From Infection to Infertility: Diagnostic, Therapeutic, and Molecular Perspectives on Postpartum Metritis and Endometritis in Dairy Cows
Simple Summary
Abstract
1. Introduction
2. Postpartum Uterine Disease
2.1. Risk Factors
2.2. Pathogens
2.3. Pathophysiological Mechanisms
2.4. Cost of Uterine Disease
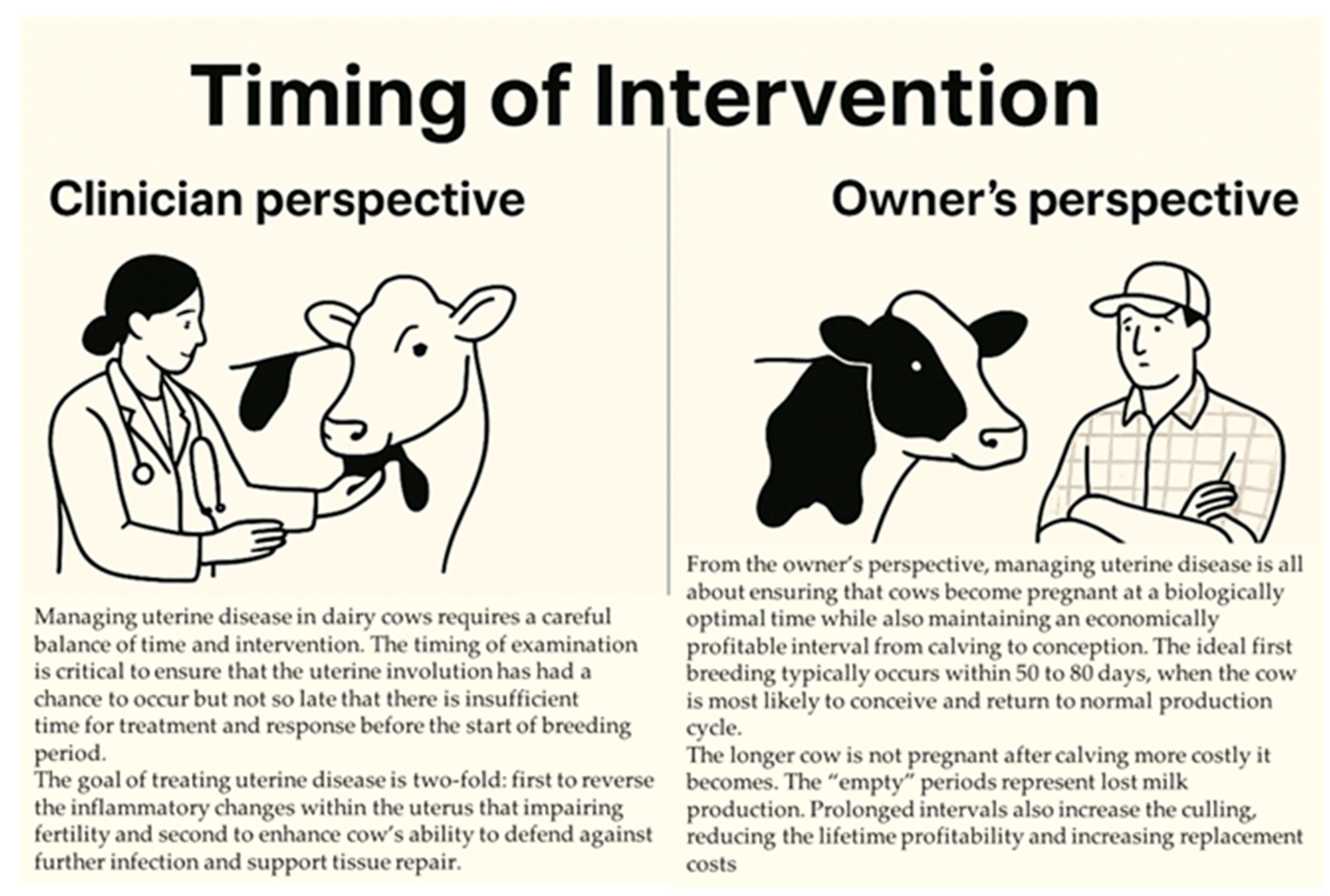
2.5. Timing of Intervention
2.5.1. Optimizing Uterine Recovery: A Veterinarian’s Approach
2.5.2. Herd Management Perspective on Uterine Disease
3. Managing Uterine Disease
3.1. Addressing Uterine Disease
3.1.1. Diagnosis
3.1.2. Treatment
3.1.3. Treatment Failure
Minimal Inhibitory Concentrations (MICs)
Choice of Antibiotics and Regulations
Bacterial Antibiotic Resistance
Biofilm Formation
Transition from Planktonic to Biofilm State
Sequestration and Support of Bacteria
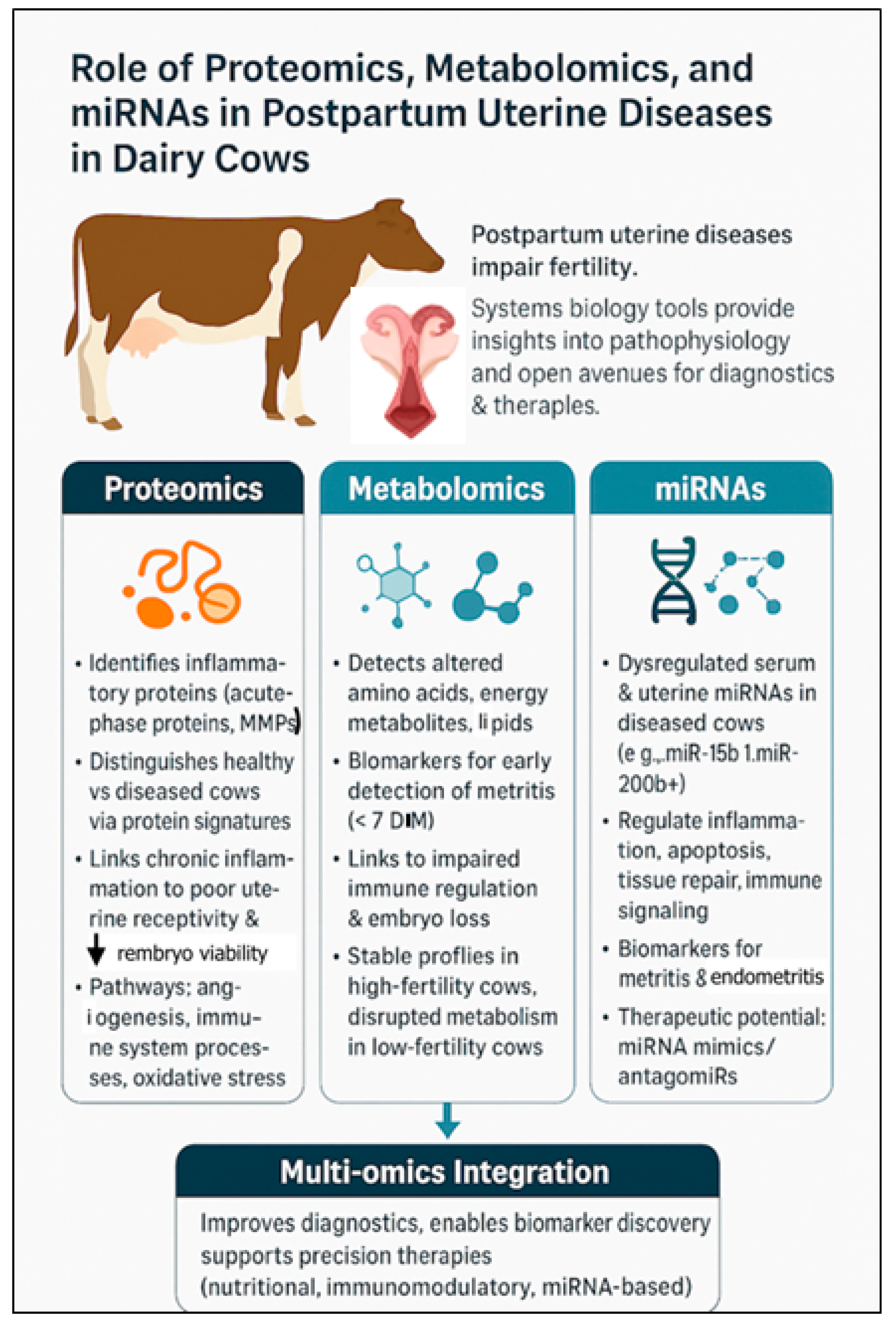
4. Role of Proteomics, Metabolomics, and miRNAs in Uterine Diseases in Postpartum Dairy Cows
4.1. Proteomics: Understanding Functional Protein Changes
4.2. Metabolomics: Capturing Biochemical Changes
4.3. Role of MicroRNAs in Postpartum Uterine Disease in Dairy Cows
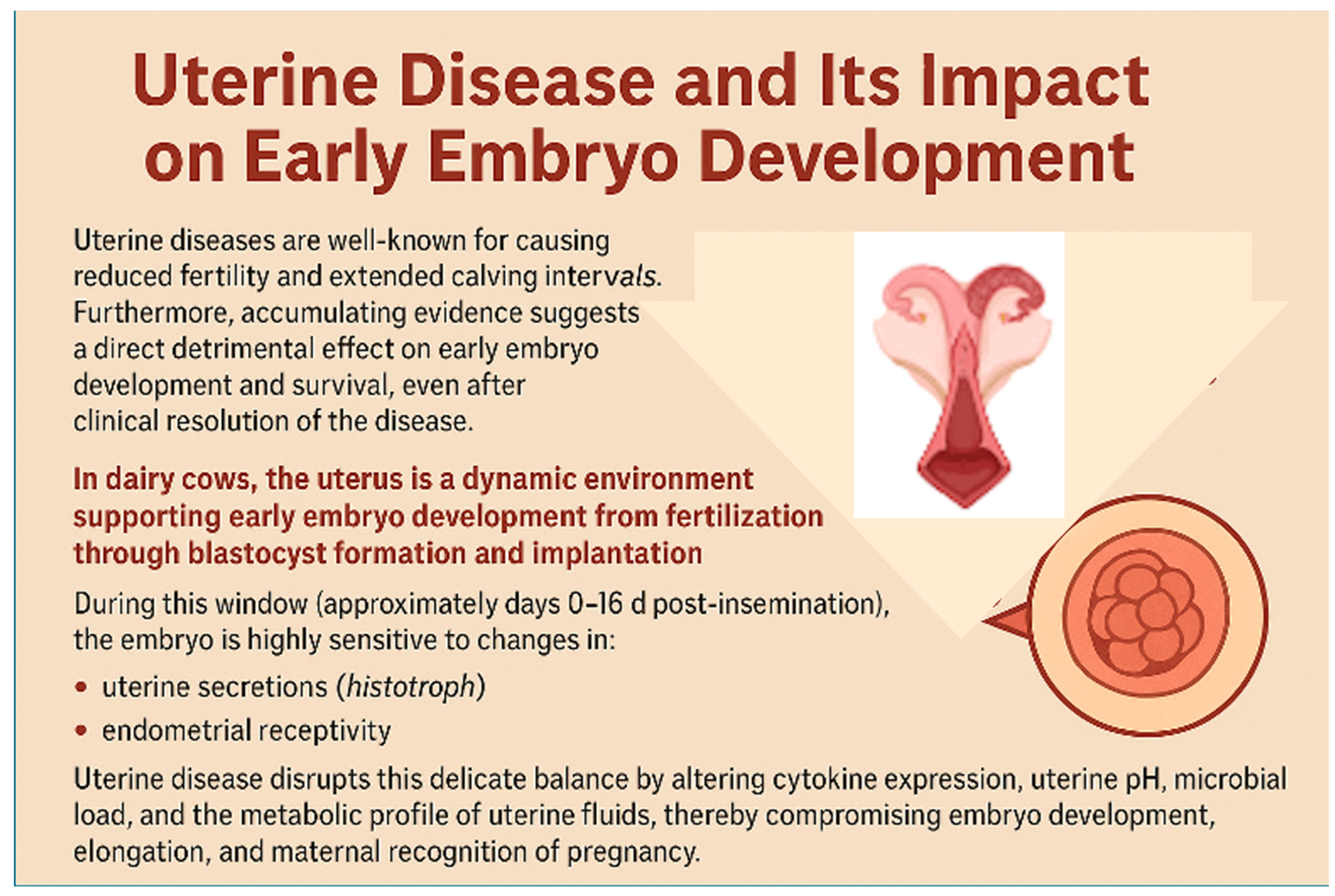
5. Uterine Disease and Impacts on Early Embryo Development
6. Nutritional Strategies to Improve Reproduction and Combat Uterine Infections in Modern Dairy Cows
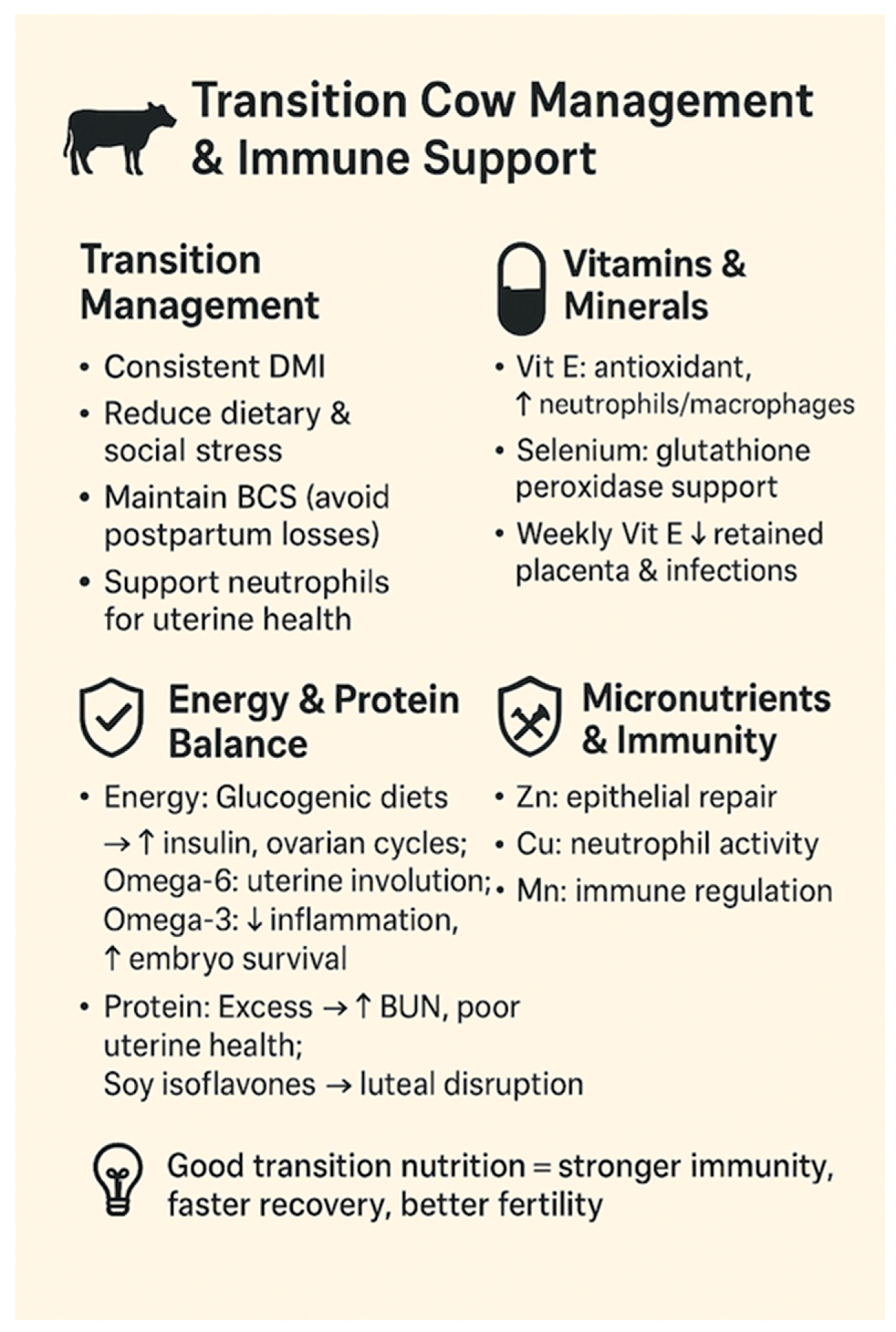
6.1. Transition Cow Management and Immune Support
6.2. Dietary Energy and Fertility
6.3. Protein and Uterine Health
6.4. Energy Balance and Uterine Immunity
6.5. Micronutrients and Uterine Immunity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Gier, H.T.; Marion, G.B. Uterus of the cow after parturition: Involutional changes. Am. J. Vet. Res. 1968, 29, 83–96. [Google Scholar]
- Földi, J.; Kulcsár, M.; Pécsi, A.; Huyghe, B.; de Sa, C.; Lohuis, J.A.C.M.; Cox, P.; Huszenicza, G. Bacterial complications of postpartum uterine involution in cattle. Anim. Reprod. Sci. 2006, 96, 265–281. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Cronin, J.G.; Sheldon, I.M. Mechanisms linking bacterial infections of the bovine endometrium to disease and infertility. Reprod. Biol. 2016, 16, 1–7. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, H.; Ahmad, M.J.; Yang, Y.; Yang, W.; Riaz, H.; Abulaiti, A.; Zhang, S.; Yang, L.; Hua, G. Postpartum uterine involution and embryonic cxevelopment pattern in Chinese holstein dairy cows. Front. Vet. Sci. 2020, 7, 604729. [Google Scholar] [CrossRef]
