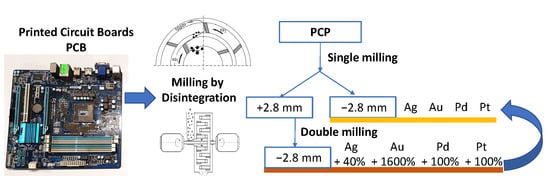
Selective Disintegration–Milling to Obtain Metal-Rich Particle Fractions from E-Waste
Abstract
:1. Introduction
2. Materials and Methods
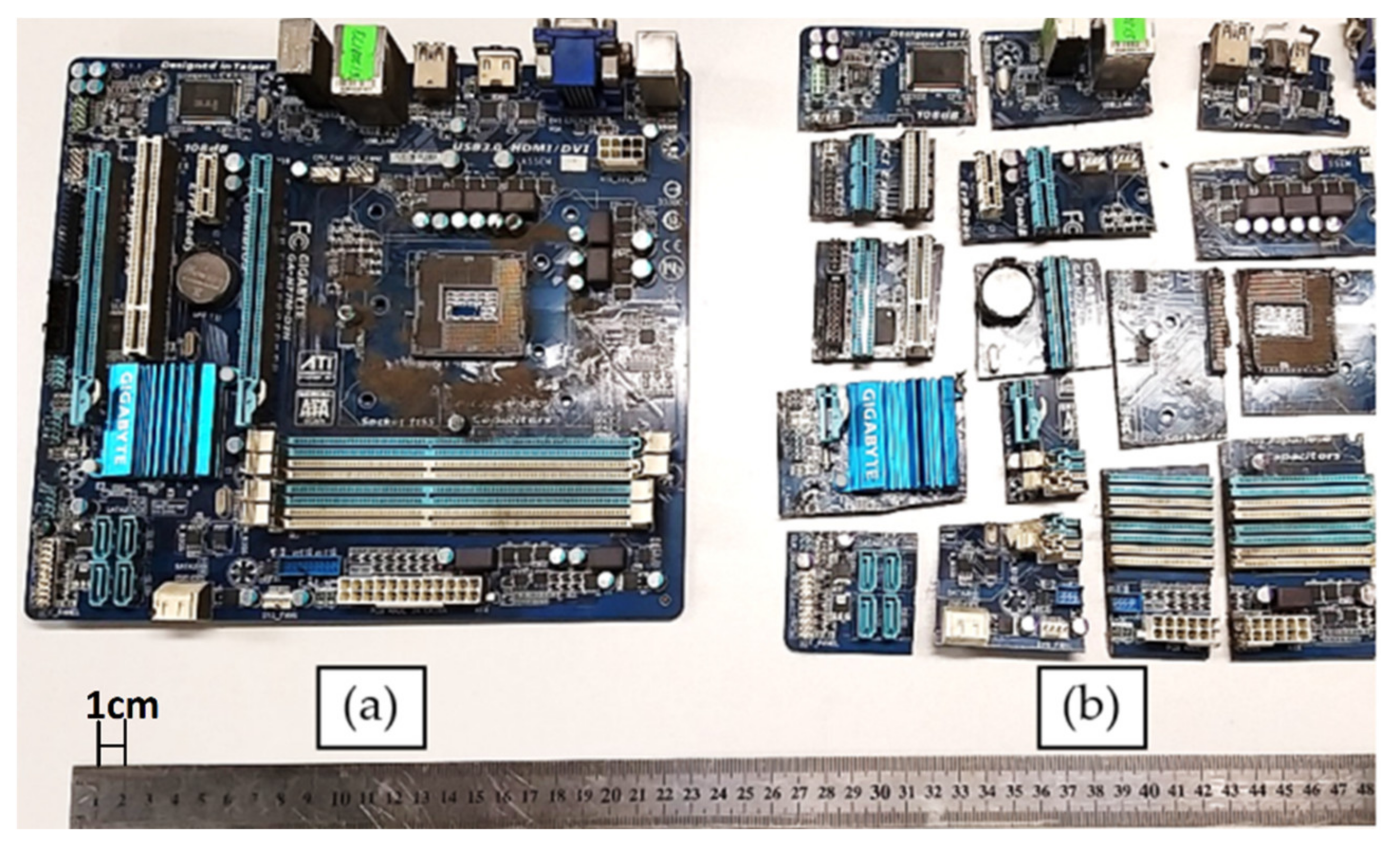
2.1. Used Materials
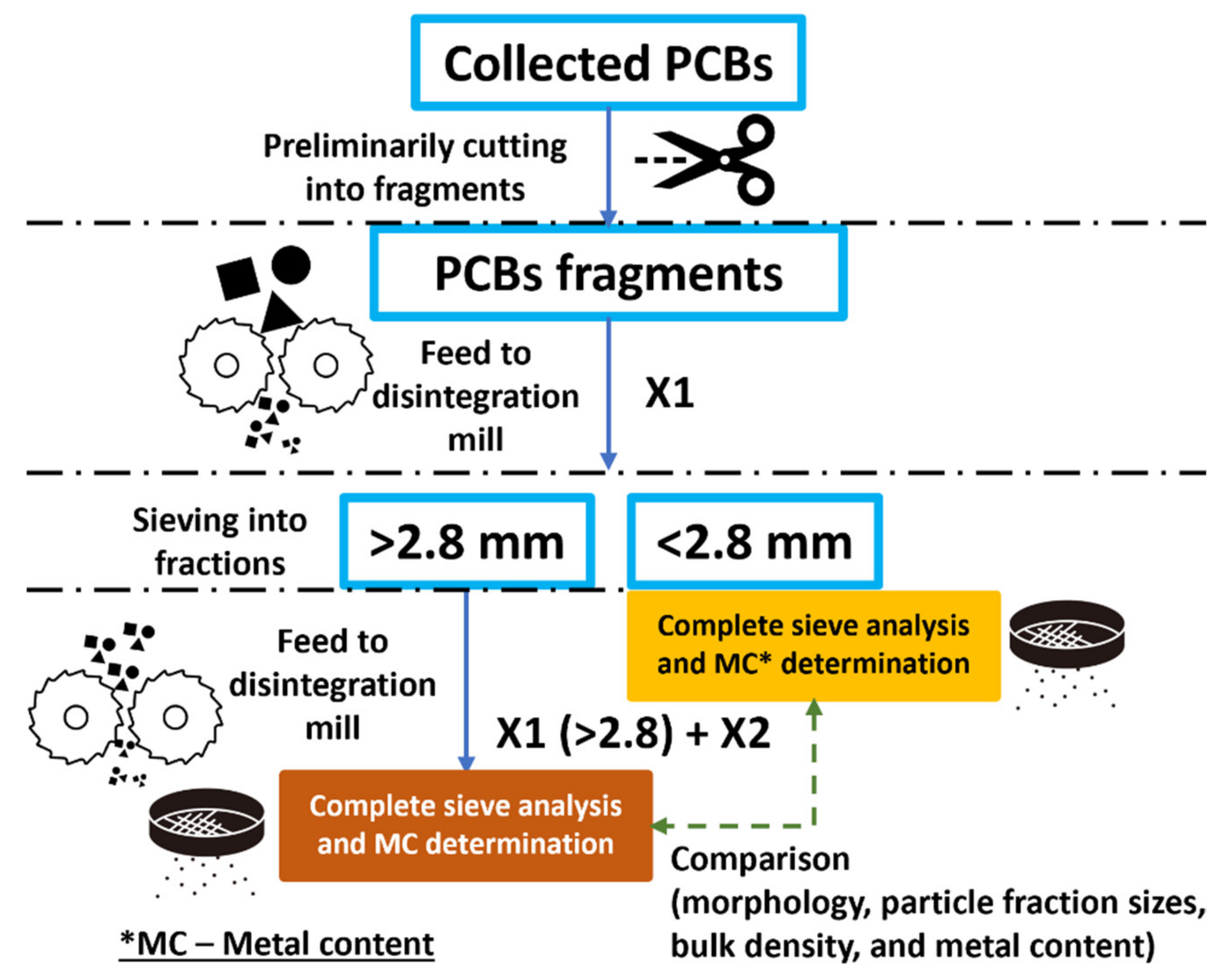
2.2. Applied Milling Procedure and Used Testing Equipment
2.3. Applied Method for Metal Content Determination
3. Results and Discussion
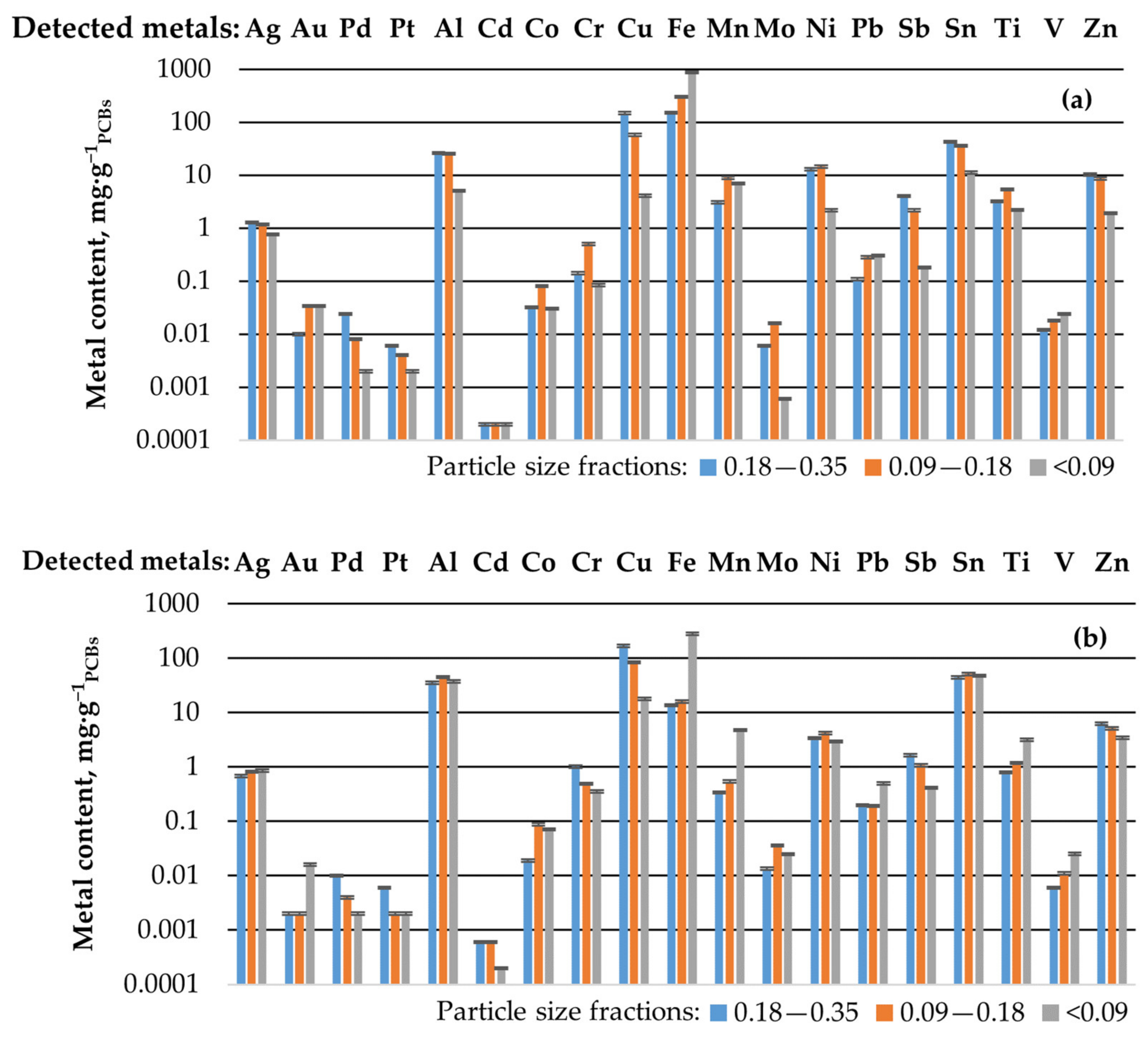
3.1. Metal Content in Disintegration-Milled PCBs Fractions
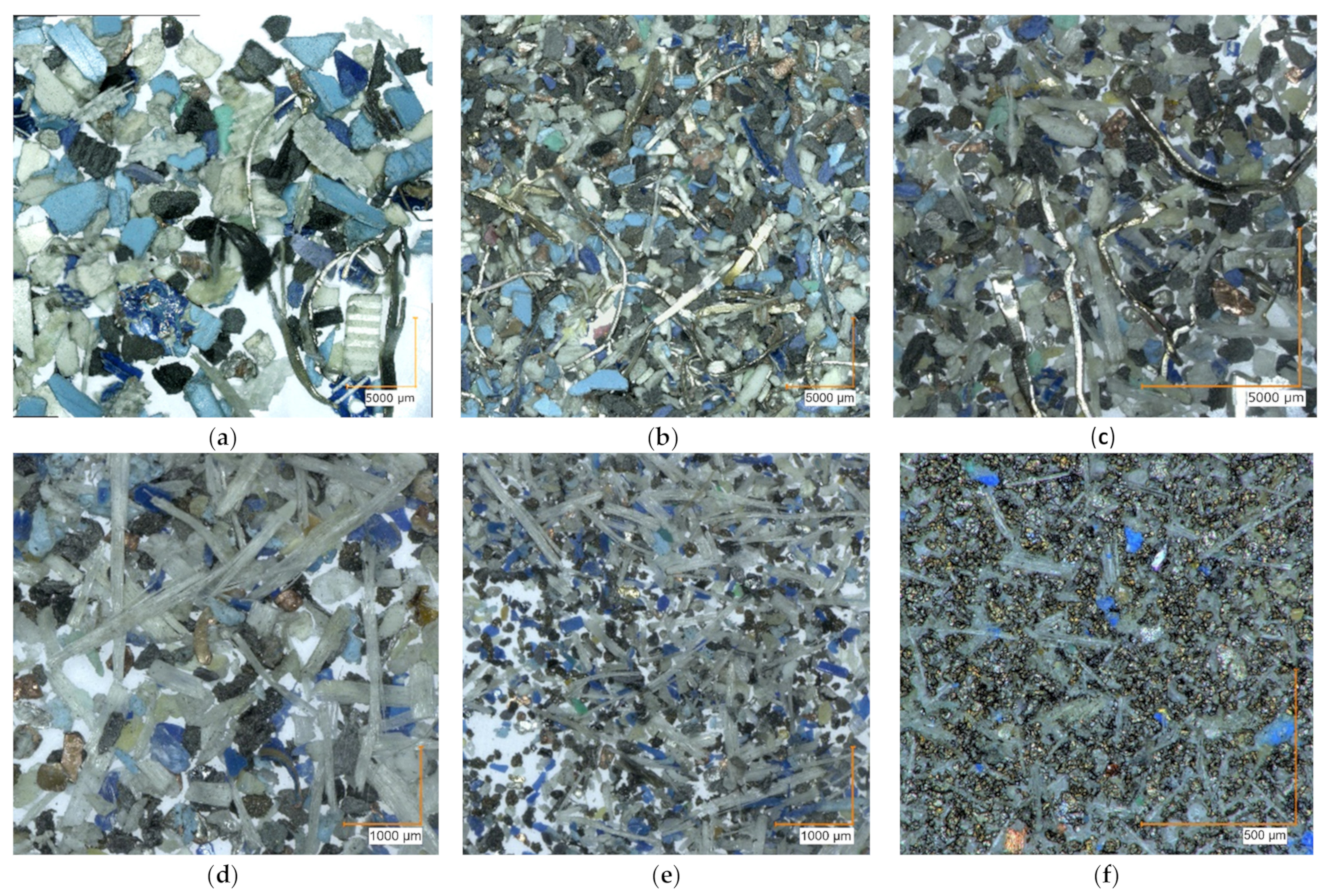
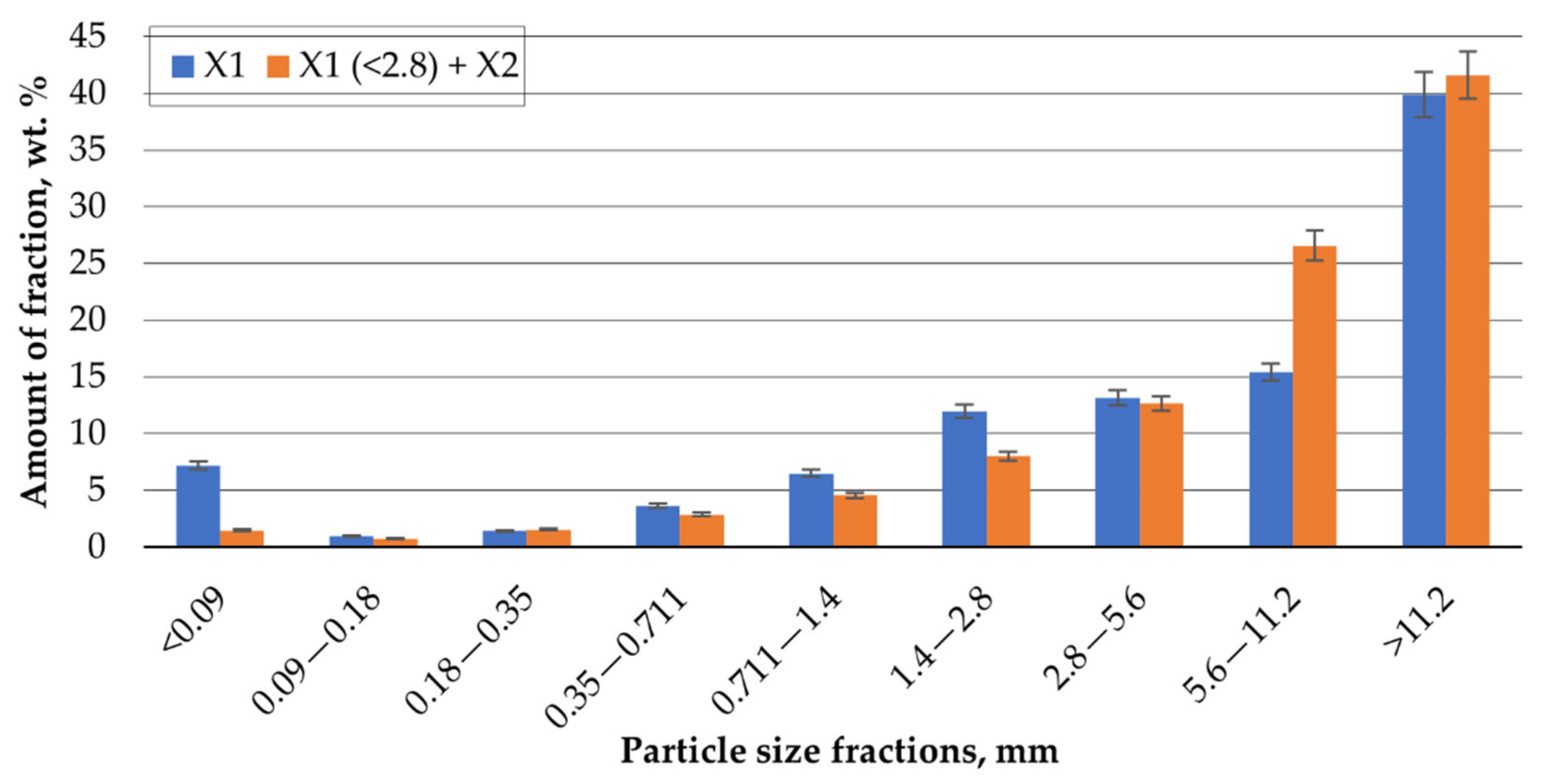
3.2. Morphology and PCBs Disintegration–Milling Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Popov, V.V.; Kudryavtseva, E.V.; Kumar Katiyar, N.; Shishkin, A.; Stepanov, S.I.; Goel, S. Industry 4.0 and Digitalisation in Healthcare. Materials 2022, 15, 2140. [Google Scholar] [CrossRef] [PubMed]
