Simple Summary
With this study, we aimed to investigate the epidemiology and genetic diversity of Spirometra tapeworms in snakes in Hunan province. The result showed that the positivity rate among snakes was 89.53%, which is the highest among other regions. Genetic diversity analysis based on concatenated sequences revealed high genetic diversity but no distinct genetic structure among Spirometra populations. Phylogenetic analysis supported the division of European and Chinese Spirometra isolates and a single species in Chinese Spirometra isolates.
Abstract
Sparganosis, caused by the plerocercoid larvae of Spirometra tapeworms, is a public health hazard worldwide. The prevalence and genetics of sparganum from snakes remain unclear. In this study, we investigated the prevalence of sparganum infection in wild snakes in Hunan province and compared the prevalence of Spirometra tapeworms in snakes worldwide. Furthermore, the genetic diversity of collected isolates was analyzed using mitochondrial cytb and cox1 genes. The result shows that the sparganum infection rate in wild snakes (89.50%, 402/449) was higher in Hunan than in other regions. Genetic diversity analysis based on concatenated sequences revealed high genetic diversity but no distinct genetic structure among Spirometra populations. Phylogenetic analysis supported the division of European and Chinese Spirometra isolates and a single species in Chinese Spirometra isolates. The prevalence of Spirometra tapeworms in snakes is serious, and the risk of sparganosis should be further publicized.
1. Introduction
The plerocercoid larvae (sparganum) of Spirometra tapeworms can parasitize humans and cause an important foodborne parasitic zoonosis known as sparganosis [1,2]. Sparganosis occurs worldwide, especially in eastern and southeastern Asian countries [3]. More than 1300 cases of sparganosis have been reported in China, and the actual number of infections may be far higher because many cases may not be recognized or reported [4].
As the most common intermediate hosts in the life cycle of Spirometra tapeworms, snakes and frogs transmit sparganosis to humans in China [5,6]. Humans can be infected by consuming raw or undercooked snake/frog meat or by using raw snake/frog flesh in traditional poultices [3]. Sparganum has been extensively isolated from frogs in different regions of China [6]. However, as a very important host, information on the prevalence of sparganosis in snakes remains scarce [7,8,9]. Therefore, knowledge regarding the prevalence of sparganum infection in snakes is valuable for preventing and controlling sparganosis in humans.
By using mitochondrial genes or the complete mitochondrial genome the sparganum isolates collected in different China locations were classified as S. erinaceieuropaei [6,10,11,12]. No other species of Spirometra has been reported as a source of human infection in China. However, two Spirometra species, S. erinaceieuropaei and S. decipiens, collected from snakes (Dinodon rufozonatum and Agkistrodon saxatilis) have been identified as species in China through morphological and genetic methods [7]. Therefore, the precise identification of Spirometra species from snakes in China requires further investigation.
Data on Spirometra tapeworm infection of snakes in Hunan province were supplemented for the first time in this study. Meanwhile, the prevalence of Spirometra tapeworm in snakes worldwide was also analyzed. Two validated markers, mitochondrial cytochrome B (cytb) and cytochrome C oxidase subunit I (cox1), which have been verified as suitable markers for inferring genetic population differences of Spirometra tapeworms [11,13,14,15], were sequenced and analyzed to infer an exhaustive genetic diversity analysis of Spirometra tapeworms in snakes from different locations in Hunan province.
2. Materials and Methods
2.1. Collection of Sparganum Isolates from Snakes
Samples were collected from field sites in 15 geographical locations of Hunan province in China from April 2018 to October 2019 (Figure 1). The presence of sparganum was examined according to the method described by Liu et al. [5]. In brief, snakes were euthanized using ethyl-ether anesthesia and skinned. The skin was peeled off from the neck to the tip of the tail, and the visceral mass was measured from the esophagus and trachea to the cloaca. Then, the number of spargana was counted. In addition, all surveys of sparganum infection in snakes from other publications were included the analysis. All collected worms were fixed in 99% ethanol and kept at −20 °C for molecular analysis.

Figure 1.
Sampling sites in Hunan province, China. The geographic location is shown in the inset. The sampling sites were added according to GPS data.
