Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden
Abstract
1. Introduction
2. Definitions of Physical Activity, Exercise, Training, Sport, and Health
3. Aerobic and Muscle-Strengthening Physical Activity
4. How does the Body Adapt to Physical Activity and Training?
5. Health Effects of Physical Activity and Training
5.1. Effects on Physical Health
5.2. Effects on Mental Health
6. How Sport Affects Health
7. Sport’s Effects on the Health of Children and Young People
7.1. Positive Aspects
7.2. Negative Aspects
7.3. Relevance of Sports
8. Sport’s Effects on the Health of Adults and the Elderly
8.1. Positive Aspects
8.2. Negative Aspects
8.3. Relevance of Sport
9. Recommendations for Healthy Sport
- 1. Plan exercise, rest, and social life. For health-promoting and healthy-aging physical activity, refer to general guidelines summarized in this paper: Aerobic exercise three times a week, muscle-strengthening exercise 2–3 times a week.
- 2. Set long-term goals.
- 3. Adopt a holistic performance development including physiological, medical, mental, and psychosocial aspects.
- 4. Monitor physiological health over time:
- ○
- a. Exercise load (time, intensity, volume);
- ○
- b. Recovery (sleep, resting heart rate, appetite, estimated fatigue, etc.);
- ○
- c. Sickness (when–where–how, type of infections, how long one is ill, etc.);
- ○
- d. Repeat type- and age-specific physical tests with relevant evaluation and feedback;
- ○
- e. Frequency of injuries and causes.
- 5. Monitor mental health over time:
- ○
- a. Motivation for training, competition, and socializing;
- ○
- b. Personal perception of stress, anxiety, depression, alienation, and self-belief;
- ○
- c. Repeat type- and age-specific psychological tests with relevant evaluation and feedback.
- 6. Register and interpret signs of overtraining, such as reduced performance over time, while maintaining or increasing exercise load.
Author Contributions
Funding
Conflicts of Interest
References
- Eime, R.M.; Young, J.A.; Harvey, J.T.; Charity, M.J.; Payne, W.R. A systematic review of the psychological and social benefits of participation in sport for children and adolescents: Informing development of a conceptual model of health through sport. Int. J. Behav. Nutr. Phys. Act. 2013, 10, 98. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.F. Amateur Sports of the Elderly: A Chance for Health and a Higher Quality of Life. Adv. Aging Res. 2014, 3, 222–229. [Google Scholar] [CrossRef]
- Fraser-Thomas, J.; Strachan, L. Personal developemnt and performance? In Health and Elite Sport: Is High Performance Sport a Healthy Pursuit? Baker, J., Safai, P., Fraser-Thomas, J., Eds.; Routledge Research in Sport, Culture and Society; Taylor & Francis Group: Londond, UK, 2015. [Google Scholar]
- Lopez Villalba, F.J.; Rodriguez Garcia, P.L.; Garcia Canto, E.; Perez Soto, J.J. Relationship between sport and physical activity and alcohol consumption among adolescents students in Murcia (Spain). Arch. Argent. Pediatr. 2016, 114, 101–106. [Google Scholar] [CrossRef]
- Elofsson, S.; Blomdahl, U.; Åkesson, M.; Lengheden, L. Dricker ungdomar i idrotsförening mindre alkohol än de som inte är med i en idrotsförening? Stockholm stads idrottsförvalnintg: Stockholm, Sweden, 2014. [Google Scholar]
- Kjonniksen, L.; Anderssen, N.; Wold, B. Organized youth sport as a predictor of physical activity in adulthood. Scand. J. Med. Sci. Sports 2009, 19, 646–654. [Google Scholar] [CrossRef]
- Khan, K.M.; Thompson, A.M.; Blair, S.N.; Sallis, J.F.; Powell, K.E.; Bull, F.C.; Bauman, A.E. Sport and exercise as contributors to the health of nations. Lancet 2012, 380, 59–64. [Google Scholar] [CrossRef]
- Howie, E.K.; McVeigh, J.A.; Smith, A.J.; Straker, L.M. Organized Sport Trajectories from Childhood to Adolescence and Health Associations. Med. Sci. Sports Exerc. 2016, 48, 1331–1339. [Google Scholar] [CrossRef]
- Rice, S.M.; Purcell, R.; De Silva, S.; Mawren, D.; McGorry, P.D.; Parker, A.G. The Mental Health of Elite Athletes: A Narrative Systematic Review. Sports Med. 2016, 46, 1333–1353. [Google Scholar] [CrossRef]
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hagglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef]
- Soligard, T.; Schwellnus, M.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.; Gleeson, M.; Hagglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 1) International Olympic Committee consensus statement on load in sport and risk of injury. Br. J. Sports Med. 2016, 50, 1030–1041. [Google Scholar] [CrossRef] [PubMed]
- Joy, E.; Kussman, A.; Nattiv, A. 2016 update on eating disorders in athletes: A comprehensive narrative review with a focus on clinical assessment and management. Br. J. Sports Med. 2016, 50, 154–162. [Google Scholar] [CrossRef]
- Brenner, J.S. Overuse injuries, overtraining, and burnout in child and adolescent athletes. Pediatrics 2007, 119, 1242–1245. [Google Scholar] [CrossRef]
- Clark, A.; Mach, N. Exercise-induced stress behavior, gut-microbiota-brain axis and diet: A systematic review for athletes. J. Int. Soc. Sports Nutr. 2016, 13, 43. [Google Scholar] [CrossRef]
- Lang, M.; Hartill, M. Safeguarding, Child Protection and Abuse in Sport: International Perspectives in Research, Policy and Practice; Taylor & Francis: Abingdon-on-Thames, UK, 2014. [Google Scholar]
- Pontzer, H.; Durazo-Arvizu, R.; Dugas, L.R.; Plange-Rhule, J.; Bovet, P.; Forrester, T.E.; Lambert, E.V.; Cooper, R.S.; Schoeller, D.A.; Luke, A. Constrained Total Energy Expenditure and Metabolic Adaptation to Physical Activity in Adult Humans. Curr. Biol. 2016, 26, 410–417. [Google Scholar] [CrossRef]
- Engström, L.-M. Barns och ungdomars idrottsvanor i förändring. Svensk Idrottsforskning: Organ för Centrum för Idrottsforskning 2004, 4, 10–15. [Google Scholar]
- The Swedish Sports Confederation. Sport statisitcs [Idrotten i siffror}; Confederation, T.S.S., Ed.; The Swedish Sports Confederation: Stockholm, Sweden, 2015. [Google Scholar]
- SCB. Levnadsförhållanden: Fritid 2006-2007 [Living Conditions: Recreation 2006-2007]; 118; Statistics Sweden: Stockholm, Sweden, 2009.
- Swedish National Institute of Publich Health Physical Activity in the Prevention and Treatment of Disease (FYSS); Swedish National Institute of Publich Health, and Swedish Professional Associations for Physical Activity: Järna, Sweden, 2017.
- WHO. Physical activity. Available online: http://www.who.int/topics/physical_activity/en/ (accessed on 19 November 2017).
- US Department of Human Services. Physical Activity Guidelines Advisory Committee report, 2008. To the Secretary of Health and Human Services. Part A: Executive Summary; 0029-6643; US Department of Human Services: Washington, DC, USA, 2009; pp. 114–120.
- Publich Health Agency of Sweden. Vad är Fysisk Aktivitet? [What Is Physical Activity?]; Publich Health Agency of Sweden: Solna, Sweden, 2016; Volume 2016.
- Swedish Research Council for Sport Science (CIF). Sport Relevance [Idrottsrelevans]; Swedish Research Council for Sport Science (CIF): Stockholm, Sweden, 2016; Volume 2016.
- Healy, G.N.; Wijndaele, K.; Dunstan, D.W.; Shaw, J.E.; Salmon, J.; Zimmet, P.Z.; Owen, N. Objectively measured sedentary time, physical activity, and metabolic risk: The Australian Diabetes, Obesity and Lifestyle Study (AusDiab). Diabetes Care 2008, 31, 369–371. [Google Scholar] [CrossRef]
- Matthews, C.E.; George, S.M.; Moore, S.C.; Bowles, H.R.; Blair, A.; Park, Y.; Troiano, R.P.; Hollenbeck, A.; Schatzkin, A. Amount of time spent in sedentary behaviors and cause-specific mortality in US adults. Am. J. Clin. Nutr. 2012, 95, 437–445. [Google Scholar] [CrossRef]
