Identifying the Gut Virome of Diaphorina citri from Florida Groves
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Asian Citrus Psyllid Collection, Gut Dissection and Total RNA Preparation
2.2. RNA-Sequencing, Assembly, and Sequence Analyses
2.3. Verification of Viral Sequences in D. citri
2.4. Psyllid Gut Staining with DAPI
3. Results
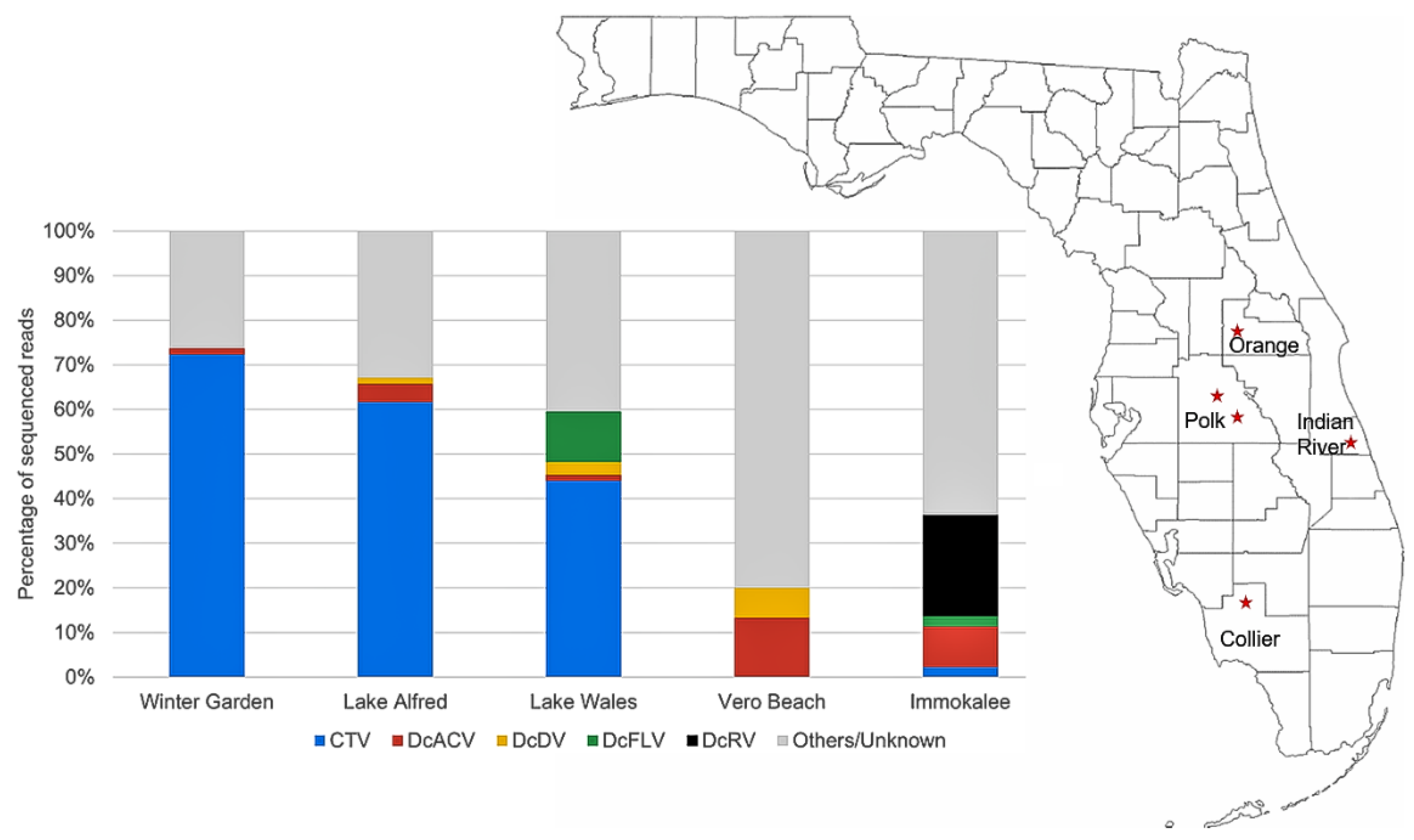
3.1. Identification of D. citri-Associated Viruses in the Gut Tissue by HTS and Validation by RT-PCR
3.2. Nuclear Damage of Psyllid Adult Guts under D. citri-Associated Virus Infection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jagoueix, S.; Bove, J.M.; Garnier, M. The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the proteobcteria. Int. J. Syst. Bacteriol. 1994, 44, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Dala-Paula, B.M.; Plotto, A.; Bai, J.; Manthey, J.A.; Baldwin, E.A.; Ferrarezi, R.S.; Gloria, M.B.A. Effect of huanglongbing or greening disease on orange juice quality: A review. Front. Plant Sci. 2019, 9, 1976. [Google Scholar] [CrossRef] [PubMed]
- McCollum, G.; Hilf, M.; Irey, M.; Luo, W.; Gottwald, T. Susceptibility of sixteen citrus genotypes to ‘Candidatus Liberibacter asiaticus’. Plant Dis. 2016, 100, 1080–1086. [Google Scholar] [CrossRef] [PubMed]
- Gottwald, T.R. Current epidemiological understanding of citrus huanglongbing. Ann. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Hall, D.G.; Richardson, M.L.; Ammar, E.D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Kunta, M.; Sétamou, M.; Skaria, M.; Rascoe, J.E.; Li, W.; Nakhla, M.K.; da Graça, J.V. First report of citrus huanglongbing in Texas. Phytopathology 2012, 102, S4–S66. [Google Scholar]
- Kumagai, L.B.; LeVesque, C.S.; Blomquist, C.L.; Madishetty, K.; Guo, Y.; Woods, P.W.; Rooney-Latham, S.; Rascoe, J.; Gallindo, T.; Schnabel, D.; et al. First report of Candidatus Liberibacter asiaticus associated with citrus huanglongbing in California. Plant Dis. 2013, 97, 283. [Google Scholar] [CrossRef]
- Oliver, J.E.; Ali, M.E.; Waliullah, S.; Price, J.; Warren, J.; Jacobs, J.; Hoppers, A.; Evans, R.; Dowdy, M.; Curry, S. Huanglongbing, caused by ‘Candidatus Liberibacter asiaticus’, Detected in new locations across Southern and Coastal Georgia. Plant Health Prog. 2020, 21, 31–35. [Google Scholar] [CrossRef]
- Blaustein, R.A.; Lorca, G.L.; Teplitski, M. Challenges for managing Candidatus Liberibacter spp. (Huanglongbing disease pathogen): Current control measures and future directions. Phytopathology 2018, 108, 424–435. [Google Scholar] [CrossRef]
