1. Introduction
Currently, most energy comes from non-renewable fossil fuels, driving climate change [
1]. The EU targets a 90% reduction in greenhouse gas emissions by 2040 and climate neutrality by 2050 [
2]. Hydrogen (H
2), with zero emissions and high energy density, is a promising alternative to fossil fuels [
3]. H
2 can find applications across sectors like transportation, electricity, and industry [
4]. However, fossil-fuel-based H
2 production releases ~830 million tons of CO
2 annually [
5].
In this scenario, microbiological H
2 production (bio-H
2) using agro-industrial waste offers a sustainable alternative, by producing H
2 independently of fossil fuels and converting waste into valuable resources, in line with circular economy principles [
6,
7]. Bio-H
2 can be produced via bio-photolysis, dark fermentation, and photofermentation using microorganisms such as microalgae, non-photosynthetic bacteria, and photosynthetic bacteria (PSB) [
8].
Integrating dark fermentation and photofermentation optimizes bio-H
2 production, showing potential for future industrial applications [
9,
10]. Dark fermentation, driven by anaerobic bacteria, generates bio-H
2, organic acids, volatile fatty acids (VFAs), and CO
2 without light [
10]. The organic acids serve as substrates for photofermentation by purple non-sulfur bacteria (PNSB), which generate bio-H
2 via nitrogenase enzyme under nitrogen-limited conditions [
11,
12]. During photofermentation, PNSB grow photoheterotrophically under anaerobic conditions, using organic acids as carbon and electron sources while harnessing light as their energy source [
12].
Agro-industrial wastewater is often rich in ammoniacal nitrogen, which inhibits nitrogenase activity and thus hampers bio-H
2 production [
13]. Substrate dilution is a common strategy to mitigate this issue, although this approach may increase substrate volumes and process costs [
14,
15].
Rhodopseudomonas palustris is a highly versatile PNSB for bio-H
2 production, as it efficiently utilizes various organic substrates, including industrial waste [
16,
17]. Its versatility is also linked to three nitrogenase isoforms (molybdenum, vanadium, and iron), each varying based on the metal in the enzyme’s active site, allowing for bio-H
2 production under different environmental conditions [
18,
19].
PNSB can produce and accumulate poly-β-hydroxybutyrate (PHB), a biodegradable polymer from the polyhydroxyalkanoates (PHA) family, suitable for bioplastics production [
9]. PHB acts as an energy reserve and stress protector, synthesized under high carbon-nitrogen (C/N) ratios and nutrient-limited conditions (e.g., sulfate, phosphate, magnesium) [
20]. While PHB biosynthesis competes with bio-H
2 production as a reductive pathway, both can be simultaneously generated [
21].
Dairy production plays a crucial role in human nutrition but generates millions of tons of waste per year, including cheese whey (CW) and second cheese whey (SCW), both with high environmental impact if not correctly managed [
22,
23,
24]. SCW, produced by heating CW during ricotta cheese production, is rich in lactose (35–50 g L
−1) and has high COD (50 g L
−1) and BOD (80 g L
−1) levels [
24]. In Italy, 15% of CW is used to obtain ricotta cheese, producing about 1 million tons of SCW per year. Though often reused as animal feed, SCW contains valuable compounds like proteins, peptides, and lactose, making it an excellent resource for biotechnological valorization [
24,
25]. Integrating dark fermentation and photofermentation, with
R. palustris, offers a sustainable method to treat SCW, reducing COD and producing bio-H
2 and/or PHB as valuable by-products.
Lactic acid bacteria (LAB) are widely used in the fermentation of dairy products due to their ability to metabolize carbohydrates into organic acids, exopolysaccharides, and other compounds, depending on the type of fermentation they perform (homo- or heterofermentative). LAB can be used to convert residual lactose present in SCW into lactate, which is the preferred carbon source for
R. palustris in the photobiological production of bio-H
2 [
26,
27].
The efficiency of bio-H
2 and PHB production in PNSB depends on the quality of light, as bacteriochlorophylls (BChls) absorb light at 590 nm and in the near-infrared range (800–880 nm) [
28]. Studies show that LEDs matched to BChl absorption significantly enhance bio-H
2 production [
29]. For instance,
R. palustris achieved higher H
2 yields under 590 nm light compared to other wavelengths [
30], while filtering light above 760 nm reduced H
2 production in
Rhodobacter sphaeroides [
31].
This study evaluates the potential for producing Bio-H2 and PHB from SCW using R. palustris strain 42OL in a two-stage process within a 5 L bioreactor. The first step involves lactic fermentation to enrich the substrate with lactic acid, followed by photofermentation under two types of LED lighting: white LEDs (WL) and selected LEDs (SL) emitting in the yellow (593 nm) and infrared (860 nm) ranges. The aim is to assess the feasibility of SCW valorization while analyzing the effects of light on R. palustris growth, BChl production, bio-H2 generation, and PHB synthesis.
