Effects of Tributyrin on Antioxidant Capacity, Immune Function, and Liver Macrophage Polarization in Weaned Piglets Under LPS Challenge
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Materials
2.2. Experimental Design
2.3. Feeding Management and Diet Composition
2.4. Sample Collection
2.5. Index Determination
2.5.1. Determination of Growth Performance of Weaned Piglets
2.5.2. Determination of Antioxidant and Immune Indexes
2.5.3. Determination of Serum Biochemical Indices
2.5.4. mRNA Expression Analysis
2.5.5. Immunofluorescence (IF)
2.5.6. Western Blot
2.6. Data Statistics and Analysis
3. Results
3.1. Effects of Tributyrin on the Growth Performance of Weaned Piglets
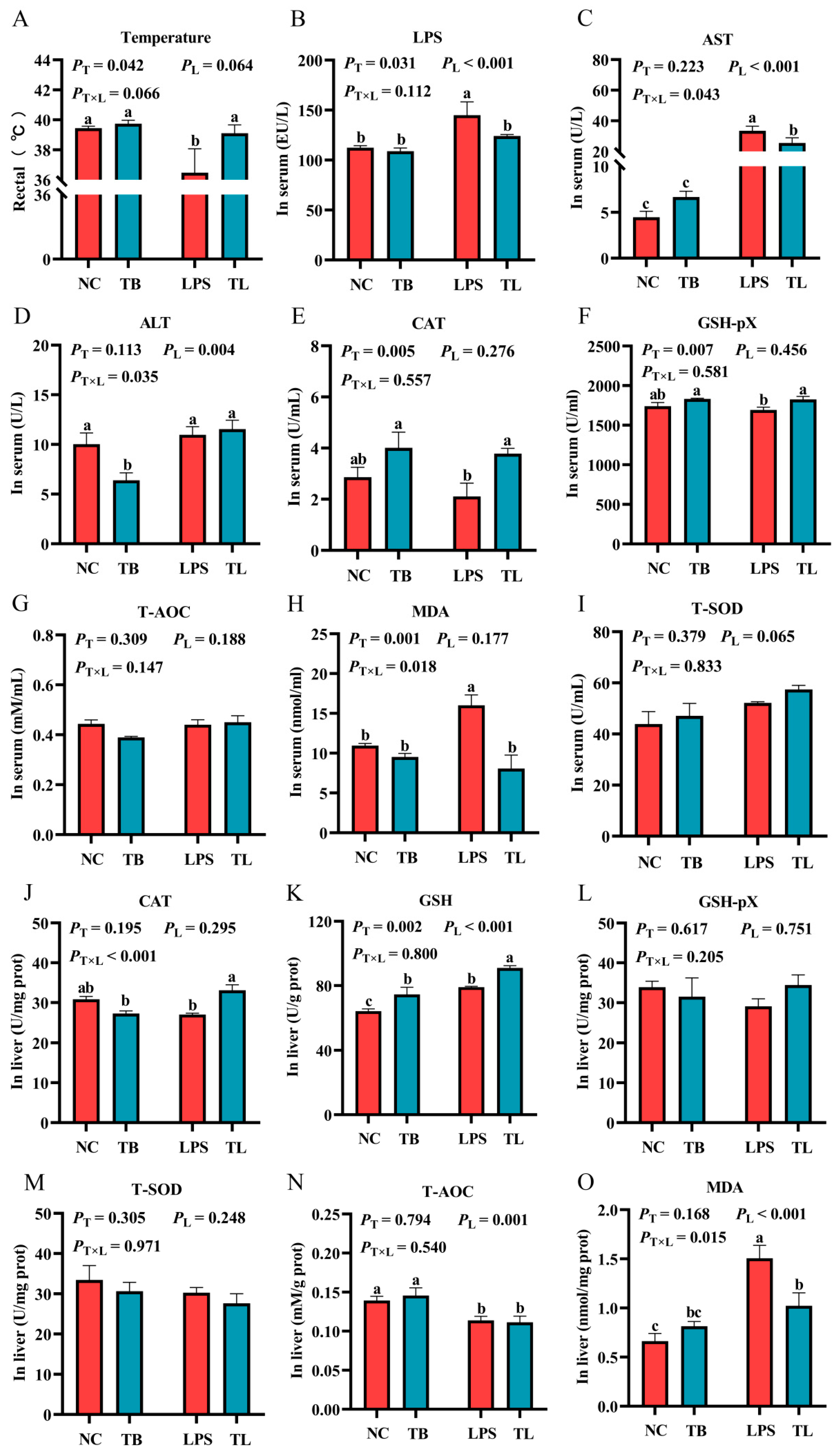
3.2. The Effects of Tributyrin on Antioxidant Function in Serum and Liver of Weaned Piglets Under LPS Challenge
3.3. The Effect of Tributyrin on Immune Function of Weaned Piglets Under LPS Challenge
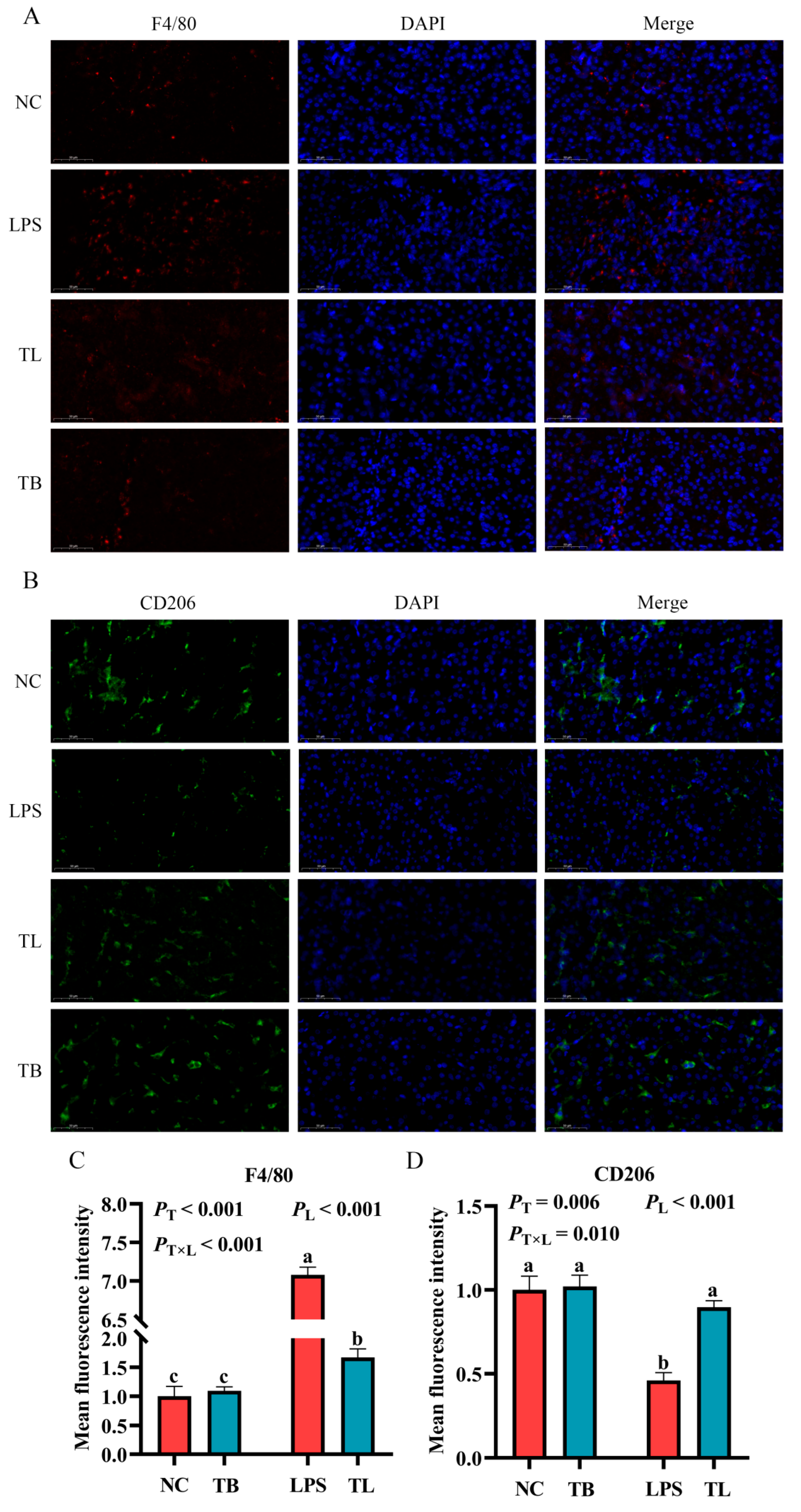
3.4. The Effect of Tributyrin on the Polarization and Infiltration of Macrophages in the Liver of Weaned Piglets Under LPS Challenge
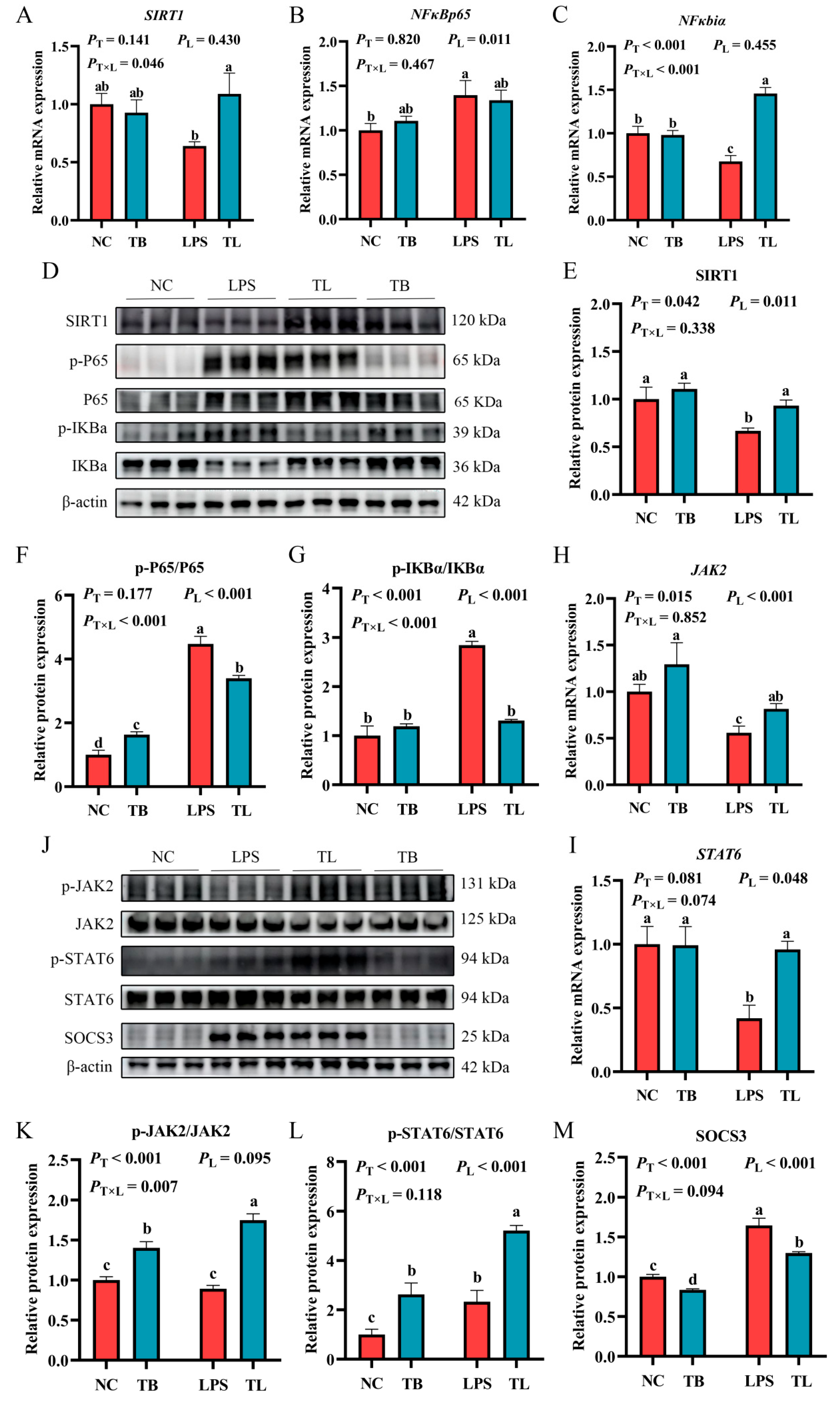
3.5. Effects of Tributyrin on the SIRT1/NF-κB and JAK2/STAT6 Signaling Pathways in the Liver of LPS Stress-Induced Weaned Piglets
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheng, L.; Zhang, X.; Wang, C.; Deng, O.; Gu, B. Whole-chain intensification of pig and chicken farming could lower emissions with economic and food production benefits. Nat. Food 2024, 5, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Van Tran, T.; Kim, Y.S.; Yun, H.H.; Nguyen, D.H.; Bui, T.T.; Van Tran, P. A blend of bacillus-fermented soybean meal, functional amino acids, and nucleotides improves nutrient digestibility, bolsters immune response, reduces diarrhea, and enhances growth performance in weaned piglets. J. Anim. Sci. 2024, 102, skae293. [Google Scholar] [CrossRef]
