon wetting (Figure 5B).
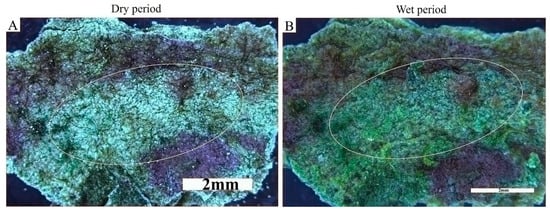
Figure 5.
The response of biocrusts to hydration. The appearance of biocrusts is different under dry conditions (A) and 15 min after wetting (B). The watering changed the appearance of the less colonized parts of the sediment (A) into a dark, continuous biofilm (B).
3.2.2. Stabilization of Loess Particles by Cyanobacteria
The SEM micrographs of biocrusts formed in the experimental chamber (Figure 6A,B) showed assemblies of various cyanobacterial colonies and their excreted polymeric substances. Superficial colonies of cyanobacteria immobilized dust particles by surrounding, binding and adhering the particles to this coherent organic layer. In this way, the dust particles that encountered the cyanobacterial biomass were stuck to the surface (Figure 6A,B, arrows), unlike the particles from the control chamber that did not interact with cyanobacteria (Figure 6C,D, arrows).
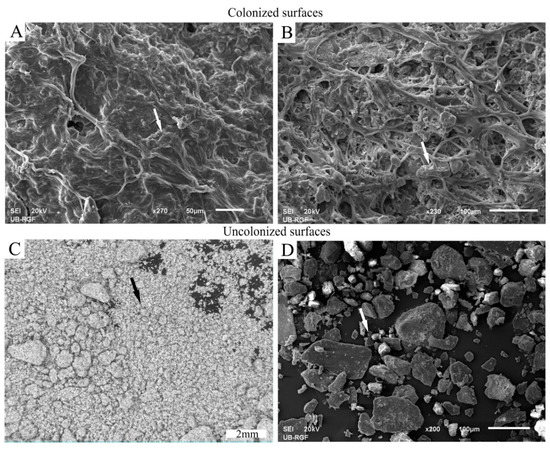
Figure 6.
Difference between colonized and uncolonized surfaces of experimental chamber (A,B) and control chamber (C,D), respectively. The scanning electron microscopy (SEM) micrographs show a cyanobacterial carpet-like structure (A) and a cyanobacterial network (B) adhering the airborne dust particles in the experimental chamber (arrows); the stereomicroscopic image (C) and the SEM micrograph (D) show loosely aggregated dust particles in the control chamber (arrows).
The accumulation and stabilization of dust particles were most visible in cross-sections of the biocrusts (Figure 7). Stereomicroscopic examination revealed a vertical stratification of biocrusts and the presence of topcrust and subcrust layers. The topcrust comprised layers of living cyanobacteria colonies associated with dust particles, where the trapping (Figure 7, arrow 1) and accumulation (Figure 7, arrow 2) of new airborne material take place. The subcrust was represented by old topcrust layers and consisted of accumulated and stabilized loess particles and residual, partially mineralized EPS (Figure 7, arrow 3). The layering and particle accumulation and stabilization are the consequence of the cyanobacterial life-style. During simulated dust storms, metabolically active cyanobacteria of the topcrust layer were trapping dust particles and their subsequent burial by the particles led to the migration of these cyanobacteria towards the light source resulting in the accumulation of trapped particles in their organic layer. Growing upwards, cyanobacteria formed a new topcrust, while the “old” EPSs remained in deeper layers (see Results section Accumulation and Stabilization of Particles) which together with accumulated particles made a stabilized subcrust layer of deposited particles.

Figure 7.
Cross-section of a biocrust sample. There were three layers within the biocrust: one showing trapping particles by superficial cyanobacterial colonies (arrow 1), another showing particle accumulation in the cyanobacterial organic layer (arrow 2), and a third one showing deposition of particles below the cyanobacterial biofilm as a result of the migration of cyanobacteria to the upper layers (arrow 3).
Trapping of Particles
The capturing and trapping of blown (or airborne) dust particles occurred during the simulated wet periods by a coherent cyanobacterial cover. Three types of particle trapping were observed, related to the morphology of the cyanobacterial colonies: 1. net-like trapping (Figure 8A,D); 2. carpet-like trapping (Figure 8B,E); and 3. trapping by pinnacles (Figure 8C,F).
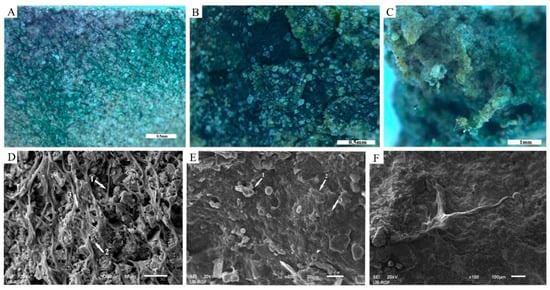
Figure 8.
Trapping of particles. Three types of particle trapping were observed: net-like trapping (A,D), carpet-like trapping (B,E) and trapping by pinnacles (C,F). Cyanobacterial network connected the particles (D, arrow 1), trapped fine particles on the exopolysaccharides (EPSs) surface (D, arrow 2) and made pores (D, arrow 3). Dense, carpet-like, cyanobacterial cover (E, arrow 3) adhered particles onto the surface of polysaccharide matrix (E, arrow 1) and grew over the overlapping particles (E, arrow 2).
Net-like trapping: the net-like trapping (Figure 8A,D) was promoted by an extensive network of cyanobacterial filaments and the EPSs that covered, surrounded, and connected the particles (Figure 8D, arrow 1). Trapping of fine particles was also observed at the top of the EPS network (Figure 8D, arrow 2). The network pores (Figure 8D, arrow 3) further supported the trapping of particles.
Carpet-like trapping: cyanobacterial colonies (Figure 8E, exemplified by arrow 3) and mostly their slime EPSs made a homogenous, amorphous surface, carpet-like biological cover by which particles were trapped (Figure 8B,E). Adhered particles were observed on the surface of the carpet-like biocover (Figure 8E, arrow 1). Wrapping of the individual particles by cyanobacterial filaments was not observed. Instead, amorphous mucilaginous cyanobacterial colonies grew above the overlapping particles. The contours of the trapped particles are visible in Figure 8E, arrow 2.
Trapping by pinnacles: vertical structures were identified in addition to the horizontal biological covers. Biocrusts that increased their own surface by joining filaments into bundles and growing upwards were observed (Figure 8C,F). The surface for trapping the particles was consequently also increased. Bundles of filaments and their sticky EPSs formed vertical bio-mineral aggregates (Figure 8C,F).
Accumulation and Stabilization of Particles
The growth of cyanobacteria above the trapped particles led to their accumulation in a lower part of the topcrust layer (Figure 7, arrow 2). Cyanobacterial cover preserved and stabilized the particles within the active topcrust layer and served as a protective cover for the particles in the subcrust layer. The weathering of loess particles was observed in the contact zone between EPS and the particles (Figure 9A–C). The weathering led to cracking and breakage of some particles (Figure 9A, black arrows). Subsequent mineral precipitation occurred on the surface of the EPS (Figure 9A, thin white arrows), leading to the formation of organomineral structures in and around the contact zone of the EPS and particles (Figure 9A, thick white arrows; Figure 9B, arrows), which could improve the stabilization of the particles during dry periods. As shown in Figure 9C, the organomineral structures strengthen the bond between the EPS and the particles and thus could contribute to particle stabilization. Mineralized residues of EPSs were also observed in the subcrust layer (Figure 9D).
