Theoretical Prediction of Structural, Mechanical, and Thermophysical Properties of the Precipitates in 2xxx Series Aluminum Alloy
Abstract
:1. Introduction
2. Model and Computational Method
| Phase | Crystal Structure | Space Group | V | Crystal Parameters | ΔH | Ecoh | DF | ||
|---|---|---|---|---|---|---|---|---|---|
| a | b | c | |||||||
| Al2Cu | Tetragonal | I4/MCM | 179.8 (179.8 a) | 6.04 (6.039 a, 6.063 b, 5.99 c) | 6.04 (6.039 a, 6.063 b, 5.99 c) | 4.928 (4.93 a, 4.872 b, 4.81 c) | −0.151 (−0.164 a, −0.203 b, −0.203 c) | −4.0 (−3.89 a, −3.99 b, −3.99 c) | 0.19 (0.19 a) |
| Al2CuMg | Orthorhombic | CMCM | 268.2 (268.1 a, 270.8 d) | 4.027 (4.026 a, 4.01 b, 3.89 c, 4.05 d, 4.01 e) | 9.319 (9.326 a, 9.25 b, 9.20 c, 9.28 d, 9.27 e) | 7.147 (7.142 a, 7.15 b, 7.16 c, 7.21 d, 7.12 e) | −0.17 (−0.186 a, −0.25 b, −0.25 c) | −3.5 (−3.35 a, −3.46 b, −3.46 c) | 0.16 (0.13 a) |
| Al3Fe | Triclinic | P63/MMC | 108.2 (108.2 f) | 5.36 (5.357 f) | 5.36 (5.357 f) | 4.354 (4.354 f) | −0.16 (−0.154 f) | −5.3 | 0.33 |
| Al3Fe2Si | Cubic | FD-3M | 1224.9 (1225 g) | 10.701 | 10.701 | 10.701 | −0.423 (−0.402 f) | −6.4 | 0.33 |
| Al5Cu2Mg8Si6 | Monoclinic | PM | 385.8 | 10.479 (10.423 h) | 4.016 (4.033 h) | 10.529 | −0.02 (−0.12 h) | −3.5 | 0.13 |
| Al7Cu2Fe | Tetragonal | P4/MNC | 590.5 | 6.324 (6.336 i, 6.338 j) | 6.324 (6.336 i, 6.338 j) | 14.763 (14.87 i, 14.83 j) | −0.266 (−0.298 j) | −4.6 | 0.14 |
| Al6Mn | Orthorhombic | CMCM | 431.4 | 6.468 (6.499 k) | 7.544 (7.555 k) | 8.841 (8.872 k) | −0.195 | −4.7 | 0.14 |
| Al3Zr_D023 | Tetragonal | I4/MMM | 280.1 | 4.020 (4.018 a, 4.015 b) | 4.020 (4.018 a, 4.015 b) | 17.332 (17.348 a, 17.454 b) | −0.489 (−0.517 a, −0.459 b) | −5.1 (5.14 a, 4.57 b) | 0.15 (0.15 a) |
