Simple Summary
Mosquito-borne pathogens require the obligate mosquito vector to shuttle the pathogen between vertebrate hosts. This typically requires the mosquito to acquire the pathogen from an initial bloodmeal, have the pathogen mature and reach the mosquito salivary glands and be transmitted to another vertebrate host in the saliva during subsequent blood feedings. Depending on the pathogen, this incubation period can be up to two weeks. Considering the short lifespan of adult mosquitoes, this means that the oldest mosquitoes are responsible for a disproportionate amount of pathogen transmission. Knowing the age structure of mosquito populations in the field could provide important insights in the likelihood of pathogen transmission occurring. Unfortunately, the current methods of age grading mosquitoes in the field are limited by accuracy, technical challenges and cost. Near-infrared spectroscopy (NIRS) has been shown to be capable of age grading large numbers of mosquitoes cost effectively, although accurate age predictions are still a challenge. In this work, we compared the ability of NIRS to age grade field-collected mosquitoes with two other methods, parity and SCP1 transcript expression. While we did not find NIRS to be suitable for predicting the precise age of individual field-collected Aedes aegypti mosquitoes, we believe that this technique has the potential to monitor changes in the age structure of Ae. aegypti populations over time.
Abstract
Given that older Aedes aegypti (L.) mosquitoes typically pose the greatest risk of pathogen transmission, the capacity to age grade wild Ae. aegypti mosquito populations would be a valuable tool in monitoring the potential risk of arboviral transmission. Here, we compared the effectiveness of near-infrared spectroscopy (NIRS) to age grade field-collected Ae. aegypti with two alternative techniques—parity analysis and transcript abundance of the age-associated gene SCP1. Using lab-reared mosquitoes of known ages from three distinct populations maintained as adults under laboratory or semi-field conditions, we developed and validated four NIRS models for predicting the age of field-collected Ae. aegypti. To assess the accuracy of these models, female Ae. aegypti mosquitoes were collected from Maricopa County, AZ, during the 2017 and 2018 monsoon season, and a subset were age graded using the three different age-grading techniques. For both years, each of the four NIRS models consistently graded parous mosquitoes as significantly older than nulliparous mosquitoes. Furthermore, a significant positive linear association occurred between SCP1 and NIRS age predictions in seven of the eight year/model combinations, although considerable variation in the predicted age of individual mosquitoes was observed. Our results suggest that although the NIRS models were not adequate in determining the age of individual field-collected mosquitoes, they have the potential to quickly and cost effectively track changes in the age structure of Ae. aegypti populations across locations and over time.
1. Introduction
Aedes aegypti (L.) mosquitoes are the principal vectors of several arboviruses of global importance, including dengue, yellow fever and Zika viruses. Efforts to prevent transmission of these viruses typically involve systematic mosquito surveillance focused on either immature or adult life stages. Unfortunately, the associations between the mosquito indices and arbovirus transmission risk are often weak, meaning mosquito density estimates alone may not predict disease outbreaks [1,2]. A mosquito surveillance approach that assessed additional factors related to vectorial capacity besides vector density could be more useful in predicting and preventing arbovirus transmission.
In mosquitoes, the processes of mating, acquiring an infectious blood meal and surviving the extrinsic incubation period account for a large portion of adult lifespan before the pathogen is capable of being transmitted to another vertebrate host. Thus, older mosquitoes are more likely to transmit human pathogens [3,4]. Because of this, the ability to quickly and accurately determine the age structure of wild mosquito populations would be a valuable tool in assessing the efficacy of vector control strategies, understanding the ecology of older mosquitoes and developing models for predicting and responding to mosquito-borne disease outbreaks. While some of this can be accomplished using mark-release-recapture studies, these are limited by logistical and safety concerns and may not be representative of what occurs in wild mosquito populations. In contrast, directly age grading field-collected mosquitoes would eliminate these difficulties and allow researchers to monitor the age structure of mosquito populations over time. Several different techniques have been developed toward this goal, but all face challenges that limit their usefulness.
Historically, parity analysis and the characterization of cuticular hydrocarbons have been used to evaluate the chronological or physiological age of mosquitoes. The Dentinova technique for parity analysis examines tracheal skeins on the surface of the ovary to determine whether a mosquito has successfully completed a reproductive cycle [5]. Intact skeins indicate that the ovaries have not expanded during egg development, and the mosquito has not completed a reproductive cycle, whereas unfurled skeins indicate that a female mosquito has completed at least one gonotrophic cycle. This technique is highly accurate for determining parity, although it does not provide a precise estimate of chronological age because mosquitoes may have completed more than one reproductive cycle or initiated their first reproductive cycle later in life. Polovodova’s method uses the number of dilations in the ovariole follicular tube to estimate the number of reproductive cycles completed [6]. However, this technique is technically challenging and often inaccurate, especially as the number of reproductive cycles increases [7,8]. Recently, a modification to this method using oil injection improved the accuracy of this approach but also increased the processing time and technical difficulty of the technique [9].
The relative abundance of specific cuticular hydrocarbons was shown to be effective at determining the age of individual mosquitoes, first in Culex quinquefasciatus Say and later in Aedes aegypti [10,11,12]. Subsequently, the ratio of two cuticular hydrocarbons was used successfully to age grade Anopheles stephensi females [13]. The drawbacks of cuticular hydrocarbon analyses are that they are time consuming and technically challenging, which limits the number of individual mosquitoes that can be feasibly analyzed.
More recently, considerable work has focused on identifying age-associated genes whose transcript levels change in an age-associated manner. Cook et al. [14] identified ten genes in Ae. aegypti whose expression changed in a predictable manner, as the chronological age of a mosquito increased. Using a combination of these genes, they were able to age grade individual mosquitoes with greater accuracy than the cuticular hydrocarbon approach. This work was successfully replicated for age predictions in Anopheles gambiae, and a subsequent screen for age-associated genes using microarray analysis identified thousands of transcripts that increased or decreased as a mosquito aged [15,16]. Several of these transcripts changed independent of most physiological events (e.g., nutrition, reproduction). One of the most predictive transcripts for both species was sarcoplasmic calcium-binding protein 1 (SCP1), for which the expression decreased in a predictable manner as the mosquitoes aged. We previously utilized this gene to develop an age-grading technique for classifying individual field-collected Ae. aegypti mosquitoes into those incapable of transmitting dengue (<5 d), with the potential to transmit dengue (5–14 d) and those at high risk of transmitting dengue (15+ d) [17]. Using this age-grading technique, we were able to determine that Ae. aegypti females in a dengue-endemic area of northern Mexico were generally older than females in a nearby region where dengue transmission was rare [18]. Recently, Weeraratne et al. used three age-associated genes, including SCP1, to age grade Ae. aegypti and Ae. albopictus mosquitoes in Sri Lanka [19]. While this approach is reasonably accurate, its greatest shortcomings include high cost, long processing times and destruction of mosquito samples, all of which limit the ability to screen and validate a large number of mosquitoes.
A different approach for age grading individual mosquitoes, near-infrared spectroscopy (NIRS), uses near-infrared electromagnetic waves to interact with cuticular molecules containing C-H, N-H, S-H or O-H bonds [20,21]. In this technique, the spectra of the head and thorax of adult female mosquitoes are scanned from 350 to 2500 nm and the near-infrared reflectance compared with models developed from the spectra of hundreds of mosquitoes of known age. The advantages of this approach include rapid analysis, minimal cost after the original equipment purchase and non-destructive sampling that allows for validation with other age-grading techniques. Studies in both anophelene and culicine mosquitoes demonstrated that this approach could reliably predict the age of individual mosquitoes from homogeneous populations reared under controlled laboratory conditions [22,23,24,25,26]. However, there are few studies that investigate the efficacy of this approach on field-collected mosquitoes exposed to a variety of environmental and physiological conditions. Studies that address the usefulness of NIRS for individual field-collected mosquitoes have been hampered by a lack of alternative age-grading techniques to validate the approach. For example, a recent study of Anopheles gambiae mosquitoes that used parity analysis and the presence of Plasmodium sporozoites to identify older mosquitoes could not accurately age grade field-collected mosquitoes using NIRS [27]. Similarly, researchers had limited success using NIRS to age grade Aedes albopictus collected as pupae from the wild and reared to adulthood under controlled, semi-field conditions [28]. In this work, we aim to ascertain the usefulness of NIRS for age grading Ae. aegypti populations by comparing the NIRS age prediction with results from parity analysis and our previously developed SCP1 transcript expression analysis [17].
