Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains
2.2. Fermentation Trials
2.3. Analytical Procedures
2.4. Sensory Analysis
2.5. Statistical Analysis
3. Results
3.1. Biomass Evolution and Sugar Consumption
3.2. Main Oenological Characters and Volatile Compounds of S. cerevisiae Pure Fermentations
3.3. Main Oenological Characters and Volatile Compounds of T. delbrueckii/S. cerevisiae DiSVA 708 Sequential Fermentations
3.4. Principal Component Analysis of the By-Products and Main Volatile Compounds
3.5. Sensory Analysis
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vigentini, I.; Fabrizio, V.; Faccincani, M.; Picozzi, C.; Comasio, A.; Foschino, R. Dynamics of Saccharomyces cerevisiae populations in controlled and spontaneous fermentations for Franciacorta D.O.C.G. base wine production. Ann. Microbiol. 2013, 64, 639–651. [Google Scholar] [CrossRef]
- Comitini, F.; Gobbi, M.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Selected non-Saccharomyces wine yeasts in controlled multistarter fermentations with Saccharomyces cerevisiae. Food Microbiol. 2011, 28, 873–882. [Google Scholar] [CrossRef]
- Jolly, N.P.; Varela, C.; Pretorius, I.S. Not your ordinary yeast: Non-Saccharomycesyeasts in wine production uncovered. FEMS Yeast Res. 2013, 14, 215–237. [Google Scholar] [CrossRef] [PubMed]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2014, 31, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.-T.; Lu, L.; Duan, C.-Q.; Yan, G.-L. The contribution of indigenous non-Saccharomyces wine yeast to improved aromatic quality of Cabernet Sauvignon wines by spontaneous fermentation. LWT 2016, 71, 356–363. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef]
- Englezos, V.; Rantsiou, K.; Cravero, F.; Torchio, F.; Pollon, M.; Fracassetti, D.; Ortiz-Julien, A.; Gerbi, V.; Rolle, L.; Cocolin, L.; et al. Volatile profile of white wines fermented with sequential inoculation of Starmerella bacillaris and Saccharomyces cerevisiae. Food Chem. 2018, 257, 350–360. [Google Scholar] [CrossRef]
- Binati, R.L.; Innocente, G.; Gatto, V.; Celebrin, A.; Polo, M.; Felis, G.E.; Torriani, S. Exploring the diversity of a collection of native non-Saccharomyces yeasts to develop co-starter cultures for winemaking. Food Res. Int. 2019, 122, 432–442. [Google Scholar] [CrossRef]
- Ciani, M.; Comitini, F. Use of Non-Saccharomyces Yeasts in Red Winemaking. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 51–68. [Google Scholar]
- Binati, R.L.; Junior, W.J.L.; Luzzini, G.; Slaghenaufi, D.; Ugliano, M.; Torriani, S. Contribution of non-Saccharomyces yeasts to wine volatile and sensory diversity: A study on Lachancea thermotolerans, Metschnikowia spp. and Starmerella bacillaris strains isolated in Italy. Int. J. Food Microbiol. 2020, 318, 108470. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Metschnikowia pulcherrima Selected Strain for Ethanol Reduction in Wine: Influence of Cell Immobilization and Aeration Condition. Foods 2019, 8, 378. [Google Scholar] [CrossRef]
- Velázquez, R.; Zamora, E.; Álvarez, M.L.; Hernández, L.M.; Ramírez, M. Effects of new Torulaspora delbrueckii killer yeasts on the must fermentation kinetics and aroma compounds of white table wine. Front. Microbiol. 2015, 6, 1222. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, M.; Velázquez, R.; Maqueda, M.; Lopez-Piñeiro, A.; Ribas, J.C. A new wine Torulaspora delbrueckii killer strain with broad antifungal activity and its toxin-encoding double-stranded RNA virus. Front. Microbiol. 2015, 6, 983. [Google Scholar] [CrossRef] [PubMed]
- Ciani, M.; Comitini, F. Yeast interactions in multi-starter wine fermentation. Curr. Opin. Food Sci. 2015, 1, 1–6. [Google Scholar] [CrossRef]
