Abstract
Environmental pollution is caused by the unsustainable use of nitrogen (N) fertilizers and pesticides. Biochar (BC) is a carbon-based material applied to remove excess nutrients and pesticides from the environment. In pot experimental research, N fertilizer and pesticides alone and different biochar types were applied in the soil to evaluate cauliflower growth, soil quality, and leaching of agricultural contaminants. BC addition had increased nutrient availability based on feedstock origin. The surface structure results by SEM showed that the BC pore size was equal to 8.94 and 7.24 µm for mixed biochar and wood biochar, respectively. Nitrate concentrations in percolation water were 43.78 and 76.82 mg/L in mixed biochar and wood biochar, respectively. In soil treated with fertilizer and pesticides, was equal to 106.76 mg/L. Biochar’s binding with pesticides depends on its nature and structure. Adding wood biochar significantly reduced the leaching of fungicide compared to unamended soil, with a contraction of 327.86 and 3576 ng/L. Mixed biochar was more efficient for herbicide mitigation. FTIR was used to identify the functional groups on biochar-amended soil that play a role in the adsorption of agricultural compounds. Research shows that the BC application greatly affects the pesticide fate and N compounds of agricultural origin in soil.
1. Introduction
Nitrogen fertilizers and pesticides are commonly used in agriculture to ensure crop yield [1]. At the same time, the compounds from agricultural sources cause environmental problems including soil quality loss, acidification, soil salinization, greenhouse gases, and water pollution. Enriching the environment with nitrogenous nutrients, such as nitrate, causes pollution of surface and groundwater [2]. High levels of nitrate in water can affect human health. Therefore, reducing excess nitrate in the environment is a significant ecological and social challenge.
Agricultural pollutants include pesticides widely used to resist plant pests. Excessive and inappropriate use of pesticides can result in significant environmental and food damage. Pesticides are harmful to human health, causing neuropathy, skin irritation, and allergic reactions in acute poisoning [3,4]. Pesticide pollution alters the physicochemical properties of soils, negatively affecting microbial activities and the degradation process of organic matter [5]. These compounds are potentially toxic to other organisms, including humans, and must be used safely and disposed of properly. According to the latest report on pesticide analysis, pesticide use trends in fruits increased from 25% to 111% from 2011 to 2020 [6]. Out of all the substances discovered through analytical activities, the European Commission has classified 53 as “more dangerous pesticides” to human health and/or the environment based on specific criteria determined by the authorities during the approval process [7]. Among these, we mention Bromoxynil, Pendimethalin, and Quinoxyfen for their ability to bioaccumulate, Chlorantraniliprole, DDT, Ethofenprox, Pirimicarb, Triallate, for resistance in the water, soil, and sediment, Azinphos-ethyl, Azinphos-methyl, Chlorfenvinphos, Cypermethrin, Deltamethrin, Dimethoate, Hexachlorobenzene, Imidacloprid, Methamidophos, Omethoate, Phosmet, Spinosad and other because they are dangerous for ecosystem services and highly toxic for bees [8]. Due to its properties, biochar has been aimed as a sustainable and economical soil improver that can be applied to reduce agricultural pollution and enhance yield. Recent studies have shown the biochar’s ability to mitigate the leaching of organic and inorganic pollutants, as well as the adsorption of plant protection products [9].
Biochar (BC) is a stable carbon-rich product derived from the thermal decomposition of biomass (i.e., agricultural and animal wastes) under anoxic conditions. Its richness in aromatic carbon makes it a valuable soil amendment [10]. BC is a carbonaceous material consisting of a highly porous structure and an outer layer rich in carboxyl and phenolic functional groups, which confer high biochemical and thermal stability [11]. Biochar porosity varies from pores of a few nanometers to micrometers. The structure of biochar’s fine pores characterizes the strong adsorption capacity, low solubility, stable physical and chemical properties, and large specific surface area [12]. Also, the high presence of functional groups such as carbonyl groups, carboxylate, phenolic hydroxy group, pyrone, lactone, etc. confers on the biochar a high adsorption capacity of soil inorganic ions and polar and non-polar organic compounds. Due to its high removal efficiency, economic, and environmental advantages, biochar is a promising ecological strategy for removing inorganic and organic pollutants from soil and water. Biochar has the potential to reduce environmental pollution and improve agricultural performance through amendment practice [13]. BC surface, waste material types, matrix pH value, pyrolysis temperature, and the interfering substances of the environmental system are among the factors that influence the rate of adsorption.
The excessive application of N fertilizers in the intensive vegetable production system stimulates nitrification, leading to the accumulation of nitrates in the soil [14], and a reduction in crop yield. N absorbed by crops in intensive agriculture usually accounts for about 10% of used N fertilizer [15]. The adsorption of N contaminants (ammonium and nitrate) is favored mainly by the functional carbonyl, carboxylic, phenolic hydroxyl, and hydroxyl groups of the biochar. A study by Wang et al. [16], shows that the removal efficiency of ammonium () and is positively correlated with the number of acid and base functional groups, respectively. Several types of research have highlighted the potential of biochar in the mitigation of pollution, with a percentage of N removal of about 98% with bamboo biochar [17] and 25–73% with biochar of palm rachis [18].
As shown in Table 1, adding biochar to soil reduces pesticide pollution and human exposure to contaminants due to biochar’s high binding capacity for pesticides. The biochar application in soil, also, reduces pesticide adsorption by plants, probably associated with the high porosity of carbonaceous material [19].

Table 1.
Effect of biochar application on pesticide reduction in soil. I = Insecticide; H = Herbicide; F = Fungicide.
As for bioremediation processes, biochar mitigates pesticide pollution in agricultural soils through the adsorption of the contaminant, promoting its subsequent degradation. Adsorption is a fundamental process in the fate of pesticides in soils and associated ecosystems and in the determination of the accumulation of agricultural contaminants [30]. This method is generally the first physical process that begins as soon as the introduction of pesticides into the soil occurs. Thus, the ability of BC to adsorb pesticides greatly affects chemical transport, leaching, bioavailability, and adverse effects on non-target organisms [31]. As shown in Table 1, biochar adsorbs several pesticide groups, with percentages that differ according to the physical-chemical properties of biochar [32]. Biochar absorption affinity for pesticides depends on both the number and the size of micropores and mesoporous [33]. The high absorption capacity of biochar for pesticides is due to the hydrogen bonds between the pollutant and oxygen groups of biochar [34]. Sun et al. [35] showed that the ability of biochar absorption for atrazine is based on aryl carbon numbers. The addition of organic substrates increases microbial biomass, leading to pesticide biodegradation through bacterial metabolic processes.
The present work is aimed at studying the role of two different types of biochar in nitrogen leaching mitigation and pesticide adsorption due to the necessity of researching sustainable technologies that are fundamental for mitigating agricultural pollution. Biochar’s influence on the degradation of azoxystrobin and spinosad, two pesticides used to protect cauliflower plants, was investigated. The present research aims to aid in understanding the combined effect of nitrogen fertilizers and new types of biochar on cauliflower yield, as well as to highlight new results behind the reuse of waste material in agriculture.
2. Materials and Methods
2.1. Experimental Setup
Greenhouse experimental set-up was conducted in collaboration with Reagri S.r.l. (Massafra, Italy) from October 2021 to February 2022. The research was carried out on late vegetative cycle sprout plants (Rafaele Variety, Vivai La Malfa Antonio and Figli, Policoro, Matera), one of the most cultivated cruciferous plants in the central-southern regions of Italy. Seedlings of Brassica oleracea L. var. botrytis were transplanted into pots about three weeks after the germination phase. Every single pot was 40 × 37 cm large, and each experimental thesis was conducted for four replicates. The soil was composed of neutral sphagnum peat. The main physicochemical characteristics of the soil are as follows: sand 70%, silt 30%, organic matter 25%, total N 0.3%, total C 0.3%, pH 5.8, electrical conductivity 0.514 dS/cm. The plants were irrigated with a system of micro drip spraying and covered with a transparent plastic sheet to avoid rain addition (Figure 1). Each plant had a plastic bottle for collecting leached water to analytical purposes. Vegetative growth occurred under a natural photoperiod.

Figure 1.
Experimental set-up of cauliflower mesocosms. A plastic sheet was used to avoid the rain addition. Plastic bottles were placed under each pot for collecting percolation water.
