Titanium Alloy Fabricated by Additive Manufacturing for Medical Applications: Obtaining, Characterization and Application—Review
Abstract
1. Introduction
2. Metal Additive Manufacturing Process Used in Medical Treatment
2.1. Binder Jetting
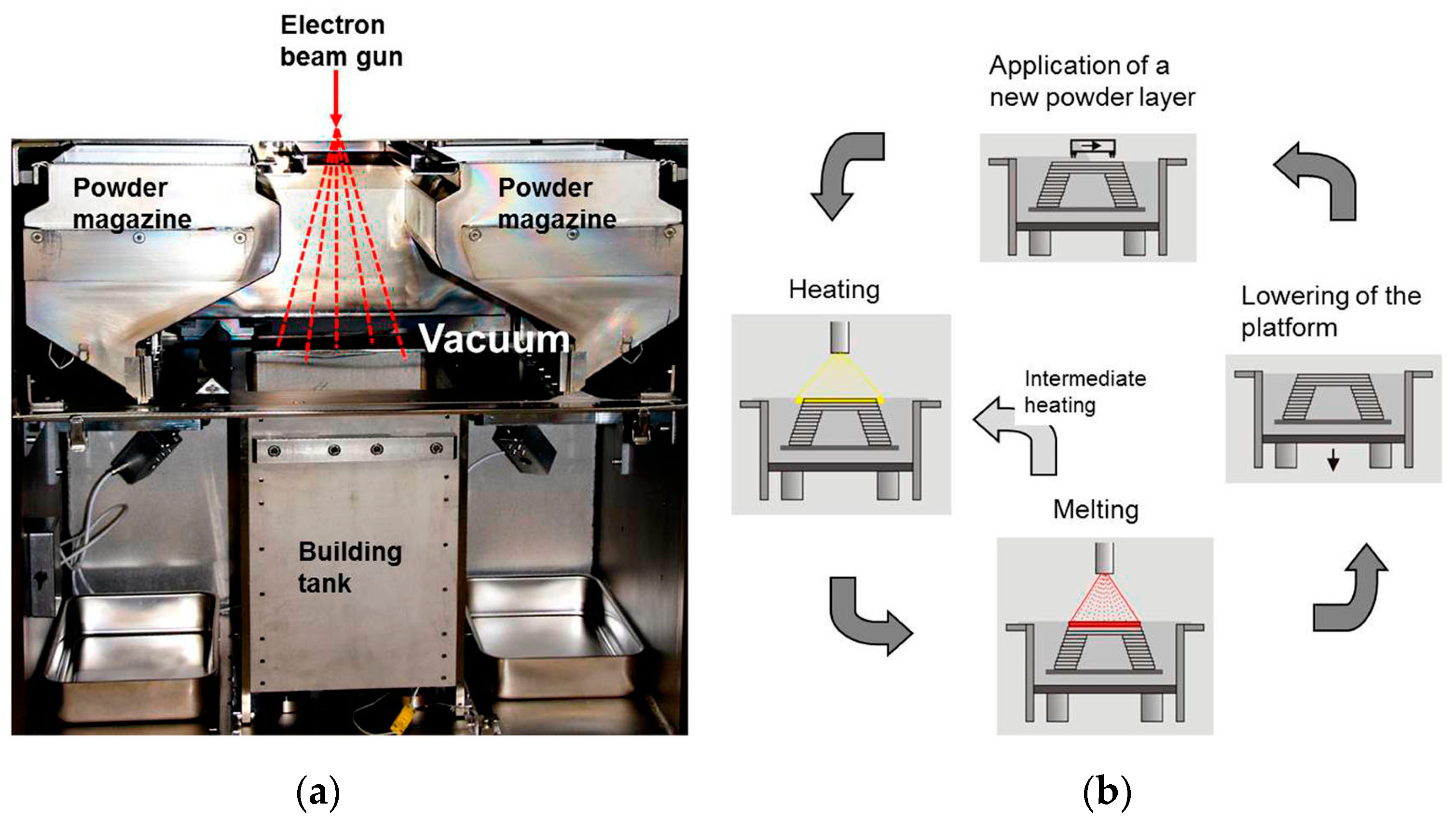
2.2. Powder Bed Fusion
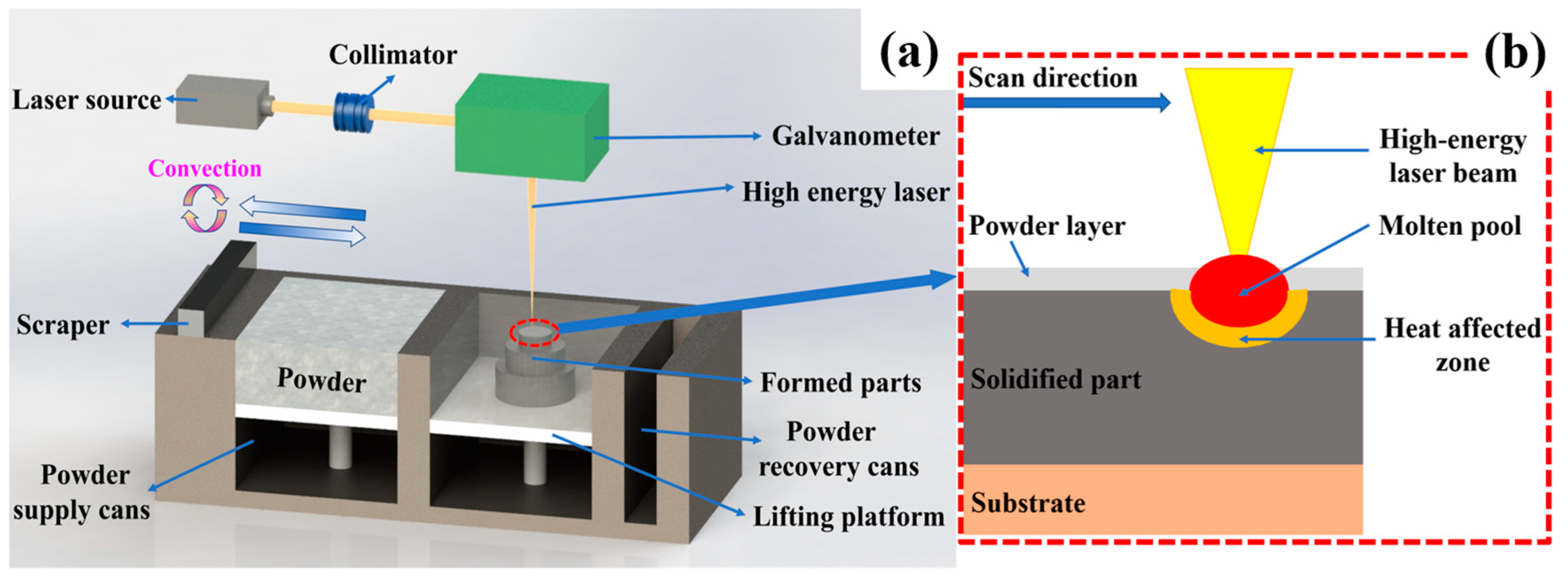
2.3. Directed Energy Deposition
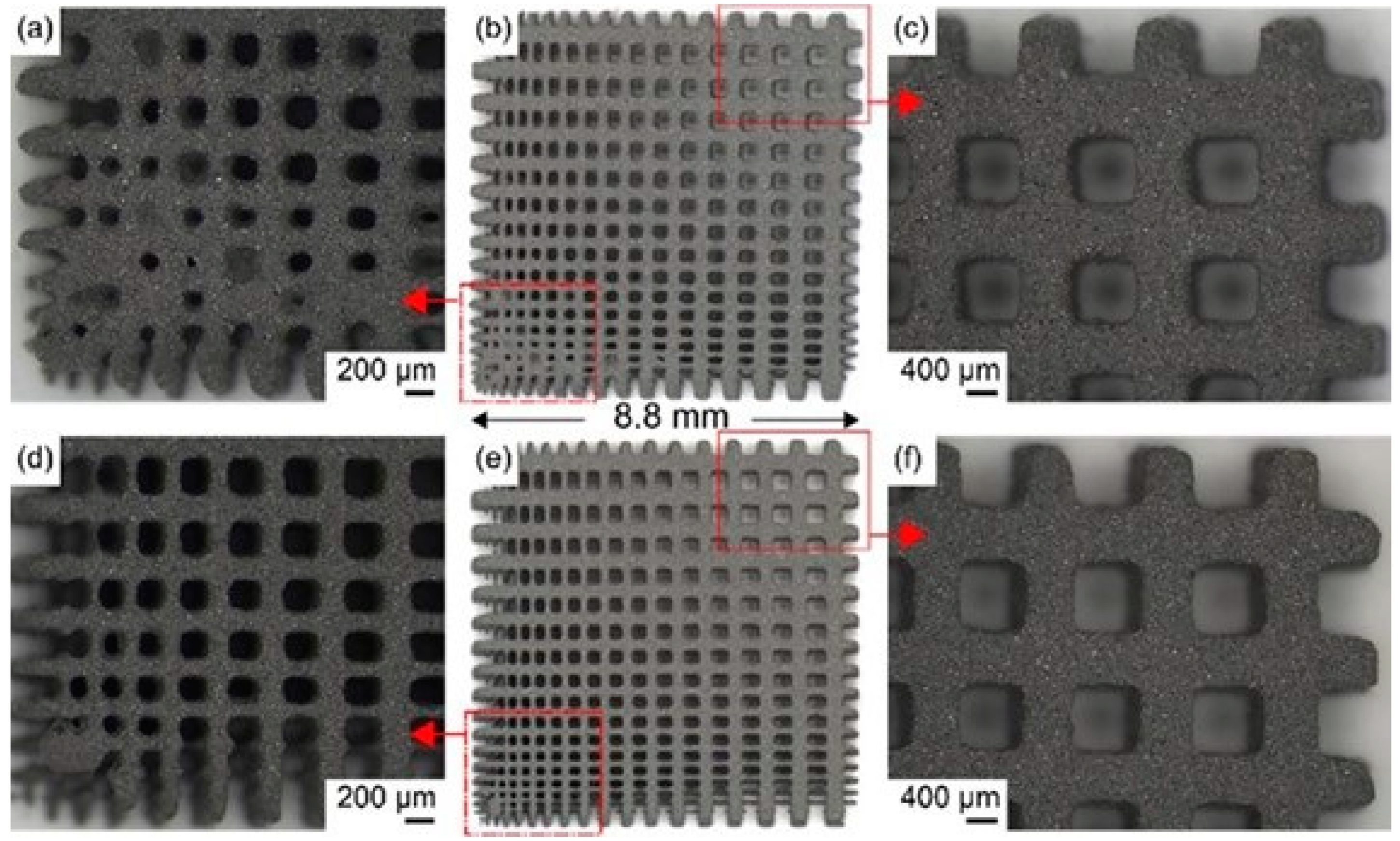
3. Key Issues of Titanium Alloys in Medical Additive Manufacturing
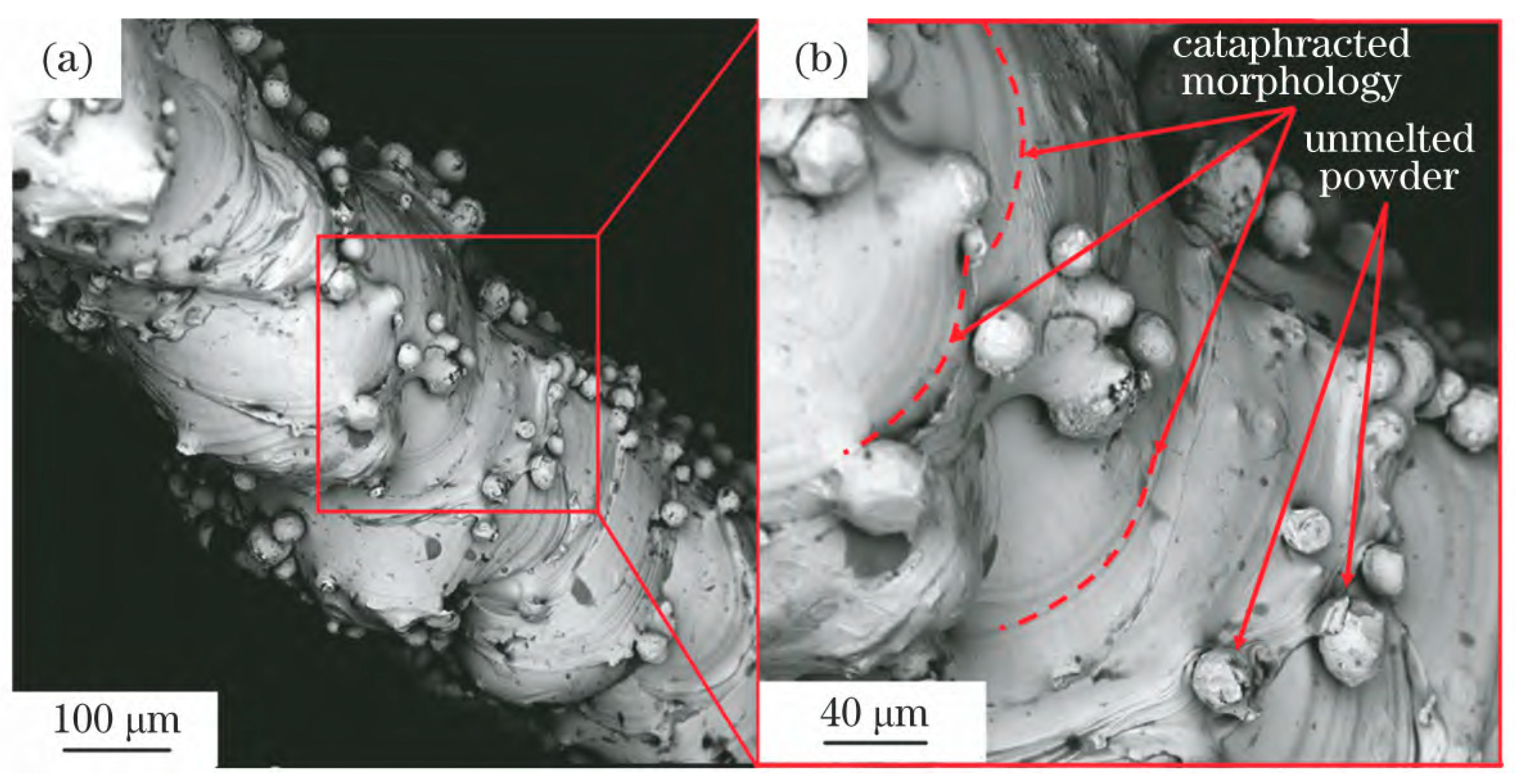
3.1. Cause of Powder Adhesion
3.2. Adhering Powder Removal Method
3.2.1. Ultrasonic Cleaning
3.2.2. Solid Medium Spray
3.2.3. Chemical Cleaning
3.2.4. Acoustic Dry Cleaning
4. General Characterization Method
4.1. Optical Inspection Method
4.2. Microscopy
4.3. Micro-CT Examination
4.4. Sample Weighing Method—Codification
4.5. Surface Roughness Measurement
5. Application of Additive Manufacturing Titanium Alloy in Medical Field
5.1. Orthopedic Implants
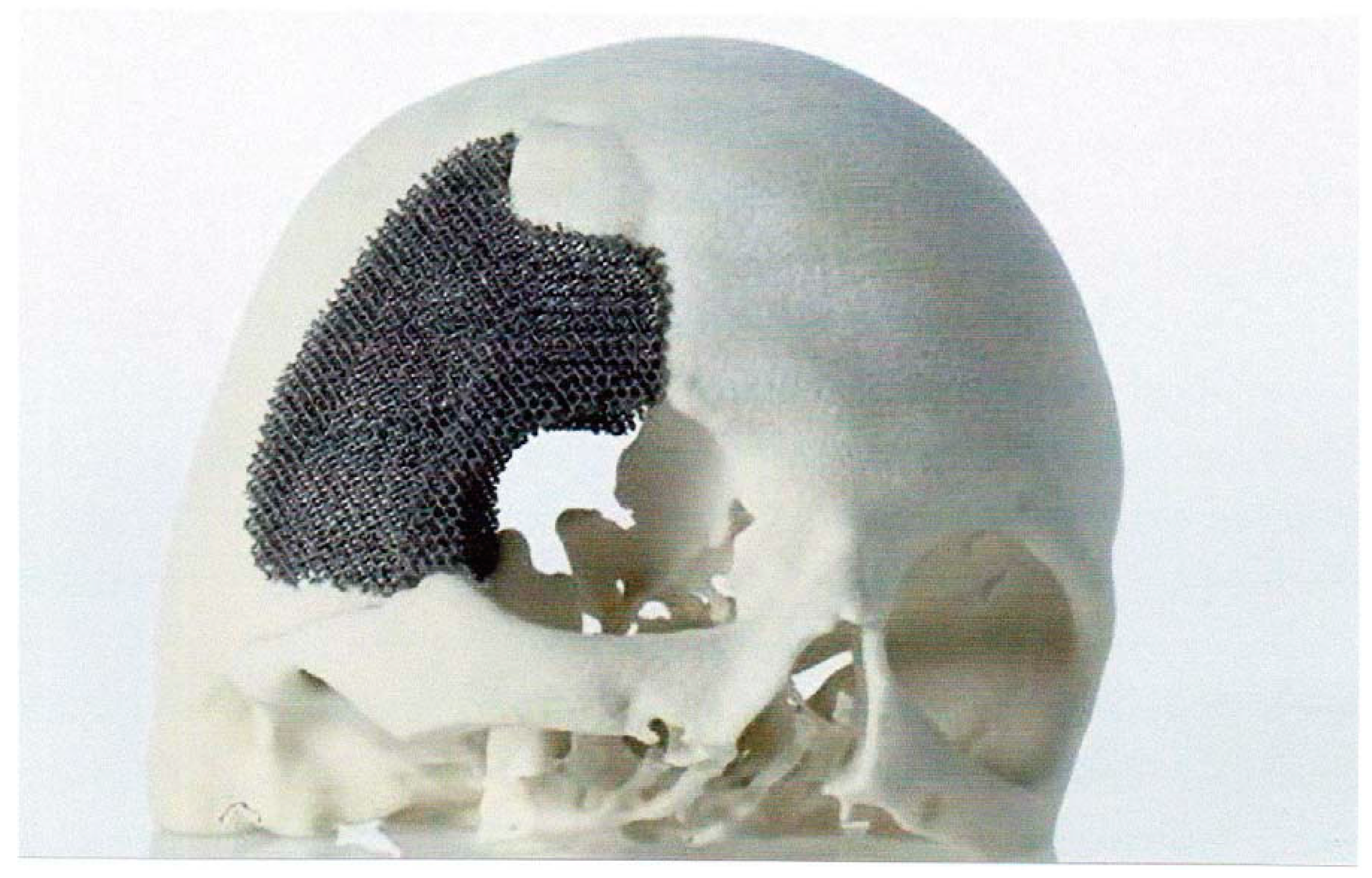
5.1.1. Skull Implants
5.1.2. Jaw Implants
5.1.3. Dental Implant
5.1.4. Spinal Implants
5.1.5. Chest Implants
5.1.6. Pelvic Implants
5.2. Medical Devices
6. Summary and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bandyopadhyay, A.; Zhang, Y.; Bose, S. Recent Developments in Metal Additive Manufacturing. Curr. Opin. Chem. Eng. 2020, 28, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S. Research progress of metal additive manufacturing technology. China Metal Bulletin. 2022, 4, 186–188. [Google Scholar]
- Xiong, J.; Yang, J.; Zhang, J.; Guo, J. Research progress of metal additive manufacturing. China Mater. Technol. 2021, 30, 107. [Google Scholar]
- Dong, C.; Tan, J.; Lin, Z.; Wang, C.; Zhao, Y. Research progress on additive manufacturing technology of titanium alloy. Met. Process. (Hot Process.) 2020, 07, 16–21. [Google Scholar]
- Dang, X.; Wang, J. Research Status and Prospect of Additive Manufacturing Technology at Home and Abroad. Aviat. Precis. Manuf. Technol. 2020, 56, 35–38. [Google Scholar]
- Kermavnar, T.; Shannon, A.; O’Sullivan, L. The application of additive manufacturing/3D printing in ergonomic aspects of product design: A systematic review. Appl. Ergon. 2021, 97, 103528. [Google Scholar] [CrossRef]
- Mohanavel, V.; Ashraff, K.; Ranganathan, S.; Allen, J.; Ravikumar, M.; Rajkumar, S. The roles and applications of additive manufacturing in the aerospace and automobile sector. Mater. Today Proc. 2021, 47, 405–409. [Google Scholar] [CrossRef]
- Schutz, R.; Watkins, H. Recent developments in titanium alloy application in the energy industry. Mater. Sci. Eng. A 1998, 243, 305–315. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, S.; Yang, X.; Wang, Y.; Shi, M.; Zhang, G. Application and Prospect of Additive Manufacturing in Porous Metal Implants. Therm. Process. 2019, 48, 1–5. [Google Scholar]
- Attarilar, S.; Salehi, M.T.; Fadhalah, K. Functionally Graded Titanium Implants: Characteristic Enhancement Induced by Combined Severe Plastic Deformation. PLoS ONE 2019, 14, e0221491. [Google Scholar] [CrossRef]
- Spataru, M.-C.; Cojocaru, F.D.; Sandu, A.V.; Solcan, C.; Duceac, I.A.; Baltatu, M.S.; Voiculescu, I.; Geanta, V.; Vizureanu, P. Assessment of the Effects of Si Addition to a New TiMoZrTa System. Materials 2021, 14, 7610. [Google Scholar] [CrossRef]
- Bălţatu, M.S.; Vizureanu, P.; Geantă, V.; Nejneru, C.; Țugui, C.A.; Focşăneanu, S.C. Obtaining and Mechanical Properties of Ti-Mo-Zr-Ta Alloys. IOP Conf. Ser. Mater. Sci. Eng. 2017, 209, 012019. [Google Scholar] [CrossRef]
- Roach, P.; Eglin, D.; Rohde, K. Modern Biomaterials:A Review Bulk Properties and Implications of Surface Modifications. J. Mater. Sci. Mater. Med. 2007, 18, 1263–1277. [Google Scholar] [CrossRef]
- Triyono, J.; Alfiansyah, R.; Sukanto, H. Fabrication and Characterization of Porous Bone Scaffold of Bovine Hydroxyapatite-glycerin by 3D Printing Technology. Bioprinting 2020, 18, e00078. [Google Scholar] [CrossRef]
- Parthasarathy, L.; Starly, B.; Raman, S.; Christensen, A. Mechanical evaluation of porous titanium (Ti6Al4V) structures with electron beam melting (EBM). J. Mech. Behav. Biomed. Mater. 2010, 3, 249–259. [Google Scholar] [CrossRef]
- Basalah, A.; Esmaeili, S.; Toyserkani, E. On the influence of sintering protocols and layer thickness on the physical and mechanical properties of additive manufactured titanium porous bio-structures. Mater. Process. Technol. 2016, 238, 341–351. [Google Scholar] [CrossRef]
- Bansiddhi, A.; Dunand, D. 7—Titanium and NiTi foams for bone replacement. In Bone Substitute Biomaterials; Woodhead Publishing: Cambridge, UK, 2014; pp. 142–179. [Google Scholar]
- Nouri, A. 5—Titanium foam scaffolds for dental applications. In Metallic Foam Bone; Woodhead Publishing: Cambridge, UK, 2017; pp. 131–160. [Google Scholar]
- Kokubo, T.; Yamaguchi, S. Novel bioactive materials developed by simulated body fluid evaluation: Surface-modified Ti metal and its alloys. Acta Biomater. 2016, 44, 16–30. [Google Scholar] [CrossRef]
- Xu, W.; Brandt, W.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K. Additive manufacturing of strong and ductile Ti–6Al–4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Rani, V.D.; Vinoth-Kumar, L.; Anitha, V.; Manzoor, K.; Deepthy, K.; Shantikumar, V. Osteointegration of titanium implant is sensitive to specific nanostructure morphology. Acta Biomater. 2012, 8, 1976–1989. [Google Scholar] [CrossRef]