- Pérez-Báez, J.; Silva, T.V.; Risco, C.A.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic cost of metritis in dairy herds. J. Dairy Sci. 2021, 104, 3158–3168. [Google Scholar] [CrossRef] [PubMed]
- Drillich, M.; Wagener, K. Pathogenesis of uterine diseases in dairy cattle and implications for fertility. Anim. Reprod. 2018, 15 (Suppl. S1), 879–885. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, L.R.; Karstrup, C.C.; Pedersen, H.G.; Angen, Ø.; Agerholm, J.S.; Rasmussen, E.L.; Jensen, T.K.; Klitgaard, K. An investigation of the microbiota in uterine flush samples and endometrial biopsies from dairy cows during the first 7 weeks postpartum. Theriogenology 2016, 86, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Galvao, K.N.; Bicalho, R.C.; Jeon, S.J. Symposium review: The uterine microbiome associated with the development of uterine disease in dairy cows. J. Dairy Sci. 2019, 102, 11786–11797. [Google Scholar] [CrossRef]
- Santos, T.M.; Bicalho, R.C. Diversity and succession of bacterial communities in the uterine fluid of postpartum metritic, endometritic and healthy dairy cows. PLoS ONE 2012, 7, e53048. [Google Scholar] [CrossRef]
- Bicalho, M.L.; Machado, V.S.; Oikonomou, G.; Gilbert, R.O.; Bicalho, R.C. Association between virulence factors of Escherichia coli, Fusobacterium necrophorum, and Arcanobacterium pyogenes and uterine diseases of dairy cows. Vet. Microbiol. 2012, 157, 125–131. [Google Scholar] [CrossRef]
- Fischer, C.; Drillich, M.; Odau, S.; Heuwieser, W.; Einspanier, R.; Gabler, C. Selected pro-inflammatory factor transcripts in bovine endometrial epithelial cells are regulated during the oestrous cycle and elevated in case of subclinical or clinical endometritis. Reprod. Fertil. Dev. 2010, 22, 818–829. [Google Scholar] [CrossRef]
- Chapwanya, A.; Meade, K.G.; Doherty, M.L.; Callanan, J.J.; Mee, J.F.; O’Farrelly, C. Histopathological and molecular evaluation of Holstein-Friesian cows postpartum: Toward an improved understanding of uterine innate immunity. Theriogenology 2009, 71, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Barlund, C.S.; Carruthers, T.D.; Waldner, C.L.; Palmer, C.W. A comparison of diagnostic techniques for postpartum endometritis in dairy cattle. Theriogenology 2008, 69, 714–723. [Google Scholar] [CrossRef] [PubMed]
- Várhidi, Z.; Csikó, G.; Bajcsy, Á.C.; Jurkovich, V. Uterine disease in dairy cows: A comprehensive review highlighting new research areas. Vet. Sci. 2024, 11, 66. [Google Scholar] [CrossRef]
- FAO. Animal Production. Available online: https://www.fao.org/antimicrobial-resistance/key-sectors/animal-production/en/ (accessed on 25 May 2025).