The level of N fertilizer was equal to 180 kg N ha−1 and was applied as ammonium nitrate to 34% in two phases: flower induction phase and enlargement of the inflorescence, about seven and twelve weeks, respectively from the beginning of the experiment [36]. The fertilization was manual. At the same time, two types of pesticides were sprayed for a time of 8 s per plant. Specifically, an insecticide (480 g spinosad/L) and a fungicide (250 g azoxystrobin/L) were applied in doses reported on the label. Before the transplantation phase, the biochar was applied to a depth of 0–20 cm of the soil. The experimental activity involved the application of two types of biochar: wood biochar (WB) and mixed biochar (MB). The chemical properties of the wood biochar were total N = 0.5%; total K = 0.4%; total P = 0.3%; total Ca = 1.1%; total Na = 0.2%; total Mg = 0.2%; organic carbon content = 68%; pH = 11.3; electrical conductivity = 500 mS/m. WB was in powder. On the contrary, mixed biochar was in the flake form and was constituted by waste agricultural of different origins (agricultural waste, olive pomace, pomace, bran, hazelnuts, fruit shells, and wood processing waste). MB chemical properties were total N = 0.5%; total K = 0.3%; total P = 0.034%; total Ca = 0.9%; total Na = 0.03%; total Mg = 0.08%; organic carbon content = 65%; pH = 9.85; electrical conductivity = 110 mS/m. The chemical properties of both types of biochar are those shown on the label. 5% of the total biochar volume was manually mixed for both types of biochar tested. Four experimental trials were set up in greenhouses: soil without treatment (C), fertilized soil and treated with pesticides (FP), soil FP with the application of wood biochar (FPWB), and soil FP with the application of mixed biochar (FPMB). The experiment ended at the cauliflower harvesting stage for market sale.
2.2. Nitrate and Ammonium Quantification in Environmental Matrices
Soil samples were collected from between 0 and 20 cm soil layers at the end of the cauliflower experiment. Soil samples were conserved at 4 °C and processed within 1 month. Water samples were collected about 10 days after treatment from each plastic bottle and combined for each treatment. Samples were filtered through 0.45 μm pore size membrane filters for chemical analysis. Inorganic N was extracted from the soil with 2 M KCl [37] on a shaker for 1 h at room temperature (20 °C). Tubes were centrifuged (4500× g, 10 min) and the supernatant was decanted into clean cylinders. Aliquots were taken for the quantification of nitrate and ammonium. Nitrate was quantified by ionic chromatography with the Metrohm930 compact ICflex (Metrohm, Herisau, Switzerland), and ammonium by spectrophotometry of the soil and water samples with the PerkinElmer spectrometer Lambda 950.
2.3. Pesticides Extraction and Quantification in Environmental Matrices and Cauliflower Curd
Azoxystrobin and spinosad (isomers A and D) molecules were extracted by adding 10 g of soil to 50 mL centrifuge tubes and mixing it with 40 mL of HPLC-grade acetonitrile (Fisher Scientific, Leicestershire, UK). The tubes were shaken for 1 h, left for 30 min to settle, and centrifuged at 4000 rpm for 2 min. Two mL of the supernatant was decanted into a 2 mL screw-top glass HPLC vial [37] and aliquots of 100 µL for each sample were directly injected into the system by an auto-sampler (VH-A10-A, Thermo Fisher Scientific, Waltham, MA, USA). The instrumental analysis was conducted with a triple quadrupole mass-spectrometer system (TSQ Altis, Thermo Scientific, Waltham, MA, USA) equipped with an Electro Spray Ionisation (ESI) source and coupled to a Vanquish Horizon UHPLC System (Thermo Scientific, Waltham, MA, USA). The operating software was Trace Finder EFS™ 4.1 Thermo Fischer Scientific.
For the analysis of water samples, any extraction was necessary but a large volume injection of aliquots of 100 µL of water was sufficient for the sensitivity.
For cauliflower analysis, the samples were first ground with vibromulin (Retsch MM 301) and then dried in the oven at 30 °C. The collected samples were analyzed using the fast, easy, economical, effective, robust, and safe extraction technique (QuEChERS) [38].
The official QuEChERS AOAC 2007.01 method includes the extraction/partitioning phase where 15 g of the homogenized sample was weighed in a 50 mL tube and was added 15 mL of 1% acetic acid in acetonitril. The solution was shaken and vortexed for 1 min on a 2500 rpm vortex mixer. In the same phase, the liquid-liquid separation from the water in the sample was salted with MgSO4, 6 g of anhydrous MgSO4 and 1.5 g of sodium acetate were inserted into the test tube. Then, it was shaken vigorously for 30 s and centrifuged at 5000 rpm for 5 min.
Solid dispersive phase extraction (dispersive-SPE) follows. It is carried out to remove organic acids, excess water, and other components with a combination of primary secondary amine (PSA) sorbent and MgSO4. The extracts are diluted with water (ratio 1:4, v:v. Optional) and transferred to LC vial for instrumental analysis (volume injection 100 µL).
The chromatographic separation was performed using an Accucore aQ C18 Thermo Scientific column and a gradient of solvent A (95% water, 5% methanol, 0.1% formic acid, ammonium formate buffer 5 mM) and B (95% methanol, 5% water, 0.1% formic acid, ammonium buffer formate 5 mM) and with a flow rate of 0.3 mL/minute.
The triple quadrupole analyzer was operated in SRM (Selected Reaction Monitoring) mode with 0.7 resolution for Q1 and Q3. The analysis was performed with positive ionization of the analytes with an optimized setting of the instrumental parameters obtained by direct infusion of CRM (Certificate Reference Material) standard solutions. The ion source was operated with a spray voltage of 3500 V, a capillary temperature of 325 °C, and a vaporizer temperature of 350 °C. Nitrogen was used as sheath gas (30), sweep gas (1), and auxiliary gas (6); argon was used as collision gas. For each compound, three ion transitions, one quantifier (Q), and two qualifiers (q1,2) were used for the quantification as follows: azoxystrobin (Q) m/z 404.07/329.054, (q1) m/z 404.07/344.111 and (q2) 404.07/372.111; spinosad A (Q) m/z 732.432/98.071, (q1) m/z 732.432/ 99.014(q2) and 732.432/142.111; spinosad D (Q) m/z 746.588/98.095, (q1) m/z 746.588/142.155 and (q2) 746.588/145.
The limit of quantification (LOQ) of the analytical method was 0.005 µg/L and 0.025 µg/L for azoxystrobin and spinosad, respectively. To ensure the quality of the analysis, Initial Precision and Recovery (IPR) was performed before processing real samples. It consists of blank soil samples fortified with both compounds (azoxystrobin and spinosad) at the LOQ and 10 × LOQ concentration levels to check the performance of recovery of the extraction. The extraction was considered satisfactory if the recoveries were in the range of 70–120%.
2.4. Soil Physicochemical Analyses
Soil physicochemical properties were investigated at the experiment end. The soil was sampled at 0–20 cm depth from each pot and mixed manually. The analyses of soil pH, water content percentage, and electrical conductivity were carried out based on the Italian Official Methods of Soil Chemistry approved by the Minister for Agricultural Policies [36]. Available phosphorus analysis was performed spectrophotometrically from an aqueous soil extract by the Olsen Method [39]. Total organic carbon was determined by a TOC analyzer (TOC-L, Shimadzu, Kyoto, Japan). Finally, the percentage of C and N in soil and the C: N ratio were quantified utilizing an elemental analyzer (Thermo Flash 2000, CHNS-O Analyzer, Thermo Scientific, Eindhoven, The Netherlands).
2.5. SEM Analyses
Scanning Electron Microscopy (HITACHI TM 3000 Tabletop, Tokyo, Japan) was utilized to characterize morphological soil structures about agronomic treatments and biochar application. Dried soil particles were fixed on carbon adhesive and covered with a slender layer of gold and palladium for 2 min and 10 mD to avoid charging during analysis. Samples were measured operating at 5 kV. The size of biochar pores was measured by SEM image software (Hitachi TM 3000, ver. 02-03-02, Tokyo, Japan).
2.6. ATR-FTIR Spectral Collection and Data Analysis
The infrared spectra for three replicates of each dried plant curds ground to a powder in a mortar were generated and analyzed by ATR-FTIR spectroscopy. A Nicolet Summit FTIR Spectrometer (ThermoFisher Scientific, Waltham, MA, USA) model, equipped with an Everest ATR with a diamond crystal plate and a DTGS KBr detector, was used for analyses. The IR absorption spectra of the samples were recorded from 400 to 4000 cm−1 at a resolution of 4 cm−1, hoarding 32 scans per spectrum [40]. The spectral information was studied via the OMNIC software (ThermoFisher, Waltham, MA, USA), analyzing the region from 3700 to 400 cm−1.