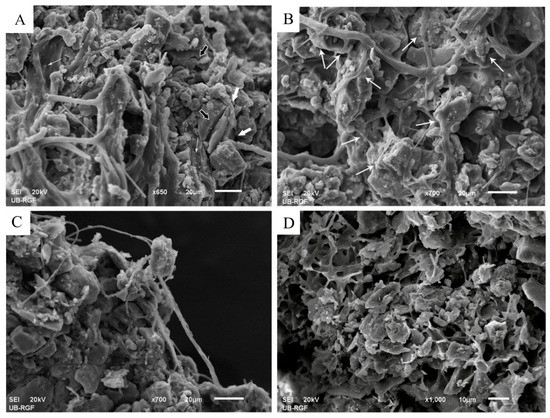
Figure 9.
Representative SEM images showing stabilization of loess particles through organomineral structures. The weathering of the particles promoted by the EPS took place in and around the contact zone of the EPS and the particles (A,B). The weathering led to the breakage of some particles (A, black arrows). The precipitation of minerals on the EPS surface (A, thin white arrows). Organomineral structures that build the mineral bond between the EPS and the particles (A, thick white arrows, and B, arrows). Although the particle has separated from the aggregate, it is still firmly attached to the tubular EPS, which is ensured by organomineral structures (C). The complex of mineralized EPS residues and weathered particles observed in the subcrust (D).
3.2.3. Chlorophyll a in the Biocrusts
The presence of Chl a in chambers examined during a wet and a dry period is presented in Figure 10. Chl a was identified only in the chamber inoculated with cyanobacteria. As shown in Figure 10, the concentration of pigment differed between the wet and dry samples in the experimental chamber, with the higher concentrations observed in the “wet” crust samples. The response of the formed biocrusts to wetting is a rapid metabolic reactivation, which is here represented as an increase in Chl a concentration.
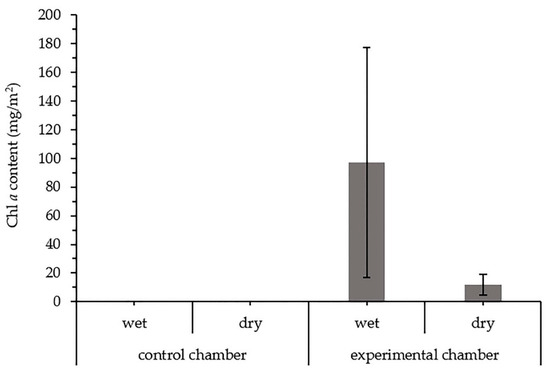
Figure 10.
Chlorophyll a content in the biocrusts (mg/m2). The pigment was analyzed in a wet and a dry state of biocrusts. The error bars indicate standard deviation.
4. Discussion
4.1. Production of Toxin, Biomass and Polysaccharide by the Cyanobacterial Strains
4.1.1. Toxicity
None of the tested cyanobacterial strains caused mortality >50% of brine shrimp larvae. A slightly higher percentage of the death of brine shrimp larvae was observed after 48 h exposure to the 16-week-old culture of Tolypothrix sp. L4, but mortality was still below 50%.
Cyanotoxins represent a large group of cyanobacterial secondary metabolites with an adverse impact on human and animal health as well as on plants [81,82,83,84]. The potential role of microcystins in carcinogenesis is also recognized [85,86,87,88]. Considering that cyanobacteria may also produce currently unknown toxins [89], we applied a biological test of toxicity to provide an estimation of overall toxicity. Biological tests are generally simple and inexpensive methods [90]. The biotests can tentatively identify strains with no or low toxicity, making these strains candidates for further studies in the restoration context. Additional analyses (e.g., genetic analyses, liquid chromatography-mass spectrometry of cyanotoxins) of these strains are recommended if toxicity is indicated by biotests. Although the strain Tolypothrix sp. L4 caused mortality below 50%, it would be advisable to test its toxicity using some additional methods.
4.1.2. Production of Biomass
Statistical analyses indicated a positive correlation between biomass production and the presence of nitrate as a source of nitrogen (Table 4). Most of the analyzed strains (four out of five) showed better growth when cultivated in a medium supplied with NaNO3 (Table 2). The only strain that showed a higher biomass production in BG11 N− medium was Nostoc sp. L6. These results are consistent with previous studies [91,92] where biomass production was increased with higher NaNO3 content in the culture medium. Higher intensity and longer illumination times e.g., [92,93,94], as well as the addition of organic carbon to the medium [94], also increase biomass production.
The absence of NaNO3 in the culture medium resulted in some strains (Chroococcidiopsis sp. L1, Nostoc sp. L5, and Nostoc sp. L6) reaching the stationary phase earlier than when cultivated in the N-containing medium (Table 2). The decrease in biomass after seven weeks of cultivation was evident in all three strains, of which Chroococcidiopsis sp. L1 strain showed the most drastic decrease. Although some Chroococcidiopsis strains can fix N2, special conditions are required, including low light density and reduced O2 tension [95,96], which were not met in this study.
4.1.3. Production of Polysaccharides
The presence of nitrogen can influence the EPS production summarized in [65,97], which does not necessarily follow the pattern of biomass production, as observed in this study (Table 3). The lowest yield of total polysaccharides was observed in Nostoc sp. L5 with the highest biomass production after seven weeks of cultivation in BG11 N+ medium. The highest yield of total polysaccharides was observed in Chroococcidiopsis sp. L1 strain cultivated in BG11 N− medium. Nevertheless, even in the stationary phase, the strain had one of the lowest observed biomass (Table 2). A few studies have shown that EPS production is enhanced upon entering the stationary growth phase [66,98,99], which could explain the higher total polysaccharide yield by our strain. In addition, Chroococcidiopsis strains can produce larger amounts of capsular polysaccharides when exposed to nitrogen deficiency [95], to create microaerobic conditions and protect the enzyme nitrogenase from being inactivated by O2 [96]. Statistical analyses (Table 4) further verified that the medium composition has an influence on the polysaccharide production in the analyzed strains.
For most of the strains, the RPS yield was in a positive correlation with the total polysaccharide yield, which indicates the importance of N in RPS synthesis. The RPS yields observed in this study (Table 3) are generally in agreement with the values observed by Richert et al. [100], even though their strains were cultivated under different cultivation conditions: permanent light, higher temperatures, aeration, and mechanical agitation. Numerous studies showed that permanent light duration and intensity, higher temperatures and aeration could significantly enhance the production of RPSs [98,101,102,103], thus further optimization of cultivation conditions could result in a higher RPS yield than observed in this study.
The production of RPS in cultures causes a progressive increase in medium viscosity [66]. The viscosity of the cell-free medium examined in this study did not show a strict positive correlation with the yield of RPSs produced. The highest viscosity was observed in BG11 N+ medium of the strain Chroococcidiopsis sp. L1, although the yield of RPSs produced by this strain was significantly higher when cultivated in BG11 N− medium. The high viscosity of the cell-free medium of another strain, Nostoc sp. L5, was observed, although the strain had the lowest yield of RPSs among all examined strains. In contrast, the lowest viscosity of the cell-free medium was measured in the strain Nostoc sp. L6 cultivated in BG11 N+ medium, which had the second-highest RPS yield. The observed results suggest that it may not be the amount of RPSs produced that play a critical role in a medium viscosity, but their structure. Whistler and Daniel [104] showed that polysaccharides of higher molecular weights and of the more linear structure have a higher viscosity, and cyanobacteria are known to produce exopolysaccharides of high molecular weights [97]. Viscosity is also affected by the presence of charged groups and the presence of protein moieties in the RPS structure [66]. Thus, even though the RPS yield was lower, the quality of RPSs in terms of molecular weight, monosaccharide content, and abundance of charged groups and proteins in their structure might have contributed to viscosity. The statistical analysis (Table 4) showed no statistically significant influence of the media composition on the viscosity, indicating that the structure of the produced polysaccharides, which is primarily a genetic characteristic of the production strain, contributes to the increase of the viscosity of the medium.