2. Materials and Methods
2.1. Mosquito Colony Establishment and Maintenance
We used the UGAL strain of Ae. aegypti for initial model development. This line was developed from mosquitoes collected at the University of Georgia and has been in continuous culture for nearly 50 years. Mosquitoes were maintained in our ACL2 insectary under a 16 h/8 h day/night cycle at 27 °C and 70% RH. Larvae were reared at a density of 150 individuals per liter of water and fed ground cat chow (Purina Complete, St. Louis, MO, USA). Adults were provided with 10% sucrose ad libitum, and human blood was used for propagating the colony.
We also developed models for a colony of Ae. aegypti originally collected in Tucson, AZ, during the summer of 2016 and a second colony established from Ae. aegypti collected directly from our experimental sites in Maricopa County, AZ (Phoenix metropolitan area), during 2017. For both colonies, we collected the eggs using simple ovitraps, returned the oviposition substrate (germination paper) to the lab and maintained the eggs under moist conditions for 48 h before allowing them to completely dry. Desiccated eggs were hatched by submerging the egg sheets in deionized water for ~2 h. Hatched larvae were reared to adulthood as described above for UGAL mosquitoes. Tucson mosquitoes were reared in our insectary for four generations and Maricopa mosquitoes for three generations prior to their use in the NIRS model development.
2.2. NIRS Model Development and Sample Analysis of Laboratory-Reared Mosquitoes
Ae. aegypti mosquitoes used to develop and test the NIRS models were reared under standardized rearing conditions for varying lengths of time following adult eclosion. Non-blood-fed adult females were collected at ages ranging from 1 to 27 days after adult emergence and frozen at −80 °C, mimicking our treatment of field-collected samples. For the UGAL lab model, mosquitoes were reared under controlled conditions (27 °C; 70% RH) in the insectary until the appropriate age. For the UGAL semi-field model, lab-reared UGAL adult females were collected within one day of adult emergence and subsequently maintained outdoors in 2.5-L cages (~100/cage) during May in Tucson, AZ, under shaded conditions to represent a typical exposure in southern Arizona. Finally, our Tucson and Maricopa NIRS models were based on females from our recently established colonies (F4 for Tucson colony and F3 for Maricopa colony) reared in our insectary to known ages and frozen at −80 °C upon collection.
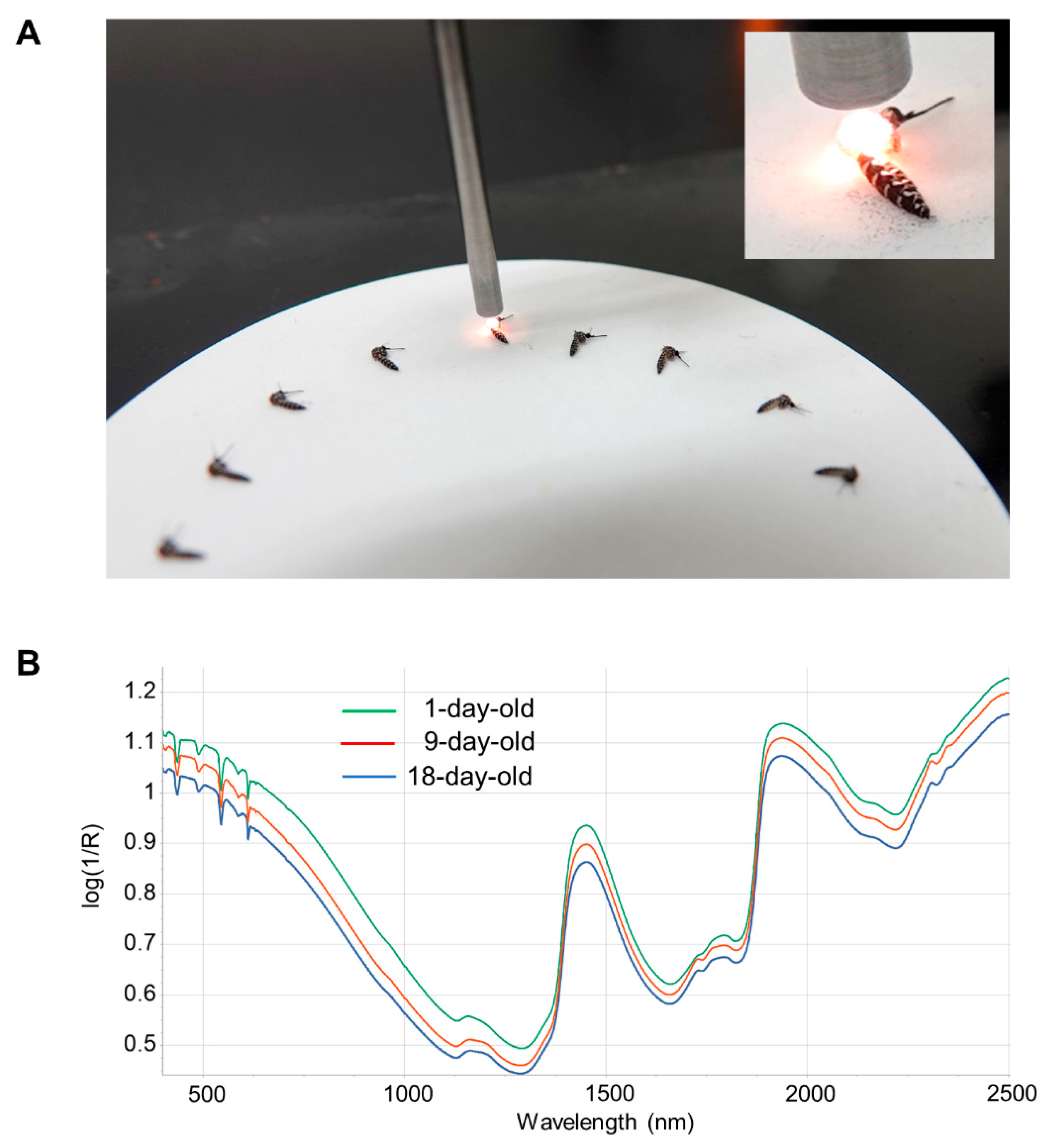
Before NIRS scanning, mosquitoes had their legs and wings removed to standardize mosquito positioning and improve the consistency of the NIRS scan (Figure 1). Frozen mosquitoes were thawed at room temperature prior to scanning. Mosquitoes were placed on a 10 cm Spectralon plate and scanned with an ASD LabSpec 4 standard resolution spectrometer (Malvern Panalytical, UK) using a bifurcated fiberoptic probe with six illumination fibers and a single collection fiber. The probe was consistently positioned 3 mm above the Spectralon plate and approximately 1.5 mm above the mosquito (Figure 1). Spectral curves from 350 to 2500 nm were collected 50 times and averaged for each mosquito. After the spectra were acquired and averaged, the ovaries were isolated for parity analysis and the head/thorax used for total RNA isolation (see below).
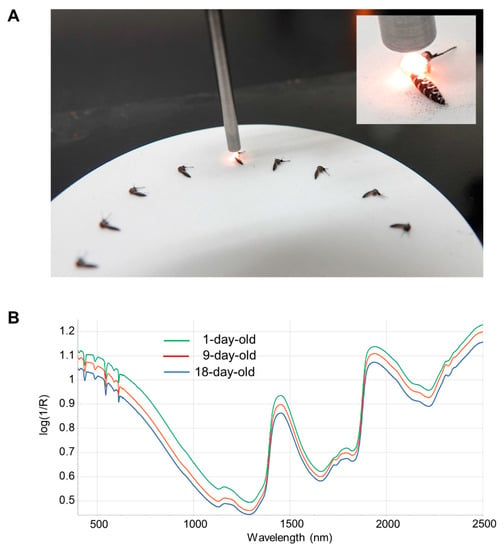
Figure 1.
NIRS scanning and typical spectra. (A). Examples of mosquito positioning under the NIRS probe. The NIRS probe was positioned 3 mm above the Spectralon plate and ~1.5 mm above the mosquito thorax. (B). Average log(1/R) spectra from 1- (green line; n = 53), 9- (red line; n = 53) and 18-day-old (blue line; n = 60) mosquitoes. Wavelength across the visible and near-infrared spectrum is indicated on the X-axis.