- Stamatakis, E.; Gale, J.; Bauman, A.; Ekelund, U.; Hamer, M.; Ding, D. Sitting Time, Physical Activity, and Risk of Mortality in Adults. J. Am. Coll. Cardiol. 2019, 73, 2062–2072. [Google Scholar] [CrossRef]
- Ratzlaff, C.R.; Doerfling, P.; Steininger, G.; Koehoorn, M.; Cibere, J.; Liang, M.; Wilson, D.R.; Esdaile, J.; Kopec, J. Lifetime trajectory of physical activity according to energy expenditure and joint force. Arthritis Care Res. 2010, 62, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Geneen, L.J.; Moore, R.A.; Clarke, C.; Martin, D.; Colvin, L.A.; Smith, B.H. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst. Rev. 2017, 4, CD011279. [Google Scholar] [CrossRef]
- Liberman, K.; Forti, L.N.; Beyer, I.; Bautmans, I. The effects of exercise on muscle strength, body composition, physical functioning and the inflammatory profile of older adults: A systematic review. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 30–53. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Wilson, J.M.; Lowery, R.P.; Krieger, J.W. Muscular adaptations in low-versus high-load resistance training: A meta-analysis. Eur. J. Sport Sci. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Muehlbauer, T.; Gollhofer, A.; Granacher, U. Associations Between Measures of Balance and Lower-Extremity Muscle Strength/Power in Healthy Individuals Across the Lifespan: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1671–1692. [Google Scholar] [CrossRef] [PubMed]
- Timperio, A.; Salmon, J.; Rosenberg, M.; Bull, F.C. Do logbooks influence recall of physical activity in validation studies? Med. Sci. Sports Exerc. 2004, 36, 1181–1186. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- McEwen, B.S. Stressed or stressed out: What is the difference? J. Psychiatry Neurosci. Jpn. 2005, 30, 315–318. [Google Scholar]
- Selye, H. Stress and the general adaptation syndrome. Br. Med. J. 1950, 1, 1383–1392. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A. Fundamentals of resistance training: Progression and exercise prescription. Med. Sci. Sports Exerc. 2004, 36, 674–688. [Google Scholar] [CrossRef] [PubMed]
- Baechle, T.R.; Earle, R.W. Essentials of Strength Training and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2008. [Google Scholar]
- Ronnestad, B.R.; Ellefsen, S.; Nygaard, H.; Zacharoff, E.E.; Vikmoen, O.; Hansen, J.; Hallen, J. Effects of 12 weeks of block periodization on performance and performance indices in well-trained cyclists. Scand. J. Med. Sci. Sports 2014, 24, 327–335. [Google Scholar] [CrossRef]
- Ahtiainen, J.P.; Walker, S.; Peltonen, H.; Holviala, J.; Sillanpaa, E.; Karavirta, L.; Sallinen, J.; Mikkola, J.; Valkeinen, H.; Mero, A.; et al. Heterogeneity in resistance training-induced muscle strength and mass responses in men and women of different ages. Age 2016, 38, 10. [Google Scholar] [CrossRef]
- Davidsen, P.K.; Gallagher, I.J.; Hartman, J.W.; Tarnopolsky, M.A.; Dela, F.; Helge, J.W.; Timmons, J.A.; Phillips, S.M. High responders to resistance exercise training demonstrate differential regulation of skeletal muscle microRNA expression. J. Appl. Physiol. (Bethesda Md. 1985) 2011, 110, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Timmons, J.A. Variability in training-induced skeletal muscle adaptation. J. Appl. Physiol. (Bethesda Md. 1985) 2011, 110, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Vollaard, N.B.; Constantin-Teodosiu, D.; Fredriksson, K.; Rooyackers, O.; Jansson, E.; Greenhaff, P.L.; Timmons, J.A.; Sundberg, C.J. Systematic analysis of adaptations in aerobic capacity and submaximal energy metabolism provides a unique insight into determinants of human aerobic performance. J. Appl. Physiol. (Bethesda Md. 1985) 2009, 106, 1479–1486. [Google Scholar] [CrossRef] [PubMed]
- Venezia, A.C.; Roth, S.M. Recent Research in the Genetics of Exercise Training Adaptation. Med. Sport Sci. 2016, 61, 29–40. [Google Scholar] [CrossRef]
- Porter, C.; Reidy, P.T.; Bhattarai, N.; Sidossis, L.S.; Rasmussen, B.B. Resistance Exercise Training Alters Mitochondrial Function in Human Skeletal Muscle. Med. Sci. Sports Exerc. 2015, 47, 1922–1931. [Google Scholar] [CrossRef]
- Verdijk, L.B.; Snijders, T.; Holloway, T.M.; J, V.A.N.K.; LJ, V.A.N.L. Resistance Training Increases Skeletal Muscle Capillarization in Healthy Older Men. Med. Sci. Sports Exerc. 2016, 48, 2157–2164. [Google Scholar] [CrossRef]
- Mujika, I.; Padilla, S. Detraining: Loss of training-induced physiological and performance adaptations. Part I: Short term insufficient training stimulus. Sports Med. 2000, 30, 79–87. [Google Scholar] [CrossRef]
- Gundersen, K. Muscle memory and a new cellular model for muscle atrophy and hypertrophy. J. Exp. Biol. 2016, 219, 235–242. [Google Scholar] [CrossRef]
- Leonard, W.R. Size counts: Evolutionary perspectives on physical activity and body size from early hominids to modern humans. J. Phys. Act. Health 2010, 7 (Suppl. 3), S284–S298. [Google Scholar] [CrossRef]
- Leonard, W.R.; Robertson, M.L. Nutritional requirements and human evolution: A bioenergetics model. Am. J. Hum. Biol. 1992, 4, 179–195. [Google Scholar] [CrossRef]
- Cordain, L.; Gotshall, R.W.; Eaton, S.B.; Eaton, S.B., 3rd. Physical activity, energy expenditure and fitness: An evolutionary perspective. Int. J. Sports Med. 1998, 19, 328–335. [Google Scholar] [CrossRef]
- SCB. Levnadsförhållanden: Fritid 1976-2002 [Levnadsförhållanden: Fritid 2006-2007 [Living conditions: Recreation 1976-2002]]; 103; Statistics Sweden: Stockholm, Sweden, 2004.
- Church, T.S.; Thomas, D.M.; Tudor-Locke, C.; Katzmarzyk, P.T.; Earnest, C.P.; Rodarte, R.Q.; Martin, C.K.; Blair, S.N.; Bouchard, C. Trends over 5 decades in U.S. occupation-related physical activity and their associations with obesity. PLoS ONE 2011, 6, e19657. [Google Scholar] [CrossRef]
- The Public Health Agency of Sweden. Children’s and Youth’s Pattern of Movement; The Public Health Agency of Sweden: Solna, Sweden 2019.
- Holt, N.L.; Neely, K.C.; Slater, L.G.; Camire, M.; Cote, J.; Fraser-Thomas, J.; MacDonald, D.; Strachan, L.; Tamminen, K.A. A grounded theory of positive youth development through sport based on results from a qualitative meta-study. Int. Rev. Sport Exerc. Psychol. 2017, 10, 1–49. [Google Scholar] [CrossRef]
- Andersen, L.B.; Mota, J.; Di Pietro, L. Update on the global pandemic of physical inactivity. Lancet 2016, 388, 1255–1256. [Google Scholar] [CrossRef]
- Das, P.; Horton, R. Physical activity-time to take it seriously and regularly. Lancet 2016, 388, 1254–1255. [Google Scholar] [CrossRef]
- Kujala, U.M.; Kaprio, J.; Sarna, S.; Koskenvuo, M. Relationship of leisure-time physical activity and mortality: The Finnish twin cohort. JAMA 1998, 279, 440–444. [Google Scholar] [CrossRef]
- Hills, A.P.; Street, S.J.; Byrne, N.M. Physical Activity and Health: “What is Old is New Again”. Adv. Food Nutr. Res. 2015, 75, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Arem, H.; Moore, S.C.; Patel, A.; Hartge, P.; Berrington de Gonzalez, A.; Visvanathan, K.; Campbell, P.T.; Freedman, M.; Weiderpass, E.; Adami, H.O.; et al. Leisure time physical activity and mortality: A detailed pooled analysis of the dose-response relationship. JAMA Internal Med. 2015, 175, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Scientific Report; U.S. Department of Health and Human Services: Washington, DC, USA, 2018.
