Abstract
(1) Background: Glycogen storage disease (GSD) represents a group of twenty-three types of metabolic disorders which damage the capacity of body to store glucose classified basing on the enzyme deficiency involved. Affected patients could present some oro-facial alterations: the purpose of this review is to catalog and characterize oral manifestations in these patients. (2) Methods: a systematic review of the literature among different search engines using PICOS criteria has been performed. The studies were included with the following criteria: tissues and anatomical structures of the oral cavity in humans, published in English, and available full text. Review articles and paper published before 1990 were excluded. (3) Results: 757 articles were identified in the initial search. In the end, 45 articles that met the selection criteria has been analyzed. The information extracted from the articles was classified according to the type of GSD (Ia; Ib; II; III; V; XIV). Oral manifestations range from dental caries to severe periodontitis in paediatric patients, from diffuses and recurrent oral ulcers in the cleft lip and palate. (4) Conclusions: Although considered a rare disease, GSD can present a varied number of oral manifestations. Therefore, it is of great importance for the oral medicine specialist to know and classify them.
1. Introduction
Glycogen storage disease (GSD) is a group of rare metabolic diseases, whose overall incidence in the population is estimated at 1 in 2000–43,000. These pathologies are caused by functional deficiency or lack of one of the enzymes that contribute to glycogen metabolism. If the enzyme activity is deficient or not optimal, the glycogen accumulates in the tissues causing alterations and dysfunctions of the organs [1]. Twenty-three types of GSDs are currently recognized, they are classified depending on the organ affected and the enzyme deficiency involved: GSD types 0, I, III, IV, VI, IX, and XI affect the liver; types II, IIIa, V, VII, IXd, X, XII, XIII, and XIV affect the muscles, and type IIA, IIb, and PRKAG2 deficiency cause myopathy/cardiomyopathy [2]. Some GSDs, types III and IXb, can affect both the liver and muscles [3]. It is possible to divide GSDs into two macro-groups, according to the affected organ and therefore to its symptomatology. The GSDs, caused by a disorder with hepatic-hypoglycemic pathophysiology, present hypoglycemia and hepatomegaly as symptoms. For this reason, the patient must not be at risk of hypoglycaemic crisis through small and frequent meals [4], causing a lowering of the salivary pH, damaging the enamel, and, consequently, favoring the development of dental caries. In the same macro-group of GSDs, particularly in the GSD Ib, neutropenia and neutrophil dysfunction may cause gingival or periodontal diseases and oral ulcers [5]. Moreover, the literature reports that some GSDs with hepatic involvement, such as the GSD I, present oral manifestations such as ulcers, periodontitis, and delay in the eruption of dental elements [6]. Disorders with muscle-energy physiopathology represent the second macro-group of GSDs. Their symptomatology mainly concerns the muscular function, with pains and cramps due to a plasma increase in the muscular enzymes, leading into the chewing muscles’ weakness and, consequently, difficulties with mastication function [5]. The purpose of this systematic review is to identify and classify oral manifestations in this group of diseases.
2. Materials and Methods
We performed a systematic review of the literature using different search engines (PubMed, ISI Web of science, and Cochrane Library). The employed MeSH terms were: glycogen storage disease, glycogenosis, oral, mouth, buccal, teeth, tooth, dental, enamel, malocclusion, malocclusions, palate, palatal, tongue, lingual, gum, gums, lip, lips, cheek, cheeks, maxilla, maxillary, mandible, mandibular, craniofacial (Supplementary material—Table S1). Search operations ended in November 2019. The review was performed following the PICOS criteria and PRISMA checklist. The populations of interest were male and female patients, of all ages, with genetically confirmed diagnosis of glycogen storage disease, characterized by alterations of soft and hard tissues of the oral cavity (intervention); comparison was no intervention. Study designs included: comparative studies, cross-sectional studies, retrospective studies, prospective studies, survey studies, case series and case reports. Our aim was to identify and classify oral manifestations in GSD. The included criteria of the examined articles were: presence of manifestations in tissues and anatomical structures of the oral cavity in humans, published in English and available full text. Review and articles published before 1990 were excluded. The selection took place in different steps: after collecting all the initial results, three reviewers (DR, AR, and DDS) read the titles and abstracts, excluded duplicates, and ruled out all those articles that did not meet the inclusion criteria during this initial analysis. Then, two reviewers (AR and DR) read in depth the full texts of the remaining articles, to better evaluate the content. Quality assessment of non-randomized studies will be based on the Risk of Bias in Non-randomized Studies of Interventions (ROBINS I) assessment tool [7]. This tool evaluates seven bias domains and Each one refers the Risk of Bias (RoB) in five grades: low, moderate, serious, critical and no information. The overall evaluation is based on the combination of these seven domains. A study based on a non-randomized design rarely presents a low level of RoB.