- Canonico, L.; Comitini, F.; Ciani, M. Influence of vintage and selected starter on Torulaspora delbrueckii/Saccharomyces cerevisiae sequential fermentation. Eur. Food Res. Technol. 2015, 241, 827–833. [Google Scholar] [CrossRef]
- Ortiz, M.J.; Barrajón, N.; Baffi, M.A.; Arévalo-Villena, M.; Briones, A. Spontaneous must fermentation: Identification and biotechnological properties of wine yeasts. LWT 2013, 50, 371–377. [Google Scholar] [CrossRef]
- Blanco, P.; Mirás-Avalos, J.M.; Pereira, E.; Fornos, D.; Orriols, I. Modulation of chemical and sensory characteristics of red wine from Mencía by using indigenous Saccharomyces cerevisiae yeast strains. OENO One 2014, 48, 63. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Ciani, M.; Comitini, F. Fitness of Selected Indigenous Saccharomyces cerevisiae Strains for White Piceno DOC Wines Production. Fermentation 2018, 4, 37. [Google Scholar] [CrossRef]
- Lorca, G.; Uribe, S.; Martínez, C.; Godoy, L.; Ganga, M.A. Screening of native S. cerevisiae strains in the production of Pajarete wine: A tradition of Atacama Region, Chile. J. Wine Res. 2018, 29, 1–13. [Google Scholar] [CrossRef]
- Çelik, Z.D.; Erten, H.; Cabaroglu, T. The Influence of Selected Autochthonous Saccharomyces cerevisiae Strains on the Physicochemical and Sensory Properties of Narince Wines. Fermentation 2019, 5, 70. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Comitini, F.; Ciani, M. Reduction of Sulfur Compounds through Genetic Improvement of Native Saccharomyces cerevisiae Useful for Organic and Sulfite-Free Wine. Foods 2020, 9, 658. [Google Scholar] [CrossRef]
- De Vero, L.; Solieri, L.; Giudici, P. Evolution-based strategy to generate non-genetically modified organisms Saccharomyces cerevisiae strains impaired in sulfate assimilation pathway. Lett. Appl. Microbiol. 2011, 53, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Z. Cross Breeding and Hybrid Identification of Sulphite-tolerant Hybrids of Saccharomyces uvarum. S. Afr. J. Enol. Vitic. 2017, 38, 125–131. [Google Scholar] [CrossRef][Green Version]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii for secondary fermentation in sparkling wine production. Food Microbiol. 2018, 74, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Canonico, L.; Comitini, F.; Ciani, M. Torulaspora delbrueckii contribution in mixed brewing fermentations with different Saccharomyces cerevisiae strains. Int. J. Food Microbiol. 2017, 259, 7–13. [Google Scholar] [CrossRef]
- EEC. Council Regulation 2870/00 laying down Community reference methods for the analysis of spirit drinks. Off. J. Eur. Comm. 2000, L333, 20–46. [Google Scholar]
- Dukes, B.C.; Butzke, C.E. Rapid determination of primaryamino acid in grape juice using ano-phthaldialdehyde/N-acetyl-L-cysteine spectrophotometric assay. Am. J. Enol. Vitic. 1998, 49, 125–134. [Google Scholar]
- Canonico, L.; Solomon, M.; Comitini, F.; Ciani, M.; Varela, C. Volatile profile of reduced alcohol wines fermented with selected non-Saccharomyces yeasts under different aeration conditions. Food Microbiol. 2019, 84, 103247. [Google Scholar] [CrossRef]
- Guillamón, J.M.; Barrio, E. Genetic Polymorphism in Wine Yeasts: Mechanisms and Methods for Its Detection. Front. Microbiol. 2017, 8, 806. [Google Scholar] [CrossRef]
- Anfang, N.; Brajkovich, M.; Goddard, M.R. Co-fermentation with Pichia kluyveri increases varietal thiol concentrations in Sauvignon Blanc. Aust. J. Grape Wine Res. 2009, 15, 1–8. [Google Scholar] [CrossRef]
