A Comparison of Generation–Retention–Expulsion in Felsic and Carbonate Laminated Shale by Semi-Open Thermal Pyrolysis: Implications for Shale Oil Exploration
Abstract
1. Introduction
2. Samples and Methods
2.1. Sample Information and Preparation
2.2. Methods
3. Results
3.1. TOC and Rock–Eval Data
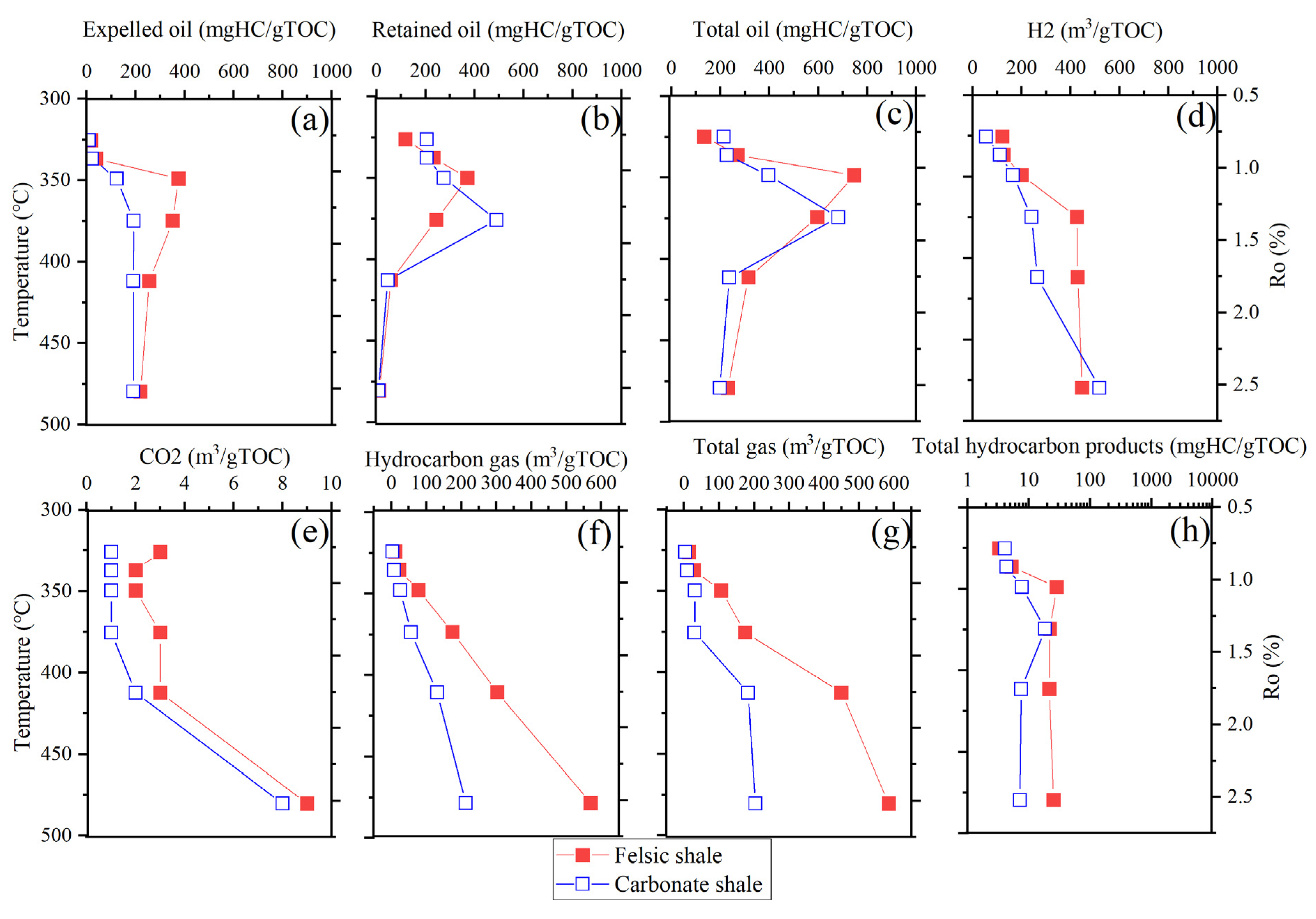
3.2. Amount of Generated, Expelled and Retained Hydrocarbon
3.3. Fraction and Molecular Composition of Generated Hydrocarbon
4. Discussion
4.1. Hydrocarbon Generation Potentials from Felsic and Carbonate Laminated Shale
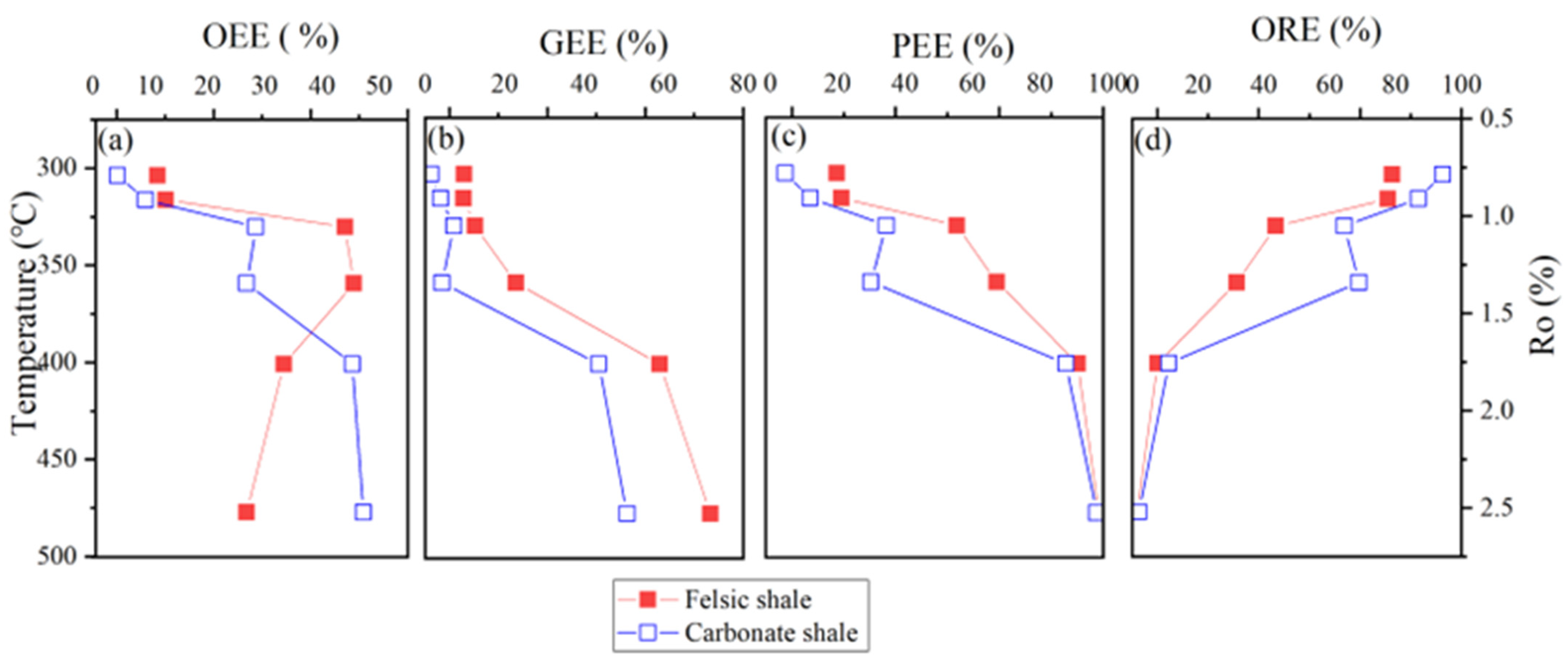
4.2. Hydrocarbon Retention-Expulsion from Felsic and Carbonate Laminated Shale
4.3. Molecular Variation of Retained Hydrocarbon with Thermal Maturity
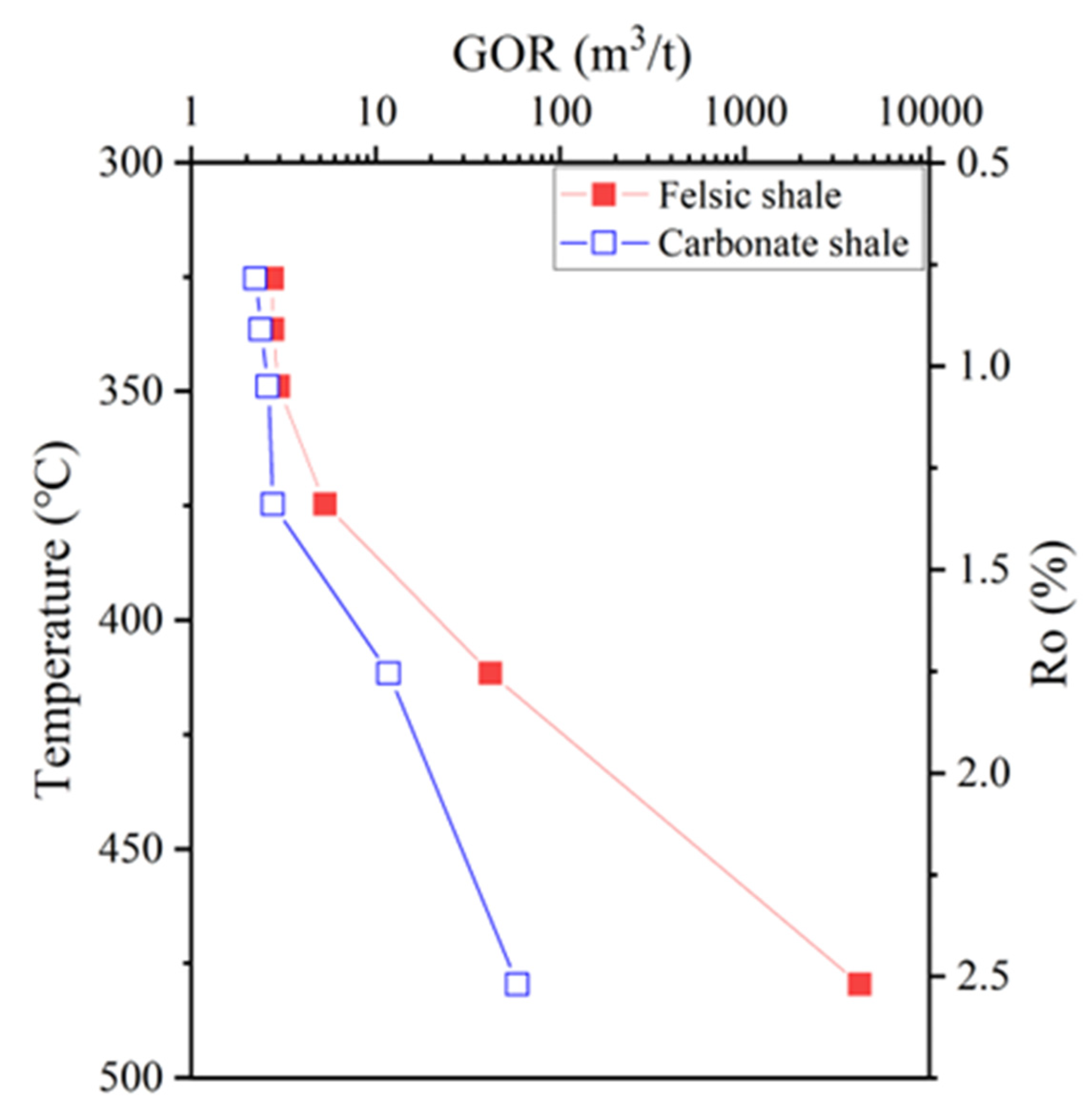
4.4. Variation of GOR During Pyrolysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, S.; Zhao, W.; Hou, L.; Yang, Z.; Zhu, R.; Wu, S.; Bai, B.; Jin, X. Development potential and technical strategy of continental shale oil in China. Pet. Explor. Dev. 2020, 47, 877–887. [Google Scholar] [CrossRef]
- Jin, Z.; Zhu, R.; Liang, X.; Shen, Y. Several issues worthy of attention in current lacustrine shale oil exploration and development. Pet. Explor. Dev. 2021, 48, 1471–1484. [Google Scholar] [CrossRef]
- Zhao, W.; Hu, S.; Hou, L.; Yang, T.; Li, X.; Guo, B.; Yang, Z. Types and resource potential of continental shale oil in China and its boundary with tight oil. Pet. Explor. Dev. 2020, 47, 1–11. [Google Scholar] [CrossRef]
- Pan, S.; Zou, C.; Li, J.; Yang, Z.; Liu, E.; Han, Y. Unconventional shale systems: A comparative study of the “in-source sweet spot” developed in the lacustrine Chang 7 Shale and the marine Barnett Shale. Mar. Pet. Geol. 2019, 100, 540–550. [Google Scholar] [CrossRef]
- Zou, C.; Yang, Z.; Dong, D.; Zhao, Q.; Chen, Z.; Feng, Y.; Li, J.; Wang, X. Formation, Distribution and Prospect of Unconventional Hydrocarbons in Source Rock Strata in China. Earth Sci. 2022, 47, 1517–1533. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, J.; He, W.; Guo, X.; Zhao, K.; Li, W. Discovery of shale oil in alkaline lacustrine basins: The Late Paleozoic Fengcheng Formation, Mahu Sag, Junggar Basin, China. Pet. Sci. 2021, 18, 1281–1293. [Google Scholar] [CrossRef]
- Pang, H.; Pang, X.; Dong, L.; Zhao, X. Factors impacting on oil retention in lacustrine shale: Permian Lucaogou Formation in Jimusaer Depression, Junggar Basin. J. Pet. Sci. Eng. 2018, 163, 79–90. [Google Scholar] [CrossRef]
- Xiang, B.; Li, E.; Gao, X.; Wang, M.; Wang, Y.; Xu, H.; Huang, P.; Yu, S. Petroleum generation kinetics for Permian lacustrine source rocks in the Junggar Basin, NW China. Org. Geochem. 2016, 98, 1–17. [Google Scholar] [CrossRef]