The acquired spectra were analyzed using the Grams IQ™ software package (Thermo Fisher Scientific, Waltham, MA, USA). Models were developed for the following four treatments: UGAL lab, UGAL semi-field, Tucson and Maricopa. Models were generated using a partial least-squares regression (PLS) in the GRAMS IQ™ spectroscopy software package (Thermo-Scientific, Waltham, MA, USA). A training set was assembled using 80% of the samples arbitrarily selected from the total sample set, with the remaining 20% used to validate the model. Multiplicative scatter correction (MSC) was performed on all models to account for the variation in spectral pathlengths and Savitzky–Golay derivative to linearize the spectra. As reported in previous studies, the visible spectrum (350–700 nm) had considerable noise and did not contribute to age identification, thus it was partially (UGAL lab model) or completely (UGAL semi-field, Tucson and Maricopa models) excluded from all models. Additionally, the UGAL lab and semi-field models were improved by excluding a central portion of the spectrum (Table 1). The final models used 10 to 14 factors and had R2 values between 0.66 and 0.79. Details of the final model parameters can be found in Table 1.

Table 1.
Summary data for the generation of the NIRS models. Detailed in this table are the number of mosquitoes utilized for the prediction (Cal.) and cross validation (Test) sets, the spectral range and band regions selected from the model, whether SG1 or multiplicative scatter correction (MSC) was used, the number of factors (Fac), R2, slope and 95% confidence interval (CI), intercept (Inter) and 95% CI, standard error of prediction (SEP) and ratio of performance deviation (RPD).
2.3. Field Collection of Aedes Aegypti Mosquitoes
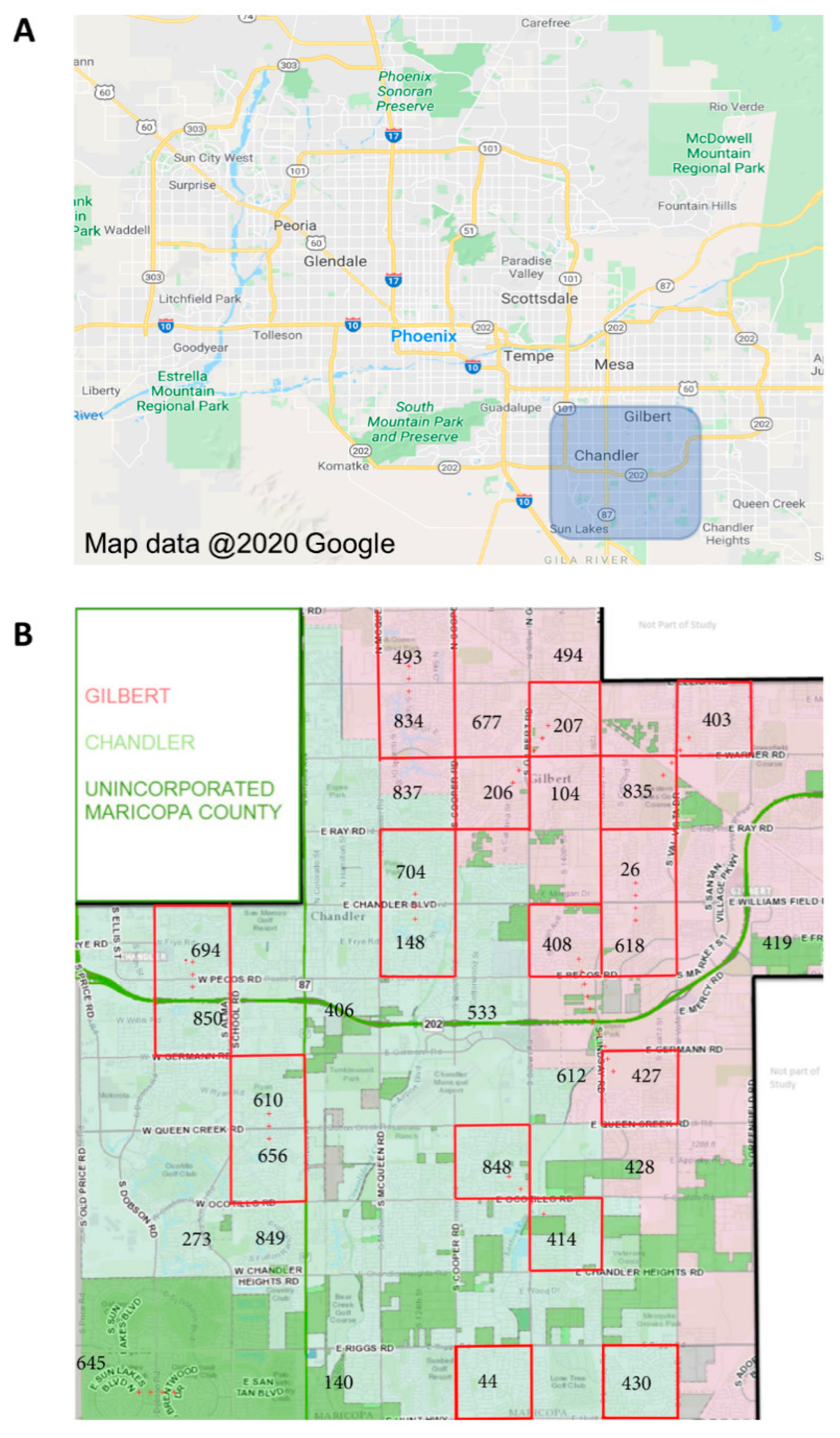
Ae. aegypti mosquitoes were collected from Gilbert, AZ, and Chandler, AZ (suburbs of Phoenix, AZ, in southern Maricopa County), during the monsoon seasons (approximately July through October) of 2017 and 2018 (Figure 2A). These mosquitoes are hereafter referred to as “field-collected mosquitoes”. A total of twenty study blocks, each one square mile, were established. Mosquito trapping sites were established at three residences in each study block, each separated by 0.5km (Figure 2B). Adult Ae. aegypti mosquitoes were collected using BG sentinel traps (3 per study block; 60 traps total) that were set out overnight (average of 18 h) once per week from July to October of each year. Live mosquitoes were sorted to species, and female Ae. aegypti mosquitoes were immediately frozen at −80 °C to inhibit RNA degradation. Frozen samples were transported on dry ice to the University of Arizona, where they were maintained at −80 °C until age-grading analysis.
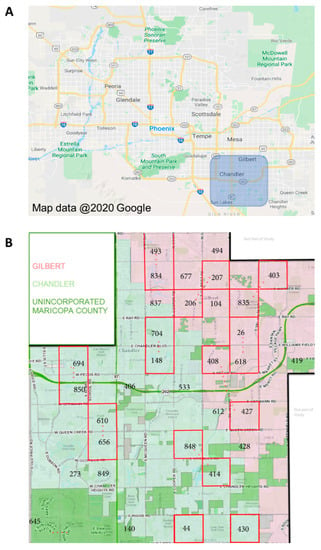
Figure 2.
Location of field collections of Ae. aegypti mosquitoes in Maricopa County, Arizona. Aedes aegypti mosquitoes were collected during the monsoon seasons (approximately July through October) in southeastern Maricopa County and included the cities of Chandler, AZ, and Gilbert, AZ (A). Mosquitoes were collected from 20 paired collection sites (B) using BG sentinel traps (3 traps distributed per site) for one night each week throughout the collection period. A total of 201 female Ae. aegypti in 2017 and 172 in 2018, representing ~15% of the total number of females collected, were used for parity analysis and SCP1/NIRS age predictions.
2.4. Parity Analysis of Field-Collected Mosquitoes
The parity status of a subset of the field-collected female Ae. aegypti was determined by examining the ovaries for the presence or absence of tracheal skeins per Dentinova [5]. Mosquitoes were arbitrarily selected for parity analysis from all trap collections based on total trap count with a maximum of 10 individuals from any single trap. Following NIRS scanning, the abdomens of field-collected mosquitoes were dissected in saline buffer (128 mM NaCl, 4.7 mM KCl and 1.9 mM CaCl2). The ovaries were gently lifted from the exposed abdominal cavity to avoid stretching of the tracheal skeins and placed onto a clean glass slide. The ovaries were allowed to air dry until they adhered to the slide and then were scored at 400× magnification with a Nikon compound microscope. The ovaries with intact tracheal skeins were considered nulliparous, and those with stretched and tangled ovarian trachea were scored as parous. Mosquitoes with residual blood in the midgut or with vitellogenin deposits in developing ovarioles were considered parous.
