Walnut Green Husk Extract Enhances Antioxidant, Anti-Inflammatory, and Immune Functions by Regulating Gut Microbiota and Metabolites in Fattening Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics
2.2. Experimental Materials
2.3. Laboratory Animals, Diet, and Husbandry Management
2.4. Fecal Microbiota Analysis
2.5. Untargeted Metabolomics Analysis
2.6. Measurement of Serum Immunity Indices, Antioxidant Indices, and Lipid Indices
2.7. Statistical Analysis
3. Results
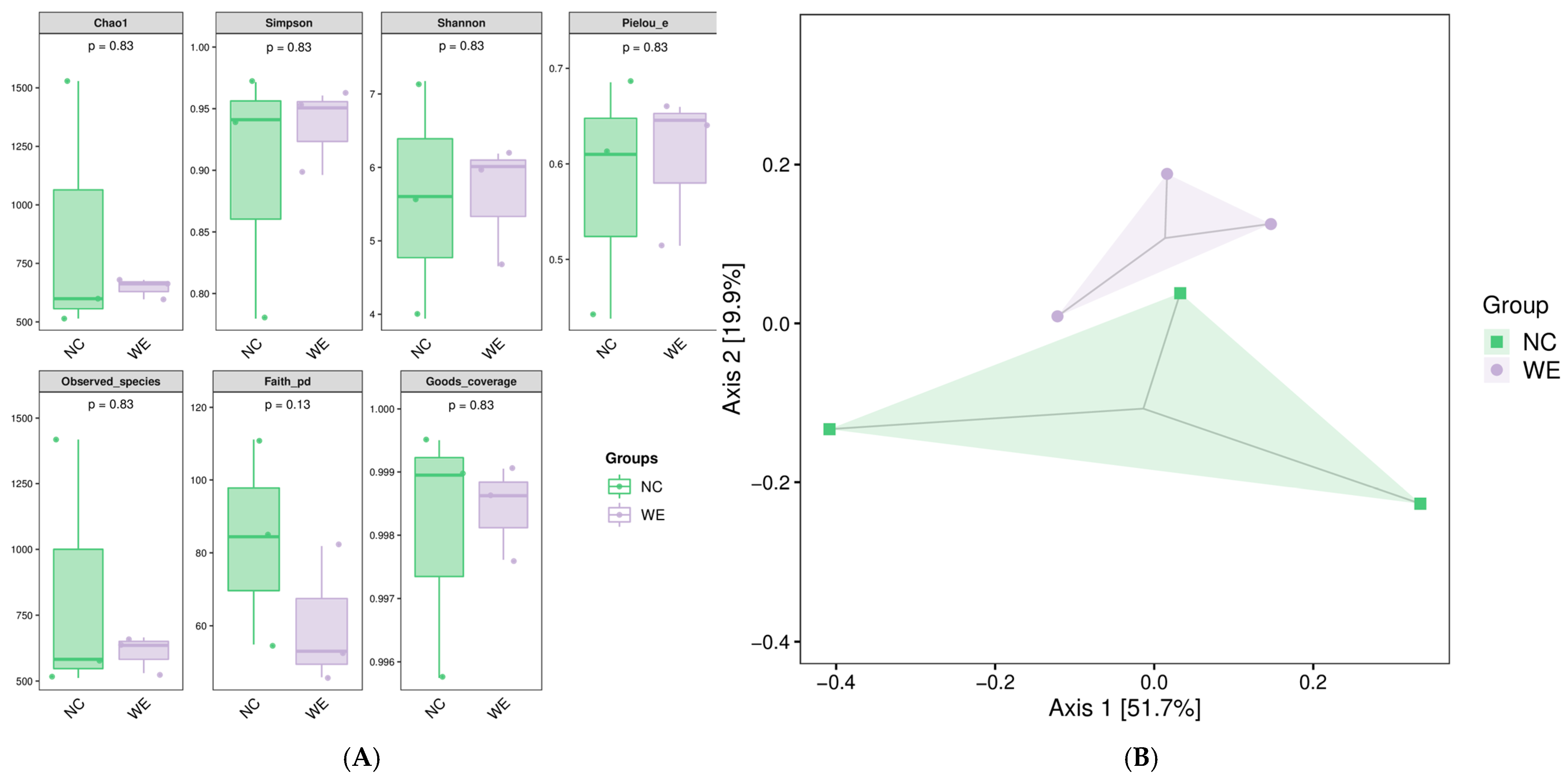
3.1. Diversity of Fecal Microbiota
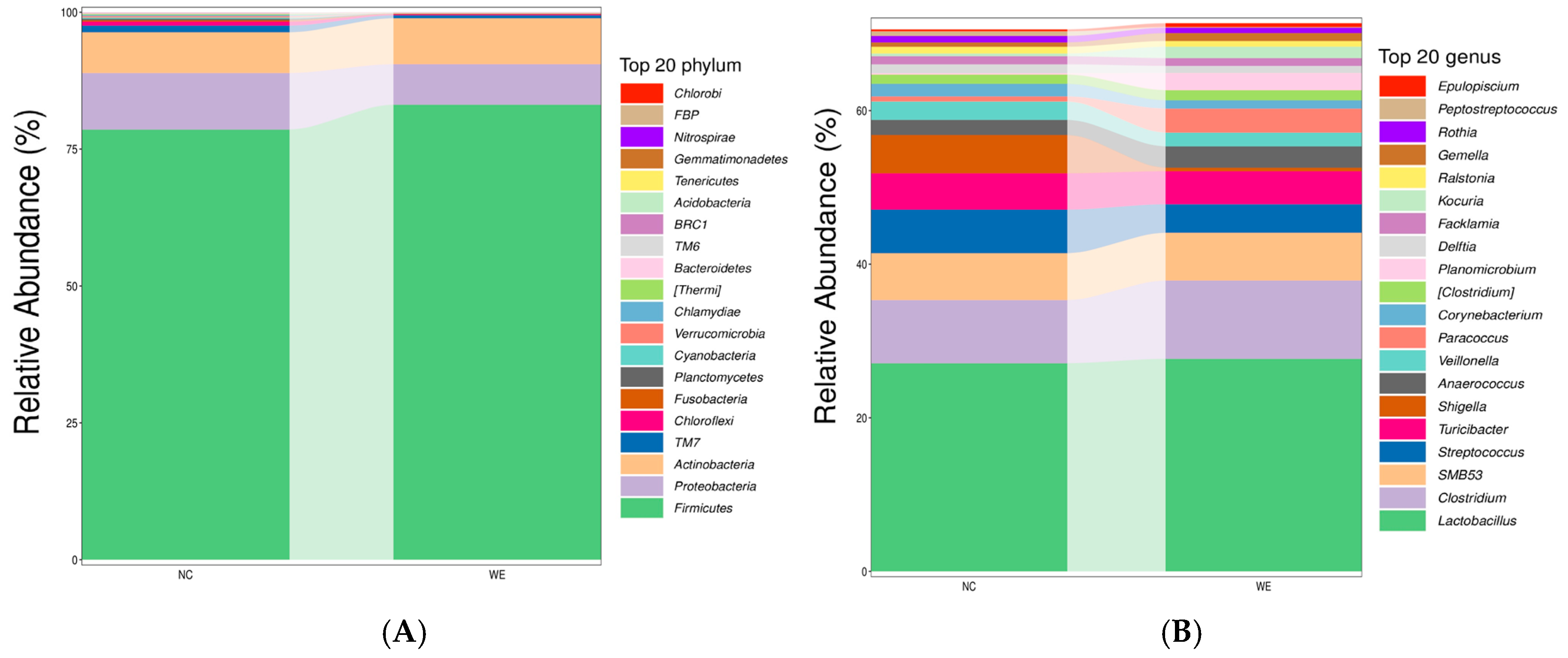
3.2. Composition of the Fecal Microbiota
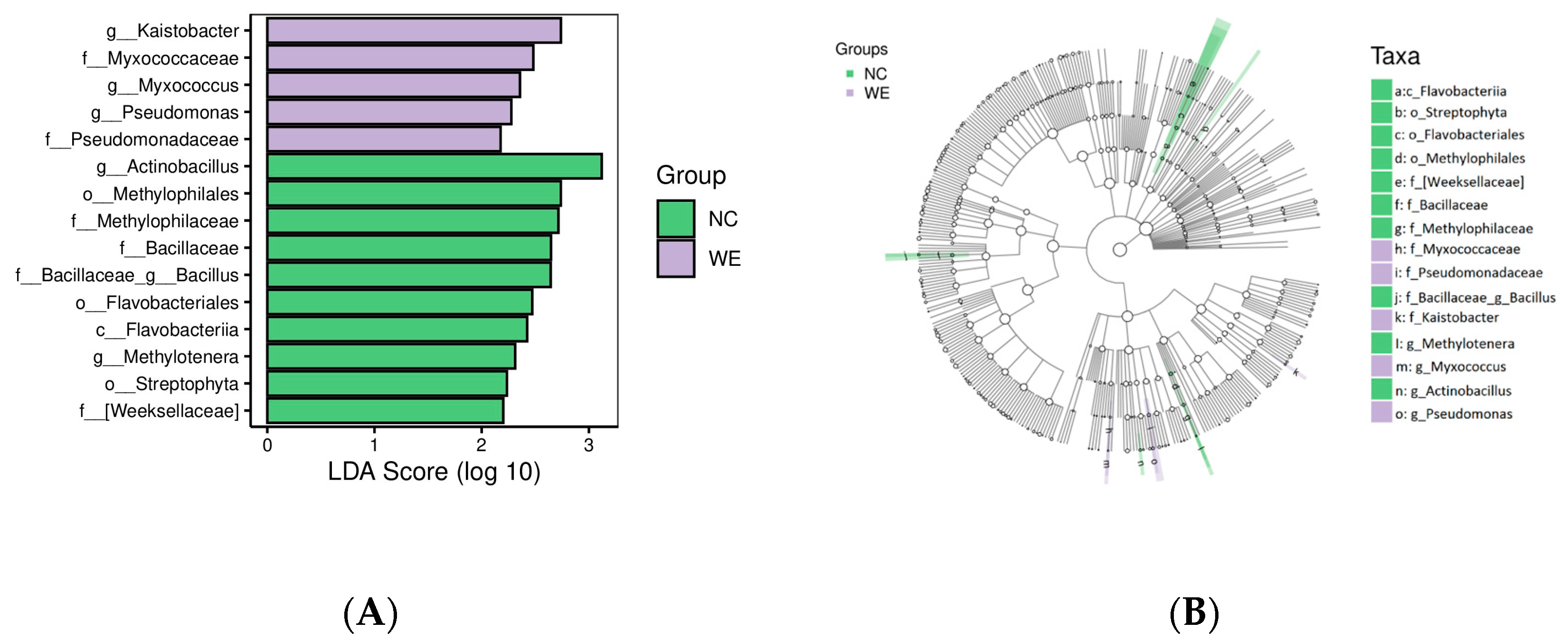
3.3. LEfSe Analysis of Fecal Microbiota
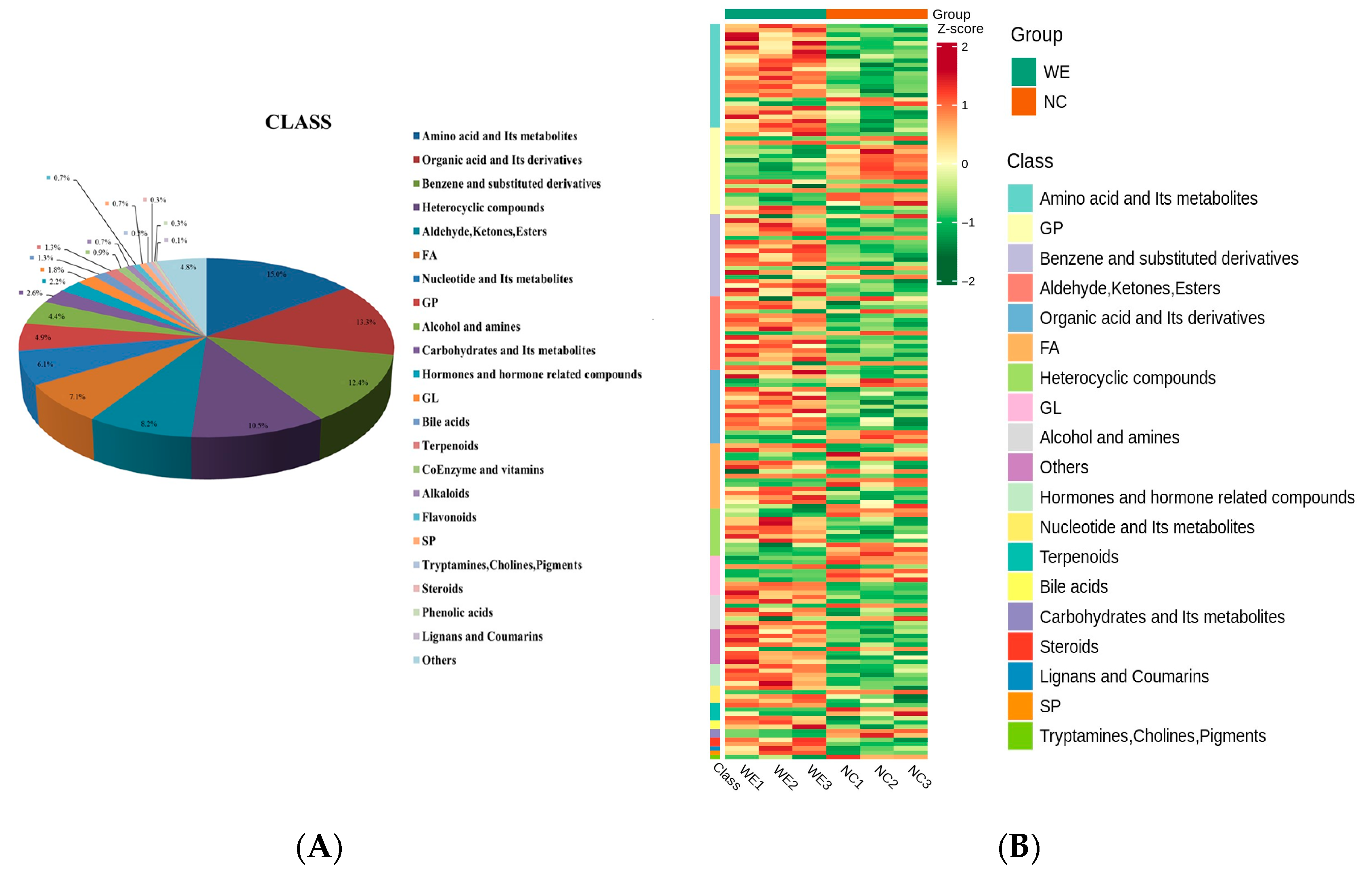
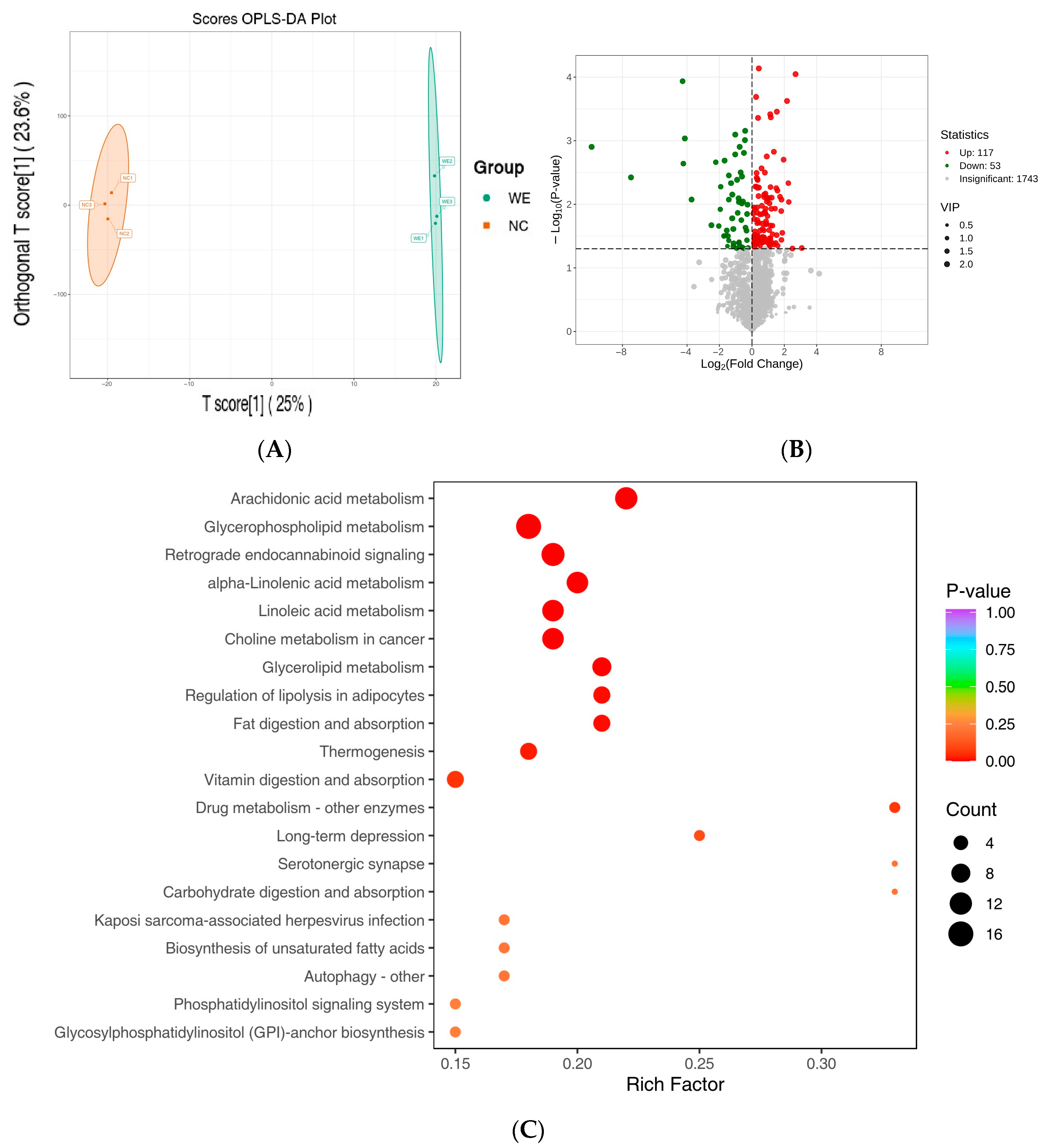
3.4. Metabolic Profiling
3.5. Differentially Accumulated Metabolite Analysis
3.6. Metabolic Pathway Analysis
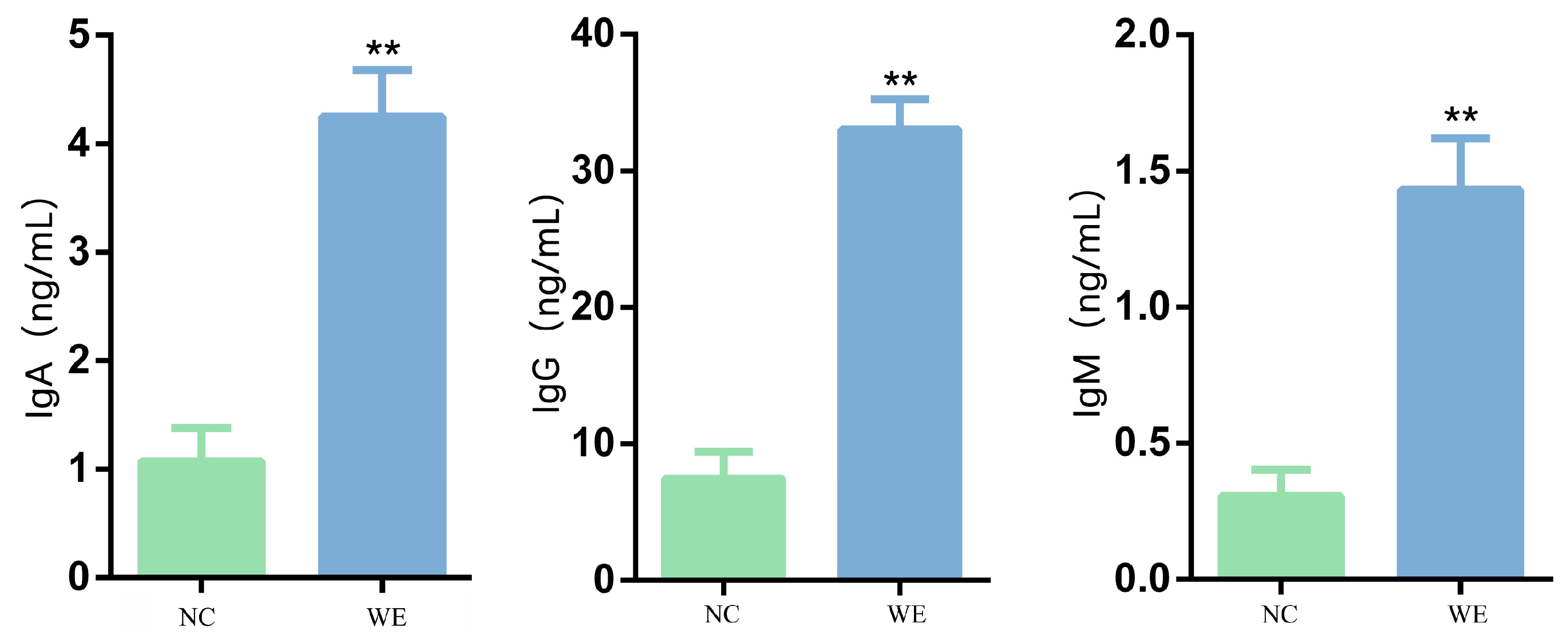
3.7. Effects of WE on Immunological Indicators in Fattening Pigs
3.8. Effects of WE on Antioxidant Activity in Fattening Pigs