- Pires, A.J.; Pereira, G.; Fangueiro, D.; Bexiga, R.; Oliveira, M. When the solution becomes the problem: A review on antimicrobial resistance in dairy cattle. Future Microbiol. 2024, 19, 903–929. [Google Scholar] [CrossRef] [PubMed]
- Choe, C.; Park, J.W.; Kim, E.S.; Lee, S.G.; Park, S.Y.; Lee, J.S.; Cho, M.J.; Kang, K.R.; Han, J.; Kang, D. Proteomic analysis of differentially expressed proteins in bovine endometrium with endometritis. Korean J. Physiol. Pharmacol. 2010, 14, 205–212. [Google Scholar] [CrossRef]
- Ledgard, A.M.; Smolenski, G.A.; Henderson, H.; Lee, R.S. Influence of pathogenic bacteria species present in the postpartum bovine uterus on proteome profiles. Reprod. Fertil. Dev. 2015, 27, 395–406. [Google Scholar] [CrossRef]
- de Oliveira, E.B.; Monteiro, H.F.; Pereira, J.M.V.; Williams, D.R.; Pereira, R.V.; Silva Del Rio, N.; Menta, P.R.; Machado, V.S.; Lima, F.S. Changes in uterine metabolome associated with metritis development and cure in lactating Holstein cows. Metabolites 2023, 13, 1156. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, T.; Gan, Z.; Li, H.; Li, Y.; Zhang, Y.; Zhao, X. Metabolomic analysis of untargeted bovine uterine secretions in dairy cows with endometritis using ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry. Res. Vet. Sci. 2021, 139, 51–58. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kastelic, J. Circulating cell-free mature microRNAs and their target gene prediction in bovine metritis. Sci. Rep. 2016, 6, 29509. [Google Scholar] [CrossRef]
- Pereira, G.; Charpigny, G.; Guo, Y.; Silva, E.; Silva, M.F.; Ye, T.; Lopes-da-Costa, L.; Humblot, P. Characterization of circulating microRNA profiles of postpartum dairy cows with persistent subclinical endometritis. J. Dairy Sci. 2023, 106, 9704–9717. [Google Scholar] [CrossRef]
- Salilew-Wondim, D.; Ibrahim, S.; Gebremedhn, S.; Tesfaye, D.; Heppelmann, M.; Bollwein, H.; Pfarrer, C.; Tholen, E.; Neuhoff, C.; Schellander, K.; et al. Clinical and subclinical endometritis induced alterations in bovine endometrial transcriptome and miRNome profile. BMC Genom. 2016, 17, 218. [Google Scholar] [CrossRef]
- Husnain, A.; Arshad, U.; Zimpel, R.; Schmitt, E.; Dickson, M.J.; Perdomo, M.C.; Marinho, M.N.; Ashrafi, N.; Graham, S.F.; Bishop, J.V.; et al. Induced endometrial inflammation compromises conceptus development in dairy cattle. Biol. Reprod. 2023, 109, 415–431. [Google Scholar] [CrossRef]
- Inversetti, A.; Zambella, E.; Guarano, A.; Dell’Avanzo, M.; Di Simone, N. Endometrial microbiota and immune tolerance in pregnancy. Int. J. Mol. Sci. 2023, 24, 2995. [Google Scholar] [CrossRef] [PubMed]
- Noakes, D.E. Physiology of the puerperium. In Veterinary Reproduction and Obstetrics, 10th ed.; Noakes, D.E., Parkinson, T.J., GCW England, Eds.; W.B. Saunders: London, UK, 2019; pp. 148–156. [Google Scholar] [CrossRef]
- Griffin, J.F.; Hartigan, P.J.; Nunn, W.R. Non-specific uterine infection and bovine fertility. I. Infection patterns and endometritis during the first seven weeks post-partum. Theriogenology 1974, 1, 91–106. [Google Scholar] [CrossRef]
- Noakes, D.E.; Wallace, L.; Smith, G.R. Bacterial flora of the uterus of cows after calving on two hygienically contrasting farms. Vet. Rec. 1991, 128, 440–442. [Google Scholar] [CrossRef]
- Hussain, A.M.; Daniel, R.C.; O’Boyle, D. Postpartum uterine flora following normal and abnormal puerperium in cows. Theriogenology 1990, 34, 291–302. [Google Scholar] [CrossRef]
- Trevisi, E.; Cattaneo, L.; Piccioli-Cappelli, F.; Mezzetti, M.; Minuti, A. International symposium on ruminant physiology: The immunometabolism of transition dairy cows from dry-off to early lactation: Lights and shadows. J. Dairy Sci. 2025, 108, 7662–7674. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Murray, R.D.; Mshelia, G.; Woldehiwet, Z. The immune status of the bovine uterus during the peripartum period. Vet. J. 2008, 175, 301–309. [Google Scholar] [CrossRef]
- Pascottini, O.B.; Van Schyndel, S.J.; Spricigo, J.F.W.; Rousseau, J.; Weese, J.S.; LeBlanc, S.J. Dynamics of uterine microbiota in postpartum dairy cows with clinical or subclinical endometritis. Sci. Rep. 2020, 10, 12353. [Google Scholar] [CrossRef] [PubMed]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Risk factors for postpartum uterine diseases in dairy cows. J. Dairy Sci. 2010, 93, 5764–5771. [Google Scholar] [CrossRef]
- Vallejo-Timaran, D.A.; Reyes, J.; Gilbert, R.O.; Lefebvre, R.C.; Palacio-Baena, L.G.; Maldonado-Estrada, J.G. Incidence, clinical patterns, and risk factors of postpartum uterine diseases in dairy cows from high-altitude tropical herds. J. Dairy Sci. 2021, 104, 9016–9026. [Google Scholar] [CrossRef]
- Sammad, A.; Khan, M.Z.; Abbas, Z.; Hu, L.; Ullah, Q.; Wang, Y.; Zhu, H.; Wang, Y. Major nutritional metabolic alterations influencing the reproductive system of postpartum dairy cows. Metabolites 2022, 12, 60. [Google Scholar] [CrossRef]
- Esposito, G.; Irons, P.C.; Webb, E.C.; Chapwanya, A. Interactions between negative energy balance, metabolic diseases, uterine health and immune response in transition dairy cows. Anim. Reprod. Sci. 2014, 144, 60–71. [Google Scholar] [CrossRef]
- Sicsic, R.; Goshen, T.; Dutta, R.; Kedem-Vaanunu, N.; Kaplan-Shabtai, V.; Pasternak, Z.; Gottlieb, Y.; Shpigel, N.Y.; Raz, T. Microbial communities and inflammatory response in the endometrium differ between normal and metritic dairy cows at 5–10 days postpartum. Vet. Res. 2018, 49, 77. [Google Scholar] [CrossRef]
- Chen, H.; Fu, K.; Pang, B.; Wang, J.; Li, H.; Jiang, Z.; Feng, Y.; Tian, W.; Cao, R. Determination of uterine bacterial community in postpartum dairy cows with metritis based on 16S rDNA sequencing. Vet. Anim. Sci. 2020, 10, 100102. [Google Scholar] [CrossRef]
- Bicalho, M.L.; Machado, V.S.; Higgins, C.H.; Lima, F.S.; Bicalho, R.C. Genetic and functional analysis of the bovine uterine microbiota Part I: Metritis versus healthy cows. J. Dairy Sci. 2017, 100, 3850–3862. [Google Scholar] [CrossRef] [PubMed]
- Bicalho, M.L.S.; Machado, V.S.; Higgins, C.H.; Lima, F.S.; Bicalho, R.C. Genetic and functional analysis of the bovine uterine microbiota. Part II: Purulent vaginal discharge versus healthy cows. J. Dairy Sci. 2017, 100, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, G.; Ravanetti, L.; Cavirani, S.; Herath, S.; Capocefalo, A.; Sheldon, I.M. Bacterial infection of endometrial stromal cells influences bovine herpesvirus 4 immediate early gene activation: A new insight into bacterial and viral interaction for uterine disease. Reproduction 2008, 136, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Peter, A.T.; Bosu, W.T.; DeDecker, R.J. Suppression of preovulatory luteinizing hormone surges in heifers after intrauterine infusions of Escherichia coli endotoxin. Am. J. Vet. Res. 1989, 50, 368–373. [Google Scholar] [CrossRef]