2.5. SCP1 Transcript Analysis of Field-Collected Mosquitoes
To validate the predicted age of NIRS scanned mosquitoes, we examined the transcript expression of a key age-associated gene, SCP1. Previously, we demonstrated that SCP1 expression decreases as Ae. aegypti mosquitoes age in a predictable manner, allowing us to classify individual, field-collected mosquitoes as non-vectors (0–5 days old), unlikely vectors (6–14 days old) and potential vectors (15+ days old) [17]. More recently, we developed a regression model for SCP1 expression to age grade these mosquitoes more accurately. We used an ordinary least-squares (OLS) regression model to relate SCP1 expression levels to log-transformed ages (in days) (R package rms; Harrell, 2016) using adult Ae. aegypti with known ages (laboratory and semi-field) [17]. A three-knot restricted cubic spline was used to model the non-linear relationship between SCP1 expression and age. This model enabled the prediction of mosquito age using transcription data in their continuous form. Following parity analysis, the heads and thorax of individual parous mosquitoes were homogenized in RLT buffer and total RNA isolated using the RNeasy Total RNA kit (Qiagen, Valencia, CA, USA). Nulliparous mosquitoes were not assayed, since our previous study demonstrated that they all scored less than 5 days old [17]. Total RNA was converted into cDNA using the High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Waltham, MA, USA). SCP1 and RPS17 (controls) transcript titers were determined using qPCR as previously described [17]. The resulting Ct values relative to RPS17 controls were used in the SCP1 linear regression model to calculate the age of individual mosquitoes.
2.6. Statistical Analysis
For mosquitoes collected in Maricopa in 2017 and 2018, a Kruskal–Wallis ANOVA followed by a Dunn’s post hoc test were used to compare NIRS age predictions from each of the four NIRS models (i.e., UGAL lab, UGAL semi-field, Tucson and Maricopa) between the parous and nulliparous mosquitoes. To evaluate the association between the age predicted by SCP1 analyses and each of the four NIRS models, we first used simple linear regression. After removing observations with Studentized residuals > |2| corresponding to unexpectedly low or high NIRS values, we used simple linear regression again to estimate the slope, intercept and their associated 95% confidence interval for analyses involving each NIRS model. Although the accuracy of SCP1 analyses remains unclear, a comparison of these slopes and intercepts provides relevant information on the relative accuracy of the NIRS models. Specifically, NIRS models with slopes close to 1 would have age predictions similar to SCP1, whereas NIRS models with lower slopes would tend to underestimate age relative to SCP1. Furthermore, NIRS models with intercepts not significantly different from 0 would have similar age predictions as SCP1 for young mosquitoes (and older ones if the slope does not differ from 1), whereas NIRS models with intercepts greater than 0 would tend to overestimate the age of young mosquitoes relative to SCP1 (and of older ones if the slope is not different from 1). For each year, we used the overlap of the 95% confidence intervals with 1 and 0 for the slope and intercept, respectively, to compare age predictions between the NIRS models and SCP1. We also used the overlap of the 95% confidence intervals associated with the slopes to compare the relative accuracy of the NIRS models.
3. Results
3.1. Generation of the NIRS Age-Grading Models
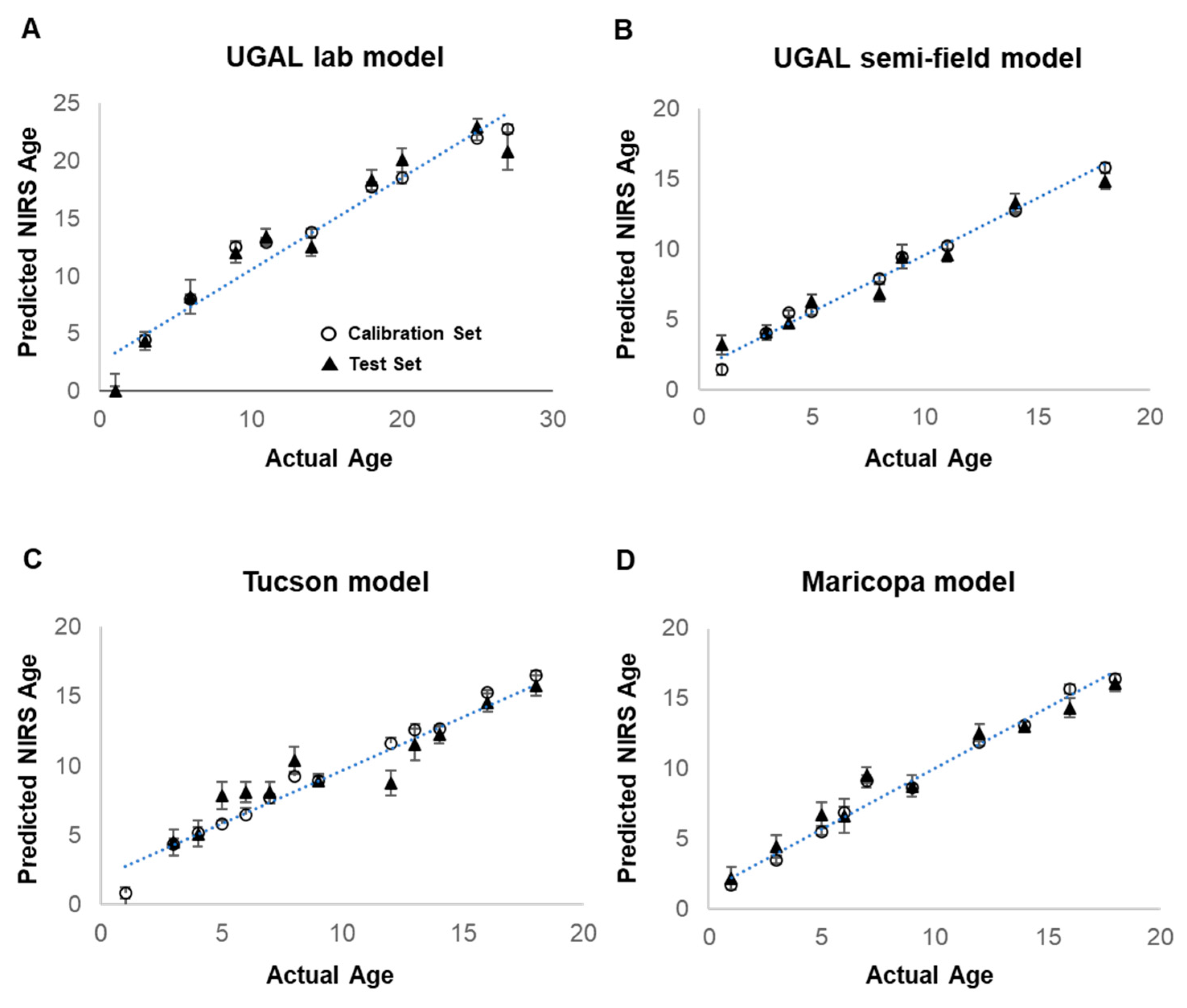
Female Ae. aegypti from laboratory-reared colonies (UGAJ, UGAL lab and UGAL semi-field models; Tucson lab colony; or Maricopa lab colony) were collected at known ages ranging from 1 to 18 days post-adult eclosion for each model (1 to 27 days for the UGAL lab model). Total sample sizes for each model ranged from 475 to 770 (Table 1; breakdown of daily n in Supplemental Table S1). All four NIRS models predict the age of laboratory-reared mosquitoes reared under controlled laboratory conditions with a reasonable degree of precision, as determined by R2 values ranging from 0.66 to 0.79 (Figure 3; Table 1). The Maricopa model has a slope and intercept that are not different from 1 and 0, respectively (based on the 95% confidence intervals), showing that it is an accurate model for estimating the age of laboratory-reared Ae. aegypti. The UGAL lab, UGAL semi-field and Tucson models have slopes significantly lower than 1, indicating that they underestimate the age of mosquitoes. Furthermore, their intercepts are significantly greater than 0, showing that they overestimate the age of newly hatched mosquitoes by about one day (Table 1).
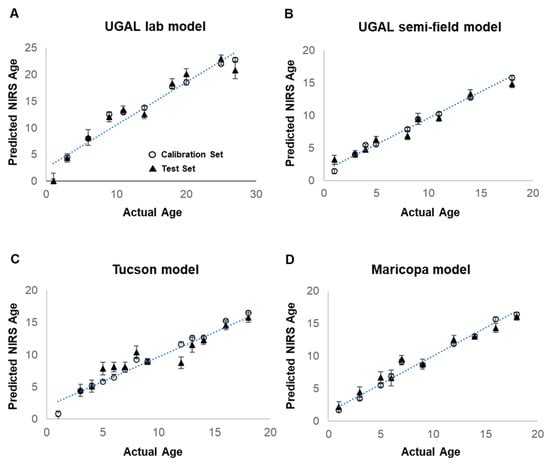
Figure 3.