- Munir, S.; He, P.; Wu, Y.; He, P.; Khan, S.; Huang, M.; Cui, W.; He, P.; He, Y. Huanglongbing control: Perhaps the end of the beginning. Microb. Ecol. 2018, 76, 192–204. [Google Scholar] [CrossRef]
- Chen, X.D.; Stelinski, L.L. Rapid detection of insecticide resistance in Diaphorina citri (Hemiptera: Liviidae) populations, using a bottle bioassay. Fla. Entomol. 2017, 100, 124–133. [Google Scholar] [CrossRef]
- Chen, X.D.; Gill, T.A.; Pelz-Stelinski, K.S.; Stelinski, L.L. Risk assessment of various insecticides used for management of Asian citrus psyllid, Diaphorina citri in Florida citrus, against honeybee, Apis mellifera. Ecotoxicology 2017, 26, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Kanga, L.H.; Eason, J.; Haseeb, M.; Qureshi, J.; Stansly, P. Monitoring for insecticide resistance in Asian citrus psyllid (Hemiptera: Psyllidae) populations in Florida. J. Econ. Entomol. 2016, 109, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Mann, R.S.; Rogers, M.E.; Stelinski, L.L. Insecticide resistance in field populations of Asian citrus psyllid in Florida. Pest Manag. Sci. 2011, 67, 1258–1268. [Google Scholar] [CrossRef] [PubMed]
- Britt, K.; Gebben, S.; Levy, A.; Rwahnih, M.A.; Batuman, O. The detection and surveillance of Asian citrus psyllid (Diaphorina citri)-associated viruses in Florida citrus grove. Front. Plant Sci. 2020, 10, 1687. [Google Scholar] [CrossRef]
- Francis, F.; Jacquemyn, H.; Delvigne, F.; Lievens, B. From diverse origins to specific targets: Role of microorganisms in indirect pest biological control. Insects 2020, 11, 533. [Google Scholar] [CrossRef]
- Niang, E.H.A.; Bassene, H.; Fenollar, F.; Mediannikov, O. Biological control of mosquito-borne diseases: The potential of Wolbachia-based interventions in an IVM framework. J. Trop. Med. 2018, 2018. [Google Scholar] [CrossRef]
- Tanzini, M.; Alves, S.; Setten, A.; Augusto, N. Compatibilidad de agent estensoactivos com Beauveria bassiana y Metarhizium anisopliae. Manejo Integr. Plagas 2001, 59, 15–18. [Google Scholar]
- Ignoffo, C.M. Development of a viral insecticide: Concept to commercialization. Exp. Parasitol. 1973, 33, 380–406. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Sosa-Gómez, D.R.; Morgado, F.S.; Corrê, R.F.T.; Silva, L.A.; Ardisson-Araújo, D.M.P.; Rodrigues, B.M.P.; Oliveira, E.E.; Aguiar, R.W.S.; Ribeiro, B.M. Entomopathogenic viruses in the Neotropics: Current status and recently discovered species. Neotrop. Entomol. 2020, 49, 315–331. [Google Scholar] [PubMed]
- Naik, N.G.; Lo, Y.W.; Wu, T.Y.; Lin, C.C.; Kuo, S.C.; Chao, Y.C. Baculovirus as an efficient vector for gene delivery into mosquitoes. Sci. Rep. 2018, 8, 17778. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.H.; Wei, S.C.; Lo, H.R.; Chao, Y.C. Baculovirus as versatile vectors for protein display and biotechnological applications. Curr. Issues Mol. Biol. 2020, 34, 231–255. [Google Scholar]