2. Materials and Methods
2.1. Bacterial Strains
For the dark fermentation tests, a microbial consortium consisting of two different species of lactic acid bacteria (LAB) was used:
Lactococcus lactis MK L84 and
Lacticaseibacillus paracasei MK L49, both belonging to the collection of Department of Agriculture, Food, Environment and Forestry (DAGRI) of the University of Florence (Italy) and previously isolated from spontaneous raw milk fermentations [
32]. The selected LAB strains were chosen for their proven fermentative efficiency, robustness, and ability to produce lactic acid from complex substrates, as reported by Galli et al. [
32].
For the photofermentation tests,
Rhodopseudomonas palustris 42OL was employed, a microorganism from the DAGRI collection isolated in 1973 from a waste treatment pond of a sugar refinery. This strain was selected due to its adaptability to different types of substrates and because it has been reported in the literature to achieve high bio-H
2 production yields [
33]. The draft genome sequence of
R. palustris 42OL is available in the NCBI database under BioProject accession number PRJNA283573 (assembly accession GCA_001020905.1).
2.2. Cultivation Media
Before being used in the dark fermentation tests, the lactic acid bacteria (LAB) were cultured for 48 h in M17 broth (Oxoid, Basingstoke, UK) with the following composition: Tryptone 5.0 g L
−1; Soy Peptone 5.0 g L
−1; Yeast Extract 2.5 g L
−1; Meat Extract 5.0 g L
−1; Sodium Glycerophosphate 19.0 g L
−1; Magnesium Sulphate 0.25 g L
−1; Ascorbic Acid 0.5 g L
−1; Lactose 5.0 g L
−1; deionized water. The pH of the medium was 6.69 ± 0.2 at 25 °C. The strains were maintained at 30 °C under constant agitation (80 rpm). Stationary phase cells were harvested, centrifuged at 3700 rpm for 15 min (Sigma Laborzentrifugen GmbH, Osterode am Harz, Germany), and resuspended in the SCW for tests. The dark fermentation process of SCW will be explored in detail in
Section 2.4.
The culture of
R. palustris 42OL was maintained at 30 °C, under a light intensity of 150 µmol (photons) m
−2 s
−1, in an RPN medium, containing DL-malic acid as the primary carbon source [
34]. The medium had the following composition: DL-malic acid 2.0 g L
−1; NH
4Cl 0.5 g L
−1; K
2HPO
4 0.5 g L
−1; KH
2PO
4 0.3 g L
−1; MgSO
4·7H
2O 0.4 g L
−1; NaCl 0.4 g L
−1; CaCl
2·2H
2O 0.075 g L
−1; Ferric citrate 0.005 g L
−1; Yeast Extract 0.4 g L
−1. Trace elements were provided by adding 10 mL per liter of a solution containing: ZnSO4·7H
2O 10 mg L
−1; MnCl
2·4H
2O 3 mg L
−1; H
3BO
3 30 mg L
−1; CoCl
2·6H
2O 20 mg L
−1; CuCl
2·2H
2O 1 mg L
−1; NiCl
2·6H
2O 2 mg L
−1; Na
2MoO
4·2H
2O 30 mg L
−1. The pH of the medium was adjusted to 6.8 with NaOH before autoclaving.
For bio-H
2 production tests,
R. palustris 42OL was activated in RPP medium for 10 days, as described by Bianchi et al. [
34]. The strain was maintained at 30 °C, under a light intensity of 150 µmol (photons) m
−2 s
−1. The medium had the following composition: DL-lactic acid 3.6 g L
−1; Na glutamate 1 g L
−1; K
2HPO
4 0.5 g L
−1; KH
2PO
4 0.3 g L
−1; MgSO
4·7H
2O 0.4 g L
−1; NaCl 0.4 g L
−1; CaCl
2·2H
2O 0.075 g L
−1; Ferric citrate 0.005 g L
−1. The trace elements were added as reported above. To promote microbial growth, 1 mL per liter of medium of a solution containing 20 mg per 100 mL of p-aminobenzoic acid (PABA) was added after autoclave. The pH of the medium was adjusted to 6.8 with NaOH before autoclaving. Activated cells were then centrifuged for 20 min at 5000 rpm (Beckman Coulter, Brea, CA, USA) and resuspended in the SCW for the photofermentation tests.