2.7. Morphologic Growth Parameters
The morphology of leaves and cauliflower curd was characterized by measuring curd length, curd fresh weight, and leaf area (LA). Leaf trait was measured on the second-stage leaves starting from the curd and collected at the experiment end. Data were determined using the ImageJ (Standard Edition 8). It is a public domain license Java-based software (Java™ 8 Platform, Standard Edition) [41] for image processing with which it is possible to calculate area and pixel value statistics of user-defined selections. We can also use it to measure distances and angles. In addition, it is possible to create density histograms and line profile plots.
2.8. Statistical Analysis
Statistical analyses were performed using OriginPro 9.0 software (OriginLab, Northampton, MA, USA). One-way ANOVA and Tukey’s test with a significant difference set at p < 0.05 were conducted. Statistical analyses were performed to evaluate physicochemical soil differences based on the treatments with agrochemical compounds and with/without biochar amendment. The change in the concentration of agrochemical compounds (N compounds and pesticides) in the percolation water was compared using One-way ANOVA with the four experimental lines, with and without biochar, as an independent variable. In addition, plant responses to biochar-based treatments were also studied via the ANOVA test. All figures were made through OriginPro software.
3. Results and Discussions
3.1. The Influence of Biochar on Soil’s Physical-Chemical Properties
Biochar amendment resulted in a strong change in the soil physical-chemical properties of cauliflower mesocosms, such as buffering the soil pH and increasing the soil elements content. The type of biochar applied to the soil is a determining factor in changing soil characteristics [42,43]. The results showed that the mixed biochar (FPMB) favors the increase in pH, available phosphorus, and total content of nitrogen (N) and carbon (C). On the contrary, a higher availability of organic C was observed in soils that were modified with wood biochar (FPWB). Research has shown that treatments with biochar do not affect the electrical conductivity of cauliflower mesocosm soils.
As shown in Table 2, treatments with and without biochar significantly affected pH changes. Soils treated with N fertilizer and pesticides (FP) showed a pH reduction compared to control soil (C), of 6.35 and 6.95, respectively. In Table 2, it is shown that biochar type influenced the changes in soil pH. In particular, the highest pH value (7.17) was measured in soil with mixed biochar. FP treatments resulted in increased Electrical Conductivity (EC), with an average value of 1492.5 µS compared to 1001.75 µS of the control soil. On the contrary, there are no significant differences between biochar-amended soil (Table 2). Biochar type affects the water content in the soil. Statistical analysis has made it possible to identify significant differences between treatments with and without BC. Wood biochar (FPWB) has led to an increase in the physical parameters of soil, followed by FP and FPMB (Table 2). The average value of WB was 191.8 g kg−1, followed by 142.22 and 112.72 g kg−1, respectively for FP and FPMB experimental lines. Significant differences in the available phosphorous (P) content were measured between the experimental treatments tested. Agricultural practices based on N fertilizer and pesticides did not affect the available P in the soils of cauliflower mesocosms. The surface of the mixed biochar (FPMB) promoted the increase in the available P in the soil, with an average value of 64.83 mg kg−1. The agricultural practices tested, and the BC type determined a significant difference in the content of organic C, with a maximum average value of 272.75 g kg−1 in FPWB soil and a minimum in the FPMB line experiment (Table 2). In addition, amended soils with mixed biochar increased the total of C and N. As reported in Table 2, in the FPMB treatment the total carbon value was 42%. The application of MB increased the total nitrogen content, with significant differences between untreated soils (C), FP-treated soils, and FPWB-treated soils.

Table 2.
Overview of the soil physicochemical characteristics of the four experimental treatments at the experiment end. Means (±SE) and p values of ANOVA testing differences between treatments are reported.
Different types of biochar, when amended in several soil types, have been shown to produce a wide range of effects on the soil’s physical properties. Soil pH increased with the application of a specific type of biochar due to the biochar feedstock [36] and surface functional groups of biochar [44]. Several studies have shown that the biochar used in the soil could determine to rise of the pH value to the increase in negative charges on the improver surface and the consequent adsorption of many cations [45,46,47]. However, other studies on the effects of biochar on the pH value are controversial. Lenz and Ippolito [48] highlighted that BC application did not alter the soil pH.
How biochar affects the dynamics of soil nutrients depending on the experimental conditions has been the subject of numerous studies in recent decades, showing that the availability of nutrients varies according to the type of soil, the biochar original material, and the pyrolysis conditions of this. Moreover, the effects of biochar on the dynamics of macronutrients (N, P, C, etc.) in soil depend on the type of culture [49,50]. About available P, inorganic fertilizer co-applied to mixed biochar improved soil P content. Analogously to our study, Adler Phares et al. [51] demonstrated a correlation between available P in soil and pH value (Table 2). Soil organic carbon influences the chemical, physical, and biological fertility of the soil and the biochar application promotes the increase in its content in agricultural soils. Biochar research has revealed that soils treated with the carbon improver show a direct increase in the organic carbon content of the soil [52]. In our experiment, only the wood biochar has increased soil organic carbon content compared to other treatments, similar to the research of Dong et al. [53]. Long-term field or potted experiments increase soil organic carbon due to the high stability of biochar [54]. However, since biochar technologies are relatively recent and agriculture-type-specific, there is limited data available on short-term carbon stabilization when using mixed plant biochar. The above analysis shows that soils with biochar influence the content of nitrogen and total carbon to different degrees, as in the study of Hui et al. [55].
SEM has been used mainly for the biochar type characterization and the detection of the enhancer pores size, a significant factor in adsorbent–adsorbate connections of agricultural origin. As shown in Figure 2, the surface morphology of wood biochar and biochar mixed after absorption was observed. The SEM image (Figure 2c) clearly shows the remains of the woody structure from WB, characterized by smooth streaks racing crosswise from top to bottom. The residual morphologies of wood cells and wood porosity remain considerable characteristics of the final biochar formulation, as shown by several studies [56,57,58]. SEM image (Figure 2d) shows soil amended with wood biochar pores that have bonded adsorbents. MB shows a larger pore size (Figure 2). The pore width was on average 8.94 and 7.24 µm for MB and WB, respectively.
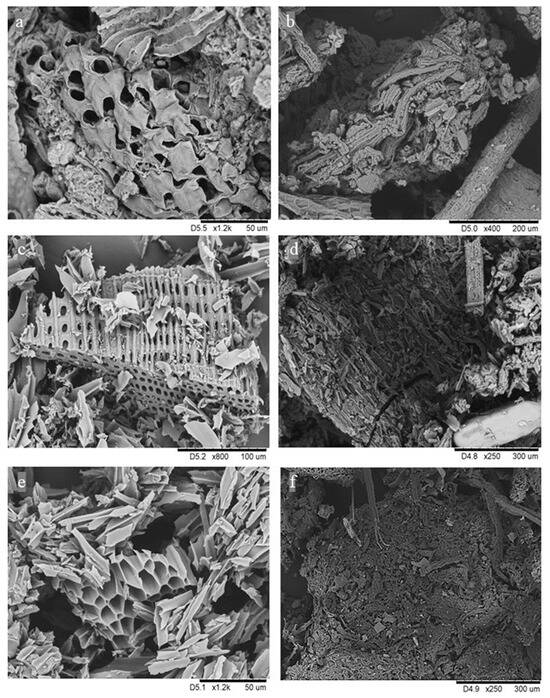
Figure 2.
Scanning Electron Microscopy (SEM) images of (a) control soil; (b) soil fertilized with pesticides; (c) soil fertilized with pesticides and wood biochar (detail of the morphological structure); (d) soil fertilized with pesticides and wood biochar; (e) soil fertilized with pesticides and mixed biochar; (f) soil fertilized with pesticides and mixed biochar (detail of the morphological structure).