- Huszenicza, G.; Fodor, M.; Gacs, M.; Kulcsar, M.; Dohmen, M.J.W.; Vamos, M. Uterine bacteriology, resumption of cyclic ovarian activity and fertility in postpartum cows kept in large-scale dairy herds. Reprod. Domest. Anim. 1999, 34, 237–245. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Noakes, D.E.; Rycroft, A.N.; Pfeiffer, D.U.; Dobson, H. Influence of uterine bacterial contamination after parturition on ovarian dominant follicle selection and follicle growth and function in cattle. Reproduction 2002, 123, 837–845. [Google Scholar] [CrossRef][Green Version]
- Kiracofe, G.H. Uterine involution: Its role in regulating postpartum intervals. J. Anim. Sci. 1980, 51 (Suppl. S2), 16–28. [Google Scholar]
- Li, X.; Yang, X.; Ma, Z.; Liu, M.; Ma, Y.; Dan, X. Comparative analysis of vaginal microflora in healthy postpartum cows and cows with incomplete uterine involution by 16S rRNA gene sequencing. Res. Vet. Sci. 2025, 193, 105813. [Google Scholar] [CrossRef] [PubMed]
- Overton, M.; Fetrow, J. Economics of postpartum uterine health. In Proceedings of the Dairy Cattle Reproduction Council, Omaha, NE, USA, 7–8 November 2008; DCRC: Hartland, WI, USA, 2008; pp. 39–44. [Google Scholar]
- Wicaksono, A.; van den Borne, B.H.P.; Aardema, H.; van Werven, T.; Hogeveen, H.; Steeneveld, W. Estimating the costs of interrelated reproductive disorders in dairy farms. J. Dairy Sci. 2025, 108, 8508–8528. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Rojas, O.A.; Pérez-Báez, J.; Casaro, S.; Chebel, R.C.; Cunha, F.; De Vries, A.; Santos, J.E.P.; Lima, F.S.; Pinedo, P.; Schuenemann, G.M.; et al. The economic impact of purulent vaginal discharge in dairy herds within a single lactation. J. Dairy Sci. 2025, 108, 2710–2720. [Google Scholar] [CrossRef]
- Dai, T.; Ma, Z.; Guo, X.; Wei, S.; Ding, B.; Ma, Y.; Dan, X. Study on the pattern of postpartum uterine involution in dairy cows. Animals 2023, 13, 3693. [Google Scholar] [CrossRef]
- Gilbert, R.O. The effects of endometritis on the establishment of pregnancy in cattle. Reprod. Fertil. Dev. 2011, 24, 252–257. [Google Scholar] [CrossRef]
- Fonseca, F.A.; Britt, J.H.; McDaniel, B.T.; Wilk, J.C.; Rakes, A.H. Reproductive traits of holsteins and jerseys. Effects of age, milk yield, and clinical abnormalities on involution of cervix and uterus, ovulation, estrous cycles, detection of estrus, conception rate, and days open. J. Dairy Sci. 1983, 66, 1128–1147. [Google Scholar] [CrossRef]
- Opsomer, G.; Gröhn, Y.T.; Hertl, J.; Coryn, M.; Deluyker, H.; de Kruif, A. Risk factors for postpartum ovarian dysfunction in high producing dairy cows in Belgium: A field study. Theriogenology 2000, 53, 841–857. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V.; Kastelic, J.P. Mucin 1 and cytokines mRNA in endometrium of dairy cows with postpartum uterine disease or repeat breeding. Theriogenology 2014, 81, 952–958.e2. [Google Scholar] [CrossRef] [PubMed]
- Spencer, T.E.; Hansen, T.R. Implantation and Establishment of Pregnancy in Ruminants. Adv. Anat. Embryol. Cell Biol. 2015, 216, 105–135. [Google Scholar] [CrossRef]
- Rhodes, F.M.; McDougall, S.; Burke, C.R.; Verkerk, G.A.; Macmillan, K.L. Invited Review: Treatment of Cows with an Extended Postpartum Anestrous Interval. J. Dairy Sci. 2003, 86, 1876–1894. [Google Scholar] [CrossRef]
- Opsomer, G.; Laevens, H.; Steegen, N.; de Kruif, A. A Descriptive Study of Postpartum Anoestrus in Nine High-Yielding Dairy Herds in Flanders. Vlaams Diergeneeskd. Tijdschr. 2000, 69, 31–37. Available online: http://hdl.handle.net/1854/LU-170974 (accessed on 28 May 2025).
- Esslemont, R.J.; Kossaibati, M.A.; Allcock, J. Economics of Fertility in Dairy Cows. In Fertility in the High Producing Dairy Cow; Diskin, M.G., Ed.; British Society of Animal Science: Edinburgh, UK, 2001; pp. 19–29. [Google Scholar]
- Ribeiro, E.S.; Galvão, K.N.; Thatcher, W.W.; Santos, J.E.P. Economic Aspects of Applying Reproductive Technologies to Dairy Herds. Anim. Reprod. 2012, 9, 370–387. [Google Scholar]
- Sheldon, I.M.; Lewis, G.S.; LeBlanc, S.; Gilbert, R.O. Defining Postpartum Uterine Disease in Cattle. Theriogenology 2006, 65, 1516–1530. [Google Scholar] [CrossRef]
- Gilbert, R.O.; Shin, S.T.; Guard, C.L.; Erb, H.N.; Frajblat, M. Prevalence of endometritis and its effects on reproductive performance of dairy cows. Theriogenology 2005, 64, 1879–1888. [Google Scholar] [CrossRef]
- Drillich, M.; Beetz, O.; Pfützner, A.; Sabin, M.; Sabin, H.J.; Kutzer, P.; Nattermann, H.; Heuwieser, W. Evaluation of a Systemic Antibiotic Treatment of Toxic Puerperal Metritis in Dairy Cows. J. Dairy Sci. 2001, 84, 2010–2017. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. Defining and Diagnosing Postpartum Clinical Endometritis and Its Impact on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. A comparison of the cytobrush and uterine lavage techniques to evaluate endometrial cytology in clinically normal postpartum dairy cows. Can. Vet. J. 2005, 46, 255–259. [Google Scholar] [PubMed]
- Bonnett, B.N.; Martin, S.W.; Gannon, V.P.; Miller, R.B.; Etherington, W.G. Endometrial Biopsy in Holstein-Friesian Dairy Cows. III. Bacteriological Analysis and Correlations with Histological Findings. Can. J. Vet. Res. 1991, 55, 168–173. [Google Scholar]
- Bromfield, J.J.; Santos, J.E.; Block, J.; Williams, R.S.; Sheldon, I.M. PHYSIOLOGY AND ENDOCRINOLOGY SYMPOSIUM: Uterine infection: Linking infection and innate immunity with infertility in the high-producing dairy cow. J. Anim. Sci. 2015, 93, 2021–2033. [Google Scholar] [CrossRef]
- Huzzey, J.M.; Duffield, T.F.; LeBlanc, S.J.; Veira, D.M.; Weary, D.M.; von Keyserlingk, M.A. Short communication: Haptoglobin as an early indicator of metritis. J. Dairy Sci. 2009, 92, 621–625. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. Endometrial Cytology and Ultrasonography for the Detection of Subclinical Endometritis in Postpartum Dairy Cows. Theriogenology 2004, 62, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, H.; Hasegawa, R.; Nishimoto, N.; Kawahata, M.; Miura, H.; Kikuchi, M.; Sakaguchi, M. Comparison of Diagnostic Methods for Uterine Health in Dairy Cattle on Different Days Postpartum. Vet. Rec. 2020, 186, 91. [Google Scholar] [CrossRef] [PubMed]
- Lection, J.; Van Syoc, E.; Miles, A.; Hamilton, J.; Martinez, M.; Bas, S.; Silverman, J.; Barragan, A.; Ganda, E. Use of Intrauterine Dextrose as an Alternative to Systemic Antibiotics for Treatment of Clinical Metritis in Dairy Cattle: A Microbiome Perspective. Front. Vet. Sci. 2024, 11, 1478288. [Google Scholar] [CrossRef] [PubMed]
- Paiano, R.B.; Morrison, E.I.; LeBlanc, S.J. Randomized Clinical Trial of Ketoprofen or Ceftiofur for Treatment of Metritis in Dairy Cows. J. Dairy Sci. 2024, 107, 8366–8377. [Google Scholar] [CrossRef]
- Adnane, M.; Whiston, R.; Tasara, T.; Bleul, U.; Chapwanya, A. Harnessing Vaginal Probiotics for Enhanced Management of Uterine Disease and Reproductive Performance in Dairy Cows: A Conceptual Review. Animals 2024, 14, 1073. [Google Scholar] [CrossRef]
- Deng, Q.; Odhiambo, J.F.; Farooq, U.; Lam, T.; Dunn, S.M.; Ametaj, B.N. Intravaginal Lactic Acid Bacteria Modulated Local and Systemic Immune Responses and Lowered the Incidence of Uterine Infections in Periparturient Dairy Cows. PLoS ONE 2015, 10, e0124167. [Google Scholar] [CrossRef]
