Modeling and Simulation of Non-Uniform Electrolytic Machining Based on Cellular Automata
Abstract
:1. Introduction
2. Theoretical Basis for Model Construction
2.1. Mass Transfer
- is the diffusion coefficient of particle (),
- is the concentration of particle (),
- is the charge number of particle ,
- is the Faraday’s constant (96,500 ),
- is the gas constant (8.313 J/mol),
- is temperature (K),
- is voltage (V), and
- is convection velocity ().
2.2. Particle Migration
- is the type of the particle,
- is particle velocity, and
- is the velocity vector of the particle.
2.3. Preconditions
- (1)
- A corrosion point already existed at the beginning of the simulation.
- (2)
- A passivation film hindered corrosion.
- (3)
- Particles moved by the concentration difference and the electric field and also obeyed the mass transfer equation of electrochemistry.
- (4)
- Electrochemical reactions were not considered during particle motion. Boundary conditions were used to simulate the dissolution of particles, the disappearance of the substrate, and the generation of product particles.
2.4. Calculation Process
3. Numerical Simulation
3.1. Physical Model
3.2. Calculation Rules
- (1)
- Free diffusion
- (2)
- Particle migration
3.3. Initial and Boundary Conditions
- (1)
- Initial conditions
- (2)
- Boundary conditions
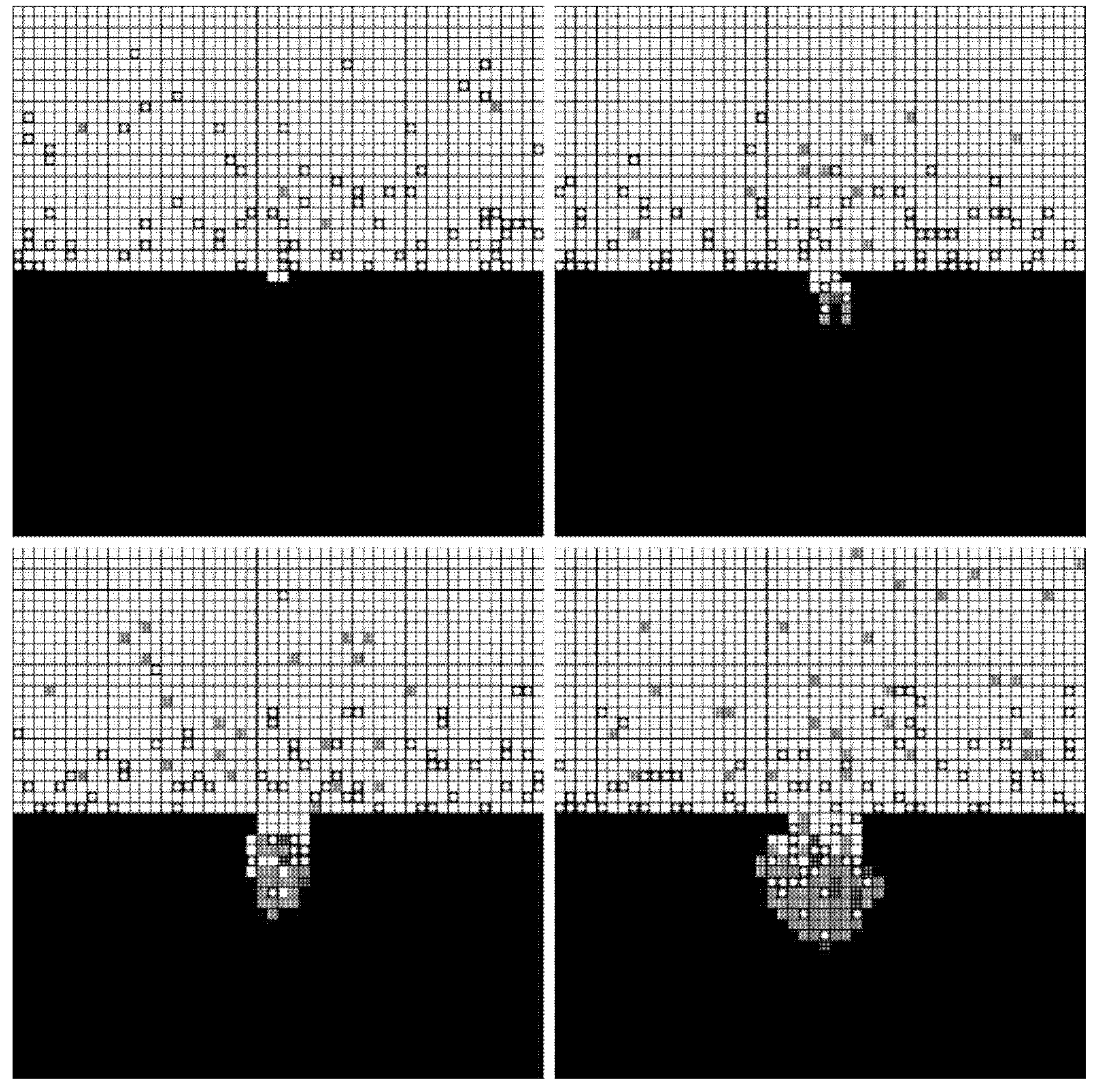
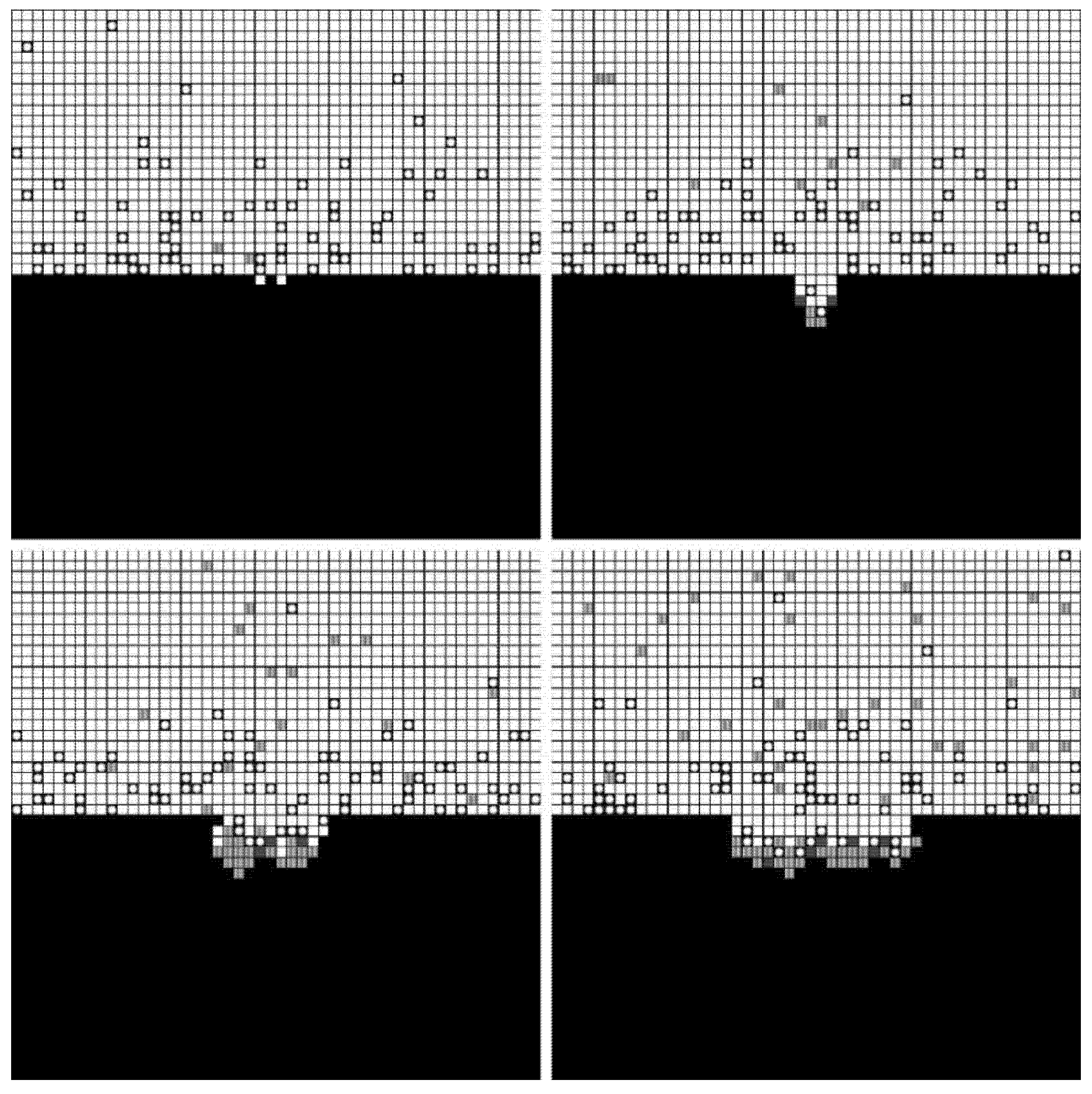
3.4. Results
4. Validation Test
4.1. Materials and Methods
4.2. Results
4.3. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, C.; Zheng, X.; Zhang, Y.; Zhang, X.; Li, Y. Multifunctional porous materials with simultaneous high water flux, antifouling and antibacterial performances from ionic liquid grafted polyethersulfone. Polymer 2021, 212, 123183. [Google Scholar] [CrossRef]
- Chen, S.; Fukazawa, K.; Inoue, Y.; Ishihara, K. Photoinduced Surface Zwitterionization for Antifouling of Porous Polymer Substrates. Langmuir 2019, 35, 1312–1319. [Google Scholar] [CrossRef]