2.3. Bioreactor Design
The photofermentation test was set up in a tubular photobioreactor designed by Biosyntex S.r.l. (Bologna, BO, Italy) and constructed by Tecnocom S.r.l. (Prato, PO, Italy). The layout of the bioreactor used in this study is reported in
Figure 1. The system consists of a skid of dimensions 515 mm × 400 mm, comprising: (1) a thermostated DURAN glass tube, measuring H 700 mm × D 140 mm and equipped with an internal chamber of D 110 mm; (2) a pH control instrument (B&C Electronics S.r.l., Carnate, MB, Italy); (3) a temperature control instrument (Gefran S.p.a., Provaglio d’Iseo, BS, Italy); (4) a mechanical stirrer model AM20-D 50 (Argolab, Carpi, MO, Italy) with manual speed control and a polypropylene (PP) rod (H 800 mm × D 8 mm); (5) two 150 W dimmable LED lamps, measuring 600 mm × 70 mm × 40 mm (Ambra Elettronica S.r.l., Bolzano Vicentino, VI, Italy); (6) an ON/OFF electric valve for thermostatic control; and (7) an electrical control and command panel.
The bioreactor makes it possible to replace white LED lamps (WL) with selected LED lamps (SL). The WL emitted light across different wavelengths with the following distribution: 19.66% in the blue region (λ = 450 nm), 15.20% in the green region (λ = 520 nm), 64.19% in the red region (λ = 660 nm), and 0.96% in the far-red region (λ = 730 nm). The SL emitted in the yellow region (λ = 593 nm) and the infrared region (λ = 860 nm), with distributions of 80% and 20%, respectively.
2.4. Dark Fermentation of SCW
This study used a SCW obtained from ricotta cheese production. The effluent was provided by the cheese factory “I Formaggi del Dottore”, located in Castelfiorentino (FI), Italy. The effluent was stored at −20 °C until use. The SCW was initially analyzed for its macro- and microelement content, as well as its ammonium, lactose, and lactic acid content. Further details on the analytical methods employed will be provided in
Section 2.6.
The SCW was initially tested to evaluate potential lactic acid production through self-fermentation, using its indigenous microflora. The tests were conducted in triplicate. Briefly, 40 mL of SCW, diluted at 25%
v/
v with non-sterile deionized water, were incubated at 30 °C for 96 h under constant agitation at 80 rpm in tightly sealed 50 mL Falcon
® tubes. Lactic acid production was quantified at the end of the experiment using High-Performance Liquid Chromatography (HPLC) analysis (see
Section 2.6)
To test the SCW as a substrate for lactic acid production, a co-inoculation was performed using the LAB microbial consortium described in
Section 2.1. The inocula of the two LAB species were prepared as outlined in
Section 2.2 and co-resuspended in 40 mL of defrosted SCW, diluted at 25%
v/
v with non-sterile deionized water, to a final concentration of 1 × 10
8 CFU mL
−1 for each strain. The test was conducted in triplicate, using tightly sealed 50 mL Falcon
® tubes, and incubated under the same conditions described above. Lactic acid production was quantified at the end of the experiment through HPLC analysis (see
Section 2.6).
After the preliminary tests in tightly sealed 50 mL Falcon® tubes, the dark fermentation experiments were continued inside the 5-litre bioreactor, as described below.
The SCW was diluted at 25% v/v with non-sterile deionized water. During the dilution, 10 mL L−1 of a phosphate-buffered solution was added, with the following composition: K2HPO4 50 g L−1; KH2PO4 30 g L−1. The LAB strains were co-inoculated into the effluent at a final concentration of 1 × 108 CFU mL−1 each.
Dark fermentation was conducted at 30 °C, with continuous stirring at 60 rpm, and lasted for 96 h. Three different pH conditions were evaluated to identify the optimal conditions for achieving the highest lactic acid yields: without pH control, pH maintained between 3.5 and 4.5, and pH maintained between 4.5 and 5.5. The pH was corrected using NaOH 4 M. The experiments were performed in duplicate for each pH condition. The lactic acid content was monitored every 24 h.
2.5. Photofermentation Tests
For the photofermentation trials conducted with
R. palustris 42OL, the dark-fermented SCW obtained with controlled pH (maintained between 3.5 and 4.5, and between 4.5 and 5.5) was combined, homogenized, and used as a culture medium. The effluent was further diluted with non-sterile deionized water at a final concentration of 18%
v/
v (compared to undiluted SCW) and supplemented with 10 mL L
−1 of the following solutions: phosphate buffer (K
2HPO
4, 50 g L
−1; KH
2PO
4, 30 g L
−1), ferric citrate (0.5 g L
−1), and MgSO
4·7H
2O (40 g L
−1), as described by Adessi et al. [
26]. The culture medium was autoclaved twice at 121 °C for 15 min, with a 48 h interval between the two cycles, to ensure the complete elimination of LAB. The pH was adjusted to 6.8 with 2 M NaOH before and after each autoclaving. Between the two autoclaving cycles, the culture medium was centrifuged under sterile conditions at 5000 rpm for 15 min (Beckman Coulter, Brea, CA, USA). This was followed by sterile filtration through filter paper under a horizontal laminar flow hood.