- Blair, S.N. Physical inactivity and cardiovascular disease risk in women. Med. Sci. Sports Exerc. 1996, 28, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Booth, F.W.; Roberts, C.K.; Laye, M.J. Lack of exercise is a major cause of chronic diseases. Compr. Physiol. 2012, 2, 1143–1211. [Google Scholar] [CrossRef]
- Wegner, M.; Helmich, I.; Machado, S.; Nardi, A.E.; Arias-Carrion, O.; Budde, H. Effects of exercise on anxiety and depression disorders: Review of meta- analyses and neurobiological mechanisms. CNS Neurol. Disorders Drug Targets 2014, 13, 1002–1014. [Google Scholar] [CrossRef]
- Bennett, K.; Manassis, K.; Duda, S.; Bagnell, A.; Bernstein, G.A.; Garland, E.J.; Miller, L.D.; Newton, A.; Thabane, L.; Wilansky, P. Preventing Child and Adolescent Anxiety Disorders: Overview of Systematic Reviews. Depress Anxiety 2015, 32, 909–918. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Hood, S.D.; Drummond, P.D. A review of lifestyle factors that contribute to important pathways associated with major depression: Diet, sleep and exercise. J. Affect. Disord. 2013, 148, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Vancampfort, D.; Rosenbaum, S.; Firth, J.; Cosco, T.; Veronese, N.; Salum, G.A.; Schuch, F.B. An examination of the anxiolytic effects of exercise for people with anxiety and stress-related disorders: A meta-analysis. Psychiatry Res. 2017, 249, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Knochel, C.; Oertel-Knochel, V.; O’Dwyer, L.; Prvulovic, D.; Alves, G.; Kollmann, B.; Hampel, H. Cognitive and behavioural effects of physical exercise in psychiatric patients. Progr. Neurobiol. 2012, 96, 46–68. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.M.; Ashcroft, S.P.; Belew, M.Y.; Stocks, B.; Currell, K.; Baar, K.; Philp, A. Utilizing small nutrient compounds as enhancers of exercise-induced mitochondrial biogenesis. Front. Physiol. 2015, 6. [Google Scholar] [CrossRef]
- Wilson, M.G.; Ellison, G.M.; Cable, N.T. Basic science behind the cardiovascular benefits of exercise. Heart 2015, 101, 758–765. [Google Scholar] [CrossRef]
- Hellsten, Y.; Nyberg, M. Cardiovascular Adaptations to Exercise Training. Compr. Physiol. 2015, 6, 1–32. [Google Scholar] [CrossRef]
- Wilson, J.M.; Loenneke, J.P.; Jo, E.; Wilson, G.J.; Zourdos, M.C.; Kim, J.S. The effects of endurance, strength, and power training on muscle fiber type shifting. J. Strength Cond. Res. 2012, 26, 1724–1729. [Google Scholar] [CrossRef]
- Cadore, E.L.; Pinto, R.S.; Bottaro, M.; Izquierdo, M. Strength and endurance training prescription in healthy and frail elderly. Aging Dis. 2014, 5, 183–195. [Google Scholar] [CrossRef]
- Tofthagen, C.; Visovsky, C.; Berry, D.L. Strength and balance training for adults with peripheral neuropathy and high risk of fall: Current evidence and implications for future research. Oncol. Nurs. Forum. 2012, 39, E416–E424. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.I.; An, D.H. Effects of a Fall Prevention Exercise Program on Muscle Strength and Balance of the Old-old Elderly. J. Phys. Ther. Sci. 2014, 26, 1771–1774. [Google Scholar] [CrossRef] [PubMed]
- Kyu, H.H.; Bachman, V.F.; Alexander, L.T.; Mumford, J.E.; Afshin, A.; Estep, K.; Veerman, J.L.; Delwiche, K.; Iannarone, M.L.; Moyer, M.L.; et al. Physical activity and risk of breast cancer, colon cancer, diabetes, ischemic heart disease, and ischemic stroke events: Systematic review and dose-response meta-analysis for the Global Burden of Disease Study 2013. BMJ 2016, 354, i3857. [Google Scholar] [CrossRef] [PubMed]
- Pearson, M.J.; Smart, N.A. Effect of exercise training on endothelial function in heart failure patients: A systematic review meta-analysis. Int. J. Cardiol. 2017, 231, 234–243. [Google Scholar] [CrossRef]
- Nielsen, J.; Gejl, K.D.; Hey-Mogensen, M.; Holmberg, H.C.; Suetta, C.; Krustrup, P.; Elemans, C.P.H.; Ortenblad, N. Plasticity in mitochondrial cristae density allows metabolic capacity modulation in human skeletal muscle. J. Physiol. 2017, 595, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Richter, E.A.; Hargreaves, M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol. Rev. 2013, 93, 993–1017. [Google Scholar] [CrossRef] [PubMed]
- Marson, E.C.; Delevatti, R.S.; Prado, A.K.; Netto, N.; Kruel, L.F. Effects of aerobic, resistance, and combined exercise training on insulin resistance markers in overweight or obese children and adolescents: A systematic review and meta-analysis. Prev. Med. 2016, 93, 211–218. [Google Scholar] [CrossRef]
- Way, K.L.; Hackett, D.A.; Baker, M.K.; Johnson, N.A. The Effect of Regular Exercise on Insulin Sensitivity in Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Diabetes Metab. J. 2016, 40, 253–271. [Google Scholar] [CrossRef] [PubMed]
- Conn, V.S.; Koopman, R.J.; Ruppar, T.M.; Phillips, L.J.; Mehr, D.R.; Hafdahl, A.R. Insulin Sensitivity Following Exercise Interventions: Systematic Review and Meta-Analysis of Outcomes Among Healthy Adults. J. Prim Care Community Health 2014, 5, 211–222. [Google Scholar] [CrossRef]
- Schuch, F.B.; Deslandes, A.C.; Stubbs, B.; Gosmann, N.P.; Silva, C.T.; Fleck, M.P. Neurobiological effects of exercise on major depressive disorder: A systematic review. Neurosci. Biobehav. Rev. 2016, 61, 1–11. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Sui, X.; Rosenbaum, S.; Firth, J.; Richards, J.; Ward, P.B.; Stubbs, B. Are lower levels of cardiorespiratory fitness associated with incident depression? A systematic review of prospective cohort studies. Prev. Med. 2016, 93, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Zahl, T.; Steinsbekk, S.; Wichstrom, L. Physical Activity, Sedentary Behavior, and Symptoms of Major Depression in Middle Childhood. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Colaianni, G.; Mongelli, T.; Colucci, S.; Cinti, S.; Grano, M. Crosstalk Between Muscle and Bone Via the Muscle-Myokine Irisin. Curr. Osteoporos. Rep. 2016, 14, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Giangregorio, L.M.; McGill, S.; Wark, J.D.; Laprade, J.; Heinonen, A.; Ashe, M.C.; MacIntyre, N.J.; Cheung, A.M.; Shipp, K.; Keller, H.; et al. Too Fit to Fracture: Outcomes of a Delphi consensus process on physical activity and exercise recommendations for adults with osteoporosis with or without vertebral fractures. Osteoporos. Int. 2015, 26, 891–910. [Google Scholar] [CrossRef] [PubMed]
- Papa, E.V.; Dong, X.; Hassan, M. Resistance training for activity limitations in older adults with skeletal muscle function deficits: a systematic review. Clin. Interv. Aging. 2017, 12, 955–961. [Google Scholar] [CrossRef] [PubMed]
- Casonatto, J.; Goessler, K.F.; Cornelissen, V.A.; Cardoso, J.R.; Polito, M.D. The blood pressure-lowering effect of a single bout of resistance exercise: A systematic review and meta-analysis of randomised controlled trials. Eur. J. Prev. Cardiol. 2016, 23, 1700–1714. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, H.V.; Johnson, B.T.; Huedo-Medina, T.B.; Livingston, J.; Forsyth, K.C.; Kraemer, W.J.; Farinatti, P.T.V.; Pescatello, L.S. Dynamic Resistance Training as Stand-Alone Antihypertensive Lifestyle Therapy: A Meta-Analysis. J. Am. Heart Assoc. 2016, 5, e003231. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef]