2.2. Sequencing of Target Genes
Genomic DNA was extracted using a Wizard® SV genomic DNA purification system (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The expression of two mitochondrial markers (cox1 and cytb) was analyzed using the primer combinations listed in Supplementary Table S1. PCR products were purified using an EasyPure PCR purification kit (Transgen, Beijing, China) and sequenced in both directions by Tsingke Company (Beijing, China).
2.3. Genetic Diversity Analysis of Isolates from Snakes
The DNA sequences of cytb and cox1 were initially aligned using the program Clustal X v.2.0 [16] and adjusted in MEGA v.7.0 according to their amino acid sequences [17]. The nucleotide composition, conserved sites, variable sites, parsimony-informative sites, and singleton sites were estimated using MEGA v.7.0. The haplotypes were inferred, and genetic diversity values per population were calculated using DnaSP v.6 [18]. The median-joining network representing the relationship among all obtained haplotypes was prepared using PopART v.1.7 [19]. Analysis of molecular variance was completed using Arlequin v.3.5.1 to detect the partitions of genetic diversity within and among populations [20]. Pairwise genetic distances were also estimated using Arlequin v.3.5.1 to explore levels of genetic differentiation among the populations. To test the demographic change of the Spirometra population, we performed neutrality tests using Arlequin v.3.5.1 with mismatch distribution using the sum of squared deviations and raggedness index (RI) between observed and expected mismatches and Fu’s FS test and Tajima’s D [21,22].
2.4. Phylogenetic Analysis
All available complete sequences of cox1 and cytb in the GenBank database were included to perform a phylogenetic analysis of Spirometra tapeworms (Supplementary Table S2). Four Dibothriocephalus species-D. nihonkaiense (Genbank accession number representing the cytb/cox1 genes: AB508837/AB015755), D. latum (AB522608/AB511963), D. dendriticum (AB522613/KC812045), and D. ditremum (AB522617/FM209182)-were used as the outgroup. The phylogeny of all collected sequences was estimated by the maximum likelihood (ML) and maximum parsimony (MP) methods. ML analysis was performed in PhyML v.3.0 [23] using models selected by jModelTest 2 under the Akaike information criterion [24]. The support of each internal branch of the phylogeny was estimated using non-parametric bootstrapping (1000 replicates). MP analyses were performed in PAUP*4b10 using heuristic searches with TBR branch swapping and 10,000 random addition sequences. The confidence of each node was assessed by bootstrapping (2000 pseudo-replicates and heuristic search of 20 random addition replicates with TBR option).
3. Results
3.1. Prevalence of Sparganum Infection in Snakes
A total of 2934 snakes belonging to 28 species were surveyed for sparganum infection. As a result, 1581 (53.89%, 1581/2934) were found to be positive (Table 1). The level of sparganum infection in wild snakes in Korea was the highest (83.04%, 235/283), followed by China (51.93%, 1152/2218) and Indonesia (50.85%, 192/378). In contrast, the infection rate among snakes from Poland was only 3.64%, which is far lower than that among snakes from Asian countries. In China, the highest prevalence was found in Zhejiang province (100%, 5/5), followed by Shanghai (93.22%, 55/59) and Hunan (89.53%, 402/449). In central China’s Hunan province, 449 wild snakes were investigated in 14 representative regions in this study. The prevalence ranged from 65% to 100%, with an infection intensity of 1-70 spargana per snake. The highest infection rate was found in snakes from Shaoyang (100%, 20/20), followed by Zhangjiajie (96.67%, 29/30) and Xiangtan (95.77%, 68/71). Generally, the infection rate in Hunan province is higher than that in other provinces in China, such as Guangdong (44.51%, 353/757), Fujian (78.79%, 25/32), Guangxi (23.17%, 38/160), Guizhou (42.16%, 85/172), Jinlin (30.80%, 134/435), and Hebei (36.90%, 55/149).

Table 1.
Prevalence of sparganum infection in snakes.
Among all 28 collected snake species, only 5 species, namely Naja kaouthia, Enhydris bocourti, Elaphe mandarinus, E. schrenkii, and E. davidi, showed no sparganum infection, indicating that these snake species were probably insensitive to sparganum infection (Table 2). The highest infection rate was found in Zaocys dhumnades (94.24%, 393/417), followed by Dinodon rufozonatum (86.89%, 126/145) and Agkistrodon saxatilis (85%, 51/60). Interestingly, the highest infection intensity of sparganum was also found in the Z. dhumnades with infection intensity of 1-294 spargana per snake, followed by D. rufozonatum, with an infection intensity of 1-291 spargana per snake; Ptyas mucosus, with an infection intensity of 1-208 spargana per snake; and Naja atra, with an infection intensity of 1-208 spargana per snake.