3.2. Biochar’s Influence on the Protection of Agricultural Pollutants
3.2.1. Effect of Biochar on Nitrogen Compounds Dynamics
Research has considerably investigated the influence of biochar on the nitrogen nutrient dynamics in soil. Several factors influence changes in nitrogen compound concentrations, including the environment, soil type, biochar raw material, and pyrolysis temperature. Our results show that when biochar was applied to silty–sandy soil, the available N content was significantly decreased as a result of increased -N adsorption. The concentration of ammonium in agricultural soils treated with agricultural compounds (FP) was equal to 41.60 mg/L, unlike biochar-amended soil with a concentration of 28.05 and 30.51 mg/L, respectively for FPMB treatment and FPWB treatment (Figure S1). The research showed that the feedstock and characteristics of the two types of biochar did not represent reasons for changes in the ammonium content in agricultural soils. In addition, statistical analysis of the -N content in soil showed that there were no significant differences in nitrate concentration between the two types of biochar tested during the research activity. On the contrary, the application of biochar compared to non-amended soils has determined the reduction of -N in the soils, more bioavailable for plant growth. Nitrate concentrations in the soil were 142.88, 96.34, and 104.78 mg/L, respectively for FP, FPMB, and FPWB treatments (Figure S1).
Biochar amendments vastly influenced the concentrations of N leached from the soils of cauliflower mesocosms (Table 3), starting from 10 days post-first nitrogen fertilization (about seven weeks after the beginning of the experiment) till the second nitrogen fertilization. concentrations in water percolation persisted significantly lower both in the experimental lines with mixed biochar (22.3 mg/L) and with wood biochar (12.74 mg/L), compared to treatments without biochar (31.08 mg/L). This study shows how the BC type has influenced the different abilities of porous structures to bind N compounds, mitigating the loss of nitrogen in the water. In addition, there were significantly lower concentrations in the percolation waters sampled from biochar treatments, compared to FP treatments, indicating lower leaching of nitrates in soil amended with biochar. Mixed biochar reduced the loss of nitrogen from soils more than wood biochar with an average concentration of nitrate of 43.78 and 76.81 mg/L, respectively (Table 3). Percolation water sampling 10 days after the second treatment with N fertilizers indicated a reduced change in the leaching of nitrogen compounds between treatments with and without biochar (Table 3).

Table 3.
N compound concentrations in water percolation at 10 days after I° and II° N fertilization in experimental lines.
Biochar’s capability in the mitigation of the nitrogen compounds leaching is demonstrated in the results reported in Table 3. The porous structure of the biochar increased the nitrogen elements availability for plants, limiting their loss via leaching. This effect was less noticeable after the second treatment because nitrogen translocation was reduced during culture growth [59]. The immobilization of nitrogen compounds in both types of biochar is likely due to electrostatic interactions with carboxyl groups [60] and O-containing functional groups (e.g., -OH and -C=O) [61]. In addition, wood biochar and mixed biochar have both demonstrated an excellent capacity for adsorbing nitrogen compounds.
3.2.2. The Impact of Biochar on Pesticide Protection in the Environment and Cauliflower
In experimental tests of cauliflower mesocosms, the leaching of azoxystrobin and spinosad is gradually decreased in the water compartment with the biochar application. Specifically, the results showed that the pesticide concentrations in the percolation water change depending on the type of biochar amended to the soil. About the first treatment with pesticides, the wood biochar has reduced the leaching of the fungicide based on azoxystrobin. In contrast, in MB treatments, a lower concentration of insecticide was determined than in FP and FPWB treatments. As shown in Figure 3, azoxystrobin concentration decreased from 339.88 ng/L in treatments without biochar to 165.32 ng/L in FPWB treatment. Regarding the determination of the content of spinosad, there were no significant differences between the different treatments (Figure S2). The amendments had a significant influence (p < 0.05) on the pesticide concentrations in percolation water sampled after the second treatment. The addition of the wood biochar significantly decreased the azoxystrobin compared to unamended soil; in fact, in FP and FPWB treatments, the pesticide concentration has gone from 3576 to 327.86 ng/L. A mixed biochar amendment led to a reduction of the herbicide from 17,991.71 to 1322.46 ng/L in FP and FPMB experimental lines, respectively (Figure S2).
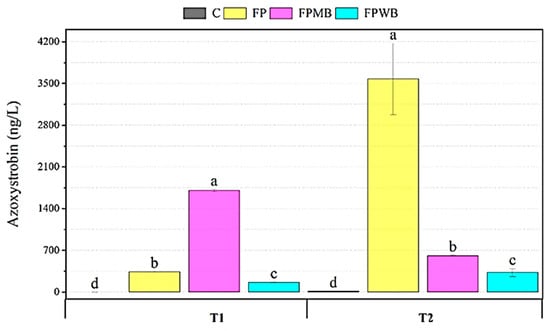
Figure 3.
Box chart of fungicide azoxystrobin-base concentration in percolation water at 10 days post-I° pesticides application (T1) and 10 days post-II° pesticides application (T2). Different letters indicate significant differences (p 0.05) in relation to soil treatments, based on one-way ANOVA and Tukey’s test. Soil without treatment (C), Fertilized soil and treated with pesticides (FP), soil FP with the application of mixed biochar (FPMB), and soil FP with the application of wood biochar (FPWB).
Several studies show that the addition of biochar to cultivated soil can reduce the leaching or outflow of pesticides. Cederlund et al. [62] noted the effect of the addition of wood biochar on the reduction of MCPA (4-cloro-2-metilfenossiacetato di sodio) and Diuron mobility. Leaching was reduced with linear retention coefficients at the thickness of the biochar layer [62]. Palangi et al. [63] have studied the effect of different doses of biochar wheat straw on the leaching of Deltamethrin, in sandy clay soils. Biochar particles strongly bind the pesticide reducing its mobility [63]. It can happen that the pores of the biochar structure undergo deformations or there may be macropores that create a strong bond with pollutants. This explains how the ability of biochar to bind pesticides can depend on the nature and structure of the biochar [64].
Also, in our experiment, we observed differences in relation to treatments with a mix biochar and wood biochar. The latter was a better strategy for azoxystrobin because it limited its leaching compared to treatment with mixed biochar for the same pesticide. As for Spinosad, there are no significant differences between the two types of biochar although, in T2, the mix biochar seems to be more successful than the wood biochar. These differences between the herbicide compared to the fungicide can probably be explained by the different structures of the phytosanitary molecules. In fact, the retention of pesticides by biochar can be affected by the polarity and aromatic character of organic molecules. The donator and acceptor interactions between organic pollutants and biochar can be affected by the aromatic character of pesticides [65]. This can affect the mobility of the pesticide and thus the absorption of the pollutant into the biochar [64].
Another important factor to consider is pH. An inversely proportional relationship has been found between the pH and the dissipation of azoxystrobin in both cultivated soils and water [66,67]. Due to the addition of biochar, soil pH may vary, so this change can result in more or less leaching of azoxystrobin in water [50].
Studies have shown that an increase in the pH unit may favor the permanence of the pesticide in the soil because it reduces the dissipation of fungicide [67]. However, the results observed in this experiment show a more excellent permanence of the pesticide azoxystrobin in the soil with the addition of wood biochar (pH plus control acid) in both application times. In the treatment with biochar mix, the pH of the soil tends to increase compared to the control but greater leaching of the fungicide in the percolation water is obtained. In the case of spinosad, as already mentioned, there are no significant differences in T1. In T2, however, we see an inverted trend compared to azoxystrobin. That is, mixed biochar shows better absorption of the herbicide than wood biochar. Probably the results of this experiment, suggest that more factors may affect the interaction between biochar and azoxystrobin or spinosad in soil. In addition to structure, we must consider the intrinsic surface factors of biochar. Yang et al. [68] have noted that the potential for pesticide absorption by biochar can be caused by the acidic environment created by the functional groups present on the surface of Biochar. This could explain the higher leaching of fungicide in water in the FPMB thesis (pH more acidic) compared to the FPWB thesis. In fact, pesticides with basic pH under acidic conditions promote absorption while neutral pesticides can be polarized to achieve absorption on a charged surface [69].