- Benito, S.; Hofmann, T.; Laier, M.; Lochbühler, B.; Schüttler, A.; Ebert, K.; Fritsch, S.; Röcker, J.; Rauhut, D. Effect on quality and composition of Riesling wines fermented by sequential inoculation with non-Saccharomyces and Saccharomyces cerevisiae. Eur. Food Res. Technol. 2015, 241, 707–717. [Google Scholar] [CrossRef]
- Wang, C.; Mas, A.; Esteve-Zarzoso, B. The Interaction between Saccharomyces cerevisiae and Non-Saccharomyces Yeast during Alcoholic Fermentation Is Species and Strain Specific. Front. Microbiol. 2016, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Benito, S. The impact of Torulaspora delbrueckii yeast in winemaking. Appl. Microbiol. Biotechnol. 2018, 102, 3081–3094. [Google Scholar] [CrossRef] [PubMed]
- Rollero, S.; Zietsman, J.; Buffetto, F.; Schückel, J.; Ortiz-Julien, A.; Divol, B. Kluyveromyces marxianus Secretes a Pectinase in Shiraz Grape Must That Impacts Technological Properties and Aroma Profile of Wine. J. Agric. Food Chem. 2018, 66, 11739–11747. [Google Scholar] [CrossRef] [PubMed]
- Roudil, L.; Russo, P.; Berbegal, C.; Albertin, W.; Spano, G.; Capozzi, V. Non-Saccharomyces Commercial Starter Cultures: Scientific Trends, Recent Patents and Innovation in the Wine Sector. Recent Patents Food Nutr. Agric. 2020, 11, 27–39. [Google Scholar] [CrossRef]
- Lin, M.M.-H.; Boss, P.K.; Walker, M.E.; Sumby, K.M.; Grbin, P.R.; Jiranek, V. Evaluation of indigenous non-Saccharomyces yeasts isolated from a South Australian vineyard for their potential as wine starter cultures. Int. J. Food Microbiol. 2019, 312, 108373. [Google Scholar] [CrossRef]
- Rapp, A.; Mandery, H. Wine aroma. Cell. Mol. Life Sci. 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Fang, Y.; Qian, M.C. Aroma compounds in Oregon Pinot Noir wine determined by aroma extract dilution analysis (AEDA). Flavour Fragr. J. 2004, 20, 22–29. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.J.; Gómez-Míguez, M.; Vicario, I.M.; Heredia, F.J. Assessment of colour and aroma in white wines vinifications: Effects of grape maturity and soil type. J. Food Eng. 2007, 79, 758–764. [Google Scholar] [CrossRef]
- Kutyna, D.R.; Borneman, A.R. Heterologous Production of Flavour and Aroma Compounds in Saccharomyces cerevisiae. Genes 2018, 9, 326. [Google Scholar] [CrossRef]
- Eder, M.; Sanchez, I.; Brice, C.; Camarasa, C.; Legras, J.-L.; Dequin, S. QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation. BMC Genom. 2018, 19, 166. [Google Scholar] [CrossRef]
- Bely, M.; Stoeckle, P.; Masneuf-Pomarède, I.; Dubourdieu, D. Impact of mixed Torulaspora delbrueckii–Saccharomyces cerevisiae culture on high-sugar fermentation. Int. J. Food Microbiol. 2008, 122, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Calderón, F. The Influence of Non-Saccharomyces Species on Wine Fermentation Quality Parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Rauhut, D.; Benito, S.; Santos, A. Influence of Torulaspora delbrueckii in varietal thiol (3-SHand4-MSP) release in wine sequential fermentations. Int. J. Food Microbiol. 2017, 257, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Dutraive, O.; Benito, S.; Fritsch, S.; Bei-Sert, B.; Patz, C.-D.; Rauhut, R. Patz Effect of Sequential Inoculation with Non-Saccharomyces and Saccharomyces Yeasts on Riesling Wine Chemical Composition. Fermentation 2019, 5, 79. [Google Scholar] [CrossRef]
 ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 (
) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 ( ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in the 2017 and 2018 vintages.
) in the 2017 and 2018 vintages.
 ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 (
) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 ( ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in the 2017 and 2018 vintages.