- Canibe, N.; Højberg, O.; Kongsted, H.; Vodolazska, D.; Lauridsen, C.; Nielsen, T.S.; Schönherz, A.A. Review on Preventive Measures to Reduce Post-Weaning Diarrhoea in Piglets. Animals 2022, 12, 2585. [Google Scholar] [CrossRef]
- Feng, K.; Sun, L.; Zhou, Z.; Zhang, J.A.; Fang, R.H.; Gao, W.; Zhang, L. Macrophage-mimicking nanodiscs for treating systemic infection caused by methicillin-resistant Staphylococcus aureus. Sci. Adv. 2025, 11, eadw7511. [Google Scholar] [CrossRef]
- Liu, H.Y.; Zhu, C.; Zhu, M.; Yuan, L.; Li, S.; Gu, F.; Hu, P.; Chen, S.; Cai, D. Alternatives to antibiotics in pig production: Looking through the lens of immunophysiology. Stress Biol. 2024, 4, 1. [Google Scholar] [CrossRef]
- Yu, C.; Luo, Y.; Shen, C.; Luo, Z.; Zhang, H.; Zhang, J.; Xu, W.; Xu, J. Effects of microbe-derived antioxidants on growth performance, hepatic oxidative stress, mitochondrial function and cell apoptosis in weaning piglets. J. Anim. Sci. Biotechnol. 2024, 15, 128. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Li, Z.; Anagnostopoulos, G.; Kong, W.T.; Zhang, S.; Chakarov, S.; Shin, A.; Qian, J.; Zhu, Y.; Bai, W.; et al. Notch signaling regulates macrophage-mediated inflammation in metabolic dysfunction-associated steatotic liver disease. Immunity 2024, 57, 2310–2327.e6. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ma, C.; Gong, L.; Guo, Y.; Fu, K.; Zhang, Y.; Zhou, H.; Li, Y. Macrophage Polarization and Its Role in Liver Disease. Front. Immunol. 2021, 12, 803037. [Google Scholar] [CrossRef]
