Simple Summary
Diarrhea often occurs in suckling piglets and milk secreted by lactating sows with metritis–vaginitis–mastitis is one of the most important contributors. Chinese herbal medicine has antibacterial and anti-inflammatory effects due to its bioactive ingredients, and it is of interest to explore whether maternal feeding with Chinese herbal medicine can increase the level of milk ingredients with anti-infectious and anti-inflammatory properties. The present study found that supplementation of fermented compound Chinese medicine feed additive to sows increased the concentration of functional ingredients, such as quercetin, pinocembrin, chlorogenic acid, methyl succinic acid, L-tryptophan, adenosine, guanine, arteannuin, inosine, guanosine, benzene-1,2,4-triol, hypoxanthine, adenine, ferulic acid, echimidine N-oxide, pogostone, kynurenine and trehalose 6-phosphate in the milk of sows, most of these functional ingredients have anti-infectious, anti-inflammatory, anti-oxidative and immune-enhancing effects. The findings of this study hint that supplementation with fermented compound Chinese medicine feed additive in sows is beneficial for the improvement of milk quality.
Abstract
Different untargeted metabolomics approaches were used to identify the differential metabolites between milk samples collected from two groups. Sows were supplemented with fermented compound Chinese medicine feed additive at levels of 0 g/d/sow (control group, n = 10) and 50 g/d/sow (experimental group, n = 10), respectively, from d 104 of gestation to d 25 of lactation, samples of colostrum and mature milk were collected. Data indicated that supplementing fermented compound Chinese medicine feed additive to sows significantly increased the concentrations of quercetin, pinocembrin, chlorogenic acid, methyl succinic acid, L-tryptophan, adenosine, guanine, arteannuin, ferulic acid, echimidine N-oxide, pogostone and kynurenine in the colostrum and inosine, guanosine, benzene-1,2,4-triol, hypoxanthine, adenine, trehalose 6-phosphate in mature milk, respectively. Seven pathways (flavone and flavanol biosynthesis, galactose metabolism, phenylpropanoid biosynthesis, stilbenoid and gingerol biosynthesis, flavonoid biosynthesis, ABC transporters and purine metabolism) in colostrum and two pathways (sucrose metabolism and retrograde endocannabinoid signaling) in mature milk were significantly enriched in the experimental group compared to control group, respectively. The supplementation of fermented compound Chinese medicine feed additive to sows increased the level of antibacterial and anti-inflammatory ingredients in milk and the findings of this study hint that supplementation with fermented compound Chinese medicine feed additive in sows is beneficial for the improvement of milk quality.
1. Introduction
Sow’s milk is a complex biological fluid and contains macro-chemicals, micro-chemicals and microbes. The composition of sow’s milk often undergoes alterations during the lactation period with the changes in breeds, parities, diets and disease status [1,2,3,4,5,6,7]. It is well known that milk is the main food for piglets during the suckling period, so the quality of sow milk has a vital impact on the survival and growth of suckling piglets [8,9,10]. There are many methods to improve the quality of sow’s milk including the use of different feeding regimens [11] or plant-derived bioactive compounds [12,13,14].
Metritis–vaginitis–mastitis of sows is one of the most prevalent and costly diseases in pig farms, milk secreted by sows with metritis–vaginitis–mastitis often contains harmful microbes and pro-inflammatory mediators, and ingestions of mastitis milk and fecal pathogens from sows are the major contributors to the high mortality of suckling piglets owing to severe diarrhea which is caused by the pathogens, allergic substances and pro-inflammatory mediators in the guts of suckling piglets [3,4,15], this is the reason why sows often have a high number of piglets born alive but a low number of piglets at weaning.
Chinese herbal medicines or extracts are often added to the diet to promote the healthy production of cow’s milk [16,17] and sow’s milk [18] because Chinese herbal medicines and extracts contain bioactive components that possess antibacterial, anti-inflammatory, anti-oxidative and immune-enhancing properties [19,20,21,22]. Some of these bioactive compounds can be transferred directly from Chinese medicine into milk, and some of them can be metabolized to other bioactive compounds by gut microbes and then enter into the milk; providing this functional milk with high levels of Chinese herb medicine-derived bioactive ingredients to suckling piglets is one of the methods to decrease diarrhea and mortality in neonates. In the previous study, we found that fermented compound Chinese medicine feed additive can effectively inhibit the growth of some bacteria associated with metritis–vaginitis–mastitis due to its bioactive metabolites [23]. Chinmedomics strategy is an integration of serum pharmco-chemistry of traditional Chinese medicine and “Omics” technology and is often performed to determine the components of Chinese medicine [24,25]; a metabolomics approach is usually applied to find out the non-Chinese medicine originated compounds [26]. In the present experiment, we applied different untargeted metabolomics approaches to characterize whether maternal feeding with fermented compound Chinese medicine feed additive has an impact on the composition of active ingredients in milk.
2. Materials and Methods
2.1. Animals and Feeding
This feeding experiment started on 20 August 2021, and twenty pregnant crossbred sows (Landrace × Large White) with similar body conditions and parities were randomly assigned to the control group and experimental group with 10 sows (10 replicates) in each group according to a randomized complete block design and fed the same basal diet (Table 1) with supplementation of fermented compound Chinese medicine feed additive from d 104 of gestation to d 25 of lactation at doses of 0 and 50 g/sow, respectively, once a day in the morning. Prior to feeding, the allowance of morning meal of each sow was divided into two parts, one part was mixed with the fermented compound Chinese medicine feed additive and then offered to the sow, and the other part was followed after the sow consumed the mixture of meal and fermented compound Chinese medicine feed additive. The information on ingredients, preparation and chemical compositions of fermented compound Chinese medicine feed additive have been reported in a published paper [23].

Table 1.
Composition and nutrient levels of the basal diet (air-dry basis) %.
2.2. Sample Collection and Preparation
Samples of milk were collected with sterile Eppendorf tubes (Hamburg, Germany), and samples of colostrum and mature milk from each sow were daily collected from d 1 to 5 and d 10 to 20 relative to parturition, respectively, all samples were stored at −20 °C. At the end of sampling, samples of colostrum and mature milk of each sow were mixed, respectively, and the mixture of samples was sub-packed with 5 mL sterile Eppendorf tubes and then stored at −20 °C before analysis.