3.9. Effects of WE on Lipid Indices in Fattening Pigs
4. Discussion
4.1. Microbial Functional Optimization Without Diversity Changes
4.2. Gut Microbiota Remodeling: Shifts in Taxa and Functional Implications
4.3. Mechanisms Underlying Flora Regulation: Roles of WE Bioactive Components
4.4. Metabolic Reprogramming: Key Metabolites and Pathways
4.5. Anti-Inflammatory and Antioxidant Effects: Metabolite–Microbiota Synergy
4.6. KEGG Pathway Enrichment: Linking Metabolism to Immunity and Lipid Regulation
4.7. Oxidative Stress and Amino Acid Metabolism
4.8. “Microbiota–Metabolite–Immunity” Cascade Regulation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meligy, A.M.A.; El-Hamid, M.I.A.; Yonis, A.E.; Elhaddad, G.Y.; Abdel-Raheem, S.M.; El-Ghareeb, W.R.; Mohamed, M.H.A.; Ismail, H.; Ibrahim, D. Liposomal encapsulated oregano, cinnamon, and clove oils enhanced the performance, bacterial metabolites antioxidant potential, and intestinal microbiota of broiler chickens. Poult. Sci. 2023, 102, 102683. [Google Scholar] [CrossRef] [PubMed]