Soil and curd samples were also analyzed to assess the potential adsorption and/or translocation of the pesticides applied to the cauliflowers into the soil and vegetable matrices. The most significant amount of both pesticide residues (>79%) resulted retained in the soil fraction in each experimental mesocosm. Concentrations of azoxystrobin equal to 204.61, 939.62, and 711.16 µg/kg were found in microcosms treated without biochar, wood, and mixed-based biochar, respectively (Table S1). High spinosad residues were also observed in the soil samples with similar values of 4309, 4720, and 4792 µg/kg detected in treatments without biochar, with wood and mixed biochar, respectively (Table S1). Differently, the presence of biochar considerably affected its translocation in the edible part. In the treated experimental microcosm, where no amendments were applied, 51.78 µg/kg representing 20% of the total azoxystrobin spread, was detected. On the contrary, the application of biochar retained more strongly the binding of the pesticides with the soil resulting in a lower uptake of them in the edible fraction of cauliflower. Indeed, concentrations of 3.17 and 3.10 µg/kg of azoxystrobin were observed in the curds with the presence of mixed and wood biochar, respectively (Figure 4). As shown in Figure 4, values five and six times greater spinosad were observed in the mesocosm where no amendment was applied (330 µg/kg) compared to the treatments with biochar (70.11 µg/kg in the mixed biochar, 79.57 µg/kg the wood biochar). Our results demonstrated how the enhanced porosity of both the biochar-based amendments influenced their ability to stabilize pesticides through their sorption with pores, thus reducing their bioavailability and bioaccumulation in a species of food interest consumed by humans. Moreover, the azoxystrobin residues detected in the cauliflower mesocosms enriched with biochar resulted in values lower than the maximum residue level (MRL) (0.01 mg/kg) [7]. As reported in previous studies, one of the meaningful properties of using biochar as a soil amendment is to reduce pesticide adverse effects [23]. Indeed, a decrement in metalaxyl uptake and translocation was observed by some authors [70] on lettuce grown on soils enriched with woodchip biochar. Other benefits of biochar application as a soil amendment evidenced an increase in microbial activity and diversity after its application accelerating, thus, the biodegradation of pesticides [71]. Moreover, the effect of biochar stimulated the enzymatic activity of soils where 2,4-D and dicamba were applied, reducing their negative effect on soil [72].
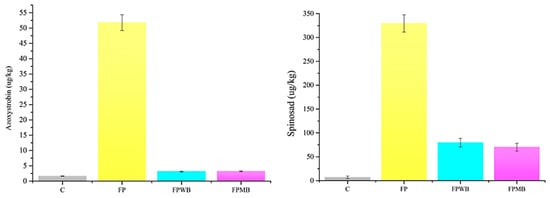
Figure 4.
Histogram of pesticide concentration in cauliflower curd at the end of the growing season. The black line represents the standard error. Spinosad concentration value represents the sum of the isomers A and D. Soil without treatment (C), Fertilized soil and treated with pesticides (FP), soil FP with the application of mixed biochar (FPMB), and soil FP with the application of wood biochar (FPWB).
3.3. Biochar Effect on Brassica oleracea L. var botrytis Growth
The statistically assessed results of the cauliflower curd size data in different N fertilizer treatments, pesticides, and with/without biochar are indicated in Figure S3. The biochar amendment was appropriate to support an ideal curd growth for market sale. However, the average cauliflower curd weight has been affected mainly by nitrogen-based treatment. The weight of curd was higher in cauliflower plants grown in FP soils (793.25 g) than in plants grown in mixed biochar amendment soils (719.5 g) or wood biochar (651.25 g). In contrast, the results for the length of the cauliflower curd showed no significant difference between the different treatments (Figure 5 and Figure S3).
Figure 5.
Cauliflower curds were sampled at the end of the experimental activity.
Biochar application has not significantly increased the leaf area of cauliflower plants grown in soils under different treatments with and without biochar. Although the average leaf area (LA) was larger in amended soil with mixed biochar (FPMB), the increase was not high enough to turn significant (Figure S4). The lowest average values of LA were determined in the control soil plants (110 m2) and amended soil with wood biochar (175 m2).
Curd N concentration of Brassica oleracea L. var. botrytis was significantly lower in both control and FP treatment without biochar but did not differ between the two types of biochar tested. The percentage of N in the cauliflower curd was 3.21, 4.46, and 4.33%, respectively for FP, FPMB, and FPWB treatments (p < 0.001). Curd C concentration was not significantly deferred between the four treatments (p = 0.3). Biochar-amended soil has increased S content in cauliflower curd with average values of 0.48 (FPMB), 0.33 (FPWB), and 0.15% (FP) (p = 0.03).
Typically, using biochar along with nitrogen fertilizers has resulted in successful cauliflower growth and yield. The curds collected from the FP lines showed a larger size, likely due to a higher nutrient accumulation in other parts of the plants [73]. Biochar from mixed waste (FPMB) increased the curd size of cauliflower when amended with nitrogen fertilizer, contrary to wood biochar (FPWB). The physical and chemical properties of soil, as well as environmental factors and experimental conditions, affect plant growth and agricultural yield [74]. For instance, You et al. [75] describe that wood biochar mixed with inorganic fertilizers did not improve the growth of Mesembryanthemum crystallinum L., as well as in studies conducted on Chenopodium quinoa by Kammann [76]. Adekiya et al. [76], instead, highlight that the best yield of sweet potato under wood biochar compared to soils without biochar was associated with an increase in the physical characteristics of the soil, such as increased porosity and moisture content.
The evaluation of biochar on the growth of cauliflower is being researched by only a few researchers; of fact, Tarar et al. [77] have evidenced a positive correlation between the application of biochar and the length of the buds and the root of three different vegetable cultures (Red Okra, cucumbers, and broccoli). Researchers show that biochar treatments enhance plant growth based on nitrogen compound dynamics, about the increased availability of nutrients and reduced loss of compounds due to leaching [77]. In line with the research of Doan et al. and Yan et al. [78,79] our study found that both nitrogen treatments and nitrogen-fertilized soils with biochar have increased the growth of cauliflower curd (Figure S3).
Spectra from the Brassica oleracea L. var. botrytis curd reflect important biochemical components characteristic of plants of the genus Brassica. The market product of cauliflower showed visible differences depending on the type of soil treatment, so both were based on treatment with nitrogen fertilizers and pesticides with or without two biochar topologies (mixed biochar and wood biochar). There were visible differences in the intensity of the peaks at 3290 cm−1 (assigned to the O-H stretching of alcohols or phenols) between untreated control plants and FPWB soil plants (Figure S5). In contrast, the comparison of FTIR spectra on the 1742 cm−1 region of carboxylic acids C=O, shows a higher intensity in curds resulting from FP and FPMB treatments (Figure S5). The absorbance intensities shown in Figure S5 reflect important differences in protein components (C=N-O) between cauliflowers grown with FP and control soil plants [40]. Conversely, the absorbance intensities of the 1000 cm−1 region, attributed to the sulfurous biochemical components typical of glucosinolates, were higher in control plants and plants grown in biochar-unamended soils.
Similarly, Tarar’s research shows that biochar shifts peak at 3344, 1725, and 1370 cm−1 due to functional group interactions [77]. The various types of biochar alter the soil’s physico-chemical properties, including porosity and cation exchange, which modifies the abundance of functional groups [80].
4. Conclusions
Biochar is a promising sustainable agriculture strategy that reduces the leaching of the main agricultural contaminants and improves soil properties. The properties and feedstock have an enormous influence on the characteristics of the resulting biochar and environmental sustainability capabilities. Our research indicates that using biochar in cauliflower mesocosm soils has improved physicochemical properties, including raising the pH value and increasing the availability of elements such as nitrogen and carbon. Research indicates that biochar application can decrease the levels of ammonium and nitrate in the soil, thus increasing the availability of nitrogen for crop growth. We can highlight the high capacity of biochar in the mitigation of nitrogen contaminants leaching from agricultural origin. In fact, in the experimental lines with wood biochar and mixed biochar, nitrate concentrations were 76.82 and 43.78 mg/L, compared to soils treated with fertilizers and pesticides (106.76 mg/L). In light of the results obtained, we can conclude that biochar showed good potentialities for food and environmental protection; in the treatment test without biochar, there is a strong translocation of both pesticides in the curd with concentrations always exceeding the maximum residue levels (MRLs). Instead, the presence of biochar is demonstrated to protect both percolation water and food. On the one hand, for azoxystrobin, the data observed in the mesocosms with both biochar are always lower than the MSLs (10 µg/kg) allowed for food. On the other hand, for spinosad, an exceeding of allowed limits is shown in each experimental line. However, the spinosad values in the curds without biochar are about six times higher than the vegetables treated with both types of biochar. According to the results, biochar-amended soil with chemical fertilizers is a suitable strategy for achieving positive crop productivity without sacrificing soil quality. Our research suggests that the growth of crops destined for the market is significantly impacted by N fertilizer and biochar-based techniques. This article highlights new information for valuing agricultural waste for environmental and agricultural sustainability.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/environments11010013/s1. Figure S1. Boxplot of NH4+-N (on the left) and NH3−-N (on the right) concentrations of soil sampled at the end of cauliflower experiment; Figure S2. Box chart of insecticide spinosad-base concentration in percolation water at 10 days post-I° pesticides application (T1) and 10 days post-II° pesticides application (T2); Table S1. Pesticides concentrations in soil sampled at the end of cauliflower experiment; Figure S3. Column graph of morphologic curd growth parameters at the end of this study; Figure S4. Effect of biochar treatment on leaf area of cauliflower plants at the experiment end; Figure S5. ATR-FTIR spectrum of Brassica oleracea L. var. botrytis curds, over the region (4000–500 cm−1).