The review was submitted and registered on PROSPERO (registration number: CRD42018108049).
3. Results
A total of 757 articles were identified in the initial search. Of those, 345 were duplicates and the other 412 were original articles. Among these, 317 did not match our selection criteria. Reading the full-text version led to the exclusion of further 50 articles. In the end, we identified 45 articles that met our criteria (Figure 1). The PICOS information about the 45 articles and their main contents is summarized in Table 1.
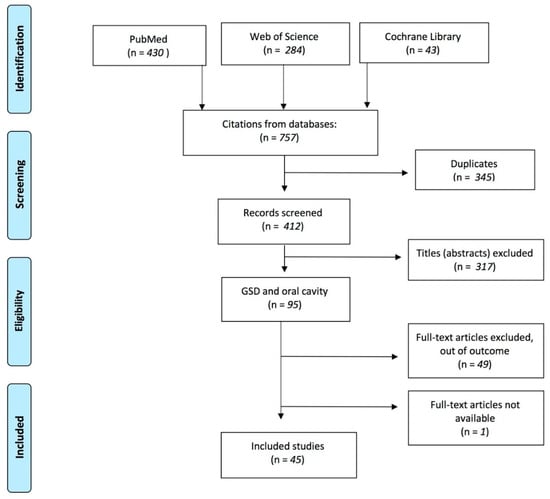
Figure 1.
The PRISMA flow diagram summarizes the steps in the selection process.

Table 1.
First author and year of publication, PICOS information and oral manifestation reported in the 45 articles.
These 45 items, selected by type of GSD found in our research, were divided into 6 groups based on the type of GSD.
3.1. Risk of Bias in Individual Studies
Through the evaluation of the 45 analyzed studies, 38 are classified as overall moderate RoB (MR) and 7 as serious overall RoB (SR). In particular, in 40 studies, the recruited patients were volunteers; in 38 studies, the biases due to deviation from the expected intent and in the measurement of the results were found to be MR because the blind was not expected in the study design by the patients, health providers and the outcome assessors. In conclusion, in the analysis of the 45 non-randomized studies, no study appears to have a critical RoB in the individual domain or in the analysis of the overall domain and, therefore, all the studies provide sound evidence (Supplementary Material—Table S2).
3.2. GSD Ia
Three articles [8,9,10] described oral manifestations in patients with GSD Ia. Avsar [8] describes a single case of a 10-year-old boy who presented a tooth eruption delay, reduced dimensions of the craniofacial complex, taurodontism and multiple caries. DeIIinger et al. [9] reported a low level of oral hygiene of the patient with the presence of multiple caries. Duplan et al. [10] evaluated dental and periodontal health in 60 patients with GSD and, among them, 25 had GSD Ia. The mean DMFT of these patients was 2.8, the prevalence of agenesis was 28 percent (n = 7) and delayed tooth eruption was observed in 2 patients. Moreover, 10 patients presented tooth shape anomalies (taurodontism, short roots and microdontia) and localized alveolar bone loss was found in 6 patients.