- Mandhwani, R.; Bhardwaz, A.; Kumar, S.; Shivhare, M.; Aich, R. Insights into Bovine Endometritis with Special Reference to Phytotherapy. Vet. World 2017, 10, 1529–1532. [Google Scholar] [CrossRef][Green Version]
- Menoud, V.; Holinger, M.; Graf-Schiller, S.; Mayer, P.; Gerber, L.; Walkenhorst, M.; Hirsbrunner, G. Comparison between Intrauterine Application of an Antibiotic and an Herbal Product to Treat Clinical Endometritis in Dairy Cattle—A Randomized Multicentre Field Study. Res. Vet. Sci. 2024, 172, 105250. [Google Scholar] [CrossRef]
- Galvão, K.N.; Flaminio, M.J.; Brittin, S.B.; Sper, R.; Fraga, M.; Caixeta, L.; Ricci, A.; Guard, C.L.; Butler, W.R.; Gilbert, R.O. Association between Uterine Disease and Indicators of Neutrophil and Systemic Energy Status in Lactating Holstein Cows. J. Dairy Sci. 2010, 93, 2926–2937. [Google Scholar] [CrossRef]
- Ospina, P.A.; Nydam, D.V.; Stokol, T.; Overton, T.R. Evaluation of Nonesterified Fatty Acids and Beta-Hydroxybutyrate in Transition Dairy Cattle in the Northeastern United States: Critical Thresholds for Prediction of Clinical Diseases. J. Dairy Sci. 2010, 93, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Espadamala, A.; Pereira, R.; Pallarés, P.; Lago, A.; Silva-Del-Río, N. Metritis Diagnosis and Treatment Practices in 45 Dairy Farms in California. J. Dairy Sci. 2018, 101, 9608–9616. [Google Scholar] [CrossRef]
- Merenda, V.R.; Ruiz-Munoz, J.; Zare, A.; Chebel, R.C. Predictive Models to Identify Holstein Cows at Risk of Metritis and Clinical Cure and Reproductive/Productive Failure Following Antimicrobial Treatment. Prev. Vet. Med. 2021, 194, 105431. [Google Scholar] [CrossRef]
- Sandals, W.C.; Curtis, R.A.; Cote, J.F.; Martin, S.W. The Effect of Retained Placenta and Metritis Complex on Reproductive Performance in Dairy Cattle—A Case Control Study. Can. Vet. J. 1979, 20, 131–135. [Google Scholar] [PubMed]
- Erb, H.N.; Smith, R.D.; Hillman, R.B.; Powers, P.A.; Smith, M.C.; White, M.E.; Pearson, E.G. Rates of Diagnosis of Six Diseases of Holstein Cows During 15-Day and 21-Day Intervals. Am. J. Vet. Res. 1984, 45, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Oltenacu, P.A.; Frick, A.; Lindhé, B. Epidemiological Study of Several Clinical Diseases, Reproductive Performance and Culling in Primiparous Swedish Cattle. Prev. Vet. Med. 1990, 9, 59–74. [Google Scholar] [CrossRef]
- Correa, M.T.; Erb, H.; Scarlett, J. Path Analysis for Seven Postpartum Disorders of Holstein Cows. J. Dairy Sci. 1993, 76, 1305–1312. [Google Scholar] [CrossRef]
- Emanuelson, U.; Oltenacu, P.A.; Gröhn, Y.T. Nonlinear Mixed Model Analyses of Five Production Disorders of Dairy Cattle. J. Dairy Sci. 1993, 76, 2765–2772. [Google Scholar] [CrossRef]
- Gautam, G.; Nakao, T.; Koike, K.; Long, S.T.; Yusuf, M.; Ranasinghe, R.M.; Hayashi, A. Spontaneous Recovery or Persistence of Postpartum Endometritis and Risk Factors for Its Persistence in Holstein Cows. Theriogenology 2010, 73, 168–179. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; LeBlanc, S.J. Definitions and Diagnosis of Postpartum Endometritis in Dairy Cows. J. Dairy Sci. 2010, 93, 5225–5233. [Google Scholar] [CrossRef]
- Runciman, D.J.; Anderson, G.A.; Malmo, J.; Davis, G.M. Use of Postpartum Vaginoscopic (Visual Vaginal) Examination of Dairy Cows for the Diagnosis of Endometritis and the Association of Endometritis with Reduced Reproductive Performance. Aust. Vet. J. 2008, 86, 205–213. [Google Scholar] [CrossRef]
- de Boer, M.W.; LeBlanc, S.J.; Dubuc, J.; Meier, S.; Heuwieser, W.; Arlt, S.; Gilbert, R.O.; McDougall, S. Invited Review: Systematic Review of Diagnostic Tests for Reproductive-Tract Infection and Inflammation in Dairy Cows. J. Dairy Sci. 2014, 97, 3983–3999. [Google Scholar] [CrossRef]
- Noakes, D.E.; Till, D.; Smith, G.R. Bovine Uterine Flora Postpartum: A Comparison of Swabbing and Biopsy. Vet. Rec. 1989, 124, 563–564. [Google Scholar] [CrossRef] [PubMed]
- Herath, S.; Lilly, S.T.; Santos, N.R.; Gilbert, R.O.; Goetze, L.; Bryant, C.E.; White, J.O.; Cronin, J.; Sheldon, I.M. Expression of genes associated with immunity in the endometrium of cattle with disparate postpartum uterine disease and fertility. Reprod. Biol. Endocrinol. 2009, 7, 55. [Google Scholar] [CrossRef]
- Bretzlaff, K. Rationale for Treatment of Endometritis in the Dairy Cow. Vet. Clin. N. Am. Food Anim. Pract. 1987, 3, 593. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarowski, M.; Malinowski, E.; Markiewicz, H. Influence of Various Treatment Methods on Bacteriological Findings in Cows with Puerperal Endometritis. Pol. J. Vet. Sci. 2004, 7, 171–174. [Google Scholar]
- Smith, B.I.; Donovan, G.A.; Risco, C.; Littell, R.; Young, C.; Stanker, L.H.; Elliott, J. Comparison of Various Antibiotic Treatments for Cows Diagnosed with Toxic Puerperal Metritis. J. Dairy Sci. 1998, 81, 1555–1562. [Google Scholar] [CrossRef] [PubMed]
- Chenault, J.R.; McAllister, J.F.; Chester, S.T., Jr.; Dame, K.J.; Kausche, F.M.; Robb, E.J. Efficacy of Ceftiofur Hydrochloride Sterile Suspension Administered Parenterally for the Treatment of Acute Postpartum Metritis in Dairy Cows. J. Am. Vet. Med. Assoc. 2004, 224, 1634–1639. [Google Scholar] [CrossRef]
- Lima, F.S.; Vieira-Neto, A.; Vasconcellos, G.S.; Mingoti, R.D.; Karakaya, E.; Solé, E.; Bisinotto, R.S.; Martinez, N.; Risco, C.A.; Galvão, K.N.; et al. Efficacy of Ampicillin Trihydrate or Ceftiofur Hydrochloride for Treatment of Metritis and Subsequent Fertility in Dairy Cows. J. Dairy Sci. 2014, 97, 5401–5414. [Google Scholar] [CrossRef] [PubMed]
- Haimerl, P.; Heuwieser, W. Invited Review: Antibiotic Treatment of Metritis in Dairy Cows: A Systematic Approach. J. Dairy Sci. 2014, 97, 6649–6661. [Google Scholar] [CrossRef]
- Reppert, E.J. Evidence for the Use of Ceftiofur for Treatment of Metritis in Dairy Cattle. Vet. Clin. N. Am. Food Anim. Pract. 2015, 31, 139–149, vii. [Google Scholar] [CrossRef]
- LeBlanc, S.J. Postpartum Uterine Disease and Dairy Herd Reproductive Performance: A Review. Vet. J. 2008, 176, 102–114. [Google Scholar] [CrossRef]
- Menta, P.R.; Fernandes, L.; Prim, J.; De Oliveira, E.; Lima, F.; Galvão, K.N.; Noyes, N.; Ballou, M.A.; Machado, V.S. randomized controlled trial evaluating the efficacy of systemic ceftiofur administration for metritis therapy in dairy cows and the effect of metritis cure on economically important outcomes. J. Dairy Sci. 2024, 107, 7092–7105. [Google Scholar] [CrossRef] [PubMed]
- Armengol, R.; Fraile, L. Comparison of two treatment strategies for cows with metritis in high-risk lactating dairy cows. Theriogenology 2015, 83, 1344–1351. [Google Scholar] [CrossRef] [PubMed]
- Iancu, I.; Popa, S.A.; Degi, J.; Gligor, A.; Popa, I.; Iorgoni, V.; Nistor, P.; Imre, K.; Nichita, I.; Herman, V. Aerobic Uterine Pathogens in Dairy Cattle: Surveillance and Antimicrobial Resistance Profiles in Postpartum Endometritis. Antibiotics 2025, 14, 650. [Google Scholar] [CrossRef] [PubMed]
- Plöntzke, J.; Madoz, L.W.; De la Sota, R.L.; Heuwieser, W.; Drillich, M. Prevalence of clinical endometritis and its impact on reproductive performance in grazing dairy cattle in Argentina. Reprod. Domest. Anim. 2011, 46, 520–526. [Google Scholar] [CrossRef]