- Biesiekierski, A.; Wang, J.; Gepreel, M.; Wen, C. A new look at biomedical Ti-based shape memory alloys. Acta Biomater. 2012, 8, 1661–1669. [Google Scholar] [CrossRef] [PubMed]
- Balaji, V.; Kumaran, S. Densification and microstructural studies of titanium-boron carbide (B4C) powder mixture during spark plasma sintering. Powder Technol. 2014, 264, 536–540. [Google Scholar] [CrossRef]
- Chen, G.; Li, N.; Fu, X.; Zhou, W. Preparation and characterization of a sodium polyacrylate/sodium silicate binder used in oxidation resistant coating for titanium alloy at high temperature. Powder Technol. 2012, 230, 134–138. [Google Scholar] [CrossRef]
- Manshadi, A.; Bermingham, M.; Dargusch, M.; StJohn, D.; Qian, M. Metal injection moulding of titanium and titanium alloys: Challenges and recent development. Powder Technol. 2017, 319, 289–301. [Google Scholar] [CrossRef]
- Hong, S.; Park, J.; Park, K.; Kim, K.; Lee, J.; Lee, K. Fabrication of titanium carbide nano-powders by a very high speed planetary ball milling with a help of process control agents. Powder Technol. 2015, 274, 393–401. [Google Scholar] [CrossRef]
- Huang, B.; Fan, C.; Pan, C.; Zheng, A.; Ma, X.; Li, Y. Synthesis and catalytic oxidation property of titanium-zirconium mixed oxide microsphere as well as titanium oxide microcube. Powder Technol. 2017, 315, 258–269. [Google Scholar] [CrossRef]
- Klisiewicz, P.; Roberts, J.; Pohlman, N. Segregation of titanium powder with polydisperse size distribution: Spectral and correlation analyses. Powder Technol. 2015, 272, 204–210. [Google Scholar] [CrossRef]
- Kumaran, S.; Sasikumar, T.; Arockiakumar, R.; Srinivasa, T. Nanostructured titanium aluminides prepared by mechanical alloying and subsequent thermal treatment. Powder Technol. 2008, 185, 124–130. [Google Scholar] [CrossRef]
- Kwon, H.; Kim, J.; Jung, S.; Suh, C.; Kil, D.; Roh, K. Premixed TiC–Ni composite powder prepared by mechanical milling of Ti–Ni alloy/graphite mixture and subsequent heat treatment. Powder Technol. 2014, 253, 681–685. [Google Scholar] [CrossRef]
- Manonukul, A.; Tange, M.; Srikudvien, P.; Denmud, N.; Wattanapornphan, P. Rheological properties of commercially pure titanium slurry for metallic foam production using replica impregnation method. Powder Technol. 2014, 266, 129–134. [Google Scholar] [CrossRef]
- Thavanayagam, G.; Pickering, K.; Swan, J.; Cao, P. Analysis of rheological behaviour of titanium feedstocks formulated with a water-soluble binder system for powder injection moulding. Powder Technol. 2015, 269, 227–232. [Google Scholar] [CrossRef]
- Vivek, A.; DeFouw, J.; Daehn, G. Dynamic compaction of titanium powder by vaporizing foil actuator assisted shearing. Powder Technol. 2014, 254, 181–186. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, F.; Cai, Y. High-velocity compaction of titanium powder and process characterization. Powder Technol. 2011, 208, 596–599. [Google Scholar] [CrossRef]
- Yan, Z.; Chen, F.; Cai, F.; Zheng, Y. Microstructure and mechanical properties of insitu synthesized TiB whiskers reinforced titanium matrix composites by highvelocity compaction. Powder Technol. 2014, 267, 309–314. [Google Scholar] [CrossRef]
- Balazic, M.; Kopac, J.; Jackson, M. Titanium and titanium alloy applications in medicine. Int. J. Nano Biomater. 2007, 1, 3–34. [Google Scholar] [CrossRef]
- Rack, H.; Qazi, J. Titanium alloys for biomedical applications. Mater. Sci. Eng. C 2006, 264, 1269–1277. [Google Scholar] [CrossRef]
- Jackson, M.; Kopac, J.; Balazic, M. Titanium and titanium alloy applications in medicine. Surg. Tools Med. Devices 2016, 1, 475–517. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Schmuki, P. Biomaterial surface modification of titanium and titanium alloys for medical applications. Nanomedicine 2014, 111, 111. [Google Scholar]
- Suard, M.; Martin, G.; Lhuissier, P. Mechanical equivalent diameter of single struts for the stiffness prediction of lattice structures produced by Electron Beam Melting. Addit. Manuf. 2015, 8, 124–131. [Google Scholar] [CrossRef]
- Drescher, P.; Reimann, T.; Seitz, H. Investigation of powder removal of net–structured titanium parts made from electron beam melting. Int. J. Rapid Manuf. 2014, 4, 81–89. [Google Scholar] [CrossRef]
- Harun, W.; Kamariah, M.; Muhamad, N. A review of powder additive manufacturing processes for metallic biomaterials. Powder Technol. 2018, 327, 128–151. [Google Scholar] [CrossRef]
- Shi, W.; Yan, T.; Liu, Y. Simulation Analysis and Experimental Study on SLM Forming Titanium Alloy Milling Hole. Metals 2022, 12, 1919. [Google Scholar] [CrossRef]
- Drescher, P.; Sarhan, M.; Seitz, H. An investigation of sintering parameters on titanium powder for electron beam melting processing optimization. Materials 2016, 9, 974. [Google Scholar] [CrossRef] [PubMed]
- Jahadakbar, A.; Nematollahi, M.; Safaei, K. Design, modeling, additive manufacturing, and polishing of stiffness-modulated porous nitinol bone fixation plates followed by thermomechanical and composition analysis. Metals 2020, 10, 151. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, S.; Zhang, C.; Wu, C.; Wang, Q.; Dong, S. Research status and prospect of laser additive manufacturing of high performance functionally gradient materials. Mater. Eng. 2020, 48, 13–23. [Google Scholar]
- Shi, Y. Industrial Application and Industrialization Development of 3D Printing Technology. Mech. Des. Manuf. Eng. 2016, 45, 11–16. [Google Scholar]