- Popov, V.V.; Lobanov, M.L.; Stepanov, S.I.; Qi, Y.; Muller-Kamskii, G.; Popova, E.N.; Katz-Demyanetz, A.; Popov, A.A. Texturing and Phase Evolution in Ti-6Al-4V: Effect of Electron Beam Melting Process, Powder Re-Using, and HIP Treatment. Materials 2021, 14, 4473. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, S.; Kibort, K.; Mioduska, J.; Lieder, M.; Małachowska, A. Waste Management in the Mining Industry of Metals Ores, Coal, Oil and Natural Gas—A Review. J. Environ. Manag. 2022, 304, 114239. [Google Scholar] [CrossRef]
- Toneatti, L.; Deluca, C.; Fraleoni-Morgera, A.; Pozzetto, D. Rationalization and Optimization of Waste Management and Treatment in Modern Cruise Ships. Waste Manag. 2020, 118, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Bumanis, G.; Vitola, L.; Stipniece, L.; Locs, J.; Korjakins, A.; Bajare, D. Evaluation of Industrial By-Products as Pozzolans: A Road Map for Use in Concrete Production. Case Stud. Constr. Mater. 2020, 13, e00424. [Google Scholar] [CrossRef]
- Sahmenko, G.; Korjakins, A.; Bajare, D. High-Performance Concrete Using Dolomite By-Products. In Concrete Durability and Service Life Planning; Kovler, K., Zhutovsky, S., Spatari, S., M. Jensen, O., Eds.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 99–103. ISBN 978-3-030-43332-1. [Google Scholar]