- Chen, Y.C.; Tsai, J.C.; Liou, Y.M.; Chan, P. Effectiveness of endurance exercise training in patients with coronary artery disease: A meta-analysis of randomised controlled trials. Eur. J. Cardiovasc. Nurs. 2017, 16, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Bachi, A.L.; Rocha, G.A.; Sprandel, M.C.; Ramos, L.R.; Gravina, C.F.; Pithon-Curi, T.C.; Vaisberg, M.; Maranhao, R.C. Exercise Training Improves Plasma Lipid and Inflammatory Profiles and Increases Cholesterol Transfer to High-Density Lipoprotein in Elderly Women. J. Am. Geriatr. Soc. 2015, 63, 1247–1249. [Google Scholar] [CrossRef]
- Climstein, M.; Walsh, J.; Debeliso, M.; Heazlewood, T.; Sevene, T.; Adams, K. Cardiovascular risk profiles of world masters games participants. J. Sports Med. Phys. Fitness 2018, 58, 489–496. [Google Scholar]
- Anstey, K.J.; Ashby-Mitchell, K.; Peters, R. Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. J. Alzheimers Dis. 2017, 56, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Stoedefalke, K. Effects of exercise training on blood lipids and lipoproteins in children and adolescents. J. Sports Sci. Med. 2007, 6, 313–318. [Google Scholar] [PubMed]
- Hvid, L.G.; Strotmeyer, E.S.; Skjodt, M.; Magnussen, L.V.; Andersen, M.; Caserotti, P. Voluntary muscle activation improves with power training and is associated with changes in gait speed in mobility-limited older adults - A randomized controlled trial. Exp. Gerontol. 2016, 80, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.M.; Davis, N.; Sands, S.A.; Whittington, R.A.; Sun, L.S. Physical Activity and Cognitive Development: A Meta-Analysis. J. Neurosurg. Anesthesiol. 2016, 28, 373–380. [Google Scholar] [CrossRef] [PubMed]
- Ludyga, S.; Gerber, M.; Brand, S.; Holsboer-Trachsler, E.; Puhse, U. Acute effects of moderate aerobic exercise on specific aspects of executive function in different age and fitness groups: A meta-analysis. Psychophysiology 2016, 53, 1611–1626. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Liu, C.S.; Sherman, C.; Chan, S.; Lanctot, K.L. The Effect of Exercise Training on Resting Concentrations of Peripheral Brain-Derived Neurotrophic Factor (BDNF): A Meta-Analysis. PLoS ONE 2016, 11, e0163037. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S. Exercise and Sleep: A Systematic Review of Previous Meta-analyses And A Meta-analysis: 284 Board #121 June 1, 9: 30 AM - 11: 00 AM. Med. Sci. Sports Exerc. 2016, 48, 68–69. [Google Scholar] [CrossRef]
- Kandola, A.; Hendrikse, J.; Lucassen, P.J.; Yucel, M. Aerobic Exercise as a Tool to Improve Hippocampal Plasticity and Function in Humans: Practical Implications for Mental Health Treatment. Front. Hum. Neurosci. 2016, 10, 373. [Google Scholar] [CrossRef]
- Smith, G.E. Healthy Cognitive Aging and Dementia Prevention. Am. Psychol. 2016, 71, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, J. Neuroplasticity and Clinical Practice: Building Brain Power for Health. Front. Psychol. 2016, 7, 1118. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.M.; Nolan, Y.M. Neuroinflammation negatively affects adult hippocampal neurogenesis and cognition: Can exercise compensate? Neurosci. Biobehav. Rev. 2016, 61, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Boraxbekk, C.J.; Salami, A.; Wahlin, A.; Nyberg, L. Physical activity over a decade modifies age-related decline in perfusion, gray matter volume, and functional connectivity of the posterior default-mode network-A multimodal approach. Neuroimage 2016, 131, 133–141. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Brodin, P.; Davis, M.M. Human immune system variation. Nat. Rev. Immunol. 2017, 17, 21–29. [Google Scholar] [CrossRef]
- Gjevestad, G.O.; Holven, K.B.; Ulven, S.M. Effects of Exercise on Gene Expression of Inflammatory Markers in Human Peripheral Blood Cells: A Systematic Review. Curr. Cardiovasc. Risk Rep. 2015, 9, 34. [Google Scholar] [CrossRef]
- Runhaar, J.; Bierma-Zeinstra, S.M. Should exercise therapy for chronic musculoskeletal conditions focus on the anti-inflammatory effects of exercise? Br. J. Sports Med. 2016. [Google Scholar] [CrossRef]
- Codella, R.; Luzi, L.; Inverardi, L.; Ricordi, C. The anti-inflammatory effects of exercise in the syndromic thread of diabetes and autoimmunity. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 3709–3722. [Google Scholar] [PubMed]
- Mika, A.; Fleshner, M. Early-life exercise may promote lasting brain and metabolic health through gut bacterial metabolites. Immunol. Cell Biol. 2016, 94, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.J.; Kenfield, S.A.; Jimenez, A. Exercise-induced biochemical changes and their potential influence on cancer: A scientific review. Br. J. Sports Med. 2016. [Google Scholar] [CrossRef]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- McKercher, C.; Sanderson, K.; Schmidt, M.D.; Otahal, P.; Patton, G.C.; Dwyer, T.; Venn, A.J. Physical activity patterns and risk of depression in young adulthood: A 20-year cohort study since childhood. Soc. Psych. Psych. Epid. 2014, 49, 1823–1834. [Google Scholar] [CrossRef] [PubMed]
- von Martels, J.Z.; Sadaghian Sadabad, M.; Bourgonje, A.R.; Blokzijl, T.; Dijkstra, G.; Faber, K.N.; Harmsen, H.J. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 2017, 44, 3–12. [Google Scholar] [CrossRef]
- Bogdanis, G.C. Effects of physical activity and inactivity on muscle fatigue. Front. Physiol. 2012, 3, 142. [Google Scholar] [CrossRef]
- Eriksson, A. Strength Training and Anabolic Steroids: A Comparative Study of the Vastus Lateralis, a Thigh Muscle and the Trapezius, a Shoulder Muscle, of Strength-Trained Athletes. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2006. [Google Scholar]
- Eriksson, A.; Kadi, F.; Malm, C.; Thornell, L.E. Skeletal muscle morphology in power-lifters with and without anabolic steroids. Histochem. Cell Biol. 2005, 124, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Stevens, G.A.; Alkema, L.; Black, R.E.; Boerma, J.T.; Collins, G.S.; Ezzati, M.; Grove, J.T.; Hogan, D.R.; Hogan, M.C.; Horton, R.; et al. Guidelines for Accurate and Transparent Health Estimates Reporting: The GATHER statement. Lancet 2016, 388, e19–e23. [Google Scholar] [CrossRef]
- Borde, R.; Hortobagyi, T.; Granacher, U. Dose-Response Relationships of Resistance Training in Healthy Old Adults: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 1693–1720. [Google Scholar] [CrossRef] [PubMed]
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Lyons, M.; Herring, M.P. The Effects of Resistance Exercise Training on Anxiety: A Meta-Analysis and Meta-Regression Analysis of Randomized Controlled Trials. Sports Med. 2017, 47, 2521–2532. [Google Scholar] [CrossRef]
- Keilani, M.; Hasenoehrl, T.; Baumann, L.; Ristl, R.; Schwarz, M.; Marhold, M.; Sedghi Komandj, T.; Crevenna, R. Effects of resistance exercise in prostate cancer patients: A meta-analysis. Support. Care Cancer 2017, 25, 2953–2968. [Google Scholar] [CrossRef]
- Yamamoto, S.; Hotta, K.; Ota, E.; Mori, R.; Matsunaga, A. Effects of resistance training on muscle strength, exercise capacity, and mobility in middle-aged and elderly patients with coronary artery disease: A meta-analysis. J. Cardiol. 2016, 68, 125–134. [Google Scholar] [CrossRef]
- Mammen, G.; Faulkner, G. Physical activity and the prevention of depression: A systematic review of prospective studies. Am. J. Prev. Med. 2013, 45, 649–657. [Google Scholar] [CrossRef]
- Csapo, R.; Alegre, L.M. Effects of resistance training with moderate vs heavy loads on muscle mass and strength in the elderly: A meta-analysis. Scand. J. Med. Sci. Sports 2016, 26, 995–1006. [Google Scholar] [CrossRef]