- Qian, H.; Liu, B.; Wu, D.; Liu, W.; Chowwanonthapunya, T.; Zhang, D. Facile fabrication of slippery lubricant-infused porous surface with pressure responsive property for anti-icing application. Colloids Surf. A Physicochem. Eng. Asp. 2021, 618, 126457. [Google Scholar] [CrossRef]
- Zou, X.; Fang, X.; Chen, M.; Zhu, D. Investigation on mass transfer and dissolution localization of wire electrochemical machining using vibratory ribbed wire tools. Precis. Eng. 2018, 51, 597–603. [Google Scholar] [CrossRef]
- He, J.; Ma, P.; Xie, A.; Gao, L.; Zhou, Z.; Yan, Y.; Dai, J.; Li, C. From black liquor to highly porous carbon adsorbents with tunable microstructure and excellent adsorption of tetracycline from water: Performance and mechanism study. J. Taiwan Inst. Chem. Eng. 2016, 63, 295–302. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, D.; Qiu, X.; Qian, Y.; Yan, M.; Li, Q. Influences of aggregation behavior of lignin on the microstructure and adsorptive properties of lignin-derived porous carbons by potassium compound activation. J. Ind. Eng. Chem. 2020, 82, 220–227. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, Y.; Hillmyer, M.A.; Francis, L.F. Processing and properties of porous poly(l-lactide)/bioactive glass composites. Biomaterials 2004, 25, 2489–2500. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Shen, J.; Zeng, Z.; Park, J.M.; Choi, Y.T.; Schell, N.; Maawad, E.; Zhou, N.; Kim, H.S. Dissimilar laser welding of a CoCrFeMnNi high entropy alloy to 316 stainless steel. Scr. Mater. 2021, 206, 114219. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Shen, J.; Escobar, J.D.; Salvador, C.A.F.; Schell, N.; Zhou, N.; Benafan, O. Laser welding of H-phase strengthened Ni-rich NiTi-20Zr high temperature shape memory alloy. Mater. Des. 2021, 202, 109533. [Google Scholar] [CrossRef]
- Aung, N.N.; Wai, W.K.; Tan, Y. A novel electrochemical method for monitoring corrosion under insulation. Anti-Corros. Methods Mater. 2006, 53, 175–179. [Google Scholar] [CrossRef]