- Sun, X.; Gao, J.; Meng, X.; Lu, X.; Zhang, L.; Chen, R. Polarized Macrophages in Periodontitis: Characteristics, Function, and Molecular Signaling. Front. Immunol. 2021, 12, 763334. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Zheng, X.; Jia, M.; Mei, Z.; Wang, Y.; Zhang, Z.; Zhou, M.; Li, C. M2-type exosomes nanoparticles for rheumatoid arthritis therapy via macrophage re-polarization. J. Control Release 2022, 341, 16–30. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, K.; Ren, G.-M.; Wang, Y.; Wang, T.; Liu, X.; Li, D.-X.; Xiao, Y.; Chen, X.; Li, Y.-T.; et al. BRISC is required for optimal activation of NF-κB in Kupffer cells induced by LPS and contributes to acute liver injury. Cell Death Dis. 2023, 14, 743. [Google Scholar] [CrossRef]
- Abbas, Z.; Ahmad, B.; Tong, Y.; Zhang, J.; Wu, S.; Wang, J.; Li, Z.; Liu, T.; Liu, Y.; Wei, X.; et al. Mulberry-derived postbiotics alleviate LPS-induced intestinal inflammation and modulate gut microbiota dysbiosis. Food Funct. 2025, 16, 4437–4450. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Wang, W.; An, R.; Wang, Y.; Ren, Q.; Xuan, J. Benefits of tributyrin on growth performance, gastrointestinal tract development, ruminal bacteria and volatile fatty acid formation of weaned Small-Tailed Han lambs. Anim. Nutr. 2023, 15, 187–196. [Google Scholar] [CrossRef]
- Rafiei, H.; Yeung, M.; Kowalski, S.; Li, M.Y.; Harris, D.; Chang, J.; Nguyen, N.; Yorke, E.; Sampath, S.; Hollman, S.; et al. Butyrate and tributyrin reduce LPS-induced inflammatory cytokine production from human visceral fat. Int. J. Obes. 2024, 48, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Ghare, S.S.; Charpentier, B.T.; Ghooray, D.T.; Zhang, J.; Vadhanam, M.V.; Reddy, S.; Joshi-Barve, S.; McClain, C.J.; Barve, S.S. Tributyrin Mitigates Ethanol-Induced Lysine Acetylation of Histone-H3 and p65-NFκB Downregulating CCL2 Expression and Consequent Liver Inflammation and Injury. Nutrients 2023, 15, 4397. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Ji, K.; Ge, X.; Xi, B.; Ren, M.; Zhang, L.; Chen, X. Tributyrin Plays an Important Role in Regulating the Growth and Health Status of Juvenile Blunt Snout Bream (Megalobrama amblycephala), as Evidenced by Pathological Examination. Front. Immunol. 2021, 12, 652294. [Google Scholar] [CrossRef]
- Li, W.; Zhang, L.; Xu, Q.; Yang, W.; Zhao, J.; Ren, Y.; Yu, Z.; Ma, L. Taxifolin Alleviates DSS-Induced Ulcerative Colitis by Acting on Gut Microbiome to Produce Butyric Acid. Nutrients 2022, 14, 1069. [Google Scholar] [CrossRef]
- Liu, K.; He, X.; Huang, J.; Yu, S.; Cui, M.; Gao, M.; Liu, L.; Qian, Y.; Xie, Y.; Hui, M.; et al. Short-chain fatty acid-butyric acid ameliorates granulosa cells inflammation through regulating METTL3-mediated N6-methyladenosine modification of FOSL2 in polycystic ovarian syndrome. Clin. Epigenetics 2023, 15, 86. [Google Scholar] [CrossRef] [PubMed]
- Dang, D.X.; Lee, H.; Lee, S.J.; Song, J.H.; Mun, S.; Lee, K.Y.; Han, K.; Kim, I.H. Tributyrin and anise mixture supplementation improves growth performance, nutrient digestibility, jejunal villus height, and fecal microbiota in weaned pigs. Front. Vet. Sci. 2023, 10, 1107149. [Google Scholar] [CrossRef]
- Huang, S.; Chen, J.; Cui, Z.; Ma, K.; Wu, D.; Luo, J.; Li, F.; Xiong, W.; Rao, S.; Xiang, Q.; et al. Lachnospiraceae-derived butyrate mediates protection of high fermentable fiber against placental inflammation in gestational diabetes mellitus. Sci. Adv. 2023, 9, eadi7337. [Google Scholar] [CrossRef] [PubMed]
- Nutrient Requirements of Swine: Eleventh Revised Edition; The National Academies Press: Washington, DC, USA, 2012; ISBN 978-0-309-22423-9.