- Cai, Y.; Han, H.; Ren, D.; Ji, H.; Lei, J. Effect of chemical etching process on surface roughness of TC4 titanium alloy formed by selective laser melting. J. Mater. Res. 2022, 36, 435–442. [Google Scholar]
- ASTM F2792-12a; Standard Terminology for Additive Manufacturing Technologies Withdrawn 2015. ASTM International: West Conshohocken, PA, USA, 2012.
- GB/T 35021-201; Standard Terminology for Additive Manufacturing Technologies China. Standards Press of China: Beijing, China, 2018.
- Shaheen, M.; Thornton, A.; Luding, S. The influence of material and process parameters on powder spreading in additive manufacturing. Powder Technol. 2021, 383, 564–583. [Google Scholar] [CrossRef]
- Oliveira, J.; LaLonde, P.; Ma, J. Processing parameters in laser powder bed fusion metal additive manufacturing. Mater. Des. 2020, 193, 108762. [Google Scholar] [CrossRef]
- Ingrassia, T.; Nigrelli, V.; Ricotta, V. Process Parameters Influence in Additive Manufacturing. In Advances on Mechanics, Design Engineering and Manufacturing; Springer: Cham, Switzerland, 2017; pp. 261–270. [Google Scholar]
- Mostafaei, A.; Elliott, A.; Barnes, J. Binder Jet 3D Printing: Process Parameters, Materials, Properties, Modeling, and Challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Mostafaei, A.; Stevens, E.; Ference, J. Binder Jetting of a Complex-Shaped Metal Partial Denture Framework. Addit. Manuf. 2018, 21, 63–68. [Google Scholar] [CrossRef]
- Gonzalez, J.; Mireles, J.; Lin, Y. Characterization of Ceramic Components Fabricated Using Binder Jetting Additive Manufacturing Technology. Ceram. Int. 2016, 42, 10559–10564. [Google Scholar] [CrossRef]
- Wei, Q.; Heng, Y.; Mao, Y.; Feng, H.; Cai, C.; Cai, D.; Li, W. The development and prospect of metal binder spray additive manufacturing technology. Packag. Eng. 2021, 42, 103–119. [Google Scholar]
- Li, M.; Du, W.; Elwany, A.; Pei, Z.; Ma, C. Metal Binder Jetting Additive Manufacturing: A Literature Review. J. Manuf. Sci. Eng. 2020, 142, 090801. [Google Scholar] [CrossRef]
- Mostafaei, A.; Vecchis, P.; Nettleship, I. Effect of Powder Size Distribution on Densification and Microstructural Evolution of Binder-Jet 3d-Printed Al- loy 625. Mater. Des. 2019, 162, 375–383. [Google Scholar] [CrossRef]
- Stevens, E.; Schloder, S.; Bono, E. Density variation in binder jetting 3D-printed and sintered Ti-6Al-4V. Addit. Manuf. 2018, 22, 746–752. [Google Scholar] [CrossRef]
- Cox, S.; Thornby, J.; Gibbons, G. 3D Printing of Porous Hydroxyapatite Scaffolds Intended for Use in Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2015, 47, 237–247. [Google Scholar] [CrossRef]
- Jurisch, M.; Studnitzky, T.; Andersen, O. 3D screen printing for the fabrication of small intricate Ti-6Al-4V parts. Powder Metall. 2015, 58, 339–343. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y. Process parameters optimization for improving surface quality and manufacturing accuracy of binder jetting additive manufacturing process. Rapid Prototyp. J. 2016, 22, 527–538. [Google Scholar] [CrossRef]
- Turker, M.; Godlinski, D.; Petzoldt, F. Effect of Production Parameters on the Properties of Ni 718 Superalloy by Three-Dimensional Printing. Mater. Charact. 2008, 59, 1728–1735. [Google Scholar] [CrossRef]
- Parteli, E.; Pöschel, T. Particle-Based Simulation of Powder Application in Additive Manufacturing. Powder Technol. 2016, 288, 96–102. [Google Scholar] [CrossRef]
- Gu, B. Application and development trend of additive manufacturing technology at home and abroad. Met. Process. (Hot Process.) 2022, 3, 1–16. [Google Scholar]
- Zhang, H.; Yang, K. A review of current status and applications of additive manufacturing. Packag. Eng. 2021, 42, 9–15. [Google Scholar]
- Zheng, Y.; Xia, D.; Chen, Y.; Liu, Y.; Xu, Y.; Wen, P.; Tian, Y.; Lai, Y. Additive manufacturing of degradable metal medical implants. Met. J. 2021, 57, 1499–1520. [Google Scholar]
- Ma, C.; Zhao, Y. Research on the development strategy of denture customization and visualization collaborative service platform based on 3D printing. China Equip. Eng. 2021, 22, 128–129. [Google Scholar]
- Shi, W.; Li, J.; Liu, Y. Experimental study on mechanism of influence of laser energy density on surface quality of Ti-6Al-4V alloy in selective laser melting. J. Cent. South Univ. 2022, 29, 3447–3462. [Google Scholar] [CrossRef]
- Zhao, C.; Li, W.; Wang, Q.; Wang, Y.; Zhao, Y.; Di, S.; Ren, D.; Ji, B. Study on Internal Defects and Their Evolution of Titanium Alloy Formed by Selective Laser Melting. Rare Met. Mater. Eng. 2021, 50, 2841–2849. [Google Scholar]
- Hou, G.; Wu, T.; Shang, Z.; Shao, Z. Application of digital surgery and additive manufacturing technology in reconstruction of mandibular defects with vascularized iliac bone flap. Chin. J. Exp. Surg. 2020, 37, 635–638. [Google Scholar]
- Majumdar, T.; Eisenstein, N.; Frith, J.E.; Cox, S.C.; Birbilis, N. Additive Manufacturing of Titanium Alloys for Orthopedic Applications: A Materials Science Viewpoint. Adv. Eng. Mater. 2018, 20, 28. [Google Scholar] [CrossRef]
- Sing, S.L.; An, J.; Yeong, W.Y.; Wiria, F.E. Laser and electron-beam powder-bed additive manufacturing of metallic implants: A review on processes, materials and designs. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2016, 34, 369–385. [Google Scholar] [CrossRef]
- Guo, J.; Xu, J.; Liu, B.; Fu, B.; Li, F. Research progress on the performance of 3D printed orthopedic titanium alloy medical devices. China Pharm. 2021, 35, 471–478. [Google Scholar]