| Al3Zr_D022 | Tetragonal | I4/MMM | 141.8 | 3.963 | 3.963 | 9.032 | −0.464 (−0.463 l) | −5.1 | 0.2 |
| Al3Zr_L12 | Cubic | PM-3M | 69.4 | 4.109 (4.111 m, 4.05 n, 4.09 o) | 4.109 (4.111 m) | 4.109 (4.111 m) | −0.461 (−0.463 l, −0.487 m, −0.47 p) | −5.1 | 0.18 |
| Al20Cu2Mn3 | Orthorhombic | BBMM | 2337.3 | 24.089 (23.98 q) | 12.601 (12.54 q) | 7.700 (7.66 q) | −0.181 (−0.156 q) | −4.64 | 0.19 |
3. Results and Discussion
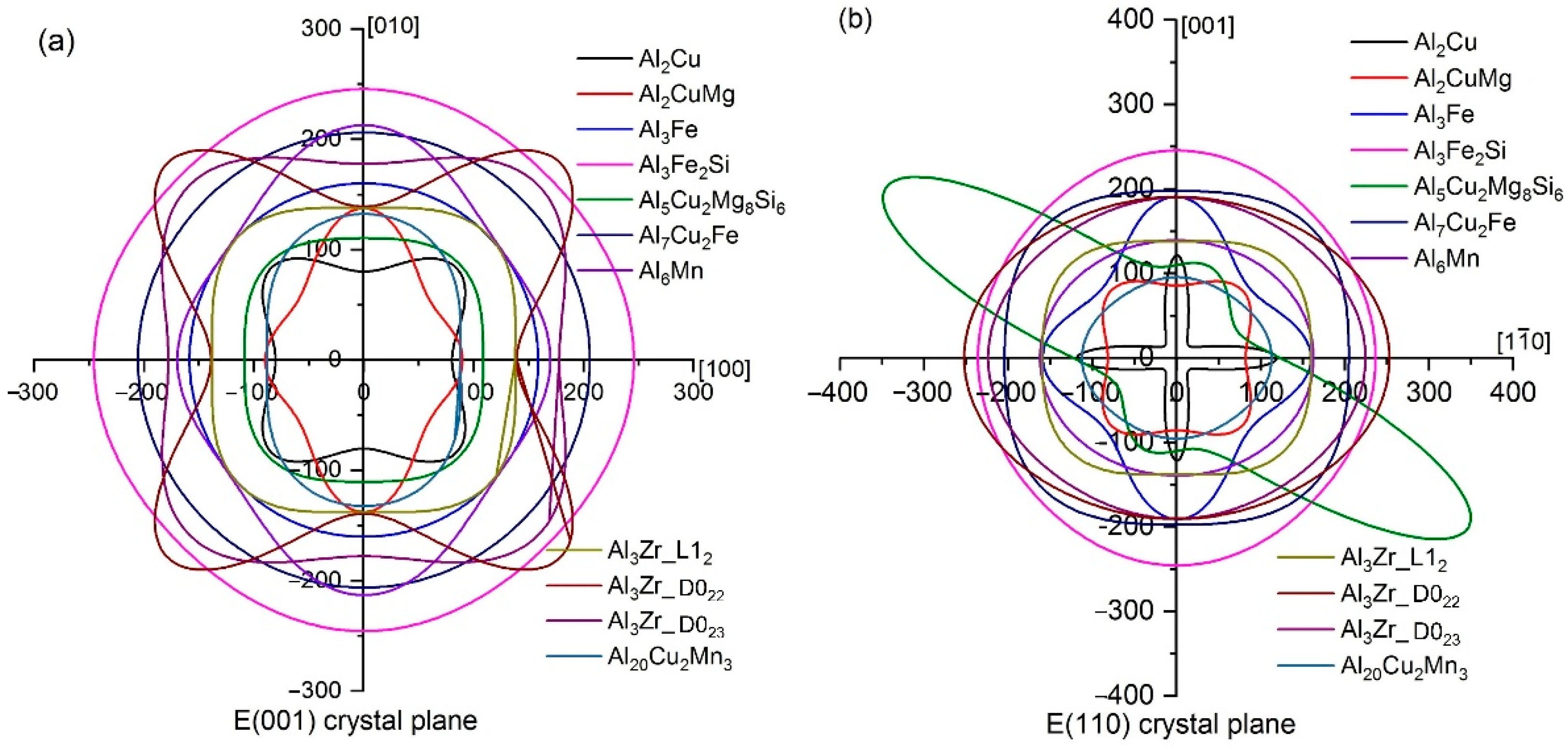
3.1. Mechanical Properties
| Phase | B | B’ | G | BH/GH | E | σ | Hv | AB | AG | AU | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BV | BR | BH | GV | GR | GH | |||||||||
| Al2Cu | 90.5 (99.4 a, 87.7 b) | 90.5 (99.4 a, 87.6 b) | 90.5 (99.4 a, 87.7 b, 108.6 c) | 4.71 | 46.1 (38.3 a, 52.1 b) | 38.2 (35.9 a, 42.3 b) | 42.1 (37.1 a, 47.2 b, 39 c) | 2.1 | 109.5 (99 a, 120 b, 104.5 c) | 0.298 (0.33 a, 0.272 b, 0.34 c) | 4.3 | 0 | 0.09 | 1.03 |
| Al2CuMg | 73.4 (80.9 b, 76.06 d) | 72.9 (80.7 b, 74.36 d) | 73.2 (80.8 b, 75.21 d, 79.48 e) | 4.639 | 45.6 (63.3 b, 51.02 d) | 41.1 (57.9 b, 45.13 d) | 43.3 (60.6 b, 48.08 d, 46.8 e) | 1.7 (1.564 d) | 108.5 (145.5 b, 118.9 d, 117.3 e) | 0.253 (0.2 b, 0.237 d, 0.254 e) | 6.8 | 0.003 | 0.052 | 0.554 (0.675 d, 0.349 e) |
| Al3Fe | 129.6 (125 f) | 129.5 (125 f) | 129.6 (125 f) | 4.156 | 54.9 (55 f) | 52.0 (51 f) | 53.4 (53 f) | 2.4 | 140.9 | 0.319 (0.31 f) | 4.3 | 0.0003 | 0.027 | 0.281 (0.35 f) |
| Al3Fe2Si | 155.9 | 155.9 | 155.9 | 4.498 | 95.6 | 95.5 | 95.6 | 1.6 | 238.1 | 0.245 | 13.3 | 0 | 0.0005 | 0.0052 |