- Cui, K.; Wang, Q.; Wang, S.Q.; Diao, Q.Y.; Zhang, N.F. The facilitating effect of tartary buckwheat flavonoids and Lactobacillus plantarum on the growth performance, nutrient digestibility, antioxidant capacity, and fecal microbiota of weaned piglets. Animals 2019, 9, 986. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.W.; Wang, H.Q.; Chen, H.Q.; Liu, Z.D.; Zhang, X.D.; Tang, H.; Wei, S.Y.; Zhou, W.T.; Yang, X.Z.; Liu, Y.H.; et al. Structural analysis and in vitro fermentation characteristics of an Avicennia marina fruit RG-I pectin as a potential prebiotic. Carbohydr. Polym. 2024, 338, 122236. [Google Scholar] [CrossRef]
- Lv, Z.P.; Fan, H.; Zhang, B.B.; Xing, K.; Guo, Y.M. Dietary genistein supplementation for breeders and their offspring improves the growth performance and immune function of broilers. Sci. Rep. 2018, 8, 5161. [Google Scholar] [CrossRef]
- Sabah Abdulameer, Y.; Abbas Alwan, I. Improvement of growth performance, biochemical blood profiles, and meat peroxidation by the inclusion of mustard seed extract in broilers’ drinking water. Arch. Razi Inst. 2022, 77, 429–437. [Google Scholar]
