On the Impact of the Intermetallic Fe2Nb Laves Phase on the Mechanical Properties of Fe-6 Al-1.25 Nb-X W/Mo Fully Ferritic Light-Weight Steels
Abstract
1. Introduction
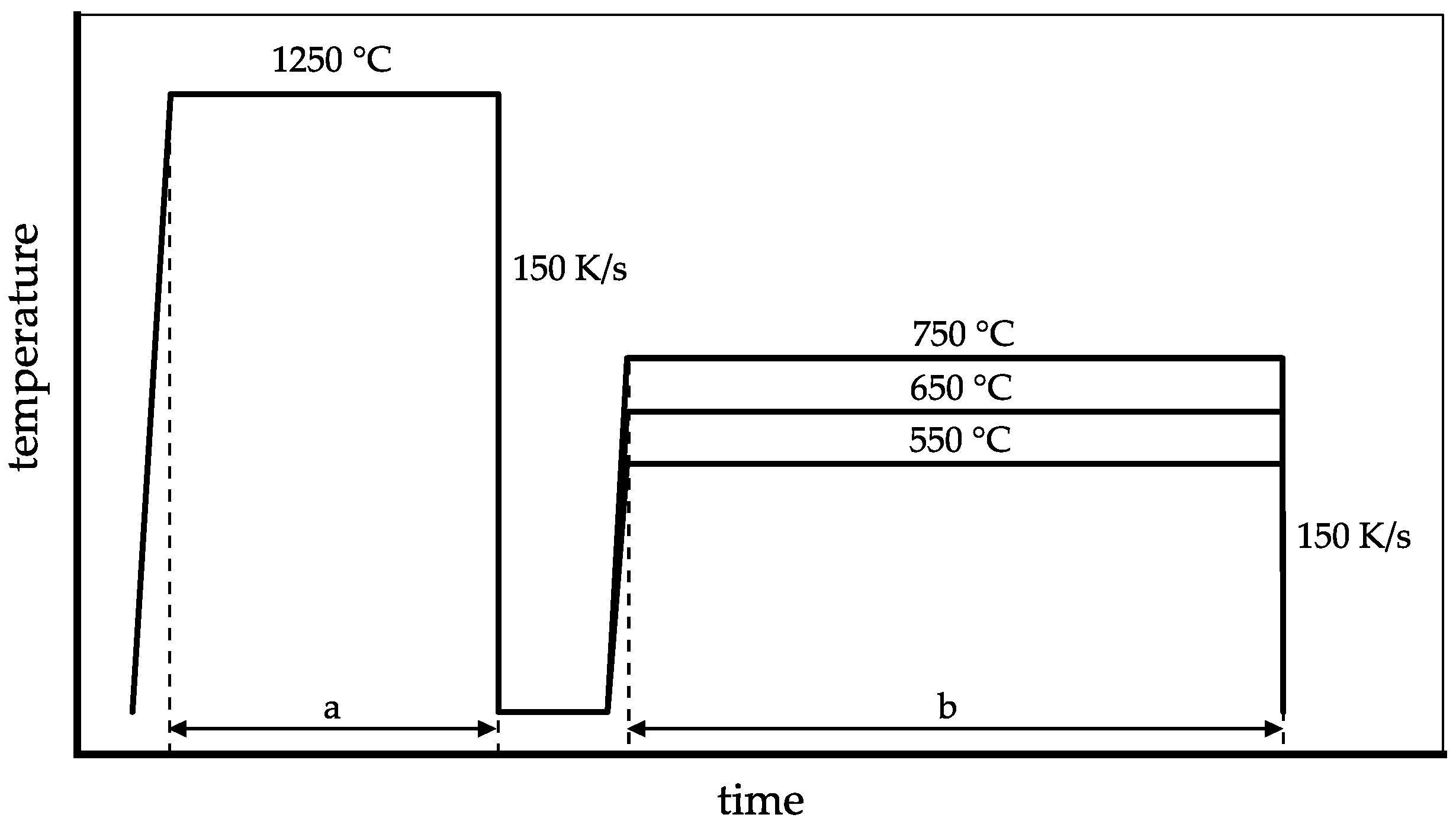
2. Materials and Methods
3. Results and Discussion
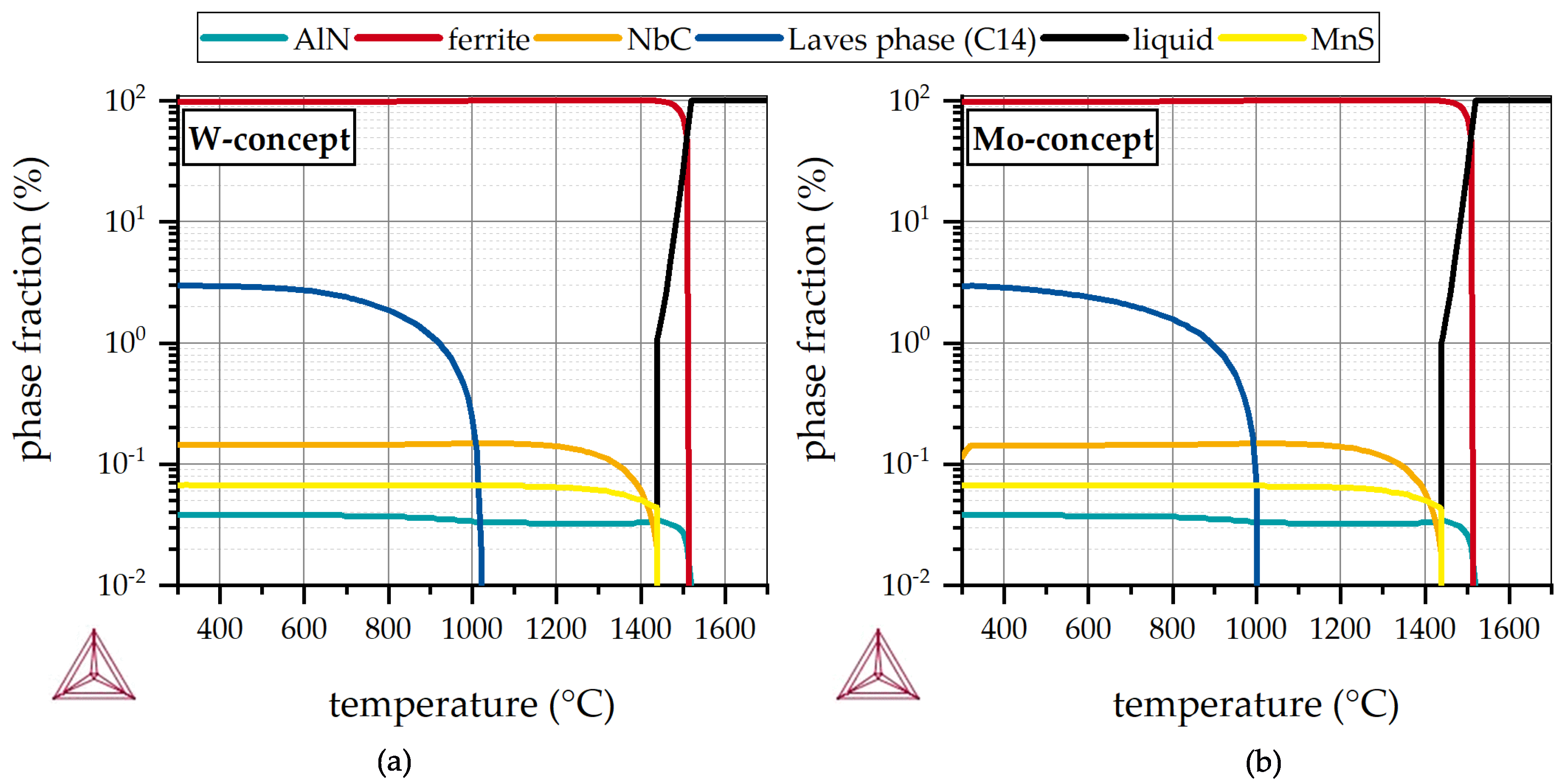
3.1. Microstructure Analysis and Thermodynamic Assessment
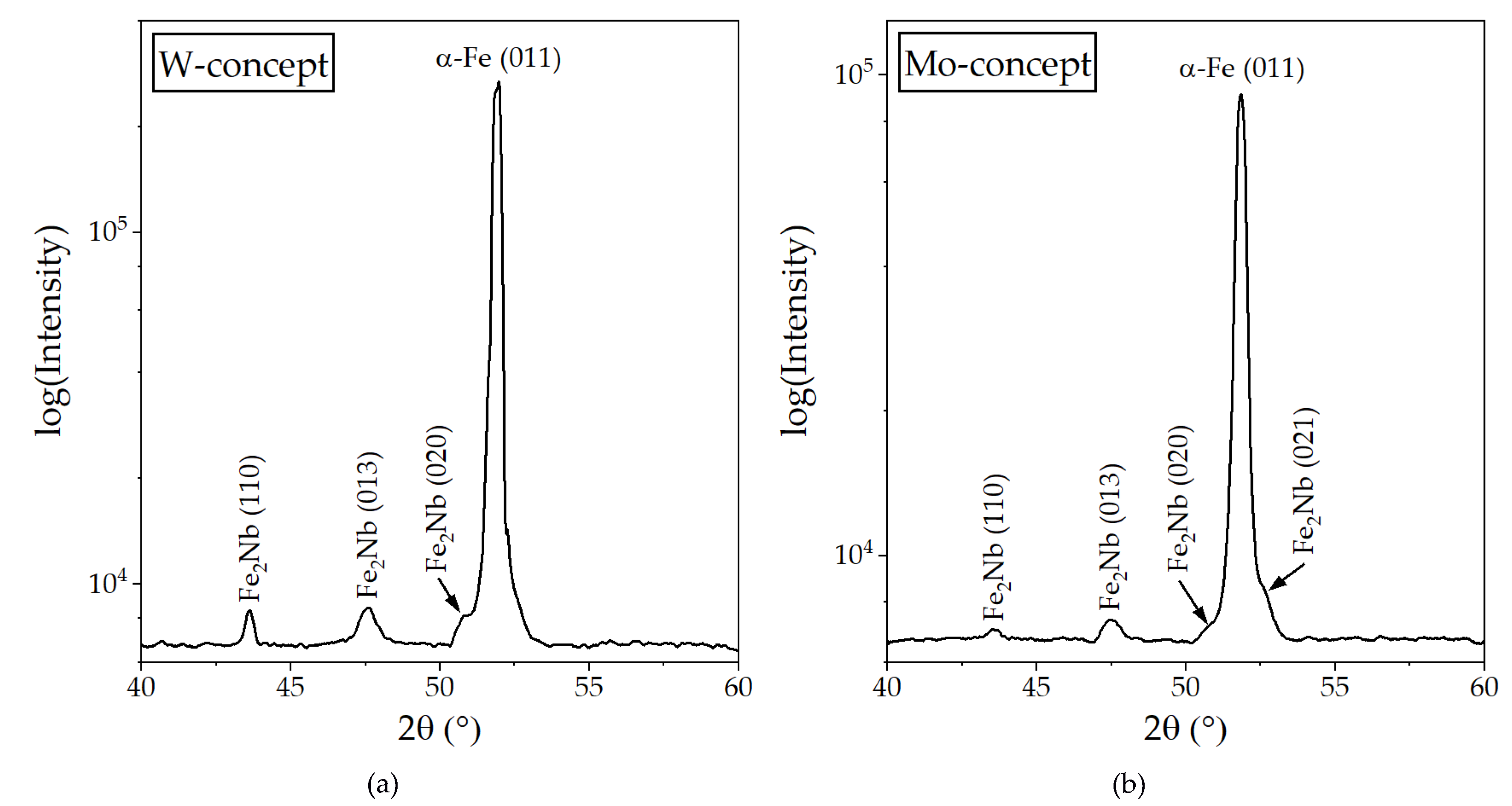
3.2. X-ray Diffraction Analysis
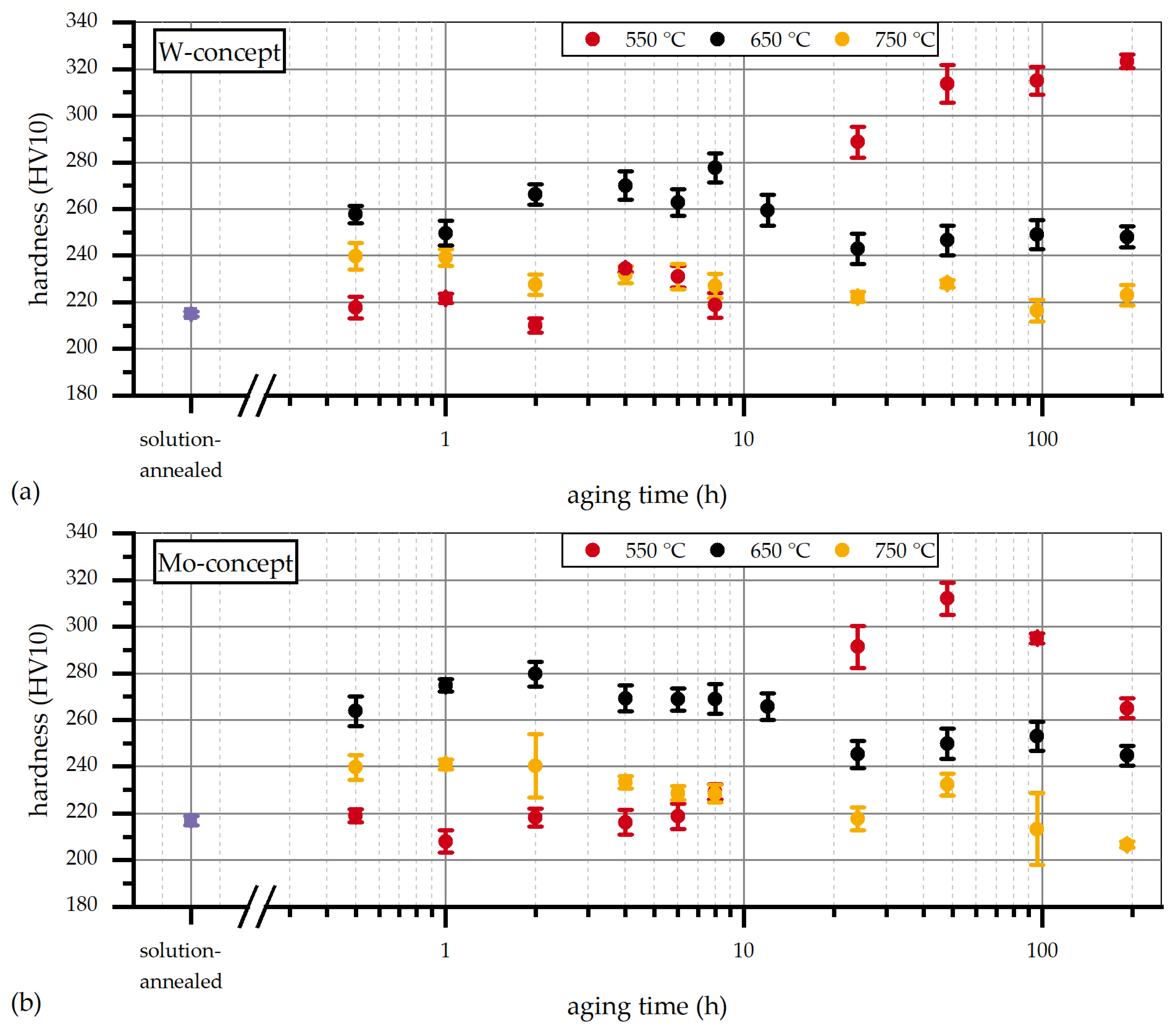