- Zhou, Q.; Gao, J.; Wu, G.; Wang, C.; Yang, Y.; Huang, T.; Wang, Y.; Yue, T.; Gao, Z.; Xie, H.; et al. Adipose progenitor cell-derived extracellular vesicles suppress macrophage M1 program to alleviate midlife obesity. Nat. Commun. 2025, 16, 2743. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- He, Z.; Liu, N.; Cai, Y.; Yang, N.; Li, G.; Xiao, Y.; Zhou, X.; Cao, S.; Qu, F.; Tang, J.; et al. Effect of Tributyrin on Growth Performance and Pathway by which Tributyrin Regulates Oligopeptide Transporter 1 in Juvenile Grass Carp (Ctenopharyngodon idellus). Animals 2022, 12, 2498. [Google Scholar] [CrossRef]
- Gong, L.; Xiao, G.; Zheng, L.; Yan, X.; Qi, Q.; Zhu, C.; Feng, X.; Huang, W.; Zhang, H. Effects of Dietary Tributyrin on Growth Performance, Biochemical Indices, and Intestinal Microbiota of Yellow-Feathered Broilers. Animals 2021, 11, 3425. [Google Scholar] [CrossRef]
- Gu, Y.; Song, Y.; Yin, H.; Lin, S.; Zhang, X.; Che, L.; Lin, Y.; Xu, S.; Feng, B.; Wu, D.; et al. Dietary supplementation with tributyrin prevented weaned pigs from growth retardation and lethal infection via modulation of inflammatory cytokines production, ileal expression, and intestinal acetate fermentation. J. Anim. Sci. 2017, 95, 226–238. [Google Scholar] [CrossRef]
- Salvi, P.S.; Cowles, R.A. Butyrate and the Intestinal Epithelium: Modulation of Proliferation and Inflammation in Homeostasis and Disease. Cells 2021, 10, 1775. [Google Scholar] [CrossRef]
- Hou, J.; Lian, L.; Lu, L.; Gu, T.; Zeng, T.; Chen, L.; Xu, W.; Li, G.; Wu, H.; Tian, Y. Effects of Dietary Bacillus coagulans and Tributyrin on Growth Performance, Serum Antioxidants, Intestinal Morphology, and Cecal Microbiota of Growing Yellow-Feathered Broilers. Animals 2023, 13, 3534. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dou, X.; Wang, R.; Jiang, Y.; Zhang, J.; Qiao, X.; Liu, Y.; Zhang, H.; Lai, C.; Chen, Y.; et al. Salvianolic Acid B Alleviates LPS-Induced Spleen Injury by Remodeling Redox Status and Suppressing NLRP3 Inflammasome. Antioxidants 2025, 14, 883. [Google Scholar] [CrossRef]
- Man, K.; Kutyavin, V.I.; Chawla, A. Tissue Immunometabolism: Development, Physiology, and Pathobiology. Cell Metab. 2017, 25, 11–26. [Google Scholar] [CrossRef]
- Dick, S.A.; Macklin, J.A.; Nejat, S.; Momen, A.; Clemente-Casares, X.; Althagafi, M.G.; Chen, J.; Kantores, C.; Hosseinzadeh, S.; Aronoff, L.; et al. Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat. Immunol. 2019, 20, 29–39. [Google Scholar] [CrossRef]
- Wang, C.; Cao, S.; Zhang, Q.; Shen, Z.; Feng, J.; Hong, Q.; Lu, J.; Xie, F.; Peng, Y.; Hu, C. Dietary Tributyrin Attenuates Intestinal Inflammation, Enhances Mitochondrial Function, and Induces Mitophagy in Piglets Challenged with Diquat. J. Agric. Food Chem. 2019, 67, 1409–1417. [Google Scholar] [CrossRef]
- Chen, C.; Qu, M.; Li, G.; Wan, G.; Liu, P.; Omar, S.M.; Mei, W.; Hu, Z.; Zhou, Q.; Xu, L. Dietary Tributyrin Improves Growth Performance, Meat Quality, Muscle Oxidative Status, and Gut Microbiota in Taihe Silky Fowls under Cyclic Heat Stress. Animals 2024, 14, 3041. [Google Scholar] [CrossRef]
- Li, J.; Hou, Y.; Yi, D.; Zhang, J.; Wang, L.; Qiu, H.; Ding, B.; Gong, J. Effects of Tributyrin on Intestinal Energy Status, Antioxidative Capacity and Immune Response to Lipopolysaccharide Challenge in Broilers. Asian-Australas. J. Anim. Sci. 2015, 28, 1784–1793. [Google Scholar] [CrossRef]
- Wang, X.; Wan, M.; Wang, Z.; Zhang, H.; Zhu, S.; Cao, X.; Xu, N.; Zheng, J.; Bu, X.; Xu, W.; et al. Effects of Tributyrin Supplementation on Growth Performance, Intestinal Digestive Enzyme Activity, Antioxidant Capacity, and Inflammation-Related Gene Expression of Large Yellow Croaker (Larimichthys crocea) Fed with a High Level of Clostridium Autoethanogenum Protein. Aquac. Nutr. 2023, 2023, 2687734. [Google Scholar] [CrossRef]