Development of four models for NIRS age grading of Ae. aegypti mosquitoes. Predictive NIRS models for the age of female Ae. aegypti were generated for four different treatment groups of laboratory-reared mosquitoes. The UGAL lab model (A) was generated from 770 laboratory-reared UGAL female Ae. aegypti maintained at 27 °C and 70% RH. The UGAL semi-field model (B) also utilized the UGAL line but maintained 748 adult mosquitoes in shaded field cages in Tucson, AZ. The Tucson model (C) was generated from 540 female Ae. aegypti from an F4 line established from eggs collected in Tucson, AZ, and maintained under laboratory conditions. Finally, the Maricopa model (D) was generated from 475 female Ae. aegypti using an F3 line established from Ae. aegypti eggs collected at our actual collection site in southeastern Maricopa County and maintained under laboratory conditions. For all models, open circles represent the calibration set and show mean predicted age at each timepoint of all mosquito spectra used in the model calibration. Solid triangles represent the test set and show the mean age prediction from mosquito spectra withheld from the model calibration and used to validate the model. The number of mosquitoes utilized for the prediction and test set, the spectral range and bend regions selected from the model, the number of factors and other parameters describing each model are shown in Table 1.
The mean age predictions of the test set (solid triangle), consisting of lab-reared mosquitoes withheld from model development, are close to the calibration set used to generate the model. However, considerable variation in the predicted age of individual mosquitoes selected for validation occurs across known ages for all models (Supplemental Figure S1). The final models have ratio of performance deviation (RPD) values of 1.70 to 2.18, with the Maricopa model having the most successful calibration. RPD values are the ratio of the standard deviation of the test set and the standard error of prediction (SEP) [29], with RPD values above 1.5 being suitable for high/low classification and above 2.0 for course quantitative prediction [30].
3.2. Parity Analysis of Field-Collected Aedes Aegypti Mosquitoes and a Comparison to NIRS Models
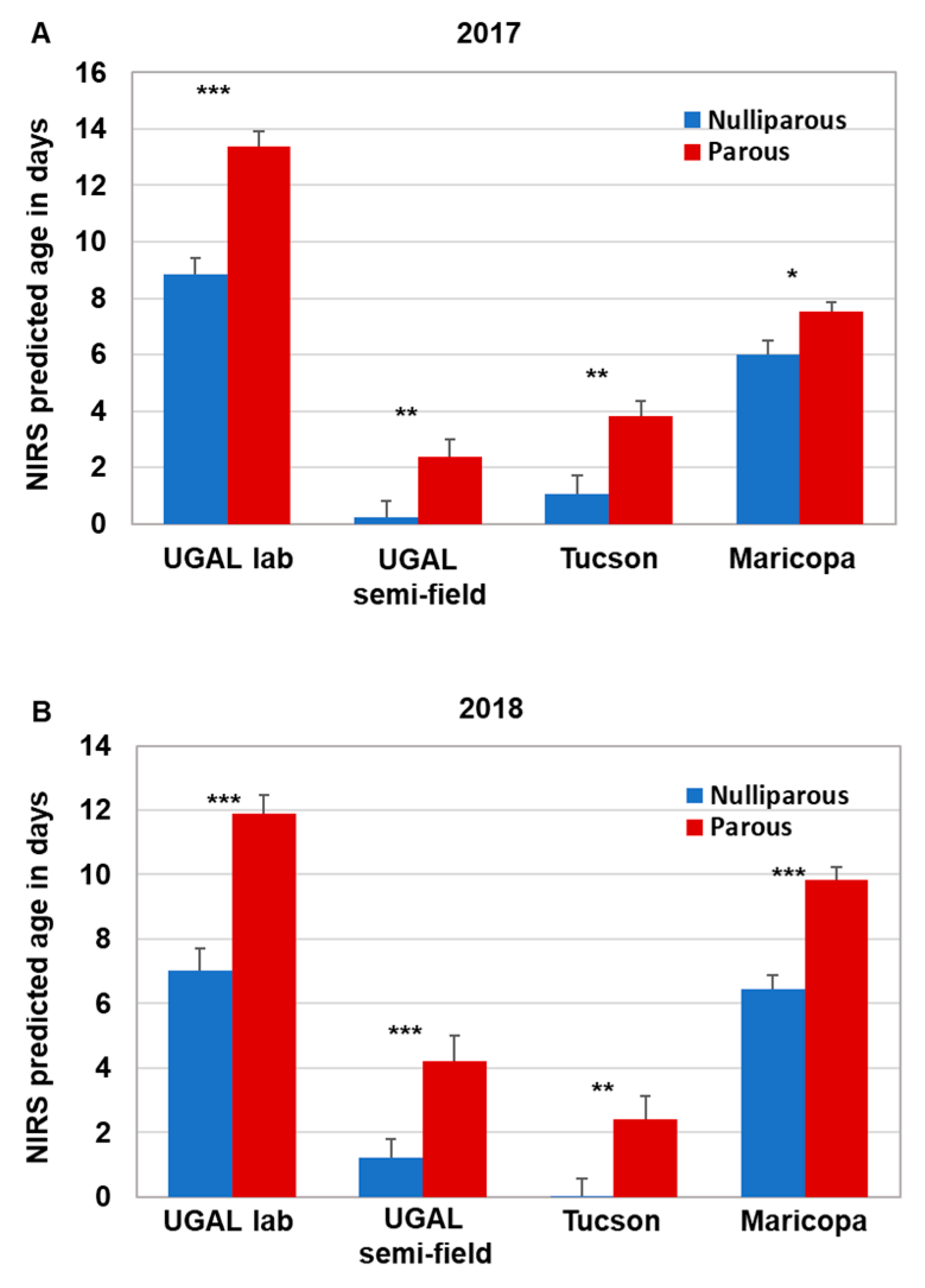
Parity analysis was conducted on a subset of the field-collected mosquitoes collected monthly during the 2017 (n = 201) and 2018 (n = 172) monsoon season in Gilbert and Chandler, AZ. This subset represents approximately 15% of the total number of female Ae. aegypti collected. Overall parity levels ranged from 49 to 82% during the monsoon seasons of 2017 and 2018. Our previous work using the SCP1 transcript to age grade field-collected Ae. aegypti suggested that the majority of nulliparous mosquitoes were less than 5 days old, although the age of parous mosquitoes varied widely [17]. Thus, we compared the parity status of individual field-collected mosquitoes with the NIRS age prediction across the four models to determine whether the NIRS age predictions conformed to parity status (i.e., nulliparous mosquitoes are young, and parous mosquitoes are older; Supplemental Data S1). Indeed, the average age of field-collected mosquitoes scored as nulliparous was significantly less than parous mosquitoes in both 2017 and 2018 across all four models (Figure 4). The actual predicted ages differed significantly between most models, with the exception of the 2017 UGAL semi-field/Tucson and 2018 UGAL lab/Maricopa models, due to variation in model construction. However, the significantly lower age of nulliparous mosquitoes across all models supports the idea that each model could be useful to assess relative changes in the population age structure over time.
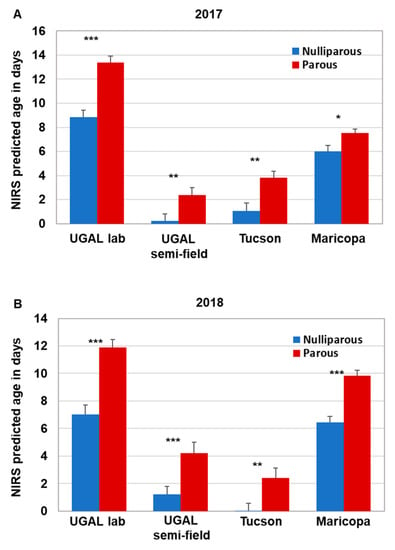
Figure 4.