) in the 2017 and 2018 vintages.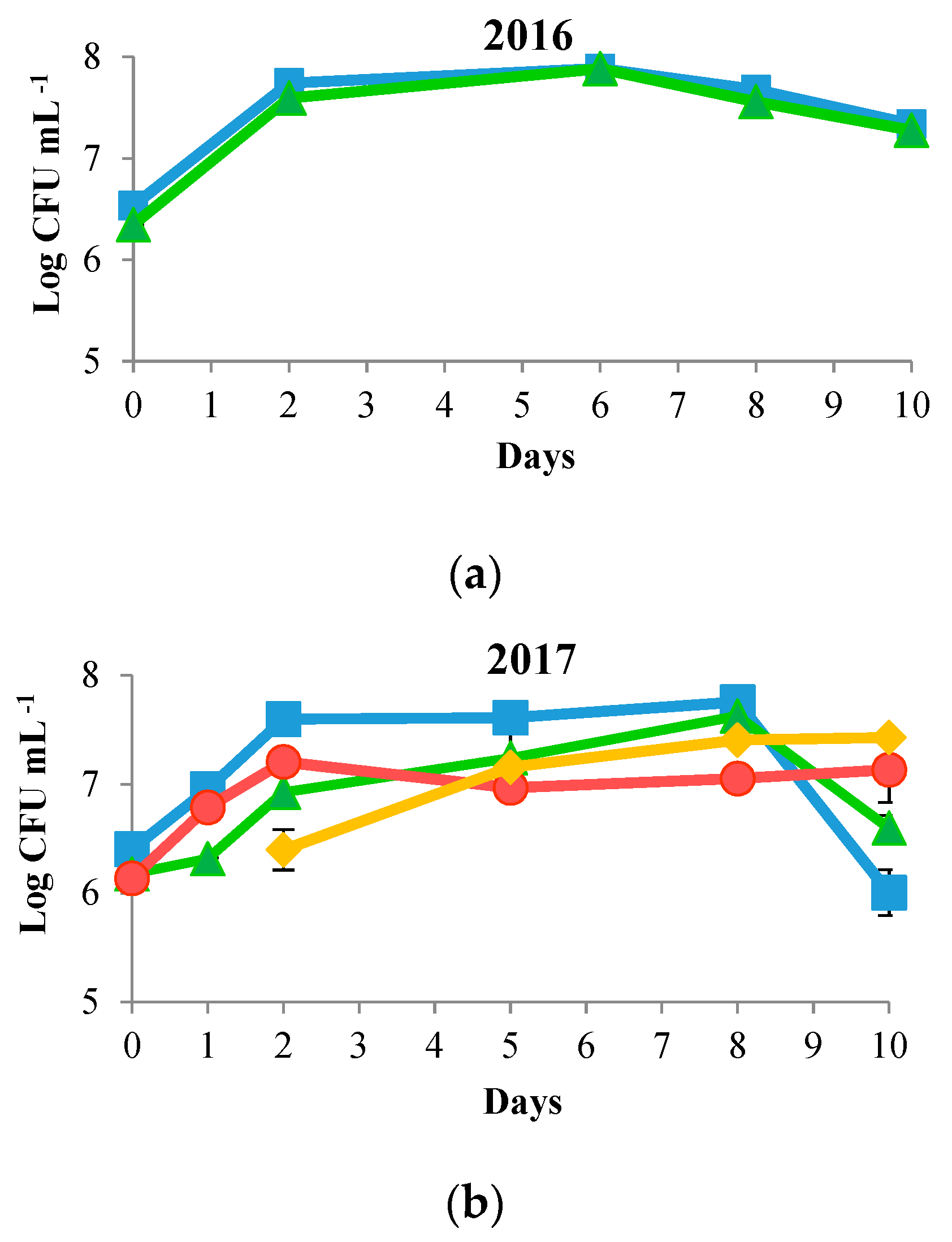

 ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 and S. cerevisiae DiSVA 708 (
) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 and S. cerevisiae DiSVA 708 ( ) in the 2017 and 2018 vintages.
) in the 2017 and 2018 vintages.
 ) and S. cerevisiae DiSVA 708 (
) and S. cerevisiae DiSVA 708 ( ) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 and S. cerevisiae DiSVA 708 (
) in each vintage. Sequential fermentations of T. delbrueckii DiSVA 130 and S. cerevisiae DiSVA 708 ( ) in the 2017 and 2018 vintages.