R. palustris 42OL, previously grown in RPP medium (described in
Section 2.2) was inoculated into the culture medium, in a final volume of 5.2 L. The initial cell concentration was equal to OD
660nm = 0.4 for each test.
Two distinct lighting conditions were tested for the culture: white LED lights (hereafter referred to as WL) and selected LED lights (hereafter referred to as SL). Detailed information about the lights used in these tests is provided in
Section 2.3.
The experiments were conducted in duplicate for each type of light, with a total duration of 336 h for each test. The culture was initially exposed to a light intensity of 150 µmol photons m−2 s−1 for the first 24 h to allow the microorganism to adapt to the bioreactor environment. This intensity was then increased to 300 µmol photons m−2 s−1 until 168 h after the start of the experiment and finally increased to 400 µmol photons m−2 s−1 for the remainder of the experiment. The adjustment in light intensity was necessary to ensure optimal photosynthetic activity. Indeed, the growth of the microorganism, with the consequent increase in the medium’s turbidity, limited light penetration in the bioreactor.
Bacterial growth was assessed by measuring bacteriochlorophyll
a (BChl
a), optical density at 660 nm (OD
660nm) every 48 h, and cellular dry weight (CDW) at 0, 168, and 336 h from the start of the experiment. Lactic acid consumption was measured every 48 h from the start of the experiment, while ammonium (NH
4+) concentration was determined at 0, 168, and 336 h from the beginning of the experiment. PHB production was determined at the end of the experiment (336 h). Further details on the analytical methods used are provided in
Section 2.6.
The bio-H
2 gas produced was collected on a calibrated column and measured by the water displacement method [
26]. The calibrated column was submerged in a CO
2-absorber solution (1.0 M NaOH, 3.4 M NaCl), as reported by Touloupakis et al. [
35].
2.6. Analytical Methods
The lactic acid concentration was determined by High-Performance Liquid Chromatography (HPLC) analysis (Varian Inc., Palo Alto, CA, USA). Before injection, each sample was centrifuged at 14,000 rpm for 10 min to ensure the complete separation of solid particles from the supernatant. Separation was obtained with a Rezex ROA organic acid H+ column (300 mm × 7.8 mm; Phenomenex, Castel Maggiore, Bologna, Italy), connected to a refractive index detector (Knauer K-2301, GmbH, Berlin, Germany) and UV detector (λ = 210). Elution was performed at 65 °C with 0.013 N H2SO4 eluent at a flow rate of 0.6 mL/min. Data were collected and analyzed by using the Galaxie software version 1.8.4.1 (Varian Inc., Palo Alto, CA, USA). Quantitative analysis was carried out by standard curve designed for lactic acid.
The concentration of metals and other inorganic compounds was determined through an inductively coupled plasma-optical emission spectrometry ICP-OES analyzer (Thermo Scientific™ iCAP™ 7400, Waltham, MA, USA), using IRSA 3010 (conventional acid mineralization) and IRSA A-3020 (determination of chemical elements by spectroscopy emission with plasma source) methods.
CDW was determined in triplicate on a 3 mL culture sample, through a filtration with 0.45 μm Mixed Cellulose Esters (MCE) membranes (Fisher Scientific International, Inc., Pittsburgh, PA, USA). Membranes were previously activated with 5 mL of distilled water. After filtration the cells were washed twice with distilled water, oven-dried at 105 °C for 3 h, and weighed on an analytical balance (Sartorius AG, Göttingen, Germany).
BChl
a content was determined according to Carlozzi and Sacchi [
36]. Briefly, a 2 mL sample was centrifugated at 7000 rpm for 10 min with a Hermle Z 167 M centrifuge (Hermle Labortechnik GmbH, Wehingen, Germany), and the supernatant was removed. BChl
a was extracted from the cell pellet, resuspending it with a 2 mL acetone/methanol 7:2
v/
v ratio solution. The sample was incubated at 4 °C for 30 min and centrifugated at 7000 rpm for 10 min. BChl
a content was determined by measuring the absorbance (A) at 775 nm (ɛ = 75 mM
−1 cm
−1) with a Varian Cary50 UV-visible spectrophotometer (Varian, Mulgrave, Australia).
The optical density was determined in triplicate on 2 mL samples at a wavelength of 660 nm (OD660nm) using a Varian Cary50 UV-visible spectrophotometer (Varian, Mulgrave, Australia). In the spectrophotometric measurement, the DF-SCW was used as the blank.