- Shi, W.; Han, Y.; Liu, Y.; Jing, Y. Selected area laser melting TC4 spheroidization splash mechanism and experimental research. Surf. Technol. 2021, 50, 75–82. [Google Scholar]
- Le, T.; Lo, Y. Effects of sulfur concentration and Marangoni convection on melt-pool formation in transition mode of selective laser melting process. Mater. Des. 2019, 179, 107866. [Google Scholar] [CrossRef]
- Yusuf, S.; Gao, N. Influence of energy density on metallurgy and properties in metal additive manufacturing. Mater. Sci. Technol. 2017, 33, 1269–1289. [Google Scholar] [CrossRef]
- Ferro, P.; Meneghello, R.; Savio, G. A modified volumetric energy density–based approach for porosity assessment in additive manufacturing process design. Int. J. Adv. Manuf. Technol. 2020, 110, 1911–1921. [Google Scholar] [CrossRef]
- Kluczyński, J.; Śnieżek, L.; Grzelak, K. The influence of exposure energy density on porosity and microhardness of the SLM additive manufactured elements. Materials 2018, 11, 2304. [Google Scholar] [CrossRef]
- Körner, C. Additive Manufacturing of Metallic Components by Selective Electron Beam Melting—A Review. Int. Mater. Rev. 2016, 61, 361–377. [Google Scholar] [CrossRef]
- Le, K.; Wong, C.; Chua, K. Discontinuity of overhanging melt track in selective laser melting process. Int. J. Heat Mass Transf. 2020, 162, 120284. [Google Scholar] [CrossRef]
- Borgman, J.M.; Wang, J.; Zani, L.; Conway, P.P.; Torres-Sanchez, C. The Effect of Energy Density and Nb Content on the Microstructure and Mechanical Properties of Selective Laser Melted Ti-(10–30 Wt.%) Nb. J. Mater. Eng. Perform. 2021, 30, 8771–8783. [Google Scholar] [CrossRef]
- Ge, J.; Yuan, B.; Zhao, L.; Yan, M.; Chen, W.; Zhang, L. Effect of Volume Energy Density on Selective Laser Melting Niti Shape Memory Alloys: Microstructural Evolution, Mechanical and Functional Properties. J. Mater. Res. Technol. 2022, 20, 2872–2888. [Google Scholar] [CrossRef]
- Gibson, I.; Rosen, D.; Stucker, B. Directed energy deposition processes. In Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; pp. 245–268. [Google Scholar]
- Ahn, D. Directed energy deposition (DED) process: State of the art. Int. J. Precis. Eng. Manuf.-Green Technol. 2021, 8, 703–742. [Google Scholar] [CrossRef]
- Oh, W.; Lee, W.; Kim, M. Repairing additive-manufactured 316L stainless steel using direct energy deposition. Opt. Laser Technol. 2019, 117, 6–17. [Google Scholar] [CrossRef]
- Fujishima, M.; Oda, Y.; Ashida, R. Study on factors for pores and cladding shape in the deposition processes of Inconel 625 by the directed energy deposition (DED) method. CIRP J. Manuf. Sci. Technol. 2017, 19, 200–204. [Google Scholar] [CrossRef]
- Shalnova, S.; Kuzminova, Y.; Evlashin, S. Effect of recycled powder content on the structure and mechanical properties of Ti-6Al-4V alloy produced by direct energy deposition. J. Alloy. Compd. 2022, 893, 162264. [Google Scholar] [CrossRef]
- Liu, Z.; He, B.; Lyu, T. A review on additive manufacturing of titanium alloys for aerospace applications: Directed energy deposition and beyond Ti-6Al-4V. JOM 2021, 73, 1804–1818. [Google Scholar] [CrossRef]
- Ren, Y.; Lin, X.; Zeng, Y.; Peng, H.; Huang, W. Long fatigue crack growth behavior of Ti–6Al–4V produced via high-power laser directed energy deposition. Mater. Sci. Eng. A 2021, 819, 141392. [Google Scholar] [CrossRef]
- Bakre, C.; Nassar, A.R.; Reutzel, E.W.; Lissenden, C.J. Ultrasonic Rayleigh Wave Interrogation of Directed Energy Deposition Ti–6Al–4V Having a Rough Surface. ASME J. Nondestruct. Eval. 2022, 5, 031008. [Google Scholar] [CrossRef]
- Li, S.; Kumar, P.; Chandra, S. Directed energy deposition of metals: Processing, microstructures, and mechanical properties. Int. Mater. Rev. 2022, 1–43. [Google Scholar] [CrossRef]
- Singh, A.; Kapil, S.; Das, M. A comprehensive review of the methods and mechanisms for powder feedstock handling in directed energy deposition. Addit. Manuf. 2020, 35, 101388. [Google Scholar] [CrossRef]
- Heigel, J.; Michaleris, P.; Reutzel, E. Thermo-mechanical model development and validation of directed energy deposition additive manufacturing of Ti–6Al–4V. Addit. Manuf. 2015, 5, 9–19. [Google Scholar] [CrossRef]
- Lia, F.; Park, J.; Tressler, J. Partitioning of laser energy during directed energy deposition. Addit. Manuf. 2017, 18, 31–39. [Google Scholar] [CrossRef]
- Shim, D.; Baek, G.; Seo, J. Effect of layer thickness setting on deposition characteristics in direct energy deposition (DED) process. Opt. Laser Technol. 2016, 86, 69–78. [Google Scholar] [CrossRef]
- Mahmoud, D.; Elbestawi, M. Lattice Structures and Functionally Graded Materials Applications in Additive Manufacturing of Orthopedic Implants: A Review. J. Manuf. Mater. Process. 2017, 1, 13. [Google Scholar] [CrossRef]
- Tilton, M.; Lewis, G.S.; Bok Wee, H.; Armstrong, A.; Hast, M.W.; Manogharan, G. Additive Manufacturing of Fracture Fixation Implants: Design, Material Characterization, Biomechanical Modeling and Experimentation. Addit. Manuf. 2020, 33, 101137. [Google Scholar] [CrossRef]
- Kuroda, D.; Niinomi, M. Design and Mechanical Properties of New β Type Titanium Alloys for Implant Materials. Mater. Sci. Eng. A 1998, 243, 244–249. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Van Rietbergen, B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin. Orthop. Rel. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]
- Wieding, J.; Wolf, A.; Bader, R. Numerical optimization of open-porous bone scaffold structures to match the elastic properties of human cortical bone. J. Mech. Behav. Biomed. Mater. 2014, 37, 56–68. [Google Scholar] [CrossRef]