2.3. Analysis of Active Ingredients Using UHPLC-QE-MS Based Untargeted Chinmedomics
Samples of milk were processed for the extraction of Chinese medicine active ingredients according to the standardized protocols (Shanghai Biotree Biotech Co., Ltd., Shanghai, China) before analysis. LC-MS/MS analysis was performed using a 1290 UPLC system (Agilent, Santa Clara, CA, USA) with a Waters UPLC BEH C18 column (1.7 μm, 2.1 × 100 mm). The flow rate was set at 0.4 mL/min and the sample injection volume was set at 5 μL. The mobile phase consisted of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). The multi-step linear elution gradient program was as follows: 0–3.5 min, 95–85% A; 3.5–6 min, 85–70% A; 6–6.5 min, 70–70% A; 6.5–12 min, 70 -30% A; 12–12.5 min, 30–30% A; 12.5–18 min, 30–0% A; 18–25 min, 0–0% A; 25–26 min, 0–95% A; 26–30 min, 95–95% A.
A Q Exactive Focus mass spectrometer (Vanquish, Thermo Fisher Scientific, Waltham, MA, USA) coupled with Xcalibur TM 3.0 software (Thermo Fisher, Waltham, MA, USA) was employed to obtain the MS and MS/MS data based on the information-dependent acquisition mode. During each acquisition cycle, the mass range was from 100 to 1500, the top three of every cycle were screened and the corresponding MS/MS data were further acquired. The ESI source was applied to analyze the chemical composition in both positive and negative ion modes with full scan/ddMS2. Sheath gas flow rate: 45 Arb, Aux gas flow rate: 15 Arb, Capillary temperature: 400 °C, Full MS resolution: 70,000, MS/MS resolution: 17,500, Collision energy: 15/30/45 in NCE mode, Spray Voltage: 4.0 kV (positive) or −3.6 kV (negative).
Raw data were processed using the XCMS package, and the qualified data were uploaded to SIMCA-P (Version 16.0.2, Sartorius Stedim Data Analytics AB, Umea, Sweden) for statistical analysis. Significantly altered metabolites were determined by t-test and a p value < 0.05 was considered statistically significant. Identification of active ingredients was performed by searching the Biotree databases and web databases (METLIN, HMDB, PubChem, and ChemSpider).
2.4. Identification of Differential Metabolites Using UHPLC-QE-MS Based Conventional Untargeted Metabolomics
Samples of milk were processed according to the standardized protocols before UHPLC-QE-MS-based conventional untargeted metabolomic analysis. LC-MS/MS analyses were performed using a 3000 UHPLC system (Vanquish, Thermo Fisher Scientific, Waltham, MA, USA) with a UPLC HSS T3 column (1.8 μm, 2.1 mm × 100 mm) coupled to a Q Exactive HFX mass spectrometer (Orbitrap MS, Thermo, Waltham, MA, USA). The mobile phase consisted of 5 mmol/L ammonium acetate and 5 mmol/L acetic acid in water (A) and acetonitrile (B). The auto-sampler temperature was 4 °C, and the injection volume was 2 μL. The QE HFX mass spectrometer was used for its ability to acquire MS/MS spectra on information-dependent acquisition mode in the control of the acquisition software (Xcalibur TM 3.0, Thermo, Waltham, MA, USA). In this mode, the acquisition software continuously evaluates the full scan MS spectrum. The ESI source conditions were set as follows: sheath gas flow rate as 30 Arb, Aux gas flow rate as 10 Arb, capillary temperature 350 °C, full MS resolution as 60,000, MS/MS resolution as 7500, collision energy as 10/30/60 in NCE mode, spray Voltage as 4.0 kV (positive) or −3.8 kV (negative), respectively.
Raw data were converted to the mzXML format using ProteoWizard and processed with an in-house program, which was developed using R and based on XCMS for peak detection, extraction, alignment and integration. Significantly altered metabolites were determined by t-test and a p value < 0.05 was considered statistically significant. An in-house MS2 database (Biotree database) was applied in metabolite annotation and the cutoff for annotation was set at 0.3.
3. Results
3.1. Differential Metabolites in Colostrum between Experimental and Control Groups Based on Chinmedomics
Twenty-six metabolites under the negative ion model and twenty-two metabolites under the positive ion model were identified in colostrum between experimental and control groups including eight flavonoids, five phenols, eight alkaloids, two phenylpropanoids, eight terpenoids, two fatty acyls, two fatty acids, one organoheterocyclic compound, two organic acids and derivatives, one organic oxygen compound, six amino acid derivatives, one carbohydrate and derivative, one aliphatic and one organooxygen compound (Table 2). The concentrations of 48 differential metabolites in the colostrum of the experimental group were numerically or significantly higher than that in the colostrum of the control group, and the colostrum of the experimental group had significantly higher concentrations of quercetin (p < 0.05), pinocembrin (p < 0.05), chlorogenic acid (p < 0.01), methyl succinic acid (p < 0.01), L-tryptophan (p < 0.01), adenosine (p < 0.05), guanine (p < 0.05) and arteannuin (p < 0.05) compared to the colostrum of the control group, respectively.

Table 2.
Differential metabolites in colostrum between experimental and control groups based on untargeted chinmedomics.
3.2. Differential Metabolites in Mature Milk between Experimental and Control Groups Based on Chinmedomics
Table 3 showed that a total of 32 metabolites were screened under positive and negative ion modes, respectively, in mature milk between the experimental and control groups including four phenols, seven phenylpropanoids, one xanthone, two sesquiterpenoids, three amino acid derivatives, four alkaloids, three flavonoids, one chalcone, four terpenoids, one organooxygen compound, one fatty acid and one carboxylic acid and derivative. The mature milk of the experimental group had numerically or significantly higher concentrations of 32 metabolites compared to the mature milk of the control group, the concentrations of ferulic acid (p < 0.05), echimidine N-oxide (p < 0.05), pogostone (p < 0.05) and kynurenine (p < 0.05) in the mature milk of the experimental group were significantly higher than that of the control group, respectively.

Table 3.
Differential metabolites in mature milk between experimental and control groups based on untargeted chinmedomics.