- Zheng, R.; Wang, Y.; Li, Z.; Zhang, Z.; Wang, G.; Zhang, H. Differences and origins of hydrocarbon generation characteristics between mudstone and shale in the Seventh Member of the Yanchang Formation, Ordos Basin, China. Int. J. Coal Geol. 2022, 257, 104012. [Google Scholar] [CrossRef]
- Ma, W.; Hou, L.; Luo, X.; Liu, J.; Tao, S.; Guan, P.; Cai, Y. Generation and expulsion process of the Chang 7 oil shale in the Ordos Basin based on temperature-based semi-open pyrolysis: Implications for in-situ conversion process. J. Pet. Sci. Eng. 2020, 190, 107035. [Google Scholar] [CrossRef]
- Guo, Q.; Yao, Y.; Hou, L.; Tang, S.; Pan, S.; Yang, F. Oil migration, retention, and differential accumulation in “sandwiched” lacustrine shale oil systems from the Chang 7 member of the Upper Triassic Yanchang Formation, Ordos Basin, China. Int. J. Coal Geol. 2022, 261, 104077. [Google Scholar] [CrossRef]
- Zou, C.; Pan, S.; Horsfield, B.; Yang, Z.; Hao, S.; Liu, E.; Zhang, L. Oil retention and intrasource migration in the organic-rich lacustrine Chang 7 shale of the Upper Triassic Yanchang Formation, Ordos Basin, central China. Am. Assoc. Pet. Geol. Bull. 2019, 103, 2627–2663. [Google Scholar] [CrossRef]
- Liu, C.; Wang, Z.; Guo, Z.; Hong, W.; Dun, C.; Zhang, X.; Li, B.; Wu, L. Enrichment and distribution of shale oil in the Cretaceous Qingshankou Formation, Songliao Basin, Northeast China. Mar. Pet. Geol. 2017, 86, 751–770. [Google Scholar] [CrossRef]
- Han, H.; Guo, C.; Zhong, N.; Pang, P.; Chen, S.; Lu, J.; Gao, Y. Pore structure evolution of lacustrine shales containing Type I organic matter from the Upper Cretaceous Qingshankou Formation, Songliao Basin, China: A study of artificial samples from hydrous pyrolysis experiments. Mar. Pet. Geol. 2019, 104, 375–388. [Google Scholar] [CrossRef]
- Li, K.; Zhao, Z.; Lu, H.; Liu, X.; Peng, P.; Hsu, C. Effects of inherent pyrite on hydrocarbon generation by thermal pyrolysis: An example of low maturity type-II kerogen from Alum shale formation, Sweden. Fuel 2022, 312, 122865. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Pu, X.; Jin, F.; Shi, Z.; Han, W.; Jiang, W.; Han, G.; Zhang, W.; Wang, H. Formation conditions and enrichment model of retained petroleum in lacustrine shale: A case study of the Paleogene in Huanghua depression, Bohai Bay Basin, China. Pet. Explor. Dev. 2020, 47, 916–930. [Google Scholar] [CrossRef]
- Ma, Y.; Qiao, Y.; Lu, P.; Zhang, X.; Chao, X.; Shi, Q. Reconsideration of shale oil development in Jiyang Depression: Based on hydrocarbon generation, hydrocarbon expulsion, hydrocarbon storage and movable hydrocarbon. Adv. Resour. Res. 2023, 3, 32–50. [Google Scholar] [CrossRef]