- Lengyel, B.; Mészáros, L.; Mészáros, G.; Fekete, É.; Janászik, F.; Szenes, I. Electrochemical methods to determine the corrosion rate of a metal protected by a paint film. Prog. Org. Coat. 1999, 36, 11–14. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic oxidation for the degradation of organic pollutants: Anode materials, operating conditions and mechanisms. A mini review. Electrochem. Commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Singh, S.; Datta, J. Influence of Nafion template on the kinetics of anodic Pt based pluri-metallic catalyst for ethanol electro-oxidation. J. Electroanal. Chem. 2021, 882, 114974. [Google Scholar] [CrossRef]
- Chuang, M.; Chien, H.; Chain, Y.; Chi, G.; Lee, S.; Woon, W.Y. Local anodic oxidation kinetics of chemical vapor deposition graphene supported on a thin oxide buffered silicon template. Carbon 2013, 54, 336–342. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, W.; Bai, Q.; Qin, J.; Zhang, Z. Alloying/dealloying mechanisms, microstructural modulation and mechanical properties of nanoporous silver via a liquid metal-assisted alloying/dealloying strategy. J. Alloys Compd. 2021, 872, 159675. [Google Scholar] [CrossRef]
- Mccue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Chen, Q.; Sieradzki, K. Mechanisms and Morphology Evolution in Dealloying. J. Electrochem. Soc. 2013, 160, C226–C231. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, Y.; Chen, M. Nanoporous metal by dealloying for electrochemical energy conversion and storage. MRS Bull. 2018, 43, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Abolusoro, O.P.; Akinlabi, E.T. Tribocorrosion Measurements and Behaviour in Aluminium Alloys: An Overview. J. Bio-Tribo-Corros. 2020, 6, 1–13. [Google Scholar] [CrossRef]
- Connolly, J.A.D.; Galvez, M.E. Electrolytic fluid speciation by Gibbs energy minimization and implications for subduction zone mass transfer. Earth Planet. Sc. Lett. 2018, 501, 90–102. [Google Scholar] [CrossRef]