- Soto-Madrid, D.; Gutiérrez-Cutiño, M.; Pozo-Martínez, J.; Zúñiga-López, M.C.; Olea-Azar, C.; Matiacevich, S. Dependence of the ripeness stage on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts from industrial by-products. Molecules 2021, 26, 2878. [Google Scholar] [CrossRef]
- Jahanban-Esfahlan, A.; Ostadrahimi, A.; Tabibiazar, M.; Amarowicz, R. A comprehensive review on the chemical constituents and functional uses of walnut (Juglans spp.) husk. Int. J. Mol. Sci. 2019, 20, 3920. [Google Scholar] [CrossRef]
- Shen, Y.; Zhao, H.B.; Wang, X.F.; Wu, S.H.; Wang, Y.L.; Wang, C.X.; Zhang, Y.; Zhao, H. Unraveling the web of defense: The crucial role of polysaccharides in immunity. Front. Immunol. 2024, 15, 1406213. [Google Scholar] [CrossRef]
- Ahmad, T.; Suzuki, Y.J. Juglone in oxidative stress and cell signaling. Antioxidants 2019, 8, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, W.J.; Li, X.Y.; Liu, J.C.; Chen, Y. Antimicrobial activity and mechanisms of walnut green husk extract. Molecules 2023, 28, 7981. [Google Scholar] [CrossRef]
- Wu, Y.; Zhai, S.H.; Fang, M.Y.; Zhang, H.L.; Chen, Y. Evaluation of the growth performance, meat quality, and gut microbiota of broilers fed diets containing walnut green husk extract. Poult. Sci. 2024, 103, 104176. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jia, M.Y.; Chen, J.F.; Ren, Q.L.; Liu, F.J.; Mo, D.L.; Zhang, J.Q.; Wang, L.; Xing, B. Effects of walnut green husks powder and its extracts on growth performance, carcass traits and meat quality of finishing pigs. China Anim. Husb. Vet. Med. 2024, 51, 2451–2459. (In Chinese) [Google Scholar] [CrossRef]
- GB/T 42304-2023; Welfare Criteria for Animals to Be Slaughtered. State Administration for Market Regulation, Standardization Administration of the People’s Republic of China: Beijing, China, 2023.
- NY/T 65-2004; Chinese National Feeding Standard of Swine. Ministry of Agriculture of the People’s Republic of China, China Agriculture Press: Beijing, China, 2004.