) in the 2017 and 2018 vintages.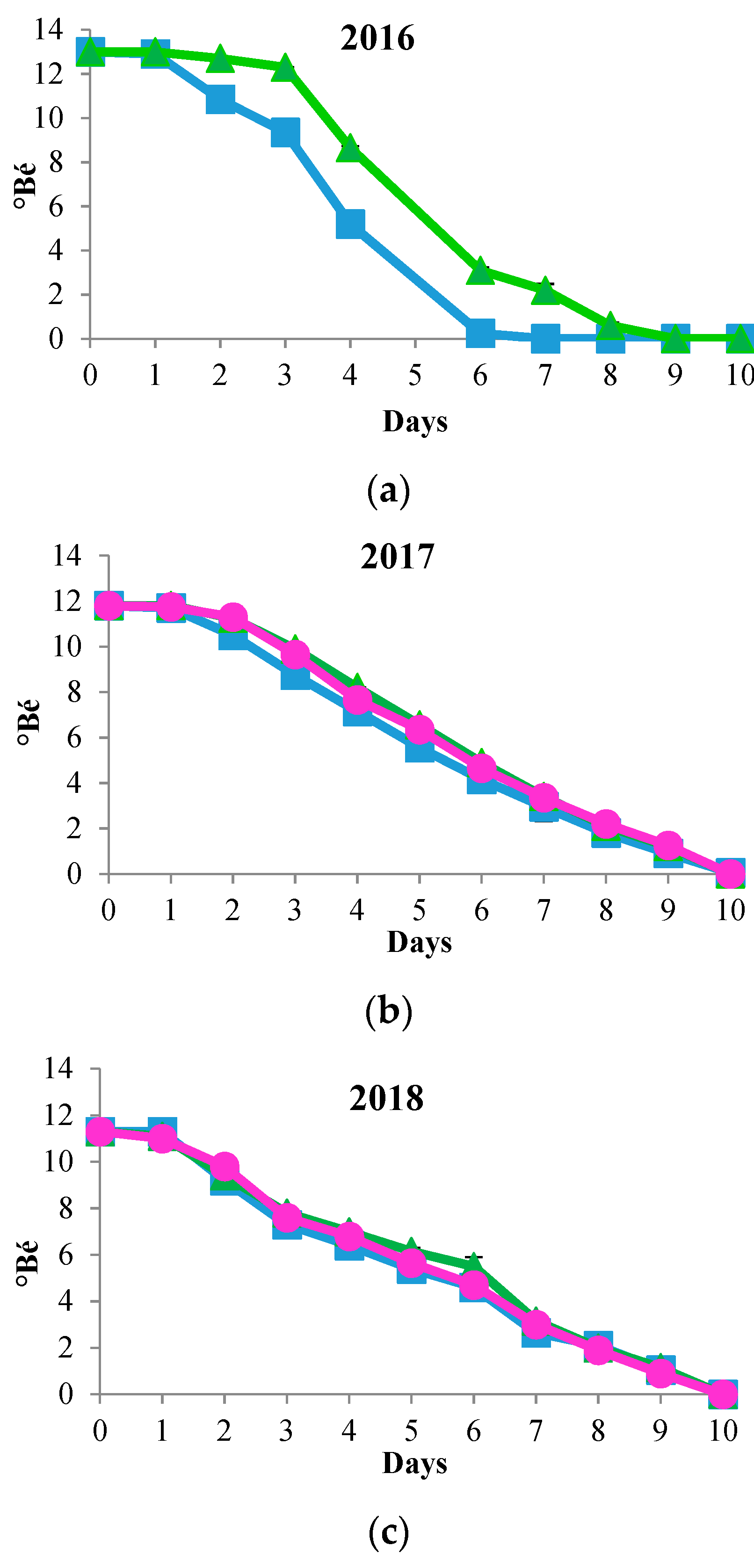

| Grape Juice Parameters | Vintage 2016 | Vintage 2017 | Vintage 2018 |
|---|---|---|---|
| Initial sugars (g L−1) | 236.00 | 212.00 | 203.00 |
| pH | 3.22 | 3.22 | 3.26 |
| Total acidity (g L−1) | 4.42 | 4.58 | 4.83 |
| Malic acid (g L−1) | 2.70 | 2.50 | 2.40 |
| Total SO2 (mg L−1) | 35.00 | 27.00 | 26.00 |
| Nitrogen content YAN * (mg N L−1) | 89.00 | 60.00 | 62.00 |
| Ethanol (% v/v) | Residual Sugars (g L−1) | Total Acidity (Tartaric Acid g L−1) | Volatile Acidity (Acetic Acid g L−1) | Glycerol (g L−1) | Malic Acid (g L−1) | Total SO2 (mg L−1) | H2S (Score) | ||
|---|---|---|---|---|---|---|---|---|---|
| 2016 | OKAY | 14.24 ± 0.07 a | 2.13 ± 0.76 c | 5.73 ± 0.03 d | 0.54 ± 0.01 a | 7.48 ± 0.27 a | 1.35 ± 0.09 b | 6.50 ± 0.71 d | 1 ± 0.0 c |
| DiSVA 708 | 13.89 ± 0.17 b | 7.01 ± 2.31 a | 6.17 ± 0.03 b | 0.49 ± 0.01 b | 6.82 ± 0.42 a | 1.60 ± 0.12 a | 18.00 ± 0.72 c | 3 ± 0.0 a | |
| 2017 | OKAY | 13.2 ± 0.12 c | 1.80 ± 0.14 c | 5.79 ± 0.12 d | 0.33 ± 0.05 d | 7.23 ± 0.19 a | 1.35 ± 0.07 b | 14.00 ± 2.83 c | 1 ± 0.0 c |
| DiSVA 708 | 13.31 ± 0.04 c | 2.1 ± 0.28 c | 6.15 ± 0.10 b | 0.42 ± 0.03 c | 7.20 ± 0.24 a | 1.1 ± 0.00 c | 39.00 ± 2.82 a | 1 ± 0.0 c | |
| Td/708 | 13.26 ± 0.07 c | 3.60 ± 0.53 b | 6.01 ± 0.01 c | 0.30 ± 0.03 d | 5.23 ± 0.18 b | 1.0 ± 0.00 c | 33.50 ± 0.71 b | 3 ± 0.0 a | |
| 2018 | OKAY | 12.57 ± 0.01 d | 1.45 ± 0.07 d | 6.11 ± 0.03 b,c | 0.14 ± 0.01 e | NA * | 1.65 ± 0.07 a | 24.00 ± 0.00 c | 2 ± 0.0 b |
| DiSVA 708 | 12.43 ± 0.01 d | 1.55 ± 0.07 d | 6.40 ± 0.09 a | 0.15 ± 0.01 e | NA | 1.55 ± 0.07 a | 33.00 ± 1.41 b | 2 ± 0.0 b | |
| Td/708 | 12.50 ± 0.02 d | 1.77 ± 0.00 c,d | 6.39 ± 0.03 a | 0.15 ± 0.01 e | NA | 1.65 ± 0.07 a | 30.50 ± 2.12 b | 3 ± 0.0 a |
| Esters (mg L−1) | 2016 | 2017 | 2018 | |||||
|---|---|---|---|---|---|---|---|---|