Author Contributions
Conceptualization, D.L.; methodology, D.L., C.C. and M.T.; software, D.L., C.C., M.T. and C.M.; validation, D.L., C.C. and M.T.; formal analysis, D.L., C.C. and M.T.; investigation, D.L., C.C. and M.T.; resources, C.M. and V.F.U.; data curation, D.L., C.C. and M.T.; writing—original draft preparation, D.L.; writing—review and editing, D.L., C.C. and M.T.; visualization, D.L.; supervision, C.M. and V.F.U.; project administration, V.F.U.; funding acquisition, V.F.U. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by project “Attuazione Direttiva 91/676/CEE relativa alla protezione delle acque dall’inquinamento provocato dai nitrati provenienti da fonti agricole-art.92 del D.Lgs. 152/2066—revisione delle Zone Vulnerabili da Nitrati di origine agricola e aggiornamento del Programma D’Azione Nitrati-Convenzione tra Regione Puglia e CNR-IRSA di Bari del 24/11/2019”.
Data Availability Statement
Data are contained within the article and in the Supplementary Material.
Acknowledgments
The authors thank Francesco Porcelli and Pasquale Trotti of the Department of Soil, Plant, and Food Sciences of the University of Bari for SEM analysis. The authors acknowledge the help of Pietro Cotugno of the Chemistry Department of the University of Bari for some chemical analysis. The authors thank Francesco Acquasanta, Fabio Fedele, Pasquale Carmignano, Stefano Convertini and all the staff of Reagri S.r.l. for the experimental setup and the agronomic support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Losacco, D.; Ancona, V.; De Paola, D.; Tumolo, M.; Massarelli, C.; Gatto, A.; Uricchio, V.F. Development of Ecological Strategies for the Recovery of the Main Nitrogen Agricultural Pollutants: A Review on Environmental Sustainability in Agroecosystems. Sustainability 2021, 13, 7163. [Google Scholar] [CrossRef]
- Corsini, E.; Sokooti, M.; Galli, C.L.; Moretto, A.; Colosio, C. Pesticide Induced Immunotoxicity in Humans: A Comprehensive Review of the Existing Evidence. Toxicology 2013, 307, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the Safety of Pesticides in Food: How Current Regulations Protect Human Health. Adv. Nutr. 2019, 10, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Arora, S.; Sahni, D.; Sehgal, M.; Srivastava, D.S.; Singh, A. Pesticides Use and Its Effect on Soil Bacteria and Fungal Populations, Microbial Biomass Carbon and Enzymatic Activity. Curr. Sci. 2019, 116, 643–649. [Google Scholar] [CrossRef]
- PAN International List of Highly Hazardous Pesticides (PAN List of HHP)—June 2013. Available online: https://pan-international.org/wp-content/uploads/PAN_HHP_List.pdf?_gl=1*q6qghn*_ga*MTI1MDQ2MDI0MC4xNjcxNjk2MDg1*_ga_PVQKRCXXT2*MTY3MTY5NjA4NS4xLjAuMTY3MTY5NjA4NS4wLjAuMA (accessed on 22 December 2022).
- European Union EUR-Lex-32009R1107-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32009R1107 (accessed on 22 December 2022).
- Van Valkenburg, S. Pan Europe. J. Geog. 1930, 29, 133–140. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Shaheen, S.M.; Rinklebe, J.; Wang, H.; Murtaza, B.; Islam, E.; Farrakh Nawaz, M.; et al. Arsenic Removal by Japanese Oak Wood Biochar in Aqueous Solutions and Well Water: Investigating Arsenic Fate Using Integrated Spectroscopic and Microscopic Techniques. Sci. Total Environ. 2018, 621, 1642–1651. [Google Scholar] [CrossRef]
- Micoli, L.; Di Rauso Simeone, G.; Turco, M.; Toscano, G.; Rao, M.A. Anaerobic Digestion of Olive Mill Wastewater in the Presence of Biochar. Energies 2023, 16, 3259. [Google Scholar] [CrossRef]
- Khorram, M.S.; Wang, Y.; Jin, X.; Fang, H.; Yu, Y. Reduced Mobility of Fomesafen through Enhanced Adsorption in Biochar-Amended Soil. Environ. Toxicol. Chem. 2015, 34, 1258–1266. [Google Scholar] [CrossRef]
- Chen, Z.; Pei, J.; Wei, Z.; Ruan, X.; Hua, Y.; Xu, W.; Zhang, C.; Liu, T.; Guo, Y. A Novel Maize Biochar-Based Compound Fertilizer for Immobilizing Cadmium and Improving Soil Quality and Maize Growth. Environ. Pollut. 2021, 277, 116455. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.; Lin, D.; Yao, Y.; Song, N.; Wang, F. Co-Application of Biochar and Nitrogen Fertilizer Promotes Rice Performance, Decreases Cadmium Availability, and Shapes Rhizosphere Bacterial Community in Paddy Soil. Environ. Pollut. 2022, 308, 119624. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Zhang, J.; Cai, Z.; Müller, C. The N Transformation Mechanisms for Rapid Nitrate Accumulation in Soils under Intensive Vegetable Cultivation. J. Soils Sediments 2011, 11, 1178–1189. [Google Scholar] [CrossRef]
- Dai, Y.; Wang, W.; Lu, L.; Yan, L.; Yu, D. Utilization of Biochar for the Removal of Nitrogen and Phosphorus. J. Clean. Prod. 2020, 257, 120573. [Google Scholar] [CrossRef]
- Wang, B.; Lehmann, J.; Hanley, K.; Hestrin, R.; Enders, A. Ammonium Retention by Oxidized Biochars Produced at Different Pyrolysis Temperatures and Residence Times. RSC Adv. 2016, 6, 41907–41913. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Liu, H.; Wu, S.; Wu, H. Nitrogen Removal Responses to Biochar Addition in Intermittent-Aerated Subsurface Flow Constructed Wetland Microcosms: Enhancing Role and Mechanism. Ecol. Eng. 2019, 128, 57–65. [Google Scholar] [CrossRef]
- Alsewaileh, A.S.; Usman, A.R.; Al-Wabel, M.I. Effects of Pyrolysis Temperature on Nitrate-Nitrogen (NO 3− -N) and Bromate (BrO 3− ) Adsorption onto Date Palm Biochar. J. Environ. Manage. 2019, 237, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Oleszczuk, P.; Rycaj, M.; Lehmann, J.; Cornelissen, G. Influence of Activated Carbon and Biochar on Phytotoxicity of Air-Dried Sewage Sludges to Lepidium Sativum. Ecotoxicol. Environ. Saf. 2012, 80, 321–326. [Google Scholar] [CrossRef]
- Deng, H.; Feng, D.; He, J.X.; Li, F.Z.; Yu, H.M.; Ge, C.J. Influence of Biochar Amendments to Soil on the Mobility of Atrazine Using Sorption-Desorption and Soil Thin-Layer Chromatography. Ecol. Eng. 2017, 99, 381–390. [Google Scholar] [CrossRef]
- Mandal, A.; Singh, N.; Purakayastha, T.J. Characterization of Pesticide Sorption Behaviour of Slow Pyrolysis Biochars as Low Cost Adsorbent for Atrazine and Imidacloprid Removal. Sci. Total Environ. 2017, 577, 376–385. [Google Scholar] [CrossRef]
- Herath, I.; Kumarathilaka, P.; Al-Wabel, M.I.; Abduljabbar, A.; Ahmad, M.; Usman, A.R.A.; Vithanage, M. Mechanistic Modeling of Glyphosate Interaction with Rice Husk Derived Engineered Biochar. Microporous Mesoporous Mater. 2016, 225, 280–288. [Google Scholar] [CrossRef]
- Zhang, P.; Sun, H.; Min, L.; Ren, C. Biochars Change the Sorption and Degradation of Thiacloprid in Soil: Insights into Chemical and Biological Mechanisms. Environ. Pollut. 2018, 236, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Weihermüller, L.; Tappe, W.; Hofmann, D.; Köppchen, S.; Laabs, V.; Vereecken, H.; Burauel, P. Sorption-Desorption Behaviour of Bentazone, Boscalid and Pyrimethanil in Biochar and Digestate Based Soil Mixtures for Biopurification Systems. Sci. Total Environ. 2016, 559, 63–73. [Google Scholar] [CrossRef]