- Lee, A.; Le Bon, M.; Connerton, I.F.; Mellits, K.H. Common colonic community indicators of the suckling pig microbiota where diversity and abundance correlate with performance. FEMS Microbiol. Ecol. 2022, 98, fiac048. [Google Scholar] [CrossRef]
- Evans, N.J.; Brown, J.M.; Murray, R.D.; Getty, B.; Birtles, R.J.; Hart, C.A.; Carter, S.D. Characterization of novel bovine gastrointestinal tract Treponema isolates and comparison with bovine digital dermatitis treponemes. Appl. Environ. Microbiol. 2011, 77, 138–147. [Google Scholar] [CrossRef]
- Pedersen, R.; Andersen, A.D.; Mølbak, L.; Stagsted, J.; Boye, M. Changes in the gut microbiota of cloned and non-cloned control pigs during development of obesity: Gut microbiota during development of obesity in cloned pigs. BMC Microbiol. 2013, 13, 30. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, W.J.; Fan, R.; Liu, Z.G.; Huang, T.; Li, J.Y.; Du, T.H.; Xiong, T. Composition and functional diversity of fecal bacterial community of wild boar, commercial pig and domestic native pig as revealed by 16S rRNA gene sequencing. Arch. Microbiol. 2020, 202, 843–857. [Google Scholar] [CrossRef]
- Shin, N.R.; Whon, T.W.; Bae, J.W. Proteobacteria: Microbial signature of dysbiosis in gut microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Ragavan, M.L.; Hemalatha, S. The functional roles of short chain fatty acids as postbiotics in human gut: Future perspectives. Food Sci. Biotechnol. 2023, 33, 275–285. [Google Scholar] [CrossRef]
- Guo, P.T.; Zhang, K.; Ma, X.; He, P.L. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef] [PubMed]
- Su, M.Q.; She, Y.H.; Deng, M.; Guo, Y.Q.; Li, Y.K.; Liu, G.B.; Zhang, H.; Sun, B.L.; Liu, D.W. The effect of capsaicin on growth performance, antioxidant capacity, immunity and gut microorganisms of calves. Animals 2023, 13, 2309. [Google Scholar] [CrossRef]
- Suh, M.J.; Kuntumalla, S.; Yu, Y.; Pieper, R. Proteomes of pathogenic Escherichia coli/Shigella group surveyed in their host environments. Expert Rev. Proteom. 2014, 11, 593–609. [Google Scholar] [CrossRef]
- Rettedal, E.; Vilain, S.; Lindblom, S.; Lehnert, K.; Scofield, C.; George, S.; Clay, S.; Kaushik, R.S.; Rosa, A.J.; Francis, D.; et al. Alteration of the ileal microbiota of weanling piglets by the growth-promoting antibiotic chlortetracycline. Appl. Environ. Microbiol. 2009, 75, 5489–5495. [Google Scholar] [CrossRef] [PubMed]
- Beyoğlu, D.; Popov, Y.V.; Idle, J.R. Metabolomic hallmarks of obesity and metabolic dysfunction-associated steatotic liver disease. Int. J. Mol. Sci. 2024, 25, 12809. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.X.; Huang, Y.T.; Zhang, Y.J.; Xie, J.Y.; Guo, Q.J.; Yang, H.F.; Yang, Y.Y.; Chen, J.; Su, L.J. Quercetin ameliorates obesity and inflammation via microbial metabolite indole-3-propionic acid in high fat diet-induced obese mice. Front. Nutr. 2025, 12, 1574792. [Google Scholar] [CrossRef]
- Yao, Y.; Cai, X.Y.; Fei, W.D.; Ye, Y.Q.; Zhao, M.D.; Zheng, C.H. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit. Rev. Food Sci. Nutr. 2022, 62, 1–12. [Google Scholar] [CrossRef]
- Liu, Z.L.; Wang, H.H.; Ma, K.Y.; Li, Q.; Wu, Y.; Qi, X.C.; Song, J.J.; Wang, C.H.; Ma, Y.J.; Li, T.T. Supplementation with Chinese herbal preparations protect the gut-liver axis of Hu sheep, promotes gut-liver circulation, regulates intestinal flora and immunity. Front. Immunol. 2024, 15, 1454334. [Google Scholar] [CrossRef]
- Walsh, M.C.; Sholly, D.M.; Hinson, R.B.; Saddoris, K.L.; Sutton, A.L.; Radcliffe, J.S.; Odgaard, R.; Murphy, J.; Richert, B.T. Effects of water and diet acidification with and without antibiotics on weanling pig growth and microbial shedding. J. Anim. Sci. 2007, 85, 1799–1808. [Google Scholar] [CrossRef]