3.3. GSD Ib
Seventeen articles [4,6,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25] described a correlation between GSD Ib and oral manifestations. Duplan et al. [10], in their study of 60 patients with GSD, examined 4 patients with GSD Ib These patients had a mean DMFT of 5.5 and all were affected by mild periodontitis. As a result of periodontitis, two patients presented severe alveolar bone loss and one patient a mild generalized alveolar bone loss. In addition, a patient had tooth shape anomalies. Prasad et al. [11], Amaral et al. [12] and Mortellaro et al. [6], in their three case reports articles, described the presence of Giant Cell Tumor (GCT) of the mandible in patients receiving Granulocyte Colony Stimulating Factor (G-CSF). However, it is currently not possible to determine whether there is a cause and effect relationship between G-CSF therapy and the development of GCT in GSD type 1b. Dagli et al. [25] reported the clinical conditions of three patients with GSD Ib in pregnancy: women simply presented oral ulcers during gestation. In a study by Dieckgraefe et al. [13] on the correlation between GSD Ib and IBD, 25 percent of patients (9 out of 36) had oral manifestations such as ulcers or periodontitis. Kishnani et al. [15] reported the presence of dry lips and “beefy red appearance” of the tongue and oral mucosa in a patient with GSD Ib suffering from nutritional deficiency. Wendel et al. [16], Schroeder et al. [17], and Ma et al. [19], in their case reports, described the presence of oral ulcers, gingival bleeding and periodontitis in three patients with GSD Ib. Salapata et al. [20], in their case report on three patients, also reported the presence of oral ulcers, gingival bleeding and periodontitis. Geographic tongue was also present in one patient. Barret et al. [21] reported oral complications in a 22-year-old patient with GSD Ib, on particular a fissure next to the right edentulous mandibular alveolar ridge, a traumatic ulceration both in the buccal extension of the lower denture and on the upper tongue. In 1995, Dougherty et al. [22] presented a case of a pediatric patient with early bone loss resulting in premature loss of dental elements. Bone defects, with a periodontal index of 4, in association with gingival bleeding and the presence of oral ulcers and candidiasis in a nine-year pediatric patient were also described by Bartoli et al. [23]. Three other case reports concerning the oral health of pediatric patients were found in our research. In the first [4], the patient had oral ulcers, gingivitis, delay in eruption time and hypodontia of primary teeth; in the second [24], the patient was affected by oral ulcers, enamel decalcification, tooth decay hard swelling in the left cheek and presence of diffuse oral mucosa inflammation in the area of the maxillary left primary canine and first primary molar. In the last case report [18], the child presented only oral ulcers.
3.4. GSD II
Fifteen articles [5,26,27,28,29,30,31,32,33,34,35,36,37,38,39] related oral manifestations and GSD II. Nine of these articles [5,14,26,27,29,30,33,34,35,36,38] described masticatory problems, swallowing problems and lingual weakness. Macroglossia is the second most common manifestation in this type of GSD [29,32,36]. A particular case report was described by Gijt et al. [39], where the patient showed an abnormal gingival overgrowth and Angle’s third-class malocclusion. Huie et al. [28] described the presence of severe and bilateral cleft lip and palate in a patient with GSD II. Milisenda et al. [37], in 2016, described unusual manifestations in a patient with GSD II, such as bright tongue, pseudohypertrophy of the tongue, and tumor on the right side of the atrophic tongue.
3.5. GSD III
The presence of oral manifestations in patients with GSD III is described in three articles [31,40,41]. Baccetti et al. [40] described dental and skeletal atypia in a child with GSD III who presented taurodontism in primary dentition and second-class malocclusion. Cleary et al. [41] described facial appearance in two pediatric patients with GSD III: the first patient was characterized by the presence of a bow-shaped upper lip with a thin vermillion, the second one by a short labial philtrum. Both patients had midface hypoplasia. Horvath et al. [31] described lingual weakness in three patients.
3.6. GSD V
Only two articles included in our study showed the presence of oral manifestations in patients with GSD V. Both articles reported painful symptoms, such as the presence of cramps, and therefore masticatory difficulty in these patients [42,43].
3.7. GSD XIV
We found a correlation between GSD XIV and oral cavity in three studies [44,45,46]. Wong et al. [44], in a study with 27 patients, described the presence of cleft palate in 19 patients and bifid uvula in other 18 patients. Loewenthal et al. [45], in their study, also described patients with cleft palate and uvula bifida. In addition, one of their patients had Pierre–Robin syndrome. The presence of cleft palate in a patient with GSD XIV is also described by Ondruskova et al. [46].