- Meng, M.; Huo, R.; Wang, Y.; Ma, N.; Shi, X.; Shen, X.; Chang, G. Lentinan inhibits oxidative stress and alleviates LPS-induced inflammation and apoptosis of BMECs by activating the Nrf2 signaling pathway. Int. J. Biol. Macromol. 2022, 222, 2375–2391. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Ma, T. Sodium butyrate protect bone mass in lipopolysaccharide-treated rats by reducing oxidative stress and inflammatory. Redox Rep. 2024, 29, 2398891. [Google Scholar] [CrossRef]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium Butyrate Inhibits Inflammation and Maintains Epithelium Barrier Integrity in a TNBS-induced Inflammatory Bowel Disease Mice Model. EBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Li, C.; Kuang, M.; Shah, A.U.; Shafiq, M.; Ahmad, M.A.; Abdalmegeed, D.; Li, L.; Wang, G. Nrf2 Activation and NF-Kb & caspase/bax signaling inhibition by sodium butyrate alleviates LPS-induced cell injury in bovine mammary epithelial cells. Mol. Immunol. 2022, 148, 54–67. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Barkema, H.W.; Gao, J.; Yang, J.; Wang, Y.; Kastelic, J.P.; Khan, S.; Liu, G.; Han, B. MicroRNA miR-223 modulates NLRP3 and Keap1, mitigating lipopolysaccharide-induced inflammation and oxidative stress in bovine mammary epithelial cells and murine mammary glands. Vet. Res. 2023, 54, 78. [Google Scholar] [CrossRef] [PubMed]
- Khaksari, M.; Pourali, M.; Rezaei Talabon, S.; Gholizadeh Navashenaq, J.; Bashiri, H.; Amiresmaili, S. Protective effects of 17-β-estradiol on liver injury: The role of TLR4 signaling pathway and inflammatory response. Cytokine 2024, 181, 156686. [Google Scholar] [CrossRef]
- Zhang, Z.; Qin, P.; Deng, Y.; Ma, Z.; Guo, H.; Guo, H.; Hou, Y.; Wang, S.; Zou, W.; Sun, Y.; et al. The novel estrogenic receptor GPR30 alleviates ischemic injury by inhibiting TLR4-mediated microglial inflammation. J. Neuroinflammation 2018, 15, 206. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, J.; Wang, X.; Ji, F.; Ronco, C.; Tian, J.; Yin, Y. Gut-liver crosstalk in sepsis-induced liver injury. Crit. Care 2020, 24, 614. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.; Saredy, J.; Yuan, Z.; Yang, X.; Wang, H. Innate-adaptive immunity interplay and redox regulation in immune response. Redox Biol. 2020, 37, 101759. [Google Scholar] [CrossRef]
- Golino, M.; Harding, D.; Del Buono, M.G.; Fanti, S.; Mohiddin, S.; Toldo, S.; Smyth, J.; Sanna, T.; Marelli-Berg, F.; Abbate, A. Innate and adaptive immunity in acute myocarditis. Int. J. Cardiol. 2024, 404, 131901. [Google Scholar] [CrossRef]
- Rydyznski Moderbacher, C.; Ramirez, S.I.; Dan, J.M.; Grifoni, A.; Hastie, K.M.; Weiskopf, D.; Belanger, S.; Abbott, R.K.; Kim, C.; Choi, J.; et al. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell 2020, 183, 996–1012.e19. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine Storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef]
- El-Shitany, N.A.; Eid, B.G. Icariin modulates carrageenan-induced acute inflammation through HO-1/Nrf2 and NF-kB signaling pathways. Biomed. Pharmacother. 2019, 120, 109567. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Zhang, L.; Bhushan, A.; Swanson, B.; Zhang, L.; Mamede, J.I.; Voigt, R.M.; Shaikh, M.; Engen, P.A.; Keshavarzian, A. The SARS-CoV-2 S1 Spike Protein Promotes MAPK and NF-kB Activation in Human Lung Cells and Inflammatory Cytokine Production in Human Lung and Intestinal Epithelial Cells. Microorganisms 2022, 10, 1996. [Google Scholar] [CrossRef]
- Jangam, A.; Tirunavalli, S.K.; Adimoolam, B.M.; Kasireddy, B.; Patnaik, S.S.; Erukkambattu, J.; Thota, J.R.; Andugulapati, S.B.; Addlagatta, A. Anti-inflammatory and antioxidant activities of Gymnema Sylvestre extract rescue acute respiratory distress syndrome in rats via modulating the NF-κB/MAPK pathway. Inflammopharmacology 2023, 31, 823–844. [Google Scholar] [CrossRef]
- Mahon, O.R.; Browe, D.C.; Gonzalez-Fernandez, T.; Pitacco, P.; Whelan, I.T.; Von Euw, S.; Hobbs, C.; Nicolosi, V.; Cunningham, K.T.; Mills, K.H.G.; et al. Nano-particle mediated M2 macrophage polarization enhances bone formation and MSC osteogenesis in an IL-10 dependent manner. Biomaterials 2020, 239, 119833. [Google Scholar] [CrossRef]
- Zhuang, H.; Ren, X.; Jiang, F.; Zhou, P. Indole-3-propionic acid alleviates chondrocytes inflammation and osteoarthritis via the AhR/NF-κB axis. Mol. Med. 2023, 29, 17. [Google Scholar] [CrossRef]