- Zou, C.; Zhang, G.; Yang, Z.; Tao, S.; Hou, L.; Zhu, R.; Yuan, X.; Ran, Q.; Li, D.; Wang, Z. Concepts, characteristics, potential and technology of unconventional hydrocarbons: On unconventional petroleum geology. Pet. Explor. Dev. 2013, 40, 413–428. [Google Scholar] [CrossRef]
- Chen, L.; Tan, J.; Cui, H.; Ma, X.; Song, X.; Yuan, Q.; Liu, J. Hydrocarbon generation mechanism of lamalginite- and telalginite-dominated source rocks in a saline lake basin: A case study of the Permian Lucaogou formation in the Jimusaer Sag, Junggar Basin. Energy Geosci. 2023, 4, 100191. [Google Scholar] [CrossRef]
- Hou, L.; Ma, W.; Luo, X.; Liu, J.; Liu, S.; Zhao, Z. Hydrocarbon generation-retention-expulsion mechanism and shale oil producibility of the permian lucaogou shale in the Junggar Basin as simulated by semi-open pyrolysis experiments. Mar. Pet. Geol. 2021, 125, 104880. [Google Scholar] [CrossRef]
- Jin, Z.; Wang, G.; Liu, G.; Gao, B.; Liu, Q.; Wang, H.; Liang, X.; Wang, R. Research progress and key scientific issues of continental shale oil in China. Acta Pet. Sin. 2021, 42, 821. [Google Scholar] [CrossRef]
- Adib, A.; Kianoush, P. Enhanced seismic hazard assessment and risk zoning in the Kashan Region, Central Iran: Insights from historical data and advanced modeling techniques. Results Earth Sci. 2025, 3, 100098. [Google Scholar] [CrossRef]
- Chen, D.; Pang, X.; Li, L.; Jiang, F.; Liu, G.; Li, M.; Pang, B.; Jiang, H.; Xu, Z.; Han, W. Organic geochemical characteristics and shale oil potential of the middle Eocene early-mature shale in the Nanpu Sag, Bohai Bay Basin, Eastern China. Mar. Pet. Geol. 2021, 133, 105248. [Google Scholar] [CrossRef]
- Chukwuma, K.; Tsikos, H.; Horsfield, B.; Schulz, H.; Harris, N.; Frazenburg, M. The effects of Jurassic igneous intrusions on the generation and retention of gas shale in the Lower Permian source-reservoir shales of Karoo Basin, South Africa. Int. J. Coal Geol. 2023, 269, 104219. [Google Scholar] [CrossRef]
- Huang, Y.; Fan, C.; Han, X.; Jiang, X. A TGA-MS investigation of the effect of heating rate and mineral matrix on the pyrolysis of kerogen in oil shale. Oil Shale 2016, 33, 125–141. [Google Scholar] [CrossRef]
- Rosenberg, Y.O.; Reznik, I.J.; Vinegar, H.J.; Feinstein, S.; Bartov, Y. Comparing natural and artificial thermal maturation of a Type II-S source rock, Late Cretaceous, Israel. Mar. Pet. Geol. 2021, 124, 104773. [Google Scholar] [CrossRef]
- Yang, S.; Wang, H.; Zheng, J.; Pan, Y.; Ji, C. Comprehensive review: Study on heating rate characteristics and coupling simulation of oil shale pyrolysis. J. Anal. Appl. Pyrolysis 2024, 177, 106289. [Google Scholar] [CrossRef]
- Shao, D.; Zhang, T.; Ko, L.; Li, Y.; Yan, J.; Zhang, L.; Luo, H.; Qiao, B. Experimental investigation of oil generation, retention, and expulsion within Type II kerogen-dominated marine shales: Insights from gold-tube nonhydrous pyrolysis of Barnett and Woodford Shales using miniature core plugs. Int. J. Coal Geol. 2020, 217, 103337. [Google Scholar] [CrossRef]