- Churchward-Venne, T.A.; Tieland, M.; Verdijk, L.B.; Leenders, M.; Dirks, M.L.; de Groot, L.C.; van Loon, L.J. There Are No Nonresponders to Resistance-Type Exercise Training in Older Men and Women. J. Am. Med. Dir. Assoc. 2015, 16, 400–411. [Google Scholar] [CrossRef]
- Garcia-Hermoso, A.; Ramirez-Velez, R.; Ramirez-Campillo, R.; Peterson, M.D.; Martinez-Vizcaino, V. Concurrent aerobic plus resistance exercise versus aerobic exercise alone to improve health outcomes in paediatric obesity: A systematic review and meta-analysis. Br. J. Sports Med. 2018, 52, 161–166. [Google Scholar] [CrossRef]
- Groot, C.; Hooghiemstra, A.M.; Raijmakers, P.G.; van Berckel, B.N.; Scheltens, P.; Scherder, E.J.; van der Flier, W.M.; Ossenkoppele, R. The effect of physical activity on cognitive function in patients with dementia: A meta-analysis of randomized control trials. Ageing Res. Rev. 2016, 25, 13–23. [Google Scholar] [CrossRef]
- Chung, C.L.; Thilarajah, S.; Tan, D. Effectiveness of resistance training on muscle strength and physical function in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2016, 30, 11–23. [Google Scholar] [CrossRef]
- Kang, H.; Lu, J.; Xu, G. The effects of whole body vibration on muscle strength and functional mobility in persons with multiple sclerosis: A systematic review and meta-analysis. Mult. Scler. Relat. Disord. 2016, 7, 1–7. [Google Scholar] [CrossRef]
- Portugal, E.M.; Vasconcelos, P.G.; Souza, R.; Lattari, E.; Monteiro-Junior, R.S.; Machado, S.; Deslandes, A.C. Aging process, cognitive decline and Alzheimer’s disease: Can strength training modulate these responses? CNS & Neurol. Disorders Drug Targets 2015, 14, 1209–1213. [Google Scholar]
- Bacchi, E.; Negri, C.; Zanolin, M.E.; Milanese, C.; Faccioli, N.; Trombetta, M.; Zoppini, G.; Cevese, A.; Bonadonna, R.C.; Schena, F.; et al. Metabolic effects of aerobic training and resistance training in type 2 diabetic subjects: A randomized controlled trial (the RAED2 study). Diabetes Care 2012, 35, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Steindorf, K.; Schmidt, M.E.; Klassen, O.; Ulrich, C.M.; Oelmann, J.; Habermann, N.; Beckhove, P.; Owen, R.; Debus, J.; Wiskemann, J.; et al. Randomized, controlled trial of resistance training in breast cancer patients receiving adjuvant radiotherapy: Results on cancer-related fatigue and quality of life. Ann. Oncol. 2014, 25, 2237–2243. [Google Scholar] [CrossRef] [PubMed]
- Ciolac, E.G.; Rodrigues-da-Silva, J.M. Resistance Training as a Tool for Preventing and Treating Musculoskeletal Disorders. Sports Med. 2016, 46, 1239–1248. [Google Scholar] [CrossRef]
- Castrogiovanni, P.; Trovato, F.M.; Szychlinska, M.A.; Nsir, H.; Imbesi, R.; Musumeci, G. The importance of physical activity in osteoporosis. From the molecular pathways to the clinical evidence. Histol. Histopathol. 2016, 31, 1183–1194. [Google Scholar] [CrossRef]
- Johansson, J.; Nordstrom, A.; Nordstrom, P. Greater Fall Risk in Elderly Women Than in Men Is Associated With Increased Gait Variability During Multitasking. J. Am. Med. Dir. Assoc. 2016, 17, 535–540. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Lamb, S.E.; Gates, S.; Cumming, R.G.; Rowe, B.H. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2009, Cd007146. [Google Scholar] [CrossRef]
- Svantesson, U.; Jones, J.; Wolbert, K.; Alricsson, M. Impact of Physical Activity on the Self-Perceived Quality of Life in Non-Frail Older Adults. J. Clin. Med. Res. 2015, 7, 585–593. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Williams, J.; Atherton, P.; Larvin, M.; Lund, J.; Narici, M. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength: A quantitative review. Front. Physiol. 2012, 3, 260. [Google Scholar] [CrossRef]
- Csapo, R.; Gormasz, C.; Baron, R. Functional performance in community-dwelling and institutionalized elderly women. Wiener Klinische Wochenschrift 2009, 121, 383–390. [Google Scholar] [CrossRef]
- Mayer, F.; Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Muller, S.; Scharhag, J. The intensity and effects of strength training in the elderly. Deutsches Arzteblatt Int. 2011, 108, 359–364. [Google Scholar] [CrossRef]
- Stewart, V.H.; Saunders, D.H.; Greig, C.A. Responsiveness of muscle size and strength to physical training in very elderly people: A systematic review. Scand. J. Med. Sci. Sports 2014, 24, e1–10. [Google Scholar] [CrossRef]
- Physical Activities Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008; Department of Health and Human Services: Washington, DC, USA, 2008.
- Olesen, J.; Gustavsson, A.; Svensson, M.; Wittchen, H.U.; Jonsson, B. The economic cost of brain disorders in Europe. Eur. J. Neurol. 2012, 19, 155–162. [Google Scholar] [CrossRef]
- Josefsson, T.; Lindwall, M.; Archer, T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scand. J. Med. Sci. Sports 2014, 24, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, S.; Tiedemann, A.; Sherrington, C.; Curtis, J.; Ward, P.B. Physical activity interventions for people with mental illness: A systematic review and meta-analysis. J. Clin. Psychiatry 2014, 75, 964–974. [Google Scholar] [CrossRef] [PubMed]
- Aberg, M.A.; Waern, M.; Nyberg, J.; Pedersen, N.L.; Bergh, Y.; Aberg, N.D.; Nilsson, M.; Kuhn, H.G.; Toren, K. Cardiovascular fitness in males at age 18 and risk of serious depression in adulthood: Swedish prospective population-based study. Br. J. Psychiatry 2012, 201, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.D.; Blanck, H.M.; Serdula, M.K.; Brown, D.R. Obesity, physical activity, and depressive symptoms in a cohort of adults aged 51 to 61. J. Aging Health 2010, 22, 384–398. [Google Scholar] [CrossRef] [PubMed]
- Adamson, B.C.; Ensari, I.; Motl, R.W. Effect of exercise on depressive symptoms in adults with neurologic disorders: A systematic review and meta-analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1329–1338. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, L.Z.; Femenia, T.; Orhan, F.; Porsmyr-Palmertz, M.; Goiny, M.; Martinez-Redondo, V.; Correia, J.C.; Izadi, M.; Bhat, M.; Schuppe-Koistinen, I.; et al. Skeletal muscle PGC-1alpha1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell 2014, 159, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Kassow, M.; Deusser, M.; Thiel, C.; Otterbein, S.; Montag, C.; Reuter, M.; Banzer, W.; Kaiser, J. Physical Exercise during Encoding Improves Vocabulary Learning in Young Female Adults: A Neuroendocrinological Study. PLoS ONE 2013, 8, e64172. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A.; et al. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef]
- Oppezzo, M.; Schwartz, D.L. Give your ideas some legs: The positive effect of walking on creative thinking. J. Exp. Psychol. 2014, 40, 1142–1152. [Google Scholar] [CrossRef]
- Kramer, A.F.; Marquez, D.X.; McAuley, E.; Kim, J.S.; Erickson, K.I.; Hu, L.; Scalf, P.E.; Prakash, R.; Colcombe, S.J.; Elavsky, S. Aerobic Exercise Training Increases Brain Volume in Aging Humans. J. Gerontol. 2006, 61, 1166–1170. [Google Scholar] [CrossRef]
- Spielman, L.J.; Little, J.P.; Klegeris, A. Physical activity and exercise attenuate neuroinflammation in neurological diseases. Brain Res. Bull. 2016, 125, 19–29. [Google Scholar] [CrossRef]
- Soundy, A.; Freeman, P.; Stubbs, B.; Probst, M.; Roskell, C.; Vancampfort, D. The Psychosocial Consequences of Sports Participation for Individuals with Severe Mental Illness: A Metasynthesis Review. Adv. Psychiatry 2015, 2015, 8. [Google Scholar] [CrossRef]
- Hassmen, P.; Koivula, N.; Uutela, A. Physical exercise and psychological well-being: A population study in Finland. Prev. Med. 2000, 30, 17–25. [Google Scholar] [CrossRef]
