Process Parameter Optimization for Selective Laser-Melted High-Nitrogen Steel and the Effects on Microstructure and Properties
Abstract
1. Introduction
2. Experimental Materials and Methods
3. Results and Discussion
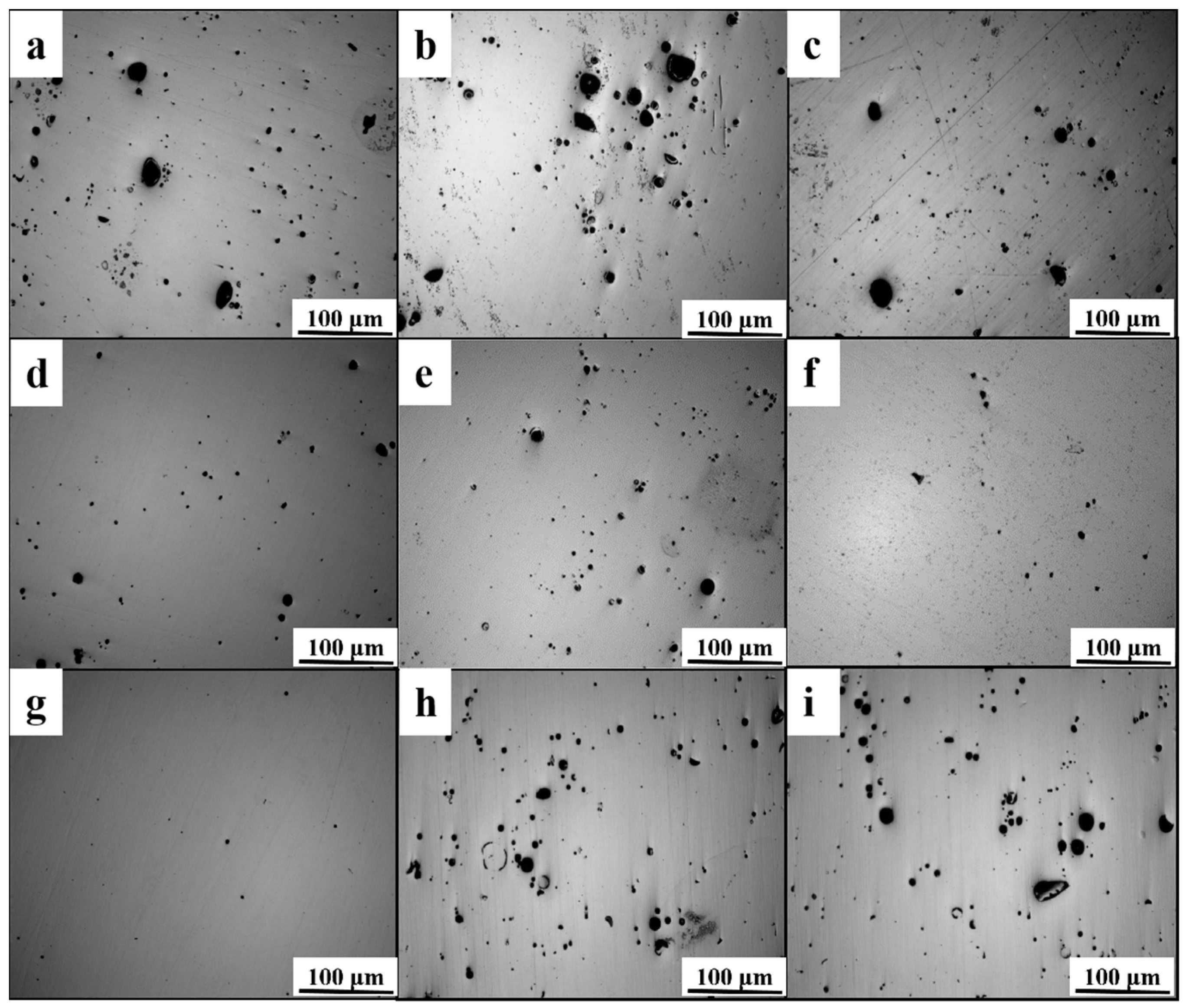
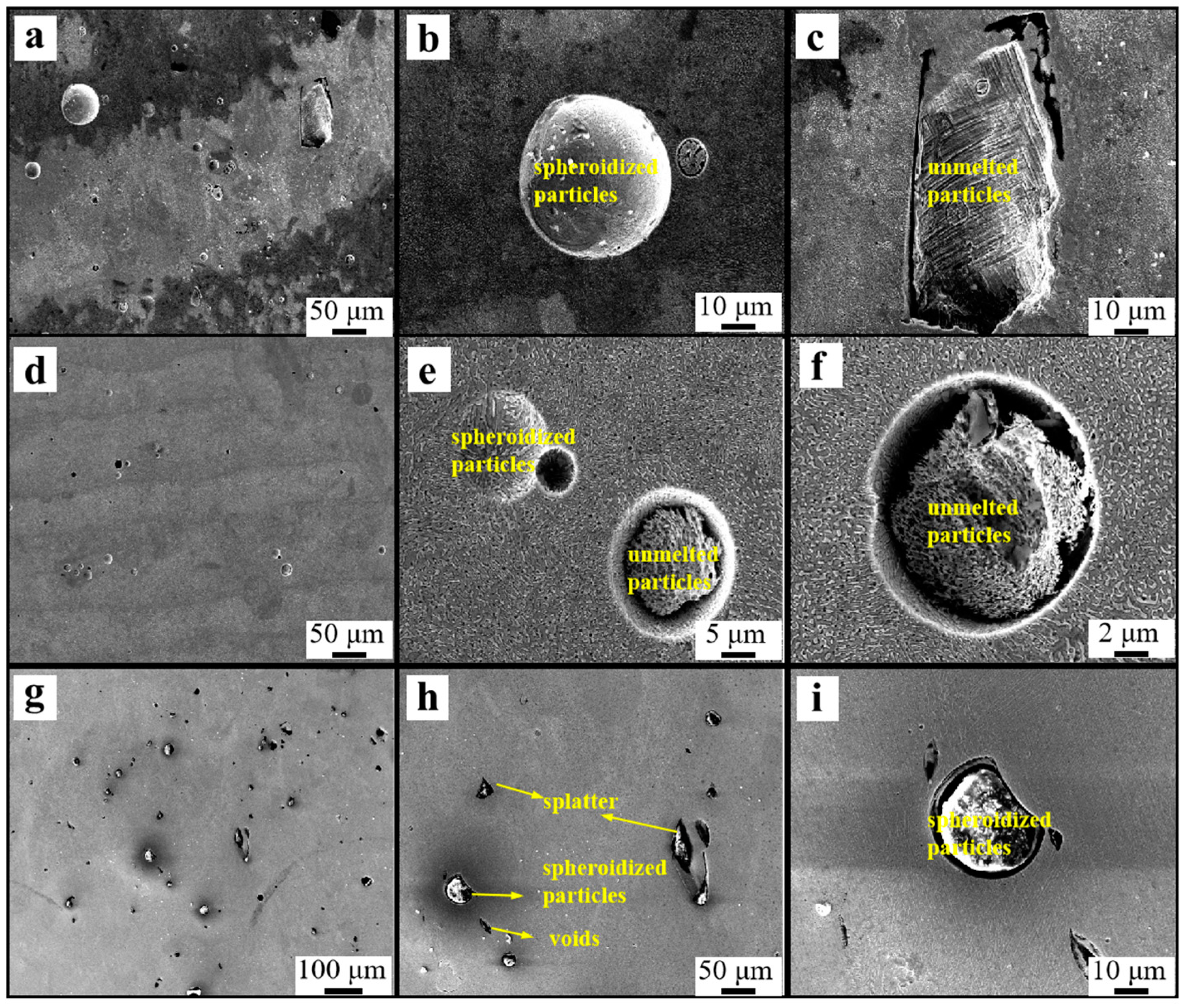
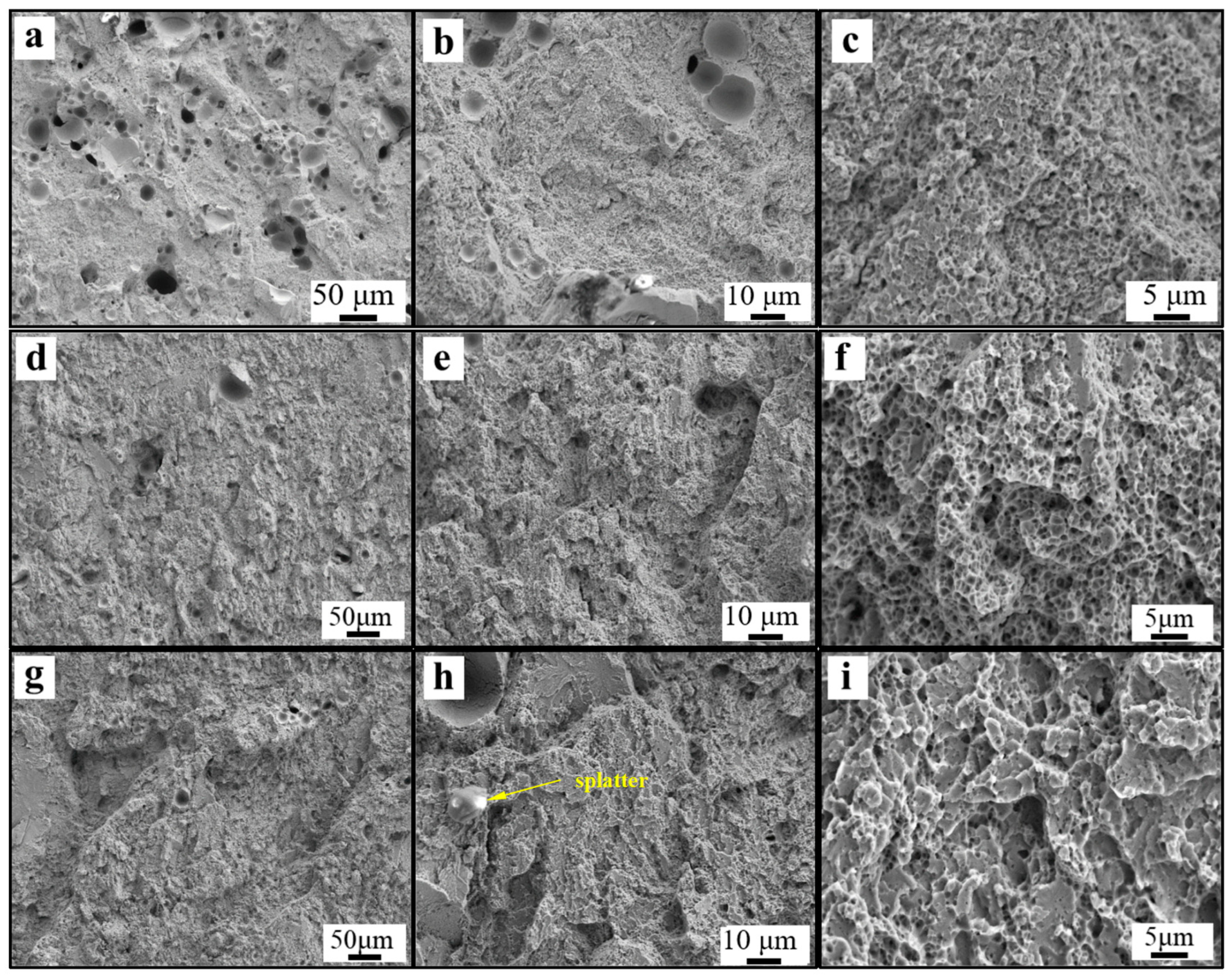
3.1. Relative Density of the High-Nitrogen Steel
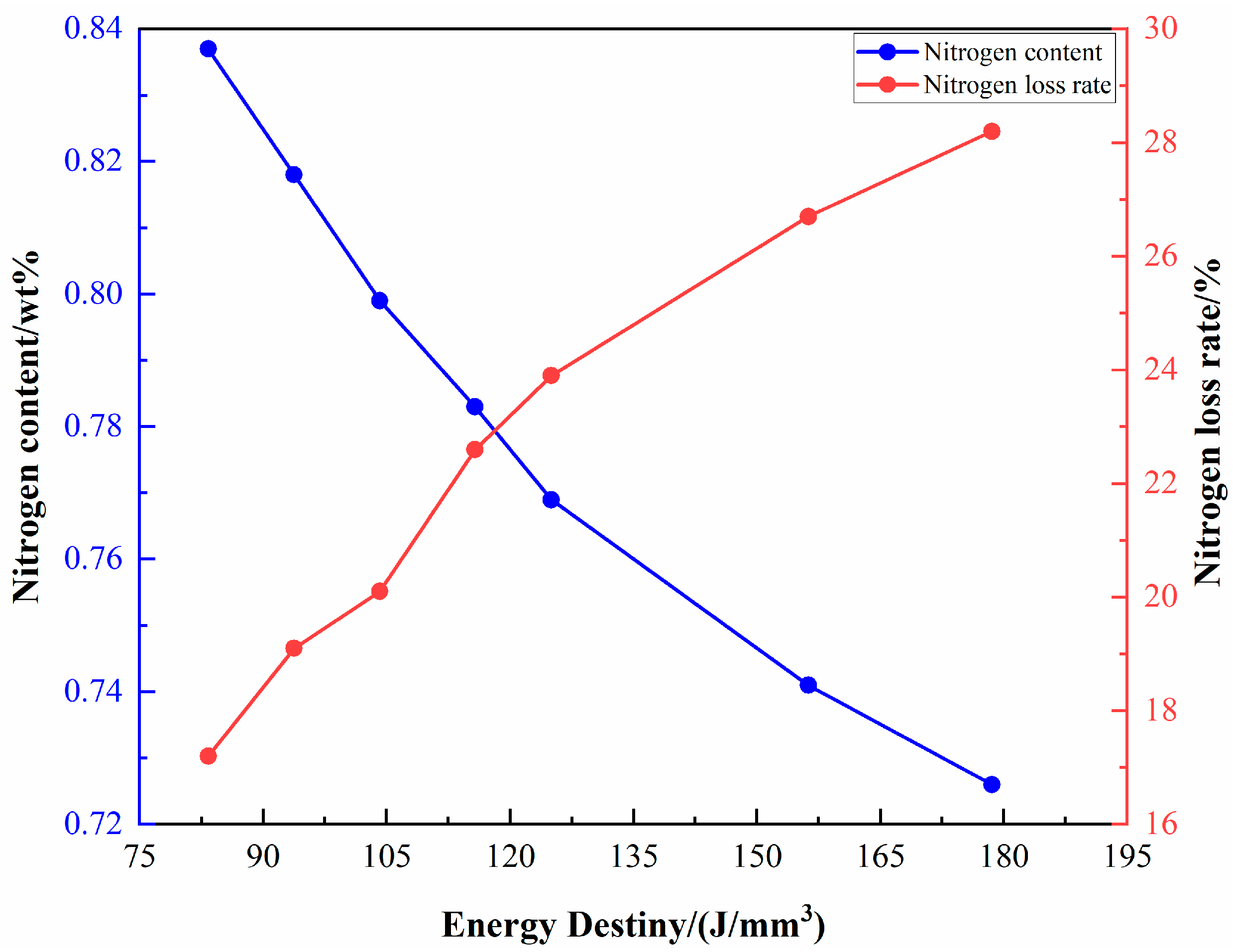
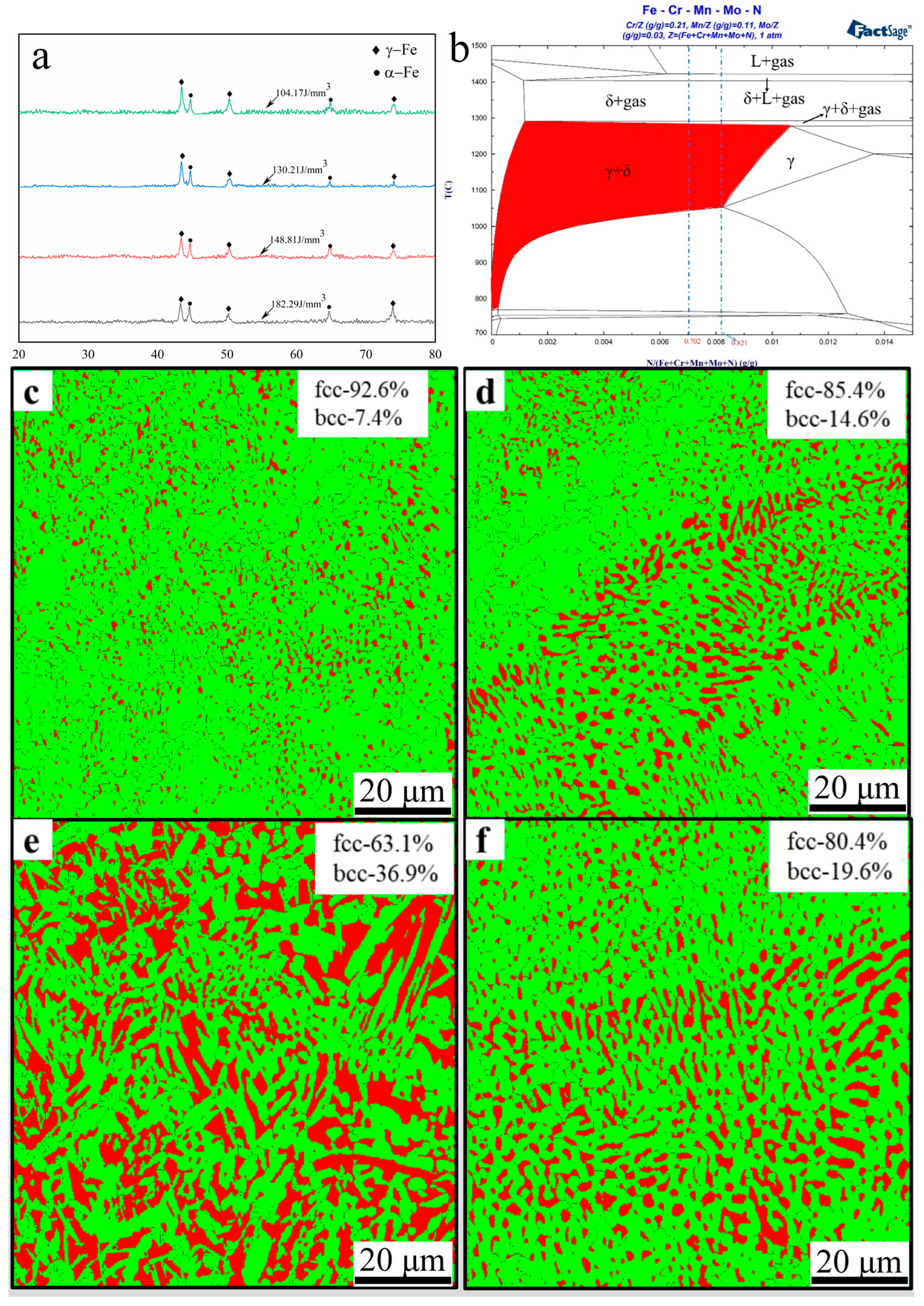
3.2. Nitrogen Concentration and Phase Evolution
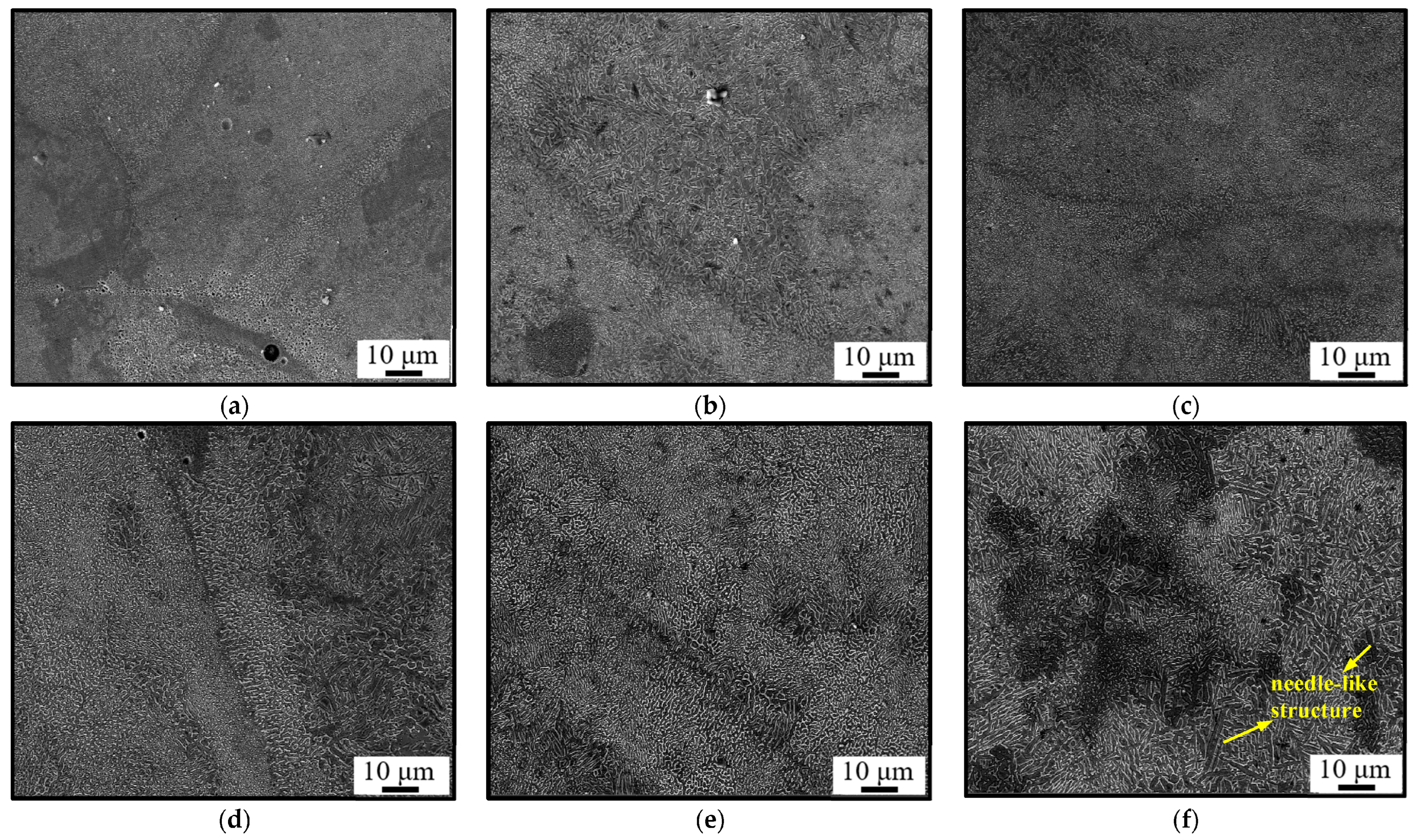
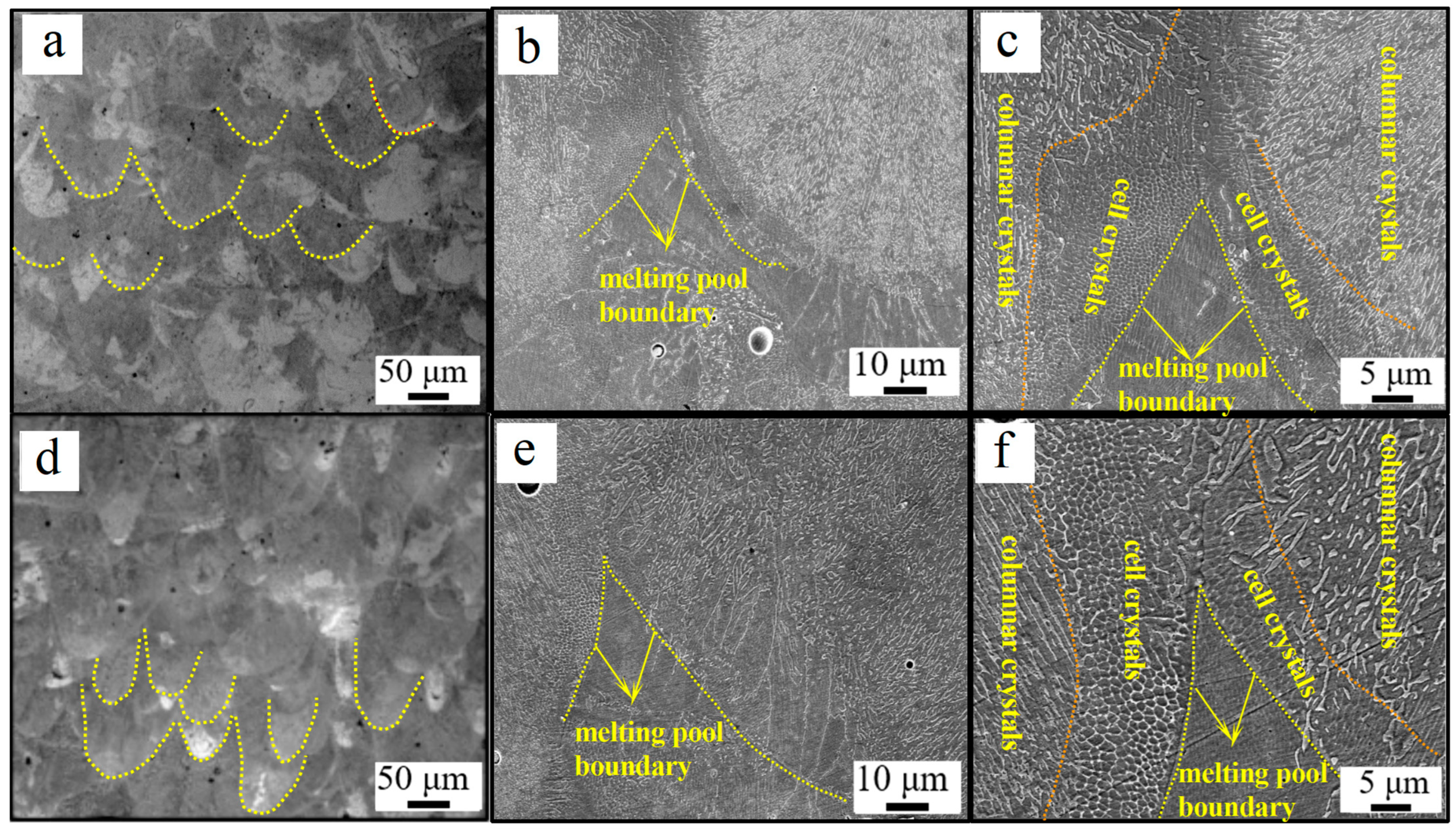
3.3. Microstructural Evolution
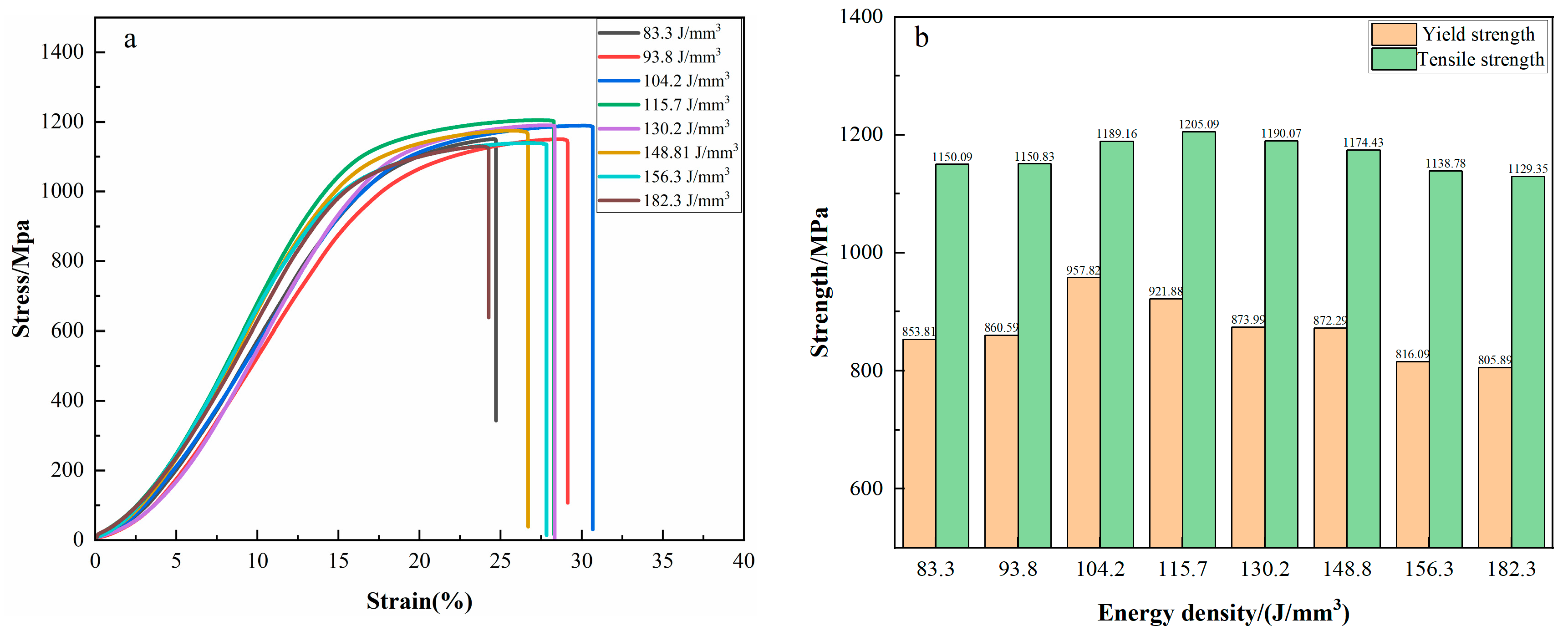
3.4. Mechanical Properties
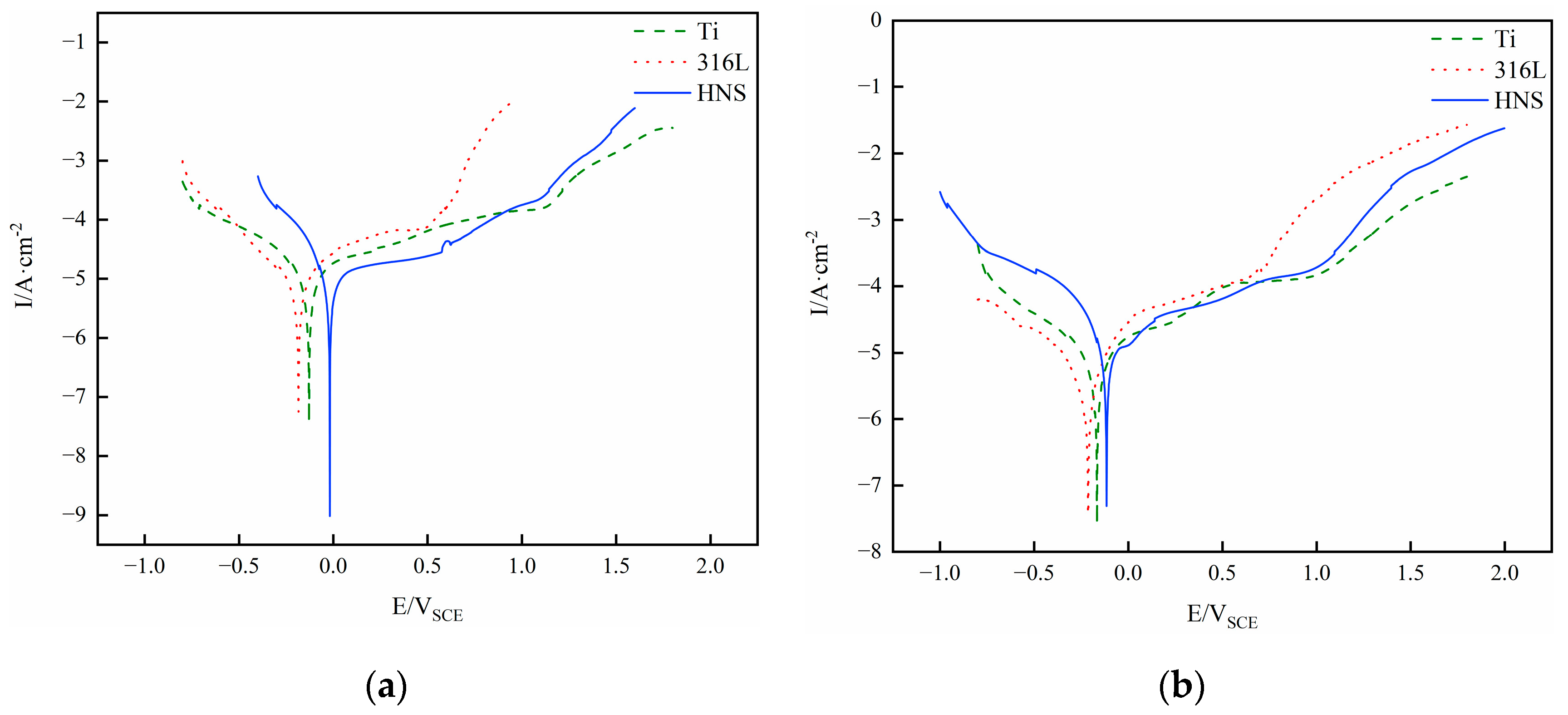
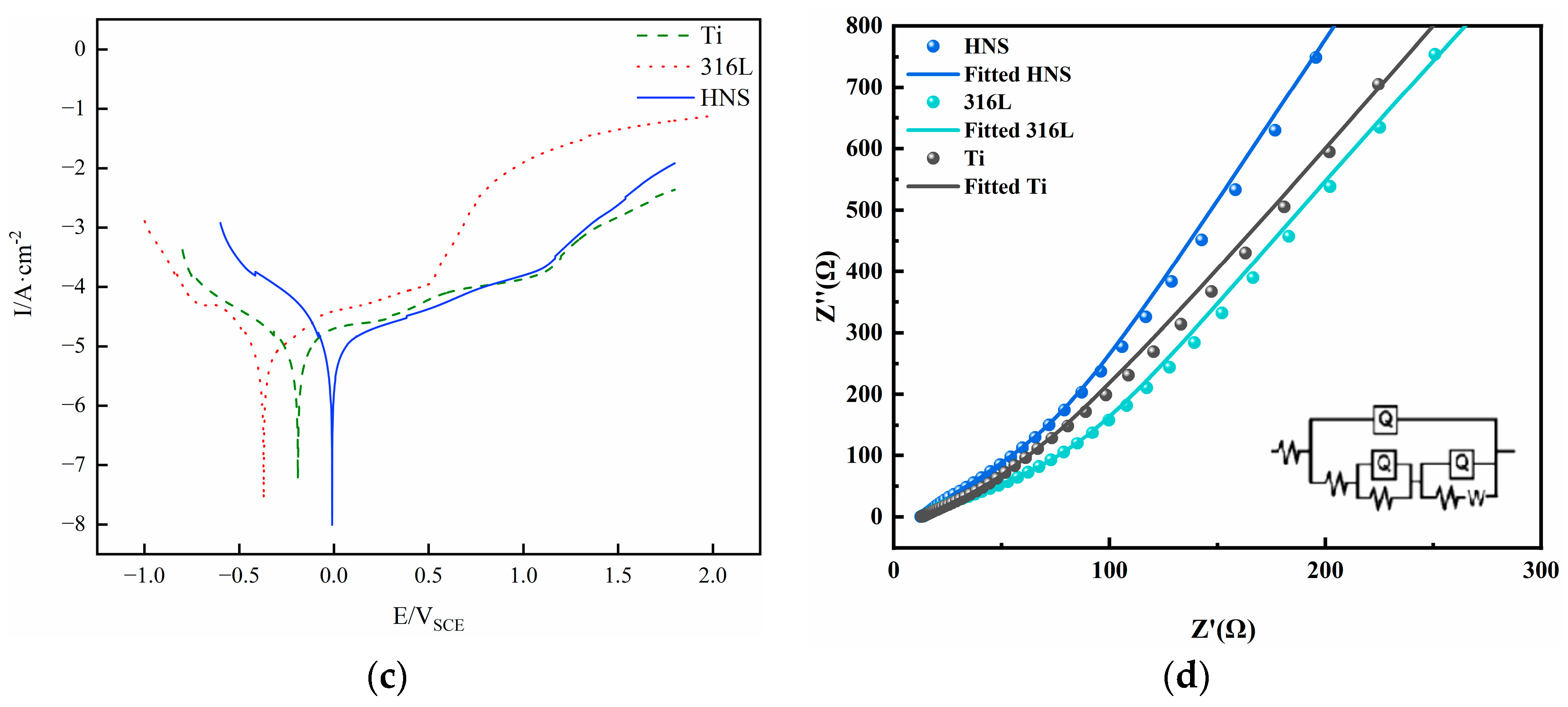