- Cao, Y.; Jin, Z.; Zhu, R.; Liu, K. Pore systems and their correlation with oil enrichment in various lithofacies of saline lacustrine shale strata. Int. J. Coal Geol. 2024, 282, 104444. [Google Scholar] [CrossRef]
- Wang, J.; Lu, S.; Zhang, P.; Zhi, Q.; Huang, H. Pore Distribution Characteristics of Different Lithofacies Shales: Evidence from Scanning Electron Microscopy. Processes 2023, 11, 1120. [Google Scholar] [CrossRef]
- Zhao, X.; Zhou, L.; Pu, X.; Jin, F.; Han, W.; Shi, Z.; Chen, C.; Jiang, W.; Guan, Q.; Xu, J.; et al. Theories, technologies and practices of lacustrine shale oil exploration and development: A case study of Paleogene Kongdian Formation in Cangdong sag, Bohai Bay Basin, China. Pet. Explor. Dev. 2022, 49, 707–718. [Google Scholar] [CrossRef]
- Chang, J.; Jiang, Z.; Gao, Z.; Zheng, G.; Zhang, Y.; Huang, L.; He, W.; Duan, L.; Chen, Z.; Song, J. Oil bearing and mobility characteristics of different lithofacies shales in Fengcheng Formation, Mahu Sag. J. Cent. South Univ. (Sci. Technol.) 2022, 53, 3354. [Google Scholar] [CrossRef]
- Ma, W.; Cao, Y.; Xi, K.; Lin, M.; Liu, J.; Wang, Y. The effect of lamina and lithofacies assemblage on molecular maturity of oil in a shale source-rock reservoir. Int. J. Coal Geol. 2023, 279, 104373. [Google Scholar] [CrossRef]
- Li, Z.; Zou, Y.; Xu, X.; Sun, J.; Li, M.; Peng, P. Adsorption of mudstone source rock for shale oil—Experiments, model and a case study. Org. Geochem. 2016, 92, 55–62. [Google Scholar] [CrossRef]
- Guo, L.; Xiao, X.; Hui, T.; Song, Z. Distinguishing gases derived from oil cracking and kerogen maturation: Insights from laboratory pyrolysis experiments. Org. Geochem. 2009, 40, 1074–1084. [Google Scholar] [CrossRef]
- Hu, C.; Tan, J.; Lyu, Q.; Zhang, Y. Evolution of organic pores in Permian low maturity shales from the Dalong Formation in the Sichuan Basin: Insights from a thermal simulation experiment. Gas Sci. Eng. 2024, 121, 205166. [Google Scholar] [CrossRef]
- Stockhausen, M.; Galimberti, R.; Elias, R.; Di, P.; Schwark, L. Expulsinator assessment of oil/gas generation and expulsion characteristics of different source rocks. Mar. Pet. Geol. 2021, 129, 105057. [Google Scholar] [CrossRef]
- Shao, X.; Pang, X.; Li, M.; Qian, M.; Hu, T.; Li, Z.; Zhang, H.; Xu, Y. Hydrocarbon retention in lacustrine shales during thermal maturation: Insights from semi-open system pyrolysis. J. Pet. Sci. Eng. 2020, 184, 106480. [Google Scholar] [CrossRef]
- Han, Y.; Mahlstedt, N.; Horsfield, B. The Barnett Shale: Compositional fractionation associated with intraformational petroleum migration, retention, and expulsion. AAPG Bull. 2015, 99, 2173–2201. [Google Scholar] [CrossRef]