The cellular concentration of lactic acid bacteria (LAB) for determining the inoculum in dairy wastewater was measured using cell counting with a Neubauer improved counting chamber (Paul Marienfeld GmbH & Co. KG, Lauda-Königshofen, Germany) with a depth of 0.01 mm.
The ammonium concentration was determined using the HI93764B-25 Ammonia HR reagents set (Hanna Instruments, Woonsocket, RI, USA), based on the Nessler method, and quantified spectrophotometrically using a Varian Cary50 UV-visible spectrophotometer (Varian, Mulgrave, Australia), as reported by Rice et al. [
37].
The intensity of the light reaching the culture surface was measured with a quantum/radiometer/photometer model DO9721 equipped with a quantum sensor model LP9021 (Delta Ohm, Padua, Italy).
Bio-H
2 was collected and quantified volumetrically using the water displacement method, as described in
Section 2.5, and subsequently analyzed by gas chromatography, as reported by Adessi et al. [
38].
Poly-β-hydroxybutyrate (PHB) production was determined at the end of the experiment (336 h), according to the method described by De Philippis et al. [
39].
2.7. Calculation and Statistical Analysis
The substrate conversion efficiency (SCE) was determined according to Equation (1) [
28], as the percentage ratio between the moles of hydrogen produced and the theoretical moles of hydrogen obtainable from the complete conversion of the substrate into hydrogen (Equation (2)) [
26].
The moles of hydrogen produced were calculated based on the hydrogen collected, applying the ideal gas law under ambient conditions (T = 25 °C, P = 1 atm).
The efficiency of light energy conversion to hydrogen (LCE) during the hydrogen production period was calculated according to Equation (3), as Adessi et al. [
26] reported, assuming that the culture absorbed all the incident light.
All analyses were conducted in experimental triplicates (N = 3); a minimum number of three instrumental replicates was always used for each measurement (n = 3). To determine whether the results were significantly different, the data were analyzed using one-way analysis of variance (ANOVA) at the 95% level of significance, followed by Tukey’s post hoc test of honest difference in significance (HSD). The results were considered statistically significant at p ≤ 0.05. Before the ANOVA, all data were checked for assumptions of normality using the D’Agostino-Pearson normality test and for homogeneity of variance using Bartlett’s test. To check for statistical differences between the mean values of the two lighting conditions, independent t-tests were performed. All analyses were conducted using R software version 4.4.1.
4. Discussion
The dairy industry produces organic-rich wastewater that, if discharged untreated into water bodies, causes acidification and eutrophication, harming ecosystems. Using PNSB to convert this wastewater into bio-H2 and PHB offers a solution, reducing organic load while generating valuable compounds.
The analysis of the raw composition of SCW used in this study revealed a high NH
4+ concentration (504.53 mg L
−1 ± 7.47). The literature widely documents that an excess of ammoniacal nitrogen inhibits bio-H
2 production by PNSB, as it interferes with the regulatory mechanisms of the nitrogenase enzyme [
40,
41]. For this reason, the effluent was initially diluted at 25%
v/
v with non-sterile deionized water to significantly reduce ammonium content while retaining enough lactose for the subsequent dark fermentation step. Although dilution increases substrate volume and management costs, it enhances light penetration in the photofermentation phase, thus shortening the bio-H
2 accumulation lag phase [
14,
15].
Lactate is the preferred carbon source for
R. palustris 42OL to maximize bio-H
2 yield [
42]. The lactate content in the raw SCW (0.31 g L
−1 ± 0.08) was approximately one order of magnitude lower than in the synthetic RPP medium (3.6 g L
−1). The subsequent dilution of the effluent further reduced the lactose concentration, necessitating a dark fermentation step to achieve lactate enrichment.