3.3. Differential Metabolites between Colostrum and Mature Milk of Experimental Group Based on Chinmedomics
A total of 23 metabolites were identified under positive and negative ion modes between the colostrum and mature milk in the experimental group including one flavonoid, one phenol, eight alkaloids, seven terpenoids, two phenylpropanoids, one coumarin and derivative, one phospholipid, one organoheterocyclic compound and one fatty acid (Table 4). Colostrum had significantly higher concentrations of bergenin (p < 0.05), 3-furfuryl 2-pyrrolecarboxylate (p < 0.05), guanosine (p < 0.01), guanine (p < 0.05), palmatine (p < 0.01), celastrol (p < 0.05), lindenenol (p < 0.05), artemisinin (p < 0.05), curcumenol (p < 0.05) and aucubin (p < 0.05) than mature milk in the experimental group, respectively.

Table 4.
Differential metabolites between colostrum and mature milk of experimental group based on untargeted chinmedomics.
3.4. Differential Metabolites between Colostrum and Mature Milk of Control Group Based on Chinmedomics
A total of 22 metabolites were found under positive and negative ion modes between colostrum and mature milk in the control group including one organic acid and derivative, one phenol, eight alkaloids, six terpenoids, two phenylpropanoids, one coumarin and derivative, one organooxygen compound, one benzene and substituted derivative and one amino acid derivative (Table 5). Colostrum had significantly higher concentrations of threonic acid (p < 0.05), bergenin (p < 0.01), boldine (p < 0.05), 3-Furfuryl 2-pyrrolecarboxylate (p < 0.05), aucubin (p < 0.05), celastrol (p < 0.01) and eudesmin (p < 0.01) than mature milk in the control group, respectively.

Table 5.
Differential metabolites between colostrum and mature milk of control group based on untargeted chinmedomics.
3.5. Differential Metabolites in Colostrum between Experimental and Control Groups Based on Conventional Untargeted Metabolomics
A total of seventy-six differential metabolites have been identified in the colostrum between experimental and control groups including 24 organic acids and derivatives, 8 organoheterocyclic compounds, 1 organooxygen compound, 4 organic oxygen compounds, 1 organic nitrogen compound, 11 nucleosides, nucleotides, and analogs, 21 lipids and lipid-like molecules and 6 benzenoids (Table 6). The colostrum of sows in the experimental group had significantly higher levels of inosine, guanosine, benzene-1,2,4-triol, hypoxanthine and adenine than the colostrum of sows in the control group (p < 0.05), respectively. Other metabolites in the colostrum of the experimental group also had numerically higher concentrations than that in the colostrum of the control group (p > 0.05), respectively.

Table 6.
Differential metabolites in colostrum between experimental and control groups based on conventional untargeted metabolomics.
3.6. Differential Metabolites in Mature Milk between Experimental and Control Groups Based on Conventional Untargeted Metabolomics
Table 7 indicated that 13 differential metabolites under the negative ion model and 10 differential metabolites under the positive ion model had been screened in mature milk between the experimental group and control group including seven organic acids and derivatives, two organoheterocyclic compounds, three organic oxygen compounds, one organic nitrogen compound, three nucleosides, nucleotides and analogs, five lipids and lipid-like molecules and two benzenoids. The concentration of trehalose 6-phosphate in the mature milk of the experimental group was significantly higher than that in the mature milk of the control group (p < 0.05), and other metabolites in the mature milk of the experimental group had numerically higher levels than those in the mature milk of the control group (p > 0.05), respectively.

Table 7.
Differential metabolites in mature milk between experimental and control groups based on conventional untargeted metabolomics.
3.7. Differential Metabolites between Colostrum and Mature Milk of Experimental Group Based on Conventional Untargeted Metabolomics
Nineteen differential metabolites under the negative ion model and thirty-two differential metabolites under the positive ion model had been found in the experimental group between colostrum and mature milk including 11 organic acids and derivatives, 5 organic oxygen compounds, 6 organoheterocyclic compounds, 1 organohalogen compound, 18 lipids and lipid-like molecules, 9 nucleosides, nucleotides and analogs and 1 benzenoid (Table 8). Thirty-five differential metabolites had significantly higher concentrations in colostrum than in mature milk and the other sixteen metabolites in colostrum also had numerically higher levels than in mature milk.

Table 8.
Differential metabolites between colostrum and mature milk of experimental group based on conventional untargeted metabolomics.
3.8. Differential Metabolites between Colostrum and Mature Milk of Control Group Based on Conventional Untargeted Metabolomics
Thirty-seven differential metabolites had been screened between colostrum and mature milk in the control group including 5 organic acids and derivatives, 12 organic oxygen compounds, 4 organoheterocyclic compounds, 1 organohalogen compound, 8 nucleosides, nucleotides and analogs, and 7 lipids and lipid-like molecules (Table 9). Twenty-seven metabolites in colostrum had significantly higher concentrations than that in mature milk and ten metabolites had numerically higher levels in colostrum than in mature milk.

Table 9.
Differential metabolites between colostrum and mature milk of control group based on conventional untargeted metabolomics.
3.9. Metabolic Pathways Enrichment
In order to identify the changes in the metabolic pathway reflected by differential metabolites, differential abundance analysis of KEGG metabolic pathways for differential metabolites screened by chinmedomics was conducted and results are shown in Figure 1. There were significant differences in the differential abundances of five metabolic pathways of differential metabolites in the colostrum between the experimental group and control group (Figure 1A), differential metabolites enriched in the pathways of flavone and flavanol biosynthesis, galactose metabolism, phenylpropanoid biosynthesis, stilbenoid and gingerol biosynthesis, flavonoid biosynthesis were upregulated in the colostrum of sows supplemented with fermented compound Chinese medicine feed additive. Two pathways of differential metabolites in mature milk had significant differences in differential abundance (Figure 1B), and differential metabolites enriched in ABC transporters and pyrimidine metabolism were downregulated in the mature milk of sows fed with fermented compound Chinese medicine feed additive. Seven pathways with differential abundances were annotated between colostrum and mature milk of sows with fermented compound Chinese medicine feed additive supplementation (Figure 1C), differential metabolites enriched in purine metabolism were significantly upregulated but in phenylpropanoid biosynthesis were significantly downregulated in colostrum. The differential abundances of metabolic pathways, biosynthesis of secondary metabolites and biosynthesis of various plant secondary metabolites were not significantly different when comparing colostrum to mature milk in sows supplemented without fermented compound Chinese medicine feed additive (Figure 1D); differential metabolites enriched in metabolic pathways, biosynthesis of secondary metabolites and biosynthesis of various plant secondary metabolites were downregulated in colostrum.