- Yavari, S.; Malakahmad, A.; Sapari, N.B.; Yavari, S. Synthesis Optimization of Oil Palm Empty Fruit Bunch and Rice Husk Biochars for Removal of Imazapic and Imazapyr Herbicides. J. Environ. Manage. 2017, 193, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Manna, S.; Singh, N.; Purakayastha, T.J.; Berns, A.E. Effect of Deashing on Physico-Chemical Properties of Wheat and Rice Straw Biochars and Potential Sorption of Pyrazosulfuron-Ethyl. Arab. J. Chem. 2020, 13, 1247–1258. [Google Scholar] [CrossRef]
- Alahabadi, A.; Moussavi, G. Preparation, Characterization and Atrazine Adsorption Potential of Mesoporous Carbonate-Induced Activated Biochar (CAB) from Calligonum Comosum Biomass: Parametric Experiments and Kinetics, Equilibrium and Thermodynamic Modeling. J. Mol. Liq. 2017, 242, 40–52. [Google Scholar] [CrossRef]
- Petter, F.A.; Ferreira, T.S.; Sinhorin, A.P.; de Lima, L.B.; de Morais, L.A.; Pacheco, L.P. Sorption and Desorption of Diuron in Oxisol under Biochar Application. Bragantia 2016, 75, 487–496. [Google Scholar] [CrossRef]
- Mayakaduwa, S.S.; Vithanage, M.; Karunarathna, A.; Mohan, D.; Ok, Y.S. Interface Interactions between Insecticide Carbofuran and Tea Waste Biochars Produced at Different Pyrolysis Temperatures. Chem. Speciat. Bioavailab. 2016, 28, 110–118. [Google Scholar] [CrossRef]
- Wu, C.; Liu, X.; Wu, X.; Dong, F.; Xu, J.; Zheng, Y. Sorption, Degradation and Bioavailability of Oxyfluorfen in Biochar-Amended Soils. Sci. Total Environ. 2019, 658, 87–94. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar Application to Soil. Agronomic and Environmental Benefits and Unintended Consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar] [CrossRef]
- García-Jaramillo, M.; Cox, L.; Cornejo, J.; Hermosín, M.C. Effect of Soil Organic Amendments on the Behavior of Bentazone and Tricyclazole. Sci. Total Environ. 2014, 466–467, 906–913. [Google Scholar] [CrossRef]
- Khalid, S.; Shahid, M.; Murtaza, B.; Bibi, I.; Natasha; Asif Naeem, M.; Niazi, N.K. A Critical Review of Different Factors Governing the Fate of Pesticides in Soil under Biochar Application. Sci. Total Environ. 2020, 711, 134645. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.B.; Ying, G.G.; Peng, P.A.; Wang, L.; Zhao, J.L.; Zhang, L.J.; Yuan, P.; He, H.P. Influence of Biochars on Plant Uptake and Dissipation of Two Pesticides in an Agricultural Soil. J. Agric. Food Chem. 2010, 58, 7915–7921. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Gao, B.; Zhang, Z.; Zhang, G.; Zhao, Y.; Xing, B. Sorption of Atrazine and Phenanthrene by Organic Matter Fractions in Soil and Sediment. Environ. Pollut. 2010, 158, 3520–3526. [Google Scholar] [CrossRef] [PubMed]
- Losacco, D.; Tumolo, M.; Cotugno, P.; Leone, N.; Massarelli, C.; Convertini, S.; Tursi, A.; Uricchio, V.F.; Ancona, V. Use of Biochar to Improve the Sustainable Crop Production of Cauliflower (Brassica Oleracea L.). Plants 2022, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Bigham: Method of Soil Analysis. Part 3. Chemical Methods. Available online: https://scholar.google.com/scholar_lookup?title=Methods+of+Soil+Analysis:+Part+3+Chemical+Methods&author=Bigham,+J.M.&publication_year=1996 (accessed on 3 January 2023).
- Stachniuk, A.; Szmagara, A.; Czeczko, R.; Fornal, E. LC-MS/MS Determination of Pesticide Residues in Fruits and Vegetables. J. Environ. Sci. Heal. Part B Pestic. Food Contam. Agric. Wastes 2017, 52, 446–457. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium bicarbonate. Sterling Robertson Olsen - Google Libros. Available online: https://books.google.it/books?hl=it&lr=&id=d-oaM88x5agC&oi=fnd&pg=PA3&ots=zZ0g-CkVXD&sig=qS9FwZLDvZrY9vshp3H6FTjt-Is&redir_esc=y#v=onepage&q&f=false (accessed on 3 January 2023).
- Losacco, D.; Campanale, C.; Tumolo, M.; Ancona, V.; Massarelli, C.; Uricchio, V.F. Evaluating the Influence of Nitrogen Fertilizers and Biochar on Brassica Oleracea L. Var. Botrytis by the Use of Fourier Transform Infrared (FTIR) Spectroscopy. Sustain. 2022, 14, 11985. [Google Scholar] [CrossRef]
- ImageJ Public Domain License. Available online: https://imagej.nih.gov/ij/docs/intro.html (accessed on 22 December 2022).
- Hossain, M.Z.; Bahar, M.M.; Sarkar, B.; Donne, S.W.; Wade, P.; Bolan, N. Assessment of the Fertilizer Potential of Biochars Produced from Slow Pyrolysis of Biosolid and Animal Manures. J. Anal. Appl. Pyrolysis 2021, 155, 105043. [Google Scholar] [CrossRef]
- Pukalchik, M.; Mercl, F.; Terekhova, V.; Tlustoš, P. Biochar, Wood Ash and Humic Substances Mitigating Trace Elements Stress in Contaminated Sandy Loam Soil: Evidence from an Integrative Approach. Chemosphere 2018, 203, 228–238. [Google Scholar] [CrossRef]
- Bandara, T.; Xu, J.; Potter, I.D.; Franks, A.; Chathurika, J.B.A.J.; Tang, C. Mechanisms for the Removal of Cd(II) and Cu(II) from Aqueous Solution and Mine Water by Biochars Derived from Agricultural Wastes. Chemosphere 2020, 254, 126745. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of Biochar on Chemical Properties of Acidic Soil. Arch. Agron. Soil Sci. 2013, 60, 393–404. [Google Scholar] [CrossRef]
- Li, X.; Song, B.; Yin, D.; Lal, M.K.; Riaz, M.; Song, X.; Huang, W. Influence of Biochar on Soil Properties and Morphophysiology of Sugar Beet Under Fomesafen Residues. J. Soil Sci. Plant Nutr. 2023, 23, 1619–1632. [Google Scholar] [CrossRef]
- Buss, W.; Shepherd, J.G.; Heal, K.V.; Mašek, O. Spatial and Temporal Microscale PH Change at the Soil-Biochar Interface. Geoderma 2018, 331, 50–52. [Google Scholar] [CrossRef]
- Lentz, R.D.; Ippolito, J.A. Biochar and Manure Affect Calcareous Soil and Corn Silage Nutrient Concentrations and Uptake. J. Environ. Qual. 2012, 41, 1033–1043. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S.; Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Agronomic Values of Greenwaste Biochar as a Soil Amendment. Soil Res. 2007, 45, 629–634. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota - A Review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Phares, C.A.; Amoakwah, E.; Danquah, A.; Akaba, S.; Frimpong, K.A.; Mensah, T.A. Improved Soil Physicochemical, Biological Properties and Net Income Following the Application of Inorganic NPK Fertilizer and Biochar for Maize Production. Acta Ecol. Sin. 2022, 42, 289–295. [Google Scholar] [CrossRef]
- Wu, J.; Jin, L.; Wang, N.; Wei, D.; Pang, M.; Li, D.; Wang, J.; Li, Y.; Sun, X.; Wang, W.; et al. Effects of Combined Application of Chemical Fertilizer and Biochar on Soil Physio-Biochemical Properties and Maize Yield. Agriculture 2023, 13, 1200. [Google Scholar] [CrossRef]