| OKAY | DiSVA 708 | OKAY | DiSVA 708 | Td/708 | OKAY | DiSVA 708 | Td/708 | |
| Ethyl butyrate | 3.05 ± 0.08 a | 0.74 ± 0.07 b,c | 1.29 ± 0.66 b | 0.96 ± 0.05 b,c | 0.82 ± 0.01 b,c | 1.44 ± 0.54 b,c | 0.59 ± 0.01 c | 0.62 ± 0.00 c |
| Ethyl acetate | 23.14 ± 0.15 b | 31.14 ± 0.15 a | 12.74 ± 0.23 c | 13.97 ± 0.40 c | 12.92 ± 0.40 c | 18.18 ± 9.07 b,c | 24.25 ± 1.30 a,b | 16.92 ± 2.43 b,c |
| Phenyl ethyl acetate | 0.25 ± 0.00 c | 0.28 ± 0.04 c | 0.21 ± 0.04 c | 0.24 ± 0.01 c | 0.33 ± 0.01 b,c | 0.22 ± 0.07 c | 0.59 ± 0.08 a,b | 0.68 ± 0.06 a |
| Ethyl hexanoate | 0.43 ± 0.07 c | 0.25 ± 0.07 c | 0.84 ± 0.07 b | 1.69 ± 0.13 a | 1.70 ± 0.02 a | 0.36 ± 0.10 c | 0.23 ± 0.07 c | 0.71 ± 0.21 b |
| Ethyl octanoate | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.00 ± 0.00 b | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.009 ± 0.012 b | 0.02 ± 0.00 a | 0 ± 0 b |
| Isoamyl acetate | 1.16 ± 0.18 c | 2.14 ± 0.31 b | 1.71 ± 0.35 c | 2.88 ± 0.36 b | 3.15 ± 0.09 a,b | 2.30 ± 0.41 b | 3.97 ± 1.18 a | 3.83 ± 1.49 a |
| Alcohols (mg L−1) | ||||||||
| n-propanol | 109.08 ± 0.31 b | 45.75 ± 0.01 e | 89.30 ± 0.47 c | 33.25 ± 0.11 f | 31.27 ± 0.60 f | 125.18 ± 4.98 a | 28.31 ± 0.74 d | 45.22 ± 1.18 e |
| Isobutanol | 13.19 ± 0.15 d,e | 23.93 ± 0.22 b | 12.76 ± 0.40 d,e | 11.74 ± 0.43 e | 20.43 ± 0.06 c | 14.79 ± 0.73 d | 28.31 ± 0.01 a | 27.78 ± 3.18 a |
| Amyl alcohol | 12.68 ± 0.17 c,d | 18.10 ± 0.44 a | 11.21 ± 0.30 e | 9.88 ± 0.09 f | 12.17 ± 0.95 d,e | 13.89 ± 0.01 b | 13.82 ± 0.35 b,c | 11.68 ± 0.89 d,e |
| Isoamyl alcohol | 116.46 ± 0.51 b,c | 151.91 ± 0.81 a | 120.78 ± 0.67 b | 64.53 ± 0.64 e | 110.11 ± 5.91 c | 114.23 ± 0.99 c | 60.36 ± 0.08 e | 82.72 ± 4.83 d |
| β-Phenyl ethanol | 12.03 ± 0.74 b,c | 16.61 ± 0.23 b | 14.22 ± 2.24 b,c | 8.32 ± 0.60 c | 12.97 ± 0.25 b,c | 29.38 ± 0.26 a | 27.86 ± 0.43 a | 29.86 ± 0.56 a |
| Carbonyl Compounds (mg L−1) | ||||||||