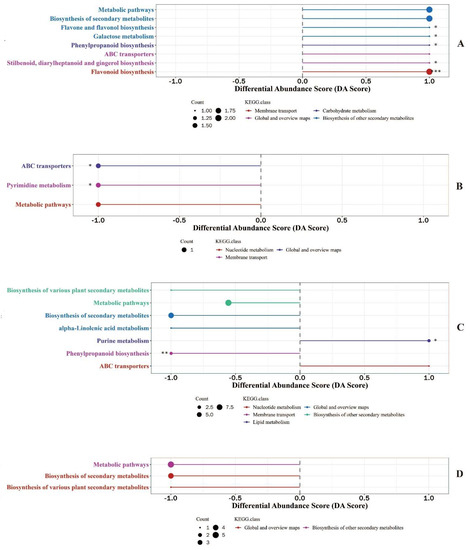
Figure 1.
Differential abundance score of metabolic pathways of metabolites identified in milk using chinmedomics approach. (A): KEGG pathways enrichment of differential metabolites in colostrum between experimental group and control group. (B): KEGG pathways enrichment of differential metabolites in mature milk between experimental group and control group. (C): KEGG pathways enrichment of differential metabolites between colostrum and mature milk of experimental group. (D): KEGG pathways enrichment of differential metabolites between colostrum and mature milk of control group. * 0.01 < p <0.05, ** 0.001 < p <0.01, *** p < 0.001.
The differential abundance score of KEGG metabolic pathways for differential metabolites in milk identified by conventional metabolomics is shown in Figure 2. Differential metabolites of colostrum between experimental and control groups were mapped to three pathways, respectively, and metabolites in the colostrum of the experimental group were significantly upregulated in ABC transporters and purine metabolism, respectively, compared to metabolites in the colostrum of the control group (Figure 2A). Differential metabolites in the mature milk between the experimental and control groups were involved in five pathways and metabolites in the mature milk of the experimental group were significantly upregulated in sucrose metabolism and retrograde endocannabinoid signaling, respectively, compared to those metabolites in the mature milk of the control group (Figure 2B). The differential abundances of 15 metabolic pathways of conventional metabolites between the colostrum and mature milk from the experimental group had significant differences (Figure 2C), differential metabolites involved in purine metabolism, biosynthesis of cofactors, thermogenesis, aldosterone synthesis and secretion, glucagon signaling pathway were upregulated in colostrum, respectively. Fifteen metabolic pathways of differential metabolites between the colostrum and mature milk of the control group had significant differences in differential abundance (Figure 2D), and differential metabolites mapped to the biosynthesis of cofactors were upregulated in the colostrum.
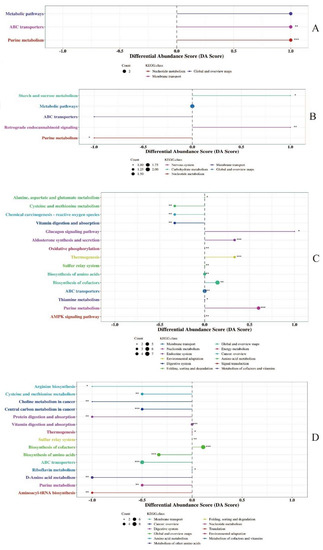
Figure 2.
Differential abundance score of metabolic pathways of metabolites identified in milk using conventional metabolomics approach. (A): KEGG pathways enrichment of differential metabolites in colostrum between experimental group and control group. (B): KEGG pathways enrichment of differential metabolites in mature milk between experimental group and control group. (C): KEGG pathways enrichment of differential metabolites between colostrum and mature milk of experimental group. (D): KEGG pathways enrichment of differential metabolites between colostrum and mature milk of control group. * 0.01 < p < 0.05, ** 0.001 < p <0.01, *** p < 0.001.
4. Discussion
Milk is the major food source of suckling piglets, and it is one of the crucial factors affecting the survival and growth of suckling piglets [27,28]. Active ingredients in milk are thought to play important roles in the prevention and control of diarrhea during the suckling period [29,30]; however, the level of these active ingredients in milk is generally low and is not enough high for the control of diarrhea, so how to increase the concentration of these active ingredients in milk is an urgent issue to be addressed.
Studies reported that Chinese medicine is a good alternative to in-feed antibiotics because lots of active ingredients in Chinese medicine have antibacterial, anti-inflammatory, antivirus, anti-oxidative and immune-enhancing effects. Our previous study also found that a fermented compound Chinese medicine feed additive had good in vitro effects in inhibiting the growth of Staphylococcus aureus, Salmonella cholerae suis, Escherichia coli and Streptococcus agalactiae, because it contained high levels of active ingredients, such as gallic acid, ellagic acid, kaempferide and adenosine [23]. Results of this feeding experiment further showed that the colostrum and mature milk of sows supplemented with fermented compound Chinese medicine feed additive had higher levels of bioactive ingredients than the milk of sows without supplementation of fermented compound Chinese medicine feed additive, particularly, the milk of sows from the experimental group had significantly higher concentrations of quercetin (p < 0.05), pinocembrin (p < 0.05), chlorogenic acid (p < 0.01), methyl succinic acid (p < 0.01), L-tryptophan (p < 0.01), adenosine (p < 0.05), guanine (p < 0.05), arteannuin (p < 0.05), inosine (p < 0.05), guanosine (p < 0.05), benzene-1,2,4-triol (p < 0.05), hypoxanthine (p < 0.05), adenine (p < 0.05), ferulic acid (p < 0.05), echimidine N-oxide (p < 0.05), pogostone (p < 0.05), kynurenine (p < 0.05) and trehalose 6-phosphate (p < 0.05) than the milk of sows from the control group.