Table 2.
Prevalence of sparganum infection in different snake species.
3.2. Genetic Diversity and Phylogenetic Pattern
All amplifications for 67 sparganum isolates were successful, with 1110-bp PCR products for cytb and 1566-bp products for cox1. These sequences detected 119 polymorphic sites (61 for cytb and 58 for cox1), with 83 parsimony-informative sites (39 for cytb and 44 for cox1) and 36 singleton sites (22 for cytb and 14 for cox1). The concatenated sequences identified 48 haplotypes within the 67 isolates, originating from 15 localities. Both individual and combined sequences for cox1 and cytb had high Hd, accompanied by low Pi (Table 3), which is consistent with previous analyses of sparganum isolates from frogs from different locations in China and isolates from different hosts in Poland [13,15].

Table 3.
Genetic diversity indices of cox1 and cytb genes in Spiromerta isolates from Hunan province, China, including sampling size (SS), number (n) of haplotypes, haplotype diversity (Hd), and nucleotide diversity (Pi).
Analysis of molecular variance indicated that most of the observed genetic variation occurred within the 14 endemic populations (70.57%), whereas the difference among the populations contributed 24.96% to the total population (Table 4). The pairwise fixation index (FST) values between specified regions were estimated to measure the population differentiation (Table 5). Across all estimated 91 pairwise FST values, only 22 exhibited statistical significance. Within these 22 statistically significant FST values, most FST values between CS and other endemic regions were above 0.25. These findings indicate high genetic differences between isolates from CS and isolates from other geographical regions in Hunan province. One reason for this observation might be that samples from CS were isolated from four different host species (Z. dhumnades, Panthera tigris, Prionailurus bengalensis, and Felis silvestris, Cat).

Table 4.
Analysis of molecular variance (AMOVA) based on mtDNA sequences of the populations of Spirometra isolates.

Table 5.
Estimates of pairwise FST of concatenated sequences between sparganum populations.
Analysis of the concatenated sequences showed no distinct genetic structure across the sampled Spirometra populations in Hunan province. In the median-joining network, all 67 sequences united in a star-like shape (Figure 2). Although the haplotypes were high (48 haplotypes), no segregation was detected by population, region, or host species. Haplotype 26 was the most prominent, represented by eight samples from two regions (CS and ZZ) and isolated from all five host species. Neutrality tests of Tajima’s D and Fu’s FS for the whole Hunan dataset showed a significant negative value of Fu’s FS (FS = −7.7694, p = 0.043) but a non-significant positive value of Tajima’s D (D = 0.6439, p = 0.798). Mismatch distribution analyses revealed multimodal frequency distributions, which did not support a demographic expansion of Spirometra populations in Hunan province (Figure 3). In addition, low values of the sum of squared deviation and RI under the demographic expansion model were found. The results of Bayesian skyline plot analyses also rejected sudden population expansion.
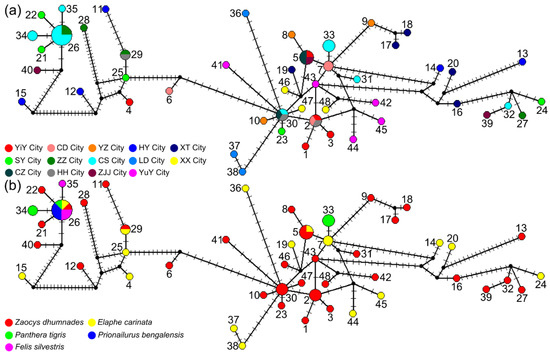
Figure 2.
Median-joining network of 67 sequences of cytb and cox1 genes in sparganum isolates colored by sampling sites (a) and host species (b) in Hunan province, China. The area of circles represents the number of individuals with that haplotype. Perpendicular short lines on the branches indicate unsampled intermediate haplotypes.