4.2. Particle Trapping and Stabilization by Cyanobacteria
After 10 months and 37 simulated dust storms that dispersed loess particles over the well-developed cyanobacterial biofilm in the experimental chamber (Figure 3A), a several millimeter-thick biocrust was formed (Figure 3B). Simultaneously, a layer of loosely aggregated loess particles formed in the control chamber (Figure 6C,D). Early-stage cyanobacterial crusts usually make a smooth crusted surface, while pinnacled crusts are usually formed by lichens and mosses [38,105]. Due to the smooth surface they made, cyanobacterial crusts are regarded as having a lower potential for dust capturing, unlike lichen and mosses [38]. However, we observed several different morphologies of biocrusts including smooth, curled, and pinnacled biocrusts, of which the latter two have an increased surface for capturing the dust particles. Assessment of species composition in different morphotypes of biocrusts formed in this study will be the next step in studying the cyanobacteria–particle interactions.
Furthermore, this study confirmed the ability of dry cyanobacterial biocrusts to reactivate their metabolic activity after wetting (Figure 5). The metabolic response to wetting is also reflected in the increased concentration of Chl a (Figure 10). It is known that cyanobacterial biocrusts can survive even under the extremely dry conditions of the Negev desert (<87 mm precipitation annually), protecting the surface from erosion and supporting the accumulation of dust particles by using the dew alone as a water source [106]. In some cases, biocrust cyanobacteria need, however, liquid water to reactivate their photosynthetic metabolism [107]. A bigger volume of biocrusts was also observed in the wet state (Figure 5), which is in line with the ability of biocrust cyanobacteria to swell rapidly up to 10 times their dry volume [108]. A more dense biological cover over the sediment observed during wet periods results from the cyanobacterial ability to follow water and glide to the biocrust surface during the wet events and to the deeper layers of biocrusts during dry periods [63].
Biocrusts have an important function in dust deposition [109], through dust particle trapping, accumulation, and consequently stabilization [9,10]. The potential of biocrust organisms to trap airborne particles is already known [38,106,110]. Cyanobacteria trap the particles mechanically through their filaments and by sticky EPSs [41]. Experiments in this study showed that the trapping of particles highly depends on the morphology of cyanobacterial colonies. Particles were either trapped within the network of cyanobacterial filaments and their tubular EPS (TB-EPS) or adhered onto the carpet-like superficial layer of amorphous cyanobacterial colonies and their slimy EPS. The same style of particle trapping was also observed by Dulić et al. [110] in the BLCs from North Iran. Our study also confirmed earlier observations of Malam Issa et al. [40] on cyanobacterial ability to form pinnacles. In our experiment, cyanobacteria formed pinnacles that rose above the biocrust surface and increased their roughness, which could significantly contribute to particle trapping. According to Williams et al. [38], the morphology of biocrusts controls the amount of dust accumulation, where biocrusts with higher surface roughness enable higher accumulation of dust.
Our observations on the mechanism of particle immobilization of coarser and finer particles are in line with earlier findings [40,41,110]. Coarser particles were mostly surrounded by the filament network, or covered by the amorphous EPS, while the finest particles were sticking to the EPS surface. We also observed that the filaments spread between the nearby particles, creating an organic bond between the adjacent loess particles (Figure 8A,D, arrow 1). Even though large pores between the particles hinder the spreading of the filaments [56], organic bonds between distant particles [51,56] reduce the space between them and allow cyanobacteria to spread more quickly and easily. Clogging the large pores with fine particles could also facilitate the spread of colonies while keeping the particles immobilized.
The airborne particles in loess landscapes are the source of essential mineral nutrients for the biocrust organisms [9]. Since in this experiment the addition of nutrient medium did not occur more than once a month, the loess particles were probably the main source of mineral nutrients. Weathering of immobilized particles through mineral dissolution is the mechanism by which mineral nutrients are acquired. As observed in this study, weathering was promoted by both TB-EPS and amorphous EPS, in and around the contact zone with the particles (Figure 9A–C). The precipitation of minerals on the EPS surface was also observed in this study. Furthermore, the precipitation of minerals in the contact zone between EPS and particles led to the formation of organomineral structures which improved the binding capacity of EPS to the particles. The same structures, referred to as cement between EPS and particles, were earlier described by Malam Issa et al. [40] and Dulić et al. [110]. We also observed that mineralized EPS structures surround and immobilize particles in the subcrust layer of induced biocrusts (Figure 9D), thereby improving the cohesion of the biocrust. Certainly, the organomineral structures observed in this and previous studies, allow a permanent immobilization, aggregation and stabilization of the loess particles [10,51].
4.3. Strain Selection Criteria and Perspectives on the Application of Cyanobacteria in the Restoration of Exposed Loess Sediments
Criteria for the selection of strains to be used as inoculum in the restoration of disturbed land surfaces should include information on as many strain characteristics as possible to ensure the formation of sustainable biocrusts that are effective in sediment stabilization. In this study, we investigated various characteristics, including toxin, biomass and polysaccharide production and the interaction between cyanobacterial colonies and loess particles. The strains used in this study were isolated from biocrust samples taken from loess sediment surfaces in Serbia, which meets the need to use native species to avoid the possible introduction of alien or invasive taxa [111].
Previous analyses of desert biocrusts indicated the potential of terrestrial cyanobacteria to produce cyanotoxins. Cox et al. [112] identified cyanotoxin presence in desert biocrusts, which was later confirmed by Metcalf et al. [113,114] and Richer et al. [115]. The studies showed that desert biocrusts from Qatar contained microcystins, anatoxin-a(S), beta-N-methylamino-L-alanine (BMAA), 2, 4-diaminobutyric acid (DAB), and N-(2-aminoethyl) glycine (AEG). In addition, possible traces of microcystins were detected in two Iranian BLCs at a concentration of a few ng/g detected using a protein phosphatase inhibition assay, while the Artemia salina mortality test indicated the presence of toxicity in one sample [110]. Toxicity assessment is a necessary step in the selection of cyanobacterial strains for land restoration because of the observed toxins. In this study, none of the strains tested caused mortality higher than 50% of the brine shrimp larvae, which condones their use in the practice of land restoration.
Ease of cultivation and efficiency in biomass production may be preferred in selection of strains for restoration. In our study, the strain Nostoc sp. L5 achieved the highest biomass after seven weeks of cultivation in BG11 N+ medium and could therefore be a strain of choice for field application. However, species with a high biomass production under culture conditions may not perform equally well under natural conditions, and this may apply in particular to strains cultivated in BG11 N+ medium. Strains that achieve higher biomass production under diazotrophic conditions should be preferred, as environmental conditions in arid areas may favour N2-fixing species. In this study, the Nostoc sp. L6 strain achieved higher biomass production when cultivated in BG11 N-, than in BG11 N+ medium. However, the biomass yield was still lower than that of the Tolypothrix sp. L4 strain, although the yields were equal in the first week of cultivation, indicating a higher rate of biomass production by Tolypothrix sp. L4 strain. In addition, field experiments by Hu et al. [116] showed that Nostoc sp. strain needed much more biomass than Microcoleus vaginatus, Phormidium tenue, or Scytonema javanicum to stabilize unconsolidated sand, which was related to the morphology of the colony. Microcoleus strains were very often selected among other strains as they are often the first colonizers of bare soil. However, the cultivation of Microcoleus proved to be very slow in terms of biomass production [117], which may affect its application in restoration practice. In view of this all, efficiency in biomass production should be supported by other characteristics when selecting a strain for inoculation.