| Al5Cu2Mg8Si6 | 63.6 (70.5 g) | 63.4 (69.7 g) | 63.5 (70.1 g) | 4.439 | 5.8 (44.5 g) | 51.8 (42.8 g) | 28.8 (43.6 g) | 2.2 | 75.0 (108.4 g) | 0.303 (0.242 g) | 2.7 | / | / | / |
| Al7Cu2Fe | 109.1 | 109.0 | 109.1 (107.8 h) | 4.42 | 91.3 | 90.8 | 91.0 (74.5 h) | 1.2 | 213.6 (181.7 h) | 0.173 (0.219 h) | 19.6 | 0.0005 | 0.0027 | 0.0285 |
| Al6Mn | 102.1 | 101.9 | 102.0 (102.6 i) | 4.022 | 67.6 | 64.1 | 65.9 | 1.5 | 126.6 | 0.234 | 10.9 | 0.001 | 0.0266 | 0.275 |
| Al3Zr_D023 | 101.6 (123.9 j) | 101.4 (117.4 j) | 101.5 (120.6 j, 100.2 k) | 3.977 | 84.1 (88.4 j) | 82.8 (85.8 j) | 83.4 (87.1 j, 77.1 k) | 1.2 | 196.5 (210.6 j, 184.1 k) | 0.177 (0.195 k) | 18.1 | 0.001 | 0.008 | 0.081 |
| Al3Zr_D022 | 101.4 (101.9 l) | 101.2 (101.6 l) | 101.3 (101.8 l) | 3.11 | 87.4 (87.9 l) | 79.3 (80.6 l) | 83.3 (84.2 l) | 1.2 | 196.2 (198 l) | 0.177 (0.176 l) | 18.1 | 0.001 | 0.0486 | 0.513 |
| Al3Zr_L12 | 101.5 | 101.5 | 101.5 (104.4 m, 103 n) | 4.134 | 63.1 | 62.2 | 62.7 (65.3 m, 64 n) | 1.6 | 156.0 (162.2 m, 159.1 n) | 0.244 (0.241 m, 0.243 n) | 9.8 | 0 | 0.0072 | 0.0723 |
| Al20Cu2Mn3 | 101.5 | 100.8 | 101.1 | 4.741 | 42.5 | 38.2 | 40.4 | 2.5 | 106.9 | 0.324 | 3.0 | 0.0035 | 0.0533 | 0.569 |
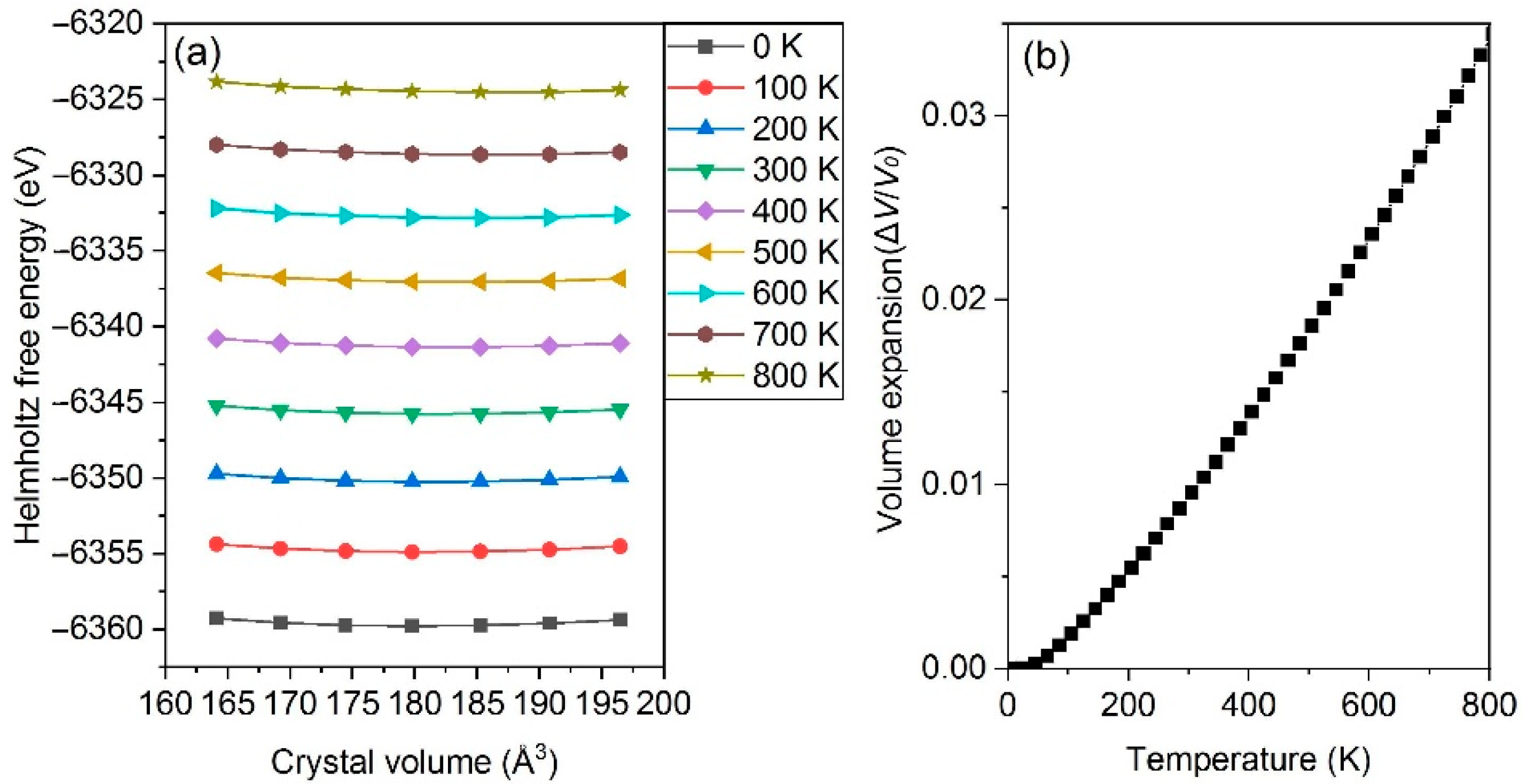
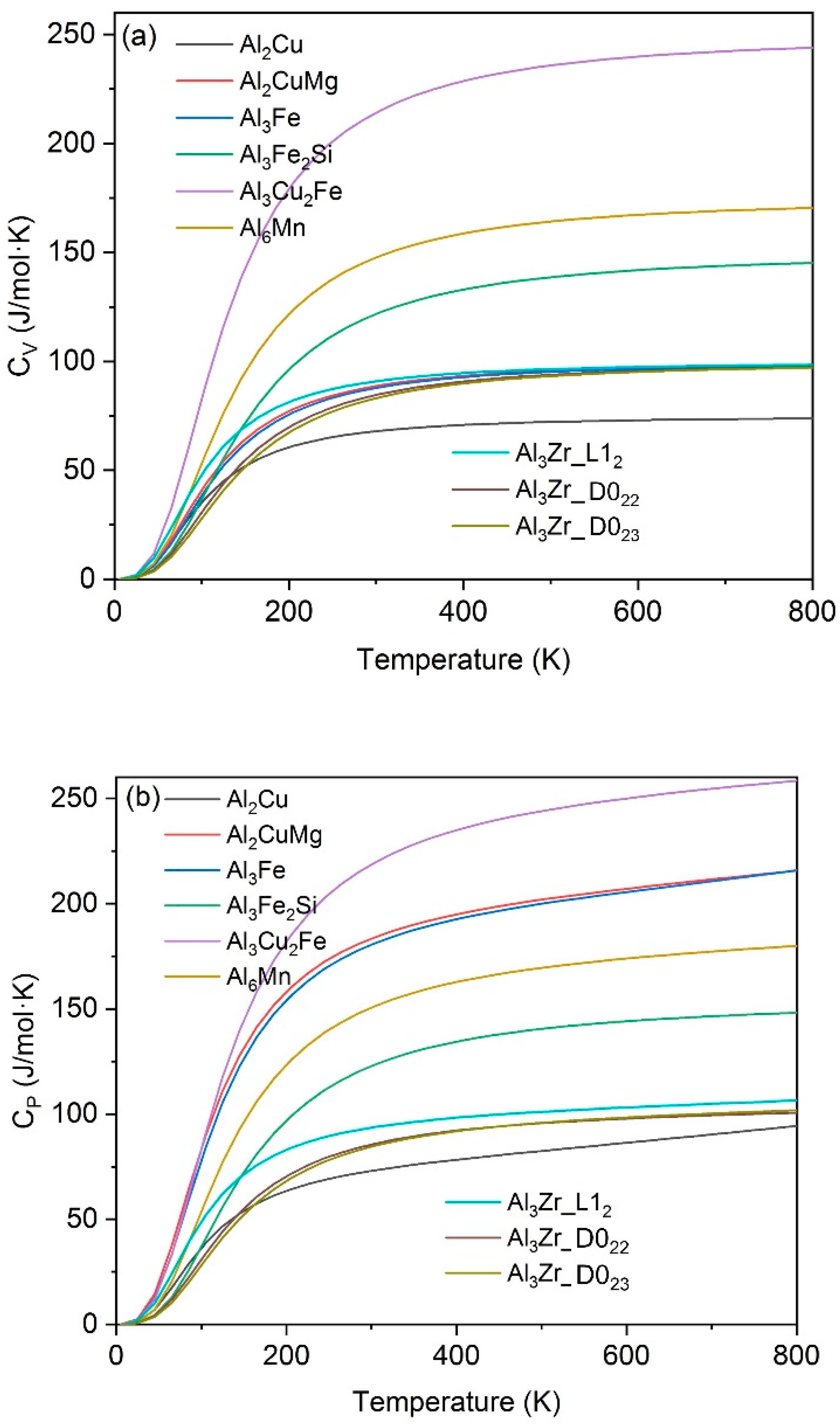
3.2. Thermophysical Properties
4. Conclusions
- The calculated formation enthalpies are −0.151, −0.17, −0.16, −0.463, −0.02, −0.266, −0.195, −0.489, −0.464, −0.461, and −0.181 eV/atom for Al2Cu, Al2CuMg, Al3Fe, Al3Fe2Si, Al5Cu2Mg8Si6, Al7Cu2Fe, Al6Mn, Al3Zr_D023, Al3Zr_D022, Al3Zr_L12, and Al20Cu2Mn3, respectively. Al5Cu2Mg8Si6 shows thermodynamic and mechanical unstable.