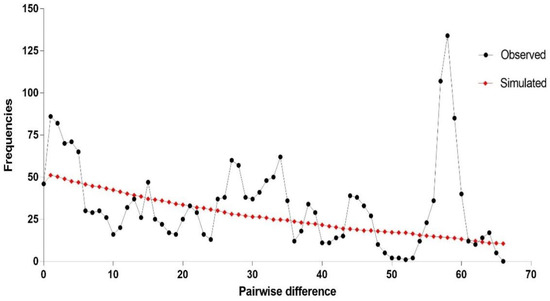
Figure 3.
Estimate of demographic expansion of sparganum isolates from Hunan province. (a) Mismatch distribution analyses. The line charts represent the observed frequencies of pairwise differences among haplotypes. (b) Bayesian skyline plot calculations. The X-axis is in units of million years in the past, and the Y-axis is Ne × μ (effective population size × mutation rate per site per generation). The median estimates are shown as thick solid lines, and the 95% HPD limits are represented by the colored areas.
3.3. Phylogenetic Pattern
In this study, we collected 67 sparganum isolates from 15 geographical locations in Hunan province to explore the phylogenetic diversity of Spirometra isolates and compare the genetic variance between the isolates collected in frogs from other geographical locations. The basic identical tree topologies were generated through phylogenetic inference based on ML and MP methods. The phylogenetic pattern based on the MP analysis is shown in Figure 4. All collected isolates were grouped into two distinct clades (Clade 1 and Clade 2) with high support values (bootstrap values = 100). Clade 1 and Clade 2 were isolates from Poland and China, respectively. However, no clear phylogenetic structure was found within both clades. Within Chinese isolates (Clade 2), although several isolates in snakes and frogs were grouped to form subclades; the support values of these subclades were too low to confirm the phylogenetic patterns, indicating that the Chinese isolates should be considered a single species.
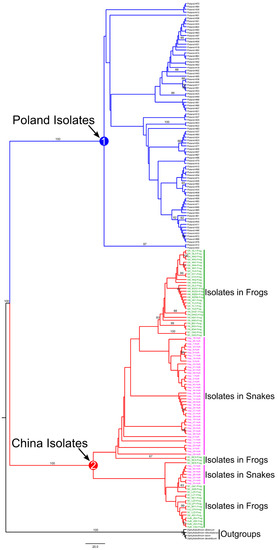
Figure 4.
Phylogenetic relationships among the examined sparganum isolates from different locations in China and Poland were inferred by maximum parsimony (MP) analysis based on the concatenated sequences of cytb and cox1. The numbers along branches indicate bootstrap values, and bootstrap values above 60 are shown. Circled numbers represent the main clades discussed in the text.
4. Discussion
China is home to the most sparganosis, accounting for 80% of the worldwide population. Sparganosis infection generally occurs in humans as a result of eating raw or undercooked frogs and snakes with sparganum [3]. In this study, we conducted a large-scale survey of sparganum infection in wild snakes from 14 geographical locations in Hunan province to understand the prevalence of Spirometra tapeworms in wild snakes. Our results showed that the average prevalence of sparganum infection in snakes in Hunan province was higher than that in Guangdong, Fujian, Guangxi, Guizhou, Jinlin, and Hebei and lower than that in Zhejiang and Shanghai. Unsurprisingly, snake infection rates are higher than frog infection rates in Hunan province (20.20%, 59/292) [12]. As a paratenic host in the life cycle of Spirometra tapeworms, snakes can enrich sparganum by preying on other infected animals, such as frogs. The prevalence of Spirometra tapeworms in snakes worldwide is generally high, suggesting that sparganum infection in snakes may be an important mode of transmission. A total of 28 species of snakes were surveyed, and Z. dhumnades showed the highest infection rate and intensity, which might be because Z. dhumnades is a large species and can therefore be parasitized by more sparganum. In the south and southwest regions of China, snakes are considered a delicacy. Notably, this study showed that the infection rate of Z. dhumnades and E. taeniura, the main edible snakes in China, is high.