Cyanobacterial EPSs have so far shown an undeniable role in particle/sediment stabilization. For this reason, EPS production is an essential feature of strains used in restoration practice. Cyanobacteria produce two different fractions of EPS in culture: hydrophobic CPS and hydrophilic, water-soluble RPS [66]. Considering sediment stabilization, the role of RPSs is still unproven. Mugnai et al. [118] and Chamizo et al. [119] showed that CPSs have a more significant role in particle fixation, while RPSs, hydrophilic in nature, potentially determine surface water repellency [118]. Therefore, RPSs could have an important role in preventing water loss and increasing water availability to biocrust cyanobacteria, which are considered the most important mechanisms for the biocrusts’ restoration [120]. In this study, the strain Tolypothrix sp. L4 had the lowest RPS yield under diazotrophic conditions, which could argue in favor of this strain to achieve better particle binding and express greater sediment stabilization. On the other hand, RPSs could play an important role in controlling the amount of water available [118], which is essential for the survival of cyanobacterial species in arid environments. In this study, the strain Chroococcidiopsis sp. L1 had the highest yield of both total polysaccharides and RPSs under diazotrophic conditions, which may be of interest for restoration studies in terms of water availability. The reasons that may have affected EPS production by this strain have been discussed above, but considering the biomass production, this strain would probably not be able to develop biocrusts after application in the field due to the high light and O2 concentrations, unless combined with another strain that has the ability to fix N2, e.g., Tolypothrix sp. L4 strain.
Inoculation with the mixture of strains that produce different yields of polysaccharide fractions indicates efficiency in the biocrust formation and sediment stabilization, and should be investigated in the future. Although this study has demonstrated the potential of cyanobacteria for the restoration of loess sediments, their large-scale use requires a comprehensive approach to the selection and preparation of the cyanobacterial inoculum. Besides the importance of the physiological properties of the cyanobacterial strains intended for inoculation to the unconsolidated substrate [121], Mugnai et al. [118] pointed out that the granulometry of the substrate plays an important role in the determination of the initial inoculum. They found that on the sand of medium grain size, compared to coarse sand, a smaller amount of the initial inoculum is necessary for the development of biocrusts with the same aggregate stability [118]. The preparation of the inoculum comprising the strains from this study and its application in the field will be investigated in the future.
The development of cyanobacterial biocrusts on loess sediments is strongly supported by their mineralogy and granulometry as well as cyanobacterial affinity to fine particles. A faster development of cyanobacterial biocrusts over the finer sand particles [56] and the formation of thicker biocrusts on finer soils compared to sandy soils [122] has been observed. The development of measurable biocrusts requires at least 4–5% clay and silt [123], while typical loess sediment consists mainly of silt (40–70%) and clay (5–20%) [2], the particles of which are finer than those of sands.
The chemical composition of mineral particles can also influence the efficiency of sediment stabilization by affecting the weathering rate and the availability and quality of cations, which can affect the formation of organomineral bonds. The diversity of mineral particles in the loess is high, with the dominance of quartz (typically 60–70%, but there are also extremes), followed by mica, feldspars, carbonates and clay minerals. Also, finer particles comprising loess are more susceptible to weathering due to their large surface area. Such loess properties support intense weathering events and subsequent mineral precipitation, which are promoted by cyanobacteria and EPS. The mineral precipitation leads to the formation of organomineral structures between the particles [10], which improves EPS-particle binding and thus sediment stabilization. In contrast to loess, desert sand is strongly dominated by coarse quartz particles, which are extremely resistant to weathering. This could hamper the formation of organomineral bonds and thus lower the efficiency of particle/sediment stabilization. Studies on weathering and mineral precipitation events within the biocrust are rare, and this study shows that in the future more attention should be paid to the interaction of cyanobacteria with sediment particles and its influence on sediment stability.
Topsoil stability and vegetation are regarded as the main concerns in combating desertification [35]. Many studies have described the role of cyanobacteria in sand stabilization through the formation of a complex network of filaments and EPSs e.g., [40,41,46,52,53,54,55,56,57,58,59,109,118,119,122,124]. Bailey et al. [125] investigated the influence of two cyanobacterial species (Oscillatoria prolifica and Nostoc commune) on aggregate stability of Peoria loess soil, the cyanobacterial potential to interact with loess particles, loess stabilization and use in loess restoration. Our study presents progress in this field to develop a strategy to combat desertification in loess landscapes. We demonstrated the formation of artificial biocrusts by initiating an interaction between dust particles as abiotic components and inoculated cyanobacterial cells as biotic components. This close relationship between biotic and abiotic components in direct contact with each other was recently recognized as synergosis [10]. Therefore, we suggest that a potential solution for the restoration of exposed loess surfaces and the mitigation of the desertification process and related problems in loess regions could be achieved by initiating the formation of cyanobacterial biocrusts. Our proposal is based on the use of cyanobacteria as inoculum for the initiation of biocrust formation and the acceleration of the natural succession of biocrusts from cyanobacteria to mosses and further to vascular plants. The proposed use of cyanobacteria for stabilization and restoration of loess landscapes has its background in the BLOCDUST hypothesis [9,10]. Earlier studies by Lan et al. [35] and Hu et al. [116] support the use of cyanobacteria as inoculum to accelerate the natural succession of biocrust components and further to develop communities of vascular plants. They emphasized the role of cyanobacteria and microalgae in maintaining the cohesion and early development of biocrusts, and the role of lichens, fungi, and mosses in improving the soil structure and its physico-chemical properties.
5. Conclusions
The ongoing process of desertification represents a significant environmental threat in many parts of the world. The health and wellbeing of humans and animals are at risk in the affected areas, and the loss of arable land causes further socio-economic problems. The method frequently used in temperate zones to restore degraded land surfaces, namely revegetation, has its shortcomings in semi-arid and arid environments such as loess landscapes.
This study demonstrated the potential of loess-derived cyanobacterial strains for restoration purposes as the strains lacked toxicity but were characterized by a high production of biomass and polysaccharides. It is proposed that special attention should be paid to Chroococcidiopsis sp. L1 in the restoration context as this strain showed a high potential for EPS production.
The interaction between the cyanobacterial biofilm and airborne dust particles was found to lead to the formation of biocrusts. The mechanism of trapping, accumulation and stabilization of particles by cyanobacteria differed depending on the morphology of the colonies. The trapped dust particles were exposed to intensive weathering and subsequent mineral precipitation events, which led to the formation of organomineral structures in the contact zone between EPS and dust particles.
The interaction between the cyanobacteria and airborne dust, which eventually led to the accumulation of particles in layers of biocrusts, can be understood through the recently introduced concept of synergosis and modelled by the BLOCDUST hypothesis, which deals with the dual origin of loess. Applied studies concerning the development of the biocrusts in field conditions are still necessary but this study presents a proof-of-concept and progress in developing a strategy to combat desertification in loess landscapes.
Author Contributions
Conceptualization, Z.S., J.M.; Methodological design, Z.S., I.O., T.P.M. and T.D.; Data curation, T.P.M., Formal analyses, Z.T.; Experiments, T.P.M., T.D., Z.T., B.K., T.V. and R.M.; Writing—original draft preparation, T.P.M.; Writingreview and editing, Z.S., T.D., T.P.M., J.M., I.O., Z.T., T.V., R.M. and B.K.; Visualization, T.P.M.; Funding acquisition, Z.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors acknowledge financial support of the Ministry of Education, Science and Technological Development of the Republic of Serbia (Grant No. 451-03-68/2020-14/200125), ERASMUS+ mobility program signed between the University of Novi Sad Faculty of Sciences and Åbo Akademi University, Project Code 2015-2-FI01-KA107-022151 and Project Code 2017-1-FI01-KA107-034440, as well as the support of the Åbo Akademi University Doctoral Scholarship Programme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author T.P.M. The data are not publicly available due to later utilization in PhD theses.