3.5. Corrosion Resistance
4. Conclusions
- With increasing energy density, the relative density of high-nitrogen steel first increases and subsequently drops. The best process parameters are 250 W laser power, 700 mm/s scanning speed, with 148.8 J/cm3 energy density. The alloy had the maximum density at this period, reaching 98.8%. The amount of unmelted particles and spheroidized flaws reduces as the energy density increases, increasing the relative density.
- The high-nitrogen steel has a microstructure that is mostly made up of columnar and cellular crystals. The size of cellular and columnar grains steadily grow as energy density rises.
- The mechanical characteristics of high-nitrogen steel first rise and then fall as energy density rises. The mechanical characteristics are the highest when the process parameters are 250 W and 700 mm/s, with yield strength, tensile strength, and elongation values reaching 921.9 MPa, 1205.1 MPa, and 27%, respectively.
- The high-nitrogen steel sample at an energy density of 148.8 J/mm³ has superior corrosion resistance in human simulated liquids when compared to pure titanium and 316L steel. In human simulated liquids (0.9% NaCl solution, simulated plasma, and Hank’s solution), high-nitrogen steel has the highest open circuit corrosion potential and the slowest corrosion rate, and its passivation film stability is slightly worse than pure titanium.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Li, Y.; Ding, J.; Xie, Q.; Zhang, X.; Wang, K. Problems in Welding of High Nitrogen Steel: A Review. Metals 2022, 12, 1273. [Google Scholar] [CrossRef]