- Lee, S.M.; Koh, D.H.; Jun, D.W.; Roh, Y.J.; Kang, H.T.; Oh, J.H.; Kim, H.S. Auranofin attenuates hepatic steatosis and fibrosis in nonalcoholic fatty liver disease via NRF2 and NF-κB signaling pathways. Clin. Mol. Hepatol. 2022, 28, 827–840. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, J.; Kong, D.; Guo, X.; Li, J.; Long, Z.; Peng, J.; Wang, Z.; Wu, H.; Liu, P.; et al. Irisin drives macrophage anti-inflammatory differentiation via JAK2-STAT6-dependent activation of PPARγ and Nrf2 signaling. Free Radic. Biol. Med. 2023, 201, 98–110. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, N.; Wang, R.; Zhan, Z.; Guo, S.; Song, H.; Wiemer, E.A.C.; Ben, J.; Ma, J. Macrophage MVP regulates fracture repair by promoting M2 polarization via JAK2-STAT6 pathway. Int. Immunopharmacol. 2023, 120, 110313. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Lin, W.; Fan, W.; Zhuang, T.; Wang, X.; Li, Q.; Wei, X.; Wang, Z.; Chen, K.; et al. Panax notoginseng saponins ameliorate LPS-induced acute lung injury by promoting STAT6-mediated M2-like macrophage polarization. Phytomedicine 2025, 139, 156513. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhao, T.; Zhou, W.; Khan, A.; Cao, J.; Liu, Y.; Wang, Z.; Cheng, G. 6′-O-caffeoylarbutin attenuates D-galactose-induced brain and liver damage in aging mice via regulating SIRT1/NF-κB pathway. Phytomedicine 2025, 141, 156710. [Google Scholar] [CrossRef]
- Yu, T.; Gan, S.; Zhu, Q.; Dai, D.; Li, N.; Wang, H.; Chen, X.; Hou, D.; Wang, Y.; Pan, Q.; et al. Modulation of M2 macrophage polarization by the crosstalk between Stat6 and Trim24. Nat. Commun. 2019, 10, 4353. [Google Scholar] [CrossRef]
- Deng, C.; Huo, M.; Chu, H.; Zhuang, X.; Deng, G.; Li, W.; Wei, H.; Zeng, L.; He, Y.; Liu, H.; et al. Exosome circATP8A1 induces macrophage M2 polarization by regulating the miR-1-3p/STAT6 axis to promote gastric cancer progression. Mol. Cancer 2024, 23, 49. [Google Scholar] [CrossRef]
- Van den Bossche, J.; Baardman, J.; Otto, N.A.; van der Velden, S.; Neele, A.E.; van den Berg, S.M.; Luque-Martin, R.; Chen, H.-J.; Boshuizen, M.C.S.; Ahmed, M.; et al. Mitochondrial Dysfunction Prevents Repolarization of Inflammatory Macrophages. Cell Rep. 2016, 17, 684–696. [Google Scholar] [CrossRef]
- Vadevoo, S.M.P.; Kang, Y.; Gunassekaran, G.R.; Lee, S.-M.; Park, M.-S.; Jo, D.G.; Kim, S.-K.; Lee, H.; Kim, W.J.; Lee, B. IL4 receptor targeting enables nab-paclitaxel to enhance reprogramming of M2-type macrophages into M1-like phenotype via ROS-HMGB1-TLR4 axis and inhibition of tumor growth and metastasis. Theranostics 2024, 14, 2605–2621. [Google Scholar] [CrossRef] [PubMed]


| Early Phase (0–14 d) | Subsequent Phase (15–28 d) | ||
|---|---|---|---|
| Ingredients (%) | Ingredients (%) | ||
| Puffed corn | 15.12 | Broken rice | 10.00 |
| Corn | 10.00 | Corn | 28.79 |
| Flour | 25.00 | Flour | 25.00 |
| Peeled soybean meal | 3.00 | Peeled soybean meal | 18.00 |
| Fermented soybean meal | 2.50 | Fermented soybean meal | 1.50 |
| Puffed soybeans | 5.00 | Puffed flaxseeds | 1.00 |
| Fish meal | 5.00 | Secondary powder | 1.50 |
| Chicken intestinal membrane protein powder | 2.50 | Chicken intestinal membrane protein powder | 1.25 |
| First-grade soybean oil | 3.65 | First-grade soybean oil | 2.00 |
| Glucose | 10.00 | Glucose | 1.25 |
| Feeding biscuit powder | 6.00 | Feeding biscuit powder | 5.00 |
| Premix ① | 4.00 | Premix ① | 4.00 |
| Acidifying agent | 0.30 | Acidifying agent | 0.30 |
| Salt | 0.15 | Salt | 0.15 |
| Zinc oxide | 0.18 | Zinc oxide | 0.16 |
| Choline chloride (50%) | 0.10 | Choline chloride (50%) | 0.10 |
| Whey powder | 5.00 | Total | 100 |
| Plasma substitute | 2.50 | ||
| Total | 100 | ||
| Nutrient levels | Nutrient levels | ||
| Digestive energy/(MJ/kg) | 13.3 | Digestive energy/(MJ/kg) | 13.05 |
| Crude protein (%) | 18.95 | Crude protein (%) | 17.62 |
| Calcium (%) | 0.65 | Calcium (%) | 0.48 |
| Total phosphorus (%) | 0.49 | Total phosphorus (%) | 0.40 |
| Available phosphorus (%) | 0.35 | Available phosphorus (%) | 0.35 |
| Digestible lysine (%) | 1.35 | Digestible lysine (%) | 1.18 |
| Digestible methionine (%) | 0.41 | Digestible methionine (%) | 0.48 |
| Digestible threonine (%) | 0.78 | Digestible threonine (%) | 0.82 |
| Gene | Primer Sequence (5′ → 3′) | Accession Numbers | Size, bp |
|---|---|---|---|