- Lapovok, R.; Popov, V.V.; Qi, Y.; Kosinova, A.; Berner, A.; Xu, C.; Rabkin, E.; Kulagin, R.; Ivanisenko, J.; Baretzky, B.; et al. Architectured Hybrid Conductors: Aluminium with Embedded Copper Helix. Mater. Des. 2020, 187, 108398. [Google Scholar] [CrossRef]
- Rumbo, C.; Espina, C.C.; Popov, V.V.; Skokov, K.; Tamayo-Ramos, J.A. Toxicological Evaluation of MnAl Based Permanent Magnets Using Different in Vitro Models. Chemosphere 2021, 263, 128343. [Google Scholar] [CrossRef]
- Mori de Oliveira, C.; Bellopede, R.; Tori, A.; Marini, P. Study of Metal Recovery from Printed Circuit Boards by Physical-Mechanical Treatment Processes. Mater. Proc. 2022, 5, 121. [Google Scholar]
- Bilesan, M.R.; Makarova, I.; Wickman, B.; Repo, E. Efficient Separation of Precious Metals from Computer Waste Printed Circuit Boards by Hydrocyclone and Dilution-Gravity Methods. J. Clean. Prod. 2021, 286, 125505. [Google Scholar] [CrossRef]
- Mandot, V.; Saraswat, V.; Jaitawat, N. Recycling Technologies of PCBs. J. Sci. Approach 2017, 1, 6–11. [Google Scholar] [CrossRef]
- Kulu, P.; Goljandin, D. Retreatment of Polymer Wastes by Disintegrator Milling. In Waste Material Recycling in the Circular Economy-Challenges and Developments; Achilias, D.S., Ed.; IntechOpen: Thessaloniki, Greece, 2021; pp. 1–23. ISBN 978-1-83969-681-7. [Google Scholar]
- Wen, X.; Zhao, Y.; Duan, C.; Zhou, X.; Jiao, H.; Song, S. Study on Metals Recovery from Discarded Printed Circuit Boards by Physical Methods. In Proceedings of the 2005 IEEE International Symposium on Electronics and the Environment, New Orleans, LA, USA, 16–19 May 2005; pp. 121–128. [Google Scholar]
- Wang, H.; Song, X.; Wang, X.; Sun, F. Tribological Performance and Wear Mechanism of Smooth Ultrananocrystalline Diamond Films. J. Mater. Processing Technol. 2021, 290, 116993. [Google Scholar] [CrossRef]