- The bulk modulus of all precipitates are derived from 63.5 to 155.9 GPa, which has the trend of: Al3Fe2Si > Al3Fe > Al7Cu2Fe > Al6Mn ≈ Al3Zr ≈ Al20Cu2Mn3 > Al2Cu > Al2CuMg > Al5Cu2Mg8Si6. The results of B/G imply that Al2Cu and Al20Cu2Mn3 are ductile precipitates. The hardness of Al3Zr_ D022 and D023 phases is very high (~18 GPa); whereas Al5Cu2Mg8Si6 shows low hardness value. The common precipitates of Al2Cu and Al2CuMg show a moderate hardness of 4.3 GPa and 6.8 GPa.
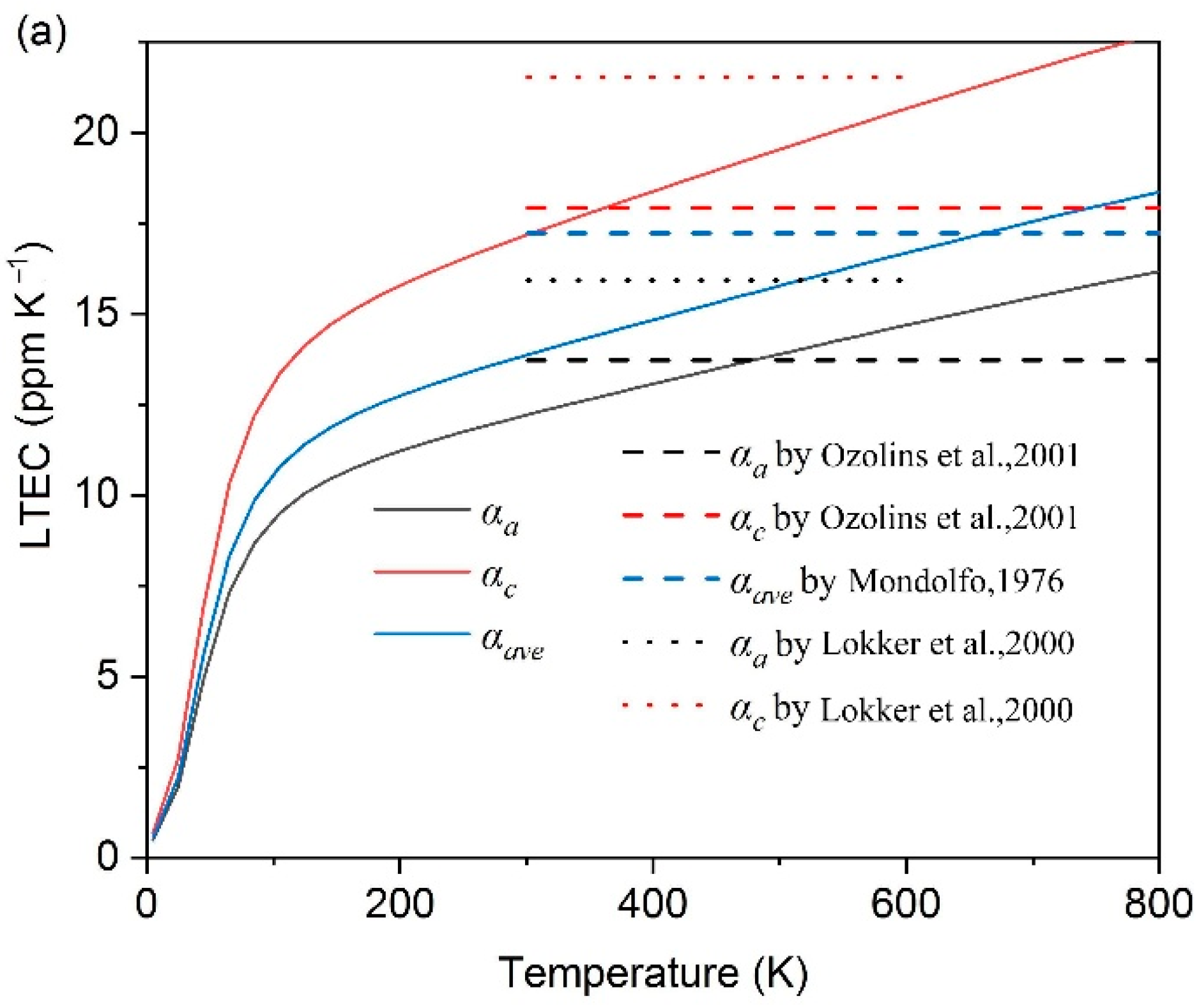
- The thermal expansion characters are also calculated based on QHA; Al2CuMg shows the highest LTEC, followed by Al3Fe, Al2Cu, Al3Zr_L12 and others, while Al3Zr_D022 is the lowest one; the discrepancy between a-Al and Al2CuMg is the smallest.