4. Discussion
GSD Ia is characterized by the deficiency of glucose-6-phosphatase activity. The main clinical manifestation is hypoglycemia, but patients also have high lactatemia, uric acid and triglyceride values. In addition, these patients have growth retardation and hepatomegaly [10]. The frequent intake of carbohydrates, to support blood glucose levels, provides a substrate for oral cariogenic bacteria by implementing the risk of developing caries. Furthermore, the generalized growth retardation of these subjects could also explain the eruption delay of the dental elements [8,9]. GSD Ib is a variant of GSD Ia; it is due to the defect of the glucose-6-phosphate transporter and presents further manifestations, such as neutropenia, altered neutrophil migration and bactericidal activity [16]. Patients are more susceptible to oral ulcers, periodontitis and oral cavity infections [16,19]. Oral hygiene seems to be less effective in patients with GSD; Kidd et al. reported that toothbrushing may be overlooked at night, when the nasogastric tube is inserted for the overnight feeds, and in the morning, because the child needs a carbohydrate intake on waking once the nasogastric tube is removed [47]. In this way, dental prevention needs to be the basis for patients with GSD. Reinforced oral hygiene education, fluoride supplementation, and frequent dental appointments should be necessary to minimize the risk of oral issues and from increased cariogenic exposures [9].
In some reported articles, GSD I is associated with other systemic diseases. In particular, the work of Dieckgraefe et al. [13] found a strong association between GSD Ib and inflammatory bowel disease (IBD). The authors reported several intraoral manifestations that could be related to the IBD, in which the oral presentations are well documented, and therefore cannot be directly attributable to GSD. Kishnani et al. [15] reported the presence of dry lips and “beefy red appearance” of the tongue and oral mucosa in a patient with GSD Ib suffering from nutritional deficiency. In this case, also, the oral manifestations could not be strictly related to the GSD, but rather to the lower serum iron, folate and vitamin B12 levels, caused by the special diet with several restrictions.
GSD III is due to a deficiency of the enzyme amylo-1,6-glucosidase; in this pathology, the most common symptoms are hepatomegaly, hypoglycaemia, recurrent diseases and infections. In addition, these patients are also affected by failure to thrive [48]. Muscle involvement, instead, is more present in GSD IIIa, a subtype of GSD III. According to Baccetti et al. [40], the cranio-facial skeletal anomalies could derive from an incorrect cervico-lumbar posture due to hepatomegaly, together with oral respiration caused by reduced lung expansion. Muscular hypotrophy could also play a decisive role in dysmorphogenesis [41]. GSD II is caused by a deficiency of the acid alpha-1,4-glucosidase enzyme, which leads to an accumulation of glycogen in the muscle tissues. This glycogen storage causes muscle weakness and macroglossia, affecting the chewing and swallowing abilities of patients. Curiously, a single case report reported a massive gingival overgrowth. The authors, even if unable to fully explain the oral manifestation, hypothesized that a mixture of chronic inflammation, dryness of the gingiva, and the slight glycogen accumulation in the fibroblasts might have performed a role in the etiology of the patient’s gingival overgrowth.
The manifestations in patients with GSD V are comparable to those with GSD II. GSD V also affects the muscles and this is due to the deficiency of muscular phosphorylase [43]. Patients show a muscular intolerance syndrome to the effort, associated with myalgia, cramps, fatigue and muscle weakness. GSD XIV is caused by phosphoglucomutase-1 deficiency; manifestations such as cleft palate, bifid uvula and Pierre–Robin syndrome are characteristic of this pathology [45]. The main critical issue of this systematic review is that most of the oral manifestations are not described by dentists, therefore the different methodological approach to the patient makes the comparison of studies particularly complex. A further problem is that two articles [47,49] describing oral manifestations in patients with different types of GSD did not specify which types of GSD the oral manifestation corresponded to. Patients are more susceptible to oral ulcers, periodontitis and oral cavity infections [16,19]. GSD III is due to a deficiency of the enzyme amylo-1,6-glucosidase; in this pathology, the most common symptoms are hepatomegaly, hypoglycaemia, recurrent diseases and infections. In addition, these patients are also affected by failure to thrive [48]. Muscle involvement, instead, is more present in GSD IIIa, a subtype of GSD III. According to Baccetti et al. [40], the cranio-facial skeletal anomalies could derive from an incorrect cervico-lumbar posture due to hepatomegaly, together with oral respiration caused by reduced lung expansion. Muscular hypotrophy could also play a decisive role in dysmorphogenesis [41]. The manifestations in patients with GSD V are comparable to those with GSD II. GSD V also affects the muscles, and this is due to the deficiency of muscular phosphorylase [43]. Patients show a muscular intolerance syndrome to the effort, associated with myalgia, cramps, fatigue and muscle weakness. GSD XIV is caused by phosphoglucomutase-1 deficiency; manifestations such as cleft palate, bifid uvula and Pierre–Robin syndrome are characteristic of this pathology [45].