- Risco, C.A.; Hernandez, J. Comparison of Ceftiofur Hydrochloride and Estradiol Cypionate for Metritis Prevention and Reproductive Performance in Dairy Cows Affected with Retained Fetal Membranes. Theriogenology 2003, 60, 47–58. [Google Scholar] [CrossRef]
- Silva, T.V.; de Oliveira, E.B.; Pérez-Báez, J.; Risco, C.A.; Chebel, R.C.; Cunha, F.; Daetz, R.; Santos, J.E.P.; Lima, F.S.; Jeong, K.C.; et al. Economic Comparison between Ceftiofur-Treated and Nontreated Dairy Cows with Metritis. J. Dairy Sci. 2021, 104, 8918–8930. [Google Scholar] [CrossRef]
- Galvão, K.N.; Frajblat, M.; Brittin, S.B.; Butler, W.R.; Guard, C.L.; Gilbert, R.O. Effect of Prostaglandin F2α on Subclinical Endometritis and Fertility in Dairy Cows. J. Dairy Sci. 2009, 92, 4906–4913. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, R.; Duffield, T.F.; Foster, R.A.; Gartley, C.J.; Leslie, K.E.; Walton, J.S.; Johnson, W.H. The Effect of a Single Administration of Cephapirin or Cloprostenol on the Reproductive Performance of Dairy Cows with Subclinical Endometritis. Theriogenology 2005, 63, 818–830. [Google Scholar] [CrossRef]
- LeBlanc, S.J.; Duffield, T.F.; Leslie, K.E.; Bateman, K.G.; Keefe, G.P.; Walton, J.S.; Johnson, W.H. The Effect of Treatment of Clinical Endometritis on Reproductive Performance in Dairy Cows. J. Dairy Sci. 2002, 85, 2237–2249. [Google Scholar] [CrossRef] [PubMed]
- López-Gatius, F. Ovarian Response to Prostaglandin F2α in Lactating Dairy Cows: A Clinical Update. J. Reprod. Dev. 2022, 68, 104–109. [Google Scholar] [CrossRef]
- Dubuc, J.; Duffield, T.F.; Leslie, K.E.; Walton, J.S.; Leblanc, S.J. Randomized Clinical Trial of Antibiotic and Prostaglandin Treatments for Uterine Health and Reproductive Performance in Dairy Cows. J. Dairy Sci. 2011, 94, 1325–1338. [Google Scholar] [CrossRef]
- Kowalska-Krochmal, B.; Dudek-Wicher, R. The Minimum Inhibitory Concentration of Antibiotics: Methods, Interpretation, Clinical Relevance. Pathogens 2021, 10, 165. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Bushnell, M.; Montgomery, J.; Rycroft, A.N. Minimum inhibitory concentrations of some antimicrobial drugs against bacteria causing uterine infections in cattle. Vet. Rec. 2004, 155, 383–387. [Google Scholar] [CrossRef]
- La Rosa, R.; Johansen, H.K.; Molin, S. Persistent Bacterial Infections, Antibiotic Treatment Failure, and Microbial Adaptive Evolution. Antibiotics 2022, 11, 419. [Google Scholar] [CrossRef]
- Drillich, M.; Arlt, S.; Kersting, S.; Bergwerff, A.A.; Scherpenisse, P.; Heuwieser, W. Ceftiofur derivatives in serum, uterine tissues, cotyledons, and lochia after fetal membrane retention. J. Dairy Sci. 2006, 89, 3431–3438. [Google Scholar] [CrossRef] [PubMed]
- von Krueger, X.; Scherpenisse, P.; Roiger, S.; Heuwieser, W. Determination of ceftiofur derivatives in serum, endometrial tissue, and lochia in puerperal dairy cows with fever or acute puerperal metritis after subcutaneous administration of ceftiofur crystalline free acid. J. Dairy Sci. 2013, 96, 1054–1062. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, U. Antimicrobial Use and Resistance in Food-Producing Animals—How Can We Protect the Efficacy of Antibiotics for Reproductive Diseases? Reprod. Domest. Anim. 2022, 57 (Suppl. S4), 13–20. [Google Scholar] [CrossRef]
- Kasimanickam, V.; Kasimanickam, M.; Kasimanickam, R. Antibiotics Use in Food Animal Production: Escalation of Antimicrobial Resistance: Where Are We Now in Combating AMR? Med. Sci. 2021, 9, 14. [Google Scholar] [CrossRef]
- Elkholly, D.; Fraser, A.; Booth, R.; O’Neill, D.; Mateus, A.; Brunton, L.; Brodbelt, D. Antimicrobial Usage in Farm Animal Practices in the UK: A Mixed-Methods Approach. Prev. Vet. Med. 2023, 213, 105870. [Google Scholar] [CrossRef]
- Speksnijder, D.C.; Jaarsma, A.D.; van der Gugten, A.C.; Verheij, T.J.; Wagenaar, J.A. Determinants Associated with Veterinary Antimicrobial Prescribing in Farm Animals in the Netherlands: A Qualitative Study. Zoonoses Public Health 2015, 62 (Suppl. S1), 39–51. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Umeokoli, V.C.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Moraes, J.G.N.; Gull, T.; Ericsson, A.C.; Caldeira, M.O.; Evans, T.J.; Poock, S.E.; Lucy, M.C. Evaluating differences in uterine microbiome and inflammatory status at 1 month postpartum associated with metritis and antibiotic treatment. J. Dairy Sci. 2025. advance online publication. [Google Scholar] [CrossRef]
- Hathroubi, S.; Mekni, M.A.; Domenico, P.; Nguyen, D.; Jacques, M. Biofilms: Microbial Shelters Against Antibiotics. Microb. Drug Resist. 2017, 23, 147–156. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.F. Molecular Mechanisms of Biofilm-Based Antibiotic Resistance and Tolerance in Pathogenic Bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Kasimanickam, V.R.; Owen, K.; Kasimanickam, R.K. Detection of Genes Encoding Multidrug Resistance and Biofilm Virulence Factor in Uterine Pathogenic Bacteria in Postpartum Dairy Cows. Theriogenology 2016, 85, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Rzewuska, M.; Kwiecień, E.; Chrobak-Chmiel, D.; Kizerwetter-Świda, M.; Stefańska, I.; Gieryńska, M. Pathogenicity and Virulence of Trueperella pyogenes: A Review. Int. J. Mol. Sci. 2019, 20, 2737. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Silva, A.R.; Fernandes, R.; Serra, P.; Barros, M.M.; Campos, A.M.; Oliveira, R.; Silva, S.; Almeida, C.; Castro, J. Emerging Approaches for Mitigating Biofilm-Formation-Associated Infections in Farm, Wild, and Companion Animals. Pathogens 2024, 13, 320. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial Biofilm: A Review on Formation, Infection, Antibiotic Resistance, Control Measures, and Innovative Treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Uruén, C.; Chopo-Escuin, G.; Tommassen, J.; Mainar-Jaime, R.C.; Arenas, J. Biofilms as Promoters of Bacterial Antibiotic Resistance and Tolerance. Antibiotics 2020, 10, 3. [Google Scholar] [CrossRef]
- Van Saun, R.J.; Sniffen, C.J. Transition Cow Nutrition and Feeding Management for Disease Prevention. Vet. Clin. N. Am. Food Anim. Pract. 2014, 30, 689–719. [Google Scholar] [CrossRef]
- Tras, B.; Dinç, D.A.; Üney, K. The Effect of N-Acetylcysteine on the Treatment of Clinical Endometritis and Pregnancy Rate in Dairy Cows. Eurasian J. Vet. Sci. 2014, 30, 133. [Google Scholar] [CrossRef]
- Dinicola, S.; De Grazia, S.; Carlomagno, G.; Pintucci, J.P. N-Acetylcysteine as Powerful Molecule to Destroy Bacterial Biofilms: A Systematic Review. Eur. Rev. Med. Pharmacol. Sci. 2014, 18, 2942–2948. [Google Scholar]
- Brick, T.A.; Schuenemann, G.M.; Bas, S.; Daniels, J.B.; Pinto, C.R.; Rings, D.M.; Rajala-Schultz, P.J. Effect of Intrauterine Dextrose or Antibiotic Therapy on Reproductive Performance of Lactating Dairy Cows Diagnosed with Clinical Endometritis. J. Dairy Sci. 2012, 95, 1894–1905. [Google Scholar] [CrossRef]