- Coakley, J. Youth Sports: What Counts as “Positive Development?”. J. Sport Soc. Issues 2011, 35, 306–324. [Google Scholar] [CrossRef]
- Seligman, M.E.P. Positive Health. Appl. Psychol. 2008, 57, 3–18. [Google Scholar] [CrossRef]
- Rongen, F.; Cobley, S.; McKenna, J.; Till, K. Talent identification and development. In Health and Elite Sport: Is High Performance Sport a Healthy Pursuit? Baker, J., Safai, P., Fraser-Thomas, J., Eds.; Routledge Research in Sport, Culture and Society; Taylor & Francis Group: London, UK, 2015. [Google Scholar]
- Baker, J.; Fraser-Thomas, J.; Dionigi, R.A.; Horton, S. Sport participation and positive development in older persons. Eur. Rev. Aging Phys. Act. 2010, 7, 3–12. [Google Scholar] [CrossRef]
- Walsh, D.W. Sport as a Resource Caravan: Examining the Role and Efficacy of Sport as a Resource Provider for Adults in Transition. Ph.D. Thesis, University of Texas at Austin, Austin, TX, USA, 2014. [Google Scholar]
- Rehn, A.; Möller, A. Den organiserade idrottens betydelse för spontanidrott; Gymnastik- och Idrottshögskolan: Stockholm, Sweden, 2011. [Google Scholar]
- Ratzlaff, C.R. Good news, bad news: Sports matter but occupational and household activity really matter - sport and recreation unlikely to be a panacea for public health. Br. J. Sports Med. 2012, 46, 699–701. [Google Scholar] [CrossRef]
- Lewis, C.J.; Reeves, M.J.; Roberts, S.J. Improving the physical and mental well-being of typically hard-to-reach men: An investigation of the impact of the Active Rovers project. Sport Soc. 2017, 20, 258–268. [Google Scholar] [CrossRef]
- Dunleavy, N. Proposed cuts to sport and recreation could hinder health of northern communities. CMAJ. 2008, 178, 1129. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.; Henderson, K.A.; Han, A.; Park, S.H. Serious engagement in sport and health benefits among Korean immigrants in the USA. Int. J. Qual. Stud. Health Well-Being 2016, 11, 31340. [Google Scholar] [CrossRef]
- Perrier, M.J.; Shirazipour, C.H.; Latimer-Cheung, A.E. Sport participation among individuals with acquired physical disabilities: Group differences on demographic, disability, and Health Action Process Approach constructs. Disabil. Health J. 2015, 8, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Engström, L.-M. Who is physically active? Cultural capital and sports participation from adolescence to middle age—A 38-year follow-up study. Phys. Educ. Sport Pedagog. 2008, 13, 319–343. [Google Scholar] [CrossRef]
- Brenner, J.S.; Council On Sports Medicine and Fitness. Sports Specialization and Intensive Training in Young Athletes. Pediatrics 2016, 138. [Google Scholar] [CrossRef]
- LaPrade, R.F.; Agel, J.; Baker, J.; Brenner, J.S.; Cordasco, F.A.; Cote, J.; Engebretsen, L.; Feeley, B.T.; Gould, D.; Hainline, B.; et al. AOSSM Early Sport Specialization Consensus Statement. Orthop. J. Sports Med. 2016, 4, 2325967116644241. [Google Scholar] [CrossRef]
- Tan, V.P.; Macdonald, H.M.; Kim, S.; Nettlefold, L.; Gabel, L.; Ashe, M.C.; McKay, H.A. Influence of physical activity on bone strength in children and adolescents: A systematic review and narrative synthesis. J. Bone Miner. Res. 2014, 29, 2161–2181. [Google Scholar] [CrossRef] [PubMed]
- Timmons, B.W.; Leblanc, A.G.; Carson, V.; Connor Gorber, S.; Dillman, C.; Janssen, I.; Kho, M.E.; Spence, J.C.; Stearns, J.A.; Tremblay, M.S. Systematic review of physical activity and health in the early years (aged 0-4 years). Appl. Physiol. Nutr. Metab. 2012, 37, 773–792. [Google Scholar] [CrossRef] [PubMed]
- Janssen, I.; Roberts, K.C.; Thompson, W. Is adherence to the Canadian 24-Hour Movement Behaviour Guidelines for Children and Youth associated with improved indicators of physical, mental, and social health? Appl. Physiol. Nutr. Metab. 2017, 42, 725–731. [Google Scholar] [CrossRef]
- Fedewa, M.V.; Gist, N.H.; Evans, E.M.; Dishman, R.K. Exercise and insulin resistance in youth: A meta-analysis. Pediatrics 2014, 133, e163–e174. [Google Scholar] [CrossRef]
- Jayanthi, N.; Pinkham, C.; Dugas, L.; Patrick, B.; Labella, C. Sports specialization in young athletes: Evidence-based recommendations. Sports Health 2013, 5, 251–257. [Google Scholar] [CrossRef]
- Wattie, N.; Schorer, J.; Baker, J. The relative age effect in sport: A developmental systems model. Sports Med. 2015, 45, 83–94. [Google Scholar] [CrossRef]
- Delorme, N.; Raspaud, M. The relative age effect in young French basketball players: A study on the whole population. Scand. J. Med. Sci. Sports 2009, 19, 235–242. [Google Scholar] [CrossRef]
- Fumarco, L.; Gibbs, B.G.; Jarvis, J.A.; Rossi, G. The relative age effect reversal among the National Hockey League elite. PLoS ONE 2017, 12, e0182827. [Google Scholar] [CrossRef]
- Geithner, C.A.; Molenaar, C.; Henriksson, T.; Fjellman-Wiklund, A. Relative Age Effects in Women’s Ice Hockey. Women Sport Phys. Act. J. 2018, 26, 124–133. [Google Scholar] [CrossRef]
- Gerdin, G.; Hedberg, M.; Hageskog, C.A. Relative Age Effect in Swedish Male and Female Tennis Players Born in 1998(-)2001. Sports 2018, 6, 38. [Google Scholar] [CrossRef]
- Jones, C.; Visek, A.J.; Barron, M.J.; Hyman, M.; Chandran, A. Association between relative age effect and organisational practices of American youth football. J. Sports Sci. 2018, 1–8. [Google Scholar] [CrossRef]
- Edwards, L.C.; Bryant, A.S.; Keegan, R.J.; Morgan, K.; Cooper, S.M.; Jones, A.M. ‘Measuring’ Physical Literacy and Related Constructs: A Systematic Review of Empirical Findings. Sports Med. 2017. [Google Scholar] [CrossRef]
- Bahr, R. Demise of the fittest: Are we destroying our biggest talents? Br. J. Sports Med. 2014, 48, 1265–1267. [Google Scholar] [CrossRef]
- Kenttä, G.; Svensson, M. Idrottarens återhämtningsbok, fysiologiska, psykologiska och näringsmässiga fakta för snabb och effektiv återhämtning; SISU Idrottsböcker: Stockholm, Sweden, 2008. [Google Scholar]
- Blair, S.N.; Kohl, H.W., 3rd; Barlow, C.E.; Paffenbarger, R.S., Jr.; Gibbons, L.W.; Macera, C.A. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA 1995, 273, 1093–1098. [Google Scholar] [CrossRef]
- Paffenbarger, R.S., Jr.; Kampert, J.B.; Lee, I.M.; Hyde, R.T.; Leung, R.W.; Wing, A.L. Changes in physical activity and other lifeway patterns influencing longevity. Med. Sci. Sports Exerc. 1994, 26, 857–865. [Google Scholar] [CrossRef]
- Rivera-Torres, S.; Fahey, T.D.; Rivera, M.A. Adherence to Exercise Programs in Older Adults: Informative Report. Gerontol. Geriatr. Med. 2019, 5, 2333721418823604. [Google Scholar] [CrossRef]
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef]
- Hamer, M.; Lavoie, K.L.; Bacon, S.L. Taking up physical activity in later life and healthy ageing: The English longitudinal study of ageing. Br. J. Sports Med. 2014, 48, 239–243. [Google Scholar] [CrossRef]
- Cobley, S.; Baker, J.; Wattie, N.; McKenna, J. Annual age-grouping and athlete development: A meta-analytical review of relative age effects in sport. Sports Med. 2009, 39, 235–256. [Google Scholar] [CrossRef]
- Unhjem, R.; Nygard, M.; van den Hoven, L.T.; Sidhu, S.K.; Hoff, J.; Wang, E. Lifelong strength training mitigates the age-related decline in efferent drive. J. Appl. Physiol. (Bethesda Md. 1985) 2016, 121, 415–423. [Google Scholar] [CrossRef]
- Power, G.A.; Allen, M.D.; Gilmore, K.J.; Stashuk, D.W.; Doherty, T.J.; Hepple, R.T.; Taivassalo, T.; Rice, C.L. Motor unit number and transmission stability in octogenarian world class athletes: Can age-related deficits be outrun? J. Appl. Physiol. (Bethesda Md. 1985) 2016, 121, 1013–1020. [Google Scholar] [CrossRef]