- Ahmadi, S.; Yavari, S.; Wauthle, R.; Pouran, B.; Schrooten, J.; Weinans, H.; Zadpoor, A. Additively manufactured open-cell porous biomaterials made from six different space-filling unit cells: The mechanical and morphological properties. Materials 2015, 8, 1871–1896. [Google Scholar] [CrossRef]
- Bobbert, F.S.L.; Lietaert, K.; Eftekhari, A.A.; Pouran, B.; Ahmadi, S.M.; Weinans, H.; Zadpoor, A.A. Additively manufactured metallic porous biomaterials based on minimal surfaces: A unique combination of topological, mechanical, and mass transport properties. Acta Biomater. 2017, 53, 572–584. [Google Scholar] [CrossRef]
- Onal, E.; Frith, J.E.; Jurg, M.; Wu, X.; Molotnikov, A. Mechanical Properties and in Vitro Behavior of Additively Manufactured and Functionally Graded Ti6Al4V Porous Scaffolds. Metals 2018, 8, 200. [Google Scholar] [CrossRef]
- Bai, L.; Gong, C.; Chen, X.; Sun, Y.; Zhang, J.; Cai, L.; Zhu, S.; Xie, S.Q. Additive Manufacturing of Customized Metallic Orthopedic Implants: Materials, Structures, and Surface Modifications. Metals 2019, 9, 1004. [Google Scholar] [CrossRef]
- An, L.; Wang, D.; Zhu, D. Combined Electrochemical and Mechanical Polishing of Interior Channels in Parts Made by Additive Manufacturing. Addit. Manuf. 2022, 51, 102638. [Google Scholar] [CrossRef]
- Ozdemir, Z.; Ozdemir, A.; Basim, G.B. Application of Chemical Mechanical Polishing Process on Titanium Based Implants. Mater. Sci. Eng. C 2016, 68, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, J.; Yuan, M.; Li, Q.; Liu, Y.; Lin, Y. Experimental Study on the Influence Mechanism of Micro-Abrasive Air Jet Machining on the Surface Quality of Ti-6Al-4V Titanium Alloy Formed by Selective Laser Melting. Mater. Today Commun. 2022, 33, 104429. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, R. Basic principles of additive manufacturing: Different additive manufacturing technologies. In Additive Manufacturing; Woodhead Publishing: Cambridge, UK, 2021; pp. 17–35. [Google Scholar]
- Bhardwaj, T.; Shukla, M.; Paul, C. Direct energy deposition-laser additive manufacturing of titanium-molybdenum alloy: Parametric studies, microstructure and mechanical properties. J. Alloy. Compd. 2019, 787, 1238–1248. [Google Scholar] [CrossRef]
- Fan, Z. Study on the Structural Properties of Titanium Alloy Gradient Porous Bionic Bone by Selective Laser Melting; Chongqing University: Chongqing, China, 2021. [Google Scholar]
- Van Hooreweder, B.; Lietaert, K.; Neirinck, B.; Lippiatt, N.; Wevers, M. CoCr F75 scaffolds produced by additive manufacturing: Influence of chemical etching on powder removal and mechanical performance. J. Mech. Behav. Biomed. Mater. 2017, 70, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Bagherifard, S.; Hickey, D.J.; de Luca, A.C.; Malheiro, V.N.; Markaki, A.E.; Guagliano, M.; Webster, T.J. The influence of nanostructured features on bacterial adhesion and bone cell functions on severely shot peened 316L stainless steel. Biomaterials 2015, 73, 185–197. [Google Scholar] [CrossRef]
- Zebrowski, R.; Walczak, M.; Klepka, T.; Pasierbiewicz, K. Effect of the shot peening on surface properties of ti-6al-4v alloy produced by means of DMLS technology. Eksploat. Niezawodn. 2019, 21, 46–53. [Google Scholar] [CrossRef]
- Ma, C.; Andani, M.T.; Qin, H.; Moghaddam, N.S.; Ibrahim, H.; Jahadakbar, A.; Amerinatanzi, A.; Ren, Z.; Zhang, H.; Doll, G.L.; et al. Improving surface finish and wear resistance of additive manufactured nickel-titanium by ultrasonic nano-crystal surface modification. J. Mater. Process. Technol. 2017, 249, 433–440. [Google Scholar] [CrossRef]
- Wu, J. Effect of semi-automatic ultrasonic cleaner on cleaning medical devices. Med. Equip. 2018, 31, 65–66. [Google Scholar]
- Tan, W.X.; Tan, K.W.; Tan, K.L. Developing high intensity ultrasonic cleaning (HIUC) for post-processing additively manufactured metal components. Ultrasonics 2022, 126, 106829. [Google Scholar] [CrossRef]
- Łyczkowska, E.; Szymczyk, P.; Dybała, B. Chemical polishing of scaffolds made of Ti-6Al-7Nb alloy by additive manufacturing. Arch. Civ. Mech. Eng. 2014, 14, 586–594. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Z.; Zhao, Y. The effect of nonionic surfactants on the cleaning of aluminum alloy after polishing under the synergistic effect of ultrasound. J. Shanghai Jiao Tong Univ. 2018, 52, 582–586. [Google Scholar]
- Vayre, B.; Vignat, F.; Villeneuve, F. Identification on some design key parameters for additive manufacturing: Application on electron beam melting. Procedia CIRP 2013, 7, 264–269. [Google Scholar] [CrossRef]
- Uhlmann, E. Flexible manufacturing with an additive process chain design, production and surface finish. In Proceedings of the ASPE Spring Topical Meeting—Achieving Precision Tolerances in Additive Manufacturing (Proceedings), Raleigh, NC, USA, 26–29 April 2015. [Google Scholar]
- Adam, G.; Zimmer, D. On design for additive manufacturing: Evaluating geometrical limitations. Rapid Prototyp. 2015, 21, 662–670. [Google Scholar] [CrossRef]
- Moon, S.; Ma, R.; Attardo, R. Impact of surface and pore characteristics on fatigue life of laser powder bed fusion Ti–6Al–4V alloy described by neural network models. Sci. Rep. 2021, 11, 20424. [Google Scholar] [CrossRef]
- Máša, V.; Horňák, D.; Petrilák, D. Industrial use of dry ice blasting in surface cleaning. J. Clean. Prod. 2021, 329, 129630. [Google Scholar] [CrossRef]