Acknowledgments
The authors thank Slobodan Marković for his support during this research.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pécsi, M. The role of principles and methods in loess-paleosol investigations. GeoJournal 1995, 36, 117–131. [Google Scholar] [CrossRef]
- Pécsi, M. Loess is not just the accumulation of dust. Quat. Int. 1990, 7, 1–21. [Google Scholar] [CrossRef]
- Smalley, I.J.; Marković, S.B.; Svirčev, Z. Loess is [almost totally formed by] the accumulation of dust. Quat. Int. 2011, 240, 4–11. [Google Scholar] [CrossRef]
- Guo, Z.T.; Ruddiman, W.T.; Hao, Q.Z.; Wu, H.B.; Qiao, Y.S.; Zhu, R.X.; Peng, S.Z.; Wei, J.J.; Yuan, B.Y.; Liu, T.S. Onset of Asian desertification by 22 Myr ago inferred from loess deposits in China. Nature 2002, 416, 159–163. [Google Scholar] [CrossRef]
- Markovic, S.B.; Stevens, T.; Kukla, G.J.; Hambach, U.; Fitzsimmons, K.E.; Gibbard, P.; Buggle, B.; Zech, M.; Guo, Z.T.; Hao, Q.Z.; et al. Danube loess stratigraphy—Towards a pan-European loess stratigraphic model. Earth Sci. Rev. 2015, 148, 228–258. [Google Scholar] [CrossRef]
- Obreht, I.; Zeeden, C.; Hambach, U.; Veres, D.; Markovic, S.B.; Lehmkuhl, F. A critical reevaluation of palaeoclimate proxy records from loess in the Carpathian Basin. Earth Sci. Rev. 2019, 190, 498–520. [Google Scholar] [CrossRef]
- Sprafke, T.; Obreht, I. Loess: Rock, sediment or soil: What is missing for its definition? Quat Int. 2016, 399, 198–207. [Google Scholar] [CrossRef]
- Smalley, I.J.; Marković, S.B. Loessification and hydroconsolidation: There is a connection. Catena 2014, 117, 94–99. [Google Scholar] [CrossRef]
- Svirčev, Z.; Marković, S.B.; Stevens, T.; Codd, G.A.; Smalley, I.J.; Simeunović, J.; Obreht, I.; Dulić, T.; Pantelić, D.; Hambach, U. Importance of biological loess crusts for loess formation in semi-arid environments. Quat. Int. 2013, 296, 206–215. [Google Scholar] [CrossRef]
- Svirčev, Z.; Dulić, T.; Obreht, I.; Codd, G.A.; Lehmkuhl, F.; Marković, S.B.; Hambach, U.; Meriluoto, J. Cyanobacteria and loess-an underestimated interaction. Plant Soil. 2019. [Google Scholar] [CrossRef]
- United Nations Convention to Combat Desertification (UNCCD). United Nations Convention to Combat Desertification in Those Countries Experiencing Serious Drought and/or Desertification Particularly in Africa; United Nations: Paris, France, 1994. [Google Scholar]
- Burrell, A.L.; Evans, J.P.; De Kauwe, M.G. Anthropogenic climate change has driven over 5 million km2 of drylands towards desertification. Nat. Commun. 2020, 11, 3853. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, G.; Zhang, Y.; Guan, X.; Wei, Y.; Guo, R. Global desertification vulnerability to climate change and human activities. Land Degrad. Dev. 2020, 31, 1380–1391. [Google Scholar] [CrossRef]
- Maestre, F.T.; Quero, J.L.; Gotelli, N.J.; Escudero, A.; Ochoa, V.; Delgado-Baquerizo, M.; García-Gómez, M.; Bowker, M.A.; Soliveres, S.; Escolar, C.; et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 2012, 335, 214–218. [Google Scholar] [CrossRef] [PubMed]
- United Nations Convention to Combat Desertification (UNCCD). Desertification, Land Degradation and Drought (DLDD): Some Global Facts and Figures; United Nations: Bonn, Germany, 2015. [Google Scholar]
- Rashki, A.; Eriksson, P.G.; Rautenbach, C.D.; Kaskaoutis, D.G.; Grote, W.; Dykstra, J. Assessment of chemical and mineralogical characteristics of airborne dust in the Sistan region, Iran. Chemosphere 2013, 90, 227–236. [Google Scholar] [CrossRef]
- Mostern, R. Loess is More: The Spatial and Ecological History of Erosion on China’s Northwest Frontier. J. Econ. Soc. Hist. Orient 2019, 62, 560–598. [Google Scholar] [CrossRef]
- Sweeney, M.R.; Mason, J.A. Mechanisms of dust emission from Pleistocene loess deposits, Nebraska, USA. J. Geophys. Res. Earth Surf. 2013, 118, 1–12. [Google Scholar] [CrossRef]
- Munson, S.; Belnap, J.; Okin, G.S. Responses of wind erosion to climate-induced vegetation changes on the Colorado Plateau. Proc. Natl. Acad. Sci. USA 2011, 108, 3854–3859. [Google Scholar] [CrossRef]
- National Development and Reform Commission (NDRC); Ministry of Water Resources (MWR); Ministry of Agriculture (MA); State Forestry Administration (SFA). People’s Republic of China. In Programming for Comprehensive Management of the Loess Plateau (2010–2030); 2010; pp. 4–14. [Google Scholar]
- Shi, H.; Shao, M. Soil and water loss from the Loess Plateau in China. J. Arid Environ. 2000, 45, 9–20. [Google Scholar] [CrossRef]
- Cai, Q. The relationships between soil erosion and human activities on the Loess Plateau. In Proceedings of the 12th ISCO Conference, Beijing, China, 26–31 May 2002. [Google Scholar]
- Goudie, A.S. Desert dust and human health disorders. Environ. Int. 2014, 63, 101–113. [Google Scholar] [CrossRef]
- Raghimi, M.; Ramezani Mojaveri, M. Investigation of esophageal cancer with medical geology aspect in Golestan Province, Iran. Chin. J. Geochem. 2006, 25, 58–59. [Google Scholar] [CrossRef]
- Jabbari, A.; Besharat, S.; Semnani, S. Role of silis in esophageal cancer. World J. Gastroenterol. 2008, 14, 3106–3107. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Chen, L.; Shankman, D.; Wang, C.; Wang, X.; Zhang, H. Excessive reliance on afforestation in China’s arid and semi-arid regions: Lessons in ecological restoration. Earth-Sci. Rev. 2011, 104, 240–245. [Google Scholar] [CrossRef]
- Ostwald, M.; Moberg, J.; Persson, M.; Xu, J. The Chinese Grain for Green Program-assessing the sequestered carbon from the land reform. In Proceedings of the World Rewable Energy Congress, Linköping, Sweden, 8–13 May 2011. [Google Scholar]
- Gao, L.; Bowker, M.A.; Xu, M.; Sun, H.; Tuo, D.; Zhao, Y. Biological soil crusts decrease erodibility by modifying inherent soil properties on the Loess Plateau, China. Soil Biol. Biochem. 2017, 105, 49–58. [Google Scholar] [CrossRef]
- Cao, S.; Chen, L.; Yu, X. Impact of China’s Grain for Green Project on the landscape of vulnerable arid and semiarid agricultural regions: A case study in northern Shaanxi Province. J. Appl. Ecol. 2009, 46, 536–543. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Hasi, E.; Dong, Z. Has the Three Norths Forest Shelterbelt Program solved the desertification and dust storm problems in arid and semiarid China? J. Arid Environ. 2010, 74, 13–22. [Google Scholar] [CrossRef]
- Lamb, D.; Erskine, P.D.; Parrotta, J.A. Restoration of degraded tropical forest landscapes. Science 2005, 310, 1628–1632. [Google Scholar] [CrossRef]
- Thomas, D.G. Science and the desertification debate. J. Arid Environ. 1997, 37, 599–608. [Google Scholar] [CrossRef]