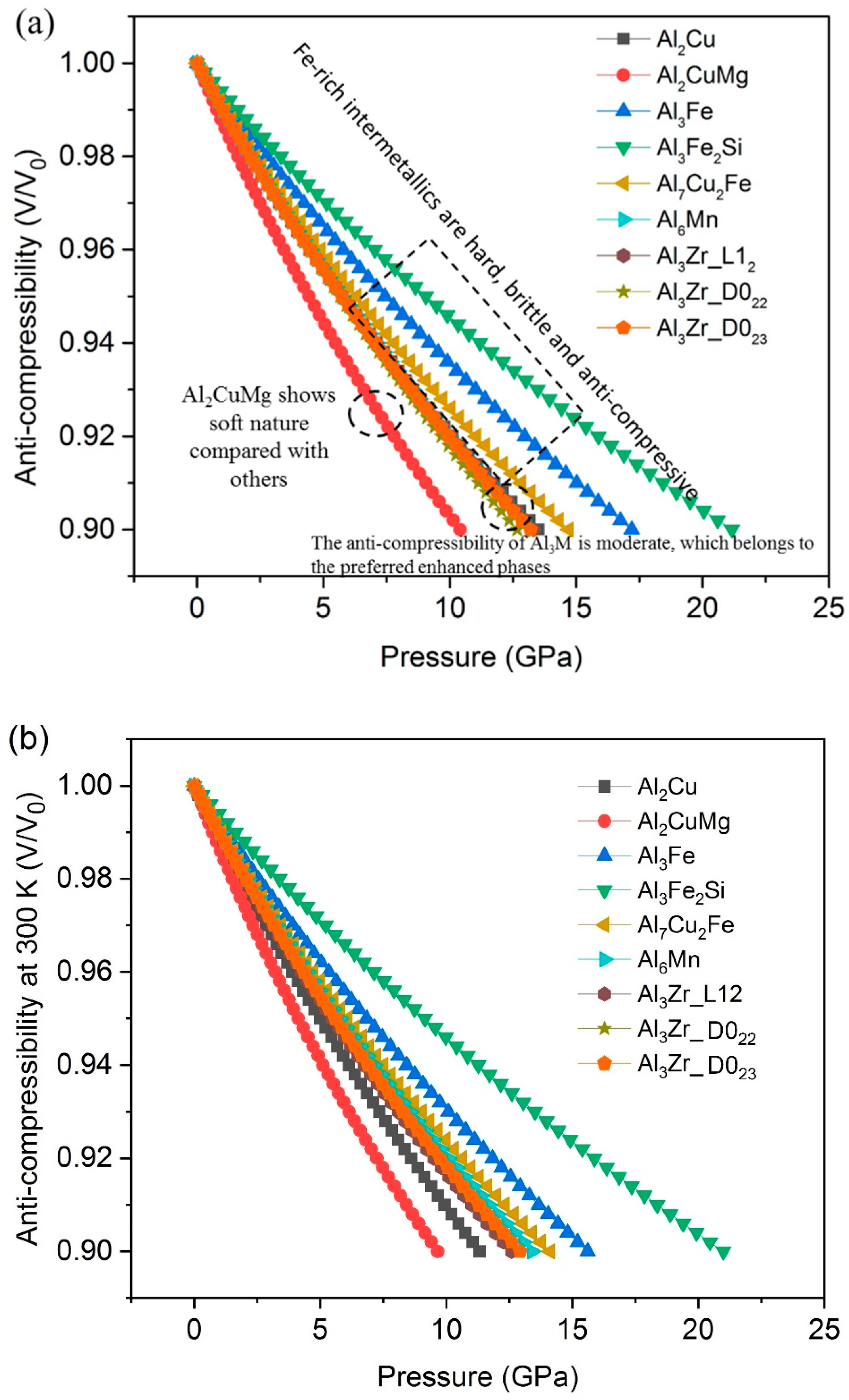
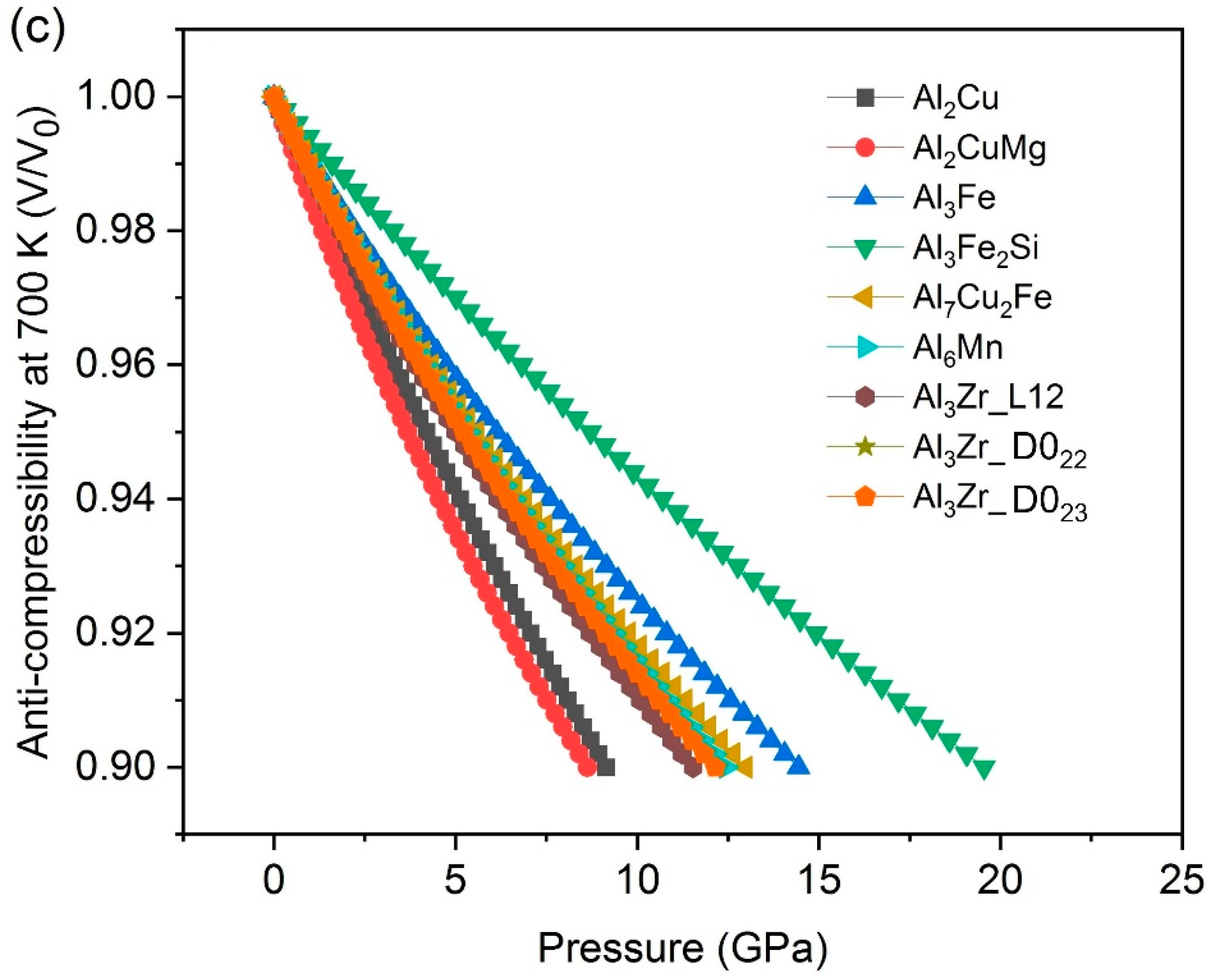
- The results of compressibility indicate Al3Fe, Al3Fe2Si and Al7Cu2Fe are hard and anti-compressive, while Al2CuMg is the softest one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuyan, M.; Borah, A. Investigating the effect of water cooling on hardness of 2xxx series aluminium alloy micro-alloyed with 0.02 and 0.04 wt% of Titanium. Mater. Today Proc. 2021, 43, 689–693. [Google Scholar] [CrossRef]
- Kumar, N.S.; Dhruthi; Pramod, G.; Samrat, P.; Sadashiva, M. A Critical Review on Heat Treatment of Aluminium Alloys. Mater. Today Proc. 2022, 58, 71–79. [Google Scholar] [CrossRef]
- Schuster, M.; De Luca, A.; Widmer, R.; Maeder, X.; Leinenbach, C. Processability, microstructure and precipitation of a Zr-modified 2618 aluminium alloy fabricated by laser powder bed fusion. J. Alloys Compd. 2022, 913, 165346. [Google Scholar] [CrossRef]
- Kairy, S.; Rouxel, B.; Dumbre, J.; Lamb, J.; Langan, T.; Dorin, T.; Birbilis, N. Simultaneous improvement in corrosion resistance and hardness of a model 2xxx series Al-Cu alloy with the microstructural variation caused by Sc and Zr additions. Corros. Sci. 2019, 158, 108095. [Google Scholar] [CrossRef]
- Moon, K.I.; Chang, K.Y.; Lee, K.S. The effect of ternary addition on the formation and the thermal stability of L12 Al3Zr alloy with nanocrystalline structure by mechanical alloying. J. Alloys Compd. 2000, 312, 273–283. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J. Precipitates and intermetallic phases in precipitation hardening Al–Cu–Mg–(Li) based alloys. Inter-Natl. Mater. Rev. 2005, 50, 193–215. [Google Scholar] [CrossRef] [Green Version]
- Kertz, J.E.; Gouma, P.I.; Buchheit, R.G. Localized corrosion susceptibility of Al-Li-Cu-Mg-Zn alloy AF/C458 due to interrupted quenching from solutionizing temperature. Met. Mater. Trans. A 2001, 32, 2561–2573. [Google Scholar] [CrossRef]
- Feng, Z.; Yang, Y.; Huang, B.; Li, M.; Chen, Y.; Ru, J. Crystal substructures of the rotation-twinned T (Al20Cu2Mn3) phase in 2024 aluminum alloy. J. Alloys Compd. 2014, 583, 445–451. [Google Scholar] [CrossRef]
- Shen, Z.; Liu, C.; Ding, Q.; Wang, S.; Wei, X.; Chen, L.; Li, J.; Zhang, Z. The structure determination of Al20Cu2Mn3 by near atomic resolution chemical mapping. J. Alloys Compd. 2014, 601, 25–30. [Google Scholar] [CrossRef]
- Huang, Y.-C.; Li, Y.; Xiao, Z.-B.; Szewczyk, R.; Yang, J. First-principles Study of Structure, Elastic and Electronic Properties of Precipitates Al3Fe, Al6Mn and Mg2Si in Al-Mg Alloys. In Proceedings of the 2nd Annual 2016 International Workshop on Materials Science and Engineering (IWMSE 2016), Guangzhou, China, 12–14 August 2016; pp. 876–883. [Google Scholar] [CrossRef]
- Zhu, X.Z.; Dong, X.X.; Blake, P.; Ji, S.X. Improvement in as-cast strength of high pressure die-cast Al–Si–Cu–Mg alloys by syn-ergistic effect of Q-Al5Cu2Mg8Si6 and θ-Al2Cu phases. Mater. Sci. Eng. A 2021, 802, 140612. [Google Scholar] [CrossRef]
- Tian, J.; Zhao, Y.; Wen, Z.; Hou, H.; Han, P. Physical properties and Debye temperature of Al7Cu2Fe alloy under various pressures analyzed by first-principles. Solid State Commun. 2017, 257, 6–10. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Segall, M.D.; Lindan, P.J.D.; Probert, M.J.; Pickard, C.J.; Clark, S.J.; Payne, M.C. First-principles simulation: Ideas, illustrations and the CASTEP code. J. Phys. Condens. Matter 2002, 14, 2717. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Martin, R.M. First-Principles Calculation of Stress. Phys. Rev. Lett. 1983, 50, 697–700. [Google Scholar] [CrossRef]
- Kern, G.; Kresse, G.; Hafner, J. Ab initio calculation of the lattice dynamics and phase diagram of boron nitride. Phys. Rev. B 1999, 59, 8551. [Google Scholar] [CrossRef]