- Dong, X.; Singh, B.P.; Li, G.; Lin, Q.; Zhao, X. Biochar Application Constrained Native Soil Organic Carbon Accumulation from Wheat Residue Inputs in a Long-Term Wheat-Maize Cropping System. Agric. Ecosyst. Environ. 2018, 252, 200–207. [Google Scholar] [CrossRef]
- Gross, A.; Bromm, T.; Glaser, B. Soil Organic Carbon Sequestration after Biochar Application: A Global Meta-Analysis. Agronomy 2021, 11, 2474. [Google Scholar] [CrossRef]
- Hui, D. Effects of Biochar Application on Soil Properties, Plant Biomass Production, and Soil Greenhouse Gas Emissions: A Mini-Review. Agric. Sci. 2021, 12, 213–236. [Google Scholar] [CrossRef]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar Applications and Modern Techniques for Characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, P.; Sarswat, A.; Steele, P.H.; Pittman, C.U. Lead Sorptive Removal Using Magnetic and Nonmagnetic Fast Pyrolysis Energy Cane Biochars. J. Colloid Interface Sci. 2015, 448, 238–250. [Google Scholar] [CrossRef]
- Chia, C.H.; Gong, B.; Joseph, S.D.; Marjo, C.E.; Munroe, P.; Rich, A.M. Imaging of Mineral-Enriched Biochar by FTIR, Raman and SEM-EDX. Vib. Spectrosc. 2012, 62, 248–257. [Google Scholar] [CrossRef]
- Gao, X.; Yang, J.; Liu, W.; Li, X.; Zhang, W.; Wang, A. Effects of Alkaline Biochar on Nitrogen Transformation with Fertilizer in Agricultural Soil. Environ. Res. 2023, 233, 116084. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Wang, H.; Zhao, X.; Cui, H.; Wei, Z. Reducing Nitrogen Loss and Phytotoxicity during Beer Vinasse Composting with Biochar Addition. Waste Manag. 2017, 61, 150–156. [Google Scholar] [CrossRef]
- Heaney, N.; Ukpong, E.; Lin, C. Low-Molecular-Weight Organic Acids Enable Biochar to Immobilize Nitrate. Chemosphere 2020, 240, 124872. [Google Scholar] [CrossRef]
- Cederlund, H.; Börjesson, E.; Stenström, J. Effects of a Wood-Based Biochar on the Leaching of Pesticides Chlorpyrifos, Diuron, Glyphosate and MCPA. J. Environ. Manage. 2017, 191, 28–34. [Google Scholar] [CrossRef]
- Palangi, S.; Bahmani, O.; Atlassi-pak, V. Effects of Wheat Straw Biochar Amendments to Soil on the Fate of Deltamethrin and Soil Properties. Environ. Technol. Innov. 2021, 23, 101681. [Google Scholar] [CrossRef]
- Cara, I.G.; Țopa, D.; Puiu, I.; Jităreanu, G. Biochar a Promising Strategy for Pesticide-Contaminated Soils. Agriculture 2022, 12, 1579. [Google Scholar] [CrossRef]
- Dai, Y.; Zhang, N.; Xing, C.; Cui, Q.; Sun, Q. The Adsorption, Regeneration and Engineering Applications of Biochar for Removal Organic Pollutants: A Review. Chemosphere 2019, 223, 12–27. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.B.; Mukerjee, I.; Gupta, S.; Gajbhiye, V.T.; Sharma, P.K.; Goel, M.; Dureja, P. Metabolism of 14C-Azoxystrobin in Water at Different PH. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2010, 45, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Bending, G.D.; Lincoln, S.D.; Edmondson, R.N. Spatial Variation in the Degradation Rate of the Pesticides Isoproturon, Azoxystrobin and Diflufenican in Soil and Its Relationship with Chemical and Microbial Properties. Environ. Pollut. 2006, 139, 279–287. [Google Scholar] [CrossRef]
- Yang, Y.; Chun, Y.; Shang, G.; Huang, M. PH-Dependence of Pesticide Adsorption by Wheat-Residue-Derived Black Carbon. Langmuir 2004, 20, 6736–6741. [Google Scholar] [CrossRef] [PubMed]
- Rasool, S.; Rasool, T.; Gani, K.M. A Review of Interactions of Pesticides within Various Interfaces of Intrinsic and Organic Residue Amended Soil Environment. Chem. Eng. J. Adv. 2022, 11, 100301. [Google Scholar] [CrossRef]
- You, X.; Zheng, H.; Ge, J.; Fang, S.; Suo, F.; Kong, Q.; Zhao, P.; Zhang, G.; Zhang, C.; Li, Y. Effect of Biochar on the Enantioselective Soil Dissipation and Lettuce Uptake and Translocation of the Chiral Pesticide Metalaxyl in Contaminated Soil. J. Agric. Food Chem. 2019, 67, 13550–13557. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Jabbarov, Z.; Arora, N.K.; Wirth, S.; Bellingrath-Kimura, S.D. Biochar Mitigates Effects of Pesticides on Soil Biological Activities. Environ. Sustain. 2021, 4, 335–342. [Google Scholar] [CrossRef]
- Oleszczuk, P.; Jośko, I.; Futa, B.; Pasieczna-Patkowska, S.; Pałys, E.; Kraska, P. Effect of Pesticides on Microorganisms, Enzymatic Activity and Plant in Biochar-Amended Soil. Geoderma 2014, 214–215, 10–18. [Google Scholar] [CrossRef]
- Rafael, R.B.A.; Fernández-Marcos, M.L.; Cocco, S.; Ruello, M.L.; Fornasier, F.; Corti, G. Benefits of Biochars and NPK Fertilizers for Soil Quality and Growth of Cowpea (Vigna Unguiculata L. Walp.) in an Acid Arenosol. Pedosphere 2019, 29, 311–333. [Google Scholar] [CrossRef]
- Hussain, F.; Hussain, I.; Khan, A.H.A.; Muhammad, Y.S.; Iqbal, M.; Soja, G.; Reichenauer, T.G.; Zeshan; Yousaf, S. Combined Application of Biochar, Compost, and Bacterial Consortia with Italian Ryegrass Enhanced Phytoremediation of Petroleum Hydrocarbon Contaminated Soil. Environ. Exp. Bot. 2018, 153, 80–88. [Google Scholar] [CrossRef]
- You, X.; Yin, S.; Suo, F.; Xu, Z.; Chu, D.; Kong, Q.; Zhang, C.; Li, Y.; Liu, L. Biochar and Fertilizer Improved the Growth and Quality of the Ice Plant (Mesembryanthemum Crystallinum L.) Shoots in a Coastal Soil of Yellow River Delta, China. Sci. Total Environ. 2021, 775, 144893. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.W.; Conte, P.; Stephen, J. Plant Growth Improvement Mediated by Nitrate Capture in Co-Composted Biochar. Sci. Rep. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Tarar, O.F.; Asghar, A.; Qayyum, S.A.; Kanwal, H.; Lateef, A.; Nazir, R.; Imam Abidi, S.H.; Naeem, M.K.; Shahid, B. Synthesis and Surface Morphology of Banana Biochar-Based Nano-Fertilizer and Its Effect on First Stages of Growth Parameters of Cucumber, Broccoli, and Red Okra. J. Saudi Soc. Agric. Sci. 2023, 22, 535–545. [Google Scholar] [CrossRef]
- Yan, B.; Zhang, Y.; Wang, Y.; Rong, X.; Peng, J.; Fei, J.; Luo, G. Biochar Amendments Combined with Organic Fertilizer Improve Maize Productivity and Mitigate Nutrient Loss by Regulating the C–N–P Stoichiometry of Soil, Microbiome, and Enzymes. Chemosphere 2023, 324, 138293. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.T.; Henry-Des-Tureaux, T.; Rumpel, C.; Janeau, J.L.; Jouquet, P. Impact of Compost, Vermicompost and Biochar on Soil Fertility, Maize Yield and Soil Erosion in Northern Vietnam: A Three Year Mesocosm Experiment. Sci. Total Environ. 2015, 514, 147–154. [Google Scholar] [CrossRef]
- Pan, X.; Zhang, Y.; Wang, X.; Liu, G. Effect of Adding Biochar with Wood Vinegar on the Growth of Cucumber. IOP Conf. Ser. Earth Environ. Sci. 2017, 61, 012149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).