- Arbuthnott, D.; Levin, T.C.; Promislow, D.E. The impacts of Wolbachia and the microbiome on mate choice in Drosophila melanogaster. J. Evol. Biol. 2016, 29, 461–468. [Google Scholar] [CrossRef]
- Ben-Yosef, M.; Pasternak, Z.; Jurkevitch, E.; Yuval, B. Symbiotic bacteria enable olive flies (Bactrocera oleae) to exploit intractable sources of nitrogen. J. Evol. Biol. 2014, 27, 2695–2705. [Google Scholar]
- Engel, P.; Moran, N.A. The gut microbiota of insects–diversity in structure and function. FEMS Microbiol. Rev. 2013, 37, 699–735. [Google Scholar] [CrossRef] [PubMed]
- Engl, T.; Kaltenpoth, M. Influence of microbial symbionts on insect pheromones. Nat. Prod. Rep. 2018, 35, 386–397. [Google Scholar] [PubMed]
- Gupta, A.; Nair, S. Dynamics of insect–microbiome interaction influence host and microbial symbiont. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Bonning, B.C. The insect virome: Opportunities and challenges. Curr. Issues Mol. Biol. 2019, 34, 1–12. [Google Scholar]
- Huang, H.J.; Ye, Z.X.; Wang, X.; Yan, X.T.; Zhang, Y.; He, Y.J.; Qi, Y.H.; Zhang, X.D.; Zhuo, J.C.; Lu, G.; et al. Diversity and infectivity of the RNA virome among different cryptic species of an agriculturally important insect vector: Whitefly Bemisia tabaci. Npj Biofilms Microbiomes 2021, 7. [Google Scholar] [CrossRef]
- Muñoz-Benavent, M.; Pérez-Cobas, A.E.; García-Ferris, C.; Moya, A.; Latorre, A. Insects’ potential: Understanding the functional role of their gut microbiome. J. Pharm. Biomed. 2021, 194, 113787. [Google Scholar]
- Ng, T.F.F.; Willner, D.L.; Lim, Y.W.; Schmieder, R.; Chau, B.; Nilsson, C.; Anthony, S.; Ruan, Y.; Rohwer, F.; Breitbart, M. Broad surveys of DNA viral diversity obtained through viral metagenomics of mosquitoes. PLoS ONE 2011, 6, e20579. [Google Scholar]
- Kuo, Y.W.; Matsumura, E.E.; Nigg, J.C.; Chen, Q.; Henry, E.; Nouri, S.; Godfrey, K.E.; Falk, B.W. ACP are full of viruses. Can we use them against HLB? Citrograph 2020, 11, 52–56. [Google Scholar]
- Marutani-Hert, M.; Hunter, W.B.; Katsar, C.S.; Sinisterra, X.H.; Hall, D.G.; Powell, C.A. Reovirus-like sequences isolated from adult Asian citrus psyllid, (Hemiptera: Psyllidae: Diaphorina citri). Fla. Entomol. 2009, 92, 314–320. [Google Scholar] [CrossRef]
- Nouri, S.; Salem, N.; Falk, B.W. Complete genome sequence of Diaphorina citri-associated C virus, a novel putative RNA virus of the Asian citrus psyllid, Diaphorina citri. Genome Announc. 2016, 4, e00639-16. [Google Scholar]