- Ariana, M.; Désilets, M.; Proulx, P. On the analysis of ionic mass transfer in the electrolytic bath of an aluminum reduction cell. Can. J. Chem. Eng. 2014, 92, 1951–1964. [Google Scholar] [CrossRef]
- Gomez-Gallegos, A.A.; Mill, F.; Mount, A.R. Surface finish control by electrochemical polishing in stainless steel 316 pipes. J. Manuf. Process. 2016, 23, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Klocke, F.; Harst, S.; Ehle, L.; Zeis, M.; Klink, A. Surface integrity in electrochemical machining processes: An analysis on material modifications occurring during electrochemical machining. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 578–585. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Choi, W.; Lee, E. The optimum condition selection of electrochemical polishing and surface analysis of the stainless steel 316L by the Taguchi method. Int. J. Adv. Manuf. Technol. 2016, 82, 1933–1939. [Google Scholar] [CrossRef]
- Kumar, M.R.P.; Prakasan, K.; Kalaichelvan, K. Experimental Investigation and Multiphysics Simulation on the Influence of Micro Tools with Various End Profiles on Diametrical Overcut of Holes Machined Using Electrochemical Micromachining for a Predetermined Optimum Combination of Process Parameters. Russ. J. Electrochem. 2015, 10, 949–954. [Google Scholar] [CrossRef]
- Danilov, I.; Hackert-Oschätzchen, M.; Zinecker, M.; Meichsner, G.; Edelmann, J.; Schubert, A. Process Understanding of Plasma Electrolytic Polishing through Multiphysics Simulation and Inline Metrology. Micromachines 2019, 10, 214. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Xiaogang, L.; Chaofang, D.; Kui, X.; Lin, L. Cellular automata modeling on pitting current transients. Electrochem. Commun. 2009, 11, 1826–1829. [Google Scholar] [CrossRef]
- Leese, R.; Ivanov, A. Electrochemical micromachining: Review of factors affecting the process applicability in micro-manufacturing. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 195–207. [Google Scholar] [CrossRef]