- Jia, W.; Wang, Q.; Liu, J.; Peng, P.; Li, B.; Lu, J. The effect of oil expulsion or retention on further thermal degradation of kerogen at the high maturity stage: A pyrolysis study of type II kerogen from Pingliang shale, China. Org. Geochem. 2014, 71, 17–29. [Google Scholar] [CrossRef]
- Sun, J.; Xiao, X.; Cheng, P.; Wang, M.; Tian, H. The relationship between oil generation, expulsion and retention of lacustrine shales: Based on pyrolysis simulation experiments. J. Pet. Sci. Eng. 2021, 196, 107625. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.; Wang, Q.; Zheng, L. Change in diagnostic ratios in expelled oils and residual extracts during semi-open pyrolysis experiments of an organic-rich shale. Environ. Pollut. 2022, 302, 119058. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Ma, W.; Luo, X.; Tao, S.; Guan, P.; Liu, J. Chemical structure changes of lacustrine Type-II kerogen under semi-open pyrolysis as investigated by solid-state 13C NMR and FT-IR spectroscopy. Mar. Pet. Geol. 2020, 116, 104348. [Google Scholar] [CrossRef]
- Shao, D.; Ellis, G.; Li, Y.; Zhang, T. Experimental investigation of the role of rock fabric in gas generation and expulsion during thermal maturation: Anhydrous closed-system pyrolysis of a bitumen-rich Eagle Ford Shale. Org. Geochem. 2018, 119, 22–35. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Z.; Wu, Y.; Wang, Z.; Shen, Q. Features of liquid hydrocarbon and biomarker maturity ratios during HTHP semi-open system pyrolysis of type II and III source rocks. Pet. Sci. Technol. 2017, 35, 1063–1069. [Google Scholar] [CrossRef]
- Wang, X.; Tian, H.; Gai, H.; Wu, Z.; Zhou, Q.; Ji, S.; Li, T.; Xiao, X. Generation and geochemical signatures of natural gas from solid bitumen: Implications from closed and stepwise semi-open pyrolysis experiments. Mar. Pet. Geol. 2022, 145, 105889. [Google Scholar] [CrossRef]
- Han, W.; Luo, X.; Lin, S.; Zhao, Z.; Liu, J.; Wang, Q. Geochemical parameters of thermal simulation of gas generation on lacustrine Type II shales in semi-open pyrolysis system. Geoenergy Sci. Eng. 2023, 231, 212178. [Google Scholar] [CrossRef]
- Singh, D.; Hazra, B.; Wood, D.; Singh, P. Hydrocarbon generation and kinetics: A case study of Permian shales, India. J. Asian Earth Sci. 2021, 222, 104960. [Google Scholar] [CrossRef]
- Li, J.; Wang, W.; Cao, Q.; Shi, Y.; Yan, X.; Tian, S. Impact of hydrocarbon expulsion efficiency of continental shale upon shale oil accumulations in eastern China. Mar. Pet. Geol. 2015, 59, 467–479. [Google Scholar] [CrossRef]