- Nouri, S.; Salem, N.; Nigg, J.C.; Falk, B.W. Diversity array of new viral sequences identified in worldwide populations of the Asian citrus psyllid (Diaphorina citri) using viral metagenomics. J. Virol. 2016, 90, 2434–2445. [Google Scholar]
- Nouri, S.; Matsumura, E.E.; Kuo, Y.W.; Falk, B.W. Insect-specific viruses: From discovery to potential translational applications. Curr. Opin. Virol. 2018, 33, 33–41. [Google Scholar]
- Britt, K.; Stevens, K.; Gebben, S.; Levy, A.; Al Rwahnih, M.; Batuman, O. Partial genome sequence of a novel Reo-like virus detected in Asian citrus psyllid (Diaphorina citri) populations from Florida citrus groves. Microbiol. Resour. Announc. 2021, 10, e00563-21. [Google Scholar]
- Britt, K.; Gebben, S.; Levy, A.; Achor, D.; Sieburth, P.; Stevens, K.; Al Rwahnih, M.; Batuman, O. Analysis of Citrus tristeza virus incidences within Asian citrus psyllid (Diaphorina citri) populations in Florida via high-throughput sequencing. Insects 2022, 13, 275. [Google Scholar]
- Wu, F.; Huang, M.; Fox, E.G.P.; Huang, J.; Cen, Y.; Deng, X.; Xu, M. Preliminary report on the acquisition, persistence, and potential transmission of Citrus tristeza virus by Diaphorina citri. Insect 2021, 12, 735. [Google Scholar] [CrossRef]
- Ammar, E.D.; Shatters Jr, R.G.; Hall, D.G. Localization of Candidatus Liberibacter asiaticus, associated with citrus huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 2011, 159, 726–734. [Google Scholar] [CrossRef]
- Ghanim, M.; Fattah-Hosseini, S.; Levy, A.; Cilia, M. Morphological abnormalities and cell death in the Asian citrus psyllid (Diaphorina citri) midgut associated with Candidatus Liberibacter asiaticus. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Ghanim, M.; Achor, D.; Ghosh, S.; Kontsedalov, S.; Lebedev, G.; Levy, A. ‘Candidatus Liberibacter asiaticus’ accumulates inside endoplasmic reticulum associated vacuoles in the gut cells of Diaphorina citri. Sci. Rep. 2017, 7, 16945. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Achor, D.; Levy, A. Intracellular life cycle of ‘Candidatus Liberibacter asiaticus’ inside psyllid gut cells. Phytopathology 2022, 112, 145–153. [Google Scholar] [CrossRef]
- Rashidi, M.; Lin, C.Y.; Britt, K.; Batuman, O.; Al Rwahnih, M.; Achor, D.; Levy, A. Diaphorina citri flavi-like virus localization, transmission, and association with Candidatus Liberibacter asiaticus in its psyllid host. Virology 2022, 567, 47–56. [Google Scholar] [PubMed]