- Qu, N.; Chen, X.; Li, H.; Zeng, Y. Electrochemical micromachining of micro-dimple arrays on cylindrical inner surfaces using a dry-film photoresist. Chin. J. Chem. Eng. 2014, 27, 1030–1036. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Shi, H.; Ren, S. Cellular Automata Simulations of Random Pitting Process on Steel Reinforcement Surface. Comput. Model. Eng. Sci. 2021, 128, 967–983. [Google Scholar] [CrossRef]
- Li, W.; Quandai, W.; Xiuqing, H.; Yucheng, D.; Bingheng, L. Finite element simulation and experimental study on the through-mask electrochemical micromachining (EMM) process. Int. J. Adv. Manuf. Technol. 2010, 51, 155–162. [Google Scholar] [CrossRef]
- Wang, H.T.; Han, E.H. Cellular automata simulation of interactions between metastable corrosion pits on stainless steel. Mater. Corros. 2015, 66, 925–930. [Google Scholar] [CrossRef]
- Wang, W.; Guan, B.; Wei, X.; Lu, J.; Ding, J. Cellular automata simulation on the corrosion behavior of Ni-base alloy in chloride molten salt. Sol. Energy Mater. Sol. Cells 2019, 203, 110170. [Google Scholar] [CrossRef]
- Wei, H.; Wang, G.; Liu, G.; Guo, Z. Formation of porous hydrophobic stainless steel surfaces by maskless electrochemical machining. Surf. Eng. 2016, 32, 132–138. [Google Scholar] [CrossRef]
- Volgin, V.M.; Lyubimov, V.V.; Davydov, A.D. Modeling and numerical simulation of electrochemical micromachining. Chem. Eng. Sci. 2016, 140, 252–260. [Google Scholar] [CrossRef]
- Rusyn, B.P.; Tors’ka, R.V.; Pokhmurs’kyi, A.Y. Modeling of the Evolution of Corrosion Pitting with the Use of Cellular Automata. Mater. Sci. 2015, 50, 706–713. [Google Scholar] [CrossRef]
- Li, Y.; Guan, L.; Wei, H.; Guo, Z.; Wang, G. Preparation of cell adhesive micropores by one-step potentiostatic polarization on 304 stainless steel. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2019, 233, 1914–1919. [Google Scholar] [CrossRef]