- Baroni, S.; Giannozzi, P.; Isaev, E. Density-Functional Perturbation Theory for Quasi-Harmonic Calculations. Rev. Miner. Geochem. 2010, 71, 39–57. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.A.; Francisco, E.; Luaña, V. GIBBS: Isothermal-isobaric thermodynamics of solids from energy curves using a quasi-harmonic Debye model. Comput. Phys. Commun. 2004, 158, 57–72. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Huang, C.; Shao, H.B.; Ma, Y.L.; Huang, Y.C.; Xiao, Z.B. First-principles calculations of stability, electromic and elastic properties of the precipitates present in 7075 aluminum alloy. Int. J. Mod. Phys. B 2018, 32, 1850104. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.N.; Mao, C.; Peng, P. Structural, elastic and electronic properties of θ (Al2Cu) and S(Al2CuMg) strengthening precipitates in Al–Cu–Mg series alloys: First-principles calculations. Solid State Commun. 2012, 152, 2100–2104. [Google Scholar] [CrossRef]
- Li, C.M.; Zeng, S.M.; Chen, Z.Q.; Cheng, N.P.; Chen, T.X. First-principles calculations of elastic and thermodynamic properties of the four main intermetallic phases in Al–Zn–Mg–Cu alloys. Comput. Mater. Sci. 2014, 93, 210–220. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, Y.; Liu, F.; Lu, T.; Tao, X.; Du, Y.; He, Y. First-principles investigation of the mechanical, electronic and ther-mophysical properties of Q-phase in Al–Mg–Si–Cu alloys. Comput. Mater. Sci. 2013, 67, 334–340. [Google Scholar] [CrossRef]
- Zuo, L.; Ye, B.; Feng, J.; Kong, X.; Jiang, H.; Ding, W. Effect of Q-Al5Cu2Mg8Si6 phase on mechanical properties of Al-Si-Cu-Mg alloy at elevated temperature. Mater. Sci. Eng. A 2017, 693, 26–32. [Google Scholar] [CrossRef]
- Liu, Y.; Wen, J.C.; Zhang, X.Y.; Huang, Y.C. A comparative study on heterogeneous nucleation and mechanical properties of the fcc-Al/L12-Al3M (M = Sc, Ti, V, Y, Zr, Nb) interface from first-principles calculations. Phys. Chem. Chem. Phys. 2021, 23, 4718. [Google Scholar] [CrossRef]
- Yang, T.; Han, X.; Ding, Z.; Wang, Y.; Li, J. Electronic and structural properties of low-index L12–Al3Zr surfaces by first-principle calculations. Calphad 2019, 66, 101645. [Google Scholar] [CrossRef]
- Villars, P.; Calvert, L.D. Pearson’s Handbook of Crystallographic Data for Intermetallic Phases; ASM: Cleveland, OH, USA, 1985. [Google Scholar]
- Heying, B.; Hoffmann, R.D.; Pöttgen, R. Structure Refinement of the S-Phase Precipitate MgCuAl2. Z. Für Nat. B 2005, 60, 491–494. [Google Scholar] [CrossRef]
- Bown, M.G.; Brown, P.J. The structure of FeCu2Al7 and T (CoCuAl). Acta Crystallogr. 1956, 9, 911–914. [Google Scholar] [CrossRef]
- Mondolfo, L. Aluminum Alloys, Structure and Properties; Butterworth: London, UK, 1976. [Google Scholar]
- Ryum, N. Precipitation and recrystallization in an A1-0.5 WT% Zr-alloy. Acta Metall. 1969, 17, 269–278. [Google Scholar] [CrossRef]
- Desch, P.; Schwarz, R.; Nash, P. Formation of metastable L12 phases in Al3Zr and Al-12.5%X-25%Zr (X ≡ Li, Cr, Fe, Ni, Cu). J. Less Common Met. 1991, 168, 69–80. [Google Scholar] [CrossRef]
- Nicol, A.D.I. The structure of MnAl6. Acta Crystallogr. 1953, 6, 285–293. [Google Scholar] [CrossRef] [Green Version]
- Kontio, A.; Coppens, P. New study of the structure of MnAl6. Acta Crystallogr. 1981, B37, 433–435. [Google Scholar] [CrossRef]
- Panda, K.; Chandran, K.R. First principles determination of elastic constants and chemical bonding of titanium boride (TiB) on the basis of density functional theory. Acta Mater. 2006, 54, 1641–1657. [Google Scholar] [CrossRef]
- Hill, R. The Elastic Behaviour of a Crystalline Aggregate. Proc. Phys. Soc. Sect. A 1952, 65, 349. [Google Scholar] [CrossRef]