- Al Rwahnih, M.; Rowhani, A.; Westrick, N.; Stevens, K.; Diaz-Lara, A.; Trouillas, F.P.; Preece, J.; Kallsen, C.; Farrar, K.; Golino, D. Discovery of viruses and virus-like pathogens in pistachio using high-throughput sequencing. Plant Dis. 2018, 102, 1419–1425. [Google Scholar]
- Hong, C.; Manimaran, S.; Shen, Y.; Perez-Rogers, J.F.; Byrd, A.L.; Castro-Nallar, E.; Crandall, K.A.; Johnson, W.E. PathoScope 2.0: A complete computational framework for strain identification in environmental or clinical sequencing samples. Microbiome 2014, 2, 33. [Google Scholar]
- Tatusova, T.A.; Madden, T.L. BLAST 2 Sequences, a new tool for comparing protein and nucleotide sequences. FEMS Microbiol. Lett. 1999, 174, 247–250. [Google Scholar]
- Harper, S.J.; Cowell, S.J. The past and present status of Citrus tristeza virus in Florida. J. Citrus Pathol. 2016, 3. [Google Scholar] [CrossRef]
- van Lenteren, J.C.; Bolckmans, K.; Köhl, J.; Ravensberg, W.J.; Urbaneja, A. Biological control using invertebrates and microorganisms: Plenty of new opportunities. BioControl 2018, 63, 39–59. [Google Scholar]
- Hajek, A.E.; Gardescu, S.; Delalibera Jr., I. Summary of classical biological control introductions of entomopathogens and nematodes for insect control. BioControl 2021, 66, 167–180. [Google Scholar] [CrossRef]
- Deka, B.; Baruah, C.; Babu, A. Entomopathogenic microorganisms: Their role in insect pest management. Egypt. J. Biol. Pest Control. 2021, 31, 121. [Google Scholar] [CrossRef]
- Carvalho, V.L.; Long, M.T. Perspectives on new vaccines against Arboviruses using insect-specific viruses as platforms. Vaccines 2021, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.I.; Villinger, J.; Muthoni, J.N.; Dobel-Ober, L.; Hughes, G.L. Exploiting insect-specific viruses as a novel strategy to control vector-borne disease. Curr. Opin. Insect Sci. 2020, 39, 50–56. [Google Scholar] [CrossRef]
- Roundy, C.M.; Azar, S.R.; Rossi, S.L.; Weaver, S.C.; Vasilakis, N. Insect-specific viruses: A historical overview and recent developments. Adv. Virus Res. 2017, 98, 119–146. [Google Scholar]
- Sarkar, P.; Ghanim, M. Unravelling the pathogenesis and molecular interactions of Liberibacter phytopathogens with their psyllid vectors. Agronomy 2020, 10, 1132. [Google Scholar] [CrossRef]
- Hobson-Peters, J.; Harrison, J.J.; Watterson, D.; Hazlewood, J.E.; Vet, L.J.; Newton, N.D.; Warrilow, D.; Colmant, A.M.; Taylor, C.; Huang, B.; et al. A recombinant platform for flavivirus vaccines and diagnostics using chimeras of a new insect-specific virus. Sci. Transl. Med. 2019, 11, eaax7888. [Google Scholar] [CrossRef]
- Szewczyk, B.; Hoyos-Carvajal, L.; Paluszek, M.; Skrzecz, I.; De Souza, M.L. Baculoviruses-re-emerging biopesticides. Biotechnol. Adv. 2006, 24, 143–160. [Google Scholar] [CrossRef]