- Chen, Y.; Tang, H. Desertification in North China: Background, anthropogenic impacts and failures in combating it. Land Degrad. Develop. 2005, 16, 367–376. [Google Scholar] [CrossRef]
- Normile, D. Getting at the roots of killer dust storms. Science 2007, 317, 314–316. [Google Scholar] [CrossRef]
- Lan, S.; Zhang, Q.; Wu, L.; Liu, Y.; Zhang, D.; Hu, C. Artificially accelerating the reversal of desertification: Cyanobacterial inoculation facilitates the succession of vegetation communities. Environ. Sci. Technol. 2014, 48, 307–315. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, M. Runoff and soil loss from revegetated grasslands in the Hilly Loess Plateau Region, China: Influence of biocrust patches and plant canopies. J. Hydrol. Eng. 2013, 18, 387–393. [Google Scholar] [CrossRef]
- Eldridge, D.J.; Greene, R.S. Microbiotic soil crusts: A review of their roles in soil and ecological processes in the rangelands of Australia. Aust. J. Soil Res. 1994, 32, 389–415. [Google Scholar] [CrossRef]
- Williams, A.J.; Buck, B.J.; Beyene, M.A. Biological soil crusts in the Mojave Desert, USA: Micromorphology and pedogenesis. Soil Sci. Soc. Am. J. 2012, 76, 1685–1695. [Google Scholar] [CrossRef]
- Svirčev, Z.; Nikolić, B.; Vukić, V.; Marković, S.; Gavrilov, M.; Ian, S.; Obreht, I.; Vukotić, B.; Meriluoto, J. Loess and life out of Earth? Quat. Int. 2016, 399, 208–217. [Google Scholar] [CrossRef]
- Malam Issa, O.; Trichet, J.; De’farge, C.; Coute, A.; Valentin, C. Morphology and microstructure of microbiotic soil crusts on a tiger bush sequence (Niger, Sahel). Catena 1999, 37, 175–196. [Google Scholar] [CrossRef]
- Malam Issa, O.; Le Bissonnais, Y.; De´farge, C.; Trichet, J. Role of a cyanobacterial cover on structural stability of sandy soils in the Sahelian part of western Niger. Geoderma 2001, 101, 15–30. [Google Scholar] [CrossRef]
- Xiao, B.; Wang, Q.H.; Zhao, Y.G.; Shao, M.A. Artificial culture of biological soil crusts and its effects on overland flow and infiltration under simulated rainfall. Appl. Soil Ecol. 2011, 48, 11–17. [Google Scholar] [CrossRef]
- Lan, S.B.; Wu, L.; Zhang, D.L.; Hu, C.X. Assessing level of development and successional stages in biological soil crusts with biological indicators. Microb. Ecol. 2013, 66, 394–403. [Google Scholar] [CrossRef]
- Maestre, F.T.; Bowker, M.A.; Cantón, Y.; Castillo-Monroy, A.P.; Cortina, J.; Escolar, C.; Escudero, A.; Lázaro, R.; Martínez, I. Ecology and functional roles of biological soil crusts in semi-arid ecosystems of Spain. J. Arid Environ. 2011, 75, 1282–1291. [Google Scholar] [CrossRef]
- Belnap, J. The world at your feet: Desert biological soil crusts. Fron. Ecol. Environ. 2003, 1, 181–189. [Google Scholar] [CrossRef]
- Wang, W.B.; Liu, Y.D.; Li, D.H.; Hu, C.X.; Rao, B.Q. Feasibility of cyanobacterial inoculation for biological soil crusts formation in desert area. Soil Biol. Biochem. 2009, 41, 926–929. [Google Scholar] [CrossRef]
- Li, X.R.; Tian, F.; Jia, R.L.; Zhang, Z.S.; Liu, L.C. Do biological soil crusts determine vegetation changes in sandy deserts? Implications for managing artificial vegetation. Hydrol. Process. 2010, 24, 3621–3630. [Google Scholar] [CrossRef]
- Hu, C.X.; Liu, Y.D. Primary succession of Algal community structure in desert soil. Acta Bot. Sin. 2003, 45, 917–924. [Google Scholar]
- Chiquoine, L.P. Restoration of Biological Soil Crust on Disturbed Gypsiferous Soils in Lake Mead National Recreation Area, Eastern Mojave Desert. Master’s Thesis, Las Vegas, University of Nevada, Reno, NV, USA, 2012. [Google Scholar]
- LeQuire, E. Biological soil crusts: A crucial component of arid ecosystems. J. Fire Sci. Brief 2009, 85, 1–6. [Google Scholar]
- Malam Issa, O.; Défarge, C.; Le Bissonais, Y.; Marin, B.; Duval, O.; Bruand, A.; D’Acqui, L.P.; Nordenberg, S.; Annerman, M. Effects of the inoculation of cyanobacteria on the microstructure and the structural stability of a tropical soil. Plant Soil 2007, 290, 209–219. [Google Scholar] [CrossRef]
- Chen, L.; Xie, Z.; Hu, C.; Li, D.; Wang, G.; Liu, Y. Man-made desert algal crusts as affected by environmental factors in Inner Mongolia, China. J. Arid Environ. 2006, 67, 521–527. [Google Scholar] [CrossRef]
- Xie, Z.; Liu, Y.; Hu, C.; Chen, L.; Li, D. Relationships between the biomass of algal crusts in fields and their compressive strength. Soil Biol. Biochem. 2007, 39, 567–572. [Google Scholar] [CrossRef]
- Lan, S.B.; Wu, L.; Zhang, D.L.; Hu, C.X.; Liu, Y.D. Effects of drought and salt stresses on man-made cyanobacterial crusts. Eur. J. Soil Biol. 2010, 46, 381–386. [Google Scholar] [CrossRef]
- Wu, Y.; Rao, B.; Wu, P.; Liu, Y.; Li, G.; Li, D. Development of artificially induced biological soil crusts in fields and their effects on top soil. Plant Soil 2013, 370, 115–124. [Google Scholar] [CrossRef]
- Rozenstein, O.; Zaady, E.; Katra, I.; Karnieli, A.; Adamowski, J.; Yizhaq, H. The effect of sand grain size on the development of cyanobacterial biocrusts. Aeolian Res. 2014, 15, 217–226. [Google Scholar] [CrossRef]
- Mugnai, G.; Rossi, R.; Felde, V.J.M.N.L.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. Development of the polysaccharidic matrix in biocrusts induced by a cyanobacterium inoculated in sand microcosms. Biol. Fertil. Soils. 2017, 54, 27–40. [Google Scholar] [CrossRef]
- Mugnai, G.; Rossi, F.; Felde, V.J.M.N.L.; Colesie, C.; Büdel, B.; Peth, S.; Kaplan, A.; De Philippis, R. The potential of the cyanobacterium Leptolyngbya ohadii as inoculum for stabilizing bare sandy substrates. Soil Biol. Biochem. 2018, 127, 318–328. [Google Scholar] [CrossRef]
- Park, C.H.; Li, X.R.; Zhao, Y.; Jia, R.L.; Hur, J.S. Rapid development of cyanobacterial crust in the field for combating desertification. PLoS ONE 2017, 12, e0179903. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Q.; Li, P.; Zhao, L.; Wang, L.; Zheng, X.; Ma, H. Effects of artificially cultivated biological soil crusts on soil nutrients and biological activities in the Loess Plateau. J. Arid Land. 2014, 6, 742–752. [Google Scholar] [CrossRef]
- Xiao, B.; Zhao, Y.; Wang, B.; Li, C. Development of artificial moss-dominated biological soil crusts and their effects on runoff and soil water content in a semi-arid environment. J. Arid Environ. 2015, 11, 75–83. [Google Scholar] [CrossRef]
- Belnap, J.; Lange, L.O. Biological Soil Crusts: Structure, Function and Management; Springer Verlag: Berlin, Germany, 2003. [Google Scholar]
- Garcia-Pichel, F.; Pringault, O. Cyanobacteria track water in desert soils. Nature 2001, 413, 380–381. [Google Scholar] [CrossRef]