- Patil, S.K.R.; Khare, S.V.; Tuttle, B.R.; Bording, J.D. Kodambaka, Mechanical stability of possible structures of PtN investigated using first-principles calculations S. Phys. Rev. B 2006, 73, 104118. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.J.; Zhao, E.J.; Xiang, H.P.; Hao, X.F.; Liu, X.J. Meng, Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles, J. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, M.Q.; Wu, X.Z.; Jia, Z.H.; Wang, R.; Li, W.G.; Liu, Q. The structural stability, mechanical properties and stacking fault energy of Al3Zr precipitates in Al-Cu-Zr alloys: HRTEM observations and first-principles calculations. J. Alloys Compd. 2016, 681, 96–108. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, L.; Li, B.; Song, Q.; Wu, P. Structural, Elastic, and Electronic Properties of Al-Cu Intermetallics from First-Principles Calculations. J. Electron. Mater. 2008, 38, 356–364. [Google Scholar] [CrossRef]
- Zhang, G.W.; Sun, F.; Liu, H.P.; Ren, X.Y.; Xu, H.; Wang, M.J.; Fu, Y.Z. Exploration of D022-Type Al3TM (TM=Sc, Ti, V, Zr, Nb, Hf, Ta): Elastic Anisotropy, Electronic Structures, Work Function and Experimental Design. Materials 2021, 14, 2206. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; Wang, X.; Li, W. Ab initio calculations on elastic properties in L12 structure Al3X and X3Al-type (X=transition or main group metal) intermetallic compounds. Solid State Commun. 2013, 156, 69–75. [Google Scholar] [CrossRef]
- Ringer, S.P.; Sofyan, B.T.; Prasad, K.S.; Quan, G.C. Precipitation reactions in Al–4.0Cu–0.3Mg (wt.%) alloy. Acta Mater. 2008, 56, 2147–2160. [Google Scholar] [CrossRef]
- Herper, H.C.; Hoffmann, E.; Entel, P. Ab initio full-potential study of the structural and magnetic phase stability of iron. Phys. Rev. B 1999, 60, 3839. [Google Scholar] [CrossRef]
- Bannikov, V.V.; Shein, I.R.; Ivanovskii, A.L. Electronic structure, chemical bonding and elastic properties of the first thorium–containing nitride perovskite TaThN3. Phys. Status Solidi (RRL) 2007, 1, 89–91. [Google Scholar] [CrossRef]
- Chen, X.Q.; Niu, H.Y.; Li, D.Z.; Li, Y.Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef] [Green Version]
- Westbrook, J.H.; Fleischer, R.L. Basic Mechanical Properties and Lattice Defects of Intermetallic Compounds; Wiley: New York, NY, USA, 2000. [Google Scholar]
- Shang, S.; Wang, J.; Wang, Y.; Du, Y.; Liu, Z. Phonon and thermodynamic properties of Al–Mn compounds: A first-principles study. Comput. Mater. Sci. 2011, 50, 2096–2103. [Google Scholar] [CrossRef]
- Wang, S.C.; Starink, M.J. The assessment of GPB2/S’’ structures in Al-Cu-Mg alloys. Mater. Sci. Eng. A 2004, 386, 156–163. [Google Scholar] [CrossRef]
- Nye, J.F. Physical Properties of Crystals; Oxford University Press: Oxford, UK, 1985. [Google Scholar]
- Ranganathan, S.I.; Ostoja-Starzewski, M. Universal Elastic Anisotropy Index. Phys. Rev. Lett. 2008, 101, 055504. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W. Anisotropy in elasticity and thermal conductivity of monazite–type REPO4 (RE= La, Ce, Nd, Sm, Eu and Gd) from first–principles calculations. Acta Mater. 2013, 61, 7364–7383. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Manghnani, M.H.; Fisher, E.S.; Brower, W.S.J. Temperature dependence of the elastic constants of single-crystal rutile between 4° and 583°K. J. Phys. Chem. Solids 1972, 33, 2149–2159. [Google Scholar] [CrossRef]
- Saha, S.; Todorova, T.Z.; Zwanziger, J.W. Temperature dependent lattice misfit and coherency of Al3X (X=Sc, Zr, Ti and Nb) particles in an Al matrix. Acta Mater. 2015, 89, 109–115. [Google Scholar] [CrossRef]
- Gautam, G.; Kumar, N.; Mohan, A.; Mohan, S.; Singh, D. ZrB2 nanoparticles transmuting tribological properties of Al3Zr/AA5052 composite. J. Braz. Soc. Mech. Sci. Eng. 2019, 41, 469. [Google Scholar] [CrossRef]