The distinct genetic separation of the Polish and Chinese populations of Spirometra isolates was identified by Kołodziej-Sobocińska, who suggested that Polish and Chinese should be two species [15]. Using a global full-length DNA sequence dataset of cox1, Kuchta performed a comprehensive phylogenetic analysis of Spirometra tapeworms [14] and suggested that there are at least six distinct lineages of the genus:
- S. mansoni lineage (corresponding to most of the isolates from Asia, Australia, Romania, and a single sequence from Tanzania);
- Spirometra sp. 1 lineage (corresponding to a few specimens from Korea and Japan);
- Spirometra folium lineage (corresponding to most specimens from Africa, such as Sudan and Ethiopia and the remaining specimens from Tanzania);
- S. erinaceieuropaei lineage (corresponding to the majority of European specimens);
- S. decipiens complex 1 lineage; and
- S. decipiens complex 2 lineages.
Yamasaki re-examined Spirometra samples from Asia based on cox1 DNA sequence data and suggested two distinct Spirometra species [35]. Type I is genetically diverse and widely distributed; however, Type II has only been found in Japan and Korea. In this study, phylogenetic analysis supported the division of Polish and Chinese Spirometra isolates, which supports the findings of Kołodziej-Sobocińska et al. and Kuchta et al. Regarding Chinese Spirometra isolates, Zhang explored the genetic diversity of sparganum isolates in frogs from eastern, central, southern, and southwest China [36]. They found that the Chinese Spirometra population probably comprises two subgroups. However, in this study, we found no distinct genetic structure of cytb and cox1 genes among Spirometra populations in snakes from Hunan province. One possible reason could be that the geographic distances between populations studied here and the sampling size were relatively small. In addition, the results also show that all isolates in snakes and frogs belong to one species, which provides support for a single species in Chinese Spirometra isolates. Until now, definitive morphological criteria for distinguishing Spirometra species, including sparganum and adult worms, have not been established [35]. Therefore, the taxonomy of Spirometra and their phylogenetic relationships remain ambiguous, and more morphological and molecular studies are warranted to clarify the systematics of the genus.
5. Conclusions
The survey results showed that sparganum infection rates in wild snakes in Asian countries were higher than in Europe. Most snake species (82.14%, 23/28) were sensitive to sparganum infection, and the most frequently infected species was Z. dhumnades, followed by D. rufozonatum and A. saxatilis. The sparganum infection rates in wild snakes in several regions of China were still high, especially in Zhejiang, Shanghai, and Hunan. Genetic diversity analysis based on cytb and cox1 genes revealed no distinct genetic structure among Spirometra populations in Hunan province. Phylogenetic analysis supported the division of European and Chinese Spirometra isolates and a single species in Chinese Spirometra isolates.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani12091216/s1. Table S1: Primers were used to amplify the sequences studied [37]; Table S2: Spirometra isolates were included in the molecular analysis.
Author Contributions
T.G., X.Z. and W.L. (Wei Liu). designed the research study. T.G., X.S., F.L., J.H., S.C. and X.Z. carried out the data acquisition, analysis, and interpretation. T.G., X.S., W.L. (Wenchao Li), X.X. and X.Z. wrote the manuscript. F.L., Y.L. and W.L. (Wei Liu). edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This project was supported by the Scientific Research Fund of Hunan Provincial Education Department, China (21A0141); the Natural Science Foundation of the Hunan Province, China (2021JJ30335); the Natural Science Foundation of Henan Province of China (212300410070); and the Research and Innovation Project of Hunan Agricultural University (202147).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving animals were approved by the Animal Ethics Committee of Hunan Agricultural University, Changsha, China (43321503).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study’s design; in the collection, analyses, or interpretation of data in the writing of the manuscript, or in the decision to publish the results.