- Matelot, D.; Schnell, F.; Kervio, G.; Ridard, C.; Thillaye du Boullay, N.; Wilson, M.; Carre, F. Cardiovascular Benefits of Endurance Training in Seniors: 40 is not too Late to Start. Int. J. Sports Med. 2016, 37, 625–632. [Google Scholar] [CrossRef]
- Lepers, R.; Stapley, P.J. Master Athletes Are Extending the Limits of Human Endurance. Front. Physiol. 2016, 7, 613. [Google Scholar] [CrossRef]
- Parkkari, J.; Natri, A.; Kannus, P.; Manttari, A.; Laukkanen, R.; Haapasalo, H.; Nenonen, A.; Pasanen, M.; Oja, P.; Vuori, I. A controlled trial of the health benefits of regular walking on a golf course. Am. J. Med. 2000, 109, 102–108. [Google Scholar] [CrossRef]
- Broman, G.; Johnsson, L.; Kaijser, L. Golf: A high intensity interval activity for elderly men. Aging Clin. Exp. Res. 2004, 16, 375–381. [Google Scholar] [CrossRef]
- Kiss, O.; Sydo, N.; Vargha, P.; Edes, E.; Merkely, G.; Sydo, T.; Merkely, B. Prevalence of physiological and pathological electrocardiographic findings in Hungarian athletes. Acta Physiol. Hung. 2015, 102, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Shapero, K.; Deluca, J.; Contursi, M.; Wasfy, M.; Weiner, R.B.; Lewis, G.D.; Hutter, A.; Baggish, A.L. Cardiovascular Risk and Disease Among Masters Endurance Athletes: Insights from the Boston MASTER (Masters Athletes Survey to Evaluate Risk) Initiative. Sports Med. Open 2016, 2, 29. [Google Scholar] [CrossRef] [PubMed]
- Yankelson, L.; Sadeh, B.; Gershovitz, L.; Werthein, J.; Heller, K.; Halpern, P.; Halkin, A.; Adler, A.; Steinvil, A.; Viskin, S. Life-threatening events during endurance sports: Is heat stroke more prevalent than arrhythmic death? J. Am. Coll. Cardiol. 2014, 64, 463–469. [Google Scholar] [CrossRef]
- Strimel, W.J.; O’Riordan, M.J. Sudden cardiac arrest in long distance races: Considering the full context. J. Am. Coll. Cardiol. 2015, 65, 407–408. [Google Scholar] [CrossRef][Green Version]
- Piasecki, M.; Ireland, A.; Coulson, J.; Stashuk, D.W.; Hamilton-Wright, A.; Swiecicka, A.; Rutter, M.K.; McPhee, J.S.; Jones, D.A. Motor unit number estimates and neuromuscular transmission in the tibialis anterior of master athletes: Evidence that athletic older people are not spared from age-related motor unit remodeling. Physiol. Rep. 2016, 4. [Google Scholar] [CrossRef]
- Moreira, N.B.; Mazzardo, O.; Vagetti, G.C.; De Oliveira, V.; De Campos, W. Quality of life perception of basketball master athletes: Association with physical activity level and sports injuries. J. Sports Sci. 2016, 34, 988–996. [Google Scholar] [CrossRef]
- Haigh, E.A.P.; Bogucki, O.E.; Sigmon, S.T.; Blazer, D.G. Depression Among Older Adults: A 20-Year Update on Five Common Myths and Misconceptions. Am. J. Geriatr. Psychiatry 2018, 26, 107–122. [Google Scholar] [CrossRef]
- Alfini, A.J.; Weiss, L.R.; Leitner, B.P.; Smith, T.J.; Hagberg, J.M.; Smith, J.C. Hippocampal and Cerebral Blood Flow after Exercise Cessation in Master Athletes. Front. Aging Neurosci. 2016, 8, 184. [Google Scholar] [CrossRef]
- Lazarus, N.R.; Harridge, S.D.R. Declining performance of master athletes: Silhouettes of the trajectory of healthy human ageing? J. Physiol. 2017, 595, 2941–2948. [Google Scholar] [CrossRef]
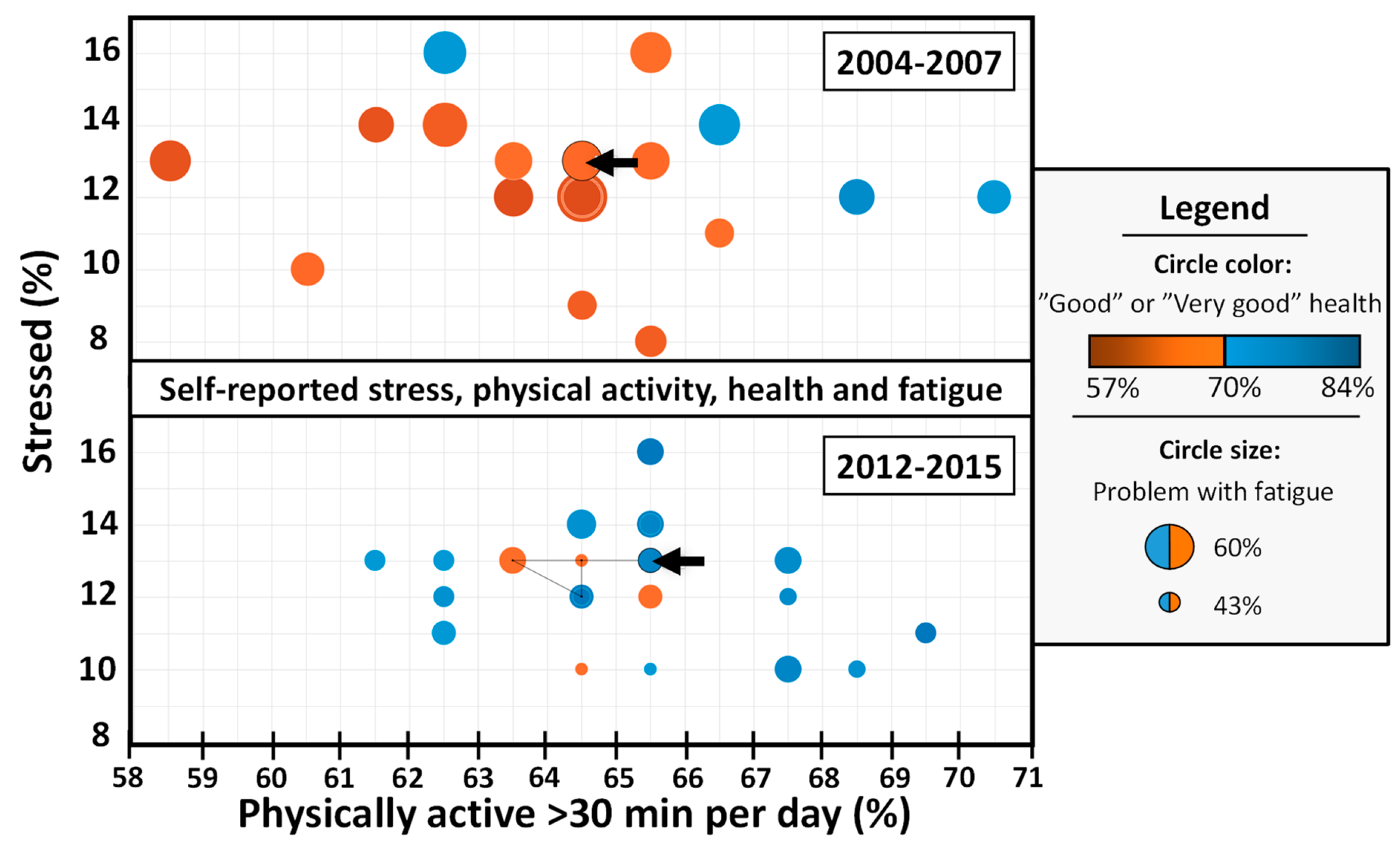
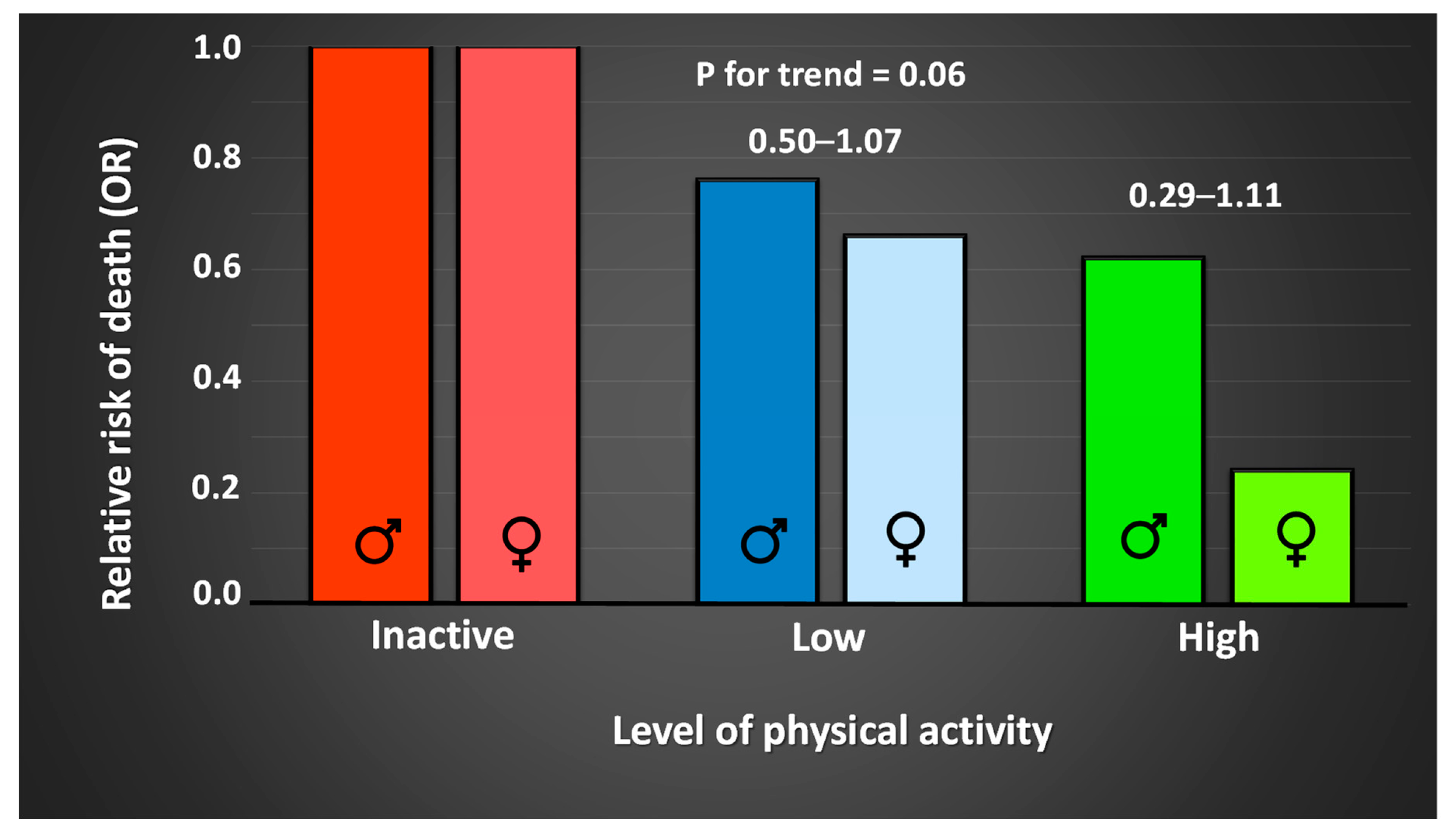
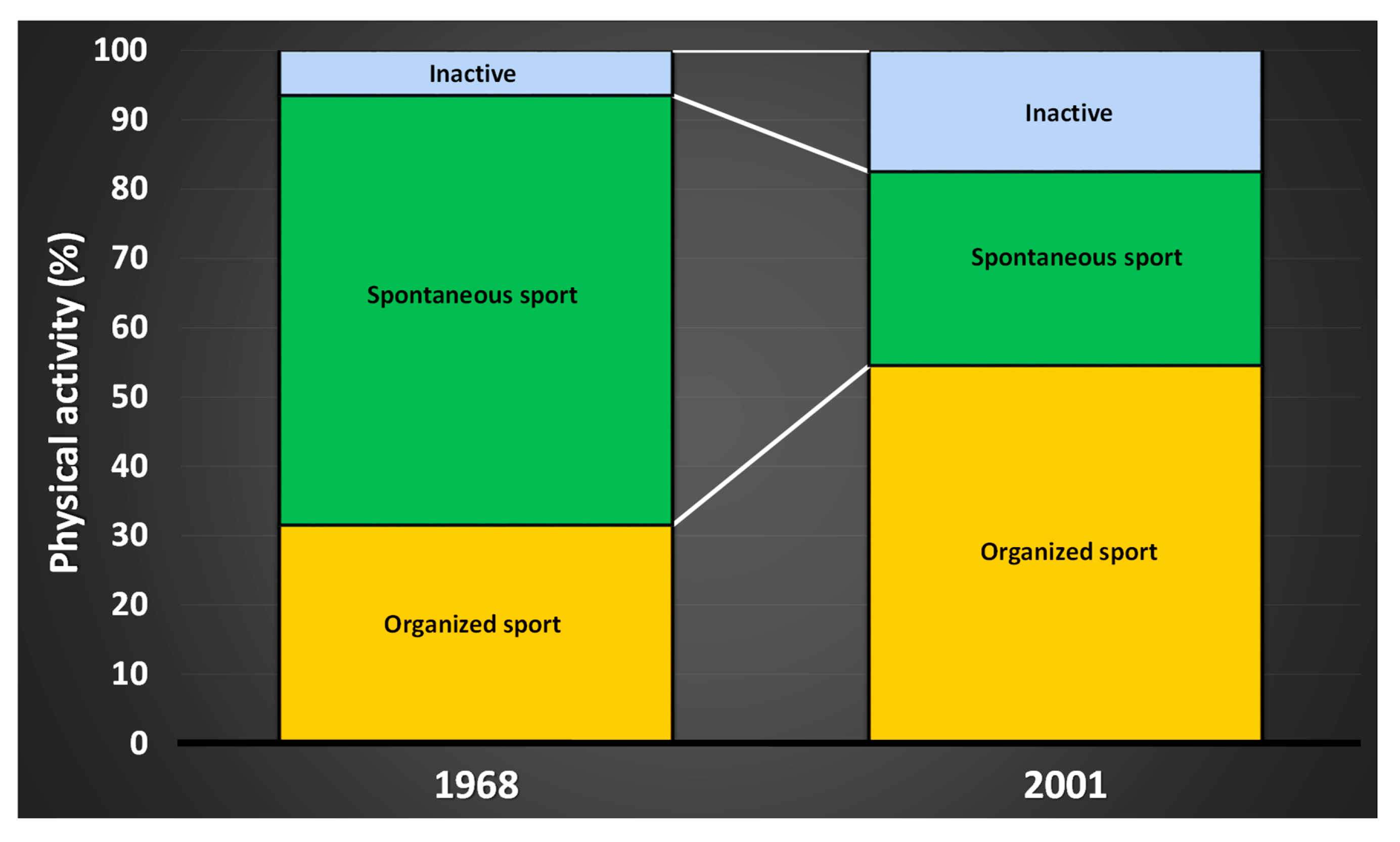
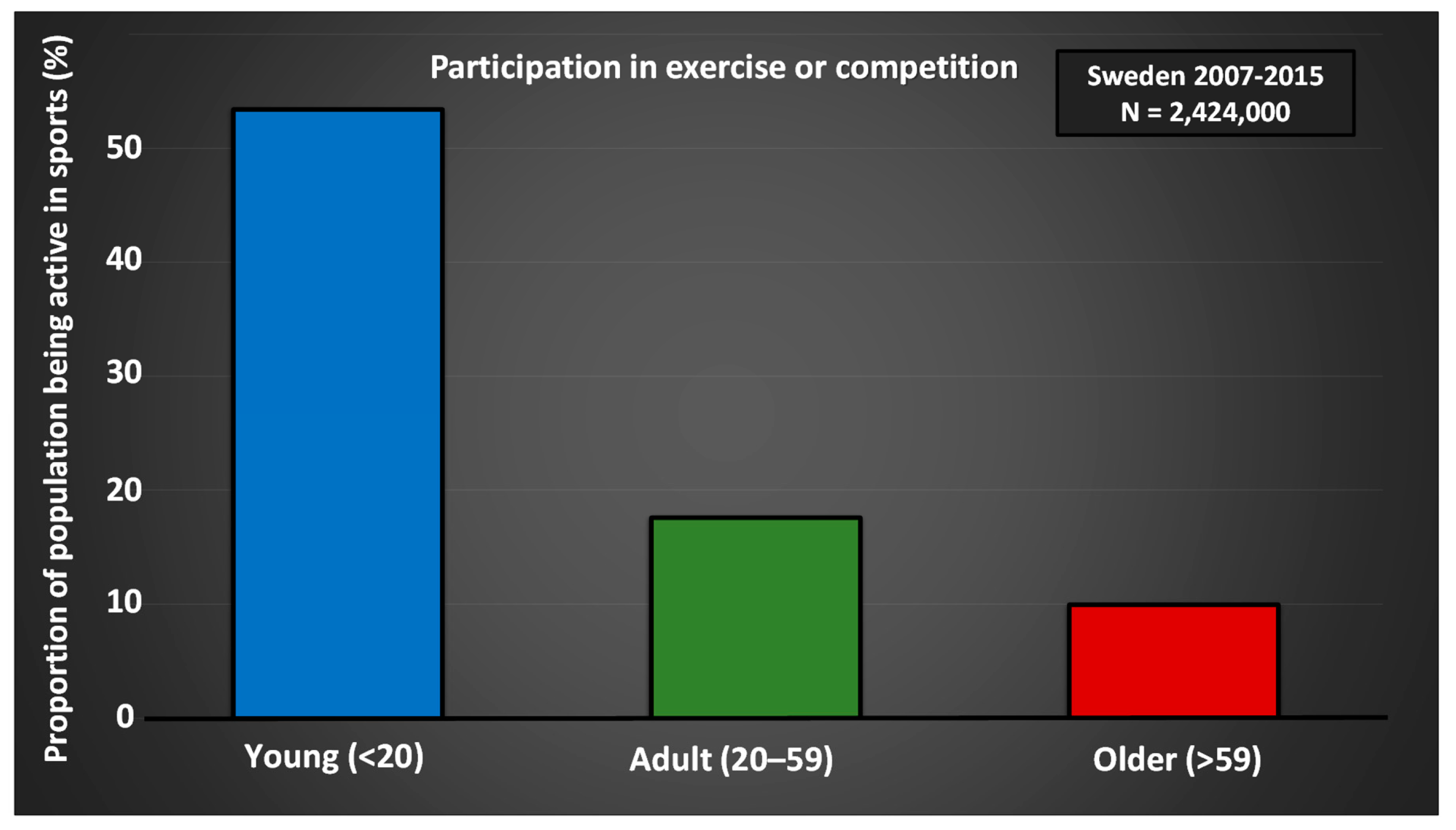
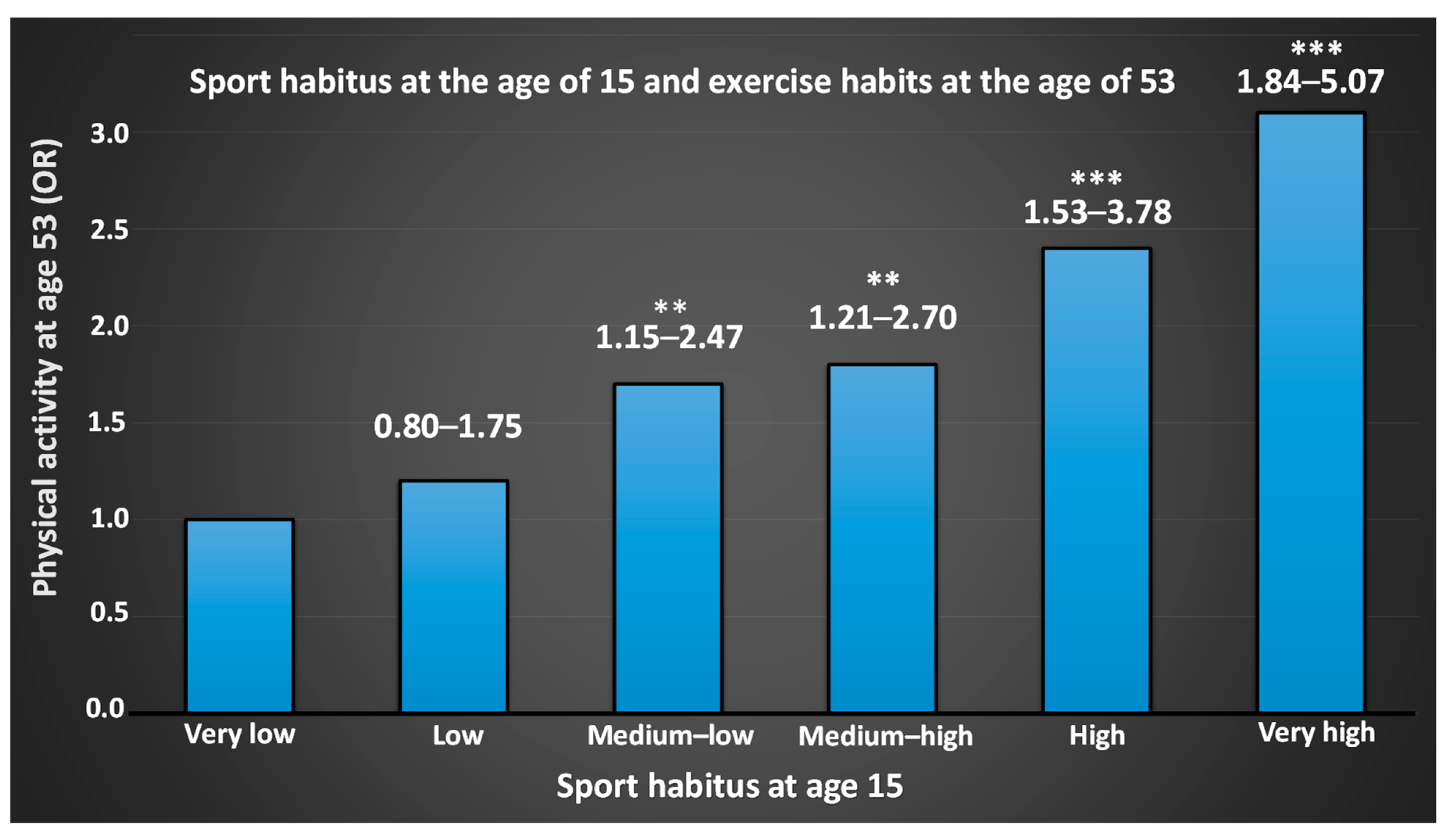
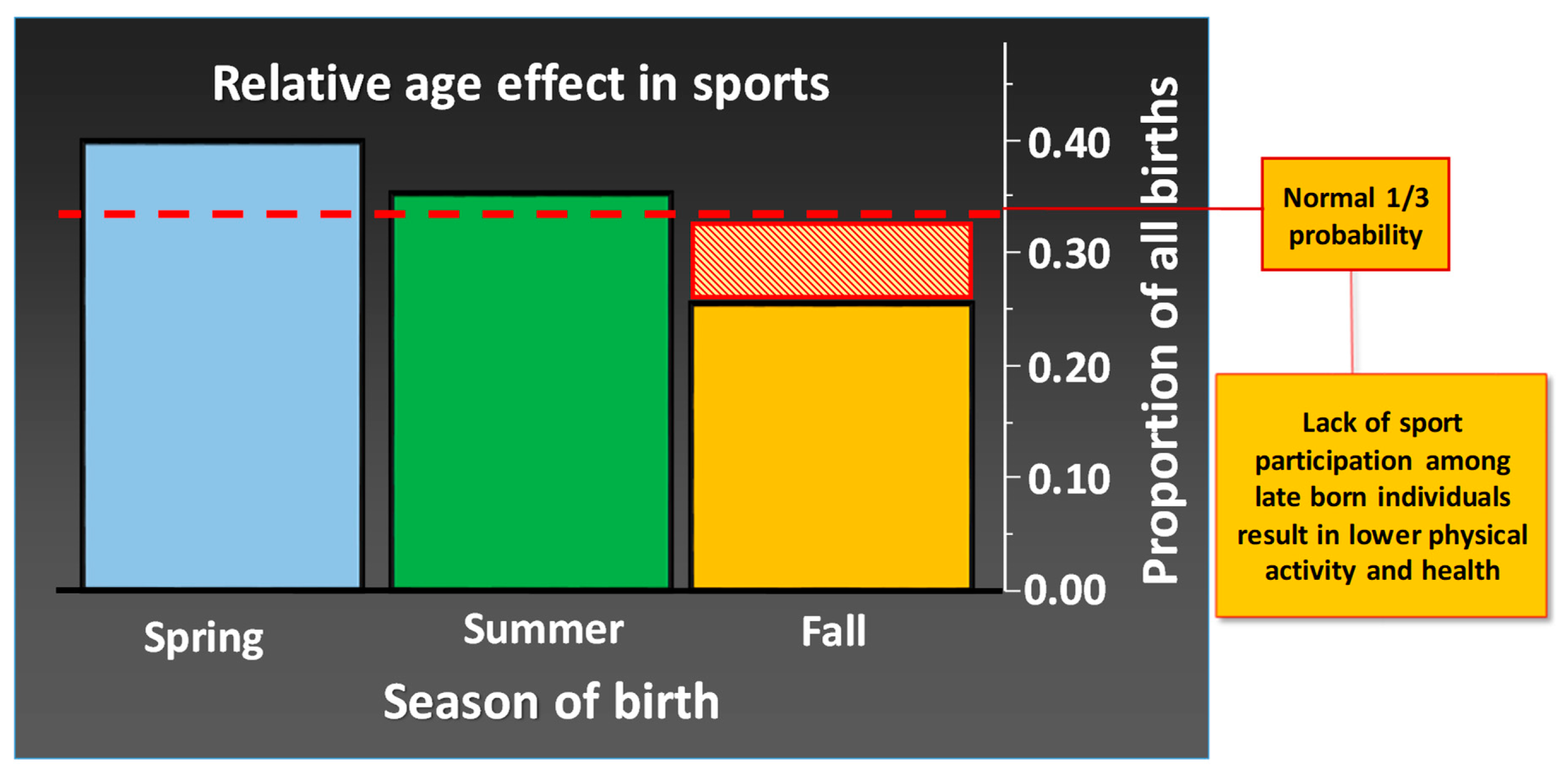
| Target Group | Recommendations | Purpose |
|---|---|---|
| Children and youth Age 6–17 years | All children and adolescents are recommended at least 60 minutes daily physical activity. Longer is better. The physical activity should be primarily of aerobic nature and the intensity moderate (easy/medium pulse increase) to high (marked pulse increase). Aerobic physical activity at high intensity at least 3 times a week. Muscle-strengthening physical activity 3 times a week. Weight-bearing activity, such as running and jumping, is positive for bone mineral density. The physical activity level will gradually be adapted to the individual’s biological and psychosocial maturation. | Development of muscles and skeletal and nervous system. Maintain a healthy weight and a good mental health. Social development, integration, good self-esteem, and self-confidence. Enhanced learning ability. Recommendations are universal, but for individuals with illness, there may be special recommendations. |
| Adults Age 18–64 | All adults from 18 years of age and above are recommended to be aerobically physically active at least 150 minutes a week at a moderate intensity (medium pulse increase), or at least 75 minutes per week at vigorous intensity (marked pulse increase). The activities should be distributed over at least three separate days. Muscle-strengthening physical activity at least twice a week should be performed. | Improvements in aerobic work capacity and muscle strength. Recommendations are universal, but for individuals with illness, there may be special recommendations. Profits from carrying out the activity are lower risk of disease, such as disturbed metabolism and certain cancers and bone fractures. |
| Elderly Age >64 | Same recommendations as adults. Muscle strengthening exercises should be performed at a high velocity, if possible. Balance training should be incorporated prior to aerobic and muscle strengthening training. Individuals with impaired ability should perform as much exercise as possible. | Improvements in aerobic work capacity, muscle strength, and balance. Recommendations are universal, but for individuals with illness, there may be special recommendations. Medical advice may be required before exercise commences. Benefits of carrying out the activity are the same as for adults, and better functional health and independence. |
| Effects on the Body | Health Effects | Aerobic | Strength |
|---|---|---|---|
| Larger proportion slow-twitch fibers [70,71] | Lower risk for metabolic syndrome with increased exchange of gases and nutrition [71,72] |  |  |
| Larger proportion slow-twitch [73] | Increased strength, coordination and balance in elderly [74] and in sickness [75], lower risk for fall [76] |  |  |
| Formation of new capillaries [71] | Increased aerobic capacity [71] |  |  |
| Improved endothelial function [71] | Lower risk for cardiovascular disease [77], improved function in heart disease [78] |  |  |
| Increased mitochondrial volume [46] | Increased aerobic capacity [79] |  |  |
| Improved glucose transport [80] | Lower risk or metabolic syndrome/Type-2 diabetes [81] |  |  |
| Improved insulin sensitivity [82] | Improved health in people with Type-2 diabetes [82], prevention of Typ-2 diabetes [83] |  |  |
| Increased heart capacity [71] | Lower risk for cardiovascular disease [77], fewer depressions [84,85], also in children [86] |  |  |
| Increased skeletal volume and mineral content [87] | Improved skeletal health [88,89] |  |  |
| Improved body composition [30] | Lower risk for metabolic syndrome [81] |  |  |
| Improved blood pressure regulation [90,91] | Lower risk for cardiopulmonary disease [92] |  |  |
| Improved blood lipid profile [93] | Lower risk for cardiopulmonary disease in elderly [94,95] and Alzheimer’s [96] No effect on blood lipid profiles in children and adolescents [97] |  |  |