- Ozolins, V.; Wolverton, C. Entropically Favored Ordering: The Metallurgy of Al2Cu Revisited. Phys. Rev. Letters. 2001, 86, 5518–5521. [Google Scholar] [CrossRef]
- Lokker, J.P.; Van Der Pers, N.M.; Verbruggen, A.H.; Janssen, G.C.A.M.; Jongste, J.F.; Radelaar, S. Localized stress near and the thermal expansion of Al2Cu precipitates in an Al thin film matrix. J. Appl. Phys. 2000, 87, 682–688. [Google Scholar] [CrossRef]
- Grimval, G. Thermophysical Properties of Materials; North Holland: Amsterdam, Netherlands, 1999. [Google Scholar]




| Phase | C11 | C12 | C13 | C22 | C23 | C33 | C44 | C66 |
|---|---|---|---|---|---|---|---|---|
| Al2Cu | 150.2 (150.3 a, 179.7 b, 163.8 c) | 97.4 (86.1 a, 72.7 b, 78.2 c) | 59.1 (62.6 a, 75.7 b, 14.7 c) | - | - | 211.3 (171.7 a, 170.2 b, 246.7 c) | 34.5 (29.4 a, 28.0 b, 33.8 c) | 41.3 (45.5 a, 44.7 b, 37.3 c) |
| Al2CuMg | 124.7 (156.4 c, 115.9 d, 133.6 e) | 22.5 (33.4 c, 35.3 d, 42.1 e) | 66.5 (62.6 c, 46.8 d, 49.9 e) | 150.5 (175.9 c, 174.1 d, 138.8 e) | 40.4 (17.7 c, 38.7 d, 58.0 e) | 126.6 (168.8 c, 153.1 d, 145.2 e) | 41.2 (43.7 c, 50.9 d, 39.0 e) | 32.6 (50.7 c, 26.6 d, 37.7 e) |
| Al3Fe | 213.2 (211 f) | 100.0 (93 f) | 76.7 (73 f) | - | - | 228.8 (228 f) | 41.3 (39 f) | 57.4 (59 f) |
| Al3Fe2Si | 288.2 | 89.8 | - | - | - | 93.2 | - | |
| Al5Cu2Mg8Si6 | 126.8 (146.3 g) | 39.7 (42.8 g) | 30.8 (33.3 g) | - | - | 126.3 (123.3 g) | <0 | <0 |
| Al7Cu2Fe | 225.0 (206 h) | 50.7 (50.6 h) | 52.6 (65.7 h) | - | - | 217.8 (194 h) | 99.5 (80.9 h) | 86.5 (71.1 h) |
| Al6Mn | 200.3 | 36.0 | 71.3 | 229.7 | 51.6 | 171.4 | 48.1 | 67.4 |
| Al3Zr_D023 | 203.4 (284.3 i, 206.7 j, 201.3 k) | 65.6 (67.8 i, 52.3 j, 70.5 k) | 43.1 (58.8 i, 50.7 j, 49.1 k) | - | - | 204.0 (175.9 i, 182.6 j, 196.7 k) | 83.0 (79.2 i, 81.4 j, 80.8 k) | 101.4 (97.2 i, 75.9 j) |
| Al3Zr_D022 | 183.2 (185.96 l) | 87.4 (85.34 l) | 42.0 (43.13 l) | - | - | 203.7 (202.08 l) | 89.0 (90 l) | 126.0 (125.22 l) |
| Al3Zr_L12 | 173.6 (182.8 m, 179 n) | 65.4 (65.2 m, 66 n) | - | - | - | - | 69.2 (70.1 m, 69 n) | - |
| Al20Cu2Mn3 | 138.0 | 69.6 | 81.0 | 177.5 | 73.0 | 150.4 | 24.6 | 46.8 |
| Phase | νl | νt | νm | ΘD |
|---|---|---|---|---|
| Al2Cu | 5.812 | 3.114 | 3.478 | 420 |
| Al2CuMg | 6.105 | 3.512 | 3.9 | 438 |
| Al3Fe | 6.992 | 3.622 | 4.055 | 484 |
| Al3Fe2Si | 7.7 | 4.499 | 4.989 | 622 |
| Al7Cu2Fe | 6.863 | 4.02 | 4.458 | 532 |
| Al6Mn | 7.637 | 4.553 | 5.041 | 557 |
| Al3Zr_D023 | 7.184 | 4.484 | 4.94 | 584 |
| Al3Zr_D022 | 7.017 | 4.258 | 4.705 | 554 |
| Al3Zr_L12 | 6.128 | 3.105 | 3.48 | 387 |
| Al20Cu2Mn3 | 6.472 | 3.303 | 3.701 | 424 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Li, Y.; Zheng, Q.; Guo, J.; Yang, Y.; Ding, W.; Ma, C.; He, K.; Su, N.; Jiang, J.; et al. Theoretical Prediction of Structural, Mechanical, and Thermophysical Properties of the Precipitates in 2xxx Series Aluminum Alloy. Metals 2022, 12, 2178. https://doi.org/10.3390/met12122178
Fang X, Li Y, Zheng Q, Guo J, Yang Y, Ding W, Ma C, He K, Su N, Jiang J, et al. Theoretical Prediction of Structural, Mechanical, and Thermophysical Properties of the Precipitates in 2xxx Series Aluminum Alloy. Metals. 2022; 12(12):2178. https://doi.org/10.3390/met12122178
Chicago/Turabian StyleFang, Xuewei, Yefei Li, Qiaoling Zheng, Jianye Guo, Yanmei Yang, Weiyun Ding, Chunhui Ma, Ke He, Ningning Su, Jingyi Jiang, and et al. 2022. "Theoretical Prediction of Structural, Mechanical, and Thermophysical Properties of the Precipitates in 2xxx Series Aluminum Alloy" Metals 12, no. 12: 2178. https://doi.org/10.3390/met12122178
APA StyleFang, X., Li, Y., Zheng, Q., Guo, J., Yang, Y., Ding, W., Ma, C., He, K., Su, N., Jiang, J., Chen, X., & Wang, H. (2022). Theoretical Prediction of Structural, Mechanical, and Thermophysical Properties of the Precipitates in 2xxx Series Aluminum Alloy. Metals, 12(12), 2178. https://doi.org/10.3390/met12122178