- Luo, X.; Lou, J.; He, H.; Wu, C.; Huang, Y.; Su, N.; Li, S. Effects of Carbon Content on the Properties of Novel Nitrogen-Free Austenitic Stainless Steel with High Hardness Prepared via Metal Injection Molding. Metals 2023, 13, 403. [Google Scholar] [CrossRef]
- Paton, B.E.; Medovar, B.I.; Saenko, V. Role of Electroslag Technology in Production of Massively Nitrogen-Alloyed Steels [Previously Titled: The Place of Electroslag Technology in Production of Super-High- Nitrogen Steels]. Adv. Spec. Electrometall. 1990, 6, 193–200. [Google Scholar]
- Mudali, U.K. High Nitrogen Steels and Stainless Steels: Manufacturing, Properties and Applications; Alpha Science: Rowland Heights, CA, USA, 2004. [Google Scholar]
- Yang, D.; Huang, Y.; Fan, J.; Jin, M.; Peng, Y.; Wang, K. Effect of N2 Content in Shielding Gas on Formation Quality and Microstructure of High Nitrogen Austenitic Stainless Steel Fabricated by Wire and Arc Additive Manufacturing. J. Manuf. Process. 2021, 61, 261–269. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Wang, Z. Enhancing Yield Strength of High Nitrogen Austenitic Stainless Steel. J. Constr. Steel Res. 2021, 187, 106927. [Google Scholar] [CrossRef]
- Wang, M.; You, B.; Wu, Y.; Liang, B.; Gao, X.; Li, W.; Wei, Q. Effect of Cr, Mo, and V Elements on the Microstructure and Thermal Fatigue Properties of the Chromium Hot-Work Steels Processed by Selective Laser Melting. Metals 2022, 12, 735. [Google Scholar] [CrossRef]
- Fang, X.; Xia, W.; Wei, Q.; Wu, Y.; Lv, W.; Guo, W. Preparation of Cu-Cr-Zr Alloy by Laser Powder Bed Fusion: Parameter Optimization, Microstructure, Mechanical and Thermal Properties for Microelectronic Applications. Metals 2021, 11, 1410. [Google Scholar] [CrossRef]
- Frazier, W.E. Metal Additive Manufacturing: A Review. J. Mater. Eng. Perform. 2014, 23, 1917–1928. [Google Scholar] [CrossRef]
- Murr, L.E.; Martinez, E.; Amato, K.N.; Gaytan, S.M.; Hernandez, J.; Ramirez, D.A.; Shindo, P.W.; Medina, F.; Wicker, R.B. Fabrication of Metal and Alloy Components by Additive Manufacturing: Examples of 3D Materials Science. J. Mater. Res. Technol. 2012, 1, 42–54. [Google Scholar] [CrossRef]
- The High Temperature Flow Behavior of Additively Manufactured Inconel 625 Superalloy—IOPscience. Available online: https://iopscience.iop.org/article/10.1088/2053-1591/ab44f6/meta (accessed on 2 July 2023).