Previous studies evidenced that lots of active ingredients have functions in killing or inhibiting pathogens, alleviating inflammation, enhancing immunity and repairing intestinal barrier function. Quercetin [31], pinocembrin [32,33], pogostone [34], adenosine [35], ferulic acid [36], echimidine-N-oxide [37], purines including adenine, guanine, xanthine and hypoxanthine [38,39,40] have strong antibacterial or antifungal effects on diarrheal pathogens, such as Escherichia coli, Salmonella, Staphylococcus aureus, Dysentery bacilli, Pseudomonas aeruginosa, Streptococcus and Clostridioides difficile, in addition, hypoxanthine can speed the excretion of fecal harmful microbiota and toxic substances via shorting gastrointestinal transit time [41]. Pro-inflammatory substances can also cause diarrhea by impairing the intestinal mucosal barrier with pro-inflammatory cytokines [42], but quercetin [31], arteannuin and kynurenine [43,44], ferulic acid [45], purines [46], inosine [47,48], guanosine [49] and benzene-1,2,4-triol [50] can reduce diarrhea by increasing IFN-γ level or inhibiting the production of pro-inflammatory cytokines [48,51,52,53]. Supplementation with Perilla frutescens leaf to Holstein cows changed the composition of differential metabolites in the milk and many differential metabolites with antibacterial and anti-inflammatory effects had been identified [17], results of our experiment also indicated that maternal supplementation with fermented compound Chinese medicine feed additive accumulated lots of high-level differential metabolites in milk which have antibacterial, anti-inflammatory and immune enhancing properties.
Differential metabolites in colostrum between the experimental group and control group were significantly enriched in the KEGG pathways of flavone and flavanol biosynthesis, galactose metabolism, phenylpropanoid biosynthesis, stilbenoid and gingerol biosynthesis, flavonoid biosynthesis, ABC transporters and purine metabolism, respectively; the colostrum of sows from the experimental group had significantly higher enrichment abundance of differential metabolites than the colostrum of sows from the control group, this means that the colostrum of the experimental group had better antibacterial and anti-inflammatory effects than the colostrum of the control group; differential metabolites in the colostrum of the experimental group can produce other active ingredients in the digestive tract of suckling piglets via these enriched KEGG pathways. It is reported that quercetin can be metabolized into other flavonoids, such as quercitrin, isoquercitrin and myricetin when fermented with some bacteria [54,55]. Adenine, hypoxanthine and guanine can be converted to xanthine and further catabolized to uric acid and allantoin under the action of bacterial fermentation [56,57]. Differential metabolites in mature milk between the experimental group and control group were significantly enriched in the KEGG pathways of starch and sucrose metabolism, and retrograde endocannabinoid signaling; the mature milk of sows from the experimental group had a significantly higher enrichment abundance of differential metabolites than that of sows from the control group. In addition, trehalose-6-phosphate can be mapped onto the pathways of starch and sucrose metabolism and retrograde endocannabinoid signaling. It could be estimated that the mature milk of the experimental group had better functions than the mature milk of the control group in improving the digestion of starter feed and the gut health of suckling piglets; trehalose-6-phosphate can promote the rapid fermentation of carbohydrates, especially glucose and lactose through the pathway of starch and sucrose metabolism to produce volatile fatty acids [58], the rapid fermentation of carbohydrate is particularly important to suckling piglets during the ingestion of starter feed, because mature milk with high trehalose-6-phosphate can quickly lower the intestinal pH of piglets; this is beneficial to the control of gut pathogens and the digestion of nutrients in artificial diets. Retrograde endocannabinoid signaling takes part in the regulation of inflammatory factor release and gut permeability [59], and trehalose-6-phosphate identified in the mature milk of the experimental group is also involved in the pathway of retrograde endocannabinoid signaling, this implies that trehalose-6-phosphate can function in anti-inflammation and intestinal permeability maintenance. Maternal feeding with fermented compound Chinese medicine feed additive had impacts on KEGG pathways and pathway abundances of differential metabolites between colostrum and mature milk. Compared to without supplementation of fermented compound Chinese medicine feed additive, supplementation of fermented compound Chinese medicine feed additive to sows from d 104 of gestation to d 25 of lactation elevated the number of enriched KEGG pathways; increased the enrichment abundance of purine metabolism, the glucagon signaling pathway, aldosterone synthesis and secretion, and the thermogenesis of differential metabolites in colostrum metabolism, but decreased the enrichment abundance of protein digestion and absorption, biosynthesis of amino acid, D-amino acid metabolism and purine metabolism of differential metabolites in mature milk metabolism. Increasing the enrichment abundance of glucagon signaling and the thermogenesis pathways could metabolize nutrients to produce more heat to raise the cold resistance of newborn animals [60,61], it is very important for newborn piglets, because the increased cold resistance can decrease the morbidity of newborn piglets.
5. Conclusions
Maternal feeding with fermented Chinese medicine feed additive elevated the concentrations of functional ingredients in the colostrum and mature milk of sows, and most of these functional ingredients have anti-infectious, anti-inflammatory, anti-oxidative and immune-enhancing effects.
Author Contributions
Design, Y.H. and W.L.; Experiments, W.Z., L.D., H.W. and Z.L.; Data analysis, W.Z.; Manuscript writing, Y.H. and W.Z.; Writing review, W.L. and Y.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the Key Research and Development Plan of Jiangxi Province (20192BBFL60021).
Institutional Review Board Statement
The animal study protocol was approved by the Ethics Committee of Jiangxi Agricultural University (JXAULL-202122, 15/08/2021).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in [Untargeted Metabolomics Profiling Reveals Beneficial Changes in Milk of Sows Supplemented with Fermented Compound Chinese Medicine Feed Additive].