To select the best conditions to produce lactate with a minimum number of treatments, we initially considered using the effluent’s indigenous microflora to ferment lactose into lactate. However, trials yielded only a minimal increase in lactate, reaching a final concentration of 0.74 g L
−1 (±0.07), still substantially below the level in the RPP synthetic medium. This limited lactic acid production was probably due to the freezing process applied to the effluent, which may have altered and reduced the indigenous microflora. Therefore, we investigated the effect of a LAB co-inoculum, which resulted in a significantly higher lactic acid yield of 2.06 g L
−1 (±0.09), nearly tripling the yield obtained by the indigenous microflora. However, this lactate concentration was still insufficient for photofermentation, remaining below that of the synthetic RPP medium. At the end of the LAB co-inoculation tests, the pH reached 3.37 (±0.11), likely limiting lactate yield, as its production is minimal in acidic conditions [
43]. Therefore, our focus shifted towards identifying the optimal substrate pH to maximize lactate production by LAB co-inoculum in a 5 L photobioreactor. To prevent the pH from dropping too quickly and complicating manual control, a phosphate buffer was added to the substrate (see
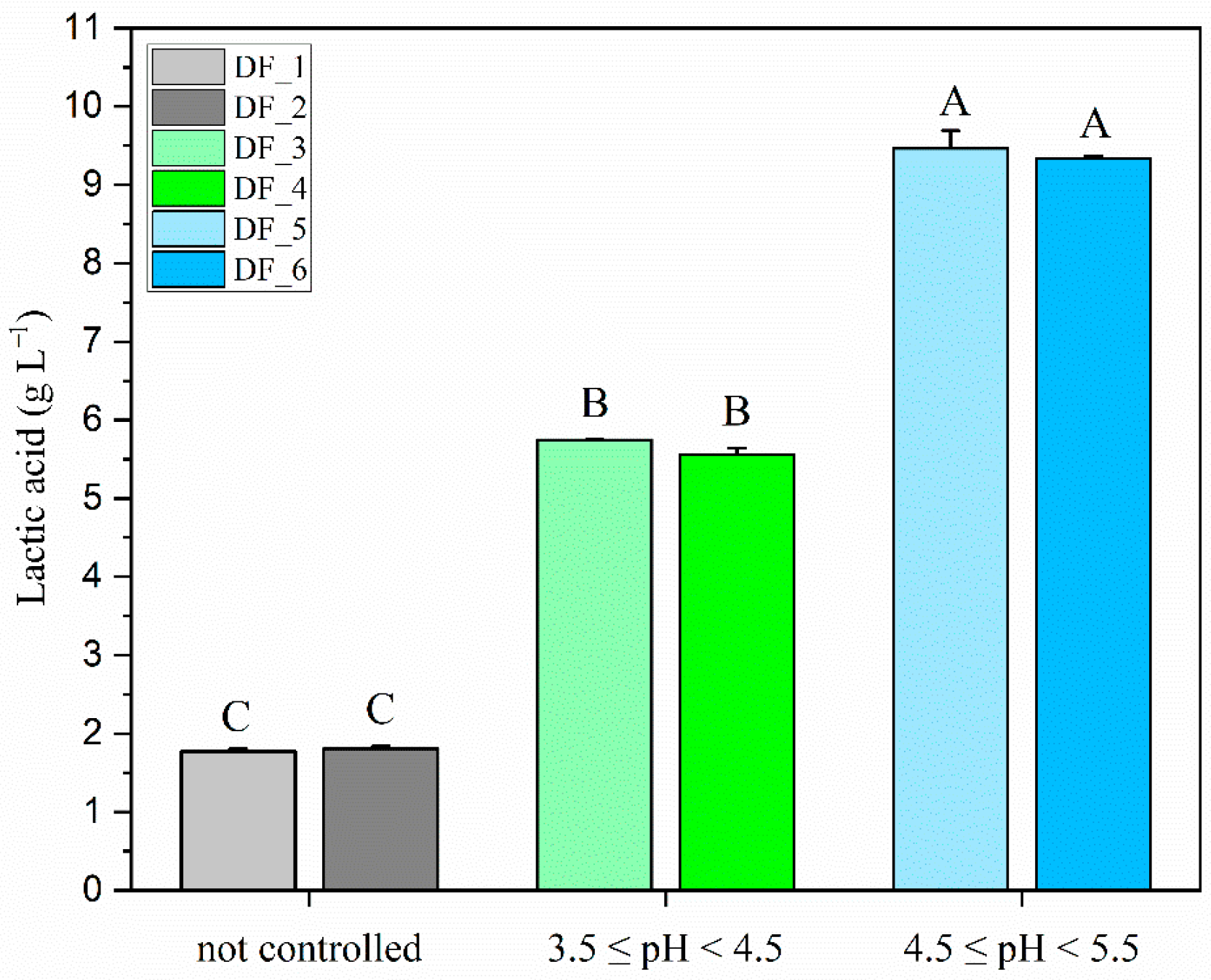
Section 2.4). The highest yields were achieved at pH between 4.5 and 5.5 (
Figure 2), with an average lactate concentration of 9.40 g L
−1 (±0.09), which was 1.7 and 5.2 times higher than the results obtained at pH levels between 3.5 and 4.5 and with uncontrolled pH, respectively. This result is supported by studies showing that the optimal pH for lactic acid production lies between 5 and 6, where LAB activity peaks, enabling efficient fermentation [
43,
44,
45]. Moradi et al. [
46] produced 14.2 g L
−1 lactic acid using
Lactobacillus delbrueckii subsp.
lactis (PTCC: 1743) on a substrate consisting of DW and glucose, with a fermentation time of 41.41 h at 37 °C and an enzymatic hydrolysis step. Our co-inoculum produced approximately 34% less lactic acid, but with significant advantages that simplify the process and reduce overall costs: reduced production time, a lower temperature (30 °C), and the absence of expensive carbon sources or additional enzymatic treatments.