- Murugan, R.V.; Bharat, S.; Deshpande, A.P.; Varughese, S.; Haridoss, P. Milling and Separation of the Multi-Component Printed Circuit Board Materials and the Analysis of Elutriation Based on a Single Particle Model. Powder Technol. 2008, 183, 169–176. [Google Scholar] [CrossRef]
- Goljandin, D.; Kulu, P. Disintegrators and Disintegrator Treatment of Materials; LAP LAMBERT Academic Publishing: Saarbrücken, Germany, 2015; ISBN 3659647683. [Google Scholar]
- Oliveira, P.C.; Taborda, F.C.; Nogueira, C.A.; Margarido, F. The Effect of Shredding and Particle Size in Physical and Chemical Processing of Printed Circuit Boards Waste. Mater. Sci. Forum 2012, 730–732, 653–658. [Google Scholar] [CrossRef]
- Kers, J.; Kulu, P.; Goljandin, D.; Kaasik, M.; Ventsel, T.; Vilsaar, K.; Mikli, V. Recycling of Electronic Wastes by Disintegrator Mills and Study of the Separation Technique of Different Materials. Medziagotyra 2008, 14, 296–300. [Google Scholar]
- Perdigones, F.; Quero, J. Printed Circuit Boards: The Layers’ Functions for Electronic and Biomedical Engineering. Micromachines 2022, 13, 460. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, M.; Shi, Q.; Kuang, X.; Qi, H.J.; Wang, T. Recycling Waste Circuit Board Efficiently and Environmentally Friendly through Small-Molecule Assisted Dissolution. Sci. Rep. 2019, 9, 17902. [Google Scholar] [CrossRef]
- Bukhari, M.; Mohd Noor, N.; Nan, N.M.M.; Shamsul, J.B. The Application of PCB, Mounted-Components and Solder Paste in Surface Mount Technology Assembly (SMTA). In Proceedings of the 1st National Conference on Electronic DesignAt: Putra Palace Hotel, Kangar, Malaysia, 18–19 May 2005; pp. 145–151. [Google Scholar]
- Khandpur, R.S. Printed Circuit Boards. Design, Fabrication, Assembly, and Testing; Tata McGraw-Hill Education: New York, NY, USA, 2006; ISBN 0070588147. [Google Scholar]
- Tilsley, G.M.; Axon, F.J. Comparison of Dry Film and Liquid Photo-Imageable Solder Masks for Surface-Mount Assemblies. Microelectron. Reliab. 1988, 28, 659. [Google Scholar] [CrossRef]
- PCBCart PCB Board Material, PCB Material Type. Available online: https://www.pcbcart.com/pcb-capability/pcb-materials.html (accessed on 15 June 2022).