- Gray, S.M.; Cilia, M.; Ghanim, M. Circulative, “nonpropagative” virus transmission: An orchestra of virus, insect and plant derived instruments. Adv. Virus Res. 2014, 89, 141–199. [Google Scholar]
- Liu, S.; Sivakumar, S.; Sparks, W.O.; Miller, W.A.; Bonning, B.C. A peptide that binds the pea aphid gut impedes entry of Pea enation mosaic virus into the aphid hemocoel. Virology 2010, 401, 107–116. [Google Scholar] [CrossRef]
- Vasilakis, N.; Tesh, R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Airs, P.M.; Bartholomay, L.C. RNA interference for mosquito and mosquito-borne disease control. Insect 2017, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Adelman, Z.N.; Blair, C.D.; Carlson, J.O.; Beaty, B.J.; Olson, K.E. Sindbis virus-induced silencing of dengue viruses in mosquitoes. Insect Mol. Biol. 2001, 10, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Travieso, T.; Li, J.; Mahesh, S.; Mello, J.D.F.R.E.; Blasi, M. The use of viral vectors in vaccine development. Vaccines 2022, 7. [Google Scholar] [CrossRef] [PubMed]
- Cicero, J.M.; Fisher, T.W.; Brown, J.K. Localization of ‘Candidatus Liberibacter solanacearum’ and evidence for surface appendages in the potato psyllid vector. Phytopathology 2016, 106, 142–154. [Google Scholar] [CrossRef] [PubMed]

| Virus 1 | Primers | Length of Amplicon | Tm (°C) | Accession Number | Reference |
|---|---|---|---|---|---|
| DcACV | F-5′ GCCGCACGAAACTAGTGATAAACGCA 3′ | 473 bp | 50 | KX235518.1 | [15] |
| R-5′ GGATCGGTGGTGCACGAGTATGTAAGTA 3′ | |||||
| DcCLV | F-5′ ATTTAGGGCCATGTGCAAAG 3′ R-3′ CCAACACACCGAGCATACAC 3′ | 526 bp | 62 | MZ484733.1 | |
| DcDV | F-5′ AGTCGGTGAGACTGATATCTTCGAGACC 3′ | 1068 bp | 60 | KX165268 | |
| R-5′ GTTTAGTTCGCTTGTCGGTTACACAGG 3′ | |||||
| DcFLV | F-5′ AGGCGAGTACTCCCATCGGATACATT 3′ | 1391 bp | 58 | KX267823.1 | |
| R-5′ GAGGGCCGCTAAGTCTGTAGGACATATT 3′ | |||||
| DcPLV | F-5′ TAGGTGAACGTGATAATCCTGGTAT 3′ | 698 bp | 62 | KT698837.1 | |
| R-5′ CAGAACGTCTGTTATGAATCGGAC 3′ | |||||
| DcRV | F-5′ TTTTCCCAGGTACATCGA 3′ | 900 bp | 50 | KT698831.1 | [36] |
| R-5′ ACCATTCAGCCAGTCCTA 3′ | |||||
| CTV | F-5′ ACCGGAGCTGGCTTGACTGAT 3′ | 113 bp | 60 | MZ670758.1 | [49] |
| R-5′ CCAAGCTGCCTGACATTAGTAA 3′ | |||||
| Probe: 6-Fam/AGAGTGTGCTGTGTACATACAAGCTAAAGA |
| County | Region | No. of Read Sequences | Megabases | No. of Contigs | BLASTN Virus Contigs | Known Viruses Detected |
|---|---|---|---|---|---|---|
| Orange | Winter Garden | 16,748,063 | 1256 | 46,344 | 115 | 17 |
| Collier | Immokalee | 26,004,558 | 1950 | 22,670 | 21 | 12 |
| Polk | Lake Alfred | 20,340,968 | 1526 | 49,651 | 58 | 19 |