- Huang, Y.S.; Huang, W.C.; Li, C.W.; Chuang, L.T. Eicosadienoic acid differentially modulates production of pro-inflammatory modulators in murine macrophages. Mol. Cell. Biochem. 2011, 358, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Sakowski, T.; Grodkowski, G.; Gołebiewski, M.; Slósarz, J.; Kostusiak, P.; Solarczyk, P.; Puppel, K. Genetic and environmental determinants of beef quality—A review. Front. Vet. Sci. 2022, 9, 819605. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, F.F.; Xu, H.J.; Yang, C.M.; Song, X.Q.; Zhou, Y.M.; Zhou, X.L.; Liu, X.D.; Miao, J.L. Cinnamaldehyde microcapsules enhance bioavailability and regulate intestinal flora in mice. Food Chem. X 2022, 15, 100441. [Google Scholar] [CrossRef]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021, 338, 127773. [Google Scholar] [CrossRef]
- Murakami, Y.; Kawata, A.; Suzuki, S.; Fujisawa, S. Cytotoxicity and pro-/anti-inflammatory properties of cinnamates, acrylates and methacrylates against RAW264.7 cells. In Vivo 2018, 32, 1309–1322. [Google Scholar] [CrossRef]
- E, L.; Li, W.; Hu, Y.; Deng, L.; Yao, J.; Zhou, X. Methyl cinnamate protects against dextran sulfate sodium-induced colitis in mice by inhibiting the MAPK signaling pathway. Acta Biochim. Biophys. Sin. 2023, 55, 1806–1818. [Google Scholar] [CrossRef]
- Zhu, Q.; Wu, Y.L.; Mai, J.X.; Guo, G.J.; Meng, J.X.; Fang, X.H.; Chen, X.P.; Liu, C.; Zhong, S.L. Comprehensive metabolic profiling of inflammation indicated key roles of glycerophospholipid and arginine metabolism in coronary artery disease. Front. Immunol. 2022, 13, 829425. [Google Scholar] [CrossRef]
- Qiu, Y.Q.; Liu, S.L.; Hou, L.; Li, K.B.; Wang, L.; Gao, K.G.; Yang, X.F.; Jiang, Z.Y. Supplemental choline modulates growth performance and gut inflammation by altering the gut microbiota and lipid metabolism in weaned piglets. J. Nutr. 2021, 151, 20–29. [Google Scholar] [CrossRef]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650. [Google Scholar] [CrossRef]
- Zhu, M.M.; Zhang, T.T.; Xu, D.H.; Zhou, B.J.; Wang, K.G.; Liao, C.S.; Cheng, Z.T.; Li, P.; Chen, C. Impact of fermented wine lees on gut microbiota and metabolic responses in Guanling crossbred cattle. BMC Microbiol. 2024, 24, 421. [Google Scholar] [CrossRef]
- Cai, L.J.; Wang, Y.; Tan, J.F.; Zhou, H.X.; Liang, S.J.; Wu, Y.; Zhang, J. Impact of arsenic exposure on the hepatic metabolic molecular network in obese pregnant mice using metabolomics and proteomics. Se Pu 2025, 43, 50–59. [Google Scholar] [CrossRef]
- Chen, L.; He, X.G.; Han, Y.F.; Huang, Y.J.; Li, J.; Li, J.; Yu, X.T.; Yun, X.Y.; Wu, J.D.; Sha, R.N.; et al. Lipidomics analysis of adipose depots at differently aged Sunit sheep. Food Chem. 2025, 467, 142243. [Google Scholar] [CrossRef]
- Martins, T.S.; Sanglard, L.M.; Silva, W.; Chizzotti, M.L.; Rennó, L.N.; Serão, N.V.; Silva, F.F.; Guimarães, S.E.; Ladeira, M.M.; Dodson, M.V.; et al. Molecular factors underlying the deposition of intramuscular fat and collagen in skeletal muscle of Nellore and Angus cattle. PLoS ONE 2015, 10, e0139943. [Google Scholar] [CrossRef]
- Ordás, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef] [PubMed]
- Jin, E.S.; Malloy, C.R.; Sharma, G.; Finn, E.; Fuller, K.N.Z.; Reyes, Y.G.; Lovell, M.A.; Derderian, S.C.; Schoen, J.A.; Inge, T.H.; et al. Glycerol as a precursor for hepatic de novo glutathione synthesis in human liver. Redox Biol. 2023, 63, 102749. [Google Scholar] [CrossRef] [PubMed]
- Anand, S.; Mande, S.S. Host-microbiome interactions: Gut-liver axis and its connection with other organs. NPJ Biofilms Microbiomes 2022, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Chen, S.; Xie, K.; Duhun, S.A.; Ortiz-Cerda, T. Evaluating the anti-inflammatory and antioxidant efficacy of complementary and alternative medicines (CAM) used for management of inflammatory bowel disease: A comprehensive review. Redox Rep. 2025, 30, 2471737. [Google Scholar] [CrossRef]
- Cui, Z.X.; Wang, H.Z.; Qin, L.; Yuan, Y.; Xue, J.W.; An, Y.L.; Sun, L.; Zhu, R.F.; Li, Q.Y.; Wang, Y.; et al. Probing the structural elements of polysaccharide adjuvants for enhancing respiratory mucosal response: From surmounting multi-obstacles to eliciting cascade immunity. ACS Nano 2025, 19, 11012–11028. [Google Scholar] [CrossRef]