Prior to each bio-H
2 production experiment, the photofermentation substrate was supplemented, as nutrient levels were insufficient due to prior dilution. For this purpose, iron citrate and magnesium sulfate were added, based on Adessi et al. [
26], who showed these nutrients enhance bio-H
2 production by
R. palustris 42OL grown on fermented bread effluent. Iron and sulfate are essential for nitrogenase synthesis [
38], while magnesium is involved in BChls synthesis [
47].
Growth, bio-H2, and PHB production in R. palustris 42OL were then evaluated on the dark-fermented SCW, comparing white light (WL) LEDs with specific wavelength LEDs (SL, 593 nm, and 860 nm) matching the absorption peaks of BChls in R. palustris 42OL. This approach clarified how specific wavelengths enhance photofermentative efficiency and high-value molecule yields.
Light intensity was gradually raised throughout the experiment to maintain adequate light penetration as cell density increased. Initially, it was set to 150 µmol photons m−2 s−1 for the first 24 h to minimize cellular stress and support cell adaptation. It was then increased to 300 µmol photons m−2 s−1 for the following 144 h and finally elevated to 400 µmol photons m−2 s−1 until the end of the experiment.
The first bio-H2 production experiment in the 5 L photobioreactor under white light (WL), using substrate diluted at 25% (v/v), showed no gas accumulation during the first 168 h post-inoculation. This result was attributed to the presence of ammonium at concentrations exceeding inhibitory levels for R. palustris 42OL.
The presence of ammonium ions above a threshold concentration of approximately 2 mM (corresponding to approximately 36 mg L
−1 NH
4+) has been reported to inhibit bio-H
2 production by PNSB. However, this inhibitory threshold may vary depending on the specific PNSB strain and operating conditions [
48]. Consequently, to reduce ammonium inhibition while simultaneously limiting substrate dilution and water consumption, the dark-fermented substrate was diluted to achieve an ammonium concentration below 100 mg L
−1.
The substrate diluted to this level (18%
v/
v relative to the raw wastewater) was used in the subsequent four photofermentation experiments. The initial lactate and ammonium concentrations for each photofermentation test are reported in
Table 2.
The cumulative bio-H
2 production trends under WL and SL conditions are shown in
Figure 4C. In the WL runs, bio-H
2 production began 48 h after inoculation. On the contrary, the SL runs showed a reduced lag phase; in SL_1, bio-H
2 production started after 24 h, while in SL_2, it began immediately. The difference in lag phases between the two SL trials is likely connected to the lower ammonium concentration in SL_2 compared to SL_1, due to the heterogeneity of the substrate.
Table 3 shows the total bio-H
2 and PHB production at the end of the experiment. On average, the SL condition produced 35.5% more H
2 than the WL condition. Similarly, PHB production increased significantly in the SL condition, almost tripling the values obtained with WL.
The higher production of H
2 and PHB under selected light wavelengths is likely due to higher light utilization efficiency. BChl
a, in PNSB, has absorption peaks around 590–600 nm and 850–870 nm [
28], making selected wavelengths more effective than broad-spectrum WL. Targeted light absorption enhances photophosphorylation, generating ATP and reducing power (NADPH) essential for PHB and H
2 biosynthesis [
21]. In contrast, exposure to broad-spectrum light may increase energy dispersion and photo-oxidative stress due to inefficiently absorbed wavelengths.
The higher efficiency of the SL compared to WL is evident also in the growth profiles of
R. palustris 42OL (
Figure 3). During the first 168 h, growth proceeds similarly in both lighting conditions, with a steady increase in CDW, OD
660nm, and BChl
a levels over time. Under SL conditions, a higher lactic acid consumption is observed (
Figure 4A), mainly due to the higher biomass concentration achieved under SL, since the specific H
2 production rates are the same (not significantly different) under SL and WL conditions (
Table 3). After 168 h, the increase in light intensity from 300 to 400 µmol photons m
−2 s
−1 marked a critical point: bio-H
2 production decreased drastically (
Figure 4C), and ammonium consumption stabilized (
Figure 4B). This is followed by a 48 h phase in which both lighting conditions show a steady and comparable increase in OD
660nm, along with stabilization in BChl
a synthesis, likely as an adaptation to the new light conditions. From 216 h onwards, the conditions diverge markedly; under SL, a significant increase in BChl
a and OD
660nm is observed, likely due to greater efficiency in photon utilization under this condition. In contrast, WL leads to high energy dissipation and photooxidative stress, halting growth and limiting BChl
a accumulation. Despite sustained cellular growth under SL, ammonium consumption stabilization appears contradictory, as nitrogen is essential for building cellular biomass. However, the Nessler method used for its quantification (see
Section 2.6) detects only free ammonium, not nitrogen in amino groups of amino acids [
49]. Dairy wastewaters contain protein and amino acid residues that
R. palustris 42OL can use as a nitrogen and carbon source [
17,
24]. The increase in light intensity, with the consequent greater availability of energy, may have favored the use of amino acids, which provide a combined source of nitrogen and carbon, advantageous under high-energy conditions. Indeed, amino acid degradation requires additional metabolic pathways and specialized enzyme systems that consume substantial amounts of ATP, helping to stabilize the cell’s energy balance. However, further studies will be needed to demonstrate the higher utilization of amino acids as a source of carbon and nitrogen under high light conditions compared to ammoniacal nitrogen.