- Zhang, P.; Yin, Y.; Lu, S.; Wang, J.; Zhang, J.; Zhi, Q.; Huang, H. Key factors controlling oil contents in different lithofacies shales from the Funing Formation, Subei Basin: Evidence from scanning electron microscopy. Geoenergy Sci. Eng. 2023, 229, 212115. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, W.; Pan, J.; Zhu, C.; Deng, S. In-situ pyrolysis of oil shale in pressured semi-closed system: Insights into products characteristics and pyrolysis mechanism. Energy 2024, 286, 129608. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, C.; Cui, J.; Mi, J.; Li, H.; He, F. Kinetic simulation of hydrocarbon generation and its application to in-situ conversion of shale oil. Pet. Explor. Dev. 2019, 46, 1288–1296. [Google Scholar] [CrossRef]
- Liao, L.; Wang, Y.; Chen, C.; Shi, S.; Deng, R. Kinetic study of marine and lacustrine shale grains using Rock-Eval pyrolysis: Implications to hydrocarbon generation, retention and expulsion. Mar. Pet. Geol. 2018, 89, 164–173. [Google Scholar] [CrossRef]
- Gai, H.; Tian, H.; Xiao, X. Late gas generation potential for different types of shale source rocks: Implications from pyrolysis experiments. Int. J. Coal Geol. 2018, 193, 16–29. [Google Scholar] [CrossRef]
- Donadelli, J.; Pineda, J.; Comerio, M.; Smal, C.; Erra, G.; Acosta, R. Natural and laboratory-induced maturation of kerogen from the Vaca Muerta Formation: A comparison study. Org. Geochem. 2023, 185, 104690. [Google Scholar] [CrossRef]
- Hu, T.; Pang, X.; Jiang, S.; Wang, Q.; Zheng, X.; Ding, X.; Zhao, Y.; Zhu, C.; Li, H. Oil content evaluation of lacustrine organic-rich shale with strong heterogeneity: A case study of the Middle Permian Lucaogou Formation in Jimusaer Sag, Junggar Basin, NW China. Fuel 2018, 221, 196–205. [Google Scholar] [CrossRef]
- Hou, L.; Huang, H.; Yang, C.; Ma, W. Experimental Simulation of Hydrocarbon Expulsion in Semi-open Systems from Variable Organic Richness Source Rocks. ACS Omega 2021, 6, 14664–14676. [Google Scholar] [CrossRef]
- Tang, Q.; Zhang, M.; Zhang, T.; Shang, H.; Lin, Y. A review on pyrolysis experimentation on hydrocarbon generation. J. Southwest Pet. Univ. (Sci. Technol. Ed.) 2013, 35, 52–62. [Google Scholar] [CrossRef]
- Zhang, Z.; Hou, L.; Luo, X.; He, K.; Zhang, Y. Hydrocarbon generation kinetics and in-situ conversion temperature conditions of Chang 7 Member shale in Ordos Basin. Nat. Gas Geosci. 2021, 32, 1849–1858. [Google Scholar] [CrossRef]
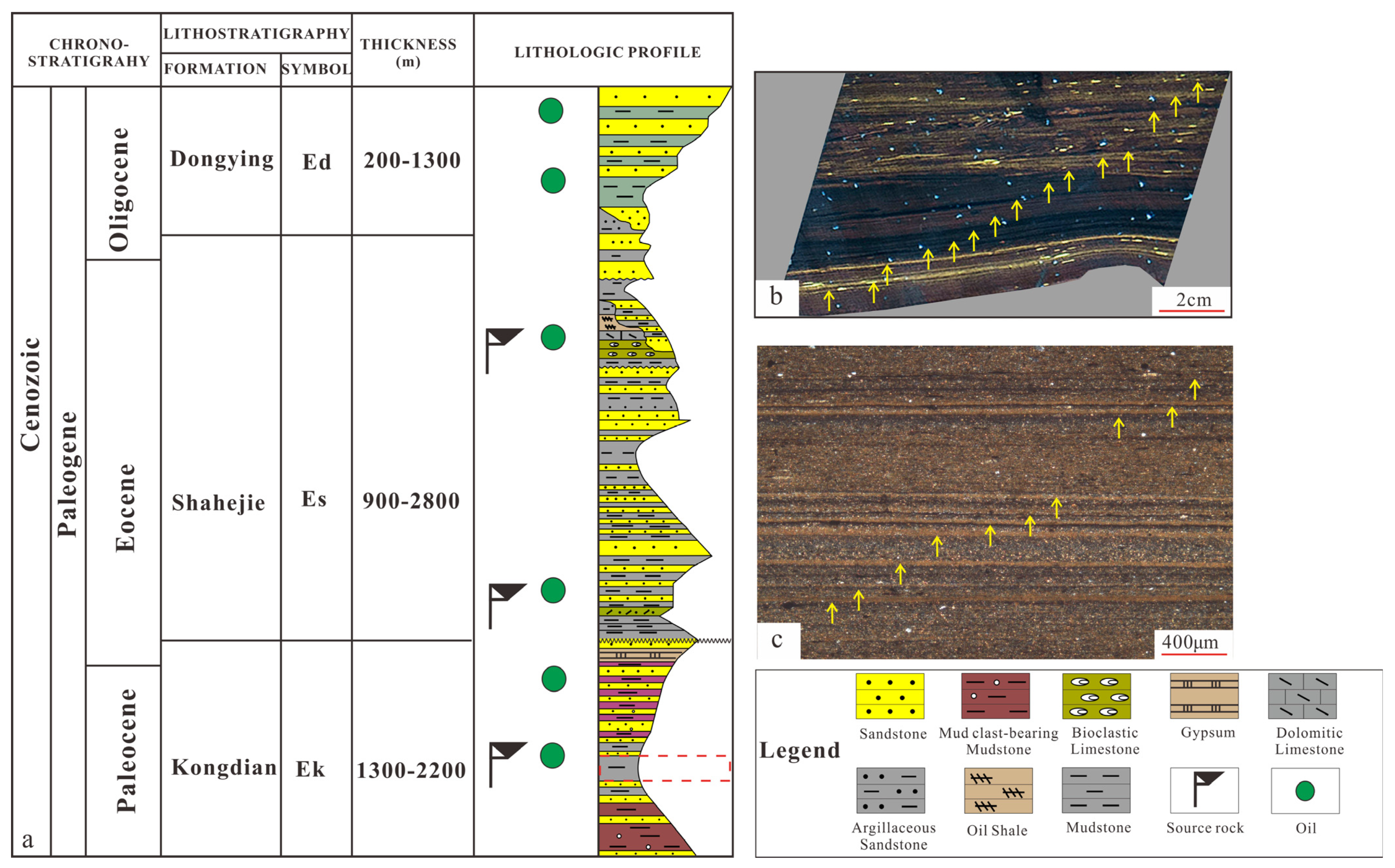







| Sample Style | Temperature, °C | Depth, m | Formation Pressure, Mpa | Lithostatic Pressure, Mpa |
|---|---|---|---|---|
| Felsic shale | OS | 2565 | 31 | 75 |
| 300 | 2575 | 31 | 75 | |
| 325 | 3116 | 37 | 88 | |
| 350 | 3449 | 39 | 94 | |
| 375 | 3651 | 45 | 109 | |
| 400 | 4538 | 49 | 117 | |
| 475 | 6000 | 60 | 144 | |
| Carbonate shale | OS | 2575 | 31 | 75 |
| 300 | 2575 | 31 | 75 | |
| 325 | 3116 | 37 | 88 | |
| 350 | 3449 | 39 | 94 | |
| 375 | 3651 | 45 | 109 | |
| 400 | 4538 | 49 | 117 | |