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Curtasu, M.V.; Theil, P.K.; Hedemann, M.S. Metabolomic profiles of colostrum and milk from lactating sows. J. Anim. Sci. 2016, 94, 272–275. [Google Scholar] [CrossRef]
- Picone, G.; Zappaterra, M.; Luise, D.; Trimigno, A.; Capozzi, F.; Motta, V.; Davoli, R.; Costa, L.N.; Bosi, P.; Trevisi, P. Metabolomics characterization of colostrum in three sow breeds and its influences on piglets’ survival and litter growth rates. J. Anim. Sci. Biotechno. 2018, 9, 23. [Google Scholar] [CrossRef]
- Laforest-Lapointe, I.; Becker, A.B.; Mandhane, P.J.; Turvey, S.E.; Moraes, T.J.; Sears, M.R.; Subbarao, P.; Sycuro, L.K.; Azad, M.B.; Arrieta, M.C. Maternal consumption of artificially sweetened beverages during pregnancy is associated with infant gut microbiota and metabolic modifications and increased infant body mass index. Gut Microbes 2021, 13, 1857513. [Google Scholar] [CrossRef]
- Fehr, K.; Moossavi, S.; Sbihi, H.; Boutin, R.C.T.; Bode, L.; Robertson, B.; Yonemitsu, C.; Field, C.J.; Becker, A.B.; Mandhane, P.J.; et al. Breastmilk feeding practices are associated with the co-occurrence of bacteria in mothers’ milk and the infant gut: The child cohort study. Cell Host Microbe 2020, 28, 285–297. [Google Scholar] [CrossRef]
- Nuntapaitoonab, M.; Juthamaneea, P.; Theilc, P.K.; Tummarukab, P. Impact of sow parity on yield and composition of colostrum and milk in Danish Landrace × Yorkshire crossbred sows. Prev. Vet. Med. 2020, 81, 105085. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Agazzi, A.; Bontempo, V.; Invernizzi, G.; Panseri, S.; Sauerwein, H.; Eckersall, P.D.; Burchmore, R.; Savoini, G. Effects of low ω6:ω3 ratio in sow diet and seaweed supplement in piglet diet on performance, colostrum and milk fatty acid profiles, and oxidative status. Animals 2020, 10, 2049. [Google Scholar] [CrossRef]
- Wang, C.; Wei, S.Y.; Liu, B.J.; Wang, F.Q.; Lu, Z.Q.; Jin, M.L.; Wang, Y.Z. Maternal consumption of a fermented diet protects offspring against intestinal inflammation by regulating the gut microbiota. Gut Microbes 2022, 14, e2057779. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef]
- Scano, P.; Murgia, A.; Demuru, M.; Consonni, R.; Caboni, P. Metabolite profiles of formula milk compared to breast milk. Food Res. Int. 2016, 87, 76–82. [Google Scholar] [CrossRef]
- Sindi, A.S.; Geddes, D.T.; Wlodek, M.E.; Muhlhausler, B.S.; Payne, M.S.; Stinson, L.F. Can we modulate the breastfed infant gut microbiota through maternal diet? FEMS Microbiol. Rev. 2021, 45, fuab011. [Google Scholar] [CrossRef]
- Rocchetti, G.; Gallo, A.; Nocetti, M.; Lucini, L.; Masoero, F. Milk metabolomics based on ultra-high-performance liquid chromatography coupled with quadrupole time-of-flight mass spectrometry to discriminate different cows feeding regimens. Food Res. Int. 2020, 134, 109279. [Google Scholar] [CrossRef] [PubMed]
- Benchaar, C.; McAllister, T.A.; Chouinard, P.Y. Digestion, ruminal fermentation, ciliate protozoal populations, and milk production from dairy cows fed cinnamaldehyde, quebracho condensed tannin, or Yucca schidigera saponin extracts. J. Dairy Sci. 2008, 91, 4765–4777. [Google Scholar] [CrossRef] [PubMed]
- Focant, M.; Froidmont, E.; Archambeau, Q.; Dang Van, Q.C.; Larondelle, Y. The effect of oak tannin (Quercus robur) and hops (Humulus lupulus) on dietary nitrogen efficiency, methane emission, and milk fatty acid composition of dairy cows fed a low-protein diet including linseed. J. Dairy Sci. 2019, 102, 1144–1159. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tu, Y.; Zhao, S.P.; Hao, Y.H.; Liu, J.X.; Liu, F.H.; Xiong, B.H.; Jiang, L.S. Effect of tea saponins on milk performance, milk fatty acids, and immune function in dairy cow. J. Dairy Sci. 2017, 100, 8043–8052. [Google Scholar] [CrossRef] [PubMed]
- Johnzon, C.F.; Dahlberg, J.; Gustafson, A.M.; Waern, I.; Moazzami, A.A.; Östensson, K.; Pejler, G. The effect of lipopolysaccharide-induced experimental bovine mastitis on clinical parameters, inflammatory markers, and the metabolome: A kinetic approach. Front. Immunol. 2018, 9, 1487. [Google Scholar] [CrossRef] [PubMed]
- Shan, C.H.; Guo, J.J.; Sun, X.S.; Li, N.; Yang, X.Y.; Gao, Y.H.; Qiu, D.R.; Li, X.M.; Wang, Y.N.; Feng, M.; et al. Effects of fermented Chinese herbal medicines on milk performance and immune function in late-lactation cows under heat stress conditions. J. Anim. Sci. 2018, 96, 4444–4457. [Google Scholar] [CrossRef]
- Wang, B.; Sun, Z.Q.; Tu, Y.; Si, B.W.; Liu, Y.L.; Yang, L.; Luo, H.L.; Yu, Z. Untargeted metabolomic investigate milk and ruminal fluid of Holstein cows supplemented with Perilla frutescens leaf. Food res. Int. 2021, 140, 110017. [Google Scholar] [CrossRef]
- Jiang, X.J.; Lin, S.; Lin, Y.; Fang, Z.F.; Xu, S.Y.; Feng, B.; Zhuo, Y.; Li, J.Y.; Che, L.Q.; Jiang, X.M.; et al. Effects of silymarin supplementation during transition and lactation on reproductive performance, milk composition and haematological parameters in sows. J. Anim. Physiol. An. N. 2020, 104, 1896–1903. [Google Scholar] [CrossRef]