Limitations of the Study
The limits regarding this review should be considered. Most of the articles selected were case reports and there is currently no way to validate the quality standard of this type of study. For this reason, we have adapted the ROBINS-I tool [7] to evaluate the quality of the selected case reports. In addition, the inclusion criteria we used in our search strategy enabled us to analyze 45 articles. In vitro, non-English and pre-1990 studies have not been selected. Nonetheless, the exclusion of these papers should be considered as a limitation of this review.
5. Conclusions
Although considered a rare disease, GSDs can present a varied number of oral manifestations. Therefore, it is of great importance for the oral medicine specialist to know and classify them. For example, patients belonging to the macro-group of GSDs with hepatic involvement showed a propensity to develop inflammatory/infectious manifestations of the oral cavity, while patients with GSD muscular problems did not present particular alterations of the oral mucosae or the dental elements. Therefore, the dentist should perform careful management and prevention work in patients with GSD with liver involvement. Further studies should be carried out in order to produce a comparable statistical analysis and increase the quality and quantity of information regarding the oral and dental management of this kind of patients.
Supplementary Materials
The following are available online at https://www.mdpi.com/2076-3417/10/19/6720/s1, Table S1: MeSH list used in the search and the combinations used in the search strategy, Table S2: risk of bias assessment for individual studies. Low risk (LR), moderate risk (MR), serious risk (SR), and critical risk (CR) or not interpretable (UR).
Author Contributions
A.R. and D.R. contributed equally to this article, so they are co-first authors. Conceptualization, D.D.S. and R.S.; methodology, A.L.; software, M.C.; validation, F.d.V., A.L. and D.D.S.; formal analysis, D.R.; investigation, A.R.; resources, R.S.; data curation, D.D.S.; writing—original draft preparation, A.R. and D.R.; writing—review and editing, D.L.; visualization, A.L.; supervision, R.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
This research received the grant from the Multidisciplinary Department of Medical-Surgical and Dental Specialties, University of Campania “L. Vanvitelli” funding program VALERE.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhang, X.; Lerman, L.O. The metabolic syndrome and chronic kidney disease. Transl. Res. 2017, 183, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.I.; Medrano, C.; Navarrete, R.; Desviat, L.R.; Merinero, B.; Rodríguez-Pombo, P.; Vitoria, I.; Ugarte, M.; Pérez-Cerdá, C.; Pérez, B. Molecular diagnosis of glycogen storage disease and disorders with overlapping clinical symptoms by massive parallel sequencing. Genet. Med. 2016, 18, 1037–1043. [Google Scholar] [CrossRef] [PubMed]
- Saudubray, J.-M.; Baumgartner, M.R.; Walter, J. Inborn Metabolic Diseases; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Farrington, F.H.; Duncan, L.L.; Roth, K.S. Looking a gift horse in the mouth: Effects of cornstarch therapy and other implications of glycogen storage disease on oral hygiene and dentition. Pediatric. Dent. 1995, 17, 311–314. [Google Scholar]