- Hino, T.; Agawa, R.; Moriya, Y.; Nishida, M.; Tsugita, Y.; Araki, T. Techniques to Separate Metal from Waste Printed Circuit Boards from Discarded Personal Computers. J. Mater. Cycles Waste Manag. 2009, 11, 42–54. [Google Scholar] [CrossRef]
- Paola, M. Recycling of Printed Circuit Boards. In Integrated Waste Management-Volume II; InTech: Houston TX, USA, 2011; ISBN 978-953-307-447-4. [Google Scholar]
- Evangelopoulos, P.; Arato, S.; Persson, H.; Kantarelis, E.; Yang, W. Reduction of Brominated Flame Retardants (BFRs) in Plastics from Waste Electrical and Electronic Equipment (WEEE) by Solvent Extraction and the Influence on Their Thermal Decomposition. Waste Manag. 2019, 94, 165–171. [Google Scholar] [CrossRef]
- Jonkers, N.; Krop, H.; van Ewijk, H.; Leonards, P.E.G. Life Cycle Assessment of Flame Retardants in an Electronics Application. Int. J. Life Cycle Assess. 2016, 21, 146–161. [Google Scholar] [CrossRef]
- Ogunniyi, I.O.; Vermaak, M.K.G.; Groot, D.R. Chemical Composition and Liberation Characterization of Printed Circuit Board Comminution Fines for Beneficiation Investigations. Waste Manag. 2009, 29, 2140–2146. [Google Scholar] [CrossRef]
- Ribeiro, P.P.M.; dos Santos, I.D.; Dutra, A.J.B. Copper and Metals Concentration from Printed Circuit Boards Using a Zig-Zag Classifier. J. Mater. Res. Technol. 2019, 8, 513–520. [Google Scholar] [CrossRef]
- Huang, T.; Zhu, J.; Huang, X.; Ruan, J.; Xu, Z. Assessment of Precious Metals Positioning in Waste Printed Circuit Boards and the Economic Benefits of Recycling. Waste Manag. 2022, 139, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Tatariants, M.; Yousef, S.; Denafas, G.; Bendikiene, R. Separation and Purification of Metal and Fiberglass Extracted from Waste Printed Circuit Boards Using Milling and Dissolution Techniques. Environ. Prog. Sustain. Energy 2018, 37, 2082–2092. [Google Scholar] [CrossRef]
- Sousa, P.M.S.; Martelo, L.M.; Marques, A.T.; Bastos, M.; Soares, H. A Closed and Zero-Waste Loop Strategy to Recycle the Main Raw Materials (Gold, Copper and Fiber Glass Layers) Constitutive of Waste Printed Circuit Boards. Chem. Eng. J. 2022, 434, 134604. [Google Scholar] [CrossRef]
- Zhou, Y.; Qiu, K. A New Technology for Recycling Materials from Waste Printed Circuit Boards. J. Hazard. Mater. 2010, 175, 823–828. [Google Scholar] [CrossRef]
- Arslan, V. Bacterial Leaching of Copper, Zinc, Nickel and Aluminum from Discarded Printed Circuit Boards Using Acidophilic Bacteria. J. Mater. Cycles Waste Manag. 2021, 23, 2005–2015. [Google Scholar] [CrossRef]
- Yazici, E.Y.; Deveci, H. Extraction of Metals from Waste Printed Circuit Boards (WPCBs) in H2SO4–CuSO4–NaCl Solutions. Hydrometallurgy 2013, 139, 30–38. [Google Scholar] [CrossRef]
- Sahin, M.; Akcil, A.; Erust, C.; Altynbek, S.; Gahan, C.S.; Tuncuk, A. A Potential Alternative for Precious Metal Recovery from E-Waste: Iodine Leaching. Sep. Sci. Technol. 2015, 50, 2587–2595. [Google Scholar] [CrossRef]
- Van Yken, J.; Cheng, K.Y.; Boxall, N.J.; Sheedy, C.; Nikoloski, A.N.; Moheimani, N.R.; Kaksonen, A.H. A Comparison of Methods for the Characterisation of Waste-Printed Circuit Boards. Metals 2021, 11, 1935. [Google Scholar] [CrossRef]
- Hanafi, J.; Jobiliong, E.; Christiani, A.; Soenarta, D.C.; Kurniawan, J.; Irawan, J. Material Recovery and Characterization of PCB from Electronic Waste. Procedia-Soc. Behav. Sci. 2012, 57, 331–338. [Google Scholar] [CrossRef]
- Zimakov, S.; Goljandin, D.; Peetsalu, P.; Kulu, P. Metallic Powders Produced by the Disintegrator Technology. Int. J. Mater. Prod. Technol. 2007, 28, 226. [Google Scholar] [CrossRef]
- Peetsalu, P.; Goljandin, D.; Kulu, P.; Mikli, V. Micropowders prozducted by disintegrator milling. Powder Metall. 2003, 3, 99–110. [Google Scholar]
- Goljandin, D.; Sarjas, H.; Kulu, P.; Käerdi, H.; Mikli, V. Metal-Matrix Hardmetal/Cermet Reinforced Composite Powders for Thermal Spray. Mater. Sci. 2012, 18, 84–89. [Google Scholar] [CrossRef]