3.3. Influence of Aging Temperature and Time on Hardness
3.3.1. Early Stages of Aging at 650 °C
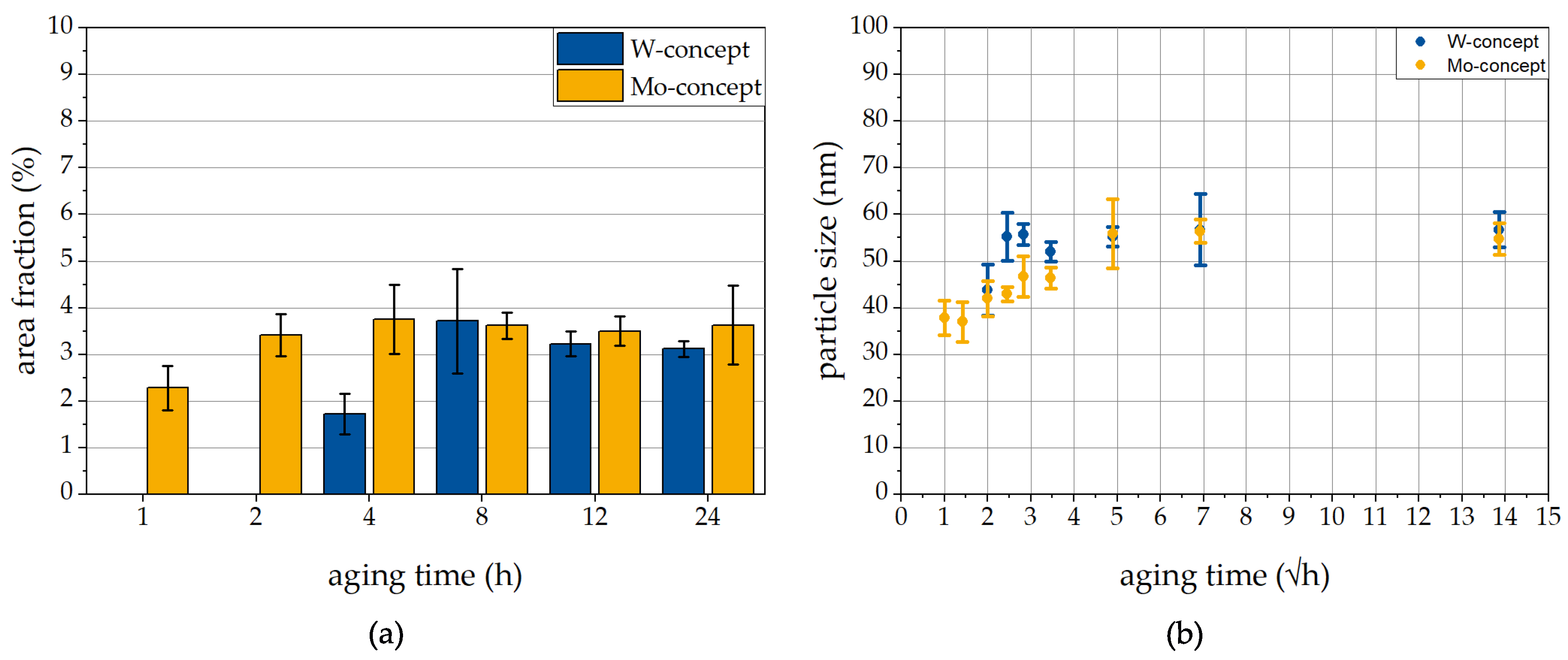
3.3.2. Evolution of Laves Phase at 650 °C
3.3.3. Hardness Profile for Aging at 550 °C
3.3.4. Hardness Profile for Aging at 750 °C
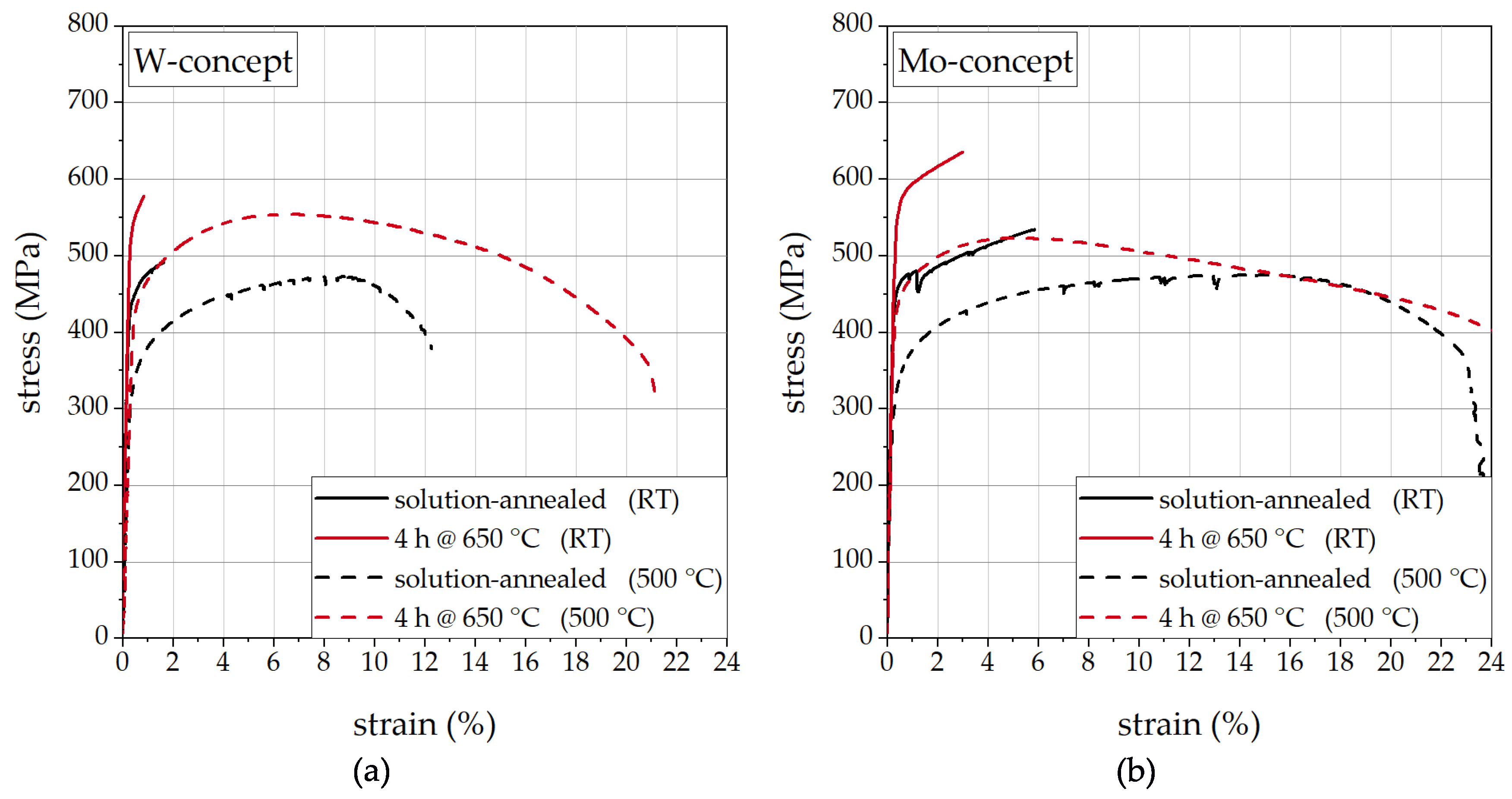
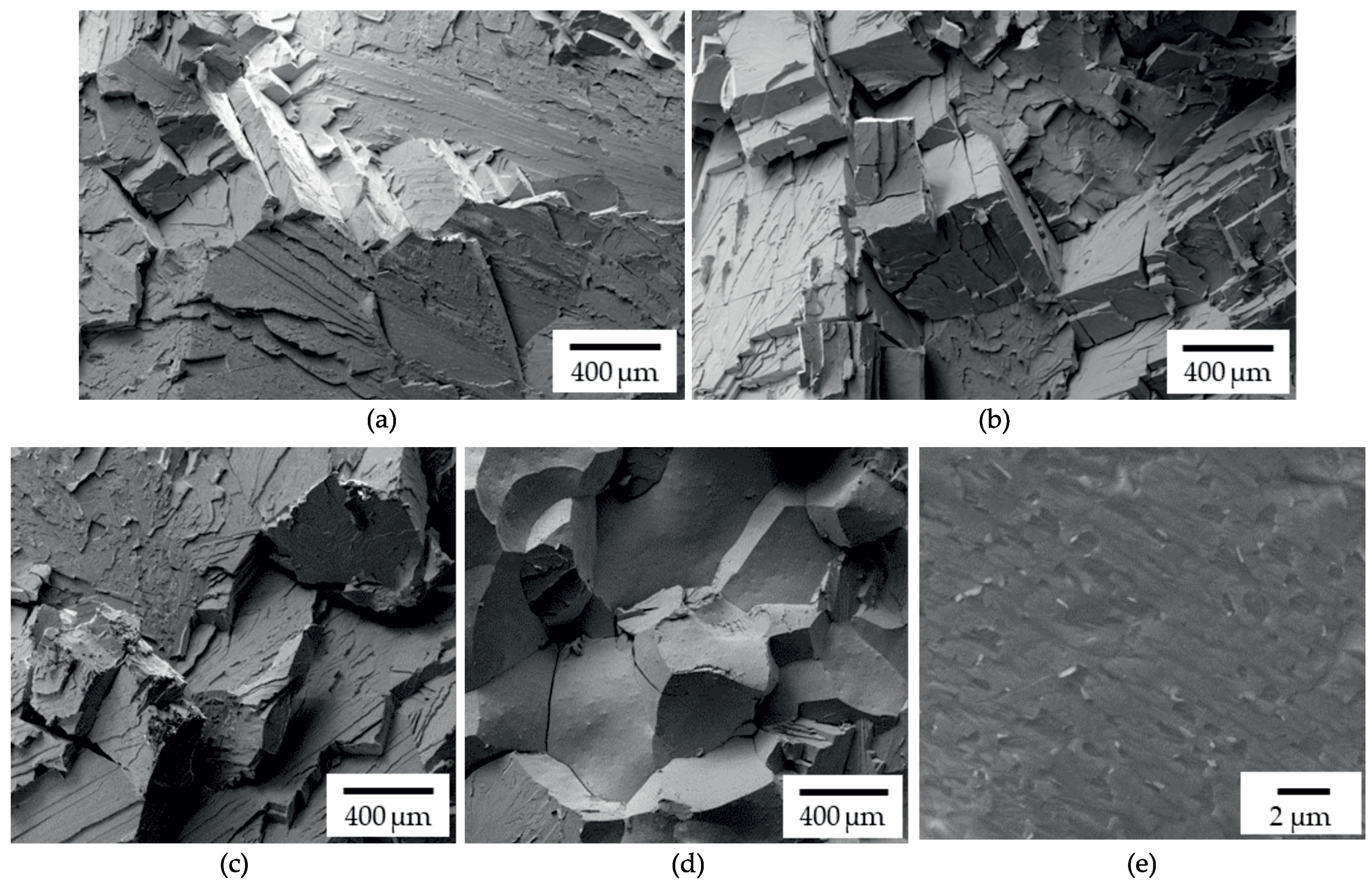
3.4. Influence of Laves Phase on Tensile Properties
4. Conclusions
- Aging at temperatures between 550 and 750 °C leads in the investigated W- and Mo-alloyed steels to the formation of the intermetallic Fe2Nb Laves phase. Aside from Fe and Nb, small amounts of W or Mo as well as Si were found to be incorporated in the Laves phase.
- The most powerfull effect of precipitation hardening occurs in both alloying concepts at 550 °C. At 650 °C, the increase in hardness is less pronounced but occurs after a significantly shorter aging time due to the higher diffusion coefficients of Nb, W, and Mo at 650 °C compared to 550 °C. Aging at 750 °C leads to no significant increase in hardness for both alloying concept since the formation and coarsening of the Laves phase is very fast at this temperature.
- At 550 °C, the precipitation hardening in the Mo-concept is less stable than in the W-concept which might be contributed to the higher diffusion coefficient of Mo and the accelerated diffusion of Nb in presence of Mo. Further investigations have to be performed to clarify the effect of W and Mo on coarsening of the Laves phase at 550 °C.