Parity status of field-collected mosquitoes relative to NIRS age predictions. Female Ae. aegypti from our field-collected samples in 2017 (A) and 2018 (B) were separated based on parity status. NIRS spectra for field-collected nulliparous and parous mosquitoes were acquired, and NIRS age predictions for the four models were determined. As expected, field-collected parous mosquitoes were scored significantly older than nulliparous mosquitoes by each of the NIRS models. Significance for each pairing was determined using the Kruskal–Wallis ANOVA followed by a Dunn’s post hoc test (significance is only shown between parous and nulliparous comparisons * = p < 0.05; ** = p < 0.01, *** p < 0.0001).
3.3. SCP1 Analysis of Field-Collected Aedes Aegypti Mosquitoes and Their Comparison to NIRS Models
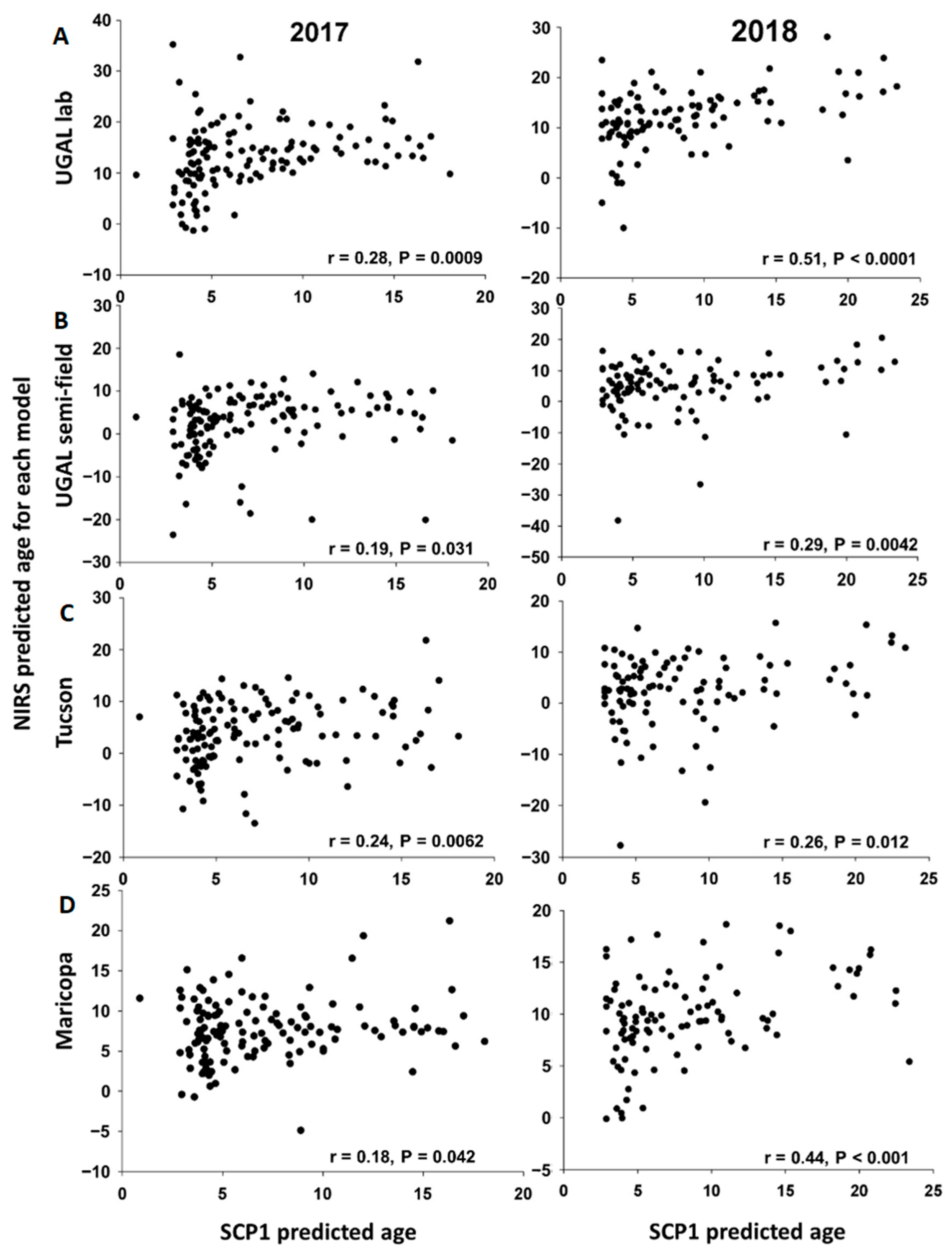
SCP1 expression levels were determined for all parous mosquitoes from the subset used for parity analysis (n = 236) and used to estimate the age of individual field-collected mosquitoes. The scatterplots for the associations between age predicted by SCP1 analyses and the four NIRS models are shown in Figure 5. For each model and year, some observations have unexpectedly low or high NIRS values, as revealed by Studentized residuals > |2|. After removing these extreme values, we estimated the slope and intercept of the association between age predicted by SCP1 and each of the four NIRS models (Table 2). With the exception of the Maricopa model in 2017, all slopes are significantly greater than 0, indicating a positive linear association between ages estimated with SCP1 analyses and the NIRS models. With the exception of the Maricopa model in 2017, the slopes do not differ significantly from each other, indicating that the NIRS models provide comparable estimates of mosquito age relative to SCP1. However, for all NIRS models, the slope is significantly lower than 1, indicating that the NIRS models tend to underestimate the age of older mosquitoes relative to the age estimated with SCP1 analyses (Table 2). Furthermore, the intercept is significantly greater than 0 for some models (notably the UGAL lab and Maricopa models), indicating that such models overestimate the age of young mosquitoes relative to results from SCP1 analyses (Table 2).
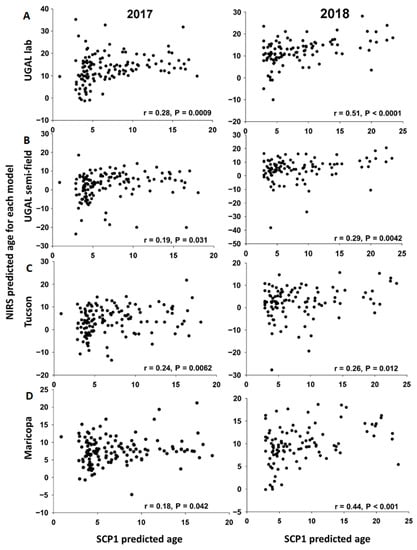
Figure 5.
Scatterplots comparing the age of individual field-collected female Ae. aegypti predicted with SCP1 or the four NIRS models. For 2017 and 2018, the scatterplots show the relationship of individual age predictions based on SCP1 (X-axis) and each of the NIRS models (Y-axis). (A). UGAL lab, (B). UGAL semi-field, (C). Tucson and (D). Maricopa models.

Table 2.
Linear associations between age predicted by SCP1 analyses and the four NIRS models in 2017 and 2018. Data points with Studentized residuals > |2| were removed before estimating intercepts, slopes and their associated 95% confidence intervals (see Figure 5). N is the number of observations used to fit simple linear regression models. 1 For each year, the slopes with different letter had non-overlapping 95% confidence intervals and are considered statistically different (p < 0.05).
4. Discussion
NIRS has considerable promise for age grading large numbers of mosquitoes quickly, cost effectively and non-destructively [22]. While this approach has proven effective for a number of different mosquito species under controlled laboratory conditions, it has provided mixed results for field-collected mosquitoes [27,28]. In this study, we used results from two well-established age-grading techniques, parity analysis and SCP1 transcript expression, to assess how four different NIRS models performed in evaluating the age of field-collected Ae. aegypti female mosquitoes. The four models generated from independent mosquito colonies reared under specific adult conditions were relatively precise (i.e., high R2 values) in predicting the average age of groups of mosquitoes reared under the same conditions, although three of the four models slightly overestimated the age of young mosquitoes (i.e., intercept > 0) and tended to underestimate the age of older mosquitoes (i.e., slope < 1) (Table 1). The effectiveness of these models is not surprising, as the mosquitoes used to train and validate the models were reared under identical conditions. Yet, even under these homogeneous rearing conditions, considerable variation in the predicted age of individual mosquitoes was observed (Supplemental Figure S1). We did find that NIRS was generally suitable for age grading field-collected mosquitoes, as each of the four models consistently graded parous mosquitoes as significantly older than nulliparous mosquitoes, and a significant positive association occurred between SCP1 age predictions and NIRS age predictions for seven of the eight analyses performed over two years. Nevertheless, the accuracy of the models differed significantly, as shown by the variation among models in the predicted age of nulliparous and parous mosquitoes. Furthermore, relative to age determined from SCP1 analyses, all NIRS models had a tendency to underestimate the age of older mosquitoes, and some models significantly overestimated the age of young mosquitoes (Table 2). This is likely due to the fact that field-collected mosquitoes developed under different environmental conditions or potentially expressed genetically based differences in their cuticular structure relative to the mosquitoes used to train the models. It is important to note that this limitation is inherent in any age-grading technique, including SCP1 transcript analysis, which is based on laboratory-reared mosquitoes for their baseline data.