| β-actin | F: CTCCAGAGCGCAAGTACTCC R: AATGCAACTAACAGTCCGCC | XM_003124280.5 | 153 |
| IL-1β | F: AGCCAGTCTTCATTGTTCAGGT R: CAGGTCATTATTGTTGTCACCGTAG | NM_214055.1 | 101 |
| TNF-α | F: TTATCGGCCCCCAGAAGGAA R: CGGCTTTGACATTGGCTACAAC | NM_214022.1 | 129 |
| IL-6 | F: AAATGTCGAGGCTGTGCAGA R: TCCACTCGTTCTGTGACTGC | XM_047753916.1 | 118 |
| IL-4 | F: ACACGACGGAGAAGGAAACC R: GTTCCTGTCAAGTCCGCTCA | NM_214123.1 | 165 |
| IL-10 | F: TCGGCCCAGTGAAGAGTTTC R: CGGCATTACGTCTTCCAGGT | NM_214041.1 | 146 |
| IL-13 | F: CTGACCACCAGCATGCAGTA R: CCCGTGGCGAAAAATCATCC | NM_213803.1 | 219 |
| iNOS | F: ACTGGGTTGAATCTGGGTGAA R: CCAGGGAGTCTGGAGATTTCTTT | NM_001143690.1 | 164 |
| CD86 | F: TGGTGCTGCCTCCTTGAAAA R: GGACACAGACGATGCTCACA | NM_214222.1 | 593 |
| Arg1 | F: TGCTAGACTGCTGAGCAACAT R: CTCCTCGTGGCTGACCC | XM_005659190.2 | 245 |
| CD206 | F: GCCCAGACTGAAGACAGCAT R: GGCATCTACCAGGCAGTTGT | NM_001255969.1 | 143 |
| SIRT1 | F: GAGAAGGAAACAATGGGCCG R: ACCAAACAGAAGGTTATCTCGGT | NM_001145750.2 | 155 |
| NFκBp65 | F: ATGTGGAGATCATTGAGCAGC R: CCTGGTCCTGTGTAGCCATT | NM_001114281.1 | 151 |
| NFκbiα | F: CAGAATCCCGACCTGGTGTC R: GTCGTCATAGGGCAGCTCAT | NM_001005150.1 | 231 |
| STAT6 | F: AGCCACTACAAACCTGAGCA R: CAGGGGCCATTCCAAGATCA | XM_013997634.2 | 151 |
| JAK2 | F: AGTAGGAGCCGAACCCACA R: TGCCTGCTTCCGAAACCC | NM_214113.1 | 125 |
| Antibodies | Dilution Ratio | Company | Company Information |
|---|---|---|---|
| CD206 | 1:500 | Proteintech | Wuhan, China |
| F4/80 | 1:200 | Cell signaling technology | Danvers, MA, USA |
| iNOS | 1:1000 | Abclonal | Wuhan, China |
| IL-1β | 1:1000 | Abcam | Cambridge, MA, USA |
| SIRT1 | 1:5000 | Proteintech | Wuhan, China |
| p-P65 | 1:1000 | Affinit biosciences | Jiangsu, China |
| P65 | 1:1000 | Cell signaling technology | Danvers, MA, USA |
| p-IKBα | 1:1000 | Cell signaling technology | Danvers, MA, USA |
| IKBα | 1:5000 | Proteintech | Wuhan, China |
| JAK2 | 1:1000 | Selleck | Houston, TX, USA |
| p-JAK2 | 1:1000 | Abclonal | Wuhan, China |
| p-STAT6 | 1:1000 | Affinit biosciences | Jiangsu, China |
| STAT6 | 1:1000 | Proteintech | Wuhan, China |
| SOCS3 | 1:1000 | Proteintech | Wuhan, China |
| β-actin | 1:10,000 | Proteintech | Wuhan, China |
| Items | Group | SEM | p-Value | |
|---|---|---|---|---|
| Control | Tributyrin | |||
| 0–14 d | ||||
| ADG, g/d | 318.25 ± 12.96 | 365.18 ± 14.27 * | 15.30 | 0.045 |
| ADFI, g/d | 428.77 ± 12.34 | 479.46 ± 25.09 | 14.82 | 0.087 |
| G:F, g/g | 0.74 ± 0.02 | 0.75 ± 0.02 | 0.01 | 0.784 |
| 15–28 d | ||||
| ADG, g/d | 530.00 ± 32.30 | 559.53 ± 20.43 | 18.75 | 0.458 |
| ADFI, g/d | 829.66 ± 29.64 | 855.43 ± 31.39 | 20.95 | 0.564 |
| G:F, g/g | 0.64 ± 0.04 | 0.66 ± 0.03 | 0.02 | 0.757 |
| 0–28 d | ||||
| ADG, g/d | 432.19 ± 11.49 | 450.82 ± 15.60 | 9.40 | 0.352 |
| ADFI, g/d | 621.60 ± 18.63 | 648.02 ± 30.29 | 17.41 | 0.475 |
| G:F, g/g | 0.70 ± 0.01 | 0.67 ± 0.01 | 0.01 | 0.198 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, M.; Ning, S.; Yu, D.; Long, F.; Li, W.; Qi, J.; Liang, Y.; Hong, C.; Tang, Y.; Liu, C.; et al. Effects of Tributyrin on Antioxidant Capacity, Immune Function, and Liver Macrophage Polarization in Weaned Piglets Under LPS Challenge. Animals 2025, 15, 2842. https://doi.org/10.3390/ani15192842
Yuan M, Ning S, Yu D, Long F, Li W, Qi J, Liang Y, Hong C, Tang Y, Liu C, et al. Effects of Tributyrin on Antioxidant Capacity, Immune Function, and Liver Macrophage Polarization in Weaned Piglets Under LPS Challenge. Animals. 2025; 15(19):2842. https://doi.org/10.3390/ani15192842
Chicago/Turabian StyleYuan, Meng, Shuai Ning, Dongming Yu, Fei Long, Weite Li, Jun Qi, Yaxu Liang, Changming Hong, Yingzhang Tang, Chunxue Liu, and et al. 2025. "Effects of Tributyrin on Antioxidant Capacity, Immune Function, and Liver Macrophage Polarization in Weaned Piglets Under LPS Challenge" Animals 15, no. 19: 2842. https://doi.org/10.3390/ani15192842
APA StyleYuan, M., Ning, S., Yu, D., Long, F., Li, W., Qi, J., Liang, Y., Hong, C., Tang, Y., Liu, C., Wang, G., Wu, B., & Zhong, X. (2025). Effects of Tributyrin on Antioxidant Capacity, Immune Function, and Liver Macrophage Polarization in Weaned Piglets Under LPS Challenge. Animals, 15(19), 2842. https://doi.org/10.3390/ani15192842