- Horvath, J.J.; Austin, S.L.; Case, L.E.; Greene, K.B.; Jones, H.N.; Soher, B.J.; Kishnani, P.S.; Bashir, M.R. Correlation between quantitative whole-body muscle magnetic resonance imaging and clinical muscle weakness in pompe disease. Muscle Nerve 2015, 51, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Mortellaro, C.; Garagiola, U.; Carbone, V.; Cerutti, F.; Marci, V.; Bonda, P.L.F. Unusual Oral Manifestations and Evolution in Glycogen Storage Disease Type Ib. J. Craniofacial Surg. Surg. 2005, 16, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Minozzi, S.; Cinquini, M.; Gianola, S.; Castellini, G.; Gerardi, C.; Banzi, R. Risk of bias in nonrandomized studies of interventions showed low inter-rater reliability and challenges in its application. J. Clin. Epidemiol. 2019, 112, 28–35. [Google Scholar] [CrossRef]
- Avsar, A. Dental findings in a child with glycogen storage disease type IA. Quintessence Int. 2007, 38, e36–e40. [Google Scholar]
- Iinger, T.M.D.; Livingston, H.M.; Holder, R.; Streckfus, C.F. Glycogen storage disease and von Willebrand’s disease implications for dental treatment: Dental management of a pediatric patient. Spec. Care Dent. 1998, 18, 243–246. [Google Scholar] [CrossRef]
- Duplan, M.B.; Hubert, A.; le Norcy, E.; Louzoun, A.; Perry, A.; Chaussain, C.; Labrune, P. Dental and periodontal manifestations of glycogen storage diseases: A case series of 60 patients. J. Inherit. Metab. Dis. 2018, 41, 947–953. [Google Scholar] [CrossRef]
- Prasad, R.; Estrella, J.; Christodoulou, J.; McKellar, G.; Tchan, M.C. A Third Case of Glycogen Storage Disease IB and Giant Cell Tumour of the Mandible: A Disease Association or Iatrogenic Complication of Therapy. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2017; Volume 42, pp. 5–8. [Google Scholar]
- do Amaral, F.R.; Carvalho, V.M.; Fraga, M.G.; Amaral, T.M.P.; Gomes, C.C.; Gomez, R.S. Oral Giant Cell Granuloma in a Patient with Glycogen Storage Disease. Open Dent. J. 2009, 3, 144–146. [Google Scholar] [CrossRef][Green Version]
- Dieckgraefe, B.; Korzenik, J.; Husain, A.; Dieruf, L. Association of glycogen storage disease 1b and Crohn disease: Results of a North American survey. Eur. J. Pediatrics 2002, 161, S88–S92. [Google Scholar] [CrossRef]
- Jones, H.N.; Muller, C.W.; Lin, M.; Banugaria, S.G.; Case, L.E.; Li, J.S.; O’Grady, G.; Heller, J.H.; Kishnani, P.S. Oropharyngeal dysphagia in infants and children with infantile Pompe disease. Dysphagia 2010, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Kishnani, P.S.; Boney, A.; Chen, Y.T. Nutritional deficiencies in a patient with glycogen storage disease type Ib. J. Inherit. Metab. Dis. 1999, 22, 795–801. [Google Scholar] [PubMed]
- Wendel, U.; Schroten, H.; Burdach, S.; Wahn, V. Glycogen storage disease type Ib: Infectious complications and measures for prevention. Eur. J. Pediatrics 1993, 152, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, T.; Hildebrandt, B.; Mayatepek, E.; Germing, U.; Haas, R. A patient with glycogen storage disease type Ib presenting with acute myeloid leukemia (AML) bearing monosomy 7 and granulocyte colony-stimulating factor (G-CSF): A case report. J. Med. Case Rep. 2008, 5, 1–5. [Google Scholar]
- Bhattacharya, K.; Heaton, N.; Rela, M.; Walter, J.H.; Lee, P.J. The benefits of liver transplantation in glycogenosis type Ib. J. Inherit. Metab. Dis. 2004, 27, 539–540. [Google Scholar] [CrossRef]
- Ma, R.; Vaziri, F.M.; Sabino, G.J.; Sarmast, N.D.; Zove, S.M.; Iacono, V.J.; Carrion, J.A. Glycogen storage disease IB and severe periodontal destruction: A case report. Dent. J. 2018, 6, 53. [Google Scholar]