While NIRS may not be ideal in predicting the age of individual field-collected mosquitoes, we suggest that it is valuable for monitoring the overall age structure of an Ae. aegypti population as it changes over time. One of the greatest strengths of NIRS is the ability to rapidly screen large numbers of mosquitoes cost effectively, after the initial expense of the NIRS equipment [22]. Parity and transcript analysis are technically challenging, time consuming and expensive, meaning that only a subset of collected mosquitoes can be screened. In contrast, it is entirely feasible for all collected Ae. aegypti to be screened with NIRS, since the technical requirements and time required to collect the spectra are minimal. Based on our comparisons between NIRS age predictions and parity status, the difference in age was remarkably robust throughout the study. Regardless of the model or year, nulliparous mosquitoes were consistently aged 3–4 days younger than parous mosquitoes. However, the estimated age of nulliparous and parous mosquitoes varied across NIRS models, with the UGAL lab and Maricopa models generally indicating older ages than the UGAL semi-field and Tucson models. Based on previous results from SCP1 analyses indicating that most field-collected nulliparous mosquitoes were less than 5 day old [15], it appears that the UGAL lab model overestimated the age of mosquitoes. This suggests that NIRS could be used to assess relative changes, including how the population age structure fluctuates throughout a typical transmission season or how effectively control measures impact the local mosquito age structure following treatment. Compared with other age-grading techniques, the variability of these data would be mitigated by the larger sample size, assuming that sufficient numbers of mosquitoes can be collected. Equally important, the non-destructive nature of NIRS allows for validation with any current or future age-grading techniques.
Of the variations found in field-collected mosquitoes discussed above, perhaps the easiest to account for is genetic diversity. Of our four models, two of them, UGAL lab and UGAL semi-field, were generated using long-established lab colonies of Ae. aegypti. The other two used recently colonized mosquitoes originally collected directly from the collection site (Maricopa) or from a population in a similar environment approximately 100 miles away (Tucson). Our assumption was that the models generated from recently collected mosquitoes directly from the study site would more accurately represent the genetic diversity of the local mosquito population. When comparing NIRS to parity, we were surprised to find a high level of consistency in the precision of the models as described above, although considerable bias was observed, particularly in the UGAL semi-field and Tucson models, which predicted both nulliparous and parous mosquitoes as likely too young for their physiological status (i.e., <1 d for nulliparous and <4 d for parous). Furthermore, when we compared NIRS and SCP1 age predictions of individual mosquitoes, we again did not discern noticeable differences in bias (i.e., difference among slopes) between the Maricopa model and others, and two models (UGAL lab and Maricopa) notably overestimated the age of young mosquitoes (i.e., had intercepts > 0). The fact that the Maricopa and Tucson models did not perform better than the UGAL lab and UGAL semi-field models does not support the hypothesis that genetic similarity could improve the accuracy of the NIRS models. Other variables, such as nutrient availability and environmental variation, are more difficult to control for, since models cannot be developed to account for all of these potential variations during mosquito development, but again, the NIRS approach has the advantage of potentially large sample sizes to mitigate these variations and their impact on the accuracy of predictions. Furthermore, advances in machine learning have the potential to improve these models over time. For example, Milali et al. have utilized artificial neural networks to improve the accuracy of age grading mosquito populations, and we are exploring these approaches for future studies [31,32].
Of the two additional methods we used to estimate the age of field-collected mosquitoes compared to the NIRS predictions, parity is the most accurate. A person skilled in ovary dissections can correctly assess parity nearly 90% of the time, although care must be taken not to stretch the ovaries and unravel some or all the skeins on the ovary [8]. In contrast, both the SCP1 and NIRS age-grading techniques are known to have varying degrees of bias and random error. Previously, we demonstrated that SCP1 age grading could correctly categorize mosquitos into one of three groups (<5 days, 6–14 days and >15 days) ~90% of the time [17]. However, in our current study, a number of parous mosquitoes were scored as less than 5 days old (Figure 5) using the SCP1 technique, which, although possible, is unlikely taking into account the time needed to find a mate, acquire a bloodmeal and complete a reproductive cycle. For NIRS, our work and others’ have consistently found that the oldest mosquitoes are typically scored younger than their actual age [33]. Thus, a variation in the accuracy of both the SCP1 and NIRS age predictions likely accounts for the discordance observed when comparing these two approaches. In all cases, however, the SCP1 age predictions are significantly positively associated with the NIRS age predictions, again suggesting that NIRS can be useful to characterize changes in age structure at the population levels if sufficient samples are available.
In summary, we developed a variety of NIRS models to attempt to age grade Ae. aegypti mosquitoes collected from the field. NIRS predicted the age of parous mosquitoes as significantly older than nulliparous mosquitoes, as would be expected for mosquitoes that have already mated and completed at least one reproductive cycle. Furthermore, when comparing NIRS age predictions with predictions based on the expression of the age-associated gene SCP1, we observed a significant positive association for all four models. However, we did observe bias in the age predictions of individual field-collected Ae. aegypti for all NIRS models. Thus, while accurate age predictions of individual mosquitoes may not currently be possible using these NIRS models, or for that matter other current age-grading techniques [8], the ability of NIRS to track changes in the population age structure of mosquitoes over time may be a valuable tool in identifying increases in the proportion of older mosquitoes that are more likely to transmit arboviruses.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/insects13040360/s1, Table S1: Number of Aedes aegypti NIRS spectra used to calibrate and test each model at each individual day; Figure S1: Variation in NIRS age predictions in mosquitoes of known age; Data S1: NIRS age predictions, SCP1 age predictions and parity status of individual mosquitoes.
Author Contributions
Conceptualization: K.W., K.E., D.H.G. and M.A.R.; Formal analysis: K.E., Y.C. and M.A.R.; Funding acquisition: K.W., K.E., D.H.G. and M.A.R.; Investigation: T.J., M.C., J.A., D.W., S.L., S.N., M.B., V.M.G., K.W., K.E., D.H.G., Y.C. and M.A.R.; Methodology: K.W., K.E., Y.C. and M.A.R.; Project administration: T.J., K.W., K.E., D.H.G. and M.A.R.; Supervision: T.J., K.W., K.E., D.H.G. and M.A.R.; Validation: T.J., K.W., K.E., Y.C. and M.A.R.; Visualization: T.J., M.C., M.B., K.W., K.E., Y.C. and M.A.R.; Writing—original draft: M.A.R.; Writing—review and editing: T.J., M.C., J.A., D.W., S.L., S.N., M.B., V.M.G., K.W., K.E., D.H.G., Y.C. and M.A.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Centers for Disease Control and Prevention contract BAA 2017-N-18041.
Institutional Review Board Statement
Anonymized human blood was obtained from the American Red Cross under IBC Approval ##2020-014.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article and supplementary material.