| Indian River | Vero Beach | 18,793,456 | 1410 | 54,646 | 8 | 14 |
| Polk | Lake Wales | 12,836,297 | 963 | 32,765 | 99 | 17 |
| Virus Name | Region 1 | Reference Accession Number | Longest Contig Length (bp) | Identity (%) | Coverage (%) |
|---|---|---|---|---|---|
| Diaphorina citri associated C virus | WG, LA, LW, VB, IK | KX235518.1-19.1 | 2376 | 98.5–99.9 | 99–100 |
| Diaphorina citri densovirus | LA, LW, VB | YP_009256210.1-11.1 | 679 | 34.6–85.5 | 21–88 |
| Diaphorina citri flavi-like virus | LW, IK | KX267823.1 | 27709 | 95.2–100 | 99–100 |
| Diaphorina citri reovirus | IK | KT698830.1-36.1 | 4266 | 94.3–98.8 | 88–100 |
| Shuangao insect virus 7 | WG, LA, VB | YP_009179392.1 | 136 | 33.0 | 26 |
| Wuhan insect virus 19 | WG, LW | YP_009342322.1 | 4134 | 37.1–41.8 | 74–98 |
| Culex mononega-like virus 2 | WG, LW | ASA47292.1 | 2079 | 44.8–46.7 | 43–81 |
| Photinus pyralis orthomyxo-like virus 2 | LA, LW, VB | AVR52573.1 | 710 | 25.1–32.5 | 32–91 |
| Liberibacter phage SGCA5-1 | LA | KX879601.1 | 36022 | 99.5 | 100 |
| Wolbachia phage WO | LA | KX522565.1 | 65653 | 90.5 | 100 |
| Trichoplusia ni TED virus | VB | YP_009507248.1 | 1084 | 46.7 | 95 |
| Hubei earwig virus 1 | LW | APG77904.1 | 758 | 52.4 | 58 |
| Lampyris noctiluca errantivirus 1 | LW | QBP37036.1 | 1096 | 49.6 | 87 |
| Citrus tristeza virus | WG, LA, LW, VB, IK | MK018120.12 | 19293 | 89.1–100 | 89–100 |
| Region | DcACV | DcCLV | DcDV | DcFLV | DcPLV | DcRV | CTV | CLas |
|---|---|---|---|---|---|---|---|---|
| Winter Garden | V 1/+ 2 | X/+ | X/− | X/− | X/− | X/− | V/+ | + |
| Lake Alfred | V/+ | X/− | X/− | X/− | X/− | X/− | V/+ | + |
| Lake Wales | V/+ | X/− | V/+ | V/+ | X/− | X/− | V/+ | + |
| Vero Beach | V/+ | X/− | V/+ | X/− | X/− | X/− | X/− | + |
| Immokalee | V/+ | X/− | X/− | V/+ | X/− | V/+ | V/+ | + |
| Avg. Percentage | 100% | 20% | 40% | 40% | 0% | 20% | 80% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Batuman, O.; Levy, A. Identifying the Gut Virome of Diaphorina citri from Florida Groves. Insects 2023, 14, 166. https://doi.org/10.3390/insects14020166
Lin C-Y, Batuman O, Levy A. Identifying the Gut Virome of Diaphorina citri from Florida Groves. Insects. 2023; 14(2):166. https://doi.org/10.3390/insects14020166
Chicago/Turabian StyleLin, Chun-Yi, Ozgur Batuman, and Amit Levy. 2023. "Identifying the Gut Virome of Diaphorina citri from Florida Groves" Insects 14, no. 2: 166. https://doi.org/10.3390/insects14020166
APA StyleLin, C.-Y., Batuman, O., & Levy, A. (2023). Identifying the Gut Virome of Diaphorina citri from Florida Groves. Insects, 14(2), 166. https://doi.org/10.3390/insects14020166