- Xiao, J.; Khan, M.Z.; Ma, Y.; Alugongo, G.M.; Ma, J.; Chen, T.; Khan, A.; Cao, Z. The Antioxidant Properties of Selenium and Vitamin E.; Their Role in Periparturient Dairy Cattle Health Regulation. Antioxidants 2021, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Silvestre, F.T.; Carvalho, T.S.; Francisco, N.; Santos, J.E.; Staples, C.R.; Jenkins, T.C.; Thatcher, W.W. Effects of Differential Supplementation of Fatty Acids during the Peripartum and Breeding Periods of Holstein Cows: I. Uterine and Metabolic Responses, Reproduction, and Lactation. J. Dairy Sci. 2011, 94, 189–204. [Google Scholar] [CrossRef]
- Mohanta, Y.K.; Chakrabartty, I.; Mishra, A.K.; Chopra, H.; Mahanta, S.; Avula, S.K.; Patowary, K.; Ahmed, R.; Mishra, B.; Mohanta, T.K.; et al. Nanotechnology in Combating Biofilm: A Smart and Promising Therapeutic Strategy. Front. Microbiol. 2023, 13, 1028086. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Olsen, J.R.; Jeffress, E.J.; Moore, D.A.; Kastelic, J.P. Associations among serum pro- and anti-inflammatory cytokines, metabolic mediators, body condition, and uterine disease in postpartum dairy cows. Reprod. Biol. Endocrinol. 2013, 11, 103. [Google Scholar] [CrossRef]
- Wagener, K.; Pothmann, H.; Prunner, I.; Peter, S.; Erber, R.; Aurich, C.; Drillich, M.; Gabler, C. Endometrial mRNA expression of selected pro-inflammatory factors and mucins in repeat breeder cows with and without subclinical endometritis. Theriogenology 2017, 90, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Horne, A.W.; Lalani, E.N.; Margara, R.A.; Ryder, T.A.; Mobberley, M.A.; White, J.O. The expression pattern of MUC1 glycoforms and other biomarkers of endometrial receptivity in fertile and infertile women. Mol. Reprod. Dev. 2005, 72, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Piibor, J.; Waldmann, A.; Prasadani, M.; Kavak, A.; Andronowska, A.; Klein, C.; Kodithuwakku, S.; Fazeli, A. Investigation of uterine fluid extracellular vesicles’ proteomic profiles provides novel diagnostic biomarkers of bovine endometritis. Biomolecules 2024, 14, 626. [Google Scholar] [CrossRef]
- Brodzki, P.; Kostro, K.; Krakowski, L.; Marczuk, J. Inflammatory cytokine and acute phase protein concentrations in the peripheral blood and uterine washings of cows with subclinical endometritis in the late postpartum period. Vet. Res. Commun. 2015, 39, 143–149. [Google Scholar] [CrossRef]
- Sitko, E.M.; Laplacette, A.; Duhatschek, D.; Rial, C.; Perez, M.M.; Tompkins, S.; Kerwin, A.L.; Giordano, J.O. Reproductive physiological outcomes of dairy cows with different genomic merit for fertility: Biomarkers, uterine health, endocrine status, estrus features, and response to ovarian synchronization. J. Dairy Sci. 2024, 107, 8670–8687. [Google Scholar] [CrossRef]
- Almughlliq, F.B.; Koh, Y.Q.; Peiris, H.N.; Vaswani, K.; McDougall, S.; Graham, E.M.; Burke, C.R.; Arachchige, B.J.; Reed, S.; Mitchell, M.D. Proteomic content of circulating exosomes in dairy cows with or without uterine infection. Theriogenology 2018, 114, 173–179. [Google Scholar] [CrossRef]
- Zhang, S.D.; Dong, S.W.; Wang, D.S.; Oguejiofor, C.F.; Fouladi-Nashta, A.A.; Yang, Z.Q.; Yan, Z.T. Differential proteomic profiling of endometrium and plasma indicate the importance of hydrolysis in bovine endometritis. J. Dairy Sci. 2017, 100, 9324–9337. [Google Scholar] [CrossRef] [PubMed]
- Aranciaga, N.; Morton, J.D.; Maes, E.; Gathercole, J.L.; Berg, D.K. Proteomic determinants of uterine receptivity for pregnancy in early and mid-postpartum dairy cows†. Biol. Reprod. 2021, 105, 1458–1473. [Google Scholar] [CrossRef]
- Barański, W.; Zduńczyk, S.; Tobolski, D.; Krupa, M. Fertility outcomes in cows with subclinical endometritis after clinical cure of clinical endometritis. Irish Vet. J. 2024, 77, 20. [Google Scholar] [CrossRef]
- Forde, N.; McGettigan, P.A.; Mehta, J.P.; O’Hara, L.; Mamo, S.; Bazer, F.W.; Spencer, T.E.; Lonergan, P. Proteomic analysis of uterine fluid during the pre-implantation period of pregnancy in cattle. Reproduction 2014, 147, 575–587. [Google Scholar] [CrossRef]
- Gegenfurtner, K.; Fröhlich, T.; Flenkenthaler, F.; Kösters, M.; Fritz, S.; Desnoës, O.; Le Bourhis, D.; Salvetti, P.; Sandra, O.; Charpigny, G.; et al. Genetic merit for fertility alters the bovine uterine luminal fluid proteome. Biol. Reprod. 2020, 102, 730–739. [Google Scholar] [CrossRef]
- D’Occhio, M.J.; Baruselli, P.S.; Campanile, G. Metabolic health, the metabolome and reproduction in female cattle: A review. Ital. J. Anim. Sci. 2019, 18, 858–867. [Google Scholar] [CrossRef]
- Ji, G.; Zhang, J.; Feng, X.; Sheng, H.; Hu, H.; Li, F.; Ma, Y.; Hu, Y.; Na, R.; Yang, W.; et al. Analysis of blood biochemistry and non-targeted metabolomics of endometritis in dairy cows. Anim. Reprod. Sci. 2024, 264, 107460. [Google Scholar] [CrossRef]
- Kasimanickam, R.; Kasimanickam, V.; Kastelic, J.P.; Ramsey, K. Metabolic Biomarkers, Body Condition, Uterine Inflammation and Response to Superovulation in Lactating Holstein Cows. Theriogenology 2020, 146, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.A.; Hidalgo, A.I.; Gutierrez, N.; Burgos, R.A.; Ratto, M. Metabolomic changes of uterine fluid of lactating cows with subclinical endometritis and treated with docosahexaenoic acid (DHA). Vet. Anim. Sci. 2025, 29, 100494. [Google Scholar] [CrossRef] [PubMed]
- Maehara, T.; Osawa, T.; Kitahara, G.; Satoh, H.; Murata, T. Profile of uterine flush lipid mediators in cows with subclinical endometritis: Pilot study. J. Vet. Med. Sci. 2024, 86, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Helfrich, A.L.; Reichenbach, H.D.; Meyerholz, M.M.; Schoon, H.A.; Arnold, G.J.; Fröhlich, T.; Weber, F.; Zerbe, H. Novel sampling procedure to characterize bovine subclinical endometritis by uterine secretions and tissue. Theriogenology 2020, 141, 186–196. [Google Scholar] [CrossRef]
- Hailemariam, D.; Ibrahim, S.; Hoelker, M.; Drillich, M.; Heuwieser, W.; Looft, C.; Cinar, M.U.; Tholen, E.; Schellander, K.; Tesfaye, D. MicroRNA-regulated molecular mechanism underlying bovine subclinical endometritis. Reprod. Fertil. Dev. 2014, 26, 898–913. [Google Scholar] [CrossRef]
- Tinning, H.; Edge, J.C.; DeBem, T.H.C.; Deligianni, F.; Giovanardi, G.; Pensabene, V.; Meirelles, F.V.; Forde, N. Review: Endometrial function in pregnancy establishment in cattle. Animal 2023, 17 (Suppl. S1), 100751. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.J.; Peterson, K.D.; Senn, L.K.; Oliver, M.A.; Ealy, A.D. Ruminant conceptus-maternal interactions: Interferon-tau and beyond. J. Anim. Sci. 2022, 100, skac123. [Google Scholar] [CrossRef]
- Mathew, D.J.; Sánchez, J.M.; Passaro, C.; Charpigny, G.; Behura, S.K.; Spencer, T.E.; Lonergan, P. Interferon tau-dependent and independent effects of the bovine conceptus on the endometrial transcriptome†. Biol. Reprod. 2019, 100, 365–380. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R. mRNA Expressions of candidate genes in gestational day 16 conceptus and Corresponding Endometrium in Repeat Breeder Dairy Cows with suboptimal uterine environment following transfer of different quality day 7 embryos. Animals 2021, 11, 1092. [Google Scholar] [CrossRef]