- Axmann, B.; Elbing, F. Strahlverfahren. Z. Für Wirtsch. Fabr. ZWF 1997, 92, 76–77. [Google Scholar]
- Hoenig, S. Cleaning surfaces with dry ice. Compress. Air Mag. 1986, 22–25. [Google Scholar]
- Sherman, R. Carbon dioxide snow cleaning. Part. Technol. 2007, 25, 37–57. [Google Scholar] [CrossRef]
- Toscano, T.; Ahmadi, G. Particle removal mechanisms in cryogenic surface cleaning. Adhesion 2003, 79, 175–201. [Google Scholar] [CrossRef]
- Liu, Y.; Hirama, D.; Matsusaka, S. Particle removal process during application of impinging dry ice jet. Powder Technol. 2012, 217, 607–613. [Google Scholar] [CrossRef]
- Spitaels, L.; Rivière-Lorphèvre, É.; Díaz, M. Surface finishing of EBM parts by (electro-) chemical etching. Procedia CIRP 2022, 108, 112–117. [Google Scholar] [CrossRef]
- Surmeneva, M.; Khrapov, D.; Prosolov, K. The influence of chemical etching on porous structure and mechanical properties of the Ti6AL4V Functionally Graded Porous Scaffolds fabricated by EBM. Mater. Chem. Phys. 2022, 275, 125217. [Google Scholar] [CrossRef]
- Buhl, L.; Drue, S.; Lind, L. Field Testing of Acoustical Cleaning of Electrostatic Precipitators; Technical University of Budapest: Budapest, Hungary, 1995. [Google Scholar]
- Gibbs, B.; Seiffert, G.; Maluski, S. Low frequency sonic cleaning of processes involving powdered material, Tenth International Congress on Sound and Vibration. In Proceedings of the Tenth International Congress on Sound and Vibration, Stockholm, Sweden, 7–10 July 2003. [Google Scholar]
- Seiffert, G.; Gibbs, B. Removal of charged powder deposits by high intensity low frequency sound: The role of inertial and drag forces. In Proceedings of the 13th International Conference on Sound and Vibration, Vienna, Austria, 2–6 July 2006; pp. 2–6. [Google Scholar]
- Predergast, P. Powder Removal from Internal Cavities of 3D Printed Parts; Massachusetts Institute of Technology: Cambridge, MA, USA, 1995. [Google Scholar]
- Pharmaceutical Industry Standards of China YY/T1809. Additive Manufacturing for Medical Applications-Verification Methods of Cleaning and Cleaning Effectiveness of Metal Powder Used in Powder-Bed Fusion Process; State Drug Administration of China: Beijing, China, 2021. [Google Scholar]
- Schllephake, H.; Lehmann, H.; Kunz, U. Ultrastructural findings in soft tissues adjacent to titanium plates used in jaw fracture treatment. Int. J. Oral Maxillofac. Surg. 1993, 22, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Hasib, H.; Harrysson, O.; West, A. Powder removal from Ti-6Al-4V cellular structures fabricated via electron beam melting. JOM 2015, 67, 639–646. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, J.; Shi, W. Study on mechanical properties of 316L stainless steel porous structure by laser powder bed forming. Chin. J. Lasers 2022, 49, 0802018. [Google Scholar]
- Wingham, J.; Turner, R.; Shepherd, J. Micro-CT for analysis of laser sintered micro-composites. Rapid Prototyp. J. 2020, 26, 649–657. [Google Scholar] [CrossRef]
- Hunter, L.; Brackett, D.; Brierley, N. Assessment of trapped powder removal and inspection strategies for powder bed fusion techniques. Int. J. Adv. Manuf. Technol. 2020, 106, 4521–4532. [Google Scholar] [CrossRef]
- Wong, P.-C.; Song, S.-M.; Tsai, P.-H.; Nien, Y.-Y.; Jang, J.S.-C.; Cheng, C.-K.; Chen, C.-H. Relationship between the Surface Roughness of Biodegradable Mg-Based Bulk Metallic Glass and the Osteogenetic Ability of MG63 Osteoblast-like Cells. Materials 2020, 13, 1188. [Google Scholar] [CrossRef]
- Damiati, L.; Eales, M.G.; Nobbs, A.H.; Su, B.; Tsimbouri, P.M.; Salmeron-Sanchez, M.; Dalby, M.J. Impact of Surface Topography and Coating on Osteogenesis and Bacterial Attachment on Titanium Implants. J. Tissue Eng. 2018, 9, 492–495. [Google Scholar] [CrossRef]
- Zhang, X.; Fang, G.; Zhou, J. Additively manufactured scaffolds for bone tissue engineering and the prediction of their mechanical behavior: A review. Materials 2017, 10, 50. [Google Scholar] [CrossRef] [PubMed]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully porous 3D printed titanium femoral stem to reduce stress-shielding following total hip arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhang, Y.; Shen, X. The fabrication and in vitro properties of antibacterial polydopamine-LL-37-POPC coatings on micro-arc oxidized titanium. Colloids Surf. B Biointerfaces 2018, 170, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Danmei, Z. Study on subchronic systemic toxicity of 3D printed orthopedic titanium alloy. J. Tissue Eng. Reconstr. Surg. 2020, 16, 6–10. [Google Scholar]
- Brydone, A.; Meek, D.; Maclaine, A.S. Bone grafting, orthopaedic biomaterials, and the clinical need for bone engineering. Proc. Inst. Mech. Eng. 2010, 224, 1329–1343. [Google Scholar] [CrossRef]
- Murr, L.E. Open-Cellular Metal Implant Design and Fabrication for Biomechanical Compatibility with Bone Using Electron Beam Melting. Mech. Behav. Biomed. Mater. 2017, 76, 164–177. [Google Scholar] [CrossRef]
- Ameen, W.; Al-Ahmari, A.; Mohammed, M. Design, finite element analysis (FEA), and fabrication of custom titanium alloy cranial implant using electron beam melting additive manufacturing. Adv. Prod. Eng. Manag. 2018, 13, 267–278. [Google Scholar] [CrossRef]
- Mazzoli, A.; Germani, M.; Raffaeli, R. Direct fabrication through electron beam melting technology of custom cranial implants designed in a PHANToM-based haptic environment. Mater. Des. 2009, 30, 3186–3192. [Google Scholar] [CrossRef]