- Rao, B.Q.; Wu, P.P.; Dauta, A.; Li, D.H.; Liu, Y.D. Effects of UV-B radiation on growth and ultrastructures of cyanobacterial crusts under greenhouse conditions. Acta Sci. Circum. 2011, 31, 649–657. [Google Scholar]
- Rossi, F.; De Philippis, R. Role of Cyanobacterial Exopolysaccharides in phototrophic biofilms and in complex microbial mats. Life 2015, 5, 1218–1238. [Google Scholar] [CrossRef]
- De Philippis, R.; Vincenzini, M. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Mazor, G.; Kidron, G.J.; Vonshak, A.; Abeliovich, A. The role of cyanobacterial exopolysaccharides in structuring desert microbial crusts. FEMS Microbiol. Ecol. 1996, 21, 121–130. [Google Scholar] [CrossRef]
- Potts, M. Desiccation tolerance: A simple process? Trends Microbiol. 2001, 9, 553–559. [Google Scholar] [CrossRef]
- Rippka, R. Isolation and purification of Cyanobacteria. Meth. Enzymol. 1988, 167, 3–27. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota 1. Chroococcales. In Süsswasserflora von Mitteleuropa 19/1; Ettl, H., Gärtner, G., Heynig, H., Mollenhauer, D., Eds.; Gustav Fischer: Jena, Germany, 1998; p. 548. [Google Scholar]
- Komárek, J.; Anagnostidis, K. Cyanoprokaryota. 2. Oscillatoriales. In Süsswasserflora von Mitteleuropa 19/2; Büdel, B., Krienitz, L., Gärtner, G., Schagerl, M., Eds.; Elsevier/Spektrum: Heidelberg, Germany, 2005; p. 759. [Google Scholar]
- Komárek, J. Cyanoprokaryota. 3. Heterocytous genera. In Süswasserflora von Mitteleuropa/Freshwater flora of Central Europe; Büdel, B., Gärtner, G., Krienitz, L., Schagerl, M., Eds.; Springer/Spektrum: Berlin/Heidelberg, Germany, 2013; p. 1130. [Google Scholar]
- Kiviranta, J.; Sivonen, K.; Niemelä, S.I.; Huovinen, K. Detection of toxicity of cyanobacteria by Artemia salina bioassay. Environ. Toxicol. Water Qual. 1991, 6, 423–436. [Google Scholar] [CrossRef]
- Tokodi, N.; Drobac, D.; Lazić, G.; Petrović, T.; Lujić, J.; Marinović, Z.; Palanački Malešević, T.; Meriluoto, J.; Svirčev, Z. Screening of cyanobacterial cultures originating from different environments for cyanotoxicity and cyanotoxins. Toxicon 2018, 154, 1–6. [Google Scholar] [CrossRef]
- Mackinney, G. Absorption of light by chlorophyll solutions. J. Biol. Chem. 1941, 140, 315–322. [Google Scholar]
- APHA. Standard Methods for the Examination of Waste and Wastewater; American Public Health Association: Washington, DC, USA, 1992. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 38, 350–356. [Google Scholar] [CrossRef]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.I.; Lee, Y.C. Carbohydrate analysis by a phenol-sulfuric acid method in a microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Castenholz, R.W. Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. J. Phycol. 1991, 27, 395–409. [Google Scholar] [CrossRef]
- Garcia-Pichel, F.; Sherry, N.D.; Castenholz, R.W. Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium. Chlorogloeopsis. Photochem. Photobiol. 1992, 56, 17–23. [Google Scholar] [CrossRef]
- Svirčev, Z.; Krstić, S.; Miladinov-Mikov, M.; Vidović, M. Freshwater cyanobacterial blooms and primary liver cancer epidemiological studies in Serbia. J. Environ. Sci. Health C 2009, 27, 36–55. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lujić, J.; Marinović, Z.; Drobac, D.; Tokodi, N.; Stojiljković, B.; Meriluoto, J. Toxicopathology induced by microcystins and nodularin: A histopathological review. J. Environ. Sci. Health C 2015, 33, 125–167. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Obradović, V.; Codd, G.A.; Marjanović, P.; Spoof, L.; Drobac, D.; Tokodi, N.; Petković, A.; Nenin, T.; Simeunović, J.; et al. Massive fish mortality and Cylindrospermopsis raciborskii bloom in Aleksandrovac Lake. Ecotoxicology 2016, 25, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, J.S.; Codd, G.A. Cyanotoxins. In Ecology of Cyanobacteria II Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer: Dordrecht, The Netherlands, 2012; pp. 651–675. [Google Scholar]
- Svirčev, Z.; Baltić, V.; Gantar, M.; Juković, M.; Stojanović, D.; Baltić, M. Molecular aspects of microcystin-induced hepatotoxicity and hepatocarcinogenesis. J. Environ. Sci. Health C 2010, 28, 39–59. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Vidović, M.; Simeunović, J.; Miladinov-Mikov, M.; Baltić, V. Epidemiology of primary liver cancer in Serbia and possible connection with cyanobacterial blooms. J. Environ. Sci. Health C 2013, 31, 181–200. [Google Scholar] [CrossRef] [PubMed]
- Svirčev, Z.; Drobac, D.; Tokodi, N.; Lužanin, Z.; Munjas, A.M.; Nikolin, B.; Vuleta, D.; Meriluoto, J. Epidemiology of cancers and in Serbia and possible connection with cyanobacterial blooms. J. Environ. Sci. Health C 2014, 32, 319–337. [Google Scholar] [CrossRef] [PubMed]
- Žegura, B.; Štraser, A.; Filipič, M. Genotoxicity and potential carcinogenicity of cyanobacterial toxins-a review. Mutat. Res. 2011, 727, 16–41. [Google Scholar] [CrossRef]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms-toxicity and risks. Interdisc. Toxicol. 2009, 2, 36–41. [Google Scholar] [CrossRef]
- Marsalek, B.; Bláha, L. Methods for detection and quantification of cyanobacterial toxins-a review. Algol. Stud. 2000, 99, 1–22. [Google Scholar]
- Yu, H.; Jia, S.; Dai, Y. Accumulation of Exopolysaccharides in Liquid Suspension Culture of Nostoc flagelliforme Cells. Appl. Bichem. Biotechnol. 2010, 160, 552–560. [Google Scholar] [CrossRef]
- Aboim, J.B.; De Oliveira, D.T.; De Mescouto, V.A.; Reis, A.S.; Rocha Filho, G.N.; Santos, A.V.; Xavier, L.P.; Santos, A.S.; Gonçalves, E.C.; Nascimento, L.A.S. Optimization of light intensity and NaNO3 concentration in Amazon Cyanobacteria cultivation to produce biodiesel. Molecules 2019, 24, 2326. [Google Scholar] [CrossRef]
- Chaneva, G.; Furnadzhieva, S.; Minkova, K.; Lukavsky, J. Effect of light and temperature on the cyanobacterium Arthronema africanum a prospective phycobiliprotein producing strain. J. Appl. Phycol. 2007, 19, 537–544. [Google Scholar] [CrossRef]
- Kovač, D.; Babić, O.; Milovanović, I.; Mišan, A.; Simeunović, J. The production of biomass and phycobiliprotein pigments in filamentous cyanobacteria: The impact of light and carbon sources. Appl. Biochem. Microbiol. 2017, 53, 539–545. [Google Scholar] [CrossRef]
- Billi, D.; Grilli Caiola, M. Effects of nitrogen limitations and starvation Chroococcidiopsis sp. (Chroococcales). New Phytol. 1996, 133, 563–571. [Google Scholar] [CrossRef]