- Kumar, N.; Pruthi, V. Potential applications of ferulic acid from natural sources. Biotechnol. Rep. 2014, 4, 86–93. [Google Scholar] [CrossRef]
- Li, D.; Rui, Y.X.; Guo, S.D.; Luan, F.; Liu, R.; Zeng, N. Ferulic acid: A review of its pharmacology, pharmacokinetics and derivatives. Life Sci. 2021, 284, 119921. [Google Scholar] [CrossRef]
- Hussain, I.; Khan, A.U.; Ullah, R.; Alsaid, M.S.; Salman, S.; Iftikhar, S.; Marwat, G.A.; Afridi, M.S.; Jan, S.; Adnan, M.; et al. Chemical composition, antioxidant and antibacterial potential of essential oil of medicinal plant Isodon rugosus. J. Essent. Oil Bear. Plants 2018, 20, 1607–1613. [Google Scholar] [CrossRef]
- Tiwari, R.; Latheef, S.K.; Ahmed, I.; Iqbal, H.M.N.; Bule, M.H.; Dhama, K.; Samad, H.A.; Karthik, K.; Alagawany, M.; El-Hack, M.E.A.; et al. Herbal immunomodulators—A remedial panacea for designing and developing effective drugs and medicines: Current scenario and future prospects. Curr. Drug Metab. 2018, 19, 264–301. [Google Scholar] [CrossRef] [PubMed]
- Zou, W.J.; Huang, H.L.; Wu, H.D.; Cao, Y.D.; Lu, W.; He, Y.Y. Preparation, antibacterial potential and antibacterial components of fermented compound Chinese medicine feed additives. Front. Vet. Sci. 2022, 9, 808846. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.J.; Zhang, A.H.; Sun, H. Future perspectives of Chinese medical formulae: Chinmedomics as an effector. OMICS 2012, 16, 414–421. [Google Scholar] [CrossRef]
- Zhang, A.H.; Yu, J.B.; Sun, H.; Kong, L.; Wang, X.Q.; Zhang, Q.Y.; Wang, X.J. Identifying quality-markers from Shengmai San protects against transgenic mouse model of Alzheimer’s disease using chinmedomics approach. Phytomedicine 2018, 45, 84–92. [Google Scholar] [CrossRef]
- Dunn, W.B.; Broadhurst, D.; Begley, P.; Zelena, E.; Francis-McIntyre, S.; Anderson, N.; Brown, M.; Knowles, J.D.; Halsall, A.; Haselden, J.N.; et al. The Human Serum Metabolome Consortium. Procedures for large-scale metabolic profiling of serum and plasma using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat. Protoc. 2011, 6, 1060–1083. [Google Scholar] [CrossRef] [PubMed]
- Kouadio, J.H.; Mobio, T.A.; Baudrimont, I.; Moukha, S.; Dano, S.D.; Creppy, E.E. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2. Toxicology 2005, 213, 56–65. [Google Scholar] [CrossRef]
- Diesing, A.K.; Nossol, C.; Panther, P.; Walk, N.; Post, A.; Kluess, J.; Kreutzmann, K.; Dänickec, S.; Rothkötter, H.J.; Kahlert, S. Mycotoxin deoxynivalenol (DON) mediates biphasic cellular response in intestinal porcine epithelial cell lines IPEC-1 and IPEC-J2. Toxicol. Lett. 2011, 200, 8–18. [Google Scholar] [CrossRef]
- Ventura, A.K.; Beauchamp, G.K.; Mennella, J.A. Infant regulation of intake: The effect of free glutamate content in infant formulas. Am. J. Clin. Nutr. 2012, 95, 875–881. [Google Scholar] [CrossRef]
- Tochitani, S. Functions of maternally-derived taurine in fetal and neonatal brain development. Adv. Exp. Med. Biol. 2017, 975, 17–25. [Google Scholar]
- Hosseini, A.; Razavi, B.M.; Banach, M.; Hosseinzadeh, H. Quercetin and metabolic syndrome: A review. Phytother. Res. 2021, 35, 5352–5364. [Google Scholar] [CrossRef] [PubMed]
- Soromou, L.W.; Zhang, Y.; Cui, Y.; Wei, M.; Chen, N.; Yang, X.; Huo, M.; Baldé, A.; Guan, S.; Deng, X. Subinhibitory concentrations of pinocembrin exert anti-Staphylococcus aureus activity by reducing alpha-toxin expression. J. Appl. Microbiol. 2013, 115, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Moulai-Hacene, F.; Boufadi, M.Y.; Keddari, S.; Homrani, A. Chemical composition and antimicrobial properties of elettaria cardamomum extract. Pharmacogn. J. 2020, 12, 1058–1063. [Google Scholar] [CrossRef]
- Peng, F.; Wan, F.; Xiong, L.; Peng, C.; Dai, M.; Chen, J.P. In vitro and in vivo antibacterial activity of pogostone. Chin. Med. J. 2014, 127, 4001–4005. [Google Scholar]
- Udono, H.; Kumanogoh, A. Introduction: Special issue-immunometabolism. Int. Immunol. 2020, 32, 433–434. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J.; Simões, M. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef]
- Kartal, M.; Kurucu, S.; Choudary, M.I. Antifungal activities of different extracts and echimidine-N-oxide from Symphytum sylvaticum Boiss. subsp. sepulcrale (Boiss. & Bal.) Greuter & Burdet var. sepulcrale. Turk. J. Med. Sci. 2001, 31, 487–492. [Google Scholar]
- Stevens, C.; Millar, T.; Clinch, J.; Kanczler, J.; Bodamyali, T.; Blake, D. Antibacterial properties of xanthine oxidase in human milk. The Lancet 2000, 356, 829–830. [Google Scholar] [CrossRef]
- Batey, R.T. Structure and mechanism of purine-binding riboswitches. Q. Rev. Biophys. 2012, 45, 345–381. [Google Scholar] [CrossRef]
- Yan, L.H.; Le Roux, A.; Boyapelly, K.; Lamontagne, A.M.; Archambault, M.A.; Picard-Jean, F.; Lalonde-Seguin, D.; St-Pierre, E.; Najmanovich, R.J.; Fortier, L.C.; et al. Purine analogs targeting the guanine riboswitch as potential antibiotics against Clostridioides difficile. Eur. J. Med. Chem. 2018, 143, 755–768. [Google Scholar] [CrossRef]
- Xiao, Y.; Louwies, T.; Smith-Edwards, K.; Beyder, A.; Linden, D.; Farrugia, G.; Kashyap, P. Bacteria-derived hypoxanthine accelerates gastrointestinal transimit. FASEB J. 2022, 36, 1–3. [Google Scholar] [CrossRef]