- Zhao, X.; Li, K.; Bai, X. Study on impact resistance of 3D printed titanium alloy personalized skull prosthesis. Integr. Technol. 2022, 11, 48–56. [Google Scholar]
- Tian, K.; Wang, L.; Wang, M.; Li, H.; Chen, R.; Zhang, K. Experimental study of 3D printed porous titanium scaffolds loaded with BMP-2 and VEGF for jaw defect repair. Beijing Stomatol. 2022, 30, 165–170. [Google Scholar]
- Yan, R.; Luo, D.; Huang, H.; Li, R.; Yu, N.; Liu, C.; Hu, M.; Rong, Q. Electron beam melting in the fabrication of three-dimensional mesh titanium mandibular prosthesis scaffold. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Moiduddin, K.; Hammad, M.; Umer, U. Reconstruction of complex zygomatic bone defects using mirroring coupled with EBM fabrication of titanium implant. Metals 2019, 9, 1250. [Google Scholar] [CrossRef]
- Mommaerts, M.Y. Additively Manufactured Sub-Periosteal Jaw Implants. Int. J. Oral Maxillofac. Surg. 2017, 46, 938–940. [Google Scholar] [CrossRef] [PubMed]
- Van den Borre, C.; Rinaldi, M.; De Neef, B. Patient-and clinician-reported outcomes for the additively manufactured sub-periosteal jaw implant (AMSJI) in the maxilla: A prospective multicentre one-year follow-up study. Int. J. Oral Maxillofac. Surg. 2022, 51, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.; Bhutta, Z.; Brown, A. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef]
- Bosshardt, D.D.; Chappuis, V.; Buser, D. Osseointegration of titanium, titanium alloy and zirconia dental implants: Current knowledge and open questions. Periodontology 2017, 73, 22–40. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Zhang, B. Cytotoxicity of 3D printed titanium alloy dental implants. Chin. J. Oral Implantol. 2019, 24, 10–13. [Google Scholar]
- Tolochko, N.; Savich, V.; Laoui, T. Dental root implants produced by the combined selective laser sintering/melting of titanium powders. J. Mater. Des. Appl. 2002, 216, 267–270. [Google Scholar] [CrossRef]
- Chan, K.; Koike, M.; Mason, R. Fatigue life of titanium alloys fabricated by additive layer manufacturing techniques for dental implants. Metall. Mater. Trans. A 2013, 44, 1010–1022. [Google Scholar] [CrossRef]
- Gonzalez, J.; Mirza-Rosca, J. Study of the corrosion behavior of titanium and some of its alloys for biomedical and dental implant applications. J. Electroanal. Chem. 1999, 471, 109–115. [Google Scholar] [CrossRef]
- Hamza, H.M.; Deen, K.M.; Haider, W. Microstructural Examination and Corrosion Behavior of Selective Laser Melted and Conventionally Manufactured Ti6Al4V for Dental Applications. Mater. Sci. Eng. C 2020, 113, 110980. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Q. 3D re-construction and SLM survey for dental implants. Mech. Med. Biol. 2017, 17, 1750084. [Google Scholar] [CrossRef]
- Takemoto, M.; Fujibayashi, S.; Neo, M.; So, K.; Akiyama, N.; Matsushita, T.; Kokubo, T.; Nakamura, T. A Porous Bioactive Titanium Implant for Spinal Interbody Fusion: An Experimental Study Using a Canine Model. J. Neurosurg. Spine 2007, 7, 435–443. [Google Scholar] [CrossRef]
- Wu, S.-H.; Li, Y.; Zhang, Y.-Q.; Li, X.-K.; Yuan, C.-F.; Hao, Y.-L.; Zhang, Z.-Y.; Guo, Z. Porous Titanium-6 Aluminum-4 Vanadium Cage Has Better Osseointegration and Less Micromotion than a Poly-Ether-Ether-Ketone Cage in Sheep Vertebral Fusion. Artif. Organs 2013, 37, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Hollander, D.A.; Von Walter, M.; Wirtz, T.; Sellei, R.; Schmidt-Rohlfing, B.; Paar, O.; Erli, H.J. Structural, mechanical and in vitro characterization of individually structured Ti-6Al-4V produced by direct laser forming. Biomaterials 2006, 27, 955–963. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Wirtz, T.; LaMarca, F.; Hollister, S.J. Structural and Mechanical Evaluations of a Topology Optimized Titanium Interbody Fusion Cage Fabricated by Selective Laser Melting Process. J. Biomed. Mater. Res. Part A 2007, 83A, 272–279. [Google Scholar] [CrossRef]
- Xu, N.; Wei, F.; Liu, X.; Jiang, L.; Cai, H.; Li, Z.; Yu, M.; Wu, F.; Liu, Z. Reconstruction of the upper cervical spine using a personalized 3D-printed vertebral body in an adolescent with ewing sarcoma. Spine 2016, 41, E50–E54. [Google Scholar] [CrossRef] [PubMed]
- Fujibayashi, S.; Takemoto, M.; Neo, M.; Matsushita, T.; Kokubo, T.; Doi, K.; Ito, T.; Shimizu, A.; Nakamura, T. A Novel Synthetic Material for Spinal Fusion: A Prospective Clinical Trial of Porous Bioactive Titanium Metal for Lumbar Interbody Fusion. Eur. Spine J. 2011, 20, 1486–1495. [Google Scholar] [CrossRef] [PubMed]
- Turna, A.; Kavakli, K.; Sapmaz, E.; Arslan, H.; Caylak, H.; Gokce, H.S.; Demirkaya, A. Reconstruction with a Patient-Specific Titanium Implant after a Wide Anterior Chest Wall Resection. Interdiscip. CardioVascular Thorac. Surg. 2013, 18, 234–236. [Google Scholar] [CrossRef]
- Alvarez, A.; Evans, P.; Dovgalski, L. Design, additive manufacture and clinical application of a patient-specific titanium implant to anatomically reconstruct a large chest wall defect. Rapid Prototyp. J. 2021, 27, 304–310. [Google Scholar] [CrossRef]
- Goldsmith, I.; Evans, P.; Goodrum, H. Chest wall reconstruction with an anatomically designed 3-D printed titanium ribs and hemi-sternum implant. 3D Print. Med. 2020, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, J.; Zhang, Y.; Lin, Q.; Wu, H.; Xu, Q.; Chen, L. The application of 3D printed titanium alloy artificial ribs and autologous rib transplantation in partial resection of ribs and sternoclavicular. Jiangxi Med. 2021, 56, 31–33+36. [Google Scholar]