- Xiao, Z.; Jun, H.; Yuqi, W.; Maosheng, Z.; Zaoxiao, Z. Simulation of Pitting Corrosion for Ni-based Alloy Using a Cellular Automata Model. Rare Met. Mater. Eng. 2015, 44, 2347–2352. [Google Scholar] [CrossRef]
- Hou, Z.; Qu, N.; Chen, X. Electrochemical micromachining of large-area micro-dimple arrays with high machining accuracy. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2018, 232, 1918–1927. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.; Qi, R. Heat and mass transfer in PEM-based electrolytic air dehumidification element with an optimized anode-side electrochemical model. Int. J. Heat Mass Transf. 2019, 135, 1152–1166. [Google Scholar] [CrossRef]


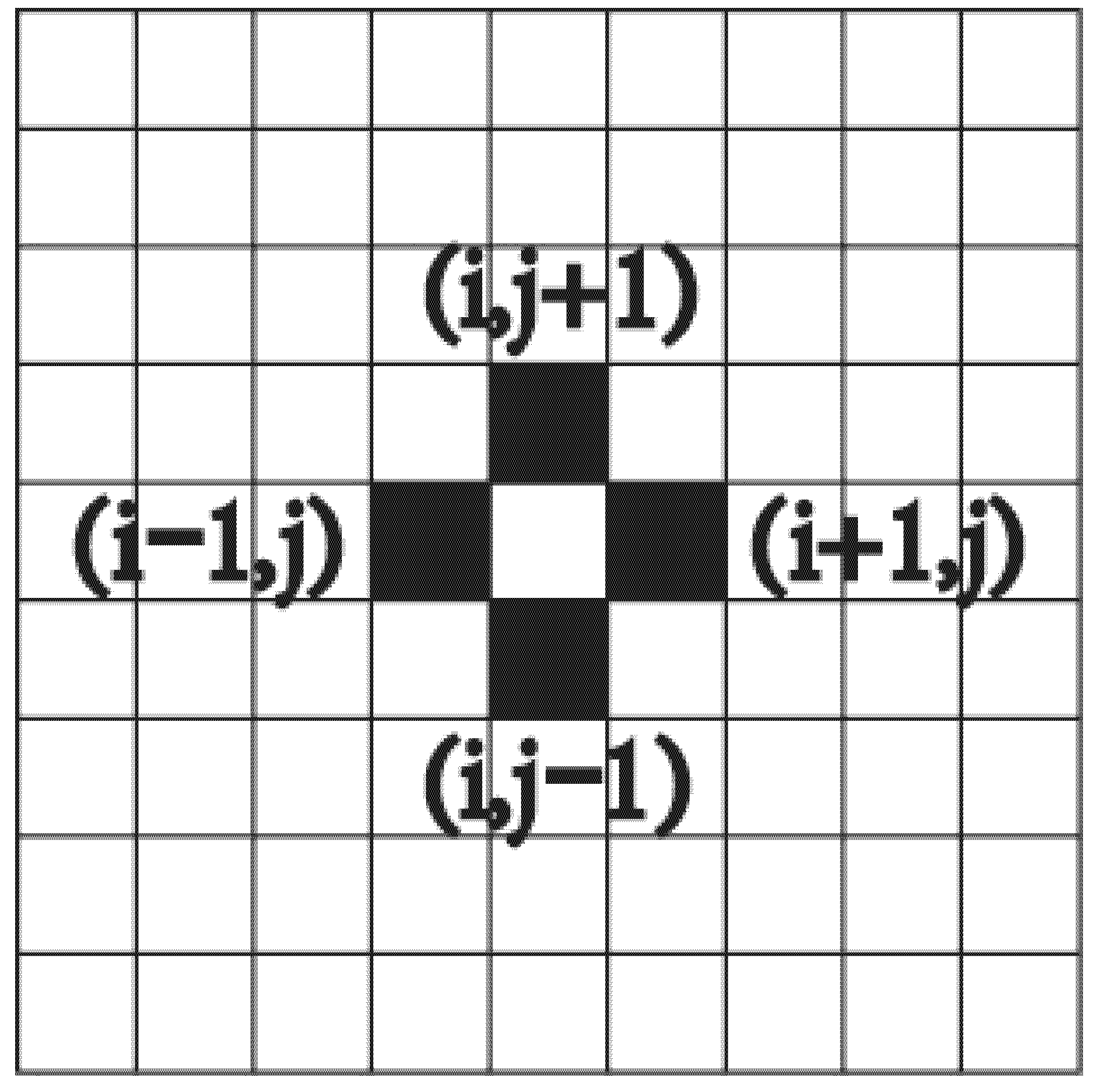


| Constant | Diffusion Coefficient of the Ternary System (cm2/s) | Temperature (K) | |
|---|---|---|---|
| Value | 7.62 × 10−9 | 6.89 | 300 |
| C | Si | Mn | P | S | Cr | Ni | Fe |
|---|---|---|---|---|---|---|---|
| 0.035 | 0.520 | 1.180 | 0.036 | 0.026 | 17.59 | 8.030 | 72.60 |
| Sample Number | 1 | 2 | 3 |
|---|---|---|---|
| Constant current (mA) | 150 | 750 | 2500 |
| Processing time (s) | 3000 | 600 | 180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, H.; Guo, Z.; Ma, Z. Modeling and Simulation of Non-Uniform Electrolytic Machining Based on Cellular Automata. Metals 2021, 11, 1694. https://doi.org/10.3390/met11111694
Wei H, Guo Z, Ma Z. Modeling and Simulation of Non-Uniform Electrolytic Machining Based on Cellular Automata. Metals. 2021; 11(11):1694. https://doi.org/10.3390/met11111694
Chicago/Turabian StyleWei, Hongyu, Zhongning Guo, and Zhiyu Ma. 2021. "Modeling and Simulation of Non-Uniform Electrolytic Machining Based on Cellular Automata" Metals 11, no. 11: 1694. https://doi.org/10.3390/met11111694
APA StyleWei, H., Guo, Z., & Ma, Z. (2021). Modeling and Simulation of Non-Uniform Electrolytic Machining Based on Cellular Automata. Metals, 11(11), 1694. https://doi.org/10.3390/met11111694