- Cui, J.; Zhang, L. Metallurgical Recovery of Metals from Electronic Waste: A Review. J. Hazard. Mater. 2008, 158, 228–256. [Google Scholar] [CrossRef] [PubMed]
- Tymanok, A.; Tamm, J.; Roes, A. Flow of Air and Particles Mixture in a Disintegrator. In Proceedings of the Estonian Academy of Sciences, Physics Mathematics, Tallinn, Estonia, 19–20 April 1994; pp. 280–292. [Google Scholar]


| Metals (~40 wt.% in Total) | wt.% of Metals | Ceramics (~30 wt.% in Total) | wt.% of Ceramics | Plastics (~30 wt.% in Total) | wt.% of Plastics |
|---|---|---|---|---|---|
| Cu | 6–27 | SiO2 | 15–30 | Polyethylene | 10–16 |
| Fe | 1.2–8 | Al2O3 | 6–9.4 | Polypropylene | 4.8 |
| Al | 2–7.2 | Alkali-earth oxides | 6 | Polystyrene | 4.8 |
| Sn | 1–5.6 | Titanates-micas | 3 | Epoxy resin | 4.8 |
| Pb | 1–4.2 | Polyvinyl chloride | 2.4 | ||
| Ni | 0.3–5.4 | Polytetrafluoroethylene | 2.4 | ||
| Zn | 0.2–2.2 | Nylon | 0.9 | ||
| Sb | 0.1–0.4 | ||||
| Au (ppm) | 250–2050 | ||||
| Ag (ppm) | 110–4500 | ||||
| Pd (ppm) | 50–4000 | ||||
| Pt (ppm) | 5–30 | ||||
| Co (ppm) | 1–4000 |
| Parameter | Value |
|---|---|
| Type of device (position of rotors) | horizontal |
| Grinding environment | air |
| Rotor system | one/two-rotor |
| Number of pins/blades roads | 1/3 |
| Rotation velocity of rotors, rpm | 2880 |
| Impact velocity, m/s | up to 180 |
| Specific energy of treatment ES, kJ·kg−1 | up to 13.6 |
| Possible operating system | direct |
| Input (max particle size), mm | 45 |
| Productivity, kg·h−1 | up to 950 |
| Fraction, mm | <0.09 | 0.09–0.18 | 0.18–0.35 | 0.35–0.711 | 0.711–1.4 | 1.4–2.8 | 2.8–5.6 |
|---|---|---|---|---|---|---|---|
| Bulk density of X1, g·cm−3 | 6.8 | 5.3 | 4.9 | 1.6 | 0.44 | 0.61 | 0.30 |
| Bulk density of X1 (>2.8) + X2, g·cm−3 | 2.23 | 0.84 | 0.81 | 0.53 | 0.58 | 0.35 | |
| MC in X1, wt.% | 95.2 ± 1.8 | 57.7 ± 1.5 | 54.6 ± 1.2 | 14.3 ± 0.9 | 8.4 ± 0.5 | ||
| MC in X1 (>2.8) + X2, wt.% | 50.6 ± 1.3 | 26.2 ± 1.6 | 33 ± 0.9 | 8 ± 0.7 | 7.2 ± 0.6 |
| Metal Content in % after One-Step Milling in Comparison with Double-Step Milling | Total MC * | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fraction, mm | Ag | Au | Pd | Pt | Al | Cd | Co | Cr | Cu | Fe | Mn | Mo | Ni | Pb | Sb | Sn | Ti | V | Zn | |
| 0.18–0.35 | 86 | 400 | 140 | 0 | −25 | −67 | 68 | −86 | −12 | 1003 | 798 | −55 | 279 | −45 | 144 | −3 | 298 | 100 | 63 | 46 |
| 0.09–0.18 | 41 | 1600 | 100 | 100 | −44 | −67 | −8 | 1 | −31 | 1770 | 1548 | −56 | 245 | 47 | 100 | −30 | 357 | 64 | 70 | 119 |
| <0.09 | −12 | 113 | 0 | 0 | −87 | 0 | −58 | −76 | −77 | 207 | 45 | −98 | −26 | −40 | −57 | −77 | −31 | −5 | −45 | 124 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumbergs, E.; Serga, V.; Shishkin, A.; Goljandin, D.; Shishko, A.; Zemcenkovs, V.; Markus, K.; Baronins, J.; Pankratov, V. Selective Disintegration–Milling to Obtain Metal-Rich Particle Fractions from E-Waste. Metals 2022, 12, 1468. https://doi.org/10.3390/met12091468
Blumbergs E, Serga V, Shishkin A, Goljandin D, Shishko A, Zemcenkovs V, Markus K, Baronins J, Pankratov V. Selective Disintegration–Milling to Obtain Metal-Rich Particle Fractions from E-Waste. Metals. 2022; 12(9):1468. https://doi.org/10.3390/met12091468
Chicago/Turabian StyleBlumbergs, Ervins, Vera Serga, Andrei Shishkin, Dmitri Goljandin, Andrej Shishko, Vjaceslavs Zemcenkovs, Karlis Markus, Janis Baronins, and Vladimir Pankratov. 2022. "Selective Disintegration–Milling to Obtain Metal-Rich Particle Fractions from E-Waste" Metals 12, no. 9: 1468. https://doi.org/10.3390/met12091468
APA StyleBlumbergs, E., Serga, V., Shishkin, A., Goljandin, D., Shishko, A., Zemcenkovs, V., Markus, K., Baronins, J., & Pankratov, V. (2022). Selective Disintegration–Milling to Obtain Metal-Rich Particle Fractions from E-Waste. Metals, 12(9), 1468. https://doi.org/10.3390/met12091468