- Sandle, G.I. Infective and inflammatory diarrhoea: Mechanisms and opportunities for novel therapies. Curr. Opin. Pharmacol. 2011, 11, 634–639. [Google Scholar] [CrossRef] [PubMed]
- Ur Rasool, J.; Sawhney, G.; Shaikh, M.; Nalli, Y.; Madishetti, S.; Ahmed, Z.; Ali, A. Site selective synthesis and anti-inflammatory evaluation of Spiro-isoxazoline stitched adducts of arteannuin B. Bioorg. Chem. 2021, 117, 105408. [Google Scholar] [CrossRef] [PubMed]
- Fila, M.; Chojnacki, J.; Pawlowska, E.; Szczepanska, J.; Chojnacki, C.; Blasiak, J. Kynurenine pathway of tryptophan metabolism in migraine and functional gastrointestinal disorders. Int. J. Mol. Sci. 2021, 22, 10134. [Google Scholar] [CrossRef]
- Szulc-Kielbik, I.; Kielbik, M.; Klink, M. Ferulic acid but not alpha-lipoic acid effectively protects THP-1-derived macrophages from oxidant and pro-inflammatory response to LPS. Immunopharm. Immunot. 2017, 39, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Wang, R.X.; Goldberg, M.S.; Clifford, G.P.; Kao, D.J.; Colgan, S.P. Microbiota-sourced purines support wound healing and mucous barrier function. iScience 2020, 23, 101226. [Google Scholar] [CrossRef] [PubMed]
- Li, D.T.; Feng, Y.; Tian, M.L.; Ji, J.F.; Hu, X.S.; Chen, F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARγ signaling activation. Microbiome 2021, 9, 83. [Google Scholar] [CrossRef]
- Mager, L.F.; Burkhard, R.; Pett, N.; Cooke, N.C.A.; Brown, K.; Ramay, H.; Paik, S.; John Stagg, J.; Groves, R.A.; Gallo, M.; et al. Microbiome-derived inosine modulates response to checkpoint inhibitor immunotherapy. Science 2020, 369, 1481–1489. [Google Scholar] [CrossRef]
- Bellaver, B.; Souza, D.G.; Bobermin, L.D.; Goncalves, C.A.; Souza, D.O.; Quincozes-Santos, A. Guanosine inhibits LPS-induced pro-inflammatory response and oxidative stress in hippocampal astrocytes through the heme oxygenase-1 pathway. Purinerg. Signal. 2015, 11, 571–580. [Google Scholar] [CrossRef]
- Hou, R.C.W.; Chen, Y.S.; Chen, C.H.; Chen, Y.H.; Jeng, K.C.G. Protective effect of 1,2,4-benzenetriol on LPS-induced NO production by BV2 microglial cells. J. Biomed. Sci. 2006, 13, 89–99. [Google Scholar] [CrossRef]
- Haskó, G.; Kuhel, D.G.; Nemeth, Z.H.; Mabley, J.G.; Stachlewitz, R.F.; Virag, L.; Lohinai, Z.; Southan, G.J.; Salzman, A.L.; Szabo, C. Inosine inhibits inflammatory cytokine production by a posttranscriptional mechanism and protects against endotoxin-induced shock. J. Immunol. 2000, 164, 1013–1019. [Google Scholar] [CrossRef]
- Nascimento, F.P.; Macedo-Júnior, S.J.; Lapa-Costa, F.R.; Cezar-dos-Santos, F.; Santos, A.R.S. Inosine as a tool to understand and treat central nervous system disorders: A neglected actor? Front. Neurosci. 2021, 15, 703783. [Google Scholar] [CrossRef] [PubMed]
- Haskó, G.; Sitkovsky, M.V.; Szabó, C. Immunomodulatory and neuroprotective effects of inosine. Trends in Pharmacol. Sci. 2004, 25, 152–157. [Google Scholar] [CrossRef]
- De Bruyn, F.; Van Brempt, M.; Maertens, J.; Van Bellegem, W.; Duchi, D.; De Mey, M. Metabolic engineering of Escherichia coli into a versatile glycosylation platform: Production of bio-active quercetin glycosides. Microbial Cell Fact. 2015, 14, 138. [Google Scholar] [CrossRef] [PubMed]
- Marín, L.; Gutiérrez-del-Río, I.; Entrialgo-Cadierno, R.; Villar, C.J.; Lombó, F. Denovo biosynthesis of myricetin, kaempferol and quercetin in Streptomyces albus and Streptomyces coelicolor. PLoS ONE 2018, 13, e0207278. [Google Scholar] [CrossRef]
- Choi, P.; Rhayat, L.; Pinloche, E.; Devillard, E.; De Paepe, E.; Vanhaecke, L.; Haesebrouck, F.; Ducatelle, R.; Immerseel, F.V.; Goossens, E. Bacillus subtilis 29784 as a feed additive for broilers shifts the intestinal microbial composition and supports the production of hypoxanthine and nicotinic acid. Animals 2021, 11, 1335. [Google Scholar] [CrossRef] [PubMed]
- Varadaiah, Y.G.C.; Sivanesan, S.; Nayak, S.B.; Thirumalarao, K.R. Purine metabolites can indicate diabetes progression. Arch. Physiol. Biochem. 2019, 3, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Vicente, R.L.; Spina, L.; Gómez, J.P.L.; Dejean, S.; Franois, J.M. Trehalose-6-phosphate promotes fermentation and glucose repression in Saccharomyces cerevisiae. Microb. Cell 2018, 5, 444–459. [Google Scholar] [CrossRef]
- Hu, K.; Liao, X.X.; Wu, X.Y.; Wang, R.; Hu, Z.W.; Liu, S.Y.; He, W.F.; Zhou, J.J. Effects of the lipid metabolites and the gut microbiota in ApoE−/− mice on atherosclerosis co-depression from the microbiota-gut-brain axis. Front. Mol. Biosci. 2022, 9, 786492. [Google Scholar] [CrossRef]
- Li, X.; Xing, B.; Liu, X.; Jiang, X.W.; Lu, H.Y.; Xu, Z.H.; Yang, Y.; Wu, Q.; Yao, D.; Zhang, Y.S.; et al. Network pharmacology-based research uncovers cold resistance and thermogenesis mechanism of Cinnamomum cassia. Fitoterapia 2021, 149, 104824. [Google Scholar]
- Contreras, C.; Nogueiras, R.; Diéguez, C.; Medina-Gómez, G.; López, M. Hypothalamus and thermogenesis: Heating the BAT, browning the WAT. Mol. Cell Endocrinol. 2016, 438, 107–115. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).