- Ahmadi, M.; Tabary, S.A.A.B.; Rahmatabadi, D.; Ebrahimi, M.S.; Abrinia, K.; Hashemi, R. Review of Selective Laser Melting of Magnesium Alloys: Advantages, Microstructure and Mechanical Characterizations, Defects, Challenges, and Applications. J. Mater. Res. Technol. 2022, 19, 1537–1562. [Google Scholar] [CrossRef]
- Azami, M.; Siahsarani, A.; Hadian, A.; Kazemi, Z.; Rahmatabadi, D.; Kashani-Bozorg, S.F.; Abrinia, K. Laser Powder Bed Fusion of Alumina/Fe–Ni Ceramic Matrix Particulate Composites Impregnated with a Polymeric Resin. J. Mater. Res. Technol. 2023, 24, 3133–3144. [Google Scholar] [CrossRef]
- Wu, T.; Liu, J.; Wang, K.; Wang, L.; Zhang, X. Microstructure and Mechanical Properties of Wire-Powder Hybrid Additive Manufacturing for High Nitrogen Steel. J. Manuf. Process. 2021, 70, 248–258. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z.; Liu, Z.; Wang, H.; Zhang, Y.; Wang, D. Influence of Shielding Gas Flow Consistency on Parts Quality Consistency during Large-Scale Laser Powder Bed Fusion. Opt. Laser Technol. 2023, 158, 108899. [Google Scholar] [CrossRef]
- Sun, X.; Ren, J.; Wang, Y.; Zhao, D.; Wang, S.; Xiong, X.; Rao, J.H. Nitriding Behaviour and Microstructure of High-Nitrogen Stainless Steel during Selective Laser Melting. Materials 2023, 16, 2505. [Google Scholar] [CrossRef]
- Lü, Y.; Tao, L.; Jie, L.; Kehong, W. Microstructure and Mechanical Properties of High-Nitrogen Stainless Steel Manufactured by Selective Laser Melting. Chin. J. Lasers 2022, 49, 2202021. [Google Scholar] [CrossRef]
- Sun, X.; Ren, J.; Wang, S.; Zhao, D. Effect of Powder Formulation and Energy Density on the Nitrogen Content, Microstructure, and Mechanical Properties of SLMed High-Nitrogen Steel. Processes 2023, 11, 1937. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Potenza, F.; Magalini, E.; Luchin, V.; Molinari, A.; Pérez-Prado, M.T. Effect of Energy Density on the Microstructure and Texture Evolution of Ti-6Al-4V Manufactured by Laser Powder Bed Fusion. Mater. Charact. 2020, 163, 110238. [Google Scholar] [CrossRef]
- Schwanekamp, T.; Müller, A.; Reuber, M.; Gobran, H.; Gdoura, N.; von Cetto, S. Investigations on Laser Powder Bed Fusion of Tungsten Heavy Alloys. Int. J. Refract. Met. Hard Mater. 2022, 109, 105959. [Google Scholar] [CrossRef]
- Wang, D.; Wu, S.; Fu, F.; Mai, S.; Yang, Y.; Liu, Y.; Song, C. Mechanisms and Characteristics of Spatter Generation in SLM Processing and Its Effect on the Properties. Mater. Des. 2017, 117, 121–130. [Google Scholar] [CrossRef]
- Svyazhin, A.; Kaputkina, L.; Smarygina, I.; Kaputkin, D. Nitrogen Steels and High-Nitrogen Steels: Industrial Technologies and Properties. Steel Res. Int. 2022, 93, 2200160. [Google Scholar] [CrossRef]
- Simmons, J.W. Overview: High-Nitrogen Alloying of Stainless Steels. Mater. Sci. Eng. A 1996, 207, 159–169. [Google Scholar] [CrossRef]
- Luo, X.; Yang, C.; Fu, Z.Q.; Liu, L.H.; Lu, H.Z.; Ma, H.W.; Wang, Z.; Li, D.D.; Zhang, L.C.; Li, Y.Y. Achieving Ultrahigh-Strength in Beta-Type Titanium Alloy by Controlling the Melt Pool Mode in Selective Laser Melting. Mater. Sci. Eng. A 2021, 823, 141731. [Google Scholar] [CrossRef]
- Shifeng, W.; Shuai, L.; Qingsong, W.; Yan, C.; Sheng, Z.; Yusheng, S. Effect of Molten Pool Boundaries on the Mechanical Properties of Selective Laser Melting Parts. J. Mater. Process. Technol. 2014, 214, 2660–2667. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, X.; Yuan, D.; Guo, C.; Huang, H.; Yang, B. Microstructure and Properties of Cu-Cr-Zr Alloy with Columnar Crystal Structure Processed by Upward Continuous Casting. J. Alloys Compd. 2021, 889, 161700. [Google Scholar] [CrossRef]
- Nyby, C.; Guo, X.; Saal, J.E.; Chien, S.-C.; Gerard, A.Y.; Ke, H.; Li, T.; Lu, P.; Oberdorfer, C.; Sahu, S.; et al. Electrochemical Metrics for Corrosion Resistant Alloys. Sci. Data 2021, 8, 58. [Google Scholar] [CrossRef]
- Effect of Relative Density on Microstructure, Corrosion Resistance and Mechanical Performance of Porous Ti–20Zr Alloys Fabricated by Powder Metallurgy|SpringerLink. Available online: https://link.springer.com/article/10.1007/s13369-023-07889-4 (accessed on 2 July 2023).