| Improved peripheral nerve function [98] | Better coordination, balance and reaction [98,99], especially in children and elderly [100] |  |  |
| Enhanced release of signaling substances [84,101] | Better sleep [102], less anxiety [68], treatment of depression [31] |  |  |
| Improved hippocampus function [103] | Improved cognition and memory [104], less medication [103] |  |  |
| Positive effects on mental capacity [105] | Counteract brain degeneration by diseases [106] and age [107] |  |  |
| Improved immune function [108] | Decreased overall risk for disease [109,110], anti-inflammatory effects [111,112] |  |  |
| Strengthening the connection between brain, metabolism and immune function [113] | Decreased risk for disease [114], improved metabolism [115], decreased risk for depression [116] |  |  |
| Improved intestinal function [14,113] | Improved health [117], mitigated metabolic syndrome, obesity, liver disease, and some cancers [115] |  |  |
| Health Condition | Risk Reduction1 or Health Improvement | Recommendations for Physical Activity2 | Dose-Response Relationship | Differences between Sex, Age, Ethnicity etc. |
|---|---|---|---|---|
| All-cause mortality | 30% (44% elderly) | General recommendations | Yes | No |
| Cardiovascular disease | 20%–35% | General recommendations | Yes | Insufficient evidence |
| Metabolic syndrome | 30%–40% | General recommendations | Yes | No |
| Type-2 diabetes | 25%–42% | General recommendations, data primarily on aerobic PA | Yes | Insufficient evidence |
| Cancer | Brain cancer: Limited evidence2; Breast cancer: 20%; Bladder cancer: 13%–15%; Colon cancer: 30%; Endometrial cancer: 17%–35%; Esophageal cancer3: 6%–21%; Gastric cancer: 19%; Head & neck cancers: 15%–22%, limited evidence; Hematological cancers: No-low effect, limited evidence3; Lung cancer: 13%–26%; Ovarian cancer: Limited/conflicting evidence; Pancreatic & prostate cancer: Limited evidence; Renal cancer: 11%–23%; Rectal cancer: No risk reduction, limited evidence; Thyroid cancer: No risk reduction | General recommendations, data primarily on aerobic PA | Renal & thyroid cancer: No. Lung, hematological, head and neck cancers: Limited evidence. Other; Yes. | Breast cancer: Weaker evidence for Hispanic and Black women. Gastric cancer: Weaker evidence for women Renal cancer: Weaker evidence for Asians Lung cancer: Greater effect for women Other: Limited evidence/No known difference |
| Overweight and obesity (weight loss) | PA alone, without diet intervention only has an effect at large volume | General recommendations, combined with diet interventions | Yes | No |
| Overweight and obesity (weight maintenance) | PA supports weight maintenance | General recommendations, stronger evidence for aerobic PA | Limited evidence | Insufficient evidence |
| Skeletal health | 36%–68% for hip fracture 1%–2% increased bone density | General recommendations including muscle- strengthening physical activity | Yes | Hip fracture: Largest effect in elderly women Bone density: Largest effect in women |
| Muscle mass | Magnitude is highly variable and mode-dependent | Weight bearing activity | Yes | Decreased effect with age |
| Functional strength/capacity (middle age and older) | 30% increased chance to counteract or postpone a decrease in functional strength/capacity 30% lower risk of falls | General recommendations including muscle- and skeletal-strengthening physical activity | Functional health: Yes Falls: No/unclear | Increased functional capacity mostly seen in older adults ages 65 or more. |
| Depression | 20%–30% lower | General recommendations | Yes | No |
| Sleep | Improved quality, sleep onset latency and total sleep time | General recommendations | No | No |
| Distress | 20%–30% lower | General recommendations | No | No |
| Dementia | 20%–30% lower | General recommendations | No | No |
| Cognition | Improved for preadolescent children and adults aged 50 years or older | General recommendations | Conflicting findings | Insufficient evidence for adolescents and adults. Ethnicity: No. |
| Physical Activity at Age 20 Years | Girls | Boys | ||||
|---|---|---|---|---|---|---|
| Sport Participation as Young | ||||||
| Participate | Quit | Never | Participate | Quit | Began late | |
| Medium/Intense physical activity | ⮉ | ⮉ | ⮋ | ⮉ | ⮉ | ⮋ |
| Current MET | ⇔ | ⇔ | ⇔ | ⮉ | ⮉ | ⮋ |
| Body stature | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Body weight | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Fat (%) | ⇔ | ⇔ | ⇔ | ⮋ | ⮉ | ⮉ |
| Fat free mass (kg) | ⮉ | ⮉ | ⮋ | ⮉ | ⮋ | ⮉ |
| Fat free mass index (kg·m-2) | ⮉ | ⮉ | ⮋ | ⮉ | ⮋ | ⮉ |
| Physical health (SF-12) | ⮉ | ⮉ | ⮋ | ⇔ | ⇔ | ⇔ |
| Mental health (SF-12) | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Depression (DASS-21) | ⇔ | ⇔ | ⇔ | ⇔ | ⮉ | ⇔ |
| Anxiety (DASS-21) | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Stress (DASS-21) | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ | ⇔ |
| Aspect | Positive | Negative |
|---|---|---|
| Personal | Better self-esteem Better academic results That endurance and hard work pay off Independence and responsibility Making wise decisions Keep a positive attitude Manage stress Set clear goals Higher assessment of skills Higher working standards Better discipline Late alcohol store Lower alcohol consumption (in most sports) Less drugs Greater social capital Better relationships with adults Uses TV/PC less Lower risk of school dropout | Emotional fatigue One-dimensional identity Risk of abuse Increased stress Injuries Temptation for doping Fear of punishment Fear of failure Feeling pressure from the surroundings Fear of disappointing surroundings Risk of burnout Risk of overtraining Poor sleep Decrepit Repeated infections Risk of self-sacrifice Risk of self-injury Increased risk of destructive decisions (doping, cheating etc.) Risk of depression in case of rejection |
| Social | The usefulness of teamwork Good communication Larger contributions to society later in life Larger contributions to the family later in life Lower crime Opportunity in developing countries Increased chance of being active in sports clubs as older Easier to reach with education | Less integrated with the family Social isolation from other society |
| Physiological | Greater physical literacy Abilities to live a healthy life as adult and elderly Less smoking Less drugs Lower body fat Larger muscle mass Beneficial metabolism Higher aerobic and anaerobic capacity Lower risk for fractures as older Reduced general disease risk | Physical fatigue Increased injury risk Risk of eating disorders Overtraining Temptation for doping Risk of abuse (physical and mental) Unilateral training and development For Para athletes, injury can be a double handicap Worse oral health |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malm, C.; Jakobsson, J.; Isaksson, A. Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports 2019, 7, 127. https://doi.org/10.3390/sports7050127
Malm C, Jakobsson J, Isaksson A. Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports. 2019; 7(5):127. https://doi.org/10.3390/sports7050127
Chicago/Turabian StyleMalm, Christer, Johan Jakobsson, and Andreas Isaksson. 2019. "Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden" Sports 7, no. 5: 127. https://doi.org/10.3390/sports7050127
APA StyleMalm, C., Jakobsson, J., & Isaksson, A. (2019). Physical Activity and Sports—Real Health Benefits: A Review with Insight into the Public Health of Sweden. Sports, 7(5), 127. https://doi.org/10.3390/sports7050127