| Acetaldehyde | 6.01 ± 0.1 d | 53.42 ± 0.27 a | 8.99 ± 0.23 c,d | 12.05 ± 0.01 c,d | 15.77 ± 0.45 c | 46.62 ± 10.47 a | 29.55 ± 0.91 b | 16.51 ± 1.87 c |
| Monoterpenes (mg L−1) | ||||||||
| Linalool | 0.04 ± 0.04 a,b | 0.00 ± 0.00 c | 0.06 ± 0.01 a,b | 0.03 ± 0.01 b,c | 0.04 ± 0.00 b,c | 0.046 ± 0.015 a,b | 0.02 ± 0.001 b,c | 0.08 ± 0.02 a |
| Geraniol | 0.01 ± 0.01 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.01 ± 0.01 b | 0.09 ± 0.02 a | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 c |
| Vintages | Trials | Smell (Score) | Taste (Score) |
|---|---|---|---|
| 2016 | OKAY | 6.78 ± 0.11 a,b | 6.34 ± 0.08 b |
| DiSVA 708 | 7.35 ± 0.12 a | 7.55 ± 0.40 a | |
| 2017 | OKAY | 5.94 ± 0.03 c | 5.89 ± 0.76 c |
| DiSVA 708 | 6.00 ± 0.10 c | 7.17 ± 0.16 a | |
| Td/708 | 5.78 ± 1.73 c | 6.66 ± 0.77 a,b | |
| 2018 | OKAY | 7.13 ± 0.52 a | 7.39 ± 1.6 a |
| DiSVA 708 | 6.29 ± 1.78 b | 6.61 ± 1.22 a,b | |
| Td/708 | 7.05 ± 0.21 a | 6.82 ± 0.63 a,b |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agarbati, A.; Canonico, L.; Comitini, F.; Ciani, M. Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites. Appl. Sci. 2020, 10, 6722. https://doi.org/10.3390/app10196722
Agarbati A, Canonico L, Comitini F, Ciani M. Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites. Applied Sciences. 2020; 10(19):6722. https://doi.org/10.3390/app10196722
Chicago/Turabian StyleAgarbati, Alice, Laura Canonico, Francesca Comitini, and Maurizio Ciani. 2020. "Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites" Applied Sciences 10, no. 19: 6722. https://doi.org/10.3390/app10196722
APA StyleAgarbati, A., Canonico, L., Comitini, F., & Ciani, M. (2020). Improved Saccharomyces cerevisiae Strain in Pure and Sequential Fermentation with Torulaspora delbrueckii for the Production of Verdicchio Wine with Reduced Sulfites. Applied Sciences, 10(19), 6722. https://doi.org/10.3390/app10196722