Acknowledgments
We would like to thank Jenet Soto-Shoumaker for her work rearing the mosquitoes needed to generate the age-grading models.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bowman, L.R.; Runge-Ranzinger, S.; McCall, P. Assessing the relationship between vector indices and dengue transmission: A systematic review of the evidence. PLoS Negl. Trop. Dis. 2014, 8, e2848. [Google Scholar] [CrossRef] [PubMed]
- Louis, V.R.; Phalkey, R.; Horstick, O.; Ratanawong, P.; Wilder-Smith, A.; Tozan, Y.; Dambach, P. Modeling tools for dengue risk mapping—A systematic review. Int. J. Health Geogr. 2014, 13, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dye, C. The analysis of parasite transmission by bloodsucking insects. Annu. Rev. Entomol. 1992, 37, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Dye, C. Epidemiological significance of vector–parasite interactions. Parasitology 1990, 101, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Dentinova, T. Age-Grouping Methods in Diptera of Medical Importance; World Health Organization: Geneva, Switzerland, 1962. [Google Scholar]
- Polovodova, V. The determination of the physiological age of female Anopheles by the number of gonotrophic cycles completed. Medskaya. Parazit. 1949, 18, 352–355. [Google Scholar]
- Hoc, T.; Charlwood, J. Age determination of Aedes cantans using the ovarian oil injection technique. Med. Vet. Entomol. 1990, 4, 227–233. [Google Scholar] [CrossRef]
- Hugo, L.E.; Quick-Miles, S.; Kay, B.; Ryan, P. Evaluations of mosquito age grading techniques based on morphological changes. J. Med. Entomol. 2014, 45, 353–369. [Google Scholar] [CrossRef] [Green Version]
- Anagonou, R.; Agossa, F.; Azondékon, R.; Agbogan, M.; Oké-Agbo, F.; Gnanguenon, V.; Badirou, K.; Agbanrin-Youssouf, R.; Attolou, R.; Padonou, G.G. Application of Polovodova’s method for the determination of physiological age and relationship between the level of parity and infectivity of Plasmodium falciparum in Anopheles gambiae s.s, south-eastern Benin. Parasites Vectors 2015, 8, 117. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Mulla, M.; March, R.; Chaney, J. Cuticular hydrocarbon patterns in Culex quinquefasciatus as influenced by age, sex, and geography. Bull. Soc. Vector Ecol. 1990, 15, 129–139. [Google Scholar]
- Desena, M.; Clark, J.; Edman, J.; Symington, S.; Scott, T.; Clark, G.; Peters, T. Potential for aging female Aedes aegypti (Diptera: Culicidae) by gas chromatographic analysis of cuticular hydrocarbons, including a field evaluation. J. Med. Entomol. 1999, 36, 811–823. [Google Scholar] [CrossRef]
- Desena, M.; Edman, J.; Clark, J.; Symington, S.; Scott, T. Aedes aegypti (Diptera: Culicidae) age determination by cuticular hydrocarbon analysis of female legs. J. Med. Entomol. 1999, 36, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Brei, B.; Edman, J.D.; Gerade, B.; Clark, J.M. Relative abundance of two cuticular hydrocarbons indicates whether a mosquito is old enough to transmit malaria parasites. J. Med. Entomol. 2004, 41, 807–809. [Google Scholar] [CrossRef] [PubMed]
- Cook, P.E.; Hugo, L.E.; Iturbe-Ormaetxe, I.; Williams, C.R.; Chenoweth, S.F.; Ritchie, S.A.; Ryan, P.A.; Kay, B.H.; Blows, M.W.; O’Neill, S.L. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc. Natl. Acad. Sci. USA 2006, 103, 18060–18065. [Google Scholar] [CrossRef] [Green Version]
- Cook, P.; Sinkins, S. Transcriptional profiling of Anopheles gambiae mosquitoes for adult age estimation. Insect Mol. Biol. 2010, 19, 745–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.-H.; Marinotti, O.; Zhong, D.; James, A.A.; Walker, E.; Guda, T.; Kweka, E.J.; Githure, J.; Yan, G. Gene expression-based biomarkers for Anopheles gambiae age grading. PLoS ONE 2013, 8, e69439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joy, T.K.; Gutierrez, E.H.J.; Ernst, K.; Walker, K.R.; Carriere, Y.; Torabi, M.; Riehle, M.A. Aging field collected Aedes aegypti to determine their capacity for dengue transmission in the southwestern United States. PLoS ONE 2012, 7, e46946. [Google Scholar] [CrossRef]
- Ernst, K.C.; Walker, K.R.; Reyes-Castro, P.; Joy, T.K.; Castro-Luque, A.L.; Diaz-Caravantes, R.E.; Gameros, M.; Haenchen, S.; Hayden, M.H.; Monaghan, A. Aedes aegypti (Diptera: Culicidae) longevity and differential emergence of dengue fever in two cities in Sonora, Mexico. J. Med. Entomol. 2017, 54, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Weeraratne, T.C.; Karunaratne, S.; Reimer, L.; de Silva, W.; Wondji, C.S. Use of transcriptional age grading technique to determine the chronological age of Sri Lankan Aedes aegypti and Aedes albopictus females. Parasites Vectors 2021, 14, 493. [Google Scholar] [CrossRef]
- Türker-Kaya, S.; Huck, C.W. A review of mid-infrared and near-infrared imaging: Principles, concepts and applications in plant tissue analysis. Molecules 2017, 22, 168. [Google Scholar] [CrossRef] [Green Version]
- Goh, B.; Ching, K.; Soares Magalhães, R.J.; Ciocchetta, S.; Edstein, M.D.; Maciel-de-Freitas, R.; Sikulu-Lord, M.T. The application of spectroscopy techniques for diagnosis of malaria parasites and arboviruses and surveillance of mosquito vectors: A systematic review and critical appraisal of evidence. PLoS Negl. Trop. Dis. 2021, 15, e0009218. [Google Scholar] [CrossRef]
- Mayagaya, V.S.; Michel, K.; Benedict, M.Q.; Killeen, G.F.; Wirtz, R.A.; Ferguson, H.M.; Dowell, F.E. Non-destructive determination of age and species of Anopheles gambiae sl using near-infrared spectroscopy. Am. J. Trop. Med. Hyg. 2009, 81, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Sikulu, M.; Killeen, G.F.; Hugo, L.E.; Ryan, P.A.; Dowell, K.M.; Wirtz, R.A.; Moore, S.J.; Dowell, F.E. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasites Vectors 2010, 3, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sikulu-Lord, M.T.; Devine, G.J.; Hugo, L.E.; Dowell, F.E. First report on the application of near-infrared spectroscopy to predict the age of Aedes albopictus Skuse. Sci. Rep. 2018, 8, 9590. [Google Scholar] [CrossRef] [PubMed]
- Sikulu-Lord, M.T.; Milali, M.P.; Henry, M.; Wirtz, R.A.; Hugo, L.E.; Dowell, F.E.; Devine, G.J. Near-infrared spectroscopy, a rapid method for predicting the age of male and female wild-type and Wolbachia infected Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0005040. [Google Scholar] [CrossRef] [PubMed]
- Liebman, K.; Swamidoss, I.; Vizcaino, L.; Lenhart, A.; Dowell, F.; Wirtz, R. The influence of diet on the use of near-infrared spectroscopy to determine the age of female Aedes aegypti mosquitoes. Am. J. Trop. Med. Hyg. 2015, 92, 1070–1075. [Google Scholar] [CrossRef] [Green Version]
- Krajacich, B.J.; Meyers, J.I.; Alout, H.; Dabiré, R.K.; Dowell, F.E.; Foy, B.D. Analysis of near infrared spectra for age-grading of wild populations of Anopheles gambiae. Parasites Vectors 2017, 10, 552. [Google Scholar] [CrossRef]
- Ong, O.T.; Kho, E.A.; Esperança, P.M.; Freebairn, C.; Dowell, F.E.; Devine, G.J.; Churcher, T.S. Ability of near-infrared spectroscopy and chemometrics to predict the age of mosquitoes reared under different conditions. Parasites Vectors 2020, 13, 160. [Google Scholar] [CrossRef]
- Williams, P.; Norris, K.H.; American Association of Cereal Chemists. Near-Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemists: St. Paul, MN, USA, 2001. [Google Scholar]
- Saeys, W.; Mouazen, A.M.; Ramon, H. Potential for onsite and online analysis of pig manure using visible and near infrared reflectance spectroscopy. Biosyst. Eng. 2005, 91, 393–402. [Google Scholar] [CrossRef]
- Milali, M.P.; Kiware, S.S.; Govella, N.J.; Okumu, F.; Bansal, N.; Bozdag, S.; Charlwood, J.D.; Maia, M.F.; Ogoma, S.B.; Dowell, F.E. An autoencoder and artificial neural network-based method to estimate parity status of wild mosquitoes from near-infrared spectra. PLoS ONE 2020, 15, e0234557. [Google Scholar] [CrossRef]
- Milali, M.P.; Sikulu-Lord, M.T.; Kiware, S.S.; Dowell, F.E.; Corliss, G.F.; Povinelli, R.J. Age grading An. gambiae and An. arabiensis using near infrared spectra and artificial neural networks. PLoS ONE 2019, 14, e0209451. [Google Scholar] [CrossRef] [Green Version]
- Lambert, B.; Sikulu-Lord, M.T.; Mayagaya, V.S.; Devine, G.; Dowell, F.; Churcher, T.S. Monitoring the age of mosquito populations using near-infrared spectroscopy. Sci. Rep. 2018, 8, 5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).