- Boison, G.; Mergel, A.; Jolkver, H.; Bothe, H. Bacterial life and dinitrogen fixation at Gypsum Rock. Appl. Environ. Microbiol. 2004, 70, 7070–7077. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexityof cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Vargas, M.A.; Olivares, H.; Rivas, J.; Guerrero, M.G. Exopolysaccharide production by the cyanobacterium Anabaena sp. ATCC 33047 in batch and continuous culture. J. Biotechnol. 1998, 60, 175–182. [Google Scholar] [CrossRef]
- Su, C.; Chi, Z.; Lu, W. Optimization of medium and cultivation conditions for enhanced exopolysaccharide yield by marine Cyanothece sp. 113. Chin. J. Oceanol. Limnol. 2007, 25, 411–417. [Google Scholar] [CrossRef]
- Richert, L.; Golubic, S.; Le Gu´ede‘s, R.; Ratiskol, J.; Payri, C.; Guezennec, J. Characterization of exopolysaccharides produced by cyanobacteria isolated from Polynesian microbial mats. Curr. Microbiol. 2005, 51, 379–384. [Google Scholar] [CrossRef]
- Nicolaus, B.; Panico, A.; Lama, L.; Romano, I.; Manca, M.C.; De Giulio, A.; Gambacorta, A. Chemical composition and production of exopolysaccharides from representative members of heterocystous and non-heterocystous cyanobacteria. Phytochemistry 1999, 52, 639–647. [Google Scholar] [CrossRef]
- Trabelsi, L.; Ouada, H.B.; Bacha, H.; Ghoul, M. Combined effect of temperature and light intensity on growth and extracellular polymeric substance production by the cyanobacterium Arthrospira platensis. J. Appl. Phycol. 2009, 21, 405–412. [Google Scholar] [CrossRef]
- Mota, R.; Guimarães, R.; Büttel, Z.; Rossi, F.; Colica, G.; Silva, C.J.; Santos, C.; Gales, L.; Zille, A.; De Philippis, R.; et al. Production and characterization of extracellular carbohydrate polymer from Cyanothece sp. CCY 0110. Carbohyd. Polym. 2013, 92, 1408–1415. [Google Scholar] [CrossRef] [PubMed]
- Whistler, R.; Daniel, R. Function of polysaccharides in foods. In Food Additives; Branen, A.L., Davidson, P.M., Salminen, S., Eds.; Marcel Dekker: New York, NY, USA, 1990. [Google Scholar]
- Lan, S.; Wu, L.; Zhang, D.; Hu, C. Successional stages of biological soil crusts and their microstructure variability in Shapotou region (China). Environ. Earth Sci. 2012, 65, 77–88. [Google Scholar] [CrossRef]
- Zaady, E.; Offer, Z.Y. Biogenic soil crusts and soil depth: A long-term case study from the Central Negev desert highland. Sedimentology 2010, 57, 351–358. [Google Scholar] [CrossRef]
- Lange, O.L. Photosynthesis of soil crust biota is dependent on environmental factors. In Biological Soil Crusts: Structure, Function and Management; Belnap, J., Lange, O.L., Eds.; Springer: Berlin, Germany, 2003; pp. 217–240. [Google Scholar]
- Verrecchia, E.; Yair, A.; Kidron, G.J.; Verrecchia, K. Physical properties of the psammophile cryptogamic crust and their consequences to the water regime of sandy soils, north-western Negev Desert, Israel. J. Arid Environ. 1995, 29, 427–437. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Wang, H.L.; Wang, X.Q.; Yang, W.K.; Zhang, D.Y. The microstructure of microbiotic crust and its influence on wind erosion for a sandy soil surface in the Gurbantunggut Desert of Northwestern China. Geoderma 2006, 132, 441–449. [Google Scholar] [CrossRef]
- Dulić, T.; Meriluoto, J.; Palanački Malešević, T.; Gajić, V.; Važić, T.; Tokodi, N.; Obreht, I.; Kostić, B.; Kosijer, P.; Khormali, F.; et al. Cyanobacterial diversity and toxicity of biocrusts from the Caspian Lowland loess deposits, North Iran. Quat. Int. 2017, 429, 74–85. [Google Scholar] [CrossRef]
- Zhao, Y.; Bowker, M.A.; Zhang, Y.; Zaady, E. Enhanced Recovery of Biological Soil Crusts after Disturbance. In Biological Soil Crusts: An Organising Principle in Drylands; Weber, B., Büdel, B., Belnap, J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 498–523. [Google Scholar]
- Cox, P.A.; Richer, R.; Metcalf, J.S.; Banack, S.A.; Codd, G.A.; Bradley, W.G. Cyanobacteria and BMAA exposure from desert dust: A possible link to sporadic ALS among Gulf War veterans. Amyotroph. Lateral. Scle. 2009, 10, 109–117. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Richer, R.; Cox, P.A.; Codd, G.A. Cyanotoxins in desert environments may present a risk to human health. Sci. Total Environ. 2012, 421–422, 118–123. [Google Scholar] [CrossRef]
- Metcalf, J.S.; Banack, S.A.; Richer, R.; Cox, P.A. Neurotoxic amino acids and their isomers in desert environments. J. Arid Environ. 2015, 112, 140–144. [Google Scholar] [CrossRef]
- Richer, R.; Banack, S.A.; Metcalf, J.S.; Cox, P.A. The persistence of cyanobacterial toxins in desert soils. J. Arid Environ. 2015, 112, 134–139. [Google Scholar] [CrossRef]
- Hu, C.; Liu, Y.; Song, L.; Zhang, D. Effect of desert soil algae on the stabilization of fine sands. J. Appl. Phycol. 2002, 14, 281–292. [Google Scholar] [CrossRef]
- Giraldo-Silva, A.; Nelson, C.; Barger, N.N.; Garcia-Pichel, F. Nursing bio-crusts: Isolation, cultivation, and fitness test of indigenous cyanobacteria. Restor. Ecol. 2019, 27, 793–803. [Google Scholar] [CrossRef]
- Mugnai, G.; Rossi, F.; Chamizo, S.; Adessi, A.; De Philippis, R. The role of grain size and inoculum amount on biocrust formation by Leptolyngbya ohadii. Catena 2020, 184, 104248. [Google Scholar] [CrossRef]
- Chamizo, S.; Adessi, A.; Mugnai, G.; Simiani, A.; De Philippis, R. Soil type and cyanobacteria species influence the macromolecular and chemical characteristics of the polysaccharidic matrix in induced biocrusts. Microb. Ecol. 2019, 78, 482–493. [Google Scholar] [CrossRef]
- Bu, C.; Wu, S.; Yang, Y.; Zheng, M. Identification of factors influencing the restoration of Cyanobacteria-dominated biological soil crusts. PLoS ONE 2014, 9, e90049. [Google Scholar] [CrossRef]
- Rossi, F.; Hua, L.; Liu, Y.; De Philippis, R. Cyanobacterial inoculation (cyanobacterisation): Perspectives for the development of a standardized multifunctional technology for soil fertilization and desertification reversal. Earth-Sci. Rev. 2017, 171, 28–43. [Google Scholar] [CrossRef]
- Chamizo, S.; Mugnai, G.; Rossi, F.; Certini, G.; De Philippis, R. Cyanobacteria inoculation improves soil stability and fertility on different textured soils: Gaining insights for applicability in soil restoration. Front. Environ. Sci. 2018, 6. [Google Scholar] [CrossRef]
- West, N.E. Structure and function of microphytic soil crusts in wildland ecosystems of arid to semi-arid regions. Adv. Ecol. Res. 1990, 20, 179–223. [Google Scholar]
- Malam Issa, O.; Défarge, C.; Trichet, J.; Valentin, C.; Rajot, J.L. Microbiotic soil crusts in the Sahel of Western Niger and their influence on soil porosity and water dynamics. Catena 2009, 77, 48–55. [Google Scholar] [CrossRef]
- Bailey, D.; Mazurak, A.P.; Rosowski, J.R. Aggregation of soil particles by algae. J. Phycol. 1973, 9, 99–101. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).