The maximum cumulative hydrogen (H
max) obtained under both lighting conditions (
Table 3) was significantly lower than the value reported by Seifert et al. [
50], who obtained an H
max of 3.23 L H
2 L
−1 of substrate by cultivating
Rhodobacter sphaeroides O.U. 001 on diluted dairy wastewater with a modified Biebel and Pfennig medium lacking a carbon source (malic acid). Optimum results were achieved at a dilution of 40% (
v/
v) and a light intensity of 9 klx.
It should be noted that, in the study by Seifert et al. [
50], the raw dairy wastewater contained 40 mg dm
−3 of ammonium nitrogen (N-NH
4+), corresponding to approximately 51.4 mg L
−1 of NH
4+. This concentration was further reduced by dilution, as photofermentation was carried out at 40% (
v/
v) wastewater. Moreover, the dairy wastewater was filtered before photofermentation, which likely reduced the particulate fraction and the contribution of protein-associated nitrogen, thereby limiting ammonium availability in the photofermentation medium. Therefore, the effective ammonium concentration in their system was substantially lower than that in the present study, which employed a dark fermentation effluent.
This substantial difference in hydrogen production can be attributed not only to differences in substrate characteristics, including ammonium availability, but also to the different experimental scales, as efficiency generally decreases with process scale-up. Indeed, while Seifert et al. [
50] conducted their experiments in small 25 mL vials filled to 12.5 mL, this study involved a 416-fold larger working volume (5200 mL). Furthermore, in the present study, the substrate was diluted with deionized water rather than with a synthetic medium, supplemented only with the minimal essential compounds required for hydrogen production.
Regarding PHB production, an important factor for optimizing its accumulation is the C/N ratio of the growth medium; ratios above 30 are generally recommended for optimal PHB synthesis [
21]. In our study, the substrate showed an average C/N ratio of 41.8 (±3.47) (
Table 2), indicating favorable conditions for PHB accumulation. However, lactate is not the preferred carbon source for PHB synthesis in PNSB; acetate and butyrate are more efficient substrates [
21], but neither was detected in our substrate. Unlike lactate, which must first be oxidized to pyruvate and subsequently converted to acetyl-CoA (the key precursor for PHB synthesis), acetate and butyrate can be directly converted to acetyl-CoA with lower energetic costs. Consequently, lactate is often directed toward metabolic pathways favoring hydrogen production rather than PHB synthesis [
21].
In agreement with this metabolic framework, Wu et al. [
50] reported that
R. palustris WP3-5 produced the highest cumulative H
2 volume when grown on lactate (131.3 mL over 114 h). However, acetate exhibited the highest SCE (14.2%), indicating a more efficient conversion of the substrate into hydrogen. When lactate was used, the SCE was lower (approximately 9.3%) and remained nearly constant regardless of PHB accumulation, suggesting limited competition between PHB synthesis and H
2 production under lactate-fed conditions.
Wu et al. [
51] also reported a PHB accumulation of 1.4% (
w/
w) in
R. palustris WP3-5 when grown on lactate, which is comparable to the values obtained in our study under SL conditions. In our experiments, higher PHB production was observed under SL conditions, which also exhibited a faster lactate consumption. This accelerated lactate utilization may also be attributed to the enhanced PHB synthesis observed under these conditions. However, since PHB was quantified only at the end of the experimental runs, it is not possible to assess its accumulation dynamics or potential intracellular reutilization over time.
Future experiments should therefore include measurements of PHB at different cultivation times to better elucidate its role in the distribution of reducing equivalents and its interaction with hydrogen production. This interpretation is consistent with the observations of Wu et al. [
51], who suggested that PHB does not merely compete with hydrogen production for reducing power, but rather plays a more complex metabolic role, particularly under stress conditions.