- The yield strength can be substantially increased by precipitation hardening with the intermetallic Laves phase. The effect of precipitation hardening is still present and not lowered at testing temperatures of 500 °C in comparison to the effect of precipitation hardening at room temperature.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- MAHLE GmbH. (Ed.) Kolben und Motorische Erprobung, 1st ed.; ATZ/MTZ-Fachbuch, Vieweg+Teubner: Wiesbaden, Germany, 2011. [Google Scholar] [CrossRef]
- Krüger, L.; Jentsch, E.; Brunke, L.; Keßler, A.; Wolf, G.; Lehnert, T.; Schubert, N.; Wagner, A.; Landgrebe, D. Development of an innovative lightweight piston through process combination “casting–forging”. Procedia Manuf. 2019, 27, 172–176. [Google Scholar] [CrossRef]
- Niu, D.; Zhang, J.; Xiong, P.; Hao, G.; Liu, S.; Guo, W. High temperature fatigue and oxidation characteristics of forged steel piston materials. Eng. Fail. Anal. 2019, 97, 220–226. [Google Scholar] [CrossRef]
- Zander, D.; Klink, A.; Harst, S.; Klocke, F.; Altenbach, C. Influence of machining processes on rim zone properties and high temperature oxidation behavior of 42CrMo4. Mater. Corros. 2019, 70, 2190–2204. [Google Scholar] [CrossRef]
- Heizmann, J.; Blau, D. High-Strength Pistons for Diesel Engines. Atzoffhighway Worldw. 2018, 11, 40–43. [Google Scholar] [CrossRef]
- Saxena, S.; Ambikesh, R.K. Design and finite element analysis of connecting rod of different materials. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2021; Volume 2341. [Google Scholar]
- Sello, M.P.; Stumpf, W.E. Laves phase precipitation and its transformation kinetics in the ferritic stainless steel type AISI 441. Mater. Sci. Eng. A 2011, 528, 1840–1847. [Google Scholar] [CrossRef]
- Reuther, F.; Mosel, A.; Freytag, P.; Lambarri, J.; Degenkolb, L.; Werner, M.; Winter, S. Numerical and experimental investigations for hot metal gas forming of stainless steel X2CrTiNb18. Procedia Manuf. 2019, 27, 112–117. [Google Scholar] [CrossRef]
- Viswanathan, R.; Bakker, W. Materials for Ultrasupercritical Coal Power Plants—Boiler Materials: Part 1. J. Mater. Eng. Perform. 2001, 10, 81–95. [Google Scholar] [CrossRef]
- Dong, J.; Yu, H.; Yoo, D.H.; Huynh, Q.; Shin, K. Effects of additional elements on the evolution of second phases in 9–12% Cr steel and resulting mechanical properties. Int. J. Mod. Phys. B 2009, 23, 1141–1147. [Google Scholar] [CrossRef]
- Hosoi, Y.; Wade, N.; Kunimitsu, S.; Urita, T. Precipitation behavior of laves phase and its effect on toughness of 9Cr-2Mo Ferritic-martensitic steel. J. Nucl. Mater. 1986, 141–143, 461–467. [Google Scholar] [CrossRef]
- Tomaszewicz, P.; Wallwork, G.R. Observations of nodule growth during the oxidation of pure binary iron-aluminum alloys. Oxid. Met. 1983, 19, 165–185. [Google Scholar] [CrossRef]
- Palm, M. Concepts derived from phase diagram studies for the strengthening of Fe–Al-based alloys. Intermetallics 2005, 13, 1286–1295. [Google Scholar] [CrossRef]
- Rana, R.; Liu, C.; Ray, R.K. Low-density low-carbon Fe–Al ferritic steels. Scr. Mater. 2013, 68, 354–359. [Google Scholar] [CrossRef]
- Falat, L.; Schneider, A.; Sauthoff, G.; Frommeyer, G. Mechanical properties of Fe–Al–M–C (M=Ti, V, Nb, Ta) alloys with strengthening carbides and Laves phase. Intermetallics 2005, 13, 1256–1262. [Google Scholar] [CrossRef]
- Morris, D.G.; Muñoz-Morris, M.A.; Requejo, L.M.; Baudin, C. Strengthening at high temperatures by precipitates in Fe–Al–Nb alloys. Intermetallics 2006, 14, 1204–1207. [Google Scholar] [CrossRef]
- Morris, D.G.; Requejo, L.M.; Muñoz-Morris, M.A. Age hardening in some Fe–Al–Nb alloys. Scr. Mater. 2006, 54, 393–397. [Google Scholar] [CrossRef]
- Morris, D.G.; Requejo, L.M.; Muñoz-Morris, M.A. A study of precipitation in DO3 ordered Fe–Al–Nb alloy. Intermetallics 2005, 13, 862–871. [Google Scholar] [CrossRef]
- Stein, F.; Schneider, A.; Frommeyer, G. Flow stress anomaly and order–disorder transitions in Fe3Al-based Fe–Al–Ti–X alloys with X=V, Cr, Nb, or Mo. Intermetallics 2003, 11, 71–82. [Google Scholar] [CrossRef]
- Kuhn, B.; Jimenez, C.A.; Niewolak, L.; Hüttel, T.; Beck, T.; Hattendorf, H.; Singheiser, L.; Quadakkers, W.J. Effect of Laves phase strengthening on the mechanical properties of high Cr ferritic steels for solid oxide fuel cell interconnect application. Mater. Sci. Eng. A 2011, 528, 5888–5899. [Google Scholar] [CrossRef]
- Kuhn, B.; Talik, M.; Niewolak, L.; Zurek, J.; Hattendorf, H.; Ennis, P.J.; Quadakkers, W.J.; Beck, T.; Singheiser, L. Development of high chromium ferritic steels strengthened by intermetallic phases. Mater. Sci. Eng. A 2014, 594, 372–380. [Google Scholar] [CrossRef]
- Talik, M. Influence of Initial Thermomechanical Treatment on High Temperature Properties of Laves Phase Strengthened Ferritic Steels. Ph.D. Thesis, RWTH Aachen University-FZ Jülich, Aachen, Germany, 2016. [Google Scholar]
- Pöpperlová, J.; Fan, X.; Kuhn, B.; Bleck, W.; Krupp, U. Impact of Tungsten on Thermomechanically Induced Precipitation of Laves Phase in High Performance Ferritic (HiperFer) Stainless Steels. Appl. Sci. 2020, 10, 4472. [Google Scholar] [CrossRef]
- Chai, Y.W.; Kato, K.; Yabu, C.; Ishikawa, S.; Kimura, Y. Disconnections and Laves (C14) precipitation in high-Cr ferritic stainless steels. Acta Mater. 2020, 198, 230–241. [Google Scholar] [CrossRef]
- Fan, X.; Kuhn, B.; Pöpperlová, J.; Bleck, W.; Krupp, U. Compositional Optimization of High-Performance Ferritic (HiperFer) Steels—Effect of Niobium and Tungsten Content. Metals 2020, 10, 1300. [Google Scholar] [CrossRef]
- Stein, F.; Palm, M.; Sauthoff, G. Structure and stability of Laves phases. Part I. Critical assessment of factors controlling Laves phase stability. Intermetallics 2004, 12, 713–720. [Google Scholar] [CrossRef]
- Maier, H.J.; Niendorf, T.; Bürgel, R. Handbuch Hochtemperatur-Werkstofftechnik: Grundlagen, Werkstoffbeanspruchungen, HochteMperaturlegierungen und -Beschichtungen, 5th ed.; Springer Fachmedien Wiesbaden: Wiesbaden, Germany, 2015. [Google Scholar] [CrossRef]
- Aghajani, A.; Richter, F.; Somsen, C.; Fries, S.G.; Steinbach, I.; Eggeler, G. On the formation and growth of Mo-rich Laves phase particles during long-term creep of a 12% chromium tempered martensite ferritic steel. Scr. Mater. 2009, 61, 1068–1071. [Google Scholar] [CrossRef]
- Isik, M.I.; Kostka, A.; Eggeler, G. On the nucleation of Laves phase particles during high-temperature exposure and creep of tempered martensite ferritic steels. Acta Mater. 2014, 81, 230–240. [Google Scholar] [CrossRef]
- Xia, Z.X.; Wang, C.Y.; Zhao, Y.F.; Zhang, G.D.; Zhang, L.; Meng, X.M. Laves Phase Formation and Its Effect on Mechanical Properties in P91 Steel. Acta Metall. Sin. 2015, 28, 1238–1246. [Google Scholar] [CrossRef][Green Version]
- α-Fe (ferrite) (Fe rt) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}1817302 (accessed on 11 October 2021).