| 475 | 6000 | 60 | 144 |
| Sample Style | Temperature, °C | TOC, % | S1, mg/g | S2, mg/g | HI, mg/g |
|---|---|---|---|---|---|
| Felsic shale | 300 | 5.51 | 0.48 | 10.61 | 596 |
| 325 | 3.02 | 2.11 | 19.88 | 659 | |
| 350 | 2.58 | 3.52 | 13.98 | 542 | |
| 375 | 1.75 | 3.24 | 3.74 | 213 | |
| 400 | 1.09 | 0.57 | 0.34 | 32 | |
| 475 | 1.07 | 0.21 | 0.04 | 4 | |
| Carbonate shale | 300 | 5.62 | 1.24 | 30.39 | 728 |
| 325 | 3.36 | 1.97 | 21.97 | 654 | |
| 350 | 3.03 | 3.98 | 15.54 | 513 | |
| 375 | 2.56 | 4.88 | 6.35 | 248 | |
| 400 | 1.53 | 0.77 | 0.78 | 51 | |
| 475 | 1.40 | 0.19 | 0.17 | 12 |
| Sample Style | Temperature, °C | Expelled Oil, mgHC/gTOC | Retained Oil, mgHC/gTOC | Total Oil, mgHC/gTOC | H2, m3/gTOC | CO2, m3/gTOC | Hydrocarbon Gas, m3/gTOC | Total Gas, m3/gTOC | Total Hydrocarbon Products, mgHC/gTOC |
|---|---|---|---|---|---|---|---|---|---|
| Felsic shale | 300 | 17 | 118 | 134 | 122 | 3 | 11 | 14 | 149 |
| 325 | 38 | 233 | 271 | 127 | 2 | 22 | 29 | 300 | |
| 350 | 375 | 371 | 746 | 200 | 2 | 78 | 106 | 852 | |
| 375 | 351 | 244 | 595 | 426 | 3 | 175 | 175 | 770 | |
| 400 | 255 | 59 | 314 | 429 | 3 | 303 | 450 | 764 | |
| 475 | 220 | 11 | 231 | 447 | 9 | 570 | 585 | 816 | |
| Carbonate shale | 300 | 9 | 205 | 214 | 54 | 1 | 3 | 3 | 217 |
| 325 | 22 | 205 | 226 | 112 | 1 | 8 | 9 | 236 | |
| 350 | 122 | 275 | 397 | 165 | 1 | 25 | 31 | 427 | |
| 375 | 191 | 490 | 681 | 240 | 1 | 56 | 30 | 711 | |
| 400 | 190 | 46 | 236 | 264 | 2 | 131 | 183 | 419 | |
| 475 | 190 | 8 | 198 | 518 | 8 | 213 | 204 | 402 |
| Sample Style | Temperature, °C | C1, mol% | C2H6, mol% | C2H4, mol% | C3H8, mol% | C3H6, mol% | iC4, mol% | nC4, mol% | C4H8, mol% | iC5, mol% | nC5, mol% | H2, mol% | CO2, mol% |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Felsic shale | 300 | 4.54 | 1.01 | 0.00 | 1.25 | 0.03 | 0.16 | 0.47 | 0.00 | 0.13 | 0.47 | 1.93 | 90.00 |
| 325 | 8.20 | 2.62 | 0.00 | 1.96 | 0.03 | 0.29 | 0.84 | 0.02 | 0.21 | 0.68 | 1.55 | 83.59 | |
| 350 | 13.91 | 5.57 | 0.00 | 4.07 | 0.00 | 0.64 | 1.93 | 0.00 | 0.48 | 1.33 | 0.78 | 71.28 | |
| 375 | 13.82 | 5.87 | 0.00 | 4.54 | 0.04 | 0.58 | 2.33 | 0.03 | 0.46 | 1.37 | 0.52 | 70.46 | |
| 400 | 16.47 | 9.08 | 0.00 | 7.97 | 0.09 | 0.94 | 4.02 | 0.05 | 0.68 | 1.92 | 0.44 | 58.34 | |
| 475 | 35.47 | 13.41 | 0.00 | 5.78 | 0.00 | 0.47 | 0.32 | 0.00 | 0.00 | 0.07 | 0.89 | 43.59 | |
| Carbonate shale | 300 | 3.34 | 0.50 | 0.00 | 0.45 | 0.00 | 0.08 | 0.17 | 0.00 | 0.06 | 0.17 | 1.06 | 94.18 |
| 325 | 3.94 | 0.97 | 0.00 | 0.69 | 0.00 | 0.11 | 0.30 | 0.00 | 0.08 | 0.23 | 0.92 | 92.77 | |
| 350 | 7.58 | 2.19 | 0.00 | 1.53 | 0.02 | 0.23 | 0.72 | 0.00 | 0.16 | 0.49 | 0.72 | 86.36 | |
| 375 | 10.85 | 3.37 | 0.00 | 2.40 | 0.01 | 0.33 | 1.14 | 0.00 | 0.23 | 0.62 | 0.35 | 80.68 | |