- Liu, J.; Dai, Y.; Yang, W.; Chen, Z.Y. Role of mushroom polysaccharides in modulation of GI homeostasis and protection of GI barrier. J. Agric. Food Chem. 2025, 73, 6416–6441. [Google Scholar] [CrossRef]
| Ingredients | Content |
|---|---|
| Corn | 58.00 |
| Soybean meal | 14.00 |
| Wheat bran | 9.00 |
| Grass meal | 15.00 |
| Premix 2 | 4.00 |
| Total | 100.00 |
| Nutrient levels 3 | |
| Digestive energy/(MJ/kg) | 11.33 |
| Crude protein | 13.13 |
| Crude fiber | 9.10 |
| Crude ash | 8.30 |
| Calcium | 0.60 |
| Phosphorus | 0.50 |
| Lysine | 0.90 |
| Type | Df | Sum of Squares | R2 | F | p-Value |
|---|---|---|---|---|---|
| Group | 1 | 0.005001 | 0.046042 | 0.193057 | 1.000000 |
| Residual | 4 | 0.103613 | 0.953958 | — | — |
| Total | 5 | 0.108614 | 1.000000 | — | — |
| Compounds | VIP | p-Value | FDR | Log2FC | Type |
|---|---|---|---|---|---|
| PC(18:3/18:3) | 1.90 | 0.05 | 0.56 | 3.08 | up |
| [(2S)-1-decanoyloxy-3-hydroxypropan-2-yl] icosanoate | 1.98 | 0.00 | 0.07 | 2.70 | up |
| Gln-Leu-Leu | 1.88 | 0.05 | 0.56 | 2.50 | up |
| N-(dodecanoyl)-sphing-4-enine-1-phosphocholine | 1.70 | 0.01 | 0.28 | 2.28 | up |
| 9,11-methane-epoxy Prostaglandin F1alpha | 1.72 | 0.00 | 0.25 | 2.25 | up |
| (2S,4S)-1-Acetoxy-16-heptadecene-2,4-diol | 1.95 | 0.00 | 0.09 | 2.17 | up |
| Palmitic amide | 1.85 | 0.00 | 0.19 | 1.96 | up |
| Gentian violet | 1.80 | 0.03 | 0.50 | 1.88 | up |
| Propionic acid | 1.68 | 0.03 | 0.52 | 1.09 | up |
| Eicosadienoic acid | 1.63 | 0.04 | 0.53 | 1.04 | up |
| Methyl cinnamate | 1.96 | 0.01 | 0.34 | 0.79 | up |
| cis-Annonacin-10-one | 2.00 | 0.00 | 0.16 | −9.91 | down |
| PC(14:0/18:4(6Z,9Z,12Z,15Z)) | 1.97 | 0.00 | 0.23 | −7.47 | down |
| 1-O-Hexadecyl-2-arachidonoyl-sn-glycero-3-phosphocholine | 1.81 | 0.00 | 0.07 | −4.28 | down |
| PC(18:3(9Z,12Z,15Z)/18:3(9Z,12Z,15Z)) | 1.75 | 0.00 | 0.19 | −4.23 | down |
| 2′-Deoxyuridine 5′-monophosphate | 1.99 | 0.00 | 0.14 | −4.14 | down |
| Panaxatriol | 1.98 | 0.02 | 0.43 | −2.49 | down |
| PC(15:0/18:3(6Z,9Z,12Z)) | 1.67 | 0.00 | 0.19 | −2.22 | down |
| Ditrans, polycis-undecaprenyl phosphate | 1.63 | 0.02 | 0.43 | −2.04 | down |
| Canthaxanthin | 1.54 | 0.01 | 0.31 | −1.94 | down |
| PE-NMe(15:0/22:2(13Z,16Z)) | 1.64 | 0.01 | 0.25 | −1.92 | down |
| Arachidonic Acid Leelamide | 1.10 | 0.03 | 0.52 | −1.64 | down |
| Items | NC | WE | p-Value |
|---|---|---|---|
| SOD, U/mL | 66.44 ± 2.83 | 69.48 ± 3.30 | 0.50 |
| GSH-Px, U/mL | 671.16 ± 17.69 | 676.50 ± 37.78 | 0.91 |
| CAT, U/mL | 102.45 ± 12.17 | 124.50 ± 8.08 | 0.20 |
| MDA, nmol/mL | 4.72 ± 0.43 | 0.81 ± 0.15 | <0.01 |
| T-AOC, U/mL | 10.29 ± 3.09 | 18.42 ± 2.91 | 0.09 |
| Items | NC | WE | p-Value |
|---|---|---|---|
| TG, mmol/L | 0.50 ± 0.01 | 0.68 ± 0.10 | 0.18 |
| TC, mmol/L | 2.32 ± 0.20 | 2.59 ± 0.07 | 0.19 |
| LDL-C, mmol/L | 1.42 ± 0.18 | 1.22 ± 0.18 | 0.47 |
| HDL-C, mmol/L | 1.01 ± 0.04 | 1.01 ± 0.08 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Jia, M.; Zhang, Q.; Yan, X.; Guo, Y.; Wang, L.; Xing, B. Walnut Green Husk Extract Enhances Antioxidant, Anti-Inflammatory, and Immune Functions by Regulating Gut Microbiota and Metabolites in Fattening Pigs. Animals 2025, 15, 2395. https://doi.org/10.3390/ani15162395
Wang J, Jia M, Zhang Q, Yan X, Guo Y, Wang L, Xing B. Walnut Green Husk Extract Enhances Antioxidant, Anti-Inflammatory, and Immune Functions by Regulating Gut Microbiota and Metabolites in Fattening Pigs. Animals. 2025; 15(16):2395. https://doi.org/10.3390/ani15162395
Chicago/Turabian StyleWang, Jing, Mingyang Jia, Qi Zhang, Xiangzhou Yan, Yaping Guo, Lei Wang, and Baosong Xing. 2025. "Walnut Green Husk Extract Enhances Antioxidant, Anti-Inflammatory, and Immune Functions by Regulating Gut Microbiota and Metabolites in Fattening Pigs" Animals 15, no. 16: 2395. https://doi.org/10.3390/ani15162395
APA StyleWang, J., Jia, M., Zhang, Q., Yan, X., Guo, Y., Wang, L., & Xing, B. (2025). Walnut Green Husk Extract Enhances Antioxidant, Anti-Inflammatory, and Immune Functions by Regulating Gut Microbiota and Metabolites in Fattening Pigs. Animals, 15(16), 2395. https://doi.org/10.3390/ani15162395