- Fe2Nb (NbFe2) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}1011276 (accessed on 11 October 2021).
- AlN Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}0533024 (accessed on 11 October 2021).
- CNb (NbC) Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}0450232 (accessed on 11 October 2021).
- Fe3Al Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}0452482 (accessed on 11 October 2021).
- FeAl Crystal Structure: Datasheet from “PAULING FILE Multinaries Edition-2012” in SpringerMaterials. Available online: https://materials.springer.com/isp/crystallographic/docs/sd{_}0312257 (accessed on 11 October 2021).
- Risanti, D.D.; Sauthoff, G. Microstructures and mechanical properties of Fe–Al–Ta alloys with strengthening Laves phase. Intermetallics 2011, 19, 1727–1736. [Google Scholar] [CrossRef]
- Morris, D.G.; Morris, M.A. Strengthening at intermediate temperatures in iron aluminides. Mater. Sci. Eng. A 1997, 239–240. [Google Scholar] [CrossRef]
- Palm, M.; Stein, F.; Dehm, G. Iron Aluminides. Annu. Rev. Mater. Res. 2019, 49, 297–326. [Google Scholar] [CrossRef]
- Stoloff, N.S. Iron aluminides: Present status and future prospects. Mater. Sci. Eng. A 1998, 258, 1–14. [Google Scholar] [CrossRef]
- Kostryzhev, A.G.; Marenych, O.O.; Killmore, C.R.; Pereloma, E.V. Strengthening Mechanisms in Thermomechanically Processed NbTi-Microalloyed Steel. Metall. Mater. Trans. A 2015, 46A, 3470–3480. [Google Scholar] [CrossRef]
- Verein Deutscher Eisenhüttenleute. (Ed.) Werkstoffkunde Stahl: Band 1: Grundlagen, 4th ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA; Tokyo, Japan, 1984. [Google Scholar]
- Lechner, C.; Seume, J. (Eds.) Stationäre Gasturbinen, 3rd ed.; VDI-Buch, Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Mintz, B.; Gunawardana, W.D.; Su, H. Al as solid solution hardener in steels. Mater. Sci. Technol. 2008, 24, 596–600. [Google Scholar] [CrossRef]
- Nabiran, N.; Klein, S.; Weber, S.; Theisen, W. Evolution of the Laves Phase in Ferritic Heat-Resistant Steels During Long-term Annealing and its Influence on the High-Temperature Strength. Metall. Mater. Trans. A 2015, 46A, 102–114. [Google Scholar] [CrossRef]
- Sim, G.M.; Ahn, J.C.; Hong, S.C.; Lee, K.J.; Lee, K.S. Effect of Nb precipitate coarsening on the high temperature strength in Nb containing ferritic stainless steels. Mater. Sci. Eng. A 2005, 396, 159–165. [Google Scholar] [CrossRef]
- Gladman, T. The Physical Metallurgy of Microalloyed Steels; Institute of Materials: London, UK, 1997. [Google Scholar]
- Li, Q. Precipitation of Fe2W Laves Phase and Modeling of Its Direct Influence on the Strength of a 12Cr-2W Steel. Metall. Mater. Trans. A 2006, 37A, 89–97. [Google Scholar] [CrossRef]
- Narita, T.; Ukai, S.; Ohtsuka, S.; Inoue, M. Effect of tungsten addition on microstructure and high temperature strength of 9CrODS ferritic steel. J. Nucl. Mater. 2011, 417, 158–161. [Google Scholar] [CrossRef]
- Majta, J.; Lenard, J.G.; Pietrzyk, M. A study of the effect of the thermomechanical history on the mechanical properties of a high niobium steel. Mater. Sci. Eng. A 1996, 208, 249–259. [Google Scholar] [CrossRef]
- Bose, S.C.; Singh, K.; Ray, A.K.; Ghosh, R.N. Effect of thermal ageing on mechanical properties and microstructures of a standard G-X 12 CrMoVWNbN 1011 grade of cast steel for turbine casing. Mater. Sci. Eng. A 2008, 476, 257–266. [Google Scholar] [CrossRef]
- Juuti, T.; Rovatti, L.; Mäkelä, A.; Karjalainen, L.P.; Porter, D. Influence of long heat treatments on the laves phase nucleation in a type 444 ferritic stainless steel. J. Alloys Compd. 2014, 616, 250–256. [Google Scholar] [CrossRef]
- Liu, H.; Wei, L.; Ma, M.; Zheng, J.; Chen, L.; Misra, R.D.K. Laves phase precipitation behavior and high-temperature strength of W-containing ferritic stainless steels. J. Mater. Res. Technol. 2020, 9, 2127–2135. [Google Scholar] [CrossRef]