- Fang, C.; Cai, H.; Kuong, E. Surgical applications of three-dimensional printing in the pelvis and acetabulum: From models and tools to implants. Der Unf. 2019, 122, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Babazadeh-Naseri, A.; Dunbar, N. Finite Element Analysis of Screw Fixation Durability under Multiple Boundary and Loading Conditions for a Custom Pelvic Implant. Med. Eng. Phys. 2022, 11, 103930. [Google Scholar] [CrossRef]
- Wong, K.C.; Kumta, S.M.; Gee, N.V.L.; Demol, J. One-step reconstruction with a 3D-printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput. Aided Surg. 2015, 20, 14–23. [Google Scholar] [CrossRef]
- Broekhuis, D.; Boyle, R.; Karunaratne, S. Custom designed and 3D-printed titanium pelvic implants for acetabular reconstruction after tumour resection. HIP Int. 2022, 11, 11207000221135068. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Park, H.; Kim, J.H.; Kim, H.M.; Yoo, C.H.; Kang, H.G. Fabrication of a Lattice Structure with Periodic Open Pores through Three-Dimensional Printing for Bone Ingrowth. Sci. Rep. 2022, 12, 17223. [Google Scholar] [CrossRef]
- Lv, Y.; Wang, B.; Liu, G.; Tang, Y.; Liu, J.; Wei, G.; Wang, L. Design of Bone-like Continuous Gradient Porous Scaffold Based on Triply Periodic Minimal Surfaces. J. Mater. Res. Technol. 2022, 21, 3650–3665. [Google Scholar] [CrossRef]
- Gupta, S.K.; Shahidsha, N.; Bahl, S.; Kedaria, D.; Singamneni, S.; Yarlagadda, P.K.D.V.; Suwas, S.; Chatterjee, K. Enhanced Biomechanical Performance of Additively Manufactured Ti-6Al-4V Bone Plates. J. Mech. Behav. Biomed. Mater. 2021, 119, 104552. [Google Scholar] [CrossRef]
- Scherer, K.; Huwer, A.; Ulber, R.; Wahl, M. Optimizing Luminous Transmittance of a Three-Dimensional-Printed Fixed Bed Photobioreactor. 3D Print. Addit. Manuf. 2022. [Google Scholar] [CrossRef]
- Weißmann, V.; Boss, C.; Schulze, C.; Hansmann, H.; Bader, R. Experimental characterization of the primary stability of acetabular press-fit cups with open-porous load-bearing structures on the surface layer. Metals 2018, 8, 839. [Google Scholar] [CrossRef]
- Murr, L.E.; Amato, K.N.; Li, S.J.; Tian, Y.X.; Cheng, X.Y.; Gaytan, S.M.; Martinez, E.; Shindo, P.W.; Medina, F.; Wicker, R.B. Microstructure and mechanical properties of open-cellular biomaterials prototypes for total knee replacement implants fabricated by electron beam melting. J. Mech. Behav. Biomed. Mater. 2011, 4, 1396–1411. [Google Scholar] [CrossRef] [PubMed]
- Herbert, N.; Simpson, D.; Spence, W.; Ion, W. A preliminary investigation into the development of 3D printing of prosthetic sockets. Rehabil. Res. Dev. 2005, 42, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Zuniga, J.M.; Dimitrios, K.; Peck, L.; Srivastava, R.; Pierce, J.E.; Dudley, D. Coactivation index of children with congenital upper limb reduction deficiencies before and after using a wrist-driven 3D printed partial hand prosthesis. J. Neuroeng. Rehabil. 2018, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Telfer, S.; Pallari, J.; Munguia, J.; Dalgarno, K.; McGeough, M.; Woodburn, J. Embracing additive manufacture: Implications for foot and ankle orthosis design. BMC Musculoskelet. Disord. 2012, 13, 84. [Google Scholar] [CrossRef]
- Paterson, A.; Bibb, R.; Campbell, R.; Bingham, G. Comparing additive manufacturing technologies for customised wrist splints. Rapid Prototyp. 2015, 21, 230–243. [Google Scholar] [CrossRef]









| Advantages | Disadvantages |
|---|---|
| Low cost | Very time consuming |
| Can be cleaned in batches | May cause damage |
| Comprehensive cleaning range | There is noise |
| Wide applicability | |
| Environmental safety |
| Advantages | Disadvantages |
|---|---|
| Simple operation | High cost |
| Thorough effect | Will introduce sand particles |
| Environmental pollution-free | Maintenance of machinery equipment |
| High efficiency | |
| Wide application range |
| Advantages | Disadvantages |
|---|---|
| Fast process speed | Operational difficulties |
| Low abrasiveness | Raw materials difficult to store |
| No secondary waste generated | There are security risks |
| Advantages | Disadvantages |
|---|---|
| Good cleaning effect | Will produce wastewater |
| Efficient | Complex operation |
| Cost is lower |
| Advantages | Disadvantages |
|---|---|
| Comprehensive cleaning | Inefficiency |
| Pollution free | Expensive equipment |
| Simplicity of operation |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Liu, S.; Liu, Y.; Guo, H.; Shi, W. Titanium Alloy Fabricated by Additive Manufacturing for Medical Applications: Obtaining, Characterization and Application—Review. Metals 2023, 13, 462. https://doi.org/10.3390/met13030462
Zhang X, Liu S, Liu Y, Guo H, Shi W. Titanium Alloy Fabricated by Additive Manufacturing for Medical Applications: Obtaining, Characterization and Application—Review. Metals. 2023; 13(3):462. https://doi.org/10.3390/met13030462
Chicago/Turabian StyleZhang, Xinjie, Shuai Liu, Yude Liu, Hanjie Guo, and Wentian Shi. 2023. "Titanium Alloy Fabricated by Additive Manufacturing for Medical Applications: Obtaining, Characterization and Application—Review" Metals 13, no. 3: 462. https://doi.org/10.3390/met13030462
APA StyleZhang, X., Liu, S., Liu, Y., Guo, H., & Shi, W. (2023). Titanium Alloy Fabricated by Additive Manufacturing for Medical Applications: Obtaining, Characterization and Application—Review. Metals, 13(3), 462. https://doi.org/10.3390/met13030462