References
- Scholz, T.; Kuchta, R.; Brabec, J. Broad tapeworms (Diphyllobothriidae), parasites of wildlife and humans: Recent progress and future challenges. Int. J. Parasitol. Parasites Wildl. 2019, 9, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.N.; Su, X.Y.; Chen, W.Q.; Yu, J.W.; Li, J.R.; Jiang, P.; Cui, J.; Wang, Z.Q.; Zhang, X. Transcriptome profiling of plerocercoid and adult developmental stages of the neglected medical tapeworm Spirometra erinaceieuropaei. Acta Trop. 2022, 232, 106483. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Li, M.W.; Wang, Z.D.; Zhao, G.H.; Zhu, X.Q. Human sparganosis, a neglected food borne zoonosis. Lancet Infect. Dis. 2015, 15, 1226–1235. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Zhang, X.; Lin, X.M.; Zhang, H.W.; Wang, Z.Q.; Chen, J.X. A neglected risk for sparganosis: Eating live tadpoles in central China. Infect. Dis. Poverty. 2017, 6, 58. [Google Scholar] [CrossRef]
- Liu, W.; Tan, L.; Huang, Y.; Li, W.C.; Liu, Y.S.; Yang, L.C. Prevalence and molecular characterization of Spirometra erinaceieuropaei spargana in snakes in Hunan Province, China. J. Helminthol. 2020, 94, e131. [Google Scholar] [CrossRef]
- Zhang, X.; Hong, X.; Liu, S.N.; Jiang, P.; Zhao, S.C.; Sun, C.X.; Wang, Z.Q.; Cui, J. Large-scale survey of a neglected agent of sparganosis Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae) in wild frogs in China. PLoS Negl. Trop. Dis. 2020, 14, e0008019. [Google Scholar] [CrossRef] [Green Version]
- Jeon, H.K.; Park, H.; Lee, D.; Choe, S.; Kim, K.H.; Sohn, W.M. Genetic Identification of Spirometra decipiens Plerocercoids in Terrestrial Snakes from Korea and China. Korean J. Parasitol. 2016, 54, 181–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, X.; Qi, R.; Han, H.J.; Liu, J.W.; Qin, X.R.; Fang, L.Z. Molecular identification and phylogenetic analysis of Cryptosporidium, Hepatozoon and Spirometra in snakes from central China. Int. J. Parasitol. Parasites Wildl. 2019, 10, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yudhana, A.; Praja, R.N.; Supriyanto, A. The medical relevance of Spirometra tapeworm infection in Indonesian Bronzeback snakes (Dendrelaphis pictus): A neglected zoonotic disease. Vet. World. 2019, 12, 844–848. [Google Scholar] [CrossRef]
- Dai, R.S.; Liu, G.H.; Song, H.Q.; Lin, R.Q.; Yuan, Z.G.; Li, M.W. Sequence variability in two mitochondrial DNA regions and internal transcribed spacer among three cestodes infecting animals and humans from China. J. Helminthol. 2012, 86, 245–251. [Google Scholar] [CrossRef]
- Hong, X.; Liu, S.N.; Xu, F.F.; Han, L.L.; Jiang, P.; Wang, Z.Q. Global genetic diversity of Spirometra tapeworms. Trop. Biomed. 2020, 37, 237–250. [Google Scholar]
- Liu, W.; Zhao, G.H.; Tan, M.Y.; Zeng, D.L.; Wang, K.Z.; Yuan, Z.G. Survey of Spirometra erinaceieuropaei spargana infection in the frog Rana nigromaculata of the Hunan Province of China. Vet. Parasitol. 2010, 173, 152–156. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Cui, J.; Jiang, P.; Fu, G.M.; Zhong, K. Characterisation of the relationship between Spirometra erinaceieuropaei and Diphyllobothrium species using complete cytb and cox1 genes. Infect. Genet. Evol. 2015, 35, 1–8. [Google Scholar] [CrossRef]
- Kuchta, R.; Kołodziej-Sobocińska, M.; Brabec, J.; Młocicki, D.; Sałamatin, R.; Scholz, T. Sparganosis (Spirometra) in Europe in the Molecular Era. Clin. Infect. Dis. 2021, 72, 882–890. [Google Scholar] [CrossRef]
- Kolodziej-Sobocinska, M.; Stojak, J.; Kondzior, E.; Ruczynska, I.; Wojcik, J.M. Genetic diversity of two mitochondrial DNA genes in Spirometra erinaceieuropaei (Cestoda: Diphyllobothridae) from Poland. J. Zool. Syst. Evol. Res. 2019, 57, 764–777. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Rozas, J.; Ferrer-Mata, A.; Sánchez-DelBarrio, J.C.; Guirao-Rico, S.; Librado, P.; Ramos-Onsins, S.E. DnaSP 6: DNA Sequence Polymorphism Analysis of Large Data Sets. Mol. Biol. Evol. 2017, 34, 3299–3302. [Google Scholar] [CrossRef]