- Salapata, Y.; Laskaris, G.; Drogari, E.; Harokopos, E.; Messaritakis, J. Oral manifestations in glycogen storage disease type 1b. J. Oral Pathol. Med. 1995, 24, 136–139. [Google Scholar]
- Barrett, A.P.; Buckley, D.J.; Katelaris, C.H. Oral complications in type 1B glycogen storage disease. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 174–176. [Google Scholar]
- Dougherty, N.; Gataletto, M.A. Oral sequelae of chronic neutrophil defects: Case report of a child with glycogen storage disease type 1b. Pediatrics Dent. 1995, 17, 224–229. [Google Scholar]
- Bartoli, A.; Bossù, A.; Sfasciotti, G.; Polimeni, A. Glycogen Storage Disease type Ib: A paediatric case report. Eur. J. Pediatrics Dent. 2006, 7, 192–198. [Google Scholar]
- Katz, J. Oral manifestations and anesthesia considerations in a child with glycogen storage disease type 1b: Case report. Pediatrics Dent. 1997, 19, 123–126. [Google Scholar]
- Dagli, A.I.; Lee, P.J.; Correia, C.E.; Rodriguez, C.; Bhattacharya, K.; Steinkrauss, L.; Stanley, C.A.; Weinstein, D.A. Pregnancy in glycogen storage disease type Ib: Gestational care and report of first successful deliveries. J. Inherit. Metab. Dis. 2010, 33, 151–157. [Google Scholar] [CrossRef]
- Jones, H.N.; Crisp, K.D.; Asrani, P.; Sloane, R.; Kishnani, P.S. Quantitative assessment of lingual strength in late-onset Pompe disease. Muscle Nerve 2015, 51, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Margolis, M.L.; Howlett, P.; Goldberg, R.; Eftychiadis, A.; Levine, S. Obstructive Sleep Apnea Syndrome in Acid Maltase Deficiency. Chest 1994, 105, 947–949. [Google Scholar] [CrossRef] [PubMed]
- Huie, M.L.; Kasper, J.S.; Arn, P.H.; Greenberg, C.R.; Hirschhorn, R. Increased occurrence of cleft lip in glycogen storage disease type II (GSDII): Exclusion of a contiguous gene syndrome in two patients by presence of intragenic mutations (Am J Med Genet 85: 5-8(1999)). Am. J. Med. Genet. Part A 2005, 137A, 114. [Google Scholar]
- Pichiecchio, A.; Rossi, M.; Cinnante, C.; Colafati, G.S.; de Icco, R.; Parini, R.; Menni, F.; Furlan, F.; Burlina, A.; Sacchini, M.; et al. Muscle MRI of classic infantile pompe patients: Fatty substitution and edema-like changes. Muscle Nerve 2017, 55, 841–848. [Google Scholar]
- Maggi, L.; Salerno, F.; Bragato, C.; Saredi, S.; Blasevich, F.; Maccagnano, E.; Pasanisi, B.; Danesino, C.; Mora, M.; Morandi, L. Familial adult-onset Pompe disease associated with unusual clinical and histological features. Acta Myol. 2013, 32, 85–90. [Google Scholar]
- Horvath, J.J.; Austin, S.L.; Jones, H.N.; Drake, E.J.; Case, L.E.; Soher, B.J.; Bashir, M.R.; Kishnani, P.S. Bulbar muscle weakness and fatty lingual in fi ltration in glycogen storage disorder type IIIa. Mol. Genet. Metab. 2012, 107, 496–500. [Google Scholar]
- Fecarotta, S.; Ascione, S.; Montefusco, G.; della Casa, R.; Villari, P.; Romano, A.; del Giudice, E.; Andria, G.; Parenti, G. Improvement of dysphagia in a child affected by Pompe disease treated with enzyme replacement therapy. Ital. J. Pediatrics 2013, 39, 30. [Google Scholar]
- Dubrovsky, A.; Corderi, J.; Lin, M.; Kishnani, P.S.; Jones, H.N. Expanding the phenotype of late-onset pompe disease: Tongue weakness: A new clinical observation. Muscle Nerve 2011, 44, 897–901. [Google Scholar] [CrossRef] [PubMed]
- Szklanny, K. Analysis of voice quality in patients with late-onset Pompe disease. Orphanet J. Rare Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- de Pinto, W.B.V.; de Souza, P.V.S.; Bortholin, T.; Naylor, F.G.M.; Oliveira, A.S.B. Abnormal tongue features as a clinical clue for late-onset Pompe’s disease. Arq. Neuro-Psiquiatria 2017, 75, 835–836. [Google Scholar] [CrossRef] [PubMed]