- Kasimanickam, R.K.; Kasimanickam, V.R.; Kumar, N.; Reisenauer, C. Day 7 embryo quality and suboptimal uterine environment influence morphometry of Day 16 conceptus in dairy cows. Theriogenology 2021, 163, 10–17. [Google Scholar] [CrossRef]
- Tamminga, S.; Luteijn, P.A.; Meijer, R.G.M. Changes in composition and energy content of liveweight loss in dairy cows with time after parturition. Livest. Prod. Sci. 1997, 52, 31–38. [Google Scholar] [CrossRef]
- Crowe, M.A.; Williams, E.J. Triennial Lactation Symposium: Effects of stress on postpartum reproduction in dairy cows. J. Anim. Sci. 2012, 90, 1722–1727. [Google Scholar] [CrossRef]
- Sheldon, I.M.; Cronin, J.G.; Pospiech, M.; Turner, M.L. Symposium review: Mechanisms linking metabolic stress with innate immunity in the endometrium. J. Dairy Sci. 2018, 101, 3655–3664. [Google Scholar] [CrossRef] [PubMed]
- Kotsampasi, B.; Karatzia, M.A.; Tsiokos, D.; Chadio, S. Nutritional Strategies to Alleviate Stress and Improve Welfare in Dairy Ruminants. Animals 2024, 14, 2573. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huo, P.; Sun, Y.; Zhang, Y. Effects of Body Condition Score Changes During Peripartum on the Postpartum Health and Production Performance of Primiparous Dairy Cows. Animals 2019, 9, 1159. [Google Scholar] [CrossRef]
- Hurley, W.L.; Doane, R.M. Recent Developments in the Roles of Vitamins and Minerals in Reproduction. J. Dairy Sci. 1989, 72, 784–804. [Google Scholar] [CrossRef]
- Pontes, G.C.; Monteiro, P.L., Jr.; Prata, A.B.; Guardieiro, M.M.; Pinto, D.A.; Fernandes, G.O.; Wiltbank, M.C.; Santos, J.E.; Sartori, R. Effect of Injectable Vitamin E on Incidence of Retained Fetal Membranes and Reproductive Performance of Dairy Cows. J. Dairy Sci. 2015, 98, 2437–2449. [Google Scholar] [CrossRef]
- Kaewlamun, W.; Grimard, B.; Duvaux-Ponter, C.; Ponter, A.A. Kick-Starting Ovarian Cyclicity by Using Dietary Glucogenic Precursors in Post-Partum Dairy Cows: A Review. Int. J. Vet. Sci. Med. 2020, 8, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.G.; Lee, W.J.; Garnsworthy, P.C.; Webb, R. Effect of Dietary-Induced Increases in Circulating Insulin Concentrations During the Early Postpartum Period on Reproductive Function in Dairy Cows. Reproduction 2002, 123, 419–427. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fouladi-Nashta, A.A.; Gutierrez, C.G.; Gong, J.G.; Garnsworthy, P.C.; Webb, R. Impact of Dietary Fatty Acids on Oocyte Quality and Development in Lactating Dairy Cows. Biol. Reprod. 2007, 77, 9–17. [Google Scholar] [CrossRef]
- Lemley, C.O.; Butler, S.T.; Butler, W.R.; Wilson, M.E. Short Communication: Insulin Alters Hepatic Progesterone Catabolic Enzymes Cytochrome P450 2C and 3A in Dairy Cows. J. Dairy Sci. 2008, 91, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Leroy, J.L.; Van Soom, A.; Opsomer, G.; Goovaerts, I.G.; Bols, P.E. Reduced Fertility in High-Yielding Dairy Cows: Are the Oocyte and Embryo in Danger? Part II. Mechanisms Linking Nutrition and Reduced Oocyte and Embryo Quality in High-Yielding Dairy Cows. Reprod. Domest. Anim. 2008, 43, 623–632. [Google Scholar] [CrossRef]
- Cools, S.; Van den Broeck, W.; Vanhaecke, L.; Heyerick, A.; Bossaert, P.; Hostens, M.; Opsomer, G. Feeding soybean meal increases the blood level of isoflavones and reduces the steroidogenic capacity in bovine corpora lutea, without affecting peripheral progesterone concentrations. Anim. Reprod. Sci. 2014, 144, 79–89. [Google Scholar] [CrossRef]
- Chen, Y.H.; Chen, Y.M.; Tu, P.A.; Lee, K.H.; Chen, J.Y.; Hsu, J.T. Effect of Supplementing Vitamin E, Selenium, Copper, Zinc, and Manganese during the Transition Period on Dairy Cow Reproductive Performance and Immune Function. Vet Sci. 2023, 10, 225. [Google Scholar] [CrossRef]
- Boyne, R.; Arthur, J.R. Effects of selenium and copper deficiency on neutrophil function in cattle. J. Comp. Pathol. 1981, 91, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Wathes, D.C.; Cheng, Z.; Chowdhury, W.; Fenwick, M.A.; Fitzpatrick, R.; Morris, D.G.; Patton, J.; Murphy, J.J. Negative energy balance alters global gene expression and immune responses in the uterus of postpartum dairy cows. Physiol. Genom. 2009, 39, 1–13. [Google Scholar] [CrossRef] [PubMed]
| Uterine Disease | Diagnostic Technique | Median Days Open | Sensitivity | Specificity | Reference |
|---|---|---|---|---|---|
| Endometritis | Palpation (>7.5 cm cervix) | 120 | 17% (Low) | 82% (High) | [63] |
| Vaginoscopy (Purulent discharge) | 120 | 21% (Low) | 91% (High) | [63] | |
| 150 | 7% (Low) | 89% (High) | [13] | ||
| Biopsy | 128 | 92% (High) | 77% (Medium) | [65] | |
| Endometrial cytology (Lavage %PMN > 8%) | 150 | 14% (Low) | 84% (High) | [13] | |
| Endometrial cytology (Cytobrush %PMN > 8%) | 150 | 13% (Low) | 91% (High) | [13,86] | |
| Endometrial cytology (Cytobrush %PMN > 6%) | 150 | 25% (Low) | 86% (High) | [86] | |
| Ultrasonography (Uterine lumen fluid > 3 mm) | 150 | 10% (Low) | 93% (High) | [13] | |
| Ultrasonography (Endometrial thickness > 7 mm) | 150 | 1% (Low) | 90% (High) | [13] | |
| Subclinical Endometritis | Endometrial cytology (%PMN > 18%) | 132 | 36% (Low) | 94% (High) | [68] |
| Ultrasonography (Uterine lumen fluid) | 132 | 56% (Medium) | 56% (Medium) | [68] |
| Pathogen | Antibiotic | MIC50 (µg/mL) | MIC90 (µg/mL) |
|---|---|---|---|
| Escherichia coli | Cefquinome | <0.06 * | <0.06 |
| Enrofloxacin | <0.06 | <0.06 | |
| Oxytetracycline | 1 | >32 | |
| Cephapirin | 4 | 8 | |
| Ceftiofur | 0.5 | 0.5 | |
| Trueperella pyogenes | Cefquinome | <0.06 | 0.125 |
| Enrofloxacin | 1 | 1 | |
| Oxytetracycline | 16 | 32 | |
| Cephapirin | <0.06 | <0.06 | |
| Ceftiofur | <0.06 | 0.125 | |
| Fusobacterium necrophorum | Cefquinome | 0.5 | >32 |
| Enrofloxacin | 8 | 8 | |
| Oxytetracycline | 1 | 16 | |
| Cephapirin | <0.06 | 2 | |
| Ceftiofur | <0.06 | 0.125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasimanickam, R.; Bhowmik, P.; Kastelic, J.; Ferreira, J.; Kasimanickam, V. From Infection to Infertility: Diagnostic, Therapeutic, and Molecular Perspectives on Postpartum Metritis and Endometritis in Dairy Cows. Animals 2025, 15, 2841. https://doi.org/10.3390/ani15192841
Kasimanickam R, Bhowmik P, Kastelic J, Ferreira J, Kasimanickam V. From Infection to Infertility: Diagnostic, Therapeutic, and Molecular Perspectives on Postpartum Metritis and Endometritis in Dairy Cows. Animals. 2025; 15(19):2841. https://doi.org/10.3390/ani15192841
Chicago/Turabian StyleKasimanickam, Ramanathan, Priunka Bhowmik, John Kastelic, Joao Ferreira, and Vanmathy Kasimanickam. 2025. "From Infection to Infertility: Diagnostic, Therapeutic, and Molecular Perspectives on Postpartum Metritis and Endometritis in Dairy Cows" Animals 15, no. 19: 2841. https://doi.org/10.3390/ani15192841
APA StyleKasimanickam, R., Bhowmik, P., Kastelic, J., Ferreira, J., & Kasimanickam, V. (2025). From Infection to Infertility: Diagnostic, Therapeutic, and Molecular Perspectives on Postpartum Metritis and Endometritis in Dairy Cows. Animals, 15(19), 2841. https://doi.org/10.3390/ani15192841