- Askeland, D.R.; Wright, W.J. Essentials of Materials Science and Engineering, 4th ed.; Cengage Learning: Boston, MA, USA, 2019. [Google Scholar]
- Hornbogen, E. Clustering in an Alpha Iron-Molybdenum Solid Solution. J. Appl. Phys. 1961, 32, 135–139. [Google Scholar] [CrossRef]
- Amara, H.; Fu, C.C.; Soisson, F.; Maugis, P. Aluminum and vacancies in α-iron: Dissolution, diffusion, and clustering. Phys. Rev. B 2010, 81, 1409. [Google Scholar] [CrossRef]
- Ferndndez Guillermet, A. The Fe-Mo (Iron-Molybdenum) System. Bull. Alloy. Phase Diagrams 1982, 3, 359–367. [Google Scholar] [CrossRef]
- Marcus, H.L.; Fine, M.E.; Schwartz, L.H. Mössbauer-Effect Study of Solid-Solution and Precipitated Fe-Rich Fe-Mo Alloys. J. Appl. Phys. 1967, 38, 4750–4758. [Google Scholar] [CrossRef]
- Ericsson, T.; Mourikis, S.; Cohen, J.B. Clustering in Fe-Mo Alloys. J. Mater. Sci. 1970, 5, 901–908. [Google Scholar] [CrossRef]
- Ustinovshikov, Y.I. Precipitation in solids. J. Mater. Sci. 1992, 27, 3993–4002. [Google Scholar] [CrossRef]
- Alam, T.; Chaturvedi, M.; Ringer, S.P.; Cairney, J.M. Precipitation and clustering in the early stages of ageing in Inconel 718. Mater. Sci. Eng. A 2010, 527, 7770–7774. [Google Scholar] [CrossRef]
- Pereloma, E.V.; Timokhina, I.B.; Russel, K.F.; Miller, M.K. Characterization of clusters and ultrafine precipitates in Nb-containing C–Mn–Si steels. Scr. Mater. 2006, 54, 471–476. [Google Scholar] [CrossRef]
- Fujita, N.; Kikuchi, M.; Ohmura, K. Expressions for Solubility Products of Fe3Nb3C carbide and Fe2Nb Laves Phase in Niobium Alloyed Ferritic Stainless Steels. ISIJ Int. 2003, 43, 1999–2006. [Google Scholar] [CrossRef]
- Takemoto, S.; Nitta, H.; Iijima, Y.; Yamazaki, Y. Diffusion of tungsten in α-iron. Philos. Mag. 2007, 87, 1619–1629. [Google Scholar] [CrossRef]
- Nitta, H.; Yamamoto, T.; Kanno, R.; Takasawa, K.; Iida, T.; Yamazaki, Y.; Ogu, S.; Iijima, Y. Diffusion of molybdenum in α-iron. Acta Mater. 2002, 50, 4117–4125. [Google Scholar] [CrossRef]
- Jeon, S.H.; Kim, S.T.; Lee, I.S.; Kim, J.S.; Kim, K.T.; Park, Y.S. Effects of W substitution on the precipitation of secondary phases and the associated pitting corrosion in hyper duplex stainless steels. J. Alloys Compd. 2012, 544, 166–172. [Google Scholar] [CrossRef]
- Oono, N.; Nitta, H.; Iijima, Y. Diffusion of niobium in α-iron. Mater. Trans. 2003, 44, 2078–2083. [Google Scholar] [CrossRef]
- Miyazaki, A.; Takao, K.; Furukimi, O. Effect of Nb on the Proof Strength of Ferritic Stainless Steels at Elevated Temperatures. ISIJ Int. 2002, 42, 916–920. [Google Scholar] [CrossRef]
- Ghosh, S. Kinetic study on the coarsening behaviour of equilibrium phases in Nb alloyed ferritic stainless steels at 700 °C. Mater. Chem. Phys. 2010, 124, 13–16. [Google Scholar] [CrossRef]
- Gottstein, G. Materialwissenschaft und Werkstofftechnik; Springer: Berlin/Heidelberg, Geramny, 2014. [Google Scholar] [CrossRef]
- Hu, P.; Yan, W.; Sha, W.; Wang, W.; Guo, Z.l.; Shan, Y.Y.; Yang, K. Study on Laves phase in an advanced heat-resistant steel. Front. Mater. Sci. China 2009, 3, 434–441. [Google Scholar] [CrossRef]
- Nabiran, N.; Weber, S.; Theisen, W. Influence of Laves Phase Precipitation and Coarsening on High-Temperature Strength of Ferritic Stainless Steels. Steel Res. Int. 2012, 83, 758–765. [Google Scholar] [CrossRef]
- Tokizane, M.; Okada, Y.; Tamura, I. The Growth of Fe2Ti-Laves Phase Precipitates in Fe-Ti Alloy during Aging*. Trans. ISIJ 1972, 12, 276–282. [Google Scholar] [CrossRef]
- Juuti, T.J.; Karjalainen, L.P.; Heikkinen, E.P. Precipitation of Si and its Influence on Mechanical Properties of Type 441 Stainless Steel. Adv. Mater. Res. 2011, 409, 690–695. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, Y.; Gong, J.; Weng, X. The influence of long-term thermal exposure on microstructural stabilization and mechanical properties in 9Cr-0.5Mo-1.8 W-VNb heat-resistant steel. Mater. Sci. Eng. A 2016, 672, 194–202. [Google Scholar] [CrossRef]