| 400 | 14.98 | 7.03 | 0.00 | 5.63 | 0.04 | 0.71 | 2.77 | 0.02 | 0.49 | 1.29 | 0.42 | 66.62 | |
| 475 | 20.66 | 5.65 | 0.00 | 2.25 | 0.00 | 0.19 | 0.09 | 0.00 | 0.00 | 0.03 | 1.05 | 70.08 |
| Sample Style | Temperature, °C | Saturated Hydrocarbons, % | Aromatic Hydrocarbons, % | Non- Hydrocarbons, % | Asphaltenes, % | Maximum Peak | ∑C21−/ ∑C22+ | OEP | Pr/nC17 | Ph/nC18 |
|---|---|---|---|---|---|---|---|---|---|---|
| Felsic shale | 300 | 25.34 | 18.62 | 47.18 | 4.76 | C21 | 1.16 | 1.29 | 0.35 | 1.53 |
| 325 | 29.79 | 21.00 | 39.87 | 7.32 | C21 | 1.03 | 1.36 | 0.16 | 0.74 | |
| 350 | 23.76 | 17.60 | 46.85 | 9.81 | C21 | 1.18 | 1.25 | 0.08 | 0.15 | |
| 375 | 31.77 | 13.48 | 46.83 | 6.56 | C17 | 1.49 | 1.03 | 0.07 | 0.10 | |
| 400 | 19.55 | 31.27 | 37.74 | 7.54 | C17 | 1.43 | 1.03 | 0.05 | 0.09 | |
| 475 | - | - | - | - | C17 | 1.43 | 0.99 | 0.08 | 0.08 | |
| Carbonate shale | 300 | 19.03 | 9.38 | 42.62 | 21.37 | C21 | 1.39 | 1.07 | 0.14 | 0.62 |
| 325 | 18.96 | 7.45 | 49.48 | 19.36 | C22 | 1.92 | 1.01 | 0.14 | 0.51 | |
| 350 | 23.54 | 13.20 | 33.83 | 24.54 | C21 | 0.92 | 1.06 | 0.14 | 0.26 | |
| 375 | 39.71 | 9.94 | 17.97 | 32.13 | C21 | 1.72 | 1.09 | 0.04 | 0.07 | |
| 400 | 48.05 | 13.21 | 22.84 | 15.76 | C21 | 2.24 | 1.05 | 0.14 | 0.04 | |
| 475 | 21.78 | 14.15 | 18.56 | 44.81 | C21 | 1.37 | 1.06 | 0.06 | 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Guan, Q.; Liu, X.; Chen, C.; Zhao, X.; Jin, F.; Jiang, W.; Pu, X.; Sun, B.; Liu, T.; Hua, Z.; et al. A Comparison of Generation–Retention–Expulsion in Felsic and Carbonate Laminated Shale by Semi-Open Thermal Pyrolysis: Implications for Shale Oil Exploration. Geosciences 2026, 16, 9. https://doi.org/10.3390/geosciences16010009
Guan Q, Liu X, Chen C, Zhao X, Jin F, Jiang W, Pu X, Sun B, Liu T, Hua Z, et al. A Comparison of Generation–Retention–Expulsion in Felsic and Carbonate Laminated Shale by Semi-Open Thermal Pyrolysis: Implications for Shale Oil Exploration. Geosciences. 2026; 16(1):9. https://doi.org/10.3390/geosciences16010009
Chicago/Turabian StyleGuan, Quansheng, Xiaoping Liu, Changwei Chen, Xianzheng Zhao, Fengming Jin, Wenya Jiang, Xiugang Pu, Biao Sun, Tian Liu, Zuxian Hua, and et al. 2026. "A Comparison of Generation–Retention–Expulsion in Felsic and Carbonate Laminated Shale by Semi-Open Thermal Pyrolysis: Implications for Shale Oil Exploration" Geosciences 16, no. 1: 9. https://doi.org/10.3390/geosciences16010009
APA StyleGuan, Q., Liu, X., Chen, C., Zhao, X., Jin, F., Jiang, W., Pu, X., Sun, B., Liu, T., Hua, Z., Peng, W., & Jia, G. (2026). A Comparison of Generation–Retention–Expulsion in Felsic and Carbonate Laminated Shale by Semi-Open Thermal Pyrolysis: Implications for Shale Oil Exploration. Geosciences, 16(1), 9. https://doi.org/10.3390/geosciences16010009