- Leigh, J.W.; Bryant, D. POPART: Full-feature software for haplotype network construction. Methods Ecol. Evol. 2015, 6, 1110–1116. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Fu, Y.X. Statistical tests of neutrality of mutations against population growth, hitchhiking and background selection. Genetics 1997, 147, 915–925. [Google Scholar] [CrossRef]
- Tajima, F. Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 1989, 123, 585–595. [Google Scholar] [CrossRef]
- Guindon, S.; Gascuel, O.A. simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhou, L.; Gong, S.; Deng, Y.; Zou, J.; Wu, J.; Liu, W.; Hou, F. Severe infection of wild-caught snakes with Spirometra erinaceieuropaei from food markets in Guangzhou, China involves a risk for zoonotic sparganosis. J. Parasitol. 2011, 97, 170–171. [Google Scholar] [CrossRef]
- Wang, F.M.; Li, W.Y.; Gong, S.P.; Wei, Y.F.; Ge, Y.; Yang, G.D.; Xiao, J.J. Spirometra erinaceieuropaei severely infect frogs and snakes from food markets in Guangdong, China: Implications a highly risk for zoonotic sparganosis. Trop. Biomed. 2018, 35, 408–412. [Google Scholar]
- Wang, F.; Li, W.; Hua, L.; Gong, S.; Xiao, J.; Hou, F.; Ge, Y.; Yang, G. Spirometra (Pseudophyllidea, Diphyllobothriidae) severely infecting wild-caught snakes from food markets in Guangzhou and Shenzhen, Guangdong, China: Implications for public health. Sci. World 2014, 2014, 874014. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.R.; Xie, P.D. A preliminary investigation of Spirometra mansoni infected by snakes in Meilie Farmers market, Sanming City, Fujian Province. Chin. J. Zoonoses 1988, 2, 55–56. [Google Scholar]
- Zhou, Q.A.; Quan, C.Y.; Zeng, Y.; Shi, Y.L.; Li, J.; Zhang, H.M.; Huang, W.Y. Investigation on plerocercoid infection in frogs and snakes in Nanning Guangxi. Progress Vet. Med. 2013, 34, 126–128. [Google Scholar]
- Zhang, X.; Cui, J.; Wei, T.; Li, L.Y.; Jiang, J.; Lu, J.C.; Jiang, P.; Liu, L.N.; Wang, Z.Q. Survey and genetic variation of Spirometra erinaceieuropaei sparganum in frogs and snakes from Guangxi of southern China. Trop. Biomed. 2014, 31, 862–870. [Google Scholar]
- Chen, Y.; Wu, Z.J.; Qiu, M.L. Infective investigation of frog and snake plerocercoids in some areas of Guizhou. J. Guizhou Normal Univ. 2008, 1, 5–6. [Google Scholar]
- Chen, J.L.; Liu, X.J.; Zhang, S.S. Harm and control of sparganum to Pit viper. Spec. Wild Econ. Anim. Plant Res. 1990, 4, 29–30. [Google Scholar]
- Xu, W.M.; Tang, Y.; Wang, J.; Yang, Y.; Fang, S.Y.; Zhu, S.J.; Jin, X.Y.; Wang, H. Survey of Sparganum mansoni infection in frogs and snakes in Hangzhou. Dis. Surveill. 2009, 24, 612–613. [Google Scholar]
- Lu, Y.; Chen, J.X.; Li, H.; Cai, Y.C.; Ai, L.; Chun, Y.H.; Song, P.; Chen, S.H. Infection of Spirometra mansoni plerocercoid in snakes from Shanghai Zoo. Chin. J. Parasitol. Parasit. Dis. 2018, 36, 593–596. [Google Scholar]
- Yamasaki, H.; Sanpool, O.; Rodpai, R.; Sadaow, L.; Laummaunwai, P.; Un, M. Spirometra species from Asia: Genetic diversity and taxonomic challenges. Parasitol. Int. 2021, 80, 102181. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; Cui, J.; Jiang, P.; Lin, M.L.; Zhang, Y.L. The phylogenetic diversity of Spirometra erinaceieuropaei isolates from southwest China revealed by multi genes. Acta Trop. 2016, 156, 108–114. [Google Scholar] [CrossRef]
- Yanagida, T.; Matsuoka, H.; Kanai, T.; Nakao, M.; Ito, A. Anomalous segmentation of Diphyllobothrium nihonkaiense. Parasitol. Int. 2010, 59, 268–270. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).