- Hall, E.O. The Deformation and Ageing of Mild Steel: III Discussion of Results. Proc. Phys. Soc. Sect. B 1951, 64, 747. [Google Scholar] [CrossRef]
- Lacy, C.E.; Gensamer, M. The tensile properties of alloyed ferrites. Trans. Am. Soc. Met. 1944, 32, 88–105. [Google Scholar]
- Sarwat, S.G.; Basu, J. Understanding Laves phase precipitation induced embrittlement of modified 9Cr–1Mo steel. SN Appl. Sci. 2019, 1, 5164. [Google Scholar] [CrossRef]
- Komazaki, S.I.; Kishi, S.; Shoji, T.; Chiba, H.; Suzuji, K. Thermal Aging Embrittlement of Tugnsten-Alloyed 9%Cr Ferritic Steels and Electrochemical Evaluation. Mater. Sci. Res. Int. 2003, 9, 42–49. [Google Scholar]

| Alloy | C * | Si | Mn | S * | Al | N | W | Mo | Nb | Fe | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| W-concept | wt. % | 0.021 | 0.50 | 0.25 | 0.002 | 5.91 | 0.007 | 1.23 | − | 1.34 | bal. |
| at. % | 0.093 | 0.94 | 0.24 | 0.003 | 11.59 | 0.026 | 0.35 | − | 0.76 | bal. | |
| Mo-concept | wt. % | 0.029 | 0.50 | 0.27 | 0.013 | 5.91 | 0.004 | − | 0.75 | 1.29 | bal. |
| at. % | 0.127 | 0.94 | 0.26 | 0.021 | 11.53 | 0.015 | − | 0.41 | 0.76 | bal. |
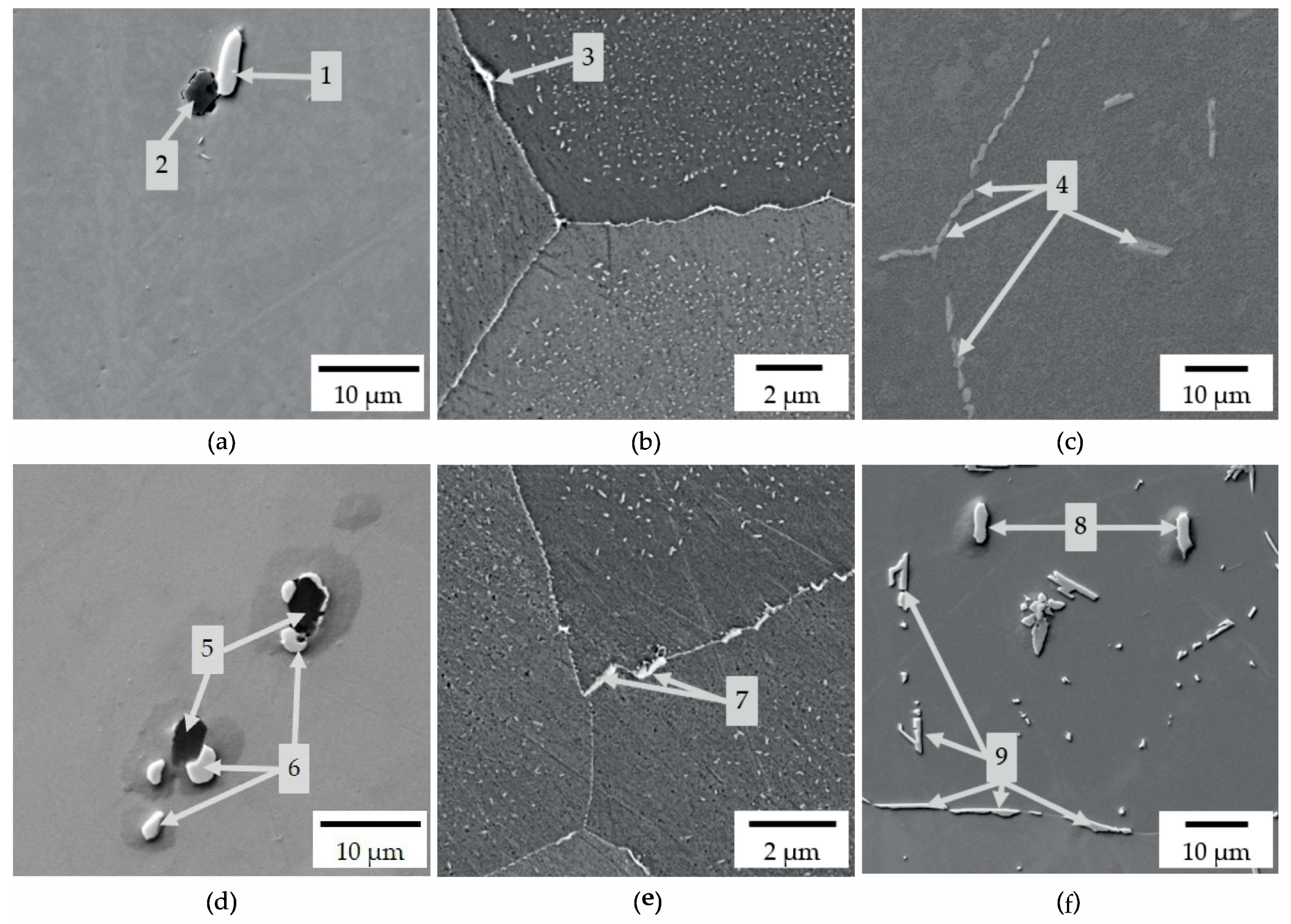
| Alloy | State | Precipitation No. | Fe | Al | Si | Nb | W | Mo | C | N |
|---|---|---|---|---|---|---|---|---|---|---|
| W-concept | solution annealed | 1 | 4.5 | − | − | 75.3 | − | − | 17.6 | 1.6 |
| 2 | 5.2 | 62.4 | − | − | − | − | 3.0 | 28.3 | ||
| 650 °C 8 h | 3 | 59.0 | − | 2.1 | 27.4 | 6.5 | − | 2.4 | − | |
| 750 °C 8 h | 4 | 55.4 | − | 3.1 | 31.6 | 0.4 | − | 1.9 | − | |
| Mo-concept | solution annealed | 5 | 2.5 | 65.0 | − | − | − | − | 2.7 | 27.3 |
| 6 | 3.4 | − | − | 77.0 | − | − | 17.1 | 1.0 | ||
| 650 °C 8 h | 7 | 55.7 | − | 4.7 | 29.9 | − | 3.9 | 3.6 | − | |
| 750 °C 8 h | 8 | 3.6 | − | − | 77.4 | − | − | 17.2 | 1.1 | |
| 9 | 51.1 | − | 4.5 | 34.3 | − | 4.1 | 3.1 | − |
| Alloy | Aging Temperature | Aging Time | Fe | Al | Si | Mn | Nb | W | Mo |
|---|---|---|---|---|---|---|---|---|---|
| W-concept | 650 °C | 1 h | 91.0 | 5.5 | 0.5 | 0.3 | 1.1 | 1.3 | − |
| 4 h | 90.7 | 5.9 | 0.5 | 0.3 | 0.7 | 1.3 | − | ||
| 12 h | 86.7 | 11.1 | 0.9 | 0.3 | 0.7 | 0.4 | − | ||
| 750 °C | 8 h | 92.1 | 5.5 | 0.4 | 0.3 | 0.1 | 1.0 | − | |
| Mo-concept | 650 °C | 1 h | 91.7 | 6.0 | 0.5 | 0.3 | 0.8 | − | 0.7 |
| 4 h | 92.4 | 5.8 | 0.5 | 0.3 | 0.6 | − | 0.5 | ||
| 12 h | 92.8 | 5.5 | 0.4 | 0.3 | 0.5 | − | 0.5 | ||
| 750 °C | 8 h | 92.7 | 6.0 | 0.4 | 0.3 | − | − | 0.4 |
| Alloy | State | Measuring Site | Fe | Al | Si | Mn | Nb | W | Mo |
|---|---|---|---|---|---|---|---|---|---|
| W-concept | solution annealed | fracture surface | 90.0 | 5.5 | 0.5 | 0.3 | 1.0 | 1.3 | − |
| 4 h at 650 °C | fracture surface | 90.4 | 5.6 | 0.4 | 0.3 | 1.0 | 1.3 | − | |
| Mo-concept | solution annealed | fracture surface | 89.8 | 6.3 | 0.6 | 0.3 | 1.2 | − | 0.7 |
| 4 h at 650 °C | fracture surface | 87.1 | 2.0 | 1.3 | 0.4 | 8.5 | − | − | |
| 4 h at 650 °C | precipitation | 79.9 | − | 2.1 | − | 13.2 | − | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Emmrich, R.; Krupp, U. On the Impact of the Intermetallic Fe2Nb Laves Phase on the Mechanical Properties of Fe-6 Al-1.25 Nb-X W/Mo Fully Ferritic Light-Weight Steels. Metals 2021, 11, 1693. https://doi.org/10.3390/met11111693
Emmrich R, Krupp U. On the Impact of the Intermetallic Fe2Nb Laves Phase on the Mechanical Properties of Fe-6 Al-1.25 Nb-X W/Mo Fully Ferritic Light-Weight Steels. Metals. 2021; 11(11):1693. https://doi.org/10.3390/met11111693
Chicago/Turabian StyleEmmrich, Robin, and Ulrich Krupp. 2021. "On the Impact of the Intermetallic Fe2Nb Laves Phase on the Mechanical Properties of Fe-6 Al-1.25 Nb-X W/Mo Fully Ferritic Light-Weight Steels" Metals 11, no. 11: 1693. https://doi.org/10.3390/met11111693
APA StyleEmmrich, R., & Krupp, U. (2021). On the Impact of the Intermetallic Fe2Nb Laves Phase on the Mechanical Properties of Fe-6 Al-1.25 Nb-X W/Mo Fully Ferritic Light-Weight Steels. Metals, 11(11), 1693. https://doi.org/10.3390/met11111693