- Liao, L.; Wan, Q.; Wang, H.; Yang, B.; Mei, Q. Irradiation Enhanced Corrosion Resistance of CrN/TiSiN Multilayers Synthesized by Cathodic Arc Ion Plating. Mater. Today Commun. 2023, 35, 106155. [Google Scholar] [CrossRef]
- Liu, C.; Bi, Q.; Leyland, A.; Matthews, A. An Electrochemical Impedance Spectroscopy Study of the Corrosion Behaviour of PVD Coated Steels in 0.5 N NaCl Aqueous Solution: Part II.: EIS Interpretation of Corrosion Behaviour. Corros. Sci. 2003, 45, 1257–1273. [Google Scholar] [CrossRef]
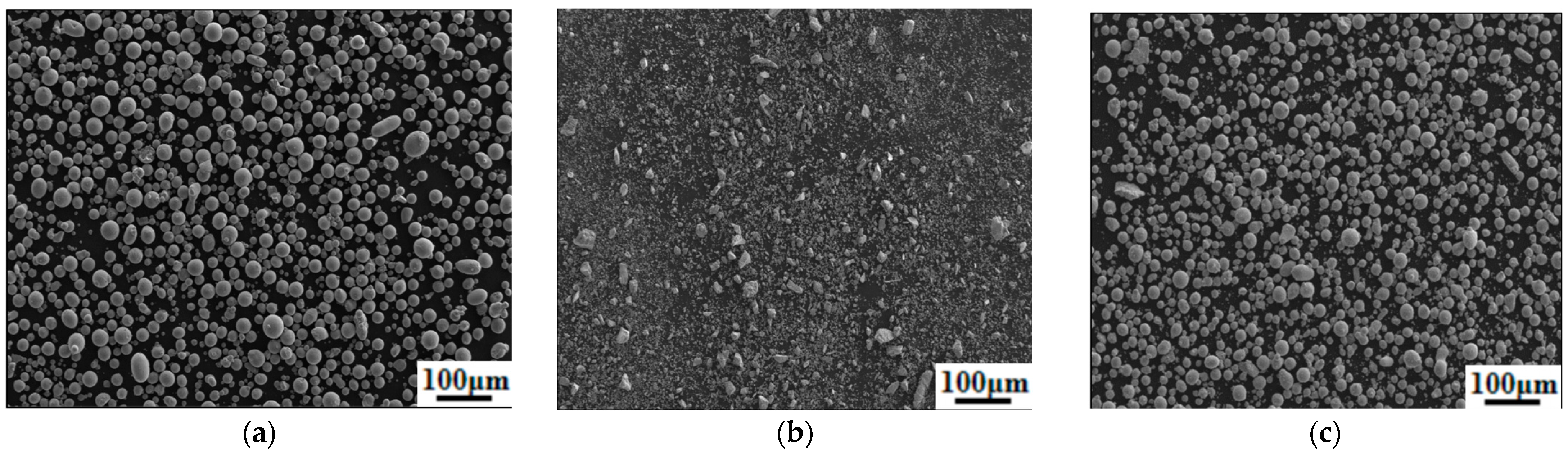


| Element | Cr | N | Mn | Mo | C | Fe |
|---|---|---|---|---|---|---|
| Original powders | 18.29 | 0.29 | 11.81 | 3.21 | 0.04 | Bal |
| Mixed powders | 21.12 | 1.01 | 11.22 | 3.04 | 0.04 | Bal |
| Powders | Size (μm) | Fluidity (s/50 g) | Apparent Density (g/cm3) | Tap Density (g/cm3) |
|---|---|---|---|---|
| Original powder | 15–53 | 21.11 | 4.24 | 4.56 |
| Mixed powder | 15–53 | 29.91 | 4.16 | 4.37 |
| Number | Laser Power (W) | Scanning Speed (mm/s) | Energy Density (J/mm3) |
|---|---|---|---|
| 1 | 200 | 1000 | 83.3 |
| 2 | 225 | 1000 | 93.8 |
| 3 | 200 | 800 | 104.2 |
| 4 | 250 | 900 | 115.7 |
| 5 | 200 | 700 | 119.1 |
| 6 | 300 | 1000 | 125.0 |
| 7 | 250 | 800 | 130.2 |
| 8 | 300 | 900 | 138.9 |
| 9 | 250 | 700 | 148.8 |
| 10 | 300 | 800 | 156.3 |
| 11 | 350 | 900 | 162.0 |
| 12 | 300 | 700 | 178.6 |
| 13 | 350 | 800 | 182.3 |
| Solute | 0.9% NaCl Solution | Simulated Plasma | Hank’s Solution |
|---|---|---|---|
| NaCl | 0.90 | 6.80 | 8.00 |
| KCl | - | 0.40 | 0.40 |
| CaCl2 | - | 0.20 | 0.14 |
| NaHCO3 | - | 2.20 | 0.35 |
| Mg4SO·7H2O | - | 0.20 | 0.06 |
| Na2HPO4 | - | 0.13 | - |
| Na2HPO4·2H2O | - | 0.03 | - |
| MgCl2·6H2O | - | - | 0.10 |
| Na2HPO4·12H2O | - | - | 0.06 |
| KH2PO4 | - | - | 0.06 |
| D-glucose | - | - | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Ren, J.; Wang, S.; Zhao, D.; Xiong, X.; Rao, J.H. Process Parameter Optimization for Selective Laser-Melted High-Nitrogen Steel and the Effects on Microstructure and Properties. Metals 2023, 13, 1242. https://doi.org/10.3390/met13071242
Sun X, Ren J, Wang S, Zhao D, Xiong X, Rao JH. Process Parameter Optimization for Selective Laser-Melted High-Nitrogen Steel and the Effects on Microstructure and Properties. Metals. 2023; 13(7):1242. https://doi.org/10.3390/met13071242
Chicago/Turabian StyleSun, Xin, Jianbiao Ren, Shuhuan Wang, Dingguo Zhao, Xiaojing Xiong, and Jeremy Heng Rao. 2023. "Process Parameter Optimization for Selective Laser-Melted High-Nitrogen Steel and the Effects on Microstructure and Properties" Metals 13, no. 7: 1242. https://doi.org/10.3390/met13071242
APA StyleSun, X., Ren, J., Wang, S., Zhao, D., Xiong, X., & Rao, J. H. (2023). Process Parameter Optimization for Selective Laser-Melted High-Nitrogen Steel and the Effects on Microstructure and Properties. Metals, 13(7), 1242. https://doi.org/10.3390/met13071242