- Felice, K.J.; Alessi, A.G.; Grunnet, M.L. Clinical variability in adult-onset acid maltase deficiency: Report of affected sibs and review of the literature. Medicine 1995, 74, 131–135. [Google Scholar] [CrossRef]
- Milisenda, J.C.; Pujol, T.; Grau, J.M. Not only bright tongue sign in Pompe disease. Neurology 2016, 87, 1629–1630. [Google Scholar] [CrossRef]
- Hobson-webb, L.D.; Jones, H.N.; Kishnani, P.S. Oropharyngeal dysphagia may occur in late-onset Pompe disease, implicating bulbar muscle involvement. Neuromuscul. Disord 2013, 23, 319–323. [Google Scholar] [CrossRef]
- de Gijt, J.P.; van Capelle, C.I.; Oosterhuis, J.W.; van der Ploeg, A.T.; van der Wal, K.G.H. Gingival Overgrowth in Pompe Disease: A Case Report. J. Oral Maxillofac. Surg. 2011, 69, 2186–2190. [Google Scholar] [CrossRef]
- Baccetti, T.; Pierleoni, L.; Filippi, L.; Donati, M.A.; Tollaro, I.; Zammarchi, E. Dental and craniofacial findings in a child affected by glycogen storage disease type III. J. Clin. Pediatrics Dent. 1994, 19, 55–60. [Google Scholar]
- Cleary, M.A.; Walter, J.H.; Kerr, B.A.; Wraith, J.E. Facial appearance in glycogen storage disease type III. Clin. Dysmorphol. 2002, 11, 117–120. [Google Scholar] [CrossRef]
- Thornhill, M.H. Masticatory muscle symptoms in a patient with McArdle’s disease. Oral Surg. Oral Med. Oral Pathol. Oral Radiol Endodontol. 1996, 81, 544–546. [Google Scholar] [CrossRef]
- Kouwenberg, C.V.; Voermans, N.C.; Quinlivan, R.; van den Engel-Hoek, L. Mastication and Oral Motor Function in McArdle Disease: Patient Reported Complaints. J. Neuromuscul. Dis. 2018, 5, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.Y.-W.; Beamer, L.J.; Gadomski, T.; Honzik, T.; Mohamed, M.; Wortmann, S.B.; Holmefjord, K.S.B.; Mork, M.; Bowling, F.; Sykut-Cegielska, J.; et al. Defining the Phenotype and Assessing Severity in Phosphoglucomutase-1 Deficiency. J. Pediatrics 2016, 175, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Loewenthal, N.; Haim, A.; Parvari, R.; Hershkovitz, E. Phosphoglucomutase-1 deficiency: Intrafamilial clinical variability and common secondary adrenal insufficiency. Am. J. Med. Genet. Part A 2015, 167, 3139–3143. [Google Scholar] [CrossRef] [PubMed]
- Nina Ondruskova, H.H.; Honzik, T.; Vondrackova, A.; Tesarova, M.; Zeman, J. Department. Glycogen storage disease-like phenotype with central nervous system involvement in a PGM1-CDG patient Nina. Neuroendocrinol. Lett 2014, 35, 137–141. [Google Scholar]
- Kidd, S.A.; Rademeyer, C.; Roberts, G.J.; Lee, P.J.; Lucas, V.S. Dental disease indices and caries-related microflora in children with glycogen storage disease. Int. J. Paediatr. Dent. 2002, 12, 8–13. [Google Scholar] [PubMed]
- Kishnani, P.S.; Austin, S.L.; Abdenur, J.E.; Arn, P.; Bali, D.S.; Boney, A.; Chung, W.K.; Dagli, A.I.; Dale, D.; Koeberl, D.; et al. Diagnosis and management of glycogen storage disease type I: A practice guideline of the American College of Medical Genetics and Genomics. Genet. Med. 2014, 16, 1–29. [Google Scholar] [CrossRef]
- Martinez, C.C.; Tonon, T.; Nalin, T.; Refosco, L.F.; de Souza, C.F.M.; Schwartz, I.V.D. Feeding Difficulties and Orofacial Myofunctional Disorder in Patients with Hepatic Glycogen Storage Diseases. In JIMD Reports; Springer: Berlin/Heidelberg, Germany, 2018; Volume 45, pp. 21–27. [Google